Abstract
Acute lymphoblastic leukemia (ALL) is the most common cancer in children. Thirdhand smoke (THS) is the residual tobacco contamination that remains after the smoke clears. We investigated the effects of THS exposure in utero and during early life in a transgenic Cdkn2a knockout mouse model that is vulnerable to the development of leukemia/lymphoma. Female mice, and their offspring, were exposed from the first day of pregnancy to weaning. Plasma cytokines, body weight and hematologic parameters were measured in the offspring. To investigate THS exposure effects on the development of leukemia/lymphoma, bone marrow was collected from control and THS-exposed mice and transplanted into bone-marrow-ablated recipient mice, which were followed for tumor development for one year. We found that in utero and early life THS exposure caused significant changes in plasma cytokine concentrations and in immune cell populations; changes appeared more pronounced in male mice. Spleen and bone marrow B-cell populations were significantly lower in THS-exposed mice. We furthermore observed that THS exposure increased the leukemia/lymphoma-free survival in bone marrow transplantation recipient mice, potentially caused by THS-induced B cell toxicity. A trend towards increased solid tumors in irradiated mice reconstituted with THS exposed bone marrow stimulates the hypothesis that the immunosuppressive effects of in utero and early-life THS exposure might contribute to carcinogenesis by lowering the host defense to other toxic exposures. Our study adds to expanding evidence that THS exposure alters the immune system and that in utero and early life developmental periods represent vulnerable windows of susceptibility for these effects.
Keywords: thirdhand smoke, leukemia, lymphoma, immune system
Introduction
Acute lymphoblastic leukemia (ALL) is the most common type of childhood malignancy, with more than 50,000 children diagnosed worldwide yearly, and 80% of these leukemias are B-lymphoblastic. In the U.S. this disease has been increasing about 1% per year for decades [1]. Although survival with childhood ALL has improved considerably in the last two decades due to more effective treatments, survivors face a life long battle with various medical conditions (e.g. hormonal, cardiovascular, and pulmonary abnormalities, osteoporosis, secondary cancer) and neuropsychological problems (e.g. neurocognitive impairment, anxiety, depression, post-traumatic distress) as a result of treatments[2, 3]. Thus, deciphering the etiology of pediatric leukemia remains an important goal.
The development of childhood ALL involves genetic and epigenetic processes, but the connection between specific environmental exposures and acquired tumor genetic and epigenetic changes in leukemia cells is inherently difficult to study in human populations. Incidence of childhood leukemia has steadily increased in the last half century, particularly among Latinos[1, 4]. This increase is mainly accounted for by one leukemia subtype – common CD10+, CD19+ childhood pre-B cell ALL. The causes for this increase have been hypothesized to include exposures to chemicals (e.g. tobacco smoke, pesticides), dietary factors, fetal growth rates, and patterns of infection [5, 6]. Here, for the first time, we assessed the effect of exposure to “thirdhand smoke” (THS), the residue left on surfaces after smoking, on the development of ALL.
Approximately 1.1 billion people are current smokers worldwide and this figure is expected to rise to over 1.6 billion by the year 2025 [7]. In many places, smoke-free laws ban smoking in public places; however, many children continue to be exposed to environmental tobacco smoke at home. Most studies on health effects of tobacco exposure in young children concentrate on passive smoking such as “secondhand smoke” (SHS), which is the aerosol present while smoking is taking place. Exposure to SHS by non-smokers, primarily through inhalation, affects immune cell numbers, levels of cytokines and is associated with an increased risk of respiratory tract infections and cancer [8]. In recent years, attention has been brought to potential adverse health effects of pollutants that remain on surfaces and in dust after tobacco has been smoked (referred to as THS). These pollutants can be re-emitted into the gas-phase, or react with other compounds in the environment to form secondary pollutants [9-11]. Evidence supports the widespread presence of THS in indoor environments, including in the U.S. [12, 13]. THS poses a potential health risk for children who tend to spend more time indoors than adults and have age-specific behaviors that bring them in closer contact with surfaces and dust. Moreover, their higher respiration rate relative to body size, larger exposed surface area to volume ratio, thinner skin, less mature immunologic systems, and lower metabolic capacity could lead to increased THS exposure levels in children compared to adults. Similar to SHS, THS exposed mice showed alterations in cytokine levels and immune cell numbers [14-16]. These prior studies focused on early-life exposure windows leaving the effects of THS exposure during the perinatal period unknown.
Abnormalities in Cdkn2a are observed in approximately one third of childhood ALL [17]. In this study we used our established Cdkn2a mouse model of childhood ALL[18] to investigate THS exposure effects during pregnancy and early life on the immune system of these leukemia-predisposed animals.
Material and Methods
Mice exposed to thirdhand smoke (THS)
All animal experimental protocols were approved by the University of California at San Francisco, Institutional Animal Care and Use Committee (UCSF-IACUC). The animal experiments were all performed in a specific pathogen free facility at the University of California, San Francisco (UCSF) and carried out in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. B-cell lymphoma development was previously described in Cdkn2a null mice [19], including the mice used in the present study [18]. In brief, our mice were derived from FVB/N Cdkn2atm2Brn mice (MGI: 2384163) containing a floxed allele of Cdkn2a crossed with FVB/n-Tg(EIIa-cre)c5379mgd/J mice (MGI:2137691) to generate Cdkn2a null animals on a pure FVB/N background. Terry cloth substrates were used as surrogates for indoor surfaces, onto which fresh SHS gases could adsorb and SHS particles deposit as previously described [20]. Briefly, clean 100% cotton terrycloth samples were repeatedly exposed to SHS in a 6-m3 stainless steel chamber for a total of 234 hrs over 1,019 days. A total of 2795 mg of total particulate material was introduced into the steel chamber, which is equivalent to the smoke from 200-350 cigarettes over 2 years and 9 months. If all THS mass deposited on the surfaces of the exposure chamber, the maximum loading of THS on each gram of cotton cloth would be 238 μg. The THS cloth was removed from the smoke, vacuum-packed in Mylar film and stored at −20°C until use. THS compounds in terry cloth substrates were analyzed following the procedures described in our previous study and the same batch of cloth was used in this study[20]. Briefly, 0.85 g THS-laden and unexposed (control) cotton cloth samples were immersed in 10 ml Dulbecco’s Modified Eagle’s Medium (DMEM). Twelve targeted THS compounds were analyzed using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Nicotine was detected at 30,600 ng/g in THS cloth compared to 14.9 ng/g in control cloth. Other THS constituents detected in THS cloth, but not control cloth included myosamine (2440 ng/g), N-formylnornicotine (998 ng/g) and cotinine (486 ng/g) were detected in THS cloth, but not in control cloth. The levels of polycyclic aromatic hydrocarbons were measured by gas chromatography coupled with mass spectrometry (GC/MS, Varian 4000, CA) after 2.5 x 5 cm specimens of the THS-laden and unexposed (control) cotton cloth samples were extracted with dichloromethane (DCM). Naphthalene (27 ng/g), 2-methyl naphthalene (27 ng/g) and pyrene (24 ng/g) were most abundant among the twelve detected PAHs in THS-laden cloth samples. PAH levels in control cloth were all below the level of quantitation.
The pregnant female Cdkn2a−/− mice (FVB/N strain) were divided into two groups: control group (30 mice) and experimental group (32 mice), one female mouse per cage. The experimental group was exposed to one THS-exposed terry cloth swatch (5x5cm2) and the control group was exposed to one sham cloth (5x5cm2) from the first day of pregnancy till the pups were weaned. THS-exposed cloth or sham cloth was added to the standard bedding in the cages and were replaced once a week. Body weight of pups was measured at age of three weeks.
Measurement of cytokine levels in mouse plasma samples
One male and one female two-day-old pups per independent litter were selected and euthanized by CO2 for 5 minutes followed by decapitation. 60-70 μL of blood was collected in a K2EDTA pediatric blood vial from the selected pup. Blood samples were centrifuged at 14,000 rpm for five minutes to collect the supernatant plasma sample (about 30 μL/sample) and saved in 1.5 mL Dnase/Rnase-free Eppendorf tubes at −80°C prior to analysis. The Luminex assay of cytokines (Table S1) was performed following the protocol of the cytokine assay kits (Bio-plex Pro™ Mouse Cytokine standard 23-Plex, Group I, Cat. M600009RDPD; Bio-plex Pro™ Mouse Cytokine standard 9-Plex, Group II, Cat. MD000000EL) purchased from Bio-rad Laboratory (Hercules, CA). Every step was performed as described in the Bio-rad protocol except for a reduction in reagent volumes (10 μL of bead mixture, 10 μL of detection Antibody cocktail) and lower sample volumes (10 μL of 4-fold diluted sample plasma) with the help of the Curiox DropArray wall-less microplate and Curiox plate washer (Curiox Biosystem, San Carlos, CA). The developed samples were suspended in 55 μL of sheath fluid and the fluorescent intensity (FI) of each sample was acquired by Bio-plex 200 plate reader system (Bio-Rad Laboratories, Inc.). The mean values of FI were calculated by comparing to the standard curve of each cytokine to determine the cytokine levels in each sample.
Bone marrow flow cytometry and transplantation
When pups were five-weeks-old, control and THS exposed mice (details noted in Table S3) were selected as the bone marrow transplantation donors. Following inhalant isoflurane anesthesia, peripheral blood samples were collected by submandibular bleeding into EDTA-coated tubes (Becton Dickinson and Company, NJ) and the complete blood cell count (CBC) including red blood cell (RBC), white blood cell (WBC), neutrophil (NE), lymphocyte (LY), monocyte (MO), and platelet (PLT) was acquired by Hematology Analyzer (HemaVet950FS, Drew Scientific, Miami Lake, FL). Live non-erythroid cells were isolated from the peripheral blood, bone marrow and spleen from 20 donor mice (10 mice from each group) following standard laboratory protocols. Subsequently, one million of those cells were incubated with fluorescent conjugated antibodies detecting subpopulations of B cells [B220+/CD19+; mature B cells (B220+/CD19+/IgK+) and immature B cells (B220+/CD19+/IgK−)], T cells (CD3+/CD4+ or CD3+/CD8+), and myeloid cells [CD19−/CD11b+; monocytes (CD19−/CDnb+/Gr-1neg-lo) and neutrophils (CD19−/CD11b+/Gr-1mod-hi)]. Antibody details are provided in Table S4. The cells were analyzed on a SP6800 spectral analyzer (Sony Biotechnology Inc.) and the percentages of cell populations were delineated with FlowJo software.
A quantity of 2x106 cells isolated from bone marrow of each donor mouse were transplanted by retro-orbital injection into one female recipient FVB/N CD45.2 congenic animal after irradiation treatment (9.5 Gy whole-body X-ray irradiation; 4.25 Gy separated by 3-6 hours). Animals received isoflurane inhalant anesthesia prior to retro-orbital injections. Reconstitution was confirmed by flow cytometry detecting the ratio of CD45.1+ cells/CD45.2+ cells in the blood at three months post-transplantation (blood collected as described above). Low FSC/SSC population (i.e. lymphoid cells) and increased FSC/SSC population (i.e. granulocytes) were predominantly donor cells in all animals (low FSC/SSC median 90% donor, mean 89%, range 78 to 95%; increased FSC/SSC median 99% donor, mean 97%, range 82 to 100%) Reconstitution was similar in recipients of control and THS exposed donor mice, as well as in recipients of male and female donor mice.
The recipient mice were followed for development of neoplasm or other illness for one year. Tissues including liver, spleen, lymph node, kidney, heart, lung, and sternum of each animal were stored in 10% formalin, embedded in paraffin (sternum following decalcification), and the pathologic findings were assessed.
Statistical Analyses
Most statistical and survival analyses were performed using SPSS version 24, with statistical tests indicated in figure legends and in tables. Competing risk analysis was performed in R as described[21]. In regard to Table S2: in order to decrease the risk of false positives as well as retain statistical power, we initially pre-selected 15 parameters for analysis by both nominal p-value and false discovery rate (FDR); these parameters are noted as “15parameters” in EXCEL worksheet labels; further analyses performed in light of the initial statistical findings are noted as “added parameters” in EXCEL worksheet labels.
Results
Experimental approach.
To investigate the effects of in utero and early-life exposure to THS on the immune system and on leukemia/lymphoma risk, we exposed pregnant Cdkn2a−/− dams to THS from the first day of pregnancy until weaning (Figure 1A). Plasma cytokine levels, body weight and hematologic parameters in bone marrow (BM), spleen (SP), and peripheral blood (PB) were measured at different time points after birth. To determine the effect of THS exposure on leukemia/lymphoma risk, bone marrow samples from THS exposed and control Cdkn2a−/− mice were transplanted into bone marrow ablated (irradiated) wild-type recipient mice, which were then followed for one year.
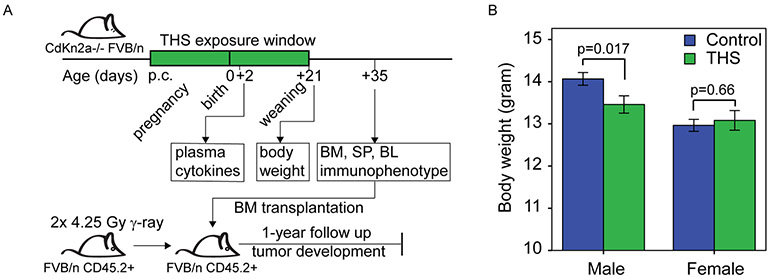
Figure 1. THS exposure altered body weight of three-week-old male pups compared to control.
A. Study design. Mice were exposed to THS starting from the first day of pregnancy (post coital, p.c.) until the pups were weaned at 3-weeks of age. Plasma cytokine levels were measured at 2 days of age. Body weight was assessed at weaning. Bone marrow (BM), spleen (SP) and peripheral blood (PB) were collected at 5 weeks of age for immunophenotyping and bone marrow was transplanted into irradiated recipients. Tumor development was monitored for one year.
B. Bars represent body weight (gram) at weaning for control and THS exposed male and female mice [n=142 pups (19 litters) in the Control group and 105 pups (15 litters) in THS-treated group]. Data are presented as the mean and error bars indicate standard error. P-values were obtained using the two-tailed t-test.
THS exposure significantly decreases body weight of male pups
We housed female Cdkn2a−/− FVB/N mice with THS exposed cotton terry cloth swatches (5 x 5 cm2) from the first day of pregnancy until the pups were weaned at three weeks of age. Mice in the control group were housed with terry cloth swatches that were not exposed to THS. All cages also contained standard bedding material. The body weight of individual pups was measured on the day of weaning and included 142 pups (19 litters) in the Control group and 105 pups (15 litters) in THS exposed group. We observed a lower mean body weight of all pups in THS group (mean±SEM: 13.27±0.16 g) compared to control group (13.63±0.12 g) and we found a statistically significant decrease in body weight of male pups in THS exposed group (13.44±0.21 g; n=55) when compared to the male pups in the Control group (14.05±0.15 g; n=87) (two-tailed T-test, p=0.017) (Figure 1B). No difference in body weight was observed in female mice between the THS-treated group (13.07±0.14 g; n=50) and the Control group (12.95±0.23 g; n=55) (T-test, p=0.66) (Figure 1B).
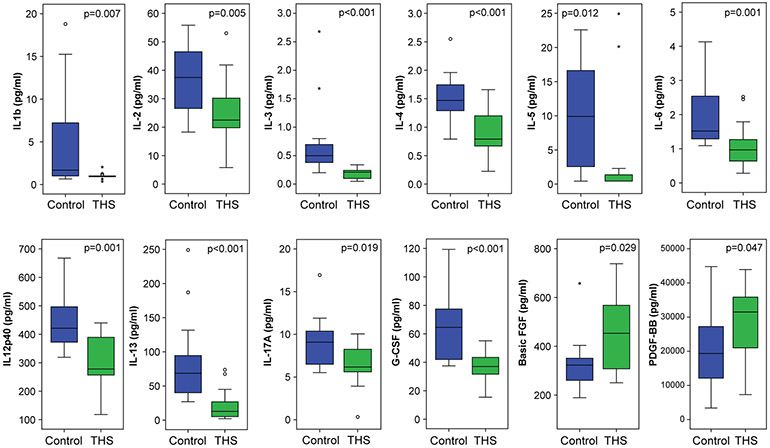
THS exposure decreases cytokine levels in two-day-old pups
To investigate the effect of THS exposure on plasma cytokine concentrations, we collected and isolated plasma from one male and one female pup at two days of age from each independent litter (n=16 for THS exposed mice and n=16 for control mice) and measured concentrations of 32 cytokines (Table S1; selected cytokines shown in Figure 2). We found that 20 out of 32 cytokines in THS exposed pups were lower than those in control mice including many interleukins (FDR <0.1). Basic fibroblast growth factor (FGF) and the B-subunit of platelet-derived growth factor (PDGF-BB) were higher in THS exposed mice compared to control. Plasma cytokine differences were observed in both male and female mice (Table S1).
Figure 2. THS exposure affects plasma cytokine levels.
Boxplots of cytokine levels in 2-day old mice exposed in utero to control (blue) or THS (green) (Control: n=16, one male and one female pup from 8 litters; THS: n=16; one male and one female pup from 8 litters). Box and whisker plots indicate median, 25th and 75th percentiles, 5th and 95th percentiles, and individual samples beyond these limits. Nominal P-values shown were obtained using the Mann-Whitney test. See also Table S1 for FDR values.
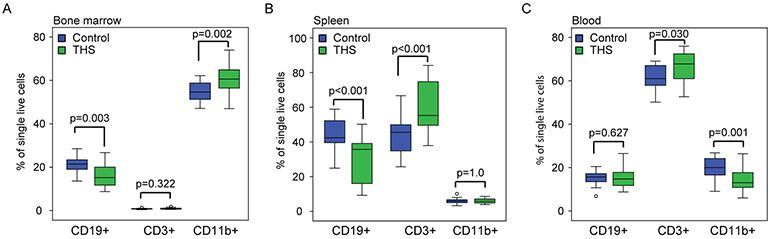
THS exposure affects the percentage of immune cell populations in bone marrow, spleen, and blood
To elucidate the potential influence of THS exposure on bone marrow, splenic, and blood cells we collected nucleated live cells of these tissues from one male and one female five-week old mouse from independent litters and measured B cell, T cell and myeloid fractions by flow cytometry (Table S2; Control: n=19, one male and one female pup from 9 litters, one male from a 10th litter; THS: n=20; one male and one female pup from 10 litters). In bone marrow, we observed a decreased percentage of B cells (FDR=0.009) and an increased percentage of myeloid cells (FDR=0.008) in THS exposed compared to control exposed mice (Figure 3A). In spleen, we found a decreased percentage of B cells (FDR=0.0005) and an increased percentage of T cells (FDR=0.0005) in THS exposed mice (Figure 3B). In blood, we found that THS exposed mice had an increase in the percentage of T cells (FDR=0.045) and a lower percentage of myeloid cells (FDR=0.0005) (Figure 3C).
Figure 3. Comparison of immune cell populations by flow cytometry in different tissues of five-week-old donor mice.
The percentage of B-cells (CD19+), T-cells (CD3+) and myeloid cells (CD19-/CD11b+) were measured by flow cytometry at 5 weeks of age in control (blue) and THS (green) exposed mice (Control: n=19, one male and one female pup from 9 litters, one male from a 10th litter; THS: n=20; one male and one female pup from 10 litters).
A. Bone marrow.
B. Spleen.
C. Blood.
Box and whisker plots indicate median, 25th and 75th percentiles, 5th and 95th percentiles, and individual samples beyond these limits. Nominal P-values shown were obtained using the Mann-Whitney test. See also Table S2 for FDR values.
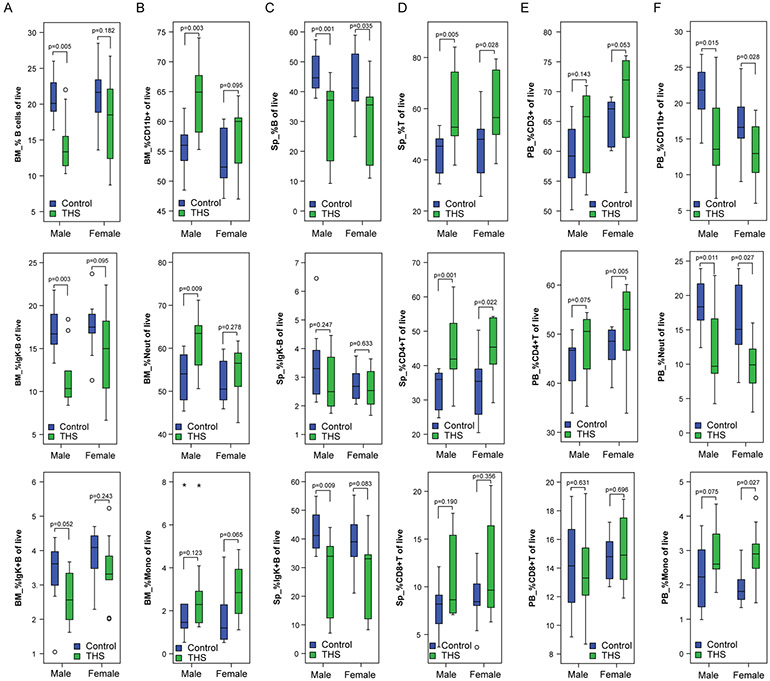
Given our observation that THS exposure particularly decreased the weight of 3-week-old male mice, the impact of sex was examined. In addition, analyses were performed to assess whether the observed differences in bone marrow, spleen, and blood were driven by changes in particular sub-populations (Table S2). The decreased percentage of bone marrow B cells and increased percentage of bone marrow myeloid cells were more pronounced in male mice (Figure 4A and B). B cell subpopulations in THS exposed as compared to control exposed mice trended lower for both immature and mature B cells (nominal p-value < 0.05 for immature marrow B cells in males), whereas myeloid sub-populations trended higher (nominal p-value < 0.05 for marrow neutrophilic cells in males). In the spleen, the decreased percentage of B cells and the increased percentage of T cells were seen in both sexes, and were driven by decreased mature B cells and by increased CD4+ T cells (Figure 4C and D). The blood showed T cell changes similar to but less pronounced than those seen in the spleen (Figure 4E, nominal p-value < 0.05 for blood CD4+ cells in females), whereas in contrast to the increased percentage of bone marrow neutrophilic cells seen in male mice, a decreased percentage of peripheral blood myeloid cells was seen in both males and females due to a decreased percentage of neutrophilic cells (Figure 4F).
Figure 4. Sex specific effects of THS on immune cell populations.
For immune cell populations identified in Figure 3 as divergent between control and THS exposed mice, males and females, as well as immune subsets, were compared as described in the legend to Figure 3.
A. Bone marrow (BM) B-cells (B220+/CD19+), mature B-cells (B220+/CD19+/IgK+) and immature B-cells (B220+/CD19+/IgK−).
B. Bone marrow myeloid cells (CD19−/CD11b+), monocytes (CD19−/CD11b+/Gr-1neg-lo) and neutrophils (CD19−/CD11b+/Gr-1mod-hi).
C. Spleen (Sp) B-cells (B220+/CD19+), mature B-cells (B220+/CD19+/IgK+) and immature B-cells (B220+/CD19+/IgK−).
D. Spleen T-cells (CD3+), T-helper cells (CD3+/CD4+) and T-suppressor cells (CD3+/CD8+).
E. Peripheral blood (PB) T-cells (CD3+), T-helper cells (CD3+/CD4+) and T-suppressor cells (CD3+/CD8+).
F. Peripheral blood myeloid cells (CD19−/CD11b+), monocytes (CD19−/CD11b+/Gr-1neg-lo) and neutrophils (CD19−/CD11b+/Gr-1mod-hi).
THS exposure alters the survival time of transplanted mice
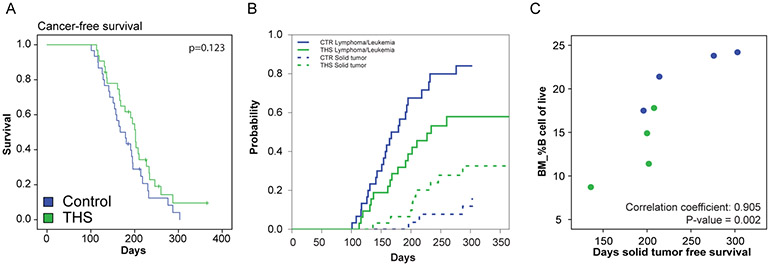
To investigate if THS promotes the development of hematopoietic tumors including leukemia/lymphoma, we transplanted bone marrow isolated from five-week-old control (n=30) and THS exposed (n=32) mice (donor mice) to irradiated FVB/N congenic CD45.2 mice (recipient mice) (Figure 1A). (We hoped with this approach to reduce the risk that mice would become ill with non-leukemia malignancies that can also develop in Cdkn2a−/− mice.) Recipient mice were followed for tumor development for one year (Table S3). We found that among the 30 recipient mice from control donor mice, 28 developed cancer within one year of transplantation. Among the 32 recipient mice from THS-exposed donor mice, 26 recipient mice developed cancer within one year. There was no significant difference in cancer-free survival between control and THS-exposed groups (Figure 5A; p=0.123). When focusing our analyses on the cause of death in the recipient animals, we observed trends towards later development of leukemia/lymphoma in THS-exposed animals and earlier development of solid tumors (Figure 5B; Control vs. THS p=0.02 for leukemia/lymphoma, p=0.13 for solid tumors). The significance of this observation was not entirely clear, and we considered the possibility that these differences in latencies reflected our particular experimental approach. One possibility was that THS immunosuppressive effects contributed to radiation-induced solid tumors in recipient animals. In our model, we used whole-body irradiation to ablate host bone marrow prior to bone-marrow transplantation. Bone-marrow-ablative radiation exposure significantly increases the risk of developing solid tumors (predominantly sarcomas). We therefore speculated that – if THS immunosuppressive effects accelerated the development of radiation induced solid tumors in recipient animals – we would find that mice receiving lower numbers of B cells would have developed solid tumors at younger ages. There were 8 recipients of bone marrow that developed such solid tumors and for which pre-transplant immunophenotyping data were available (4=control exposed donors, 4=THS exposed donors). In these animals we indeed observed a significant correlation between the percentage of B cells in donor mouse marrow and days to solid tumor development (Figure 5C; Spearman rank correlation coefficient = 0.905; p=0.002); mice that received fewer B cells at transplant appeared to develop such non-leukemia/lymphoma cancers at earlier time points. Hence, the trends seen in Figure 5B could reflect THS immunosuppression of irradiated recipient animals leading to accelerated solid tumors, THS altering lymphopoiesis to delay leukemia/lymphoma, or a combination of these effects.
Figure 5. THS exposure significantly affects leukemia and lymphoma development.
A. Cancer-free survival curves of bone marrow recipient mice which received bone marrow from control (blue; n=30) and THS (green; n=32) exposed donor mice. P value was obtained by Log-Rank Mantel-Cox test.
B. Cumulative incidence functions for competing risk of solid tumor development (dashed lines; n=5 for control, n=9 for THS) with leukemia/lymphoma (solid lines; n=24 for control, n=17 for THS) as first observed event (control mice indicated in blue and THS mice in green). Competing risk analysis: Control vs. THS p=0.02 for leukemia/lymphoma, p=0.13 for solid tumors.
C. Correlation between the percentage of transplanted B cells and tumor latency of non-leukemia-lymphoma cancers in the combined control and THS cohorts. Control mice indicated in blue and THS mice in green. P-value was obtained using Spearman Correlation.
Discussion
In this study we utilized the Cdkn2a null mouse model of childhood ALL to address in utero and early-life THS exposure effects, from the first day of pregnancy through weaning, on plasma cytokines, body weight, hematologic parameters, and leukemia/lymphoma development. We found that THS exposure caused significant changes in plasma cytokine concentrations and in bone marrow, spleen, and blood immune cell populations. We furthermore observed that THS exposure increased leukemia/lymphoma-free survival in bone marrow transplantation recipient mice.
Since FVB/N mice that lack Cdkn2a are cancer prone, primarily developing leukemia/lymphoma and sarcoma, we transplanted bone marrow of THS-exposed and control Cdkn2a null mice into histocompatible bone marrow ablated recipient animals. As expected, we observed a high penetrance of leukemia/lymphoma in the recipient mice. Interestingly, in our model system recipient mice that received THS-exposed donor bone marrow exhibited increased lymphoma/leukemia free survival compared to recipient mice receiving bone marrow from control donor mice. This result might reflect a consequence of the immunosuppressive effect observed in THS-exposed donor mice. The immunosuppressive effect results in fewer lymphoid cells from THS treated donor mice being transplanted into recipient mice compared to control donor mice effectively reducing the number of targets for oncogenic transformation and lowering the incidence of leukemia/lymphoma after THS exposure. However, an alternative explanation involving the risk of competing events has to be considered in our model since bone marrow was transplanted after myeloablative radiation therapy of recipient mice. One side effect of this treatment is the development of solid tumors, which could prevent the observation of leukemia/lymphoma. It remains possible that the immunosuppressive effect of THS resulted in an acceleration in the development of these solid tumors, possibly due to inadequate immunosurveillance. Even though the development of solid tumors was not statistically different between THS-treated and control mice, we did observe an increased number of solid tumors that occurred earlier in the THS group. The inability to definitively conclude whether THS exposure influences solid tumor development is a limitation of our work. In conclusion, THS alone was not carcinogenic in our Cdkn2a−/− leukemia/lymphoma model, but it may have contributed to radiation-exposure-associated tumor development through its immunosuppressive effects.
Interestingly, epidemiological studies have suggested a relationship between smoking and a spectrum of diseases with a significant inflammatory component; in some cases there is evidence that smoking may decrease incidence and/or severity. For example, maternal smoking during pregnancy reduces the risk of type 1 diabetes in children[22]. Also, adult smoking reduced the risk of ulcerative colitis[23], sarcoidosis[24], endometriosis[25], and Parkinson’s disease[26]. A possible biological mechanism for these observations is that nicotine, which is present in cigarette smoke and THS, is known to have immunosuppressive effects[27]. Even though our results suggest that THS exposure might, in some settings, reduce the risk of leukemia/lymphoma, we observed profound potentially detrimental impacts on the immune system, the detrimental health risks associated with maternal smoking are well-documented, and any potential health benefit from the immunosuppressive effects of exposure to THS does not outweigh the harmful effects of smoking on health.
Future studies will have to determine whether the observed adverse effects of THS on hematologic parameters are dose and genetic background dependent. Our results show that THS exposure of FVB/n Cdkn2a null mice during pregnancy and early life has a profound effect on male body weight and on immune parameters in both males and females. In a related study, investigating effects of THS on body weight and hematologic parameters in C57BL/6 mice exposed during the first three weeks of life, we showed a reduction in body weight in both male and female mice [16]. Furthermore, our prior study showed that THS exposure during the first 3 weeks of life significantly increased the percentage of B-cells in peripheral blood fourteen weeks after THS exposure [16]. In contrast, our current study showed no difference in the percentage of B-cells in peripheral blood and a significant decrease in spleen and bone marrow in THS-exposed mice at 5 weeks of age. These differences could be the result of differences in strain genetic background, exposure window, and/or the time between exposure and immune parameter measurements. Chen et al, showed that THS exposure of C57BL/6 mice for 2 months starting at 3 weeks of age resulted in a dose dependent increase in serum cytokine levels including IL-1a, IL-4, IL-10, TNFalpha and GM-CSF [15]. Similarly, Adhami et al, investigated exposure of male C57BL/6 mice to THS for 1, 2, 4, or 6 months starting at weaning and observed significant increases in serum levels of TNFalpha and GM-CSF when mice were exposed for as little as one month [14]. In our study, we also observed that THS exposure significantly altered plasma cytokine levels. However, in contrast to these previous studies showing a pro-inflammatory phenotype associated with THS exposure, we observed that perinatal exposure led to a significant decrease for the majority of cytokines assayed at 2 days of age, including IL-4, IL-10, TNFalpha and GM-CSF. The reason for these different observations could be due to differences in exposure window. Our cytokine measurements were conducted in mice exposed in utero, from the first day of pregnancy, to 2 days of age. THS exposure effects have not previously been investigated for this exposure. Development and maturation of the immune system starts early in fetal life and our results suggest that THS exposure affects this process. Differences could similarly be due to differences in mouse genetic background, experimental timing and/or exposure levels. Our study also found sex differences in the response to THS exposure emphasizing the importance of including both male and female mice in exposure studies. In general, male mice were more susceptible to THS exposure effects than female mice. Previous studies showed that male mice were found to be more sensitive than female mice to in utero exposure to SHS for lung development and an immune challenge later in life [28, 29]. These findings suggest that sex differences during fetal development play an important role in determining health risks associated with THS exposure. Collectively, these studies emphasize the need to define the window-of-susceptibility of THS-induced health outcomes. These studies can be initiated in mouse population-based model systems, which mimic the genetic and phenotypic diversity observed in the human population while allowing precise control of exposures and the ability to analyze multiple phenotypic endpoints [30].
In conclusion, our results using the Cdkn2a−/− mouse model of leukemia/lymphoma showed that THS exposure during pregnancy and early life caused substantial biological effects, including decreased regulators of the immune system at birth (cytokines) and persistent alterations of blood cells. These findings further support the growing evidence that THS exposure may have significant persistent health effects for human mothers and infants. Although our results did not demonstrate that THS exposure increased risk for leukemia/lymphoma, its immunosuppressive effects may have contributed to the carcinogenic effects of ionizing radiation. These data contribute to our understanding of the potential health risks of THS exposures, and should be useful for framing and advocating for policies against indoor smoking in the U.S.A. and worldwide.
Supplementary Material
Table S3. Cancer incidence in mice reconstituted with bone marrow of control and THS exposed donor mice.
Table S2. Hematologic parameters in control and THS exposed mice.
Table S1. Plasma cytokine concentrations in control and THS exposed mice.
Table S4. Reagents: Antibodies.
Clinical Perspectives.
We investigated the effects of in utero and early-life THS exposure on plasma cytokines, body weight, hematologic parameters and leukemia/lymphoma development using the Cdkn2a null mouse model of childhood ALL.
Our study demonstrates that in utero and early-life THS exposure is broadly immunosuppressive and increased leukemia/lymphoma-free survival in bone marrow transplantation recipient mice.
Our study adds to expanding evidence that THS exposure has profound effects on the immune system and that in utero and early life developmental periods represent vulnerable windows of susceptibility for these effects.
Acknowledgement
The authors utilized shared resources of the UCSF Helen Diller Family Comprehensive Cancer Center (Biorepository & Tissue Biomarker Technology; Computational Biology; Laboratory for Cell Analysis) in the performance of these studies.
Funding
This work was supported by a grant from NIEHS and EPA (P50-ES018172 and RD86315901) funding the Center for Integrative Research on Childhood Leukemia and the Environment, a Federally funded Children’s Environmental Health Center, as well as by a Pilot Project (T29IP0703) from the California Tobacco-Related Disease Research Program.
Abbreviations
- ALL
acute lymphoblastic leukemia
- BM
bone marrow
- FDR
false discovery rate
- PB
peripheral blood
- SHS
secondhand smoke
- SP
spleen
- THS
thirdhand smoke
Footnotes
Competing interests: The authors declare no competing financial interests.
Data Availability Statement
Datasets related to the article are included as supplementary materials, Tables S1-S4.
References
- 1.Barrington-Trimis JL, et al. , Trends in childhood leukemia incidence over two decades from 1992 to 2013. Int J Cancer, 2017. 140(5): p. 1000–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hudson MM, et al. , Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA, 2013. 309(22): p. 2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oeffinger KC, et al. , Chronic health conditions in adult survivors of childhood cancer. N Engl J Med, 2006. 355(15): p. 1572–82. [DOI] [PubMed] [Google Scholar]
- 4.Barrington-Trimis JL, et al. , Rising rates of acute lymphoblastic leukemia in Hispanic children: trends in incidence from 1992 to 2011. Blood, 2015. 125(19): p. 3033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitehead TP, et al. , Childhood Leukemia and Primary Prevention. Curr Probl Pediatr Adolesc Health Care, 2016. 46(10): p. 317–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greaves M, A causal mechanism for childhood acute lymphoblastic leukaemia. Nat Rev Cancer, 2018. 18(8): p. 471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonardi-Bee J, Jere ML, and Britton J, Exposure to parental and sibling smoking and the risk of smoking uptake in childhood and adolescence: a systematic review and meta-analysis. Thorax, 2011. 66(10): p. 847–55. [DOI] [PubMed] [Google Scholar]
- 8.Carreras G, et al. , Burden of disease attributable to second-hand smoke exposure: A systematic review. Prev Med, 2019. 129: p. 105833. [DOI] [PubMed] [Google Scholar]
- 9.Matt GE, et al. , Thirdhand tobacco smoke: emerging evidence and arguments for a multidisciplinary research agenda. Environ Health Perspect, 2011. 119(9): p. 1218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacob P 3rd, et al. , Thirdhand Smoke: New Evidence, Challenges, and Future Directions. Chem Res Toxicol, 2017. 30(1): p. 270–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winickoff JP, et al. , Beliefs about the health effects of "thirdhand" smoke and home smoking bans. Pediatrics, 2009. 123(1): p. e74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quintana PJ, et al. , Wipe sampling for nicotine as a marker of thirdhand tobacco smoke contamination on surfaces in homes, cars, and hotels. Nicotine Tob Res, 2013. 15(9): p. 1555–63. [DOI] [PubMed] [Google Scholar]
- 13.Matt GE, et al. , Thirdhand smoke and exposure in California hotels: non-smoking rooms fail to protect non-smoking hotel guests from tobacco smoke exposure. Tob Control, 2014. 23(3): p. 264–72. [DOI] [PubMed] [Google Scholar]
- 14.Adhami N, Chen Y, and Martins-Green M, Biomarkers of disease can be detected in mice as early as 4 weeks after initiation of exposure to third-hand smoke levels equivalent to those found in homes of smokers. Clin Sci (Lond), 2017. 131(19): p. 2409–2426. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Adhami N, and Martins-Green M, Biological markers of harm can be detected in mice exposed for two months to low doses of Third Hand Smoke under conditions that mimic human exposure. Food Chem Toxicol, 2018. 122: p. 95–103. [DOI] [PubMed] [Google Scholar]
- 16.Hang B, et al. , Early exposure to thirdhand cigarette smoke affects body mass and the development of immunity in mice. Sci Rep, 2017. 7: p. 41915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullighan CG, et al. , Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature, 2007. 446(7137): p. 758–64. [DOI] [PubMed] [Google Scholar]
- 18.Li M, et al. , Initially disadvantaged, TEL-AML1 cells expand and initiate leukemia in response to irradiation and cooperating mutations. Leukemia, 2013. 27(7): p. 1570–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serrano M, et al. , Role of the INK4a locus in tumor suppression and cell mortality. Cell, 1996. 85(1): p. 27–37. [DOI] [PubMed] [Google Scholar]
- 20.Hang B, et al. , Short-term early exposure to thirdhand cigarette smoke increases lung cancer incidence in mice. Clin Sci (Lond), 2018. 132(4): p. 475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scrucca L, Santucci A, and Aversa F, Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant, 2007. 40(4): p. 381–7. [DOI] [PubMed] [Google Scholar]
- 22.Magnus MC, et al. , Parental Smoking and Risk of Childhood-onset Type 1 Diabetes. Epidemiology, 2018. 29(6): p. 848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berkowitz L, et al. , Impact of Cigarette Smoking on the Gastrointestinal Tract Inflammation: Opposing Effects in Crohn's Disease and Ulcerative Colitis. Front Immunol, 2018. 9: p. 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlens C, et al. , Smoking, use of moist snuff, and risk of chronic inflammatory diseases. Am J Respir Crit Care Med, 2010. 181(11): p. 1217–22. [DOI] [PubMed] [Google Scholar]
- 25.Cramer DW, et al. , The relation of endometriosis to menstrual characteristics, smoking, and exercise. JAMA, 1986. 255(14): p. 1904–8. [PubMed] [Google Scholar]
- 26.Gallo V, et al. , Exploring causality of the association between smoking and Parkinson's disease. Int J Epidemiol, 2019. 48(3): p. 912–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sopori M, Effects of cigarette smoke on the immune system. Nat Rev Immunol, 2002. 2(5): p. 372–7. [DOI] [PubMed] [Google Scholar]
- 28.Noel A, et al. , Sex-specific lung functional changes in adult mice exposed only to second-hand smoke in utero. Respir Res, 2017. 18(1): p. 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao R, et al. , In utero exposure to second-hand smoke aggravates the response to ovalbumin in adult mice. Am J Respir Cell Mol Biol, 2013. 49(6): p. 1102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Churchill GA, et al. , The Collaborative Cross, a community resource for the genetic analysis of complex traits. Nat Genet, 2004. 36(11): p. 1133–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S3. Cancer incidence in mice reconstituted with bone marrow of control and THS exposed donor mice.
Table S2. Hematologic parameters in control and THS exposed mice.
Table S1. Plasma cytokine concentrations in control and THS exposed mice.
Table S4. Reagents: Antibodies.
Data Availability Statement
Datasets related to the article are included as supplementary materials, Tables S1-S4.