Abstract
BACKGROUND:
The daily use of low-dose aspirin may be a safe, widely available, and inexpensive intervention for reducing the risk of preterm birth. Data on the potential side effects of low-dose aspirin use during pregnancy in low- and middle-income countries are needed.
OBJECTIVE:
This study aimed to assess differences in unexpected emergency medical visits and potential maternal side effects from a randomized, double-blind, multicountry, placebo-controlled trial of low-dose aspirin use (81 mg daily, from 6 to 36 weeks’ gestation).
STUDY DESIGN:
This study was a secondary analysis of data from the Aspirin Supplementation for Pregnancy Indicated Risk Reduction In Nulliparas trial, a trial of the Global Network for Women’s and Children’s Health conducted in India (2 sites), Pakistan, Guatemala, Democratic Republic of the Congo, Kenya, and Zambia. The outcomes for this analysis were unexpected emergency medical visits and the occurrence of the following potential side effects—overall and separately—nausea, vomiting, rash or hives, diarrhea, gastritis, vaginal bleeding, allergic reaction, and any other potential side effects. Analyses were performed overall and by geographic region.
RESULTS:
Between the aspirin (n=5943) and placebo (n=5936) study groups, there was no statistically significant difference in the risk of unexpected emergency medical visits or the risk of any potential side effect (overall). Of the 8 potential side effects assessed, only 1 (rash or hives) presented a different risk by treatment group (4.2% in the aspirin group vs 3.5% in the placebo group; relative risk, 1.20; 95% confidence interval, 1.01–1.43; P=.042).
CONCLUSION:
The daily use of low-dose aspirin seems to be a safe intervention for reducing the risk of preterm birth and well tolerated by nulliparous pregnant women between 6 and 36 weeks’ gestation in low- and middle-income countries.
Keywords: low- and middle-income countries, low-dose aspirin, potential side effects, pregnancy, preterm birth, safety, unexpected emergency medical visits
Introduction
Preterm birth, defined as birth before 37 weeks’ gestation, remains a major global health issue. An estimated 15 million babies are born preterm each year, and this number is increasing.1 Globally, preterm birth complications are the leading cause of death among children under 5 years of age, responsible for approximately 1 million deaths each year.2 Higher rates of preterm birth are observed in low- and middle-income countries (LMICs),3,4 where the average preterm birth rate is 12% in LMICs compared with 9% in higher-income countries.1
Although the causes of preterm birth are complex and vary across countries and regions,5 there is evidence that low-cost interventions may be universally effective at reducing preterm births.6–9 A meta-analysis of previous low-dose aspirin (LDA) trials that included 35,212 women reported a 9% reduction in the rate of preterm birth.6 Studies in high-income countries (United States, Europe) have reported similar effects of LDA on preterm birth, in those with a high risk for preeclampsia.8,9 The Aspirin Supplementation for Pregnancy Indicated Risk Reduction In Nulliparas (ASPIRIN) trial involving nearly 12,000 women from 6 LMICs reported a reduced incidence of preterm delivery among nulliparous women with singleton pregnancies who took daily LDA.7 Specifically, women taking daily LDA were 11% less likely to deliver before the completion of pregnancy at 37 weeks’ gestation, compared with women given a placebo. In addition, there was a 25% reduction in the rate of early preterm birth (<34 weeks’ gestation).
Given the low cost of aspirin and its widespread availability, adoption of LDA to prevent preterm birth may be warranted. However, to ensure generalizability of the use of LDA, data on the side effects of its use during pregnancy in LMICs are needed. Consequently, this study aimed to assess the risk of potential side effects and unexpected emergency medical visits associated with daily LDA use among participants in the ASPIRIN trial.10 We hypothesized that the risk of potential side effects would not differ between the LDA and placebo groups.
Materials and Methods
This study was a secondary analysis of data from the ASPIRIN trial, a multinational, randomized, double-blind, placebo-controlled trial, to assess the efficacy of LDA (81 mg) administered daily and initiated early in pregnancy through 36 completed weeks for the reduction of preterm birth. The ASPIRIN trial occurred at 7 community sites in 6 countries (2 sites in India and 1 site each in Pakistan, Zambia, the Democratic Republic of the Congo, Guatemala, and Kenya) between March 23, 2016, and April 11, 2019. Nulliparous pregnant women aged 18 to 40 years (women of younger ages in Africa were permitted) and between 6 weeks and 0 days of pregnancy and 13 weeks and 6 days of pregnancy, confirmed by ultrasound for dating, who resided in the study areas were eligible to enroll in this trial. The exclusion criteria included a medical contraindication to aspirin, previous prescription of aspirin for more than 7 days during the pregnancy, multiple gestation, more than 2 first-trimester pregnancy losses, hemoglobin levels of <7.0 g/dL at screening, systolic blood pressure of ≥140 mm Hg and diastolic blood pressure of ≥90 mm Hg at screening, and other medical conditions that might be considered a contraindication based on the judgment of the site investigator. Eligible and consented women were randomly assigned (1:1) to receive aspirin or an identical-appearing placebo. Participants were prescribed a daily dose of 81 mg of aspirin or placebo from the time of randomization to completion of 36 weeks’ gestation or the time of delivery if delivery occurred before that time. The study protocol was approved by the relevant ethics committees and regulatory agencies of each country, including the ethics committees of the collaborators based in the United States and that of the Research Triangle Institute International. Written informed consent for study participation was obtained from all participants. The trial was registered with ClinicalTrials.gov (NCT02409680) and the Clinical Trials Registry-India (CTRI/2016/05/006970). Additional study details, including the study protocol, have been previously published.7,10
Study sample
The population for this analysis includes all randomized women who consumed a dose of LDA (n=5943) or placebo (n=5936) irrespective of the amount of medication received or duration of treatment (safety population).
Data collection
Eligibility was determined by performing a brief physical examination, reviewing medical history, and asking about the use of other medicines or medicinal products. Eligible participants were consented and randomized. Participants met with study staff biweekly. These visits included the monitoring of potential side effects. This process was continued until completion of 36 weeks’ gestation or until an earlier delivery occurred. During this same time frame, any unexpected or emergency medical visits reported from participants and/or documented in medical records were also documented.
Demographic, clinical, and delivery characteristics
Maternal demographic characteristics were collected at study baseline and included maternal age, number of previous pregnancies, gestational age at enrollment, highest level of education, and height and weight to calculate body mass index. Pregnancy and delivery characteristics included number of antenatal care visits, type of delivery attendant, delivery location, and delivery mode.
Outcomes
The primary outcomes for this analysis included at least 1 unexpected emergency medical visit (yes or no), at least 1 episode of any potential side effect (yes or no), and at least 1 episode of the following potential side effects (yes or no)—separately—nausea, vomiting, rash or hives, diarrhea, gastritis, vaginal bleeding, allergic reaction, and any other potential side effects. Unexpected medical visit occurrences were obtained by interview at scheduled biweekly visits and by review of available medical records. The presence or absence of potential side effects were assessed by interview at scheduled, biweekly visits. Participants were asked directly whether they had experienced any of the listed symptoms since the previous biweekly visit. Each response was read aloud to the participant. The list of symptoms was adapted from a listing used in a similar trial.8 For the side effect of allergic reaction, if the participant indicated that she experienced an allergic reaction, she was asked to specify if the reaction was mild, severe, or anaphylactic. The specific side effect linked to the allergic reaction was not collected. Potential side effects reported at an unexpected emergency medical visit were excluded from these outcomes as they would be collected differentially across participants and sites. Secondary outcomes for this analysis were the number of unexpected emergency visits, the number of biweekly visits with any potential side effect reported, and the number of biweekly visits with each potential side effect reported.
Statistical analysis
Demographic and baseline characteristics were summarized by treatment arm. Categorical variables were summarized by frequency, normally distributed continuous variables were summarized by mean and standard deviation, and nonnormally distributed continuous variables were summarized as median with 25th and 75th percentiles. Outcomes were summarized by treatment arm overall and by region.
Relative risks (RRs) and associated 95% confidence intervals (CI) of the aspirin intervention compared with the placebo intervention were obtained for outcomes via generalized linear models adjusting for site. The dichotomous event models used a log link and binomial distribution. The models for event count outcomes used a log link and Poisson distribution, offset by a log-transformed number of visits to account for the number of biweekly visits completed. In addition, the count outcome models were subset to only include participants with at least 1 episode of the outcome. This subset allowed the assessment of outcome burden among those reporting any such events. In addition, all models were run, including geographic region (Africa, India, Pakistan, Guatemala) and the region by treatment interaction to obtain region-specific effect estimates. All data were analyzed with Statistical Analysis Software (version 9.4; SAS Institute, Cary, NC). Because the analyses of these secondary ASPIRIN study outcomes were exploratory in nature, no adjustment for multiplicity was made.
Role of the funding source
Staff from the funder Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) had input into the study design, data interpretation, and reviewed and approved this report. Nonetheless, the authors’ views do not necessarily represent those of the NICHD. The corresponding author had final responsibility for the decision to submit for publication.
Results
A total of 11,879 participants were included in this analysis; 97 participants (47 in the aspirin group and 50 in the placebo group) were excluded because they were never exposed to a study treatment (Figure). The proportion of participants in each treatment arm was distributed evenly; 50% (n=5943) were in the aspirin arm and 50% (n=5936) were in the placebo arm. Characteristics of study participants are shown in Table 1. Furthermore, 41% of the participants were from India (Belagavi, Nagpur); 31% were from Africa (Democratic Republic of the Congo, Zambia, Kenya); 14% were from Pakistan; and 14% were from Guatemala. Most participants were 20 to 29 years of age (59%), had no previous pregnancy losses (91%), and completed less than 13 years of formal schooling (89%). The median gestational age at enrollment was 10 weeks (range, 6–14), and the median number of antenatal care visits was 4. Nearly three-quarters of participants delivered vaginally (72%), and more than half delivered at a hospital (60%). Characteristics were similar between the aspirin and placebo study groups (Table 1). As previously reported, 85% of women were adherent (defined as taking 90% or more of the medication or placebo).7
FIGURE. Consort for safety analysis.
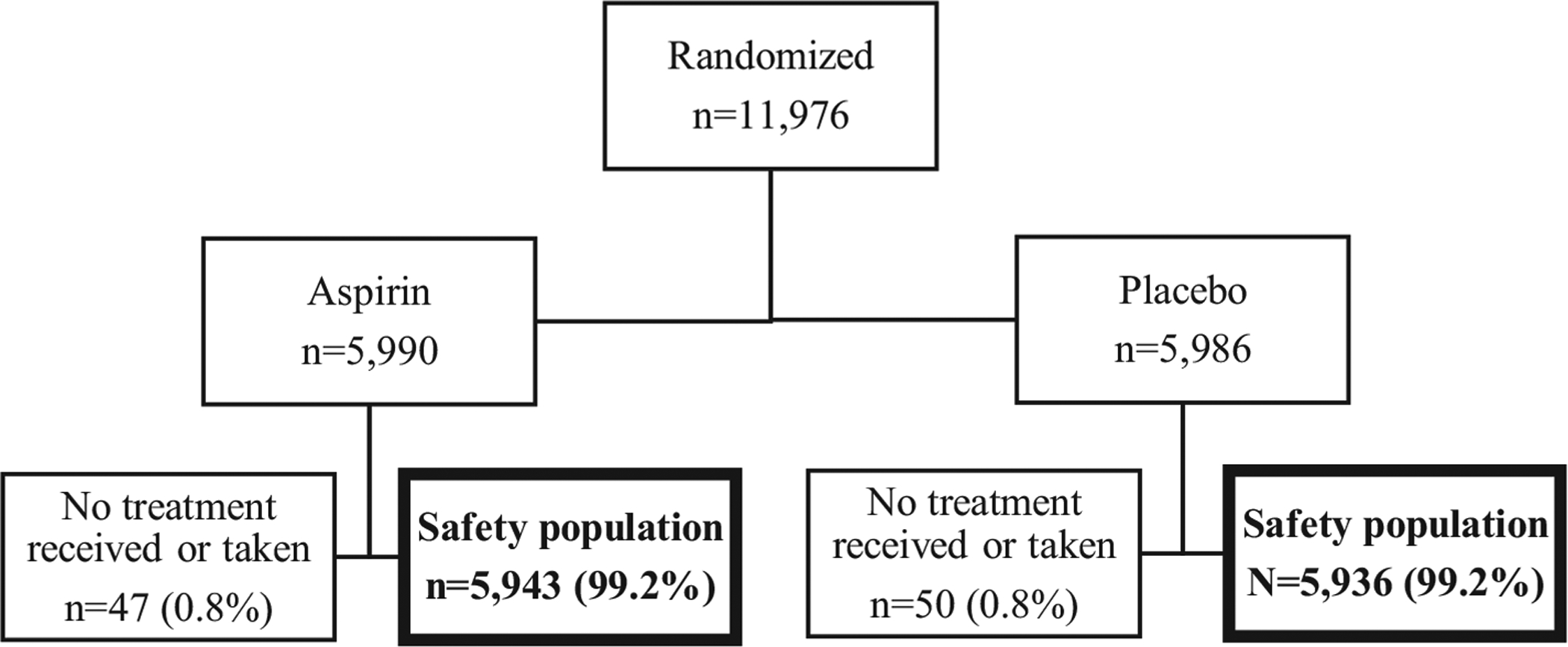
Aspirin Supplementation for Pregnancy Indicated Risk Reduction In Nulliparas trial.
TABLE 1.
Baseline demographic and site of delivery characteristics: Aspirin Supplementation for Pregnancy Indicated Risk Reduction In Nulliparas trial
| Variable | Total (N=11,879) | Aspirin (n=5943) | Placebo (n=5936) |
|---|---|---|---|
| Maternal age (y) | |||
| <20 | 4636 (39) | 2299 (39) | 2337 (39) |
| 20–29 | 6977 (59) | 3514 (59) | 3463 (58) |
| >29 | 266 (2) | 130 (2) | 136 (2) |
| Number of previous pregnancies not resulting in delivery | |||
| 0 | 10,780 (91) | 5412 (91) | 5368 (90) |
| 1 | 950 (8) | 466 (8) | 484 (8) |
| 2 | 149 (1) | 65 (1) | 84 (1) |
| Gestational age at enrollment (wk) | 10.1 (8.6–12.0) | 10.0 (8.6–12.0) | 10.1 (8.6–12.0) |
| Maternal education | |||
| No formal schooling | 1743 (15) | 874 (15) | 869 (15) |
| 1–6 y of schooling | 1757 (15) | 875 (15) | 882 (15) |
| 7–12 y of schooling | 7085 (60) | 3546 (60) | 3539 (60) |
| ≥13 y of schooling | 1292 (11) | 647 (11) | 645 (11) |
| Maternal height (cm) | 153.1 (7.0) | 153.1 (6.9) | 153.1 (7.0) |
| Maternal weight (kg) | 49.2 (8.9) | 49.3 (9.0) | 49.2 (8.7) |
| Maternal BMI (kg/m2) | 21.0 (3.8) | 21.0 (3.8) | 21.0 (3.7) |
| Antenatal care visits | 5 (4–6) | 5 (4–6) | 5 (4–6) |
| Delivery attendant | |||
| Physician | 5947 (50) | 2991 (50) | 2956 (50) |
| Nurse or nurse midwife | 4509 (38) | 2255 (38) | 2254 (38) |
| Traditional birth attendant | 947 (8) | 472 (8) | 475 (8) |
| Family, self, or other | 430 (4) | 208 (4) | 222 (4) |
| Delivery location | |||
| Hospital | 7077 (60) | 3535 (60) | 3542 (60) |
| Clinic or health center | 3625 (31) | 1836 (31) | 1789 (30) |
| Home or other | 1135 (10) | 557 (9) | 578 (10) |
| Delivery mode | |||
| Vaginal | 8573 (72) | 4252 (72) | 4321 (73) |
| Cesarean delivery | 2962 (25) | 1523 (26) | 1439 (24) |
| Miscarriage | 236 (2) | 112 (2) | 124 (2) |
| MTP | 66 (1) | 41 (1) | 25 (<1) |
| Site | |||
| DRC | 1361 (11) | 678 (11) | 683 (12) |
| Zambia | 1008 (8) | 502 (8) | 506 (9) |
| Guatemala | 1701 (14) | 850 (14) | 851 (14) |
| Belagavi, India | 2750 (23) | 1375 (23) | 1375 (23) |
| Pakistan | 1625 (14) | 821 (14) | 804 (14) |
| Nagpur, India | 2068 (17) | 1036 (17) | 1032 (17) |
| Kenya | 1366 (11) | 681 (11) | 685 (12) |
Data are presented as number (percentage), median (Q1–Q3), or median (standard deviation).
BMI, body mass index; DRC, Democratic Republic of the Congo; MTP, medical termination of pregnancy.
Short. Aspirin safety during pregnancy in low- and middle-income countries. Am J Obstet Gynecol Glob Rep 2021.
A total of 11,736 (99%) participants completed at least 1 biweekly safety interview comprising a total of 147,565 separate interviews, and most participants (90%) completed 10 or more of the maximum possible 15 biweekly visits. In addition, 7349 unexpected medical visit interviews were conducted. Among those who had at least 1 unexpected visit, most participants (55%) had only the 1 unexpected visit, 78% to 79% had 1 or 2 unexpected visits (range, 1–9 for both treatment groups).
Among participants, one-third (34%) had at least 1 unexpected visit, and about one-half (49%) had at least 1 potential side effect during the study period (Table 2). The most common reported potential side effects among all participants were nausea and vomiting. Among all participants, 29% had at least 1 episode of nausea, and 27% had at least 1 episode of vomiting. In addition, more than a quarter of participants (28%) reported at least 1 episode of an “other” potential side effect. The most common “other” side effects reported were cold/cough, fever, abdominal pain or discomfort, and headache.
TABLE 2.
Unexpected medical visit and potential side effects: Aspirin Supplementation for Pregnancy Indicated Risk Reduction In Nulliparas trial
| Variable | Total (N=11,879) | Aspirin (n=5943) | Placebo (n=5936) | RR (95% CI) | P value |
|---|---|---|---|---|---|
| Unexpected emergency medical visit | |||||
| At least 1 unexpected emergency medical visit | 3989 (33.6) | 2015 (33.9) | 1974 (33.3) | 1.02 (0.98–1.06) | .247 |
| Number of unexpected medical visits | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.97 (0.93–1.02) | .223 |
| Biweekly monitoring of potential side effects | |||||
| Any potential side effect | |||||
| At least 1 episode | 5857 (49.3) | 2947 (49.6) | 2910 (49.0) | 1.00 (0.98–1.03) | .804 |
| Number of potential side effects, median | 3 (1–7) | 3 (1–7) | 3 (1–6) | 0.98 (0.95–1.00) | .040 |
| Nausea | |||||
| At least 1 episode | 3402 (28.6) | 1691 (28.5) | 1711 (28.8) | 0.98 (0.94–1.03) | .505 |
| Number of episodes | 2 (1–3) | 2 (1–3) | 2 (1–3) | 1.01 (0.97–1.05) | .679 |
| Vomiting | |||||
| At least 1 episode | 3198 (26.9) | 1587 (26.7) | 1611 (27.1) | 0.99 (0.94–1.04) | .641 |
| Number of episodes | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.99 (0.95–1.04) | .677 |
| Rash or hives | |||||
| At least 1 episode | 458 (3.9) | 251 (4.2)a | 207 (3.5)a | 1.20 (1.01–1.43)a | .042a |
| Number of episodes | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.97 (0.83–1.13) | .674 |
| Diarrhea | |||||
| At least 1 episode | 877 (7.4) | 452 (7.6) | 425 (7.2) | 1.06 (0.94–1.20) | .342 |
| Number of episodes | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0.94 (0.83–1.05) | .279 |
| Gastritis | |||||
| At least 1 episode | 1752 (14.7) | 853 (14.4) | 899 (15.1) | 0.94 (0.87–1.02) | .149 |
| Number of episodes | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.97 (0.91–1.04) | .406 |
| Vaginal bleeding | |||||
| At least 1 episode | 457 (3.8) | 214 (3.6) | 243 (4.1) | 0.88 (0.73–1.05) | .150 |
| Number of episodes | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1.03 (0.86–1.22) | .778 |
| Allergic reaction | |||||
| At least 1 episode | 45 (0.4) | 22 (0.4) | 23 (0.4) | 0.96 (0.53–1.71) | .882 |
| Number of episodes | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0.99 (0.57–1.73) | .982 |
| Other potential side effect | |||||
| At least 1 episode | 3313 (27.9) | 1673 (28.2) | 1640 (27.6) | 1.01 (0.97–1.06) | .611 |
| Number of episodes | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.99 (0.95–1.04) | .696 |
Data are presented as number (percentage) or median (25th percentile–75th percentile). Unexpected emergency medical visits may have been related to potential side effects but are analyzed separately from potential side effects reported at biweekly visits. For “at least 1” variables, the denominator is any participant included in the safety population, and the numerator is participants with at least 1 report of the event. For “number of” variables, the denominator is any participant included in the safety population who experienced at least 1 episode of that specific event, and the numerator is the number of events of that specific type experienced by the participant. In addition, the “number of” RR models have been adjusted for the number of biweekly visits completed (range, 1–16). The most commonly reported other potential side effects include cold/cough or fever, abdominal pain or discomfort, and headache.
CI, confidence interval; RR, relative risk.
P<.05.
Short. Aspirin safety during pregnancy in low- and middle-income countries. Am J Obstet Gynecol Glob Rep 2021.
Between the aspirin and placebo study groups, there was no significant difference in the risk of unexpected medical visit. Of the 8 potential side effects assessed, only 1 (rash or hives) presented a difference in risk by treatment group (4.2% in the aspirin group vs 3.5% in the placebo group RR, 1.20; 95% CI, 1.01–1.43; P=.042).
We observed an interaction between region and treatment for the risk of participants having at least 1 episode of an unexpected medical visit and at least 1 episode of rash or hives (P=.002 and P=.007, respectively); Table 3. For participants in the African sites, those in the aspirin group were at greater risk of unexpected medical visits (RR, 1.25; 95% CI, 1.09–1.43) and rash or hives (RR, 2.18; 95% CI, 1.25–3.80). Conversely, for these same outcomes, participants in the Guatemalan site in the aspirin group were at lower risk of experiencing unexpected medical visits (RR, 0.74; 95% CI, 0.57–0.95) and rash or hives (RR, 0.58; 95% CI, 0.34–0.99). All other regions observed no difference in risk for these outcomes, and no other outcome had a significant interaction of region and treatment (Tables 3 and 4).
TABLE 3.
Unexpected medical visits and potential side effects by region: Aspirin Supplementation for Pregnancy Indicated Risk Reduction In Nulliparas trial
| Variable | Int P value | Total (n=11,879) | Africa (n=3735) | India (n=4818) | Pakistan (n=1625) | Guatemala (n=1701) |
|---|---|---|---|---|---|---|
| Participants with at least 1 unexpected emergency medical visit, RR (95% CI) | .002 | 0.98 (0.90–1.06) | 1.25 (1.09–1.43) | 1.00 (0.94–1.06) | 0.99 (0.88–1.11) | 0.74 (0.57–0.95) |
| Aspirin | 2015/5943 (33.9) | 368/1861 (19.8) | 1228/2411 (50.9) | 330/821 (40.2) | 89/850 (10.5) | |
| Placebo | 1974/5936 (33.3) | 297/1874 (15.8) | 1228/2407 (51.0) | 328/804 (40.8) | 121/851 (14.2) | |
| Biweekly monitoring of potential side effects | ||||||
| Participants with at least 1 potential side effect, RR (95% CI) | .423 | 1.01 (0.97–1.06) | 1.06 (0.97–1.17) | 0.99 (0.94–1.03) | 1.03 (0.97–1.08) | 0.98 (0.85–1.12) |
| Aspirin | 2947/5943 (49.6) | 625/1861 (33.6) | 1434/2411 (59.5) | 625/821 (76.1) | 263/850 (30.9) | |
| Placebo | 2910/5936 (49.0) | 591/1874 (31.5) | 1452/2407 (60.3) | 597/804 (74.3) | 270/851 (31.7) | |
| Participants with at least 1 episode of nausea, RR (95% CI) | .927 | 0.99 (0.92–1.06) | 1.00 (0.85–1.17) | 0.97 (0.89–1.04) | 1.00 (0.93–1.09) | 0.99 (0.80–1.22) |
| Aspirin | 1691/5943 (28.5) | 252/1861 (13.5) | 817/2411 (33.9) | 482/821 (58.7) | 140/850 (16.5) | |
| Placebo | 1711/5936 (28.8) | 255/1874 (13.6) | 844/2407 (35.1) | 470/804 (58.5) | 142/851 (16.7) | |
| Participants with at least 1 episode of vomiting, RR (95% CI) | .295 | 0.94 (0.86–1.03) | 0.94 (0.78–1.13) | 0.97 (0.90–1.05) | 1.05 (0.96–1.15) | 0.81 (0.61–1.09) |
| Aspirin | 1587/5943 (26.7) | 199/1861 (10.7) | 881/2411 (36.5) | 434/821 (52.9) | 73/850 (8.6) | |
| Placebo | 1611/5936 (27.1) | 213/1874 (11.4) | 903/2407 (37.5) | 405/804 (50.4) | 90/851 (10.6) | |
| Participants with at least 1 episode of rash or hives, RR (95% CI) | .007 | 1.19 (0.95–1.47) | 2.18 (1.25–3.80) | 1.33 (0.96–1.84) | 1.16 (0.90–1.51) | 0.58 (0.34–0.99) |
| Aspirin | 251/5943 (4.2) | 39/1861 (2.1) | 84/2411 (3.5) | 107/821 (13.0) | 21/850 (2.5) | |
| Placebo | 207/5936 (3.5) | 18/1874 (1.0) | 63/2407 (2.6) | 90/804 (11.2) | 36/851 (4.2) | |
| Participants with at least 1 episode of diarrhea, RR (95% CI) | .919 | 1.05 (0.91–1.23) | 1.15 (0.85–1.55) | 1.06 (0.89–1.26) | 0.98 (0.69–1.40) | 1.03 (0.73–1.46) |
| Aspirin | 452/5943 (7.6) | 88/1861 (4.7) | 246/2411 (10.2) | 56/821 (6.8) | 62/850 (7.3) | |
| Placebo | 425/5936 (7.2) | 77/1874 (4.1) | 232/2407 (9.6) | 56/804 (7.0) | 60/851 (7.1) | |
| Participants with at least 1 episode of gastritis, RR (95% CI) | .380 | 0.99 (0.89–1.10) | 1.07 (0.84–1.36) | 0.91 (0.80–1.02) | 0.90 (0.76–1.05) | 1.10 (0.84–1.44) |
| Aspirin | 853/5943 (14.4) | 124/1861 (6.7) | 415/2411 (17.2) | 214/821 (26.1) | 100/850 (11.8) | |
| Placebo | 899/5936 (15.1) | 117/1874 (6.2) | 457/2407 (19.0) | 234/804 (29.1) | 91/851 (10.7) | |
| Participants with at least 1 episode of vaginal bleeding, RR (95% CI) | .349 | 0.83 (0.69–1.02) | 1.05 (0.71–1.54) | 0.92 (0.69–1.21) | 0.85 (0.59–1.24) | 0.59 (0.36–0.98) |
| Aspirin | 214/5943 (3.6) | 52/1861 (2.8) | 91/2411 (3.8) | 48/821 (5.8) | 23/850 (2.7) | |
| Placebo | 243/5936 (4.1) | 50/1874 (2.7) | 99/2407 (4.1) | 55/804 (6.8) | 39/851 (4.6) | |
| Participants with at least 1 episode of allergic reaction, RR (95% CI) | .823 | 0.84 (0.39–1.78) | 1.34 (0.47–3.86) | 0.75 (0.26–2.15) | 0.49 (0.04–5.39) | 1.00 (0.35–2.84) |
| Aspirin | 22/5943 (0.4) | 8/1861 (0.4) | 6/2411 (0.2) | 1/821 (0.1) | 7/850 (0.8) | |
| Placebo | 23/5936 (0.4) | 6/1874 (0.3) | 8/2407 (0.3) | 2/804 (0.2) | 7/851 (0.8) | |
| Participants with at least 1 episode of other potential side effect, RR (95% CI) | .677 | 1.03 (0.94–1.13) | 1.10 (0.95–1.28) | 1.00 (0.94–1.07) | 0.98 (0.86–1.12) | 1.03 (0.76–1.39) |
| Aspirin | 1673/5943 (28.2) | 305/1861 (16.4) | 1013/2411 (42.0) | 277/821 (33.7) | 78/850 (9.2) | |
| Placebo | 1640/5936 (27.6) | 279/1874 (14.9) | 1008/2407 (41.9) | 277/804 (34.5) | 76/851 (8.9) | |
Data are presented number/total number (percentage), unless otherwise specified. Unexpected emergency medical visits may have been related to potential side effects but are analyzed separately from potential side effects reported at biweekly visits. The denominator is any participant included in the safety population, and the numerator is participants with at least 1 report of the event. The most commonly reported other potential side effects include cold/cough or fever, abdominal pain or discomfort, and headache.
CI, confidence interval; Int, interaction; RR, relative risk.
Short. Aspirin safety during pregnancy in low- and middle-income countries. Am J Obstet Gynecol Glob Rep 2021.
TABLE 4.
Unexpected medical visit and potential side effects counts by region: Aspirin Supplementation for Pregnancy Indicated Risk Reduction In Nulliparas trial
| Variable | Int P value | Total (n=11,879) | Africa (n=3735) | India (n=4818) | Pakistan (n=1625) | Guatemala (n=1701) |
|---|---|---|---|---|---|---|
| Number of unexpected emergency medical visit, RR (95% CI) | .785 | 0.96 (0.89–1.04) | 1.01 (0.88–1.16) | 0.99 (0.93–1.04) | 0.94 (0.84–1.06) | 0.91 (0.70–1.18) |
| Aspirin | 1 (1–2) | 1 (1–1) | 2 (1–3) | 1 (1–2) | 1 (1–1) | |
| Placebo | 1 (1–2) | 1 (1–1) | 2 (1–3) | 1 (1–2) | 1 (1–1) | |
| Biweekly monitoring of potential side effects | ||||||
| Number of potential side effects, RR (95% CI) | .003 | 0.96 (0.93–0.99) | 0.98 (0.91 −1.06) | 1.01 (0.98–1.04) | 0.92 (0.87–0.96) | 0.93 (0.85–1.02) |
| Aspirin | 3 (1–7) | 2 (1–3) | 4 (2–8) | 4 (2–8) | 2 (1–3) | |
| Placebo | 3 (1–6) | 2 (1–3) | 4 (2–8) | 4 (2–9) | 2 (1–4) | |
| Number of episodes of nausea, RR (95% CI) | .092 | 0.99 (0.93–1.05) | 1.04 (0.90–1.20) | 1.06 (0.99–1.12) | 0.95 (0.88–1.02) | 0.90 (0.76–1.08) |
| Aspirin | 2 (1–3) | 1 (1–2) | 2 (1–3) | 2 (1–4) | 1 (1–2) | |
| Placebo | 2 (1–3) | 1 (1–2) | 2 (1–3) | 2 (1–4) | 1 (1–2) | |
| Number of episodes of vomiting, RR (95% CI) | .192 | 0.98 (0.91 −1.06) | 1.09 (0.93–1.28) | 1.01 (0.96–1.08) | 0.93 (0.85–1.01) | 0.91 (0.72–1.16) |
| Aspirin | 2 (1–3) | 1 (1–2) | 2 (1–3) | 2 (1–3) | 1 (1–2) | |
| Placebo | 2 (1–3) | 1 (1–2) | 2 (1–3) | 2 (1–4) | 1 (1–2) | |
| Number of episodes of rash or hives, RR (95% CI) | .841 | 1.00 (0.83–1.21) | 0.98 (0.60–1.60) | 0.97 (0.73–1.29) | 0.91 (0.73–1.14) | 1.15 (0.74–1.79) |
| Aspirin | 1 (1–2) | 1 (1–1) | 1 (1–1) | 1 (1–2) | 1 (1–2) | |
| Placebo | 1 (1–2) | 1 (1–1) | 1 (1–1) | 1 (1–2) | 1 (1–2) | |
| Number of episodes of diarrhea, RR (95% CI) | .902 | 0.96 (0.83–1.10) | 0.94 (0.71 −1.23) | 0.91 (0.78–1.06) | 0.95 (0.68–1.34) | 1.04 (0.76–1.40) |
| Aspirin | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1 (1–1) | |
| Placebo | 1 (1–1) | 1 (1–1) | 1 (1–2) | 1 (1–1) | 1 (1–1) | |
| Number of episodes of gastritis, RR (95% CI) | .840 | 0.97 (0.89–1.05) | 0.94 (0.75–1.18) | 0.99 (0.90–1.09) | 0.93 (0.81–1.06) | 1.01 (0.83–1.23) |
| Aspirin | 1 (1–2) | 1 (1–1) | 1 (1–2) | 1 (1–2) | 1 (1–3) | |
| Placebo | 1 (1–2) | 1 (1–1) | 1 (1–2) | 1 (1–2) | 1 (1–2) | |
| Number of episodes of vaginal bleeding, RR (95% CI) | .599 | 1.02 (0.84–1.24) | 1.08 (0.74–1.57) | 0.93 (0.71 −1.22) | 1.23 (0.86–1.75) | 0.88 (0.54–1.45) |
| Aspirin | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1 (1–1) | |
| Placebo | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1 (1–1) | |
| Number of episodes of allergic reaction, RR (95% CI) | .945 | 1.04 (0.50–2.16) | 0.94 (0.36–2.45) | 0.85 (0.32–2.24) | 1.12 (0.10–12.41) | 1.29 (0.48–3.45) |
| Aspirin | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1 (1–1) | |
| Placebo | 1 (1–1) | 1 (1–2) | 1 (1–2) | 1 (1–1) | 1 (1–1) | |
| Number of episodes of other potential side effect, RR (95% CI) | .080 | 0.95 (0.88–1.04) | 0.95 (0.83–1.09) | 1.03 (0.97–1.08) | 0.87 (0.77–0.98) | 0.98 (0.76–1.28) |
| Aspirin | 2 (1–3) | 1 (1–2) | 2 (1–4) | 1 (1–2) | 1 (1–1) | |
| Placebo | 2 (1–3) | 1 (1–2) | 2 (1–3) | 1 (1–3) | 1 (1–2) | |
Data are presented as median (25th percentile–75th percentile), unless otherwise specified. Unexpected emergency medical visits may have been related to potential side effects but are analyzed separately from potential side effects reported at biweekly visits. The denominator is any participant included in the safety population who experienced at least 1 episode of that specific event, and the numerator is the number of events of that specific type experienced by the participant. In addition, the RR models have been adjusted for the number of biweekly visits completed (range, 1–16). The most commonly reported other potential side effects include cold/cough or fever, abdominal pain or discomfort, and headache.
CI, confidence interval; Int, interaction; RR, relative risk.
Short. Aspirin safety during pregnancy in low- and middle-income countries. Am J Obstet Gynecol Glob Rep 2021.
Discussion
Principal findings
In this ethnically and geographically diverse population of nearly 12,000 nulliparous women with a singleton pregnancy, daily LDA use initiated in early pregnancy was associated with no or minimal risk as assessed by unexpected medical visits and potential side effects. There was no increase in risk other than a 20% increase in the incidence of rash or hives among the LDA group. None of the rashes or hives were serious or led to hospitalization or urgent medical care. Findings suggest that daily use of LDA is a safe intervention for reducing the risk of preterm birth and well tolerated by nulliparous pregnant women in LMICs.
Results
These findings are consistent with trials of LDA initiated preconceptionally11 after the first trimester of pregnancy,12 which have shown a low incidence of harm. The safety of LDA use in our study population is further supported by the lack of a treatment effect observed in serious adverse events occurring in the ASPIRIN trial. Specifically, LDA use was not associated with major maternal (eg, bleeding complications, mortality) or fetal and neonatal (eg, fetal loss, neonatal death, congenital abnormalities) adverse events in the ASPIRIN trial.7
Strengths and limitations
Generalizability of our findings to general obstetrical populations may be limited because we enrolled only nulliparous women with singleton pregnancies in LMICs. Data collection for outcomes included in this analysis were limited to those occurring during pregnancy. Detailed information on reasons for unexpected emergency medical visits was not collected, and visits may not have been related to LDA use or pregnancy. Data pertaining to long-term infant outcomes associated with in utero aspirin exposure were not collected. Follow-up data from a previous study showed no increased risk in adverse outcomes among infants exposed in utero.12 Despite these limitations, our study has several strengths, most notably the use of data from a randomized, controlled clinical trial. High-quality data regarding unexpected medical visits and potential side effects were obtained biweekly throughout the trial, including more than 154,900 data collection opportunities. The study sample was large and diverse and represented women from 6 LMICs.
Conclusion
This study adds important information to the literature regarding LDA use during pregnancy in LMICs. The results of the ASPIRIN trial have shown that LDA given to nulliparous women from LMICs beginning early in pregnancy reduces the rate of preterm birth.7 Our supplementary findings have shown that this therapy is safe and tolerated well. Because treatment with LDA is low cost and available in LMICs, it is an ideal candidate for implementation in these environments. Given the high and increasing rate of preterm birth in LMICs, LDA use during pregnancy seems to be a low-risk intervention that could have major benefits.
AJOG MFM at a Glance.
Why was this study conducted?
The daily use of low-dose aspirin (LDA) may be a safe, widely available, and inexpensive intervention for reducing the risk of preterm birth. Data on the potential side effects of LDA use during pregnancy in low- and middle-income countries (LMICs) are needed.
Key findings
The daily use of (LDA) seems to be a safe intervention for reducing the risk of preterm birth and well tolerated by nulliparous pregnant women between 6 and 36 weeks’ gestation in LMICs.
What does this add to what is known?
Our findings were consistent with trials of LDA initiated preconceptionally and after the first trimester of pregnancy, which have shown a low incidence of harm.
ACKNOWLEDGMENTS
The authors would like to thank Geetanjali Katageri, Parth Lalakia, and Javier Chicuy Viélman for their contributions to this study.
This study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The funding source had no involvement in the study design; collection, analysis, and interpretation of data; writing of the report; and decision to submit the article for publication.
Footnotes
The authors report no conflict of interest.
The trial was registered with ClinicalTrials.gov (NCT02409680) and the Clinical Trials Registry-India (CTRI/2016/05/006970).
REFERENCES
- 1.World Health Organization. Preterm birth. Available at: https://www.who.int/news-room/fact-sheets/detail/preterm-birth. Accessed April 14, 2020.
- 2.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016;388:3027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simmons LE, Rubens CE, Darmstadt GL, Gravett MG. Preventing preterm birth and neonatal mortality: exploring the epidemiology, causes, and interventions. Semin Perinatol 2010;34:408–15. [DOI] [PubMed] [Google Scholar]
- 4.Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019;7:e37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. New global estimates of preterm birth published. Available at: https://www.who.int/reproductivehealth/global-estimates-preterm-birth/en/. Accessed April 14, 2020.
- 6.Duley L, Meher S, Hunter KE, Seidler AL, Askie LM. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev 2019;2019:CD004659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman MK, Goudar SS, Kodkany BS, et al. Low-dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (aspirin): a randomised, double-blind, placebo-controlled trial. Lancet 2020;395:285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schisterman EF, Silver RM, Lesher LL, et al. Preconception low-dose aspirin and pregnancy outcomes: results from the EAGeR randomised trial. Lancet 2014;384:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silver RM, Ahrens K, Wong LF, et al. Low-dose aspirin and preterm birth: a randomized controlled trial. Obstet Gynecol 2015;125:876–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman MK, Goudar SS, Kodkany BS, et al. A description of the methods of the aspirin supplementation for pregnancy indicated risk reduction in nulliparas (aspirin) study. BMC Pregnancy Childbirth 2017;17:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahrens KA, Silver RM, Mumford SL, et al. Complications and safety of preconception low-dose aspirin among women with prior pregnancy losses. Obstet Gynecol 2016;127: 689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2014;160:695–703. [DOI] [PubMed] [Google Scholar]


