Abstract
Background:
The COVID-19 pandemic has created increased stress and anxiety for many; however, some individuals are particularly prone to heightened anxiety. It is unclear if and how pre-stress neurocognitive factors moderate risk for anxiety during high stress situations. Enhanced error monitoring and a cognitive control strategy of more instantaneous (reactive) control have both been independently related to anxiety. We examine if a specific neurocognitive profile characterized by heightened error monitoring and a more reactive cognitive control strategy in adolescence predicts young adults’ anxiety trajectories across three early months of the COVID-19 pandemic.
Methods:
As part of a longitudinal study (N=291), data were acquired in adolescence (13 years) on error monitoring (n=124) and cognitive control strategy (n=119). In young adulthood (18 years), anxiety was assessed three times during the COVID-19 pandemic (n=162).
Results:
On average, participants experienced greater anxiety in the first COVID-19 assessment, then anxiety decreased in the following months. Error monitoring and cognitive control strategy interacted to predict anxiety trajectories, such that among adolescents with an increased reliance on reactive control, error monitoring predicted greater anxiety in the first assessment, but greater decreases the following months as stay-at-home orders were lifted and families adapted to the restrictions.
Conclusions:
Results suggest that neurocognitive profiles in adolescence predict young adults’ anxiety responses during a highly stressful period such as the initial months of the COVID-19 pandemic. Our findings have implications for the early identification of individuals at greater risk for anxiety.
Keywords: Anxiety, Error Monitoring, Cognitive Control, Neurocognitive, COVID-19 pandemic
The COVID-19 pandemic brought unprecedented changes to individuals’ lives, resulting in increased stress and anxiety for many, especially young adults (1,2). However, most young adults do not experience heightened anxiety (1), highlighting the importance of identifying risk factors that contribute to elevated symptoms of anxiety during COVID-19. Extant studies have examined psychosocial factors that influence youth’s reactivity to stress, but less is known about how pre-stress neurocognitive factors moderate risk for anxiety during stressful periods (e.g., COVID-19 pandemic). Enhanced error monitoring and a cognitive control strategy characterized by more instantaneous (reactive) control (as opposed to planful/proactive control) have both been independently related to anxiety (3-5). In the current study, we examine if a specific neurocognitive profile characterized by heightened error monitoring and a more reactive cognitive control strategy in adolescence predicts young adults’ anxiety trajectories across three early months of the COVID-19 pandemic in the United States.
A wealth of research relates cognitive control to anxiety (6). Cognitive control involves several processes, including detection and deployment (7). Detection processes involve registering the presence of salient information (e.g. detecting that an error has occurred), whereas deployment processes involve changes in attention and behavior in response to this information (e.g., planning ahead to prevent future errors) (7). Previous research relates both detection and control processes to anxiety.
For detection, extensive research links anxiety to exaggerated error monitoring (5,8), often reflected in the error-related negativity (ERN). The ERN is an event-related potential (ERP) component that reaches maximal amplitude over frontocentral recording sites within 100 ms after errors (9,10). Importantly, the ERN is not strictly implicated in cognitive control as it is thought to reflect the affective evaluation of errors (11-13). Converging evidence suggests that the ERN is generated, at least in part, within the anterior cingulate cortex (ACC) – a brain region that integrates threat, pain, and negative feedback (e.g., punishment) to guide future behavior (14,15). The relation between heightened error monitoring, as indexed by a larger (i.e., more negative) ERN, and greater anxiety has been demonstrated both concurrently and longitudinally, such that a larger ERN predicts greater anxiety later in development (16,17). Although longitudinal data remain scarce and the effects are relatively weak, the available evidence suggest that individuals with an elevated ERN are prone to heightened anxiety following high levels of stress (18,19), such as natural disasters (e.g., Hurricane Sandy (19)). Together, these findings suggest that heightened error monitoring may predict increased anxiety during the COVID-19 pandemic.
For deployment, previous models identify two cognitive control strategies with distinct chronometry: proactive and reactive control (20). Proactive control involves the early selection of goal-relevant information to prepare for future events, whereas reactive control deploys on an as-needed basis towards recently-encountered events. Research connects anxiety to reduced proactive and increased reactive control (5,20,21). For instance, training high anxious individuals to use a proactive control strategy lowers aspects of anxiety (3). Nevertheless, reactive tendencies may create risk when accompanied with increased detection (i.e., error monitoring). Indeed, we recently argued that increased detection confers risk for anxiety in a way that is moderated by strategy: relatively higher proactive control reduces, whereas relatively higher reactive control potentiates this risk (7).
This prior work generates a relatively specific hypothesis. We propose that individuals with both heightened detection and heavy reliance on reactive control fixate their attention on the source of the detected information (e.g., an error). We view this cognitive profile as supporting maladaptive behaviors like avoidance and freezing associated with anxiety. In contrast, enhanced detection is less problematic when accompanied with proactive control. Such strategies support adaptive changes in attention deployment and associated behaviors, aimed at avoiding future errors and maintaining original goal-directed behavior (7).
The current study tests this hypothesis by examining whether anxiety trajectories during the COVID-19 pandemic differ depending on neurocognitive profiles in adolescence. Specifically, we used latent growth curve modeling to characterize anxiety trajectories during three consecutive months of the pandemic. We had three hypotheses: 1) anxiety levels would increase overall during the pandemic; 2) adolescents with an enhanced ERN would display a trajectory of high initial anxiety levels and increases in anxiety during the pandemic; and 3) the effect of the ERN on anxiety would be moderated by individuals’ cognitive control strategy, such that anxiety trajectories would differ based on individuals’ neurocognitive profiles. We hypothesized that a neurocognitive profile characterized by enhanced error monitoring and a heavier reliance on reactive control strategies in adolescence would predict higher initial anxiety levels and increases in anxiety during the pandemic.
Methods and Materials
Participants
Participants were involved in an ongoing longitudinal, multi-method study of temperament and socioemotional development conducted in a large metropolitan area in the Mid-Atlantic United States. Two hundred ninety one four-month-old infants (156 female) were selected based on displays of positive and negative affect and motor reactivity to novel stimuli (22). Based on maternal report in infancy, mothers were 69.4% White, 16.5% African American, 7.2% Hispanic, 3.1% Asian, 3.4% other, and 0.3% missing. Mothers in the sample were highly educated: 35.7% graduate school graduates, 41.9% college graduates, 16.2% high school graduates, 5.5% with other forms of education, and 0.7% with missing information.
Of the original sample (N=291), 124 participants successfully completed a flanker task while EEG was collected to assess error monitoring in adolescence (Mage=13.11, SD=0.59 years). At the same assessment, 119 participants successfully completed an AX-CPT to assess cognitive control strategy (proactive vs. reactive). In young adulthood (Mage= 18.26, SD = 0.66 years), participants reported on their anxiety during three consecutive months of the COVID-19 pandemic. 155 participants completed their first assessment (Month 1) of online questionnaires between April 20 and May 15 of 2020, which was approximately one month (M=29.67 days, SD=6.01 days) after the stay-at-home order was implemented in Maryland, the state where most participants resided. Approximately a month later (M=26.48 days, SD=7.31 days), 153 participants completed their second assessment (Month 2) as gradual reopening started in Maryland and approximately a month later (M=28.86 days, SD=5.83 days), 141 participants completed their third assessment after stay at home orders were lifted and non-essential businesses reopened in Maryland. The Institutional Review Board of the University of the University of Maryland approved all study protocols. All participants were compensated for their time.
Examinations of the patterns of missing data revealed that mothers’ race and ethnicity (non-Hispanic White vs. other minority groups) was associated with missing data on the second (p=.027) and third (p=.022) assessments during the pandemic, ERN (p=.004), and cognitive control strategy (p=.013) – such that children with data on these measures were more likely to have non-Hispanic White mothers. As such, maternal ethnicity was included as a covariate in the SEM analyses. Missing data on all other variables were not associated with maternal ethnicity or education, children’s gender, error monitoring, cognitive control strategy or anxiety at any timepoint (p’s>.06).
Measures
Generalized Anxiety (18 years).
Generalized anxiety was measured using the Generalized Disorder 7-Item Scale (23) during the three assessments of the COVID-19 pandemic when participants were, on average, 18 years old. The items consisted of various anxiety symptoms and were summed to create an overall score. Higher scores indicated greater anxiety and scores ≥10 are considered to be in the clinical range. This scale has been shown to have high test-retest reliability and good convergent validity (23). The scale showed excellent internal consistency at all time points (α’s>.92) and good test re-test reliability (rs>.65). Moreover, in this sample, the GAD-7 was significantly correlated to COVID-related worries at each assessment (rrange = .48-.59), suggesting that the GAD-7 is related to pandemic-induced distress (Morales et al., under review).
Error Monitoring.
At the 13-year assessment, adolescents completed a flanker task while continuous EEG data were acquired using a 128-channel HydroCel Geodesic Sensor Net and Electrical Geodesic, Inc software. The task, data, and preprocessing pipeline have been previously described (24) and are described in detail in the supplement. In brief, EEG activity surrounding erroneous behavior during the flanker task was isolated to measure error monitoring. Participants completed the flanker task twice, once under standard flanker conditions and once under a “social” pressure manipulation. Although there was a larger ERN in the social condition compared to the standard flanker (24), these manipulations were counterbalanced across individuals, and there was no evidence that manipulation order affected the amplitude of the standard ERN (t(122)= 0.21, p = .834), nor was there evidence of any significant interaction with manipulation order to predict anxiety trajectories (all p’s > .496). In the current study, we focus on the ERN data from the standard flanker task because extensive work has documented that it is related with anxiety (5,25,26) and focusing on the standard (i.e., non-social) ERN allows for comparison with a broader array of literature. A similar approach has been used by previous studies with this sample (27).
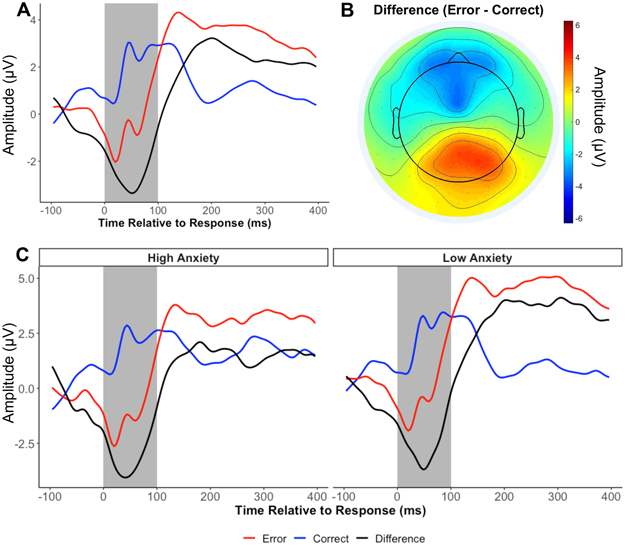
EEG data were preprocessed using MATLAB scripts involving a combination of EEGLAB toolbox (28) and custom-made scripts (24,29) (see supplement). Mean amplitudes of ERN and correct-related negativity (CRN) were calculated from a cluster of frontocentral electrodes surrounding FCz for the first 100 ms following response (Figure 1). The CRN was then subtracted from the ERN to compute the delta-ERN in order to isolate error-specific processes, which was used for all subsequent analyses. More negative values indicate a larger delta-ERN and increased error monitoring. We determined the minimum number of trials to obtain a delta-ERN estimate with average acceptable reliability (.6), using a Spearman-Brown split-half correlation procedure with multiple iterations (30,31). Results suggested that participants needed at least 10 trials for a reliable ERN and at least 15 trials for a reliable delta-ERN. Participants with at least 15 artifact-free trials were included. The delta-ERN showed good reliability (Spearman-Brown r=.84).
Figure 1.
The error-related negativity (delta-ERN) and its relation to anxiety. A) Grand average waveform for each condition and their difference; B) Topographic plot of the mean amplitude of the difference between conditions (Error- Correct) during the shaded time window (0-100 ms); C) Average waveforms for adolescents who reported high and low anxiety in the first assessments during the COVID-19 pandemic. For plotting purposes only, participants with an anxiety score 1 SD above or below the mean were plotted separately.
Cognitive Control Strategy.
At the 13-year assessment, participants completed a standard behavioral AX-CPT to generate a measure of cognitive control strategy (i.e., proactive and reactive control) (20). The task, data, and cleaning of these data have been previously described (32) and are described in detail in the supplement. In short, the AX-CPT is presented as a continuous series of letter pairs composed of 4 trial types (AX, AY, BX, and BY), which are presented at different rates. AX trials were the target trial type and required different response than the other 3 trial types. To obtain a measure of the sensitivity to the differences between target and nontarget trials while controlling for individual differences in response bias, d’ context was computed (see online supplement for details). Higher d’ context scores indicate a more proactive style of cognitive control because the participant was sensitive to cue information and used it to inform future responses.
Analytic Strategy
First, to examine the average trajectory of anxiety across three months, a latent growth curve model was conducted with lavaan (33) in R, Version 3.6 (34). The latent intercept factor, representing anxiety levels at the first COVID-19 assessment (Month 1), was estimated by constraining the paths of each month to 1. The latent slope factor, representing the linear change in anxiety across the three monthly COVID-19 assessments, was estimated by constraining the paths for each month, Month 1, Month 2, and Month 3, to 0, 1, and 2, respectively.
Second, to evaluate if the trajectories of anxiety varied as a function of error monitoring, the delta-ERN was modeled as a predictor of the intercept and slope latent factors. Third, to examine if the trajectories of anxiety varied as a function of different neurocognitive profiles, error monitoring (delta-ERN), cognitive control strategy (d’ context), and their interaction were modeled as predictors of the intercept and slope latent factors. The interaction between error monitoring and cognitive control strategy was created by first standardizing each variable and then computing their interaction term. To probe the interactions, we utilized the Johnson-Neyman procedure to examine the precise regions of the cognitive control strategy continuum in which the effect of the delta-ERN significantly predicted anxiety trajectories (i.e., intercept or slope factors) (35).
Based on the preliminary analyses described below with covariates and missing patterns and in line with previous studies with this sample (36), we controlled for maternal education, maternal ethnicity, gender, and participants’ average age during the COVID-19 assessments on the anxiety intercept and slope factors. In addition, we controlled for the date of the first assessment (Month 1; in days) since the stay-at-home order on the intercept and the date of the last assessment (Month 3; in days) since the stay-at-home order on the slope. Finally, to control for initial levels of anxiety, we controlled for anxiety levels at age 13, when error monitoring and cognitive control strategy were measured, using the Total Anxiety Score of the Screen for Child Anxiety Related Disorders (37). Missing data were handled using full information maximum likelihood estimation (FIML) to reduce potential bias in the parameter estimates (38). Due to missing data and potential departures from multivariate normality, the model was estimated using a robust maximum likelihood estimator (MLR) (39) and a scaled test chi-squared statistic.
Results
Descriptive analyses
Descriptive statistics and the correlations among all study variables are presented in Table 1. Compared to males, females displayed a smaller delta-ERN (13 years), and higher levels of anxiety at Month 1 and Month 3 during the pandemic (18 years). Maternal education and maternal ethnicity were related to the delta-ERN, such that adolescents of non-Hispanic White mothers and of more educated mothers had a smaller delta-ERN. As such, gender, maternal education, and maternal ethnicity were included as covariates in the growth curve model examining predictors of the anxiety trajectories. Finally, the delta-ERN predicted anxiety levels at Month 1 during the pandemic, such that a larger delta-ERN at age 13 was longitudinally related to greater anxiety at the first assessment during COVID-19 (Figure 1). Cognitive control strategy was not significantly related to any study variables.
Table 1.
Means, standard deviations, and correlations
| Variable | N | M | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Gender | 291 | * | |||||||||
| 2. Maternal Ethnicity | 290 | ** | −.05 | ||||||||
| 3. Maternal Education | 273 | 1.21 | 0.72 | .01 | .19 | ||||||
| 4. Anxiety (13 years) | 178 | 18.13 | 11.66 | −.33 | −.07 | −.18 | |||||
| 5. delta-ERN (13 years) | 124 | −2.53 | 2.93 | −.24 | .23 | .24 | .14 | ||||
| 6. AX-CPT d' (13 years) | 119 | 2.01 | 1.10 | −.09 | .15 | −.09 | −.06 | −.03 | |||
| 7. Anxiety T1 (18 years) | 155 | 5.69 | 5.62 | −.16 | .05 | .16 | .14 | −.22 | .07 | ||
| 8. Anxiety T2 (18 years) | 153 | 5.07 | 5.06 | −.10 | .14 | .08 | .11 | −.15 | .21 | .81 | |
| 9. Anxiety T3 (18 years) | 141 | 4.50 | 4.82 | −.17 | .13 | .07 | .27 | .00 | .06 | .65 | .71 |
Note. M and SD are used to represent mean and standard deviation, respectively. Bold indicates p < .05.
0 = Females and 1 = Males.
Non-Hispanic White = 1 and Other = 0. Maternal education was coded as High school graduate = 0, College Graduate = 1, Graduate school graduate = 2, and Other = missing. Anxiety T1, Anxiety T2, and Anxiety T3 represent the first, second, and third anxiety assessments during the COVID-19 pandemic, respectively.
Growth curve analyses
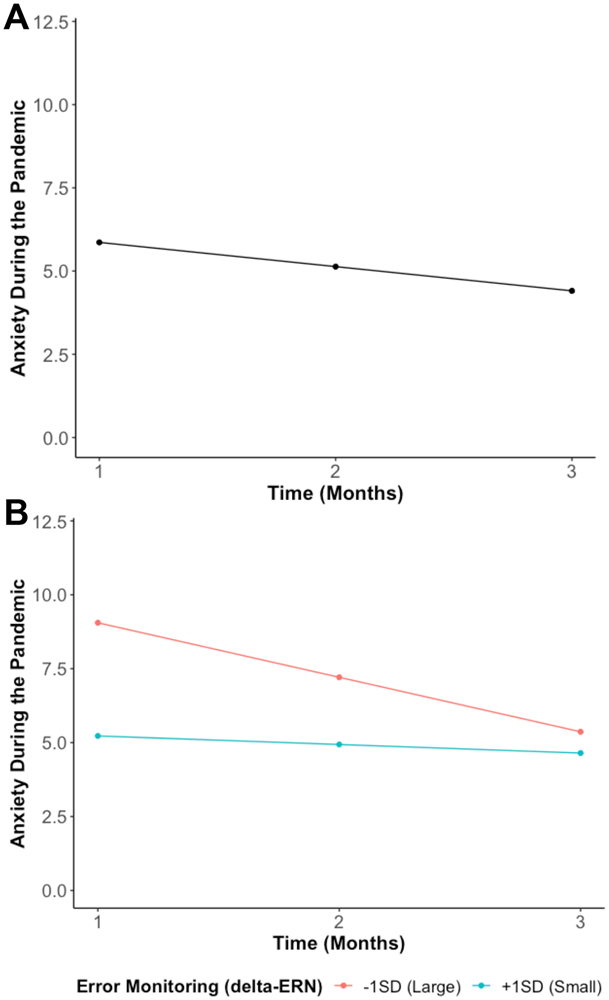
The growth curve model examining the trajectory of anxiety during the pandemic suggested that anxiety decreased across time (b=−0.73, p=.001) as the stay-at-home orders were lifted and reopening gradually occurred and/or families adapted to the pandemic-related restrictions (Figure 2A). As a reference to clinical levels, at Month 1, 20.0% of the participants reported anxiety symptoms considered to be in the clinical range. These frequencies declined to 18.3% at Month 2 and 17.0% at Month 3.
Figure 2.
A) Average trajectory of anxiety (Generalized Anxiety Disorder 7-Item Scale) from T1 (during stay-at-home orders) to T3 (re-opening). This model without predictors showed a good fit (χ2(1) = 0.01, p = .94, RMSEA = 0.00, SRMR = 0.00, CFI = 1.00). B) Shows the predicted anxiety trajectories at different levels of error monitoring (delta-ERN). More error monitoring (a larger delta-ERN) is indicated by a more negative value. This model with predictors showed a good fit (χ2(12) = 9.41, p = .67, RMSEA = 0.00, SRMR = 0.02, CFI = 1.00).
Error monitoring predicting anxiety trajectories
To examine our second hypothesis, we tested if error monitoring (delta-ERN) predicted the anxiety trajectories. As shown in Figure 2B and in line with our hypothesis, we found that adolescents with an enhanced delta-ERN displayed a larger intercept (greater anxiety in Month 1; b= −1.91, p = .008), but a more negative slope (i.e., greater decreases in anxiety across time; b= 0.78, p = .005).
Neurocognitive profiles predicting anxiety trajectories
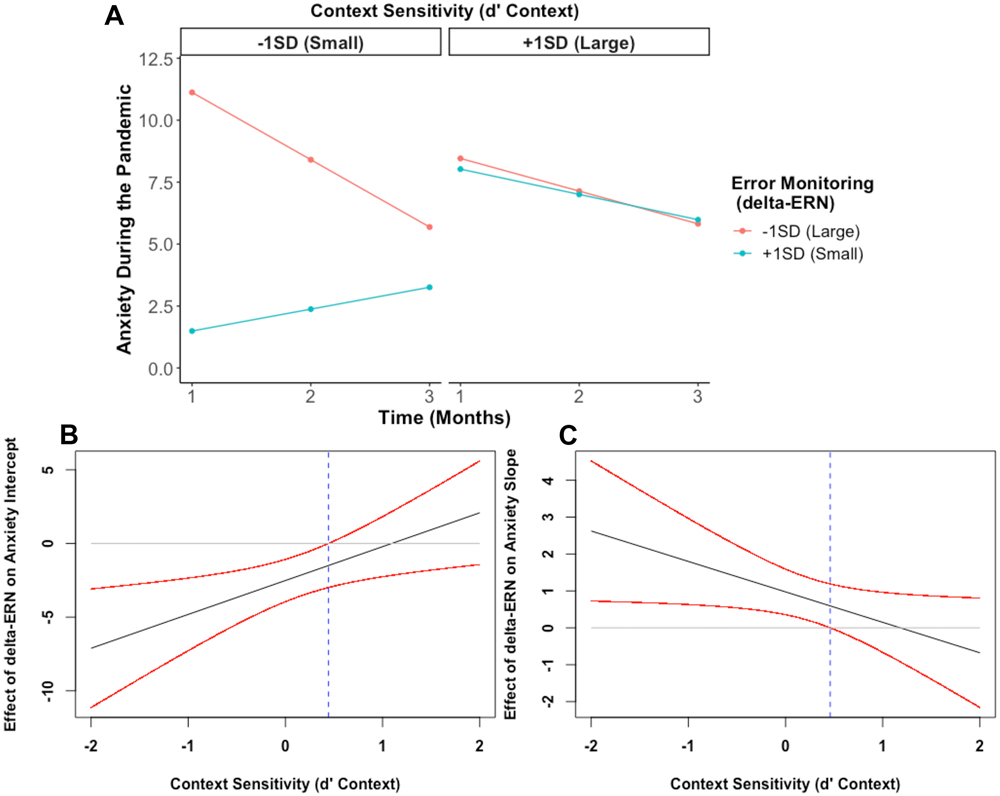
Our final model tested whether error monitoring, cognitive control strategy, and their interaction predicted anxiety trajectories. As shown in Table 2, the interaction between error monitoring (delta-ERN) and cognitive control strategy (d’ context) predicted the intercept, b= 2.30, p = .005, and the slope, b= −0.83, p = .026, of the anxiety trajectory. As shown in Figures 3A and 3B, probing this interaction yielded a significant negative association between delta-ERN and anxiety at Month 1 among individuals who showed a tendency to deploy relatively more reactive strategies (i.e., smaller d’ context scores, < 0.44 SD from the mean). This relation was not significant among those used a more proactive control strategy (i.e., larger d’ context scores). In contrast, Figures 3A and 3C, probing the interaction predicting the slope revealed a positive association between the delta-ERN and the slope of anxiety, but only among individuals who showed relatively more reactive control (i.e., < 0.46 SD from the mean; smaller d’ context scores), but not among those with relatively more proactive control (i.e., larger d’ context scores).
Table 2.
Latent growth curve analysis results for final model including neurocognitive predictors.
| Predictors/Outcome | β | b | p | CI Lower | CI Upper |
|---|---|---|---|---|---|
| Anxiety Intercept | |||||
| Maternal Education | 0.19 | 1.42 | 0.023 | 0.194 | 2.639 |
| Maternal Ethnicity | 0.09 | 1.08 | 0.298 | −0.948 | 3.100 |
| Gender | −0.15 | −1.63 | 0.082 | −3.461 | 0.207 |
| Average Age | 0.00 | −0.02 | 0.971 | −1.325 | 1.276 |
| Date of First Assessment | −0.05 | −0.04 | 0.503 | −0.164 | 0.080 |
| Anxiety (13 yrs) | 0.22 | 0.10 | 0.042 | 0.004 | 0.195 |
| Flanker Task Accuracy | −0.17 | −0.94 | 0.082 | −1.988 | 0.118 |
| delta-ERN | −0.47 | −2.52 | 0.000 | −3.828 | −1.202 |
| AX-CPT d' | 0.18 | 0.97 | 0.143 | −0.326 | 2.260 |
| delta-ERN x AX-CPT d' | 0.43 | 2.30 | 0.005 | 0.702 | 3.897 |
| Anxiety Slope | |||||
| Maternal Education | −0.14 | −0.30 | 0.204 | −0.774 | 0.165 |
| Maternal Ethnicity | 0.09 | 0.30 | 0.514 | −0.595 | 1.189 |
| Gender | 0.15 | 0.47 | 0.255 | −0.338 | 1.274 |
| Average Age | −0.01 | −0.01 | 0.966 | −0.590 | 0.564 |
| Date of Third Assessment | −0.08 | −0.02 | 0.383 | −0.075 | 0.029 |
| Anxiety (13 yrs) | 0.07 | 0.01 | 0.617 | −0.027 | 0.046 |
| Flanker Task Accuracy | 0.06 | 0.09 | 0.797 | −0.602 | 0.784 |
| delta-ERN | 0.61 | 0.97 | 0.001 | 0.410 | 1.538 |
| AX-CPT d' | −0.08 | −0.13 | 0.657 | −0.685 | 0.432 |
| delta-ERN x AX-CPT d' | −0.52 | −0.83 | 0.026 | −1.550 | −0.100 |
Note: delta-ERN = Error-related Negativity; Gender is coded as 0 = Females and 1 = Males; Maternal Ethnicity is coded as Non-Hispanic White = 1 and Other = 0. Date of First Assessment and Date of Third Assessment were measured in days since the stay-at-home orders. This model fit the data well, χ2(14) = 16.56, p = .28, RMSEA = .03, SRMR = .02, CFI = .99). Bold indicates p < .05.
Figure 3.
The impact of error monitoring and cognitive control strategy on anxiety trajectories during the COVID-19 pandemic. More error monitoring (a larger delta-ERN) is indicated by a more negative value and more proactive control is indicated by higher d’ context values. A) Shows the predicted anxiety trajectories at different levels of error monitoring (delta-ERN) and cognitive control strategy; B) Johnson-Neyman plot showing the negative effect of error monitoring on anxiety intercept is greater as children exhibit more a reactive (less proactive) cognitive control strategy; and C) Johnson-Neyman plot showing the effect of error monitoring on anxiety slope increases as children exhibit more reactive (less proactive) cognitive control.
In sum and as shown in Figure 3A, adolescents with both enhanced error monitoring (more negative delta-ERN) and an increased reliance on reactive control strategies (as opposed to planful/proactive control) displayed a larger intercept (i.e., greater anxiety at Month 1), but a more negative slope (i.e., greater decreases in anxiety across time) – compared to individuals with diminished error monitoring and more reactive control strategy use.
Sensitivity analyses demonstrated that modeling the times of assessment as continuous time based on participants’ assessment dates via multilevel modeling (MLM) yielded the same conclusions as the SEM approach that treated time of assessment as ordinal. Importantly, the MLM approach used maximum likelihood with listwise deletion on the covariates and predictors rather than FIML, suggesting that different ways of handing missing data also did not significantly impact the results. Moreover, sensitivity analyses suggest that the results were driven by the ERN rather than the CRN (see online supplement). Finally, exploratory analyses indicate that the effects of the delta-ERN or the interaction between error monitoring and cognitive control strategy were not moderated by gender (not shown).
Discussion
The COVID-19 pandemic has brought increased anxiety for many individuals, especially young adults (1,2). At the same time, not all young adults have experienced heightened anxiety during the pandemic, begging the question of which young adults are most at risk. The current study utilizes neurocognitive factors previously linked to risk for elevated anxiety, specifically error monitoring and a more reactive cognitive control strategy, as predictors of anxiety during COVID-19. Our results suggest that error monitoring and a more reactive cognitive control strategy interact with each other to predict anxiety trajectories during the COVID-19 pandemic. Our results have implications for the identification of individuals at the highest risk for anxiety and can inform prevention and intervention efforts by providing potential malleable neurocognitive processes that may serve as resilience factors during highly stressful situations, such as the COVID-19 pandemic.
Results revealed that, on average, anxiety decreased between the first and third months of the pandemic (Figure 1). This result was unexpected, but it is in line with emerging evidence indicating that some individuals, such as those who are highly educated and higher SES, are not as heavily impacted by the pandemic, especially when compared to individuals from low SES backgrounds (2,40-42). For example, a weekly survey of a US-representative sample showed, on average, a similar decrease in anxiety for caregivers and youths; however, low-income households did not show decreases in anxiety, experiencing more anxiety than the average-income household (40,41). It is important to note that participants of the current study were largely from moderate-to-high SES households and none of them were impacted directly by the pandemic during the time sampled (e.g., family members getting seriously sick or hospitalized). In a similar sample of youth in New York, US, anxiety increased through April, peaking around late April/early May, and then decreased rapidly through July (43). Likewise, for our sample of young adults, the first assessment, when stay-at-home orders were implemented and the uncertainty about the virus and its implications for individuals’ lives were highest, reflected the highest anxiety levels. As the restrictions were lifted and families adapted to the restrictions in the subsequent months, anxiety, on average, decreased.
In addition to the average response, we also observed important individual differences that varied as a function of previously measured neurocognitive factors. First, we observed that a larger delta-ERN in adolescence predicted a trajectory of increased intercept, but a steeper slope, compared to individuals with a small delta-ERN in adolescence. However, as expected and in line with our theoretical model (7), error monitoring and cognitive control strategy interacted to predict anxiety trajectories – such that the predictive effects of the delta-ERN were pronounced among individuals with increased reactive control strategies. In other words, individuals with a profile characterized by enhanced error monitoring and an increased reliance on instantaneous (reactive) control (as opposed to planful/proactive control) displayed greater anxiety in the first assessment (i.e., larger intercept). However, this profile also predicted greater anxiety decreases in subsequent months (i.e., a more negative slope). These results suggest that a neurocognitive profile of increased error monitoring and a more reactive control strategy predicted increased anxiety approximately five years later during the stay-at-home orders, the most anxiety-producing times of the pandemic. Importantly, this difference was not observed during the last assessment when stay-at-home orders were no longer in place. This suggests that the risk associated with some neurocognitive factors may only be evident during acute periods of elevated stress or at higher levels of anxiety (e.g., clinical levels). In our sample, this was during the initial assessment during the pandemic; however, as our relatively advantaged sample adapted to the pandemic and reopening occurred, the effects of this neurocognitive profile or the delta-ERN were no longer observable.
Additionally, the effects of enhanced error monitoring on anxiety were not evident in individuals relying on a more proactive, rather than reactive, control strategy. This opens the possibility of intervention or prevention efforts that could target cognitive control strategy to promote planful and proactive strategies while reducing automatic and reactive control strategies (3). Our findings also highlight the importance of distinguishing detection from control processes, rather than considering cognitive control a unitary construct (7,21). This distinction has important clinical consequences as intervention or prevention strategies will likely differ whether detection or control is targeted. For example, in contrast to control processes, detection processes (e.g., error-monitoring) are not impacted by explicit, cognitive and behavioral strategies like cognitive behavioral therapy (44-46), but are modified by implicit interventions such as attention-bias modification training (47-49) and a computerized intervention that was designed to directly reduce sensitivity to errors (50). Future studies should continue to develop and evaluate personalized intervention strategies for modifying specific components of cognitive control that may place individuals at higher risk for anxiety during adverse situations.
This study’s limitations should be considered when interpreting the results. Although our study is one of the first studies with multiple repeated assessments of anxiety symptoms during the COVID-19 pandemic, not having an assessment right before the COVID-19 outbreak did not allow us to capture the purported increases in anxiety. However, by leveraging our repeated assessments, we were able to detect anxiety levels potentially returning to pre-pandemic levels. As a longitudinal study, our study had missing data. To mitigate the impacts of missing data, we utilized statistical approaches to use all available data and reduce the biases associated with missing data. Moreover, sensitivity analyses suggest that different ways of handing missing data do not significantly impact the results (see online supplement). Because of the urgency of data collection in response to the pandemic and the longitudinal design, our sample size was not determined by an a priori power analysis – we collected data on as many participants as possible. When interpreting the results of the current study, it is also important to consider the nature of our sample – a community sample composed of relatively educated White families that were oversampled for temperamental patterns in infancy. Thus, caution is warranted when interpreting and generalizing our results and in particular the prevalence of anxiety during the COVID-19 pandemic. Moreover, because our results captured variability in anxiety as a continuum and the majority of participants experienced an adaptive response (anxiety increases followed by decreases), our findings need to be replicated in clinical and more diverse samples to better understand their implications for identifying individuals who may be at increased risk for experiencing elevated anxiety during high-stress situations as well as to inform the design of preventive or therapeutic interventions.
Overall, our findings provide evidence that neurocognitive profiles in adolescence predict young adults’ anxiety responses during a highly stressful situation such as the initial phase of the COVID-19 pandemic. Specifically, among adolescents with an increased reliance on more instantaneous (reactive) control strategies (as opposed to planful/proactive control), error monitoring predicted increased anxiety during the initial phase of the pandemic (stay-at-home orders), but greater decreases in the following months. These findings highlight the importance of considering multiple components of cognitive control. A better characterization of these neurocognitive processes has implications for the early identification of individuals at greater risk for anxiety and can ultimately inform prevention and intervention efforts as these neurocognitive processes may serve as risk and resilience factors for anxiety.
Supplementary Material
Acknowledgements
We thank the many research assistants involved in collecting and coding the data presented in this manuscript. We also thank the participating families without whom the study would not have been possible. This research was supported by grants from the National Institute of Health MH093349 and HD017899 to NAF.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Czeisler MÉ, Lane RI, Petrosky E, Wiley JF, Christensen A, Njai R, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic—United States, June 24–30, 2020. Morbidity and Mortality Weekly Report. 2020;69(32):1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGinty EE, Presskreischer R, Anderson KE, Han H, Barry CL. Psychological Distress and COVID-19–Related Stressors Reported in a Longitudinal Cohort of US Adults in April and July 2020. JAMA. 2020. Dec 22;324(24):2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birk JL, Rogers AH, Shahane AD, Urry HL. The heart of control: Proactive cognitive control training limits anxious cardiac arousal under stress. Motiv Emot. 2018. Feb 1;42(1):64–78. [Google Scholar]
- 4.Buzzell GA, Morales S, Bowers ME, Troller-Renfree SV, Chronis-Tuscano A, Pine DS, et al. Inhibitory control and set shifting describe different pathways from behavioral inhibition to socially anxious behavior. Developmental Science. 2021;24(1):e13040. [DOI] [PubMed] [Google Scholar]
- 5.Moser JS, Moran TP, Schroder HS, Donnellan MB, Yeung N. On the relationship between anxiety and error monitoring: a meta-analysis and conceptual framework. Frontiers in Human Neuroscience [Internet]. 2013. [cited 2015 Nov 2];7. Available from: http://journal.frontiersin.org/article/10.3389/fnhum.2013.00466/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7(2):336–53. [DOI] [PubMed] [Google Scholar]
- 7.Fox NA, Buzzell GA, Morales S, Valadez EA, Wilson M, Henderson HA. Understanding the Emergence of Social Anxiety in Children with Behavioral Inhibition. Biological Psychiatry [Internet]. 2020. Oct 10 [cited 2020 Oct 31]; Available from: http://www.sciencedirect.com/science/article/pii/S0006322320319892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: Toward an endophenotype. Clinical psychology review. 2008;28(8):1343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991. Jun;78(6):447–55. [DOI] [PubMed] [Google Scholar]
- 10.Gehring WJ, Goss B, Coles MG, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological science. 1993;4(6):385–90. [Google Scholar]
- 11.Dignath D, Eder AB, Steinhauser M, Kiesel A. Conflict monitoring and the affective-signaling hypothesis—An integrative review. Psychonomic Bulletin & Review. 2020;27(2):193–216. [DOI] [PubMed] [Google Scholar]
- 12.Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295(5563):2279–82. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg A, Meyer A, Hale-Rude E, Perlman G, Kotov R, Klein DN, et al. Error-related negativity (ERN) and sustained threat: Conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology. 2016;53(3):372–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buzzell GA, Richards JE, White LK, Barker TV, Pine DS, Fox NA. Development of the error-monitoring system from ages 9–35: Unique insight provided by MRI-constrained source localization of EEG. NeuroImage. 2017. Aug;157:13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011. Mar;12(3):154–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer A, Hajcak G, Torpey-Newman DC, Kujawa A, Klein DN. Enhanced error-related brain activity in children predicts the onset of anxiety disorders between the ages of 6 and 9. Journal of Abnormal Psychology. 2015;124(2):266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer A, Nelson B, Perlman G, Klein DN, Kotov R. A neural biomarker, the error-related negativity, predicts the first onset of generalized anxiety disorder in a large sample of adolescent females. Journal of Child Psychology and Psychiatry. 2018;59(11):1162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banica I, Sandre A, Shields GS, Slavich GM, Weinberg A. The error-related negativity (ERN) moderates the association between interpersonal stress and anxiety symptoms six months later. Int J Psychophysiol. 2020. Jul;153:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer A, Danielson CK, Danzig AP, Bhatia V, Black SR, Bromet E, et al. Neural biomarker and early temperament predict increased internalizing symptoms after a natural disaster. Journal of the American Academy of Child & Adolescent Psychiatry. 2017;56(5):410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends in Cognitive Sciences. 2012. Feb;16(2):106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buzzell GA, Troller-Renfree SV, Morales S, Fox NA. Relations between Behavioral Inhibition, Cognitive Control, and Anxiety: Novel Insights Provided by Parsing Subdomains of Cognitive Control. In: Pérez-Edgar K, Fox NA, editors. Behavioral Inhibition: Integrating Theory, Research, and Clinical Perspectives [Internet]. Cham: Springer International Publishing; 2018. [cited 2018 Nov 21]. p. 213–35. Available from: 10.1007/978-3-319-98077-5_10 [DOI] [Google Scholar]
- 22.Hane AA, Fox N a, Henderson H a, Marshall PJ. Behavioral reactivity and approach-withdrawal bias in infancy. Developmental psychology. 2008. Sep;44(5):1491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine. 2006;166(10):1092–7. [DOI] [PubMed] [Google Scholar]
- 24.Buzzell GA, Troller-Renfree SV, Barker TV, Bowman LC, Chronis-Tuscano A, Henderson HA, et al. A Neurobehavioral Mechanism Linking Behaviorally Inhibited Temperament and Later Adolescent Social Anxiety. Journal of the American Academy of Child & Adolescent Psychiatry. 2017. Dec;56(12):1097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hajcak G, McDonald N, Simons RF. Anxiety and error-related brain activity. Biological Psychology. 2003. Oct;64(1–2):77–90. [DOI] [PubMed] [Google Scholar]
- 26.Meyer A A biomarker of anxiety in children and adolescents: A review focusing on the error-related negativity (ERN) and anxiety across development. Developmental Cognitive Neuroscience. 2017. Oct 1;27:58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filippi CA, Subar AR, Sachs JF, Kircanski K, Buzzell G, Pagliaccio D, et al. Developmental pathways to social anxiety and irritability: The role of the ERN. Development and Psychopathology. 2020. Aug;32(3):897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of neuroscience methods. 2004;134(1):9–21. [DOI] [PubMed] [Google Scholar]
- 29.Debnath R, Buzzell GA, Morales S, Bowers ME, Leach SC, Fox NA. The Maryland analysis of developmental EEG (MADE) pipeline. Psychophysiology. 2020;57(6):e13580. [DOI] [PubMed] [Google Scholar]
- 30.Leach SC, Morales S, Bowers ME, Buzzell GA, Debnath R, Beall D, et al. Adjusting ADJUST: Optimizing the ADJUST algorithm for pediatric data using geodesic nets. Psychophysiology. 2020;n/a(n/a):e13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morales S, Bowers M, Leach S, Buzzell G, Fifer W, Elliott A, et al. Time-frequency dynamics of error monitoring in childhood: an EEG study [Internet]. PsyArXiv; 2021. [cited 2021 Mar 12]. Available from: https://psyarxiv.com/ag9s7/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Troller-Renfree SV, Buzzell GA, Pine DS, Henderson HA, Fox NA. Consequences of Not Planning Ahead: Reduced Proactive Control Moderates Longitudinal Relations Between Behavioral Inhibition and Anxiety. Journal of the American Academy of Child & Adolescent Psychiatry. 2019. Aug 1;58(8):768–775.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosseel Y Lavaan: An R package for structural equation modeling and more. Version 0.5–12 (BETA). Journal of statistical software. 2012;48(2):1–36. [Google Scholar]
- 34.R Development Core Team. R: A language and environment for statistical computing [Internet]. R Foundation for Statistical Computing, Vienna, Austria; 2008. Available from: http://www.R-project.org. [Google Scholar]
- 35.Preacher KJ, Curran PJ, Bauer DJ. Computational Tools for Probing Interactions in Multiple Linear Regression, Multilevel Modeling, and Latent Curve Analysis. Journal of Educational and Behavioral Statistics. 2006. Jan 1;31(4):437–48. [Google Scholar]
- 36.Morales S, Miller NV, Troller-Renfree SV, White LK, Degnan KA, Henderson HA, et al. Attention bias to reward predicts behavioral problems and moderates early risk to externalizing and attention problems. Development and Psychopathology. 2019;1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, et al. The screen for child anxiety related emotional disorders (SCARED): Scale construction and psychometric characteristics. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(4):545–53. [DOI] [PubMed] [Google Scholar]
- 38.Enders CK, Bandalos D. The Relative Performance of Full Information Maximum Likelihood Estimation for Missing Data in Structural Equation Models. Structural Equation Modeling: A Multidisciplinary Journal. 2001. Jul 1;8(3):430–57. [Google Scholar]
- 39.Yuan KH, Bentler PM. Three Likelihood-Based Methods for Mean and Covariance Structure Analysis with Nonnormal Missing Data. Sociological Methodology. 2000;30:165–200. [Google Scholar]
- 40.Center for Translational Neuroscience. Flattening the Other Curve [Internet]. Medium. 2020. [cited 2020 Nov 3]. Available from: https://medium.com/rapid-ec-project/flattening-the-other-curve-7be1e574b340 [Google Scholar]
- 41.Center for Translational Neuroscience. Flattening the Other Curve, Part 2 [Internet]. Medium. 2020. [cited 2020 Nov 3]. Available from: https://medium.com/rapid-ec-project/flattening-the-other-curve-part-2-5661a2d36a82 [Google Scholar]
- 42.Fancourt D, Steptoe A, Bu F. Trajectories of anxiety and depressive symptoms during enforced isolation due to COVID-19 in England: a longitudinal observational study. The Lancet Psychiatry. 2021. Feb 1;8(2):141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hawes MT, Szenczy AK, Olino TM, Nelson BD, Klein DN. Trajectories of depression, anxiety and pandemic experiences; A longitudinal study of youth in New York during the Spring-Summer of 2020. Psychiatry Research. 2021. Apr;298:113778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorka SM, Burkhouse KL, Klumpp H, Kennedy AE, Afshar K, Francis J, et al. Error-related Brain Activity as a Treatment Moderator and Index of Symptom Change during Cognitive-Behavioral Therapy or Selective Serotonin Reuptake Inhibitors. Neuropsychopharmacology. 2018. May;43(6):1355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hajcak G, Franklin ME, Foa EB, Simons RF. Increased Error-Related Brain Activity in Pediatric Obsessive-Compulsive Disorder Before and After Treatment. The American Journal of Psychiatry; Washington. 2008. Jan;165(1):116–23. [DOI] [PubMed] [Google Scholar]
- 46.Riesel A, Endrass T, Auerbach LA, Kathmann N. Overactive Performance Monitoring as an Endophenotype for Obsessive-Compulsive Disorder: Evidence From a Treatment Study. AJP. 2015. Mar 17;172(7):665–73. [DOI] [PubMed] [Google Scholar]
- 47.Nelson BD, Jackson F, Amir N, Hajcak G. Single-session attention bias modification and error-related brain activity. Cognitive, Affective, & Behavioral Neuroscience [Internet]. 2015. Jun 11 [cited 2015 Nov 2]; Available from: http://link.springer.com/10.3758/s13415-015-0365-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nelson BD, Jackson F, Amir N, Hajcak G. Attention bias modification reduces neural correlates of response monitoring. Biological Psychology. 2017. Oct 1;129:103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klawohn J, Hajcak G, Amir N, Kathmann N, Riesel A. Application of attentional bias modification training to modulate hyperactive error-monitoring in OCD. International Journal of Psychophysiology. 2020;156:79–86. [DOI] [PubMed] [Google Scholar]
- 50.Meyer A, Gibby B, Wissemann K, Klawohn J, Hajcak G, Schmidt NB. A brief, computerized intervention targeting error sensitivity reduces the error-related negativity. Cognitive, Affective, & Behavioral Neuroscience. 2020;20(1):172–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.