Abstract
Glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-2 (GLP-2) are gut hormones secreted postprandially. In healthy humans, both hormones decrease bone resorption accompanied by a rapid reduction in parathyroid hormone (PTH).
The aim of this study was to investigate whether the changes in bone turnover after meal intake and after GIP- and GLP-2 injections, respectively, are mediated via a reduction in PTH secretion. This was tested in female patients with hypoparathyroidism given a standardized liquid mixed-meal test (n = 7) followed by a peptide injection-test (n = 4) using a randomized crossover design. We observed that the meal- and GIP-, but not the GLP-2-induced changes in bone turnover markers, were preserved in the patients with hypoparathyroidism. To understand the underlying mechanisms, we examined the expression of the GIP receptor (GIPR) and the GLP-2 receptor (GLP-2R) in human osteoblasts and osteoclasts as well as in parathyroid tissue. The GIPR was expressed in both human osteoclasts and osteoblasts, whereas the GLP-2R was absent or only weakly expressed in osteoclasts. Furthermore, both GIPR and GLP-2R were expressed in parathyroid tissue. Our findings suggest that the GIP-induced effect on bone turnover may be mediated directly via GIPR expressed in osteoblasts and osteoclasts and that this may occur independent of PTH. In contrast, the effect of GLP-2 on bone turnover seems to depend on changes in PTH and may be mediated through GLP-2R in the parathyroid gland.
Keywords: GIP, GLP-2, bone turnover, osteoclasts, osteoblasts, biochemical markers of bone turnover
Introduction
Gastrointestinal hormones secreted after meal ingestion have been suggested as mediators in the gut-bone axis (1–8). In particular, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 2 (GLP-2) seem to be potent inhibitors of bone resorption in humans (2–6, 9–12). Thus, exogenously administered GIP as well as GLP-2 reduce bone resorption, as estimated from serum concentrations of collagen type 1 C-terminal telopeptide (CTX), to 60 – 50% of baseline with a maximal effect reached after approximately 90 minutes for GIP and 180 minutes for GLP-2 (12). Importantly, the bone effects of both GIP and GLP-2 are of clinical relevance since individuals with altered GIP receptor (GIPR) activity due to a specific mutation, (Glu354Gln(rs1800437)) that desensitizes the receptor (13), have decreased bone mineral density (BMD) and a more than 50% increased risk of non-vertebral fractures (14), while exogenous GLP-2 has been found to increase BMD dose-dependently in postmenopausal women with low BMD (5). In addition to its antiresorptive effect, GIP seems to induce a small increase in bone formation as reflected by increased serum levels of procollagen type 1 N-terminal propeptide (P1NP) (11, 12, 15). Thus, an important feature of GIP is its ability to uncouple bone resorption and bone formation, which are normally tightly coupled (16).
At the cellular level, GIPR expression has been described in a murine osteoclast-like cell line (17) and on human osteoblast-like cells (18), supporting the notion of a direct effect of GIP on bone cells. Expression of the GLP-2 receptor (GLP-2R) on bone cells has, so far, only been reported in a single study, where it was found on osteoblast-like cell lines (MG-63 and TE-85) derived from osteosarcomas displaying features of immature osteoblasts (19). Whether the GLP-2R is expressed on mature human osteoblasts or in osteoclasts is therefore an open question and the mechanism behind the effect of GLP-2 on bone is unexplained. The modulation of bone turnover in response to exogenous injection of GIP and GLP-2 is accompanied by a sudden and drastic decrease in parathyroid hormone (PTH); a similar decrease is observed after meal ingestion (11, 12, 20–23). The extent to which the effects of GIP or GLP-2 on bone turnover are mediated via a reduction in PTH, is unknown.
In this study, we firstly conducted a meal-test to investigate the acute responses to meal intake on the markers of bone turnover (CTX and P1NP), GIP, and GLP-2 in patients with hypoparathyroidism. Secondly, we gave injections of GIP and GLP-2 to the patients after a fasting period to investigate whether the acute effects of exogenous GIP and GLP-2 on bone turnover were dependent on changes in PTH. We included patients with surgical hypoparathyroidism (due to complete thyroidectomy) with low or undetectable levels of PTH to eliminate PTH-mediated effects on bone turnover. We hypothesized that the effect of GIP and GLP-2 on bone resorption (measured by CTX) would be lost in the patients, if it was dependent on an inhibition of PTH.
Furthermore, we examined human parathyroid tissue for expression of GIPR and GLP-2R to evaluate the possibility of direct effects of GIP and GLP-2 on PTH secretion as an explanatory factor for their effects on bone. This was combined with an examination of the expression of GIPR and GLP-2R in in vitro cultured human osteoblasts and osteoclasts.
Materials and methods
Human study participants and procedures
Participants with chronic hypoparathyroidism due to total thyroidectomy were recruited from Hvidovre Hospital, Denmark. Both men and women were eligible for inclusion. Exclusion criteria were diabetes, use of recombinant PTH replacement, anti-osteoporotic medication, corticosteroid therapy within the last year, pregnancy, prior history of malabsorptive disease (e.g. celiac, inflammatory bowel disease, or bariatric surgery), allergy to the interventional substances, or Hgb< 7 mmol/L. Participants were studied during a meal-test and thereafter they were asked to participate in peptide-test days. There were at least one week between the meal-test and the peptide-tests. The peptide-test consisted of three separate test days (in a randomized crossover design) with subcutaneous injections of exogenous GIP, GLP-2, or placebo, respectively (no meals were served during the peptide-test days) (Fig. 2A). Apart from the applied stimuli, the procedures for the test days were similar. Experiments were conducted at Hvidovre Hospital, Denmark. Participants refrained from strenuous physical activities and ingestion of alcohol for two days before each study day. On study days, participants arrived at 8.00 am after fasting at least 10 hours. Medication throughout the fasting period and during the test day was avoided if possible. Participants were resting in a hospital bed during the test day with a catheter inserted in a cubital vein for blood sampling. Blood samples were drawn at time points −10, −5, 7, 15, 30, 45, 60, 90, 120, 150, 180, 210, and 240 minutes relative to the ingestion of the Nutridrink (meal-test) or the subcutaneous injection (peptide-test).
Figure 2. Results from the peptide-test.
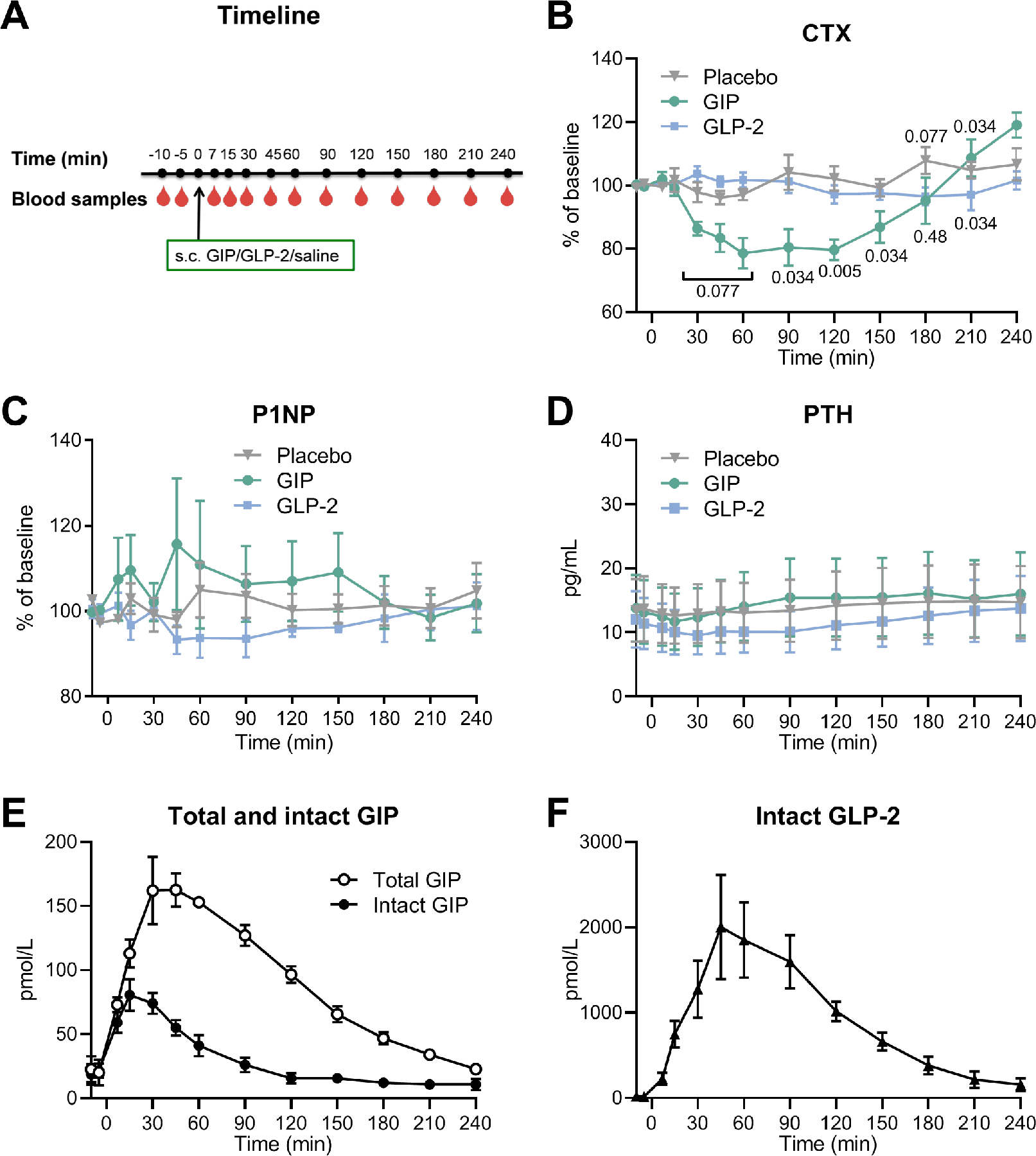
(A) Timeline for the study. Baseline blood samples were drawn from the fasting participants in the morning (after at least 10 hours of fasting) at time point t = −10 and −5 min. At t = 0 min, glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptide-2 (GLP-2), or placebo (saline) was subcutaneously injected followed by blood sampling at t = 7, 15, 30, 45, 60, 90, 120, 150, 180, 210, and 240 min. (B) serum collagen type 1 C-terminal telopeptide (CTX) presented as percentage of baseline, (C) serum procollagen type 1 N-terminal propeptide (P1NP) presented as percentage of baseline, and (D) serum parathyroid hormone (PTH) in response to GIP (green circles), GLP-2 (blue squares), and placebo (grey triangles). (E) plasma concentration of total GIP (white circles) and intact GIP (black circles), and (F) plasma concentrations of intact GLP-2 (black triangles). (B-D) the data was analyzed by a Related-samples Friedmańs Two-Way Analysis of Variance by Ranks, an if relevant, followed by post hoc pairwise comparisons between groups to evaluate differences between interventions (GIP or GLP-2) and placebo at individual time points. Actual p values (when p<0.10 or p>0.001) are indicated on the figure). Data (n = 4) are shown as mean ± SEM.
Meal-test:
A 200 mL liquid mixed meal (Nutridrink, N.V. Nutricia, Zoetemeer, Holland) containing 1260 kJ, carbohydrate 49 E%, protein 16 E%, fat 35 E%, and 91 mg calcium/100 mL was ingested at approximately 8.30 am (t = 0 min).
Peptide-test:
We conducted a cross-over study with a balanced design (a Williams design). A list of the sequences was generated by a computer and participants were randomly assigned to the sequences. All participants were studied on the three study days: GIP, GLP-2, and placebo which were separated by at least one week. Participants were blinded to the order of the injections. At approximately 8.30 am (t = 0 min), GIP 100 µg, GLP-2 400 µg, or placebo was subcutaneously injected in the umbilical region.
Synthetic human GIP(1–42) was from PolyPeptide (PolyPeptide Group, Strasbourg, France) and synthetic human GLP-2(1–33) was from Bachem (Bachem, Bubendorf, Switzerland), both with a purity above 97%. Peptides were dissolved in sodium hydrogen carbonate buffer with 0.5% human serum albumin to a final peptide concentration of 100 µg/mL for GIP and 400 µg/mL for GLP-2. One mL vials were prepared by The Capital Region Pharmacy (Herlev, Denmark) and stored at −20°C. Placebo injection was 1 mL of saline (NaCl 9 mg/mL).
Blood samples:
Blood for plasma preparation was collected in chilled EDTA tubes with added dipeptidyl peptidase-4 inhibitor (valine pyrrolidide) to a final concentration of 0.01 mmol/L and kept on ice. Blood for serum was collected in clot activator tubes and kept at room temperature 30 minutes for coagulation. Tubes were centrifuged 10 minutes at 1200 x g at 4°C. Plasma and serum samples were stored at −20°C until measurements.
Measurements:
Blood pressure and heart rate were measured using a standard blood pressure monitor (Omron M6, Intelli Sense, Omron Healthcare Europe B.V., Hoofddorp, The Netherlands). Glucose was measured with the glucose oxidase method using an YSI (YSI model 2300D STAT plus analyzer, YSI Incorporated, Yellow Springs, Ohio, USA). Serum insulin and C-peptide were measured by sandwich immunoassay (Advia Centaur XP, Siemens). Plasma concentrations of GIP and GLP-2 were measured by in-house radio-immunoassays. Total GIP was measured using an antibody directed towards the C-terminal (code no. 80867), which reacts fully with intact and N-terminally truncated GIP. Intact GIP was measured using an antibody which reacts with the N-terminal of intact GIP (code no. 98171). The standard used was human GIP (Bachem, cat no. H-5645) and the tracer was 125I-labeled human GIP (Perkin Elmer, cat no. Nex402). Intact GLP-2 was measured using an antibody specific for the intact N-terminus of GLP-2 (code no. 92160). The standard was recombinant human GLP-2 and the tracer was 125I-labeled rat GLP-2 with an Asp33 to Tyr33 substitution. Sensitivity for all assays was below 5 pmol/L and intra-assay coefficient of variation was below 10%. Serum CTX, P1NP, and intact PTH were measured using the chemiluminescence technology on an IDS-iSYS Multi-Discipline Automated System (ImmunoDiagnosticSystems, Frankfurt am Main, Germany). The reportable range for CTX (code IS-3000) is 0.33 – 6.000 ng/mL and the expected values for women is 0.0034 – 1.037 ng/mL. The reportable range for P1NP (code IS-4000) is 2 – 230 ng/mL with expected values for normal adults 27.7 – 127.6 ng/mL. The reportable range for PTH (code IS-3600) is 5 – 5000 pg/mL with expected values for normal adults 11.5 – 78.4 pg/mL.
Expression of the GIP and GLP-2 receptor in parathyroid tissue
The data regarding the relative expression of GLP-1R, GIPR, and GLP-2R among normal human parathyroid glands were derived from our previous comparative transcriptome analysis (PMID: 27760455, 21393447), deposited at Gene Expression Omnibus (GEO accession: GSE83421).
Gene expression analyses in osteoblast and osteoclast cultures
To determine the expression of GIPR and GLP-2R in osteoblasts, a well-characterized human bone marrow stromal (mesenchymal) stem cell line (hMSC-TERT) was employed to obtain osteoblast cultures (24, 25). hMSC were cultured in a standard growth medium containing minimal essential medium (MEM) (Gibco Invitrogen) supplemented with 10% FBS (Biochrom) and 1% penicillin/streptomycin (Gibco Invitrogen) at 37°C in a humidified atmosphere containing 5% CO2. The medium was changed every 3rd day until the cells became 90% confluent. For osteoblast differentiation, cells were seeded at 20,000 cells/cm2 and the day after, osteoblast differentiation was induced by supplementing the hMSC culture media with 10 mM β-glycerophosphate (Calbiochem-Merck, Germany), 50 µg/mL L-ascorbic acid-2-phosphate (Wako Chemicals GmbH, Germany), 10 nM dexamethasone (Sigma-Aldrich, Denmark), and 10 nM calcitriol (1.25-dihydroxy vitamin-D3 kindly provided by Leo Pharma, Ballerup, Denmark) (26). Gene expression analyses were done using Affymetrix Human GeneChip® U133 Plus 2.0 arrays in hMSC cultures at the baseline (Day 0), 6 and 12 days after induction of osteoblast differentiation. To determine the expression of GIPR and GLP-2R in osteoclasts, a publicly available data set (GSE107295) was employed, in which gene expression analysis was done using Affymetrix Human GeneChip® U133 Plus 2.0 Array in osteoclast cultures established by CSF 1 (33 ng/mL) and RANKL (66 ng/mL) treatment of osteoclast precursors (27). We also determined expression of GIPR and GLP-2R mRNA in mature osteoclasts, where human CD14+ monocytes were isolated from buffy coats obtained from blood donations from healthy women age 18–49 (n = 8). Monocytes were seeded in T25 cell culture flasks (1.67×106 cells/flask) stored in an incubator (37oC, 5% CO2) and differentiated into mature osteoclasts (t = 10 days) using minimal essential medium α (ThermoFischer Scientific, Waltham, USA) with 10% fetal bovine serum (FBS) and 1% Penicillin/Streptomycin (Sigma Aldrich, Seelze, Germany) and stimulated with macrophage colony-stimulating factor (MCSF) (R&D Systems, Minneapolis, USA) 25 ng/ml. Media which contained MCSF and receptor activator of nuclear κβ (RANKL) (R&D Systems, Minneapolis, USA) 25 ng/ml each were changes on day 3, 6, and 8 (28, 29).
Analyses of gene expression by RNA-sequencing
Primary mature human osteoclasts were harvested in TRIzol (Thermo Fisher). RNA was purified using Econo Spin columns (Epoch Life Sciences). RNA-sequencing was performed according to manufacturer’s instructions (TruSeq 2, Illumina) using 2 µg RNA for preparation of cDNA libraries. Sequencing reads were mapped to the human genome (hg19) using STAR (30), and tag counts were summarized at the gene level using HOMER (31). Differential gene expression was analyzed using DESeq2 (32). Gene ontology analysis was performed using goseq (33).
Statistics
Primary outcome of the human study was bone resorption measured as CTX. CTX and P1NP levels were presented as percentage of baseline (mean of t= −10 min and t= −5 min). The changes after peptide administrations were analyzed using a Related-Samples Friedmańs Two-Way Analysis of Variance by Ranks (non-parametric test), and if relevant, followed by post hoc pairwise comparison between the groups to evaluate differences at specific time points. Differences in baseline values between test days were analyzed by Friedman test (non-parametric test). Participant’s baseline characteristics are presented as median and range. Results are presented as mean ± SEM. When measurements were below the detection limit, the detection limit was entered. Two-sided p values less than 0.05 were considered statistically significant. All statistical analyses and graphs were made in SPSS Version 26 for Mac (IBM, Armonk, NY, USA), and GraphPad Prism Version 7.00 for Windows (GraphPad Software, LA, Jolla California USA). The sample size was determined based on previous studies (2, 6, 9, 12).
Study approval
The study was conducted according to Declaration of Helsinki principles. The Scientific Ethical Committee of the Capital Region of Denmark approved the study (protocol no. H-16047626), and it was registered at the Danish Data Protection Agency (journal no. SUND-2017–21), and at ClinicalTrials.gov (NCT03728959). All participants gave written informed consent prior to inclusion in the study.
Results
Seven female participants were included in the meal-test, and four of these also completed the subsequent peptide-test, where GIP, GLP-2, or placebo, respectively, was administered subcutaneously on separate study days. Patients were recruited and tested in the period from August 2019 to February 2020. Only women volunteered to participate. Blood samples were drawn in the fasting state and regularly for 240 minutes after the intervention (Fig. 2A). Baseline characteristics of participants are listed in table 1. In the meal-test, five of the seven participants were postmenopausal. In the peptide-test, three of the four participants were postmenopausal. Four participants were not able to skip all of their medication and took necessary medication (judged by their physician and the participant) the night before the test day and/or in the morning on the test day. For further information regarding medication, see table 2 and supplemental table 1.
Table 1. Baseline characteristics of study participants in the meal-test and the peptide-test.
Baseline characteristics at screening of participants in the meal-test (n = 7) and the peptide-test (n = 4) given as median and range.
| Meal-test | Peptide-test | |
|---|---|---|
| Sex (female/male) | 7/0 | 4/0 |
| Postmenopausal/pre-menopausal | 5/2 | 3/1 |
| Age (years) | 54 (40–75) | 58 (40–75) |
| Weight (kg) | 71 (58–82) | 71 (60–82) |
| Height (m) | 1.70 (1.60–1.82) | 1.65 (1.60–1.74) |
| BMI (kg/m2) | 23.6 (19.8–30.1) | 27.7 (19.8–30.1) |
| PTH intact (pg/mL) | 10.0±3.80 (<5 to 24.7) | 12.3 (<5–21.7) |
| Calcium total (mmol/L) | 2.25 (2.18–2.38) | 2.22 (2.22–2.27) |
| Calcium ion (mmol/L) | 1.21 (1.08–2.21) | 1.21 (1.08–2.21) |
| eGFR (ml/min/1.73 m2) | 77 (44–95) | 75 (44–95) |
| Phosphate (mmol/L) | 1.28 (0.95–1.48) | 1.32 (0.95–1.48) |
| Potassium (mmol/L) | 3.9 (3.6–4.8) | 3.9 (3.8–4.8) |
| Creatinine (µmol/L) | 71 (63–115) | 68 (65–115) |
| Alkaline phosphatase (U/L) | 65 (34–102) | 60 (48–66) |
| 25OHvitamin D (nmol/L) | 130 (69–172) | 113 (69–149) |
Table 2. Medication.
Four of the seven participants had to take some of their medication the night before the test day and/or in the morning on the test day.
| Medication | Test | |
|---|---|---|
| Subject 2 | Levothyroxine, alfacalcidol, calcium | Meal-test + peptide-test |
| Subject 4 | Alfacalcidol, oxycodone, enalapril | Meal-test + peptide-test |
| Subject 6 | Alfacalcidol, pregabalin, metoprolol succinate, rivaroxaban |
Meal-test |
| Subject 7 | Metoprolol succinate | Meal-test |
During the meal-test, a normal postprandial response with lowering of bone resorption was seen in patients with hypoparathyroidism
Participants in the meal-test had a median age of 54 (range 40 – 75) years and a median BMI of 23.6 (range 19.8 – 30.1) kg/m2. All participants had low basal plasma levels of intact PTH with a median of 10 ± 3.8 (range < 5 to 24.7) pg/mL (expected values for normal adults are 11.5 to 78.4 pg/mL). The clinical characteristics of participants are shown in table 1.
Plasma concentrations of total GIP and intact GLP-2 increased after the meal as expected. GIP plasma level increased from a basal level 17 ± 3.5 pmol/L (mean ± SEM) to a maximum of 69 ± 10 pmol/L reached at t = 45 min and then decreased towards the basal level towards the end of the test (Fig. 1A). GLP-2 plasma level increased from 18 ± 2.5 pmol/L to a maximum of 30 ± 4.3 pmol/L reached at t = 15 min and returned to the baseline level within one hour (Fig. 1B).
Figure 1. Results from the meal-test.
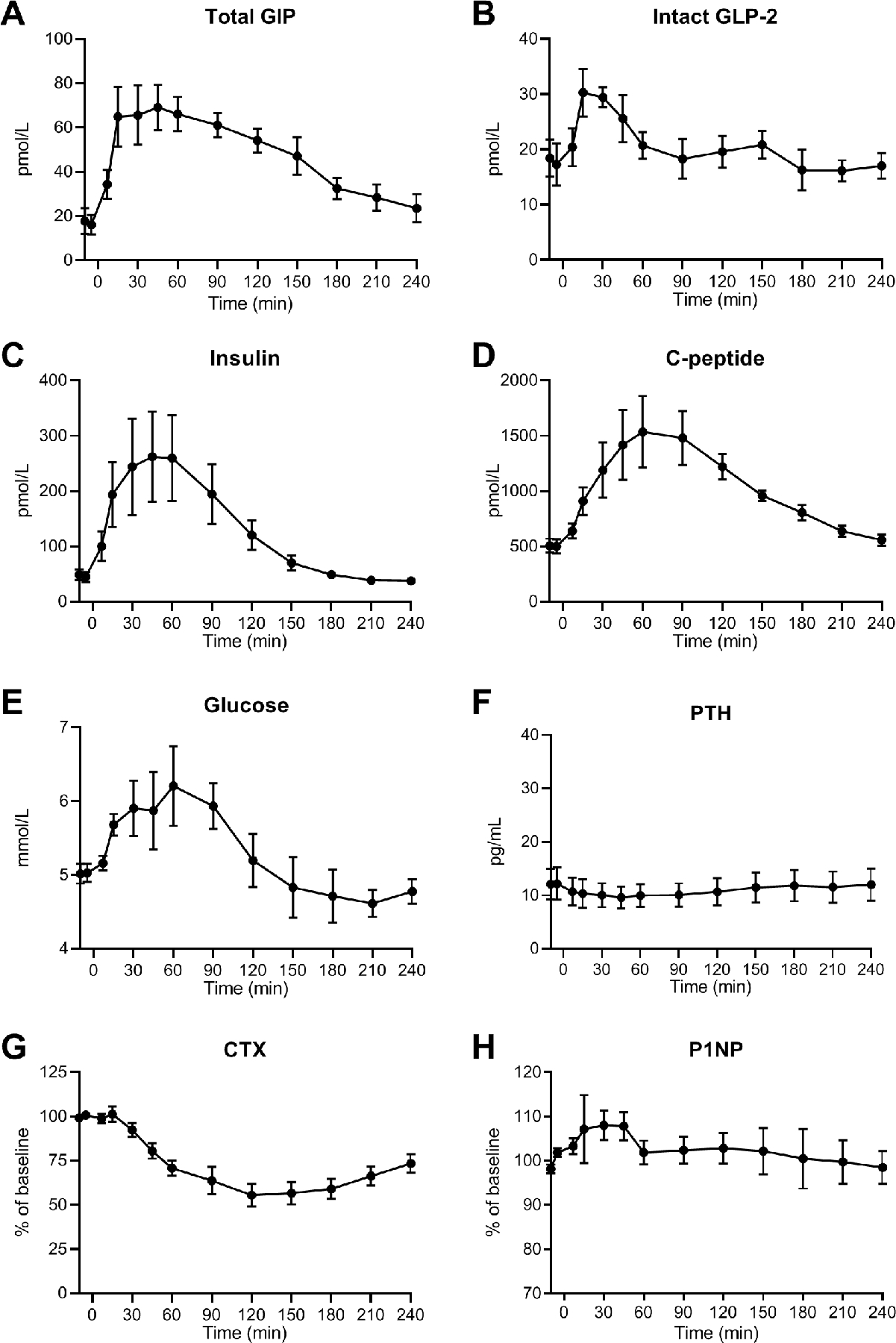
Seven female participants with hypoparathyroidism were included in the meal-test. After at least 10 hours of fasting, the participants met in the morning. After baseline blood samples, a liquid meal was ingested at time point t = 0 min followed by blood sampling regularly for 240 minutes. (A) plasma concentrations of total glucose-dependent insulinotropic polypeptide (GIP), (B) plasma concentrations of intact glucagon-like peptide-2 (GLP-2), (C) serum insulin, (D) serum C-peptide, (E) serum glucose, (F) serum parathyroid hormone (PTH), (G) serum collagen type 1 C-terminal telopeptide (CTX) presented as percentage of baseline, and (H) serum procollagen type 1 N-terminal propeptide (P1NP) presented as percentage of baseline. Data (n = 7) are shown as mean ± SEM.
Insulin and C-peptide increased after the meal with a peak value around t = 60 min (~250 pmol/L insulin and ~1500 pmol/L C-peptide) followed by a decrease to the baseline level (Fig. 1C-D). Glucose rose modestly from a basal concentration of 5.0 ± 0.1 mmol/L to a peak concentration of 6.2 ± 0.5 mmol/L at t = 60 min and then decreased to 4.6 ± 1.8 mmol/L (Fig. 1E).
Changes in blood pressure and heart rate were also observed. A decrease in systolic blood pressure from 143 ± 6.7 to 126 ± 6.1 mmHg and diastolic blood pressure from 85 ± 4.4 to 75 ± 6.8 mmHg was observed after the meal, while the heart rate increased from 66 ± 4.6 to 71 ± 4.8 bpm (Table 3).
Table 3. Insulin, C-peptide, glucose, blood pressure, and heart rate at the day of the meal-test and the peptide test.
Insulin, C-peptide, glucose, blood pressure, and heart rate at the day of the liquid meal-test and the peptide-test. Data are presented as mean±SEM baseline and peak/nadir values as relevant.
| Meal-test (n = 7) |
Peptide-test (n = 4) |
||||
|---|---|---|---|---|---|
| GIP | GLP-2 | Placebo | |||
| Insulin (pmol/L) | Baseline | 47 ± 6.3 | 53 ± 5.7 | 37 ± 3.3 | 32 ± 2.7 |
| Peak | 262 ± 82 | 77 ± 11 | 44 ± 6.8 | 32 ± 3.7 | |
| C-peptide (pmol/L) | Baseline | 501 ± 43 | 530 ± 72 | 451 ± 41 | 399 ± 29 |
| Peak | 1536 ± 325 | 639 ± 80 | 447 ± 64 | 404 ± 48 | |
| Glucose (mmol/L) | Baseline | 5.0 ± 0.1 | 5.2 ± 0.1 | 5.0 ± 0.1 | 5.0 ± 0.0 |
| Peak/Nadir | 6.2 ± 0.5 peak | 4.6 ± 0.3 nadir | 4.9 ± 0.2 nadir | 4.7 ± 0.1 nadir | |
| Systolic blood pressure (mmHg) | |||||
| Baseline | 143 ± 6.7 | 135 ± 9.1 | 130 ± 8.3 | 133 ± 5.9 | |
| Nadir | 127 ± 6.1 | 121 ± 9.2 | 118 ± 12 | 128 ± 12 | |
| Diastolic blood pressure (mmHg) | |||||
| Baseline | 85 ± 4.4 | 77 ± 4.3 | 70 ± 1.1 | 73 ± 2.6 | |
| Nadir | 75 ± 6.8 | 75 ± 8.5 | 67 ± 6.6 | 68 ± 3.8 | |
| Heart rate (bpm) | Baseline | 66 ± 4.6 | 67 ± 2.7 | 66 ± 3.1 | 62 ± 2.2 |
| Peak | 71 ± 4.8 | 71 ± 3.5 | 70 ± 3.2 | 63 ± 4.4 | |
| CTX (ng/mL) | Baseline | 0.45 ± 0.15 | 0.30 ± 0.10 | 0.37 ± 0.06 | 0.32 ± 0.09 |
| Nadir | 0.21 ± 0.05 | 0.25 ± 0.16 | 0.27 ±0.20 | 0.31 ± 0.19 | |
| P1NP (pg/mL) | Baseline | 60.9 ±13.7 | 37.3 ± 12.2 | 38.2 ± 13.5 | 39.7 ± 12.2 |
| Peak | 65.8 ± 15.4 | 44.6 ± 21.7 | 38.9 ± 14.7 | 44.5 ± 17.0 | |
| Nadir | 59.5 ± 14.0 | 26.2 ± 18.1 | 35.4 ± 10.6 | 38.9 ± 10.7 | |
Following the meal, a small and insignificant decrease in PTH to 7.5 ± 2.9 pg/mL was observed (Fig. 1F). Three of the participants had basal PTH levels below the detection limit of the assay (below 5.0 pg/mL).
Regarding the biochemical markers of bone resorption (CTX) and bone formation (P1NP), all participants responded to the meal with a marked decrease in CTX to a mean ± SEM of 55.5 ± 17.1% of baseline reached at t = 120 min and then returned towards the baseline level (Fig. 1G). Absolute basal value of CTX was 0.45 ± 0.15 ng/mL. P1NP increased to a maximum of 108 ± 8.9% of baseline reached at t = 30 min and hereafter P1NP stayed near the baseline level (Fig. 1H). Absolute basal concentration of P1NP was 60.9 ± 13.7 pg/mL.
In patients with hypoparathyroidism, exogenous GIP inhibited bone resorption whereas the effect of GLP-2 appeared to be lost
Four participants from the meal-test also volunteered to participate in the peptide-test. Their median age was 58 (range 40–75) years and the median BMI was 27.7 (range 19.8 – 30.1) kg/m2. For further characteristics of participants, see table 1 and 2.
Timeline for the study days is depicted in figure 2A. CTX decreased after GIP injection, but not after injection of GLP-2. At baseline, CTX levels were comparable with absolute concentrations of 0.30 ± 0.10, 0.37 ± 0.06, and 0.32 ± 0.09 ng/mL in the GIP, GLP-2, and placebo group, respectively. After placebo, CTX stayed near the baseline level during the 4 hours study period. GIP injection significantly decreased CTX to 80.5 ± 5.8% of baseline after 90 min compared to 104.2 ± 5.5% of baseline after placebo (p = 0.034). GLP-2 did not significantly change CTX compared to placebo (except for a small decrease at t = 210 min) (Fig. 2B). There were no effects of GIP or GLP-2 on bone formation as measured by serum levels of P1NP (Fig. 2C), and basal values were comparable on the three study days (37.3 ± 12.2, 38.2 ± 13.4, and 39.7 ± 12.2 ng/mL on the GIP, GLP-2, and placebo day, respectively).
Baseline PTH concentrations were low and below the detection limit for two of the participants in the peptide-test (<5, <5, 17.9, and 21.7 pg/mL, respectively for the four participants). For the two participants with measurable PTH levels, PTH initially decreased slightly after all three interventions (GIP, GLP-2, and placebo) with no differences between the interventions (Fig. 2D).
Plasma concentrations of GIP and GLP-2 increased after the injections, as expected. After GIP administration, total GIP increased to a peak concentration of 163 ± 13 pmol/L reached at t = 60 min. Intact GIP increased to a peak of 81 ± 12 pmol/L at t = 15 min and dropped to baseline levels at t = 120 min. After administration of GLP-2, intact GLP-2 increased to a maximum of 2004 ± 609 pmol/L at t = 45 min and concentrations remained elevated until t = 240 min (Fig. 2E-F).
Insulin, C-peptide, and glucose were all affected by GIP, but not by GLP-2. Compared to placebo, GIP significantly increased levels of insulin and C-peptide during the first 30 minutes after injection. Insulin increased from 53 ± 5.7 to 77 ± 11 pmol/L and C-peptide from 530 ± 72 to 639 ± 80 pmol/L followed by a decrease in glucose from 5.2 ± 0.1 to 4.6 ± 0.3 mmol/L. GLP-2 had no significant effect on insulin, C-peptide, or glucose levels compared to placebo (Table 3).
Regarding blood pressure and heart rate, both GIP and GLP-2 insignificantly decreased systolic blood pressure compared to placebo. There were no changes in diastolic blood pressure. Compared with placebo, the heart rate increased significantly from 67 ± 2.7 to 71 ± 3.5 bpm after GIP and from 66 ± 3.1 to 70 ± 3.2 bpm after GLP-2, similar to the changes observed in the meal-test (Table 3).
Receptor expression in human parathyroid tissue
Analysis of our previously published transcriptome array data (PMID: 27760455, 21393447) showed that GLP-1R, GIPR, and GLP-2R are expressed in normal human parathyroid glands. Moreover, the relative expression of GLP-1R, GIPR, and GLP-2R revealed comparable expression levels (Fig. 3).
Figure 3. Receptor expression in normal parathyroid glands.
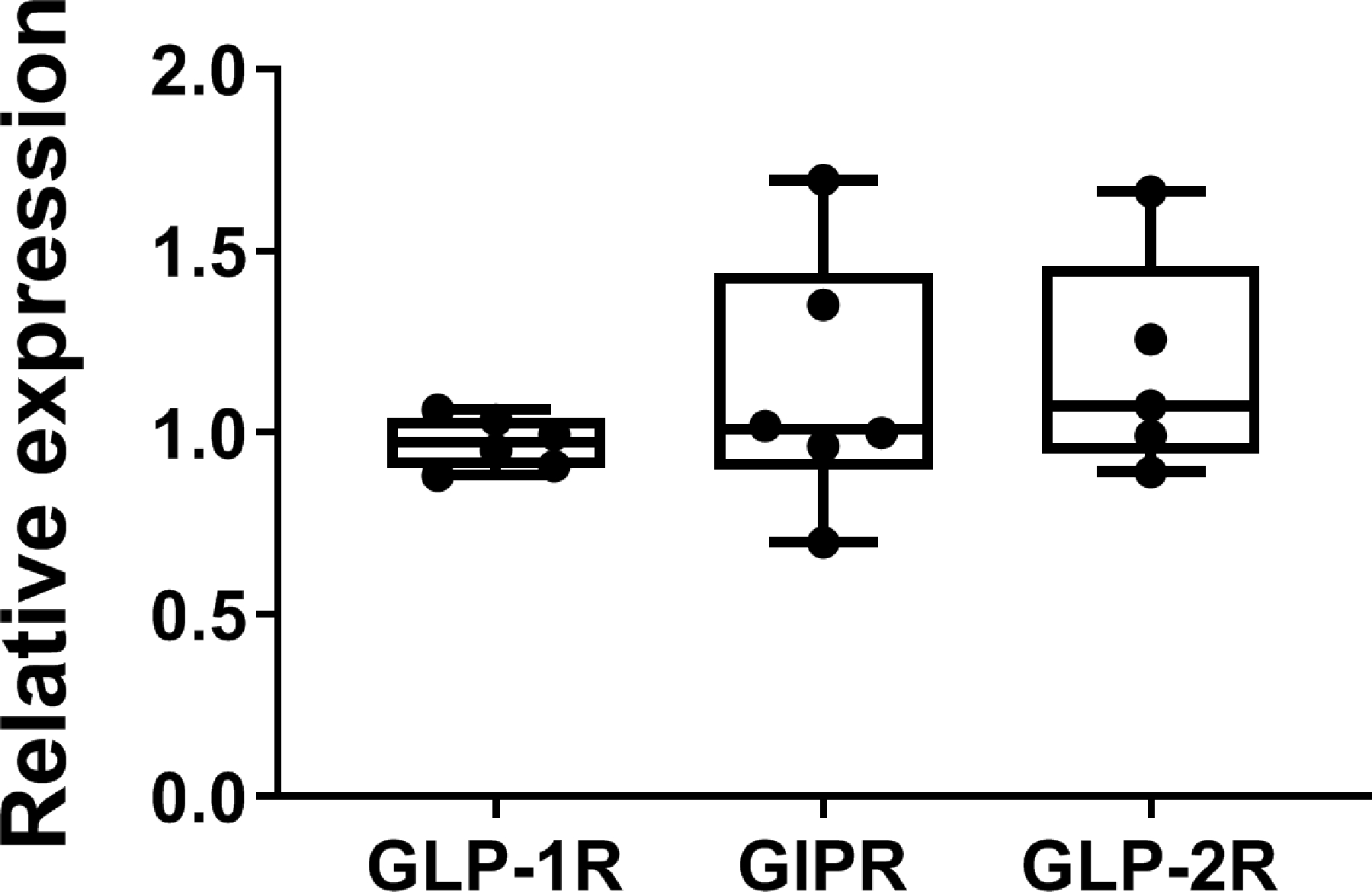
Glucagon-like peptide-1 receptor (GLP-1R), glucose-dependent insulinotropic polypeptide receptor (GIPR), and glucagon-like peptide-2 receptor (GLP-2R) are expressed in normal human parathyroid tissue. Analysis of data from GEO Series Accession: GSE83421 shows the relative expression of the GLP-1R, GIPR, and GLP-2R in normal parathyroid tissue. Grubbs’ test identified a gland as an outlier in GLP-2R expression and data was excluded from further statistical analysis by one-way ANOVA. Data are shown as boxplot (with median, interquartile range, maximum, and minimum) showing all data points.. No significant differences were found among presented genes.
Receptor expression in human osteoblasts and osteoclasts
To determine the expression of GIPR and GLP-2R in osteoblasts, cultured human bone marrow stromal (mesenchymal) stem cell line (hMSC-TERT) was induced towards osteoblast differentiation, and gene expression analysis was performed at different stages of differentiation (day 0, 6, and 12). The analysis showed expression of GIPR at all times points, whereas GLP-2R expression was not detectable at any time point (Fig. 4A). A publicly available gene expression data set (27) was employed to determine the expression of GIPR and GLP-2R in osteoclasts. Using this method, we found an increasing expression of GIPR during osteoclast differentiation, whereas GLP-2R was not detectable (Fig. 4B). We also assessed expressions of the GIPR and GLP-2R in osteoclasts differentiated from human CD14+-monocytes isolated from buffy coats obtained from anonymous blood donors (n=8). Gene expression analyses using RNA sequencing were performed on day 10, i.e. in mature osteoclasts. These analyses confirmed expression of GIPR in primary mature human osteoclasts. However, contradictory to the public available gene expression data set, we observed expression of GLP-2R in primary mature osteoclast in vitro in all donors. However, the expression of the GLP-2R was substantially weaker compared to that of the GIPR (Fig. 4C).
Figure 4. Receptor expression in osteoblasts and osteoclasts.
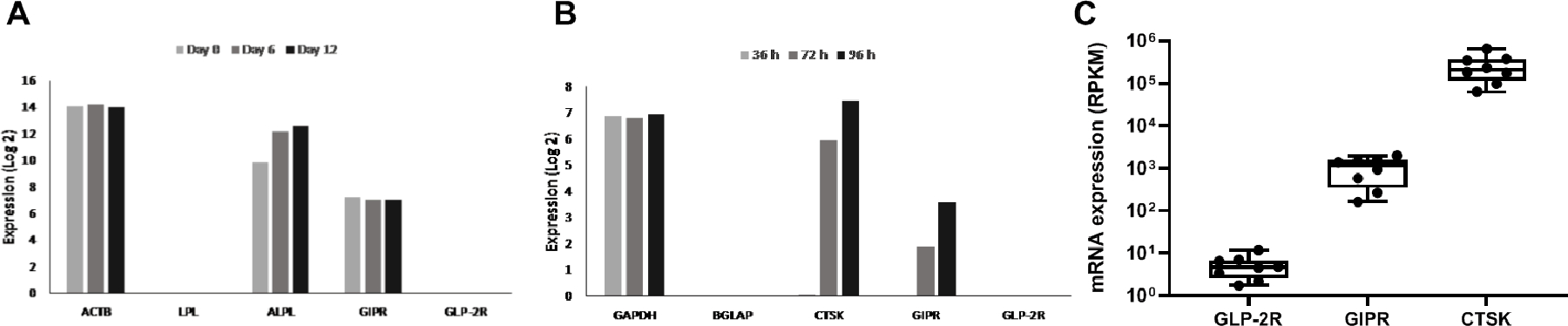
Expression of glucose-dependent insulinotropic polypeptide receptor (GIPR) and glucagon-like peptide-2 receptor (GLP-2R) in human osteoblast and osteoclasts. (A) analyses of GIPR and GLP-2R expression at different time points (day 0, 6, 12) during osteoblast differentiation of human mesenchymal stem cells (hMSC) and (B) analyses of GIPR and GLP-2R expression at different time points (36 h, 72 h, 96 h) during osteoclast differentiation of human osteoclast precursors treated with macrophage-colony stimulating factor MCSF-1 and receptor activator of nuclear κβ (RANKL). Actin beta (ACTB), lipoprotein lipase (LPL), alkaline phosphatase (ALPL), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), bone gamma-carboxyglutamate protein (BGLAP), and cathepsin K (CTSK) were used as housekeeping, negative-, and positive controls, respectively. (C) mRNA expression of GLP-2R, GIPR and cathepsin K (CTSK) (positive control) in primary mature human osteoclasts treated with MCSF and RANKL (n = 8). Each gene is normalized to gene length and the number of reads sequenced per sample (reads per kilobase million (RPKM)). (C) Data are shown asboxplot (with median, interquartile range, maximum, and minimum) showing all data points.
Discussion
The present study provides new insight into the gut-bone-axis and demonstrates basic aspects of bone turnover in response to meal ingestion and subcutaneous injection of GIP and GLP-2. We conducted a liquid mixed meal-test and injected exogenous GIP, GLP-2, and placebo to investigate the effects on bone turnover in patients with hypoparathyroidism (due to thyroidectomy) with no or very low levels of PTH. We did this in order to assess to what extent the changes in bone turnover markers (CTX and P1NP), as observed in healthy individuals (2–6, 9–12), depend on inhibition of PTH secretion, which is normally acutely lowered after meal ingestion as well as after injections of both GIP and GLP-2 (12, 23, 34). If PTH inhibition was essential, the changes in bone turnover markers would not be expected to occur in the patients with hypoparathyroidism.
During the mixed meal, total GIP and intact GLP-2 plasma concentrations increased to ~70 pmol/L and 30 pmol/L respectively, which is similar to what is seen in healthy persons (2, 23, 35). Concomitantly, CTX levels decreased to 55.5 ± 17.1% of baseline in the patients with hypoparathyroidism, similar to the meal-induced decrease to 50 – 60% of baseline observed in healthy individuals (1, 2, 7). The mixed meal also resulted in a transient increase in P1NP to 108 ± 3.7% of baseline. In regards to the effect of a meal or glucose intake on bone formation, the literature is inconsistent. Whereas some studies report decreases in markers of bone formation, others report minor increases (1, 11, 36, 37). However, some studies have shown that GIP may induce a small acute increase in P1NP (12, 15, 34). Regarding PTH, we confirmed that the participants had low levels of PTH and three of the seven participants in the meal-test even had PTH levels below the measurable concentration (<5 pg/mL). Importantly, all participants responded during the meal-test with a marked decrease in CTX, irrespective of their basal PTH levels, suggesting that the changes in bone turnover seen after meal ingestion are probably not related to changes in PTH levels. During the meal-test, insulin, C-peptide, and glucose increased as normally seen in healthy subjects (35, 38, 39).
With the peptide injections, we studied the individual effects of exogenously administered GIP and GLP-2 in our patients with hypoparathyroidism. After GIP, we observed a clear reduction in CTX to 80.5 ± 5.8% of baseline at t = 90 min compared to a value of 104.2 ± 5.5% after placebo (p = 0.034). The reduction in CTX is less than previously observed after subcutaneous injection of GIP in healthy individuals, where a maximal inhibition to ~55% of baseline was observed after 90 min (12). However, it should be noted that in the previous study, a higher dose of GIP was used (200 µg versus 100 µg in the present study) and also a greater reduction in CTX after placebo was observed (12). In the present study, P1NP increased to 116±15% of baseline after GIP administration, but this change did not reach significance. In healthy individuals and in patients with type 1 diabetes, P1NP has been found to significantly increase after GIP (11, 12, 15) indicating an acute uncoupling of bone formation from bone resorption. However, the present study was undoubtedly underpowered to detect a significant difference in P1NP since only 4 participants received the injections and the P1NP response is substantially smaller than the CTX response (12).
After subcutaneous injection of GLP-2, CTX levels remained unchanged and similar to those observed with placebo, suggesting that the effect of GLP-2 is diminished in hypoparathyroidism and may depend on changes in PTH levels. In comparison, a significant reduction to ~60% of baseline CTX is seen after GLP-2 in healthy young men (12). We have previously observed basal levels of CTX in the range of ~0.7 – 0.9 ng/mL and basal levels of P1NP of ~70 ng/mL in healthy young men (12). In the present study, the participants had basal levels of CTX ranging from 0.3 – 0.45 ng/mL and P1NP basal levels were 38 – 60 pg/mL, which was as expected since patients with hypoparathyroidism have low levels of bone turnover markers. Hypoparathyroidism is most often caused by accidental removal during thyroid surgery due to either cancer or hyperthyroidism (40, 41) and the disease is characterized by low PTH leading to a low bone turnover, low serum calcium, elevated levels of serum phosphorus, and low 1,25(OH)D3. In most patients with hypoparathyroidism, BMD is increased compared to healthy controls due to the low bone turnover, although the microarchitecture of the bone is abnormal (40, 41). Normally, BMD correlates positively with reduced fracture risk, but lower bone remodeling may result in impaired regeneration and accumulated old bone with fatigue microfractures. Whether the fracture risk is affected in hypoparathyroidism is not clear (41, 42), but it may be unchanged or lower in some patients (43).
For the two participants with measurable PTH levels, we observed a small decrease in PTH after both GIP and GLP-2, but this was also observed after placebo (absolute changes were 2.9, 2.9, and 2.1 pg/mL, respectively within 45 minutes). In healthy participants, the changes in PTH are more pronounced with more acute decreases around 9 pg/mL (from ~29 to ~20 pg/mL within 15 minutes) after GIP and GLP-2 and with no change after placebo (12). Thus, the antiresorptive effect of GIP injection seems to be independent of changes in PTH, whereas the effect of GLP-2 may depend on an inhibitory effect on PTH secretion.
Our finding that GIP decreases CTX in patients with hypoparathyroidism is consistent with our in vitro results demonstrating GIPR expression in human osteoblast and osteoclast cultures, and support that GIP may act directly on bone cells in a PTH-independent fashion. By contrast, GLP-2R was not expressed in osteoblasts and absent or only weakly expressed in osteoclasts (depending on the method used), in agreement with the indiscernible effect of GLP-2 in patients with hypoparathyroidism. However, we did find expression of GLP-2R (as well as the GIPR and GLP-1R) in human parathyroid tissue, which could explain the effect of GLP-2 on bone as an indirect effect mediated by GLP-2R in the parathyroid gland. However, further studies are now required to investigate the possible direct effect of GLP-2 on the parathyroid glands.
In a previous study, investigating the effect of GLP-2 on bone turnover in patients with short bowel syndrome (SBS), a GLP-2 injection (1600 µg) only reduced PTH and CTX in patients with ileostomy and not in patients with jejunostomy (20). This might suggest that the underlying mechanism whereby GLP-2 reduces CTX may involve reductions in PTH as well as a factor derived from the intestine e.g. a cytokine or a growth factor, or by increased calcium absorption that in turn may change the PTH level. Since the GLP-2R is expressed in enteric neurons (44), the effect of GLP-2 may also be mediated through neuronal signaling.
A limitation of the present study is the small sample size and lack of a non-hypoparathyroid control group. However, regarding the GIP response, we achieved statistically significant results regarding CTX, and we know from previous studies in healthy controls (conducted using the same methods) that subcutaneous GLP-2 injections normally result in a response of similar magnitude as that obtained after subcutaneous GIP (with CTX being reduced to around 60% of baseline) (12, 45). Our results may also be confounded by the inclusion of participants using various types of medication. The main strength of the investigation is that it was possible to carry out these studies in patients with hypoparathyroidism and that all participants served as their own controls in a cross-over design in the peptide test. Although our results strongly indicate that GIP modulates bone turnover independent of changes in PTH, and that the GLP-2 effect appears to depend on changes in PTH, we recognize that the patients with hypoparathyroidism still had some residual PTH secretion and small changes in PTH levels therefore did occur during the tests. However, we assume that these small changes in PTH are biologically insignificant; in addition, the changes in PTH were of similar magnitude after all three interventions in the peptide test (i.e. after GIP, GLP-2, and placebo). All of the patients had undergone total thyroidectomy and therefore also lacked the thyroid gland which may have affected the calcitonin responses to GIP and GLP-2, if any. Thus, a further limitation is that we did not measure calcitonin, and we also did not measure total or ionized calcium during test days. Further studies are now needed to confirm our findings.
Our findings are compatible with the notion that GIP seems to act directly on the bone cells mediated by the GIPR in a non PTH-dependent fashion, while the effect of GLP-2 may be mediated in a PTH-dependent fashion, which would explain the diminished GLP-2 effect in the patients with hypoparathyroidism in our study. The apparently different underlying mechanisms of action of GIP and GLP-2 on bone turnover may mean that a combination of the two hormones might have additive or even synergistic effects, which would be of interest from a pharmacological point of view.
Supplementary Material
Acknowledgments
This work was supported by AP Møller Fonden, Novo Nordisk Foundation, and in part by NIH grant (1R01DK088188–01), and Novo Nordisk Foundation Center for Basic Metabolic Research. Novo Nordisk Foundation Center for Basic Metabolic Research is an independent Research Center, based at the University of Copenhagen, Denmark and partially funded by an unconditional donation from the Novo Nordisk Foundation (www.cbmr.ku.dk) (Grant number NNF18CC0034900).
Footnotes
Conflicts of interest
JJH and MMR are shareholders of Antag Therapeutics. JJH, MMR and BH are shareholders of Bainan Biotech. SM Advisory boards: AstraZeneca; Boehringer Ingelheim; Eli Lilly; Intarcia Therapeutics; Merck Sharp & Dohme; Novartis; Novo Nordisk; Sanofi; Lecture fees: AstraZeneca; Boehringer Ingelheim; Merck Sharp & Dohme; Novo Nordisk; Sanofi; Research Grant Recipient: Novo Nordisk; Boehringer Ingelheim. JBJ Advisory boards: Amgen, Eli Lilly, UCB, Gedion Richter. Lectures: Amgen, Eli lilly, UCB, Gilead, Otsaka. NH has received research funding from Alexion Pharmaceuticals, Inc. Other authors have declared no conflicts of interest.
References
- 1.Clowes JA, Allen HC, Prentis DM, Eastell R, and Blumsohn A. Octreotide abolishes the acute decrease in bone turnover in response to oral glucose. J Clin Endocrinol Metab 2003;88(10):4867–73. [DOI] [PubMed] [Google Scholar]
- 2.Henriksen DB, Alexandersen P, Bjarnason NH, Vilsboll T, Hartmann B, Henriksen EE, et al. Role of gastrointestinal hormones in postprandial reduction of bone resorption. J Bone Miner Res 2003;18(12):2180–9. [DOI] [PubMed] [Google Scholar]
- 3.Henriksen DB, Alexandersen P, Byrjalsen I, Hartmann B, Bone HG, Christiansen C, et al. Reduction of nocturnal rise in bone resorption by subcutaneous GLP-2. Bone 2004;34(1):140–7. [DOI] [PubMed] [Google Scholar]
- 4.Henriksen DB, Alexandersen P, Hartmann B, Adrian CL, Byrjalsen I, Bone HG, et al. Disassociation of bone resorption and formation by GLP-2: a 14-day study in healthy postmenopausal women. Bone 2007;40(3):723–9. [DOI] [PubMed] [Google Scholar]
- 5.Henriksen DB, Alexandersen P, Hartmann B, Adrian CL, Byrjalsen I, Bone HG, et al. Four-month treatment with GLP-2 significantly increases hip BMD: a randomized, placebo-controlled, dose-ranging study in postmenopausal women with low BMD. Bone 2009;45(5):833–42. [DOI] [PubMed] [Google Scholar]
- 6.Nissen A, Christensen M, Knop FK, Vilsboll T, Holst JJ, and Hartmann B. Glucose-dependent insulinotropic polypeptide inhibits bone resorption in humans. J Clin Endocrinol Metab 2014;99(11):E2325–9. [DOI] [PubMed] [Google Scholar]
- 7.Bjarnason NH, Henriksen EE, Alexandersen P, Christgau S, Henriksen DB, and Christiansen C. Mechanism of circadian variation in bone resorption. Bone 2002;30(1):307–13. [DOI] [PubMed] [Google Scholar]
- 8.Schiellerup SP, Skov-Jeppesen K, Windelov JA, Svane MS, Holst JJ, Hartmann B, et al. Gut Hormones and Their Effect on Bone Metabolism. Potential Drug Therapies in Future Osteoporosis Treatment. Front Endocrinol (Lausanne) 2019;10:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Askov-Hansen C, Jeppesen PB, Lund P, Hartmann B, Holst JJ, and Henriksen DB. Effect of glucagon-like peptide-2 exposure on bone resorption: Effectiveness of high concentration versus prolonged exposure. Regul Pept 2013;181:4–8. [DOI] [PubMed] [Google Scholar]
- 10.Gottschalck IB, Jeppesen PB, Holst JJ, and Henriksen DB. Reduction in bone resorption by exogenous glucagon-like peptide-2 administration requires an intact gastrointestinal tract. Scand J Gastroenterol 2008;43(8):929–37. [DOI] [PubMed] [Google Scholar]
- 11.Christensen MB, Lund A, Calanna S, Jorgensen NR, Holst JJ, Vilsboll T, et al. Glucose-Dependent Insulinotropic Polypeptide (GIP) Inhibits Bone Resorption independently of Insulin and Glycemia. J Clin Endocrinol Metab 2017. [DOI] [PubMed] [Google Scholar]
- 12.Skov-Jeppesen K, Svane MS, Martinussen C, Gabe MBN, Gasbjerg LS, Veedfald S, et al. GLP-2 and GIP exert separate effects on bone turnover: A randomized, placebo-controlled, crossover study in healthy young men. Bone 2019;125:178–85. [DOI] [PubMed] [Google Scholar]
- 13.Gabe MBN, van der Velden WJC, Gadgaard S, Smit FX, Hartmann B, Brauner-Osborne H, et al. Enhanced agonist residence time, internalization rate and signalling of the GIP receptor variant [E354Q] facilitate receptor desensitization and long-term impairment of the GIP system. Basic Clin Pharmacol Toxicol 2020;126 Suppl 6:122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torekov SS, Harslof T, Rejnmark L, Eiken P, Jensen JB, Herman AP, et al. A functional amino acid substitution in the glucose-dependent insulinotropic polypeptide receptor (GIPR) gene is associated with lower bone mineral density and increased fracture risk. J Clin Endocrinol Metab 2014;99(4):E729–33. [DOI] [PubMed] [Google Scholar]
- 15.Gasbjerg LS, Hartmann B, Christensen MB, Lanng AR, Vilsboll T, Jorgensen NR, et al. GIP’s effect on bone metabolism is reduced by the selective GIP receptor antagonist GIP(3–30)NH2. Bone 2020;130:115079. [DOI] [PubMed] [Google Scholar]
- 16.Hadjidakis DJ, and Androulakis, II. Bone remodeling. Ann N Y Acad Sci 2006;1092:385–96. [DOI] [PubMed] [Google Scholar]
- 17.Zhong Q, Itokawa T, Sridhar S, Ding KH, Xie D, Kang B, et al. Effects of glucose-dependent insulinotropic peptide on osteoclast function. Am J Physiol Endocrinol Metab 2007;292(2):E543–8. [DOI] [PubMed] [Google Scholar]
- 18.Bollag RJ, Zhong Q, Phillips P, Min L, Zhong L, Cameron R, et al. Osteoblast-derived cells express functional glucose-dependent insulinotropic peptide receptors. Endocrinology 2000;141(3):1228–35. [DOI] [PubMed] [Google Scholar]
- 19.Pacheco-Pantoja EL, Ranganath LR, Gallagher JA, Wilson PJ, and Fraser WD. Receptors and effects of gut hormones in three osteoblastic cell lines 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottschalck IB, Jeppesen PB, Hartmann B, Holst JJ, and Henriksen DB. Effects of treatment with glucagon-like peptide-2 on bone resorption in colectomized patients with distal ileostomy or jejunostomy and short-bowel syndrome. Scand J Gastroenterol 2008;43(11):1304–10. [DOI] [PubMed] [Google Scholar]
- 21.Valderas JP, Padilla O, Solari S, Escalona M, and Gonzalez G. Feeding and bone turnover in gastric bypass. J Clin Endocrinol Metab 2014;99(2):491–7. [DOI] [PubMed] [Google Scholar]
- 22.Polymeris AD, Doumouchtsis KK, Giagourta I, and Karga H. Effect of an oral glucose load on PTH, 250HD3, calcium, and phosphorus homeostasis in postmenopausal women. Endocr Res 2011;36(2):45–52. [DOI] [PubMed] [Google Scholar]
- 23.Fuglsang-Nielsen R, Rakvaag E, Vestergaard P, Hartmann B, Holst JJ, Hermansen K, et al. Consumption of nutrients and insulin resistance suppress markers of bone turnover in subjects with abdominal obesity. Bone 2020;133:115230. [DOI] [PubMed] [Google Scholar]
- 24.Simonsen JL, Rosada C, Serakinci N, Justesten J, Stenderup K, Rattan SIS, et al. Telomerase expression extends lifespan and prevents senescence-associated impairment of osteoblast functions. Nature biotechnology 2002;20(6):592–6. [DOI] [PubMed] [Google Scholar]
- 25.Jafari A, Isa A, Chen L, Ditzel N, Zaher W, Harkness L, et al. TAFA2 induces skeletal (stromal) stem cell migration through activation of Rac1‐p38 signaling. Stem Cells 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jafari A, Qanie D, Andersen TL, Zhang Y, Chen L, Postert B, et al. Legumain Regulates Differentiation Fate of Human Bone Marrow Stromal Cells and Is Altered in Postmenopausal Osteoporosis. Stem Cell Reports 2017;8(2):373–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carey HA, Hildreth BE 3rd, Geisler JA, Nickel MC, Cabrera J, Ghosh S, et al. Enhancer variants reveal a conserved transcription factor network governed by PU.1 during osteoclast differentiation. Bone Res 2018;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boissy P, Andersen TL, Abdallah BM, Kassem M, Plesner T, and Delaisse JM. Resveratrol inhibits myeloma cell growth, prevents osteoclast formation, and promotes osteoblast differentiation. Cancer Res 2005;65(21):9943–52. [DOI] [PubMed] [Google Scholar]
- 29.Soe K, and Delaisse JM. Glucocorticoids maintain human osteoclasts in the active mode of their resorption cycle. J Bone Miner Res 2010;25(10):2184–92. [DOI] [PubMed] [Google Scholar]
- 30.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013;29(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 2010;38(4):576–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Love MI, Huber W, and Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young MD, Wakefield MJ, Smyth GK & Oshlack A Gene ontology analysis for RNA-seq accounting for selection bias. Genome biol 2010;11(R14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christensen MB, Lund A, Calanna S, Jørgensen NR, Holst JJ, Vilsbøll T, et al. Glucose-Dependent Insulinotropic Polypeptide (GIP) Inhibits Bone Resorption Independently of Insulin and Glycemia. J Clin Endocrinol Metab 2018;103(1):288–94. [DOI] [PubMed] [Google Scholar]
- 35.Vilsboll T, Krarup T, Sonne J, Madsbad S, Volund A, Juul AG, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab 2003;88(6):2706–13. [DOI] [PubMed] [Google Scholar]
- 36.Clowes JA, Robinson RT, Heller SR, Eastell R, and Blumsohn A. Acute changes of bone turnover and PTH induced by insulin and glucose. Euglycemic and hypoglycemic hyperinsulinemic clamp studies. The Journal of Clinical Endocrinology & Metabolism 2002;87(7):3324–9. [DOI] [PubMed] [Google Scholar]
- 37.Bergmann NC, Lund A, Gasbjerg LS, Jørgensen NR, Jessen L, Hartmann B, et al. Separate and Combined Effects of GIP and GLP-1 Infusions on Bone Metabolism in Overweight Men Without Diabetes. The Journal of Clinical Endocrinology & Metabolism 2019;104(7):2953–60. [DOI] [PubMed] [Google Scholar]
- 38.Gasbjerg LS, Helsted MM, Hartmann B, Sparre-Ulrich AH, Veedfald S, Stensen S, et al. GIP and GLP-1 Receptor Antagonism During a Meal in Healthy Individuals. J Clin Endocrinol Metab 2020;105(3). [DOI] [PubMed] [Google Scholar]
- 39.Alsalim W, Tura A, Pacini G, Omar B, Bizzotto R, Mari A, et al. Mixed meal ingestion diminishes glucose excursion in comparison with glucose ingestion via several adaptive mechanisms in people with and without type 2 diabetes. Diabetes Obes Metab 2016;18(1):24–33. [DOI] [PubMed] [Google Scholar]
- 40.Rubin MR, and Bilezikian JP. Hypoparathyroidism: clinical features, skeletal microstructure and parathyroid hormone replacement. Arquivos brasileiros de endocrinologia e metabologia 2010;54(2):220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarke BL. Bone disease in hypoparathyroidism. Arquivos brasileiros de endocrinologia e metabologia 2014;58(5):545–52. [DOI] [PubMed] [Google Scholar]
- 42.Silva BC, Rubin MR, Cusano NE, and Bilezikian JP. Bone imaging in hypoparathyroidism. Osteoporos Int 2017;28(2):463–71. [DOI] [PubMed] [Google Scholar]
- 43.Underbjerg L, Sikjaer T, Mosekilde L, and Rejnmark L. Postsurgical hypoparathyroidism--risk of fractures, psychiatric diseases, cancer, cataract, and infections. J Bone Miner Res 2014;29(11):2504–10. [DOI] [PubMed] [Google Scholar]
- 44.Bjerknes M, and Cheng H. Modulation of specific intestinal epithelial progenitors by enteric neurons. Proc Natl Acad Sci U S A 2001;98(22):12497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gabe MBN, Skov-Jeppesen K, Gasbjerg LS, Schiellerup SP, Martinussen C, Gadgaard S, et al. P12 Osteoporosis treatment with GIP and GLP-2 dual-agonists based on their synergistic actions in humans – a novel therapeutic principle in children and premenopausal women suffering from bone fragility 20. November 2020 ICCBH VIRTUAL FORUM, Bone Fragility Disorders in Children; 18–20 November 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


