Abstract
Facilitating the entry of molecules into mammalian cells is of great interest to fields as diverse as cell biology and drug delivery. The discovery of natural protein transduction domains and the development of artificial ones, including polyarginine, provides a means to achieve this goal. Here, we comment on key chemical and biological aspects of cationic peptide internalization, including the physiological relevance of this process.
Keywords: endocytosis, heparan sulfate proteoglycan, HIV–TAT, polyarginine, protein transduction domain.
Introduction
The ability of polycations to enhance the cellular uptake of macromolecules has been known for nearly fifty years [1]. Interest in this phenomenon reemerged in 1988 with the finding that exogenous TAT protein from the human immunodeficiency virus (HIV–TAT) could enter mammalian cells [2,3]. Many natural and synthetic cationic peptides likewise have this ability [4]. These peptides have been classified as “protein transduction domains” (PTDs) or “cell-penetrating peptides” (CPPs).
Rather than discuss the detailed mechanism of PTD internalization or potential applications for these peptides, which have been well-covered in the literature [5–7], we wish to take a step back and discuss more general issues about the cellular internalization of cationic molecules. We focus on one PTD — polyarginine — as the basis for this discussion, and speculate on the role of its guanidinium groups in cellular uptake. Further, we discuss whether the pathway of PTD internalization is unique to cationic peptides, or if these peptides hijack pathways that have evolved for the internalization of endogenous cationic macromolecules. It is important to note that we shall limit our discussion to experiments performed with live cells, as fixation has been shown to alter the internalization of PTDs [8].
Role of charge in PTD internalization
HIV–TAT is perhaps the best-studied PTD. Structure–function studies defined an 11-residue, basic sequence corresponding to a portion of the RNA-binding domain of HIV–TAT as being necessary for cellular uptake [9]. This region contained six arginine residues that were especially critical. This finding led several groups to examine the uptake of simple polybasic peptides, such as polyarginine and polylysine. Polyarginine was found to be superior to other homopolymeric amino acids [10], and the optimal length of polyarginine necessary for transport was found to be between 5 and 11 residues, with octa- and nonaarginine being transported most efficiently [10,11]. Other PTDs are of similar size and likewise contain a high percentage of basic residues [12–14].
Why is polyarginine internalized by cells?
What physicochemical properties of polyarginine allow for translocation into cells? Is polyarginine internalization purely a biophysical phenomenon, or have cells evolved a mechanism(s) for the uptake of cationic peptides and proteins? If so, why?
Polyarginine is natural polymer of guanidinium groups. Like its enantiomer, poly(d-arginine) enters cells efficiently, indicating that the internalization process is not stereospecific [10]. In addition, guanidinium groups displayed from numerous non-proteinogenic scaffolds can facilitate internalization [15–17]. These results indicate that a peptide scaffold is not essential for PTD uptake.
Why is the guanidinium group important for uptake? Primary guanidinium groups bear a positive charge under physiological conditions and have the potential to donate up to five hydrogen bonds to electron-rich functionalities [18], such as the carboxyl, phosphoryl, and sulfuryl groups of cell-surface carbohydrates and phospholipids (Figure 1). The favorable Coulombic component makes such hydrogen bonds especially strong [19]. Using N-methylated arginine residues, Rothbard and coworkers have elegantly demonstrated that the ability of the guanidinium group to form hydrogen bonds is indeed essential for efficient cellular uptake [20,21].
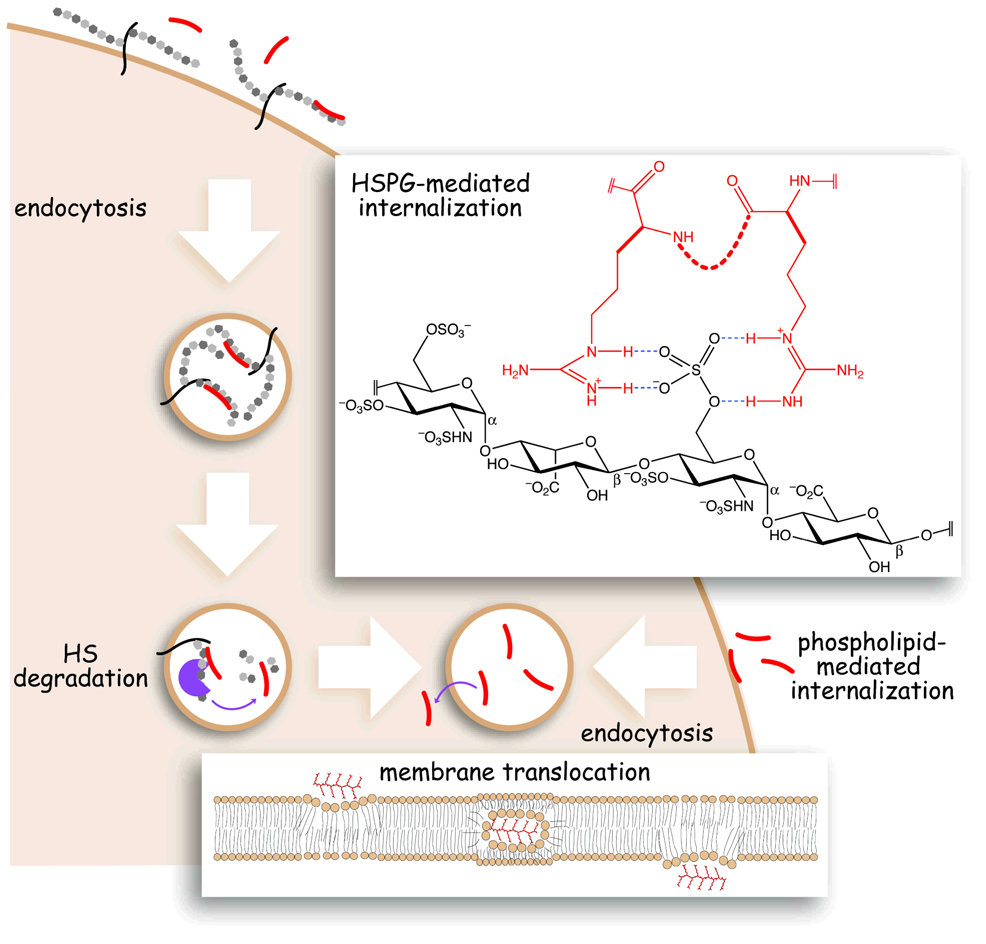
Figure 1.
Scheme of putative routes for polyarginine internalization. Polyarginine (red) interacts with either HSPGs (black/gray) or lipids (brown) at the cell surface and undergoes endocytosis. Polyarginine is released from HSPGs by glycosidase activity within endocytic compartments. Free polyarginine crosses the endocytic membrane into the cytosol. (Upper inset) Possible hydrogen bonds between two guanidinium groups of polyarginine (red) and a sulfuryl group of heparan sulfate (black). (Lower inset) Possible mechanism for the membrane translocation of polyarginine in which cationic guanidinium groups interact with anionic lipid head-groups to form a neutral complex that traverses the bilayer.
The role of the guanidinium group in PTD internalization is not understood completely. Our group has shown that polyarginine forms a tight interaction with heparin, a mimic for the heparan sulfate proteoglycans (HSPGs) on the surface of mammalian cells (Figure 1) [22,23]. HIV–TAT peptide and protein have also been shown to interact with heparin [24–27]. Many groups have shown that in GAG-deficient cell lines, internalization of PTDs is decreased [22,4,28,29]. These results suggest that the role of the guanidinium functionality in PTD uptake could be to facilitate binding to the cell surface through interactions with cell-surface HSPGs.
An alternative role for the guanidinium functionality in uptake could be to facilitate binding to cell-surface lipids (Figure 1). Rothbard and coworkers have shown that PTDs, such as polyarginine, can form neutral complexes with phospholipids within endocytic vesicles, which in turn can cross cellular membranes in the presence of a chemical gradient [21]. Likewise, Matile and coworkers have shown that cationic peptides and hydrophobic anions can form neutral complexes that translocate across bilayers [30,31]. Merkle and coworkers have reported that the cationic peptide pVEC (and its derivatives) can induce phospholipid phase transitions, suggesting another mechanism by which PTDs could reach the cytosol [32].
Do cells have a mechanism for internalizing cationic molecules?
The transport of molecules into and out of cells is a highly regulated process. Nonetheless, many cationic proteins freely enter cells. Basic fibroblast growth factor (bFGF) does so via caveolae-mediated endocytosis [33]. Bovine pancreatic ribonuclease (RNase A) and its homologs interact strongly with HSPGs [34] and are internalized by dynamin-independent endocytosis [35]. Cellular entry of the cationic growth factor, midkine (MK), is mediated by the lipoprotein receptor-related protein (LRP) [36]. (Interestingly, LRP has also been implicated as a possible mediator of HIV–TAT internalization [37].) One common link between many of these internalized cationic growth factors is that they form biologically relevant interactions with HSPGs on the cell surface. Indeed, HSPGs play an important role in the internalization of a diverse group of macromolecules, including growth factors [38], polyamines [39], and viruses [40].
There is evidence that, in addition to being found at the cell surface, HSPGs are transported to both the cytosol and the nucleus [41]. The importance of this trafficking is not understood, but suggests that some fraction of cell-surface HSPGs must be able to escape degradation in the lysosome and traffic to other regions of the cell. It is therefore conceivable, perhaps likely, that cationic proteins or peptides that associate with HSPGs could traffic with these carbohydrates.
The interaction between cationic proteins and anionic carbohydrates on the cell surface could be important for either signaling (for growth factors) or patterned gene expression (for cationic transcription factors). Belting and coworkers have proposed that extracellular transcription factors form gradients similar to those observed for morphogens, and that their biological effects are regulated by HSPG-mediated internalization [42]. PTDs could exploit this very same pathway to enter cells.
Conclusions
Much is known about the internalization of PTDs and the role of HSPGs in complex biological processes. The interaction between PTDs and HSPGs is also fairly well established. HSPGs play an important role in the internalization and trafficking of other cationic molecules. Still, the role HSPGs plays in the internalization process has not been studied extensively. Although it remains possible that PTD internalization is dictated through direct interactions with the plasma membrane, we must not discount the possibility that PTDs follow a pathway that evolved for another purpose—the uptake of cationic growth factors and other signaling molecules.
Acknowledgements
Work in the Raines laboratory on the cellular internalization of cationic peptides and proteins is supported by Grant CA73808 (NIH). S.M.F. was supported by Biotechnology Training Grant 08349 (NIH). The subtitle refers to “The Road Not Taken” by Robert Frost (1916) in Mountain Interval, p. 9, Henry Holt, New York.
References
- 1.Ryser HJ, Hancock R. Histones and basic polyamino acids stimulate the uptake of albumin by tumor cells in culture. Science. 1965;150:501–503. doi: 10.1126/science.150.3695.501. [DOI] [PubMed] [Google Scholar]
- 2.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 3.Green M, Loewenstein PM. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell. 1988;55:1179–1188. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- 4.Fischer R, Fotin-Mleczek M, Hufnagel H, Brock R. Break on through to the other side—Biophysics and cell biology shed light on cell-penetrating peptides. Chembiochem. 2005;6:2126–2142. doi: 10.1002/cbic.200500044. [DOI] [PubMed] [Google Scholar]
- 5.Leifert JA, Lindsay WhittonJ. “Translocatory proteins” and “protein transduction domains”: A critical analysis of their biological effects and underlying mechanisms. Mol. Ther. 2003;8:13–20. doi: 10.1016/s1525-0016(03)00151-5. [DOI] [PubMed] [Google Scholar]
- 6.Brooks H, Lebleu B, Vives E. Tat peptide-mediated cellular delivery: Back to basics. Adv. Drug Deliv. Rev. 2005;57:559–577. doi: 10.1016/j.addr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Fittipaldi A, Giacca M. Transcellular protein transduction using the Tat protein of HIV-1. Adv. Drug Deliv. Rev. 2005;57:597–608. doi: 10.1016/j.addr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, Chernomordik LV, Lebleu B. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J. Biol. Chem. 2003;278:585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- 9.Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell DJ, Kim DT, Steinman L, Fathman CG, Rothbard JB. Polyarginine enters cells more efficiently than other polycationic homopolymers. J. Pept. Res. 2000;56:318–325. doi: 10.1034/j.1399-3011.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki T, Futaki S, Niwa M, Tanaka S, Ueda K, Sugiura Y. Possible existence of common internalization mechanisms among arginine-rich peptides. J. Biol. Chem. 2002;277:2437–2443. doi: 10.1074/jbc.M110017200. [DOI] [PubMed] [Google Scholar]
- 12.Elmquist A, Lindgren M, Bartfai T, Langel U. VE-cadherin-derived cell-penetrating peptide, pVEC, with carrier functions. Exp. Cell Res. 2001;269:237–244. doi: 10.1006/excr.2001.5316. [DOI] [PubMed] [Google Scholar]
- 13.Ho A, Schwarze SR, Mermelstein SJ, Waksman G, Dowdy SF. Synthetic protein transduction domains: Enhanced transduction potential in vitro and in vivo. Cancer. Res. 2001;61:474–477. [PubMed] [Google Scholar]
- 14.Wadia JS, Dowdy SF. Transmembrane delivery of protein and peptide drugs by TAT-mediated transduction in the treatment of cancer. Adv. Drug Deliv. Rev. 2005;57:579–596. doi: 10.1016/j.addr.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: Peptoid molecular transporters. Proc. Natl. Acad. Sci. U.S.A. 2000;97:13003–13008. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Umezawa N, Gelman MA, Haigis MC, Raines RT, Gellman SH. Translocation of a β -peptide across cell membranes. J. Am. Chem. Soc. 2002;124:368–369. doi: 10.1021/ja017283v. [DOI] [PubMed] [Google Scholar]
- 17.Wender PA, Rothbard JB, Jessop TC, Kreider EL, Wylie BL. Oligocarbamate molecular transporters: Design, synthesis, and biological evaluation of a new class of transporters for drug delivery. J. Am. Chem. Soc. 2002;124:13382–13383. doi: 10.1021/ja0275109. [DOI] [PubMed] [Google Scholar]
- 18.Hutchings MG, Grossel MC, Merckel DAS, Chippendale AM, Kenworthy M, McGeorge G. The structure of m-xylylenediguanidinium sulfate: A putative molecular tweezer ligand for anion chelation. Cryst. Growth Des. 2001;1:339–342. [Google Scholar]
- 19.Fersht AR, Shi JP, Knill-Jones J, Lowe DM, Wilkinson AJ, Blow DM, Brick P, Carter P, Waye MM, Winter G. Hydrogen bonding and biological specificity analysed by protein engineering. Nature. 1985;314:235–238. doi: 10.1038/314235a0. [DOI] [PubMed] [Google Scholar]
- 20.Rothbard JB, Jessop TC, Lewis RS, Murray BA, Wender PA. Role of membrane potential and hydrogen bonding in the mechanism of translocation of guanidinium-rich peptides into cells. J. Am. Chem. Soc. 2004;126:9506–9507. doi: 10.1021/ja0482536. [DOI] [PubMed] [Google Scholar]
- 21.Rothbard JB, Jessop TC, Wender PA. Adaptive translocation: The role of hydrogen bonding and membrane potential in the uptake of guanidinium-rich transporters into cells. Adv. Drug Deliv. Rev. 2005;57:495–504. doi: 10.1016/j.addr.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Fuchs SM, Raines RT. Pathway for polyarginine entry into mammalian cell. Biochemistry. 2004;43:2438–2444. doi: 10.1021/bi035933x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuchs SM, Raines RT. Polyarginine as a multifunctional fusion tag. Protein Sci. 2005;14:1538–1544. doi: 10.1110/ps.051393805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rusnati M, Coltrini D, Oreste P, Zoppetti G, Albini A, Noonan D, d’Adda di Fagagna F, Giacca M, Presta M. Interaction of HIV-1 Tat protein with heparin. Role of the backbone structure, sulfation, and size. J. Biol. Chem. 1997;272:11313–11320. doi: 10.1074/jbc.272.17.11313. [DOI] [PubMed] [Google Scholar]
- 25.Hakansson S, Jacobs A, Caffrey M. Heparin binding by the HIV-1 tat protein transduction domain. Protein Sci. 2001;10:2138–2139. doi: 10.1110/ps.23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyagi M, Rusnati M, Presta M, Giacca M. Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. J. Biol. Chem. 2001;276:3254–3261. doi: 10.1074/jbc.M006701200. [DOI] [PubMed] [Google Scholar]
- 27.Ziegler A, Seelig J. Interaction of the protein transduction domain of HIV-1 TAT with heparan sulfate: Binding mechanism and thermodynamic parameters. Biophys. J. 2004;86:254–263. doi: 10.1016/S0006-3495(04)74101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fotin-Mleczek M, Fischer R, Brock R. Endocytosis and cationic cell-penetrating peptides—A merger of concepts and methods. Curr. Pharm. Des. 2005;11:3613–3628. doi: 10.2174/138161205774580778. [DOI] [PubMed] [Google Scholar]
- 29.Richard JP, Melikov K, Brooks H, Prevot P, Lebleu B, Chernomordik LV. Cellular uptake of unconjugated TAT peptide involves clathrin-dependent endocytosis and heparan sulfate receptors. J. Biol. Chem. 2005;280:15300–15306. doi: 10.1074/jbc.M401604200. [DOI] [PubMed] [Google Scholar]
- 30.Sakai N, Matile S. Anion-mediated transfer of polyarginine across liquid and bilayer membranes. J. Am. Chem. Soc. 2003;125:14348–14356. doi: 10.1021/ja037601l. [DOI] [PubMed] [Google Scholar]
- 31.Nishihara M, Perret F, Takeuchi T, Futaki S, Lazar AN, Coleman AW, Sakai N, Matile S. Arginine magic with new counterions up the sleeve. Org. Biomol. Chem. 2005;3:1659–1669. doi: 10.1039/b501472g. [DOI] [PubMed] [Google Scholar]
- 32.Herbig ME, Assi F, Textor M, Merkle HP. The cell-penetrating peptides pVEC and W2-pVEC induce transformation of gel phase domains in phospholipid bilayers without affecting their integrity. Biochemistry. 2006;45:3598–3609. doi: 10.1021/bi050923c. [DOI] [PubMed] [Google Scholar]
- 33.Gleizes PE, Noaillac-Depeyre J, Amalric F, Gas N. Basic fibroblast growth factor (FGF-2) internalization through the heparan sulfate proteoglycans-mediated pathway: An ultrastructural approach. Eur. J. Cell Biol. 1995;66:47–59. [PubMed] [Google Scholar]
- 34.Soncin F, Strydom DJ, Shapiro R. Interaction of heparin with human angiogenin. J. Biol. Chem. 1997;272:9818–9824. doi: 10.1074/jbc.272.15.9818. [DOI] [PubMed] [Google Scholar]
- 35.Haigis MC, Raines RT. Secretory ribonucleases are internalized by a dynamin-independent endocytic pathway. J. Cell Sci. 2003;116:313–324. doi: 10.1242/jcs.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shibata Y, Muramatsu T, Hirai M, Inui T, Kimura T, Saito H, McCormick LM, Bu G, Kadomatsu K. Nuclear targeting by the growth factor midkine. Mol. Cell. Biol. 2002;22:6788–6796. doi: 10.1128/MCB.22.19.6788-6796.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat. Med. 2000;6:1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- 38.Wiedlocha A, Sorensen V. Signaling, internalization, and intracellular activity of fibroblast growth factor. Curr. Top. Microbiol. Immunol. 2004;286:45–79. doi: 10.1007/978-3-540-69494-6_3. [DOI] [PubMed] [Google Scholar]
- 39.Belting M, Persson S, Fransson LA. Proteoglycan involvement in polyamine uptake. Biochem. J. 1999;338:317–323. [PMC free article] [PubMed] [Google Scholar]
- 40.Spear PG, Shieh MT, Herold BC, WuDunn D, Koshy TI. Heparan sulfate glycosaminoglycans as primary cell surface receptors for herpes simplex virus. Adv. Exp. Med. Biol. 1992;313:341–353. doi: 10.1007/978-1-4899-2444-5_33. [DOI] [PubMed] [Google Scholar]
- 41.Kolset SO, Prydz K, Pejler G. Intracellular proteoglycans. Biochem. J. 2004;379:217–227. doi: 10.1042/BJ20031230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belting M, Sandgren S, Wittrup A. Nuclear delivery of macromolecules: Barriers and carriers. Adv. Drug Deliv. Rev. 2005;57:505–527. doi: 10.1016/j.addr.2004.10.004. [DOI] [PubMed] [Google Scholar]