Abstract
Cancer screening rates declined sharply early in the COVID-19 pandemic. The impact of the pandemic may have exacerbated existing disparities in cancer screening due to the disproportionate burden of illness and job loss among racial/ ethnic minorities, and potentially, uneven resumption of care between different racial/ ethnic groups. Using electronic health record data from Mass General Brigham (MGB), we assessed changes in rates of breast, cervical, colorectal and lung cancer screening before and during the pandemic. Among patients who received primary care in an MGB-affiliated primary care practice, cancer screening rates were calculated as the number of individuals who received a screening test for each cancer type over the number of individuals due for each test, during each month between April 2019-November 2020. We conducted an interrupted time-series analysis to test for changes in screening rates by race/ethnicity before and during the pandemic. Prior to the pandemic, relative to White individuals, Asian women were less likely to receive breast cancer screening (p<0.001), and Latinx and Black individuals were less likely to screen for lung cancer (p<0.001 and p=0.02). Our results did not show significant improvement or worsening of racial/ethnic disparities for any cancer screening type as screening resumed. However, as of November 2020 rates of screening for breast cancer were lower than pre-pandemic levels for Latinx individuals, and lung cancer screening rates were higher than baseline for Latinx, Black or White individuals. Further monitoring of disparities in cancer screening is warranted as the pandemic evolves.
Keywords: cancer screening, disparities, COVID-19
Introduction
Timely screening for breast, cervical, colorectal (CRC) and lung cancer reduces cancer morbidity and mortality and is endorsed by national guidelines for age-appropriate populations.1–4 The timeliness of cancer screening becomes especially important during periods when the provision of care is constrained. Prior studies have shown that cancer screening deteriorates as a result of natural disasters and conflicts,5–7 and that delays in screening in these instances may result in higher stage of disease among those subsequently diagnosed with cancer.8
The COVID-19 pandemic presents challenges to the delivery of cancer screening. Cancer screening rates sharply declined in the U.S. early in the COVID-19 pandemic.9–12 Concerns about preservation of personal protective equipment, avoidance of undue exposure to the virus in health care facilities, re-deployment of clinical staff, and government regulations affected cancer screening rates with many facilities closed for preventive services throughout Spring 2020. While COVID-19-related disruptions in care were widespread, the total burden of COVID-19 on cancer screening may not impact all groups equally, as racial/ethnic minorities have been disproportionately affected by the health, social, and economic consequences of the pandemic, and given the significant inequalities in cancer screening prior to the pandemic.
Racial and ethnic minorities in the US have experienced disparities in cancer screening, incidence and mortality. Latinx women have the highest incidence of cervical cancer, while non-Latinx Black (hereafter called Black) men and women have the highest cancer mortality of all racial and ethnic groups in the U.S. for lung, colorectal, breast, and cervical cancers, and for all cancers combined.13 The observed disparities in cancer incidence and mortality reflect, in part, racial/ethnic disparities in the use of cancer screening. Black, Latinx and Asian individuals are also less likely to receive timely colorectal cancer screening.14 Latinx women are less likely to use mammography than non-Latinx White (hereafter called White) women.15,16 For cervical cancer screening, the findings for Latinx and non-Latinx Asian (hereafter called Asian) women are mixed with some studies suggesting lower rates of timely screening compared to White women but others showing comparable or even higher rates.16–19 In summary, prior to the pandemic, racial/ethnic minorities were often less likely to receive timely breast, cervical, colorectal and lung cancer screening.
While the pandemic has highlighted the pre-pandemic disparities in health and health care in the U.S. and has fueled many new initiatives to promote health equity, concern remains that the COVID-19 pandemic may worsen racial/ethnic disparities in care, including the use of cancer screening,11 particularly since racial/ethnic minorities have been disproportionately affected by COVID-19 related illness, and may be more hesitant to accept the coronavirus vaccine.20,21
The purpose of this study is to assess the trajectories of cancer screening by race/ ethnicity before and during the initial period of the pandemic. Because COVID-19 has led to disproportionate illness and job loss among racial/ ethnic minorities,22–24 we hypothesize that any disparities in cancer screening that were present before the pandemic have worsened over the course of 2020 as White individuals resumed their screening more quickly than Black, Latinx and Asian individuals.
Methods
Study Design and Population
This is a retrospective cohort study using electronic medical record data from Mass General Brigham (MGB), a large academic health system in eastern Massachusetts. To assess screening use in a population receiving primary care at MGB, individuals eligible for screening during each month of the study period were identified among those who had at least one visit to a primary care provider (PCP) in the prior 3 years relative to each month between April 1, 2019 and November 30, 2020. Because our focus was on screening use by race/ ethnicity, we excluded racial/ ethnic groups that were too small to analyze or had unknown race/ ethnicity. To focus on individuals with average screening risk, we excluded individuals with prior cancer for each cancer type. For each month from April 2019 to November 2020, eligibility for screening was determined on a monthly basis by assessing whether a patient was alive at the beginning of the month and met cancer screening eligibility based on the United States Preventive Services Task Force (USPSTF) recommendations for each type of cancer screening test at the beginning of the month,1–4 specifically: 1) For breast cancer screening, females 40–75 years of age without a documented mammogram, including digital breast tomosynthesis, in the previous two years; 2) For cervical cancer screening, females 21–65 years of age without a documented Papanicolaou (Pap) test in the past 3 years; 3) For colorectal cancer screening, males and females 50–75 years of age without a colonoscopy in the past 10 years or a fecal immunohistochemistry test (FIT) in the past year; 4) For lung cancer screening, males and females age 55–80 years without a low dose computed tomography of the chest (LDCT) in the past year who currently smoke or quit smoking within the prior 15 years and the quit date was available. We did not include pack-years of smoking in our eligibility definition due to a high degree of missing data on smoking duration.
Study Period
The pre-COVID-19 period was defined as April 1, 2019 to March 1, 2020, the time of the initial COVID-19 surge in Massachusetts. The period during COVID-19 was divided into two phases: 1) a COVID-19 surge period between March 1, 2020 and May 31, 2020 when lockdown measures were instituted in Massachusetts, and 2) the time following this initial COVID-19 surge defined as occurring from June 1, 2020 through November 30, 2020, when ambulatory services resumed albeit at lower capacity than prior to the pandemic.
Screening Use
For patients who were eligible for each type of screening in each month, we determined whether s/he had received a USPSTF recommended screening test (mammogram for breast cancer, Pap test for cervical cancer, colonoscopy or FIT for colorectal cancer, or LDCT for lung cancer) based on their electronic health record.
Statistical Analysis
Descriptive demographic characteristics were summarized at the individual level for each of the four screening cohorts. These variables included age, sex, race/ethnicity (White, Black, Latinx, Asian), highest education level (completed high school or less, college degree, graduate degree, unknown), and health insurance coverage (private, Medicare, Medicaid/uninsured).
We conducted an interrupted time-series analysis to evaluate the impact of the pandemic on screening rates by race/ ethnicity.25,26 For each screening type, a separate Poisson generalized linear model was fit to analyze expected screening rates according to the specification:
where Ytj and Ntj denote the number of screenings and eligible patients in the t-th month and j-th racial/ethnic group, for t=0, 1, …, 19 and j=0, 1, 2, 3. Here Tt = t is a linear trend that initiates from the beginning of the study period (April 2019), and is a linear trend initiating from pandemic onset time τ (March 2020). Pt is an indicator of the pandemic period (t ≥ τ) and Rj is a vector of 3 indicators for whether the j-th group corresponds to Black, Latinx, and Asian groups, relative to the White group. The β4 and β5 parameters estimate differences in mean screening rates, on the log scale, at the beginning of the study period and in their slope between racial/ethnic groups in the pre-pandemic period. The β6 and β7 parameters estimate how these disparities changed during the pandemic. We used two-sided Wald tests to test against the null hypotheses β4 = β5 = 0 (jointly for any minority race/ethnicity and separately for each race/ethnicity) to assess for the presence of any disparities relative to White individuals in the pre-pandemic period. We then assessed whether disparities have shifted during the pandemic by testing against β6 = β7 = 0. Finally, we tested whether expected screening rates, under the estimated model, in November 2020 differed from those in November 2019 based on testing for other linear restrictions on the coefficient parameters by Wald tests. Heteroskedasticity and autocorrelation consistent standard errors were used to account for overdispersion and residual autocorrelation after modeling linear time trends.27,28 We also further stratified these regression analyses by known highest education level and insurance status to assess for potential differences in disparities across these sociodemographic subgroups. P-values were two-sided. Data management was done in SAS version X and data analysis was done in R version 4.0.2.
Results
Across eligible patients included in the analysis for any screening type, the mean age was 51.1 years (SD 15.9); mean age was youngest for those eligible for cervical cancer screening (46.8 years) and oldest for those eligible for lung cancer screening (65.7 years; Table 1). Overall, most patients were White (78.4%), followed by Black (8.4%), Asian (7.0%), and Latinx (6.2%). Most patients were female (59.4%), had a college degree (44.6%), and had private health insurance (67.3%). Similar trends in education and insurance were observed in patients eligible for breast, cervical and colon cancer screening. Among those eligible for lung cancer screening, there were fewer patients who were Asian (2.7%), had graduated from college (29.6%), or had private insurance (47.1%) than the other cancer screening populations.
Table 1.
Description of eligible patients for each cancer screening type.
| Cancer Screening Type | ||||
|---|---|---|---|---|
| Breast | Cervical | Colon | Lung | |
| N | 29,081 | 51,436 | 24,706 | 10,697 |
| Age (mean) (SD) | 55.1 (10.6) | 46.8 (12.8) | 59.5 (8.0) | 64.7 (6.6) |
| n(%) | n(%) | n(%) | n(%) | |
| Female | - | - | 14,913 (60.4) | 5,248 (49.1) |
| Race | ||||
| White, non-Latinx | 21,707 (74.6) | 38,775 (75.4) | 19,746 (79.9) | 8,926 (83.4) |
| Black, non-Latinx | 3,116 (10.7) | 4,911 (9.6) | 2,167 (8.8) | 955 (8.9) |
| Latinx | 1,981 (6.8) | 3,511 (6.8) | 1,279 (5.2) | 524 (4.9) |
| Asian, non-Latinx | 2,277 (7.8) | 4,239 (8.2) | 1,514 (6.1) | 292 (2.7) |
| Education | ||||
| High school or less | 7,745 (26.6) | 12,934 (25.1) | 7,270 (29.4) | 4,671 (43.7) |
| College degree | 12,984 (44.6) | 24,151 (47.0) | 10,247 (41.5) | 3,169 (29.6) |
| Graduate degree | 3,075 (10.6) | 6,022 (11.7) | 2,551 (10.3) | 725 (6.8) |
| Unknown | 5,277 (18.1) | 8,329 (16.2) | 4,638 (18.8) | 2,132 (19.9) |
| Health insurance | ||||
| Private | 18,696 (64.3) | 38,780 (75.4) | 15,602 (63.2) | 5,037 (47.1) |
| Medicare | 5,360 (18.4) | 3,616 (7.0) | 5,508 (22.3) | 4,099 (38.3) |
| Medicaid/no insurance | 5,025 (17.3) | 9,040 (17.6) | 3,596 (14.6) | 1,561 (14.6) |
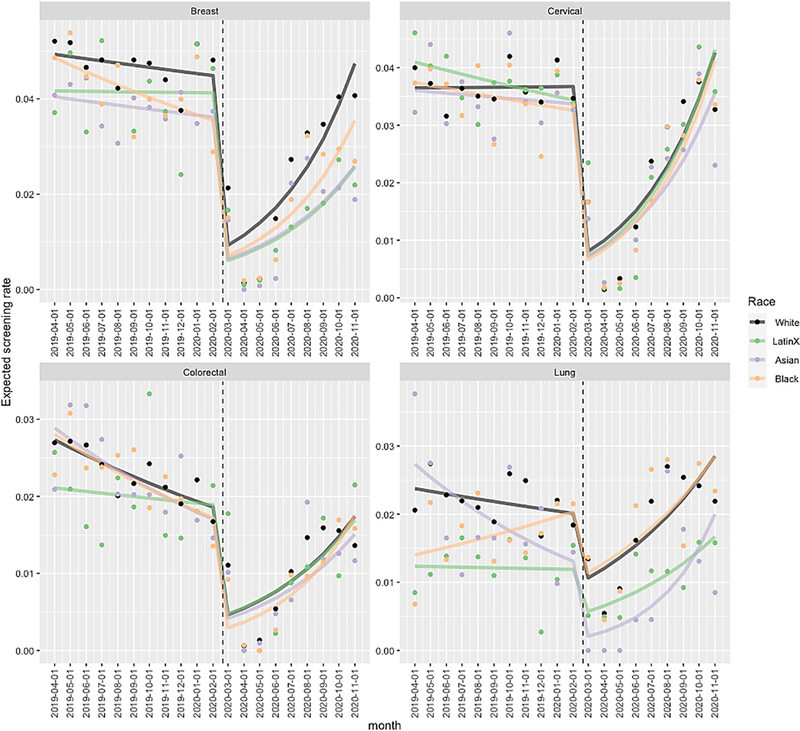
At the beginning of the study period in April 2019, rates of mammography for breast cancer screening were lower for Asian compared to White women (incidence rate ratio (IRR) 0.82, 95% CI 0.74–0.91, p<0.001; Table 2 and Figure), as were rates of LDCT for Black and Latinx individuals compared to White individuals (IRR 0.59, 95% CI 0.40–0.88, p=0.01; IRR 0.52, 95% CI 0.38–0.71, p<0.001 respectively). In the pre-COVID-19 period, Latinx, Black and Asian individuals in aggregate had lower rates of screening compared to White individuals for breast (p<0.001, “Overall” in Table 3) and lung cancer screening (p<0.001). When specific race/ethnicities were compared to White individuals, Asian individuals also had lower rates of breast cancer screening (p<0.001), while Latinx individuals had lower rates of lung cancer screening (p<0.001). Expected screening rates plummeted for all cancer screening types and all racial/ ethnic groups during the COVID-19 surge (March 2020 through May 2020), as shown in the Figure. This was followed by a period of recovery occurring at different rates for different racial/ethnic groups.
Table 2.
Poisson generalized linear model coefficient estimates
| Breast | Cervical | Colon | Lung | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRRa | 95% CI | P-value | IRRa | 95% CI | P-value | IRRa | 95% CI | P-value | IRRa | 95% CI | P-value | |||||
| Time (month)b | 0.99 | 0.97 | 1.00 | 0.32 | 1.00 | 0.98 | 1.02 | 0.94 | 0.96 | 0.95 | 0.98 | <0.001 | 0.98 | 0.96 | 1.01 | 0.22 |
| Before-After Pandemic Onsetc (ref: Before) | 0.21 | 0.08 | 0.57 | 0.002 | 0.22 | 0.09 | 0.53 | <0.001 | 0.26 | 0.09 | 0.79 | 0.02 | 0.54 | 0.33 | 0.89 | 0.02 |
| LatinX (ref: White) | 0.85 | 0.66 | 1.08 | 0.17 | 1.12 | 0.98 | 1.28 | 0.10 | 0.77 | 0.59 | 1.01 | 0.05 | 0.52 | 0.38 | 0.71 | <0.001 |
| Asian | 0.82 | 0.74 | 0.91 | <0.001 | 0.99 | 0.82 | 1.20 | 0.91 | 1.06 | 0.80 | 1.39 | 0.70 | 1.15 | 0.75 | 1.77 | 0.52 |
| Black | 0.99 | 0.86 | 1.14 | 0.84 | 1.03 | 0.90 | 1.17 | 0.69 | 1.03 | 0.85 | 1.24 | 0.78 | 0.59 | 0.40 | 0.88 | 0.01 |
| Timeb*Onset | 1.24 | 1.06 | 1.44 | 0.01 | 1.23 | 1.07 | 1.42 | 0.004 | 1.23 | 1.03 | 1.46 | 0.02 | 1.15 | 1.03 | 1.25 | 0.001 |
| Time*LatinX | 1.01 | 0.96 | 1.06 | 0.72 | 0.98 | 0.96 | 1.01 | 0.14 | 1.03 | 0.99 | 1.07 | 0.19 | 1.01 | 0.95 | 1.09 | 0.72 |
| Time*Asian | 1.00 | 0.98 | 1.02 | 0.89 | 0.99 | 0.97 | 1.02 | 0.61 | 0.99 | 0.94 | 1.03 | 0.55 | 0.95 | 0.88 | 1.01 | 0.11 |
| Time*Black | 0.98 | 0.94 | 1.02 | 0.24 | 0.99 | 0.96 | 1.01 | 0.20 | 0.99 | 0.96 | 1.02 | 0.51 | 1.06 | 1.00 | 1.11 | 0.05 |
| Onset c *LatinX | 0.71 | 0.15 | 3.35 | 0.67 | 0.98 | 0.25 | 3.5 | 0.973 | 0.99 | 0.11 | 9.10 | 0.99 | 0.90 | 0.37 | 2.19 | 0.82 |
| Onset*Asian | 0.87 | 0.27 | 2.81 | 0.81 | 0.98 | 0.44 | 2.21 | 0.97 | 1.00 | 0.2 | 5.02 | 1.00 | 0.32 | 0.07 | 1.54 | 0.15 |
| Onset*Black | 0.98 | 0.51 | 1.89 | 0.95 | 0.94 | 0.57 | 1.56 | 0.81 | 0.69 | 0.22 | 2.17 | 0.53 | 1.01 | 0.60 | 1.67 | 0.99 |
| Timeb*Onset c *LatinX | 0.97 | 0.77 | 1.22 | 0.78 | 1.03 | 0.82 | 1.30 | 0.78 | 0.97 | 0.67 | 1.39 | 0.85 | 1.00 | 0.88 | 1.13 | 0.97 |
| Time*Onset*Asian | 0.97 | 0.81 | 1.16 | 0.75 | 1.0 | 0.86 | 1.16 | 0.98 | 1.01 | 0.79 | 1.30 | 0.94 | 1.24 | 0.98 | 1.58 | 0.08 |
| Time*Onset*Black | 1.02 | 0.92 | 1.13 | 0.69 | 1.03 | 0.95 | 1.13 | 0.42 | 1.07 | 0.90 | 1.27 | 0.44 | 0.94 | 0.86 | 1.03 | 0.17 |
| Test for changes in disparities | 0.26 | 0.71 | 0.89 | 0.34 | ||||||||||||
IRR = incidence rate ratio; CI – confidence interval
Time refers to linear trend in months (IRR refers to rate ratio for each month relative to prior)
Onset refers to before or after onset of the COVID-19 pandemic defined as March 2020
NOTE: p-values from Wald test for hypothesis β6 = β7 = 0
Figure.
Expected screening rates estimated by the Poisson model over time by race/ethnicity, separately for each cancer type
NOTES: Solid point represents raw monthly screening rates by race
Separate Poisson generalized linear models are fit for each screening type
Table 3.
Screening incidence rates by race vs. White in pre-pandemic period
| IRR Point Estimates | ||||
|---|---|---|---|---|
| Cancer | Group | Race vs. White (β4) | Time*Race Interaction (β5) | p-value |
| Breast | Latinx | 0.845 | 1.008 | 0.176 |
| Breast | Asian | 0.819 | 0.998 | <0.001 |
| Breast | Black | 0.985 | 0.978 | 0.099 |
| Breast | Overall | - | - | <0.001 |
| Cervical | Latinx | 1.124 | 0.982 | 0.246 |
| Cervical | Asian | 0.989 | 0.993 | 0.508 |
| Cervical | Black | 1.027 | 0.985 | 0.292 |
| Cervical | Overall | - | - | 0.494 |
| Colorectal | Latinx | 0.772 | 1.028 | 0.156 |
| Colorectal | Asian | 1.056 | 0.986 | 0.794 |
| Colorectal | Black | 1.026 | 0.990 | 0.713 |
| Colorectal | Overall | - | - | 0.58 |
| Lung | Latinx | 0.521 | 1.013 | <0.001 |
| Lung | Asian | 1.152 | 0.945 | 0.098 |
| Lung | Black | 0.591 | 1.055 | 0.02 |
| Lung | Overall | - | - | <0.001 |
NOTE:
[1] Wald p-value based on testing for joint null hypothesis for beta4 = beta5 = 0 (for each race and for all races in overall)
[2] Race vs. White IRR estimates refer to rates of screening in April 2019
[3] Time* Race interaction refer to the ratio of year-to-year OR for each race relative to white
By November 2020 rates of breast, cervical and colorectal cancer screening overall were similar to screening rates prior to the pandemic in November 2019, and lung cancer screening rates significantly differed (“joint test” in Table 4). Specifically for Latinx individuals, rates of breast cancer screening by November 2020 were still lower than those in November 2019, and rates of lung cancer screening were higher. (Table 4 and Figure). For both Black and White individuals, lung cancer screening rates were also higher by November 2020 than they had been in November 2019. As screening rates began to recover following the COVID surge, there were no significant changes in disparities ascertained in the pre-COVID-19 period after the onset of the pandemic (p=0.26 for breast, p=0.71 for cervical, p=0.89 for CRC, p=0.34 for lung; Table 2). There were similarly no clear changes in disparities by race/ ethnicity after stratification by education level and insurance (data not shown).
Table 4.
Differences in expected screening rates in November 2020 vs. November 2019 by race
| Cancer | Race | Nov 2019 Rate | Nov 2020 Rate | IRR | p-value |
|---|---|---|---|---|---|
| Breast | Latinx | 0.041 | 0.026 | 0.621 | 0.024 |
| Breast | Asian | 0.037 | 0.026 | 0.694 | 0.094 |
| Breast | Black | 0.039 | 0.035 | 0.908 | 0.633 |
| Breast | White | 0.046 | 0.047 | 1.027 | 0.856 |
| Breast | Overall | - | - | - | 0.192 |
| Cervical | Latinx | 0.036 | 0.043 | 1.190 | 0.402 |
| Cervical | Asian | 0.034 | 0.036 | 1.034 | 0.897 |
| Cervical | Black | 0.034 | 0.041 | 1.213 | 0.285 |
| Cervical | White | 0.037 | 0.043 | 1.163 | 0.393 |
| Cervical | Overall | - | - | - | 0.398 |
| Colorectal | Latinx | 0.020 | 0.017 | 0.865 | 0.668 |
| Colorectal | Asian | 0.020 | 0.015 | 0.756 | 0.244 |
| Colorectal | Black | 0.020 | 0.018 | 0.882 | 0.492 |
| Colorectal | White | 0.021 | 0.017 | 0.833 | 0.361 |
| Colorectal | Overall | - | - | - | 0.666 |
| Lung | Latinx | 0.012 | 0.017 | 1.386 | 0.054 |
| Lung | Asian | 0.016 | 0.020 | 1.224 | 0.645 |
| Lung | Black | 0.018 | 0.028 | 1.562 | 0.006 |
| Lung | White | 0.021 | 0.028 | 1.348 | 0.054 |
| Lung | Overall | - | - | - | 0.014 |
NOTE:
[1] Wald p-value based on testing for null hypothesis that expected rates in November 2020 differs from those in November 2019 (for each race and overall)
[2] Nov 2019 and Nov 2020 rates refer to expected incidence rates of screening based on the model
[3] Ratio refers to IRR for Nov 2020 rate by the Nov 2019 rate
Discussion
Our findings support prior work that demonstrates that cancer screening rates plummeted at the start of the pandemic.9–11 This work extends what is known about the effect of the pandemic on cancer screening in the US by specifically looking at the trajectory of disparities in cancer screening early in the pandemic and showed no significant worsening or improvement in racial/ethnic disparities early in the initial COVID-19 recovery period compared to prior to the pandemic. Yet, racial/ethnic disparities in cancer screening present prior to the pandemic were still observed after the COVID-19 pandemic began.
While prior work suggests that other crises that have disrupted cancer screening may ultimately result in delays in cancer diagnosis,6 our work is unique in examining the effect of a catastrophic disruption in care on disparities. Kanjanvaikoon et al. showed that women diagnosed with cervical cancer had higher stage of disease among those diagnosed with cancer after Hurricane Katrina in 2005, likely due to a decrease in cervical screening utilization.8 Preliminary estimates from pandemic-associated disruptions in national cancer screening programs in the Netherlands and the United Kingdom also raise concern for delays in cancer diagnoses and their effect on mortality.29,30
The pandemic has brought renewed attention to disparities in health and health care in the US. As health care systems and public health departments will face financial stresses and the ongoing challenges of COVID-19 interventions should be considered to prevent or improve disparities in cancer screening to thwart any ultimate worsening of the disparities in health that have contributed to the adverse impact and outcomes of the pandemic.31 As we move forward, the following considerations merit further discussion: 1) Whether COVID-19 related financial stresses on healthcare systems allow equal resumption of robust screening programs across the population; 2) Whether shifts to telemedicine will generate differences in who will request or be referred for screening, who will receive and complete active outreach, or who will schedule in-person follow-up testing; 3) Whether the pandemic’s economic ramifications will exacerbate existing national sociodemographic differences in healthcare access and outcomes; 4) Whether differences in vaccine uptake or concerns about the possibility of health care associated exposure to coronavirus will result in differential return to screening and other preventive services over time by race/ethnic groups; 5) Whether having insurance coverage will lead to preferential resumption of cancer screening The resumption of routine healthcare, including cancer screening, must incorporate intentional strategies to monitor for and minimize health disparities.
Although our study did not show worsened disparities in cancer screening early in the pandemic, the trends observed may widen or narrow depending on whether measures are taken to mitigate pre-COVID-19 cancer screening inequities. Our results nevertheless indicated that in many cases disparities were maintained as screening exams resumed and the rates of recovery were uneven across different race/ethnicities. It remains to be seen whether these differences will worsen as the pandemic continues and eventually subsides. Worsened disparities in cancer screening are likely to result in delayed cancer detection, more advanced stages of malignancy at diagnosis, and loss of life-years among those with cancer.
Our study is not without limitations. Our study is restricted to individuals with a primary care provider at a large academic health system in one region of the United States, with few disparities in access to care prior to the pandemic. As a result, there may be selection effects that make it difficult to generalize the results to other patient populations. Due to the limited number of screenings observed each month within racial/ethnic groups by cancer type, this study may have limited power to detect subtle shifts in disparities after the onset of the pandemic. This analysis is also restricted to time periods covering the first surge and subsequent recovery. Further studies are needed to investigate the cancer diagnoses/cancer stage migration due to delays in screening as the COVID-19 pandemic evolves.
The pandemic has documented the necessity of countering racial/ethnic disparities in care and provides opportunity for innovative cancer screening strategies including FIT testing and fecal DNA testing for colorectal cancer screening and potentially self-sampling HPV testing for cervical cancer screening. Real-time data monitoring of trends in screening and other preventive care by race/ ethnicity should be considered to mitigate the challenge of resuming cancer screening during the pandemic without worsening disparities.
Highlights.
Cancer screening rates declined sharply early in the COVID-19 pandemic.
While our results did not show significant improvement or worsening of racial/ethnic disparities for any cancer screening type as screening resumed, as of November 2020 rates of screening for breast cancer were lower than pre-pandemic levels for Latinx individuals, but lung cancer screening rates were higher than baseline for Latinx, Black and White individuals.
Further monitoring of disparities in cancer screening is warranted as the pandemic evolves.
Acknowledgements:
Funders: This study was conducted as part of the National Cancer Institute (NCI)-funded consortium, METRICS (Multi-level Optimization of the Cervical Cancer Screening Process in Diverse Settings & Populations; Grant number 1UM1CA221940).
Footnotes
Declaration of competing interest
None of the authors have any financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Curry SJ, Krist AH, Owens DK, et al. Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(7):674–686. [DOI] [PubMed] [Google Scholar]
- 2.Screening for Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Annals of internal medicine. 2008;149(9):627–637. [DOI] [PubMed] [Google Scholar]
- 3.Moyer VA. Screening for Lung Cancer: U.S. Preventive Services Task Force Recommendation Statement. Annals of internal medicine. 2014;160(5):330–338. [DOI] [PubMed] [Google Scholar]
- 4.Siu AL. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Annals of internal medicine. 2016;164(4):279–296. [DOI] [PubMed] [Google Scholar]
- 5.Man RX-G, Lack DA, Wyatt CE, Murray V. The effect of natural disasters on cancer care: a systematic review. The lancet oncology. 2018;19(9):e482–e499. [DOI] [PubMed] [Google Scholar]
- 6.El Saghir NS, Soto Pérez de Celis E, Fares JE, Sullivan R. Cancer Care for Refugees and Displaced Populations: Middle East Conflicts and Global Natural Disasters. American Society of Clinical Oncology educational book. 2018;38(38):433–440. [DOI] [PubMed] [Google Scholar]
- 7.Nelson RL, Dollear T, Freels S, Persky V. The relation of age, race, and gender to the subsite location of colorectal carcinoma. Cancer. 1997;80(2):193–197. [DOI] [PubMed] [Google Scholar]
- 8.Kanjanvaikoon P, Gerber D, Robison WR. Long term impact of natural disaster on cervical cancer demographics. Gynecologic oncology. 2011;123(2):446–446. [Google Scholar]
- 9.Kaufman HW, Chen Z, Niles J, Fesko Y. Changes in the Number of US Patients With Newly Identified Cancer Before and During the Coronavirus Disease 2019 (COVID-19) Pandemic. JAMA network open. 2020;3(8):e2017267–e2017267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mast C, Munoz del Rio A. Delayed Cancer Screenings—A Second Look. https://ehrn.org/articles/delayed-cancer-screenings-a-second-look/. Published 2020. Accessed December 10, 2020.
- 11.Corley DA, Sedki M, Ritzwoller DP, et al. Cancer Screening during COVID-19: A Perspective from NCI’s PROSPR consortium. Gastroenterology (New York, NY 1943). 2020. [Google Scholar]
- 12.Miller MJ, Xu L, Qin J, et al. Impact of COVID-19 on Cervical Cancer Screening Rates Among Women Aged 21–65 Years in a Large Integrated Health Care System - Southern California, January 1-September 30, 2019, and January 1-September 30, 2020. MMWR. 2021;70(4):109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on 2019 submission data (1999–2017): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; www.cdc.gov/cancer/dataviz, released in June 2020. 2017. [Google Scholar]
- 14.Beydoun HA, Beydoun MA. Predictors of colorectal cancer screening behaviors among average-risk older adults in the United States. Cancer causes & control. 2007;19(4):339–359. [DOI] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics. Table 33. use of mammography among women aged 40 and over, by selected characteristics: United States, selected years 1987–2015. 2019.
- 16.White A, Thompson TD, White MC, et al. Cancer Screening Test Use - United States, 2015. MMWR. 2017;66(8):201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee S, Gupta D, Caputo TA, Holcomb K. Disparities in Gynecological Malignancies. Frontiers in oncology. 2016;6:36–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musselwhite LW, Oliveira CM, Kwaramba T, et al. Racial/Ethnic Disparities in Cervical Cancer Screening and Outcomes. Acta cytologica. 2016;60(6):518–526. [DOI] [PubMed] [Google Scholar]
- 19.Hall IJ, Tangka FKL, Sabatino SA, Thompson TD, Graubard BI, Breen N. Patterns and Trends in Cancer Screening in the United States. Prev Chronic Dis. 2018;15:E97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreps S, Prasad S, Brownstein JS, et al. Factors Associated With US Adults’ Likelihood of Accepting COVID-19 Vaccination. JAMA Network Open. 2020;3(10):e2025594–e2025594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.COVID Collaborative, Coronavirus Vaccine Hesitancy in Black and Latinx Communities. https://www.covidcollaborative.us/content/vaccine-treatments/coronavirus-vaccine-hesitancy-in-black-and-latinx-communities. Published 2020. Accessed March 21, 2021.
- 22.Couch KA, Fairlie RW, Xu H. Early evidence of the impacts of COVID-19 on minority unemployment. Journal of public economics. 2020;192:104287–104287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gold JAW, Wong KK, Szablewski CM, et al. Characteristics and Clinical Outcomes of Adult Patients Hospitalized with COVID-19 - Georgia, March 2020. MMWR. 2020;69(18):545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and Mortality among Black Patients and White Patients with Covid-19. The NEJM. 2020;382(26):2534–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. International journal of epidemiology. 2017;46(1):348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. Journal of clinical pharmacy and therapeutics. 2002;27(4):299–309. [DOI] [PubMed] [Google Scholar]
- 27.Donald WKA. Heteroskedasticity and Autocorrelation Consistent Covariance Matrix Estimation. Econometrica. 1991;59(3):817–858. [Google Scholar]
- 28.Zeileis A Object-Oriented Computation of Sandwich Estimators. Journal of statistical software. 2006;16(9):1–16. [Google Scholar]
- 29.Dinmohamed AG, Visser O, Verhoeven RHA, et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. The lancet oncology. 2020;21(6):750–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. The lancet oncology. 2020;21(8):1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wen LS, Sadeghi NB. Addressing Racial Health Disparities In The COVID-19 Pandemic: Immediate And Long-Term Policy Solutions. In. Health Affairs Blog 2020. [Google Scholar]