Abstract
Background:
P-wave signal-averaged electrocardiography (P-SAECG) quantifies atrial electrical activity. P-SAECG measures and their clinical correlates and heritability have had limited characterization in community-based cohorts.
Objectives:
to 1) Establish reference values; 2) Identify clinical risk factors associated with P-SAECG; and 3) Estimate genetic heritability for P-SAECG traits.
Methods:
We performed P-SAECG in two generations of Framingham Heart Study participants. We performed backward elimination regression models to assess associations of clinical factors with each SAECG trait (P-wave (PW) duration, Root Mean Square Voltage in terminal 40 ms (RMS40), RMS30, RMS20, RMS PW, and PW integral). We estimated the adjusted genetic heritability of P-SAECG measures using the SOLAR program.
Results:
We included 4307 participants (age 55±14 years, 56% female). The reference values were derived from 1752 participants without cardiovascular risk factors. The median (2.5th percentile; 97.5th percentile) total PW duration was 118ms (93;146) in women, and 128ms (104;158) in men in the reference sample, and 121ms (94;151) in women and 129ms (103;159) in the entire study cohort (broad sample). In the broad sample, after adjusting for age and sex, total PW duration was positively associated with height, weight, prevalent heart failure, history of AF, and AV-node blockers, and negatively associated with smoking, waist circumference, heart rate, and diabetes. The estimated heritability of P-SAECG traits was moderate, ranging from 11.9% for RMS30 to 24.9% for PW integral.
Conclusion:
P-SAECG traits are associated with multiple AF-related risk factors and are moderately heritable.
Keywords: electrocardiography, epidemiology, heritable traits, range reference, P wave, signal average ECG
Introduction
The P-wave SAECG (P-SAECG) is a noninvasive, high fidelity measurement of atrial electric function. It has shown potential to predict atrial fibrillation (AF) risk after myocardial infarction,1 cardiothoracic surgery,2 cardioversion,3 and catheter ablation.4 P-wave (PW) indices and PR interval are commonly used ECG measures reflecting intermediate phenotypes for AF.5 Longer PW duration and lower PW amplitude are associated with incident AF,6,7 as well as with increased rates of cardiovascular and all-cause mortality.8 A meta-analysis of PW indices showed limited improvement in risk prediction for AF compared to a robust risk score for the condition, initially derived from multiple community-based cohorts.7
However, the results are inconsistent,9–11 and most of P-SAECG studies are limited by small-to-modest sample sizes with incomplete anthropometric and clinical characterization,12,13 or technical issues.14,15 Also, it is unclear which clinical risk factors are associated with P-SAECG measurements, and the heritability of P-SAECG measurements remain known.
The objective of our investigation was to ascertain P-SAECG assessments in a large, multigenerational cohort that has had extensive characterization of clinical risk factors. We studied P-SAECG traits 1) to establish reference values; 2) to identify associated clinical risk factors; and 3) to estimate the genetic heritability, as well as genetic and environmental correlations between traits.
Methods
Study population
The Framingham Heart Study (FHS) is an observational epidemiological study located in Framingham, Massachusetts. It was initiated by the US Public Health Service in 1948 to identify risk factors for cardiovascular disease. The study enrolled Framingham residents in the Original cohort (n=5209), Offspring cohort – children of the Original cohort and their spouses (n=5124), and Third Generation cohort – adult children from the Offspring cohort (n=4095). To reflect the more diverse nature of contemporary Framingham, two multiethnic cohorts, were recruited with examination 4 of the Offspring (Omni 1) and examination 1 of the Third Generation (Omni 2). Offspring and Third Generation, and Omni 1 and 2 multi-ethnic cohorts were studied every four to eight years with standardized FHS examinations. At each examination, data on medical history, physical examination, and laboratory tests were collected. Regular health-status updates for cardiovascular disease included requests for hospital admission or outpatient records and ECGs.
SAECG was assessed in participants from the Offspring (Exam 9, 2011 – 2014), Omni 1 (Exam 4, 2011 – 2014), Offspring Spouse (Exam 2, 2008 – 2011), Generation 3 (Exam 2, 2008 – 2011), and Omni 2 (Exam 2, 2009 – 2011) cohorts (Figures 1–2). Exclusion of individuals with SAECG was based on criteria presented in Supplemental Tables 1–3. Reference values were analyzed in a subgroup of individuals without cardiovascular disease or risk factors (e.g., without obesity, smoking, hypertension, AF, diabetes, and cardiac medication use).
Figure 1.
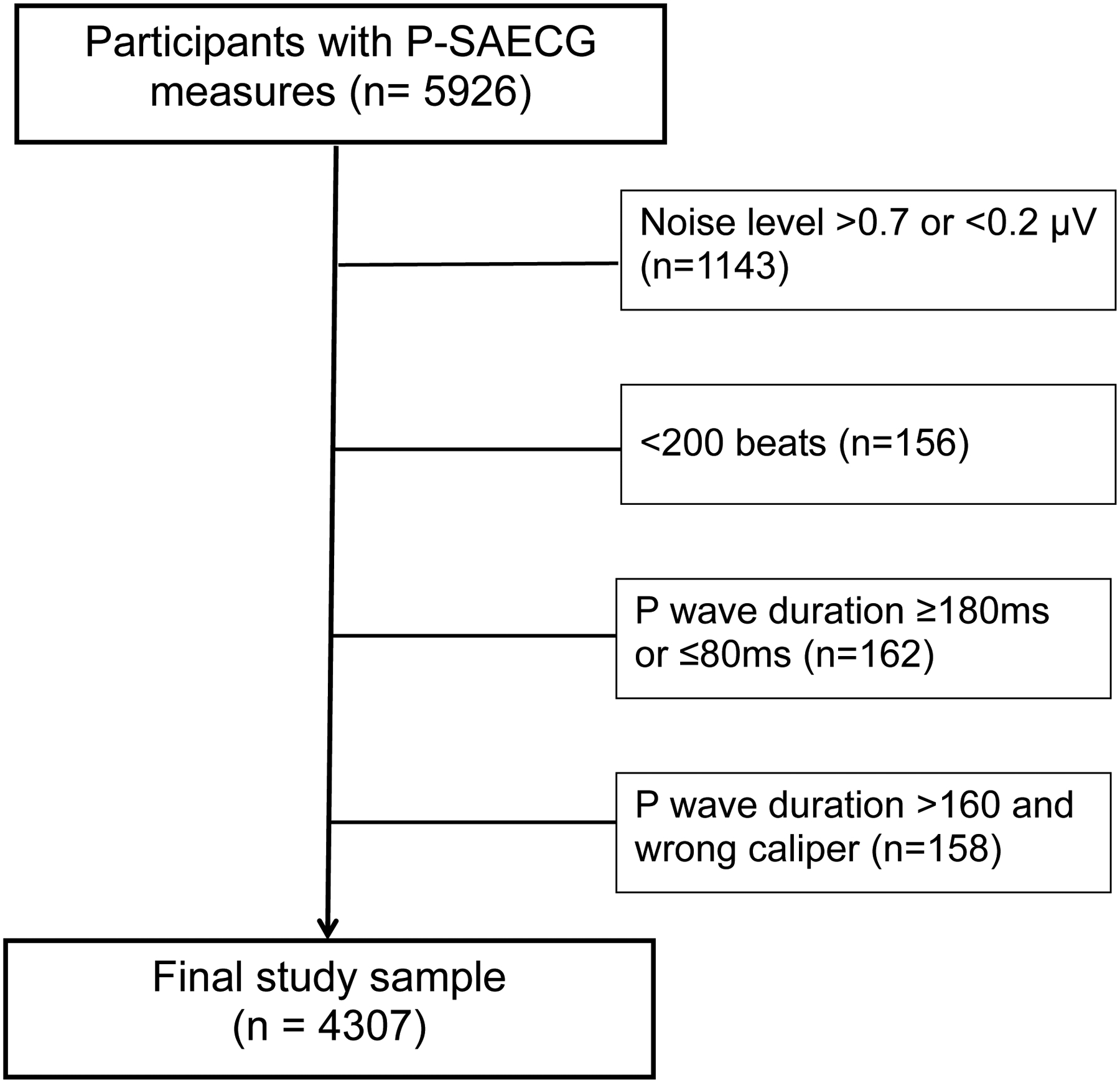
Representative P-SAECG in the Framingham Heart Study
Figure 2.
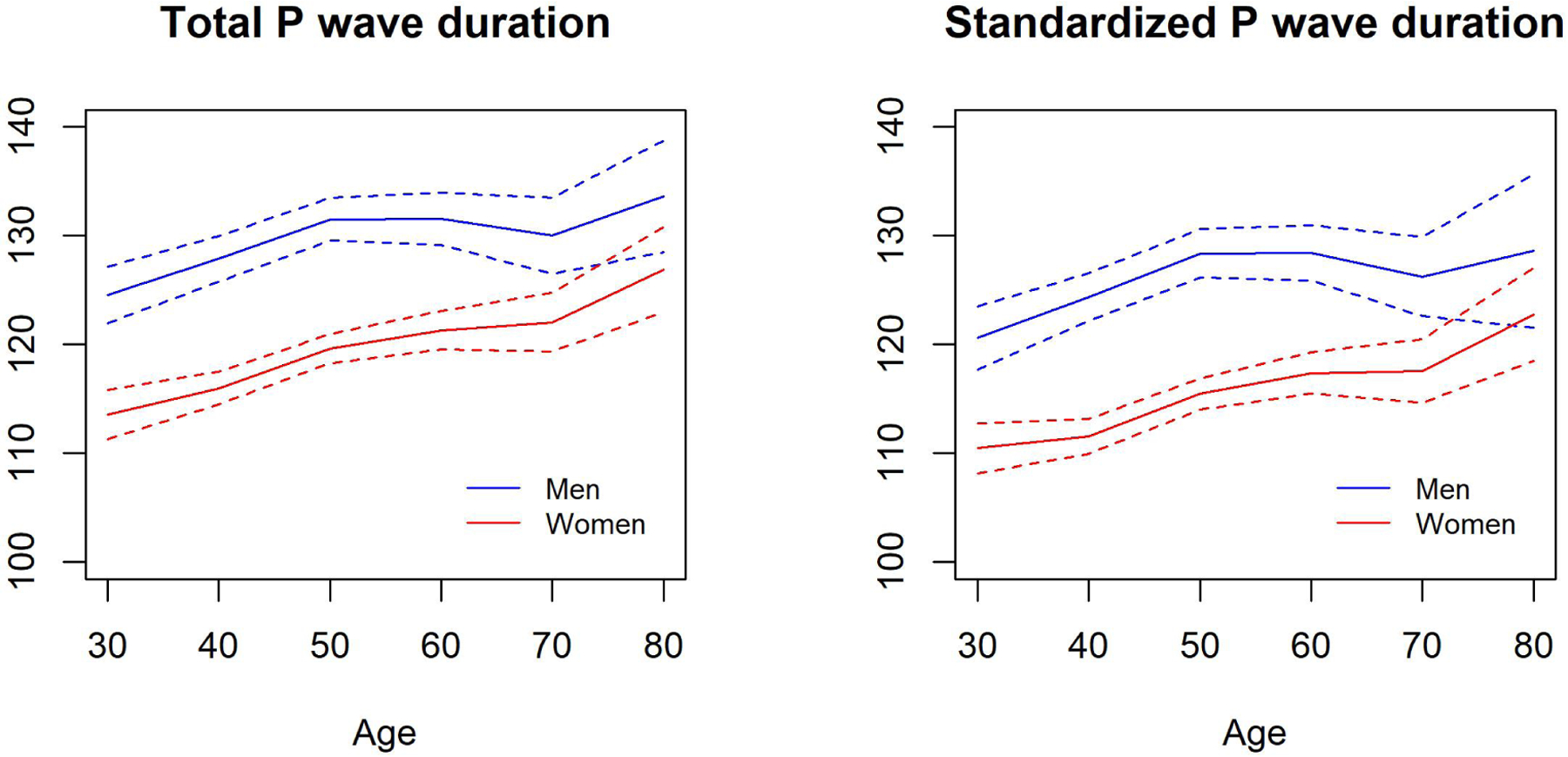
Flowchart of study cohort
The FHS protocol was approved by the Boston University Medical Campus Institutional Review Board and participants signed informed consent.
P-SAECG protocol and heritability estimation
Detailed P-SAECG protocol and heritability estimation are provided in the Supplement.
Covariate measurement
Research staff recorded age, sex, height, weight, body mass index, diabetes, systolic and diastolic blood pressure (BP), and self-reported smoking status, moderate/severe alcohol consumption, and antihypertensive medication use during each examination. Triglycerides and TC/HDL ratio were measured at the same examination when SAECG analysis was performed. Moderate/severe alcohol use was defined by self-report as weekly consumption of >14 drinks for men or more than >7 drinks for women. Heart rate was measured at rest from the 12-channel ECG performed on the day of SAECG analysis. If needed, further information was obtained from records supplied by hospital, attending physician, pathologist, medical examiner, or family. At least two clinical researchers reviewed FHS research examination results and outside clinician and hospital records to adjudicate a history of heart failure and myocardial infarction, as previously described.16
Statistical analysis
Reference values were defined as the median (2.5th and 97.5th percentiles). Linear mixed models were used to study the association of P-SAECG traits with multiple clinical factors, including age, sex, cohort, height, weight, smoking, systolic and diastolic BP, triglycerides, total/HDL cholesterol, waist, heart rate, alcohol consumption, diabetes, hypertension treatment, history of myocardial infarction, heart failure, and AF, atrioventricular (AV) node blockers (beta blockers and calcium antagonists of non-dihydropyridine type).
We examined the association of multiple clinical factors with P-SAECG traits by backward elimination. The model started with all clinical factors, and the association of each clinical factor with P-SAECG traits was assessed. The one with the least significant association was eliminated and the association of the remaining clinical factors with P-SAECG traits were reassessed. The procedure continued until all the remaining clinical factors were at least nominally two-sided significant (P<0.05). All models were adjusted for age, sex, and cohort.
All the statistical analyses were performed using R software version 3.6.0 (https://www.r-project.org/).
Results
Study population
In total, 4307 individuals were included in the analysis. The clinical characteristics of the study sample are presented in Table 1. The mean age of the study cohort was 55±14 years, 56% were women, 31% received antihypertensive treatment, 4% had prior myocardial infarction, and 3% prevalent AF. The P-SAECG traits are presented in Table 2.
Table 1.
Baseline characteristics of the whole study sample.
| Clinical characteristics+ | n=4307 |
|---|---|
| Age, years | 55 ± 14 |
| Female, n (%) | 2417 (56.1) |
| Systolic BP, mmHg | 120 ± 16 |
| Diastolic BP, mmHg | 74 ± 9 |
| Height, inches | 66 ± 4 |
| Weight, pounds | 174 ± 40 |
| Current smoking, n (%) | 338 (7.8) |
| Triglycerides, mg/dl | 113 ± 73 |
| Total/HDL cholesterol | 3.3. ± 1.1 |
| Waist circumference, inches / cm | 39 ± 6 / 98 ± 14 |
| Heart rate, beats/min | 64± 9 |
| Moderate/severe alcohol consumption, n (%) | 657 (15.3) |
| Diabetes, n (%) | 370 (8.6) |
| Hypertension treatment, n (%) | 1324 (30.7) |
| Prevalent myocardial infarction, n (%) | 182 (4.2) |
| Prevalent heart failure, n (%) | 28 (0.7) |
| Prevalent AF, n (%) | 109 (2.5) |
| AV node blockers, n (%) | 769 (17.9) |
Values are presented as n (%) for dichotomous variables and mean ± standard deviation or median (25th, 75th percentiles) for continuous variables
P value was calculated by the Fisher’ exact test for categorical variables, or Mann-Whitney U test for continuous variables.
Table 2.
Values of P-SAECG traits in the reference sample without cardiovascular disease or risk factors
| P-SAECG traits | Women (n=1079) Men (n=673) |
2.5% | 25% | 50% | 75% | 97.5% |
|---|---|---|---|---|---|---|
| Total P wave duration, ms | Women | 93 | 111 | 118 | 127 | 146 |
| Men | 104 | 120 | 128 | 140 | 158 | |
| Standardized P wave duration, ms | Women | 90 | 105 | 114 | 123 | 146 |
| Men | 101 | 115 | 124 | 138 | 157 | |
| Integral of P wave, μVms | Women | 252 | 504 | 637 | 798 | 1116 |
| Men | 286 | 512 | 666 | 810 | 1170 | |
| Root mean square voltage in terminal 20, mV | Women | 1 | 2 | 3 | 4 | 8 |
| Men | 1 | 2 | 3 | 4 | 8 | |
| Root mean square voltage in terminal 30 ms,−mV | Women | 1 | 3 | 4 | 6 | 9 |
| Men | 1 | 2 | 4 | 5 | 10 | |
| Root mean square voltage in terminal 40 ms, mV | Women | 2 | 4 | 5 | 7 | 10 |
| Men | 2 | 3 | 4 | 6 | 11 | |
| Root mean square voltage in the entire P wave, mV | Women | 3 | 6 | 7 | 9 | 12 |
| Men | 3 | 5 | 7 | 8 | 12 |
Reference values of P-SAECG traits
We derived the reference values of P-SAECG traits from 1752 participants without cardiovascular risk factors. As shown in Table 2, for the total PW duration, the median (2.5th percentile; 97.5th percentile) value was 118 ms (93; 146) in women, and 128 ms (104; 158) in men. For the standardized PW duration, the median (2.5th percentile; 97.5th percentile) value was 114 ms (90; 146) in women, and 124 ms (101; 157) in men. Whereas PW durations were significantly higher in men, all three RMS voltage traits in the terminal portion of the signal-averaged P wave (20, 30, and 40 ms) were higher in women (all P<0.001). Men and women had similar PW integrals. Distribution of total and standardized PW durations across different age groups are presented in Supplemental Table 4 and Figure 3. The means of both traits were higher with advancing age (P<0.001).
Figure 3.
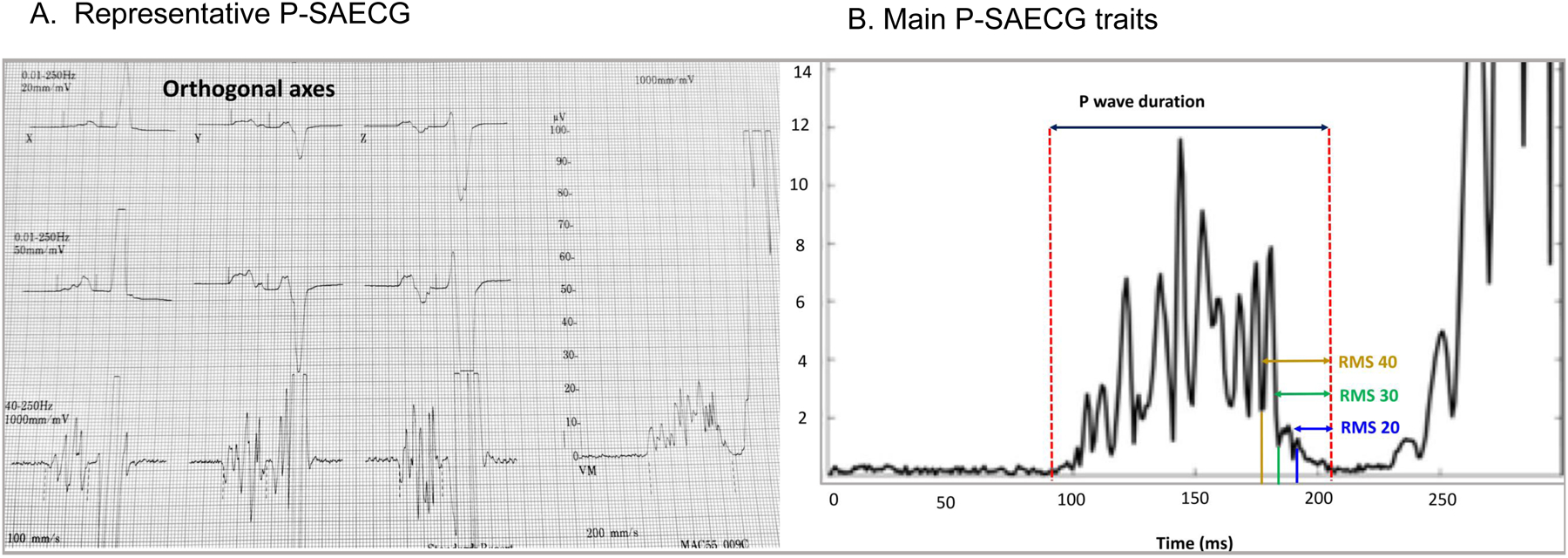
Distribution of total and standardized PW durations across different age in healthy participants.
The P-SAECG traits’ values in the entire broad study sample are presented in Supplemental Table 5.
Association of P-SAECG traits with clinical factors and resting ECG traits
Table 3 shows clinical factors significantly associated with each P-SAECG trait. Total and standardized PW durations were both positively associated with height, weight, history of heart failure, history of AF, and AV node blockers, and negatively associated with smoking, waist circumference, heart rate, and diabetes. The RMS PW was positively associated with smoking, and heart rate, and negatively associated with weight, total cholesterol, and history of AF. PW integral was positively associated with smoking and diastolic BP, and negatively associated with weight and total cholesterol. All three RMS traits in the terminal portion of the signal-averaged P wave (20, 30, and 40 ms) were positively associated with waist circumference and heart rate, and negatively associated with weight. RMS20 was additionally associated with smoking. There was no evidence of association between systolic BP, triglycerides, alcohol consumption, hypertension treatment, and history of MI with any SAECG traits in multivariable models. The association of each P-SAECG trait with each individual clinical factor is shown in Supplemental Table 6.
Table 3.
Multiple regression coefficients for SAECG traits
| Total P-wave duration | Standardized P-wave duration | Integral of P wave | RMS20 | RMS30 | RMS40 | RMS PW | |
|---|---|---|---|---|---|---|---|
| Height, inches | 0.30 | 0.24 | |||||
| Weight, pounds | 0.09 | 0.10 | −0.72 | −0.01 | −0.01 | −0.01 | −0.01 |
| Current smoking | −1.58 | −1.70 | 36.63 | 0.28 | 0.41 | ||
| Diastolic BP, mmHg | 0.92 | ||||||
| Total/HDL cholesterol | −11.68 | −0.10 | |||||
| Waist, inches | −0.32 | −0.33 | 0.04 | 0.04 | 0.04 | ||
| Heart rate, beat/min | −0.30 | −0.46 | 0.02 | 0.03 | 0.03 | 0.02 | |
| Diabetes | −1.96 | −2.40 | |||||
| Prevalent heart failure | 6.82 | 6.45 | |||||
| Prevalent AF | 4.55 | 5.08 | −0.52 | ||||
| AV node blockers | 2.03 | 1.93 |
Blank cells represent clinical factors that were not significant and thus eliminated into the final backward models. Regression coefficients per unit (continuous variables) or presence of condition (binary variables). Age, sex, and cohort were forced in all the models.
The following factors were not retained in any of the models: Systolic blood pressure, triglycerides, moderate/severe alcohol consumption, hypertension treatment, and prevalence myocardial infarction.
Abbreviations: SAECG – signal averaged electrocardiogram; RMSV20 – root mean square voltage in terminal 20 ms; RMS30 – root mean square voltage in terminal 30 ms; RMS40 – root mean square voltage in terminal 40 ms; RMS PW – root mean square voltage of the entire P wave; BP – blood pressure.
The associations adjusted for age, sex, and cohort between P-SAECG and 12-leads resting ECG traits are shown in Supplemental Table 7. Total and standardized PW durations were both positively associated with PR interval in 12-leads ECG, whereas RMS20, RMS30, RMS40, and RMS PW were negatively associated with PR interval.
Heritability
Table 4 shows the heritability estimation for each P-SAECG trait. In the simple model that was adjusted for age, sex, and cohort, total PW duration, standardized PW duration, PW integral, and RMS PW all showed modest heritability, whereas PW integral had the highest heritability of 26.1%. All three RMS traits in the terminal portion of the signal-averaged P wave (20, 30, and 40 ms) had limited but still significant heritability (all with P value<0.0001). The heritabilities were slightly attenuated in the multivariable model. PW integral remained the most heritable trait with 24.9% heritability.
Table 4.
Heritability and correlation between traits.
| Heritability | Total P-wave duration | Standardized P-wave duration | Integral of P wave | RMSV20 | RMSV30 | RMSV40 | RMS PW | ||
|---|---|---|---|---|---|---|---|---|---|
| Simple model* | Multivariable model+ | ||||||||
| Total P-wave duration | 24.6% | 21.6% | - | 0.99±0.01 | 0.20±0.11 | −0.41±0.13 | −0.42±0.13 | −0.29±0.15 | −0.05±0.12 |
| Standardized P-wave duration | 20.8% | 18.0% | 0.93±0.00 | - | 0.14±0.12 | −0.51±0.13 | −0.57±0.12 | −0.42±0.14 | −0.11±0.12 |
| Integral of P-wave | 26.1% | 24.9% | 0.12±0.04 | 0.03±0.04 | - | 0.22±0.03 | 0.38±0.03 | 0.50±0.03 | 0.50±0.03 |
| RMS20 | 15.0% | 14.3% | −0.54±0.03 | −0.52±0.03 | 0.34±0.15 | - | 0.84±0.01 | 0.72±0.02 | 0.34±0.03 |
| RMS30 | 12.7% | 11.9% | −0.49±0.03 | −0.48±0.03 | 0.44±0.14 | 0.98±0.04 | - | 0.89±0.01 | 0.49±0.03 |
| RMS40 | 12.2% | 12.3% | −0.45±0.03 | −0.45±0.03 | 0.72±0.10 | 0.76±0.10 | 0.88±0.05 | - | 0.60±0.02 |
| RMS PW | 24.7% | 23.0% | −0.13±0.04 | −0.19±0.04 | 0.72±0.10 | 0.44±0.14 | 0.56±0.12 | 0.80±0.09 | - |
Abbreviations: See Table 2.
Upper diagonal displays genetic correlations, and . Values are presented as correlation coefficients ± standard error
Simple model: Adjusted for age, sex, and cohort
Multivariable model: additionally adjusted for height, weight, smoking, systolic and diastolic blood pressure, triglycerides, total/HDL cholesterol, waist, heart rate, moderate/severe alcohol consumption, diabetes, hypertension treatment, prevalent myocardial infarction, prevalent AF, prevalent heart failure, and AV node blockers.
Table 4 also shows the genetic and environmental correlations between P-SAECG traits pairs. Strong genetic correlation (0.99) and environmental correlation (0.93) were observed between total and standardized PW durations. Both measures were negatively associated with RMS20, RMS30, RMS30, RMS PW, and positively associated with PW integral. For most trait pairs, similar patterns were observed between genetic and environmental correlations, and the genetic correlations tended to be slightly stronger than environmental correlations.
Discussion
In the current study we established reference values for P-SAECG traits in a large community-based cohort. This assessment of P-SAECG in a highly phenotyped and genotyped, multigenerational community-based cohort represents the most extensive assessment of these measures to date. In the whole study sample P-SAECG traits were associated with multiple AF-related risk factors such as diabetes, obesity, smoking, and history of heart failure. The traits were also moderately heritable; the highest heritability was observed for RMS PW.
Comparison with previous studies
Previous studies analyzed PW mostly using standardized ECG techniques. However, accuracy of PW analysis was limited as very low electric potentials in atrial myocardium were suppressed by 12-lead ECG filters.15 Therefore, high-resolution SAECG was introduced to record very low (microvolt) amplitude signals to increase the accuracy of PW measurement. The main issue behind P-SAECG was identification of individuals at high risk for AF and with AF-related outcomes (especially thromboembolic complications). Also, it was important to determine predisposing endophenotypes that can be dissected for mechanistic insights, including genetic risk, with the hope of identifying risk in the community.
A summary of clinical P-SAECG studies is presented in Supplemental Table 8. First attempts to use P-SAECG in AF were performed in late 1980s and were mainly negative probably due to small sample sizes, inaccurate PW sampling and SAECG data processing.14 Later technical improvements allowed the transferring from QRS-triggered to PW-triggered testing, and “P-wave layout” was introduced.17,18 Beside a filtered PW duration analysis, SAECG allows more advanced characteristics of the PW traits such as the amplitude of the terminal part of atrial depolarization (i.e., the terminal 40, 30, or 20 ms of the PW).19 In a first prospective study analyzing AF prediction, P-SAECG was significantly correlated with incident AF in patients undergoing cardiac surgery.2 Although PW duration was not prolonged in the 12-lead resting ECG, filtered PW duration using SAECG was significantly prolonged in individuals developing AF. Furthermore, AF incidence was ~4-fold higher in patients with P-SAECG >140ms. Later SAECG studies demonstrated an association between PW indices with recurrent AF after cardioversion3,20 or progression to chronic AF.21,22
We observed that all P-SAECG traits were associated with at least one clinical risk factor related with AF. PW duration and PR interval in 12-lead ECG were significantly higher in men compared to women, which could be partly explained by the larger heart and body size in men. In contrast, PW terminal depolarization traits were significantly higher in women, possibly due to the impact of neurohormonal processes.
Heritability
Heritability of ECG traits is essential for understanding population variance in disease presentation. Several common variants modulate heart rate, PR interval, and QRS complex durations.23–25 Heritability of ECG traits was analyzed using 12-lead resting ECGs, which can be considered as a 10 sec “snapshot” of cardiac electrical activity. Others have analyzed ECG heritability using 24h Holter monitoring.26 Heritability of different ECG traits vary from high (e.g., for RR interval reaching 40–98%) to moderate (e.g., for QT/QTc with 25–67%, PR interval with 34–46%, and QRS with 33–43%).23,27,28
To date, our analysis is the largest study investigating heritability of PW traits using P-SAECG. In contrast to previous studies analyzing heritability among different 12-lead ECG traits,27 we found only mild heritability among P-SAECG traits. The heritability of PW duration was only 22%. Nevertheless, the knowledge of P-SAECG heritability underscores genetic contributions to PW endophenotypes. Understanding the genetic predispotion to endophenotypes for complex diseases such as AF, may lead to future targeted medical therapy for AF prevention or treatment. Nevertheless, the knowledge of P-SAECG heritability underscores genetic contributions to PW endophenotypes. Understanding the genetic predisposing to endophenotypes for complex diseases such as AF, may lead to future targeted medical therapy for AF prevention or treatment. Furthermore, the genetic and environmental correlation contribute understanding, whether some traits are co-regulated by genetic factors or environmental factors.
Clinical implication
Atrial cardiomyopathy describes an atrial dysfunction due to structural (anatomical) and electrical remodeling.29 Although atrial cardiomyopathy can be a cause of AF, it may exist without AF as well.30 Thus, atrial premature beats or atrial tachycardia are markers of underlying atrial substrate and are associated with increased risk of stroke beyond AF.31 Therefore, invasive or non-invasive atrial electrocardiograms may help identifying individuals with underlying atrial cardiomyopathy and consequently with increased risk for cerebrovascular thromboembolic complications.
Currently, the CHA2DS2-VASc score is widely used to identify increased risk for thromboembolic complications in individuals with AF. However, those individuals at increased risk for stroke but without a history of AF are usually not considered for oral anticoagulation. In such cases, identification or detection of an atrial cardiomyopathy may enhance risk assessment for thromboembolic stroke or identify individuals meriting increased routine screening for unrecognized AF. In contrast, individuals without features of an atrial cardiomyopathy may not require oral coagulation independent on their CHA2DS2-VASc score. In contrast, identification of atrial cardiomyopathy after apparently successful catheter ablation (e.g., no arrhythmia recurrences but with features of atrial cardiomyopathy) would indicate necessity of continuation of oral anticoagulation. Therefore, P-SAECG as a high-fidelity analysis of P-wave might be useful to predict and prevent adverse outcomes in individuals at risk.
Strengths and limitations
The current study represents the largest epidemiological cohort with extensive measures of P-SAECG. With P-SAECG measures in over 4000 participants, we characterized distributions of different P-SAECG traits and identified multiple clinical factors associated with P-SAECG traits. The pedigree structure in FHS also allows the heritability estimation of P-SAECG traits, which may motivate the identification of genetic variants associated with P-SAECG traits and enable future targeted medical therapy.
We also acknowledge several limitations. First, the vast majority of the study participants are of European ancestry. The generalizability to other races/ethnicities is thus unknown. Also, the study population at the time of SAECG analysis was relatively young (mean age 55 years). Therefore, the reference ranges and heritability results might differ in older samples. The study was cross-sectional, and observational in nature; we cannot exclude residual confounding in our analyses of factors associated with the SAECG traits (e.g., sleep apnea, not routinely ascertained during exams). Advantages of SAECG include the incorporation of information from hundreds of data points and essential improvement of the reliability and accuracy. However, mentioning disadvantages, SAECG requires highly specialized equipment that is not in wide clinical usage. Also, to reach at least 250 signals for the qualitative registration and accurate analysis, acquisition times of up to 10 minutes are necessary for each individual. Furthermore, the nominal significance cutoff in our study was used to define significant associations, such that the association of some of clinical risk factors with P-SAECG traits may be by chance. Also, the difference in P-SAECG values was significant, but modest. Finally, more informed knowledge about the SAECG and facility of its application may increase its clinical usage, as the ECG is widely available, non-invasive, and low-cost assessment. Clinical significance needs to be established in future large-scale cohorts.
Conclusions
In our large community-based cohort, we observed that P-SAECG traits are associated with multiple AF-related risk factors such as height, weight, smoking, blood pressure, and prevalent heart failure. The P-SAECG traits were moderately heritable, and the highest heritability was observed for RMS PW. Further studies analyzing the impact of P-SAECG predicting incident AF and AF-related complications are needed. Also, the role of P-SAECG evaluating association with atrial cardiomyopathy in individuals at risk remain a clinical unmet need.
Supplementary Material
Acknowledgment
We acknowledge the dedication of the FHS study participants without whom this research would not be possible. We thank the GE representatives Robert M. Farrell and Michael J. Mysliwy for their constructive advice and help during manuscript revision addressing technical aspects of P-SAECG analysis.
Conflict of interests:
The Framingham Heart Study acknowledges the support of contracts NO1-HC-25195, HHSN268201500001I and 75N92019D00031 from the National Heart, Lung and Blood Institute. Dr. Kornej received funding from the Marie Sklodowska-Curie Actions under the European Union’s Horizon 2020 research and innovation programme (Agreement No 838259). Dr. Magnani is supported by NIH R01HL143010 and R33HL144669. Dr. Preis is supported by NIH grant 5R01HL128914-04. Dr. Trinquart is supported by the American Heart Association (18SFRN34150007). Dr. Ko is supported by American College of Cardiology Foundation/Merck Research Fellowship in Cardiovascular Diseases and Cardiometabolic Disorders. Dr. Benjamin was supported by NIH 2R01 HL092577; 1R01 HL141434 01A1; 2U54HL120163; 1R01AG066010; American Heart Association, AHA_18SFRN34110082. Dr. Lin is supported by the European Commission Grant (Agreement No 847770).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Rosiak M, Ruta J, Bolinska H. Usefulness of prolonged P-wave duration on signal averaged ECG in predicting atrial fibrillation in acute myocardial infarction patients. Med Sci Monit 2003;9:MT85–8. [PubMed] [Google Scholar]
- 2.Steinberg JS, Zelenkofske S, Wong SC, Gelernt M, Sciacca R, Menchavez E. Value of the P-wave signal-averaged ECG for predicting atrial fibrillation after cardiac surgery. Circulation 1993;88:2618–22. [DOI] [PubMed] [Google Scholar]
- 3.Budeus M, Hennersdorf M, Perings C, Wieneke H, Erbel R, Sack S. Prediction of the recurrence of atrial fibrillation after successful cardioversion with P wave signal-averaged ECG. Annals of noninvasive electrocardiology : the official journal of the International Society for Holter and Noninvasive Electrocardiology, Inc 2005;10:414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogawa M, Kumagai K, Vakulenko M, et al. Reduction of P-wave duration and successful pulmonary vein isolation in patients with atrial fibrillation. J Cardiovasc Electrophysiol 2007;18:931–8. [DOI] [PubMed] [Google Scholar]
- 5.Schumacher K, Dagres N, Hindricks G, Husser D, Bollmann A, Kornej J. Characteristics of PR interval as predictor for atrial fibrillation: association with biomarkers and outcomes. Clin Res Cardiol 2017;106:767–75. [DOI] [PubMed] [Google Scholar]
- 6.Magnani JW, Johnson VM, Sullivan LM, et al. P wave duration and risk of longitudinal atrial fibrillation in persons >/= 60 years old (from the Framingham Heart Study). Am J Cardiol 2011;107:917–21 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magnani JW, Zhu L, Lopez F, et al. P-wave indices and atrial fibrillation: cross-cohort assessments from the Framingham Heart Study (FHS) and Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2015;169:53–61 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magnani JW, Gorodeski EZ, Johnson VM, et al. P wave duration is associated with cardiovascular and all-cause mortality outcomes: the National Health and Nutrition Examination Survey. Heart Rhythm 2011;8:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arroja JD, Burri H, Park CI, Giraudet P, Zimmermann M. Electrophysiological abnormalities in patients with paroxysmal atrial fibrillation in the absence of overt structural heart disease. Indian Pacing Electrophysiol J 2016;16:152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babaev AA, Vloka ME, Sadurski R, Steinberg JS. Influence of age on atrial activation as measured by the P-wave signal-averaged electrocardiogram. Am J Cardiol 2000;86:692–5, A9. [DOI] [PubMed] [Google Scholar]
- 11.Gang Y, Hnatkova K, Mandal K, Ghuran A, Malik M. Preoperative electrocardiographic risk assessment of atrial fibrillation after coronary artery bypass grafting. J Cardiovasc Electrophysiol 2004;15:1379–86. [DOI] [PubMed] [Google Scholar]
- 12.Barbosa EC, Barbosa PR, Ginefra P, et al. The frequency analysis of signal-averaged ECG of P wave as predictor of efficacy of class III antiarrhythmic drugs to maintain sinus rhythm in recurrent idiopathic atrial fibrillation. Ann Noninvasive Electrocardiol 2001;6:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbosa EC, Benchimol-Barbosa PR, Bomfim Ade S, Rocha PJ, Boghossian SH, Albuquerque DC. Reversal atrial electrical remodeling following cardioversion of long-standing lone atrial fibrillation. Arq Bras Cardiol 2009;93:213–20. [DOI] [PubMed] [Google Scholar]
- 14.Engel TR, Vallone N, Windle J. Signal-averaged electrocardiograms in patients with atrial fibrillation or flutter. Am Heart J 1988;115:592–7. [DOI] [PubMed] [Google Scholar]
- 15.Palano F, Adduci C, Cosentino P, Silvetti G, Boldini F, Francia P. Assessing Atrial Fibrillation Substrates by P Wave Analysis: A Comprehensive Review. High Blood Press Cardiovasc Prev 2020. [DOI] [PubMed] [Google Scholar]
- 16.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med 2002;347:305–13. [DOI] [PubMed] [Google Scholar]
- 17.Michelucci A, Padeletti L, Chelucci A, et al. Influence of age, lead axis, frequency of arrhythmic episodes, and atrial dimensions on P wave triggered SAECG in patients with lone paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 1996;19:758–67. [DOI] [PubMed] [Google Scholar]
- 18.Stafford PJ, Turner I, Vincent R. Quantitative analysis of signal-averaged P waves in idiopathic paroxysmal atrial fibrillation. Am J Cardiol 1991;68:751–5. [DOI] [PubMed] [Google Scholar]
- 19.Fukunami M, Yamada T, Ohmori M, et al. Detection of patients at risk for paroxysmal atrial fibrillation during sinus rhythm by P wave - triggered signal-averaged electrocardiogram. Circulation 1991;83:162–9. [DOI] [PubMed] [Google Scholar]
- 20.Dixen U, Joens C, Parner J, Rasmussen V, Pehrson SM, Jensen GB. Prolonged signal-averaged P wave duration after elective cardioversion increases the risk of recurrent atrial fibrillation. Scand Cardiovasc J 2004;38:147–51. [DOI] [PubMed] [Google Scholar]
- 21.Budeus M, Felix O, Hennersdorf M, Wieneke H, Erbel R, Sack S. Prediction of conversion from paroxysmal to permanent atrial fibrillation. Pacing Clin Electrophysiol 2007;30:243–52. [DOI] [PubMed] [Google Scholar]
- 22.Dixen U, Larsen MV, Ravn L, Parner J, Jensen GB. Signal-averaged P wave duration and the long-term risk of permanent atrial fibrillation. Scand Cardiovasc J 2008;42:31–7. [DOI] [PubMed] [Google Scholar]
- 23.Holm H, Gudbjartsson DF, Arnar DO, et al. Several common variants modulate heart rate, PR interval and QRS duration. Nat Genet 2010;42:117–22. [DOI] [PubMed] [Google Scholar]
- 24.Pfeufer A, van Noord C, Marciante KD, et al. Genome-wide association study of PR interval. Nat Genet 2010;42:153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darbar D, Hardy A, Haines JL, Roden DM. Prolonged signal-averaged P-wave duration as an intermediate phenotype for familial atrial fibrillation. J Am Coll Cardiol 2008;51:1083–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodkinson EC, Neijts M, Sadrieh A, et al. Heritability of ECG Biomarkers in the Netherlands Twin Registry Measured from Holter ECGs. Front Physiol 2016;7:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva CT, Kors JA, Amin N, et al. Heritabilities, proportions of heritabilities explained by GWAS findings, and implications of cross-phenotype effects on PR interval. Hum Genet 2015;134:1211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith JG, Lowe JK, Kovvali S, et al. Genome-wide association study of electrocardiographic conduction measures in an isolated founder population: Kosrae. Heart Rhythm 2009;6:634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zipes DP. Atrial fibrillation. A tachycardia-induced atrial cardiomyopathy. Circulation 1997;95:562–4. [DOI] [PubMed] [Google Scholar]
- 30.Kottkamp H. Fibrotic atrial cardiomyopathy: a specific disease/syndrome supplying substrates for atrial fibrillation, atrial tachycardia, sinus node disease, AV node disease, and thromboembolic complications. J Cardiovasc Electrophysiol 2012;23:797–9. [DOI] [PubMed] [Google Scholar]
- 31.Larsen BS, Kumarathurai P, Falkenberg J, Nielsen OW, Sajadieh A. Excessive Atrial Ectopy and Short Atrial Runs Increase the Risk of Stroke Beyond Incident Atrial Fibrillation. J Am Coll Cardiol 2015;66:232–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


