Abstract
Background -
Carbohydrate responsive element binding protein (ChREBP) is a transcription factor that responds to sugar consumption. Sugar-sweetened beverage (SSB) consumption and genetic variants in the CHREBP locus have separately been linked to high-density lipoprotein cholesterol (HDL-C) and triglyceride (TG) concentrations. We hypothesized that SSB consumption would modify the association between genetic variants in the CHREBP locus and dyslipidemia.
Methods -
Data from 11 cohorts from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium (N=63,599) and the UK Biobank (UKB) (N=59,220) were used to quantify associations of SSB consumption, genetic variants, and their interaction on HDL-C and TG concentrations using linear regression models. A total of 1,606 single-nucleotide polymorphisms (SNPs) within or near CHREBP were considered. SSB consumption was estimated from validated questionnaires and participants were grouped by their estimated intake.
Results -
In a meta-analysis, rs71556729 was significantly associated with higher HDL-C concentrations only among the highest SSB consumers (β, 2.12 [95% CI, 1.16–3.07] mg/dL per allele; P<0.0001), but not significantly among the lowest SSB consumers (p=0.81; pDiff<0.0001). Similar results were observed for two additional variants (rs35709627 and rs71556736). For TG, rs55673514 was positively associated with TG concentrations only among the highest SSB consumers (β, 0.06 [95% CI, 0.02–0.09] ln-mg/dL per allele, P=0.001), but not the lowest SSB consumers (p=0.84; pDiff=0.0005).
Conclusions -
Our results identified genetic variants in the CHREBP locus that may protect against SSB-associated reductions in HDL-C and other variants that may exacerbate SSB-associated increases in TG concentrations.
Clinical Trial Registration -
Some participating cohorts were registered at URL: https://www.clinicaltrials.gov/ with unique identifiers: NCT00005131 (Atherosclerosis Risk in Communities), NCT00005133 (Cardiovascular Health Study), NCT00005121 (Framingham Offspring Study), NCT00005487 (Multi-Ethnic Study of Atherosclerosis), and NCT00000479 (Women’s Health Study: parent study of the Women’s Genome Health Study).
Keywords: sugars, genetics, carbohydrates, dyslipidemia, triglyceride, epidemiology, nutrition
Introduction
Low circulating high-density lipoprotein cholesterol (HDL-C) and elevated fasting triglyceride (TG) concentrations are positively associated with risk of type 2 diabetes (T2D) and cardiovascular disease (CVD).1–5 Both genetic and environmental factors, including diet, are important determinants of HDL-C and TG concentrations.5–7 Genetic determinants of HDL-C and TG concentrations have been identified in genome-wide association studies (GWAS),8–12 but the extent to which genetic variants interact with environmental exposures is unknown. It is plausible that unrecognized genetic variants or genetic effects may be suppressed or exacerbated by environmental factors, such as diet.
Carbohydrate Responsive Element Binding Protein (ChREBP) is a transcription factor that regulates glucose and lipid metabolism in response to sugar consumption, including sugar from sugar sweetened beverages (SSB).13,14 GWAS have consistently observed an association between single nucleotide polymorphisms (SNPs) in the CHREBP locus (also known as MLXIPL), and HDL-C and TG concentrations.8,9,15,16 In animal studies, hepatic ChREBP is robustly activated by dietary fructose, a major constituent of SSB, and potentiates hepatic lipogenesis and TG secretion.14,17–20 These findings are consistent with large population-based studies in which high SSB consumption has been associated with elevated fasting plasma TG and reduced HDL-C concentrations,21–24 and increased T2D25–27 and CVD21 risk. Thus, SNPs within the CHREBP locus present promising candidates for gene-SSB interactions on circulating HDL-C and TG concentrations.
These pieces of biological, epidemiological and genetic evidence suggest that SSB consumption may modify how genetic variants within the CHREBP locus influence plasma lipid concentrations in some individuals. Although reduction of SSB consumption is increasingly being encouraged globally,28 public health efforts to reduce SSB consumption have achieved limited success and SSB consumption remains a modifiable dietary exposure that contributes substantially to the burden of T2D and CVD worldwide.29,30 A better understanding of the mechanisms underlying the SSB-ChREBP-lipid relationship may reveal novel mechanisms that contribute to the pathogenesis of T2D and CVD risk. Understanding these mechanisms may provide alternative strategies and approaches to reduce metabolic disease that may complement or facilitate dietary interventions.
The present study aimed to examine whether SSB consumption may modify the association of genetic variants within the CHREBP locus on HDL-C and TG concentrations in aggregated data from cohorts who are part of the Cohorts for Heart and Aging Research in Genetic Epidemiology (CHARGE) consortium.31 Descriptions of the CHARGE cohorts are included in the supplemental material, Table I. We further used data from the UK Biobank (UKB) to assess the reproducibility of these findings in an independent cohort.32
Methods
Methods are available in the Supplemental Material. The data that support the findings of this study are available from the corresponding author upon reasonable request. All study participants provided written informed consent, and approval for all study protocols was granted by local institutional review boards and/or oversight committees.
Results
General characteristics and mean dietary intakes for the eleven CHARGE cohorts are shown in Table 1. Replication of previous findings on associations of SSB consumption and SNPs with lipid traits in the CHARGE cohorts are presented in the Supplemental Results in the Supplemental Material.
Table 1.
General characteristics of participating CHARGE consortium cohorts*
|
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Raine Study | ARIC | FHS | NEO | Fenland | YFS | WGHS | WHI | MESA | CHS | RS | |
| Characteristics | |||||||||||
| Country | Australia | USA | USA | Netherlands | UK | Finland | USA | USA | USA | USA | Netherlands |
| n | 617 | 10,924 | 6,382 | 5,694 | 10,022 | 1,782 | 16,284 | 1,109 | 1,805 | 3,196 | 5,784 |
| Age (years) | 20 (1) | 55 (6) | 49 (14) | 56 (6) | 49 (7) | 38 (5) | 55 (7) | 65 (7) | 70 (10) | 72 (5) | 66 (8) |
| Sex (% women) | 52.4 | 52.7 | 54.3 | 52.0 | 53.3 | 55.9 | 100 | 100 | 51.2 | 61.0 | 57.8 |
| Body Mass Index (kg/m2) | 24.5 (5.2) | 27.0 (4.8) | 27.4 (5.5) | 30.0 (4.8) | 26.9 (4.8) | 25.9 (4.6) | 25.9 (4.9) | 28.6 (5.7) | 28.0 (5.3) | 26.3 (4.4) | 26.5 (3.7) |
| Current Smoker (%) | 13.5 | 24.2 | 13.4 | 16.0 | 12.0 | 27.6 | 11.7 | 10.1 | 7.0 | 11.4 | 23.4 |
| Completed High School (%) | 81.5 | 84.9 | 98.0 | 93.0 | 81.8 | 75.4 | 100 | 94.7 | 96.5 | 75.1 | 60.8 |
| Fasting HDL-C (mg/dl) | 51 (13) | 51 (17) | 54 (17) | 55 (16) | 59 (16) | 52 (13) | 54 (15) | 58 (15) | 57 (18) | 55 (16) | 53 (14) |
| Fasting TG (mg/dl) | 85 (2) | 137 (90) | 117 (87) | 130 (85) | 106 (81) | 122 (82) | 119 (89) | 156 (92) | 107 (59) | 140 (76) | 137 (71.0) |
| Dietary Intakes | |||||||||||
| SSB intake (servings/d) | 0.7 (1.0) | 0.5 (0.9) | 0.4 (0.8) | 0.4 (0.8) | 0.3 (0.6) | 0.3 (0.5) | 0.3 (0.6) | 0.2 (0.6) | 0.1 (0.5) | 0.1 (0.3) | 0.1 (0.2) |
| <1 serving/month (%) | 13.6 | 35.7 | 33.9 | 49.4 | 35.8 | 23.6 | 44.8 | 58.0 | 70.0 | 63.4 | 71.9 |
| 1-4 serving/month (%) | 14.4 | 16.3 | 24.3 | 13.8 | 24.6 | 31.9 | 22.0 | 19.3 | 12.4 | 16.9 | 13.5 |
| 1-2 serving/week (%) | 23.8 | 12.1 | 9.76 | 14.1 | 14.0 | 17.1 | 13.1 | 3.5 | 2.2 | 0.06 | 6.4 |
| 3-7 serving/week (%) | 29.2 | 25.7 | 21.3 | 11.7 | 15.2 | 21.0 | 15.1 | 15.3 | 8.6 | 18.7 | 7.5 |
| >1 serving/day (%) | 19.0 | 10.3 | 10.8 | 11.0 | 10.4 | 6.3 | 5.0 | 3.9 | 2.3 | 0.9 | 0.8 |
| Energy Intake (kcal/d) | 1,857 (850) | 1,644 (599) | 1,956 (645) | 2,291 (763) | 1,935 (578) | 2,381 (762) | 1,732 (524) | 1,698 (670) | 1708 (734) | 2,024 (654) | 2,046 (1,409) |
| Saturated Fat Intake (% total energy) | 16.1 (3.1) | 12.2 (3.1) | 11.1 (2.9) | 12.4 (2.9) | 12.5 (3.0) | 11.8 (2.4) | 10.2 (2.5) | 11.6 (3.3) | 11.3 (3.3) | 10.4 (2.2) | 14.4 (3.1) |
| Fruit intake (servings/d) | 1.9 (1.3) | 1.5 (1.3) | 1.1 (1.0) | 1.1 (0.9) | 2.7 (2.2) | 3.4 (3.1) | 1.9 (1.2) | 1.8 (1.2) | 2.1 (1.7) | 2.7 (1.5) | 1.2 (1.0) |
| Vegetable Intake (servings/d) | 1.7 (0.9) | 1.7 (1.2) | 2.0 (1.1) | 2.8 (1.5) | 5.0 (2.5) | 1.4 (1.8) | 3.9 (2.3) | 2.2 (1.3) | 2.4 (1.5) | 2.8 (1.5) | 2.8 (2.1) |
| Whole Grain Intake (servings/d) | 0.8 (1.0) | 1.1 (1.1) | 1.2 (1.2) | NA | 1.8 (1.4) | 3.2 (1.9) | 1.5 (1.2) | 1.2 (0.8) | 1.0 (0.8) | 1.0 (0.7) | 3.4 (2.9) |
| Fish Intake (servings/d) | 0.4 (0.6) | 0.3 (0.3) | 0.4 (0.4) | 0.2 (0.2) | 0.4 (0.3) | 1.3 (0.9) | 0.3 (0.2) | 0.2 (0.2) | 0.3 (0.3) | 0.3 (0.3) | 0.1 (0.2) |
| Nuts/Seeds Intake (servings/d) | 0.1 (0.2) | 0.4 (0.6) | 0.6 (0.9) | 0.8 (1.0) | 0.2 (0.3) | 0.1 (0.1) | 0.3 (0.4) | 0.2 (0.3) | 0.5 (0.6) | 0.2 (0.3) | 0.2 (2.1) |
| Alcohol Intake (g/d) | 7.8 (8.9) | 6.7 (13.5) | 10.5 (14.8) | 15.5 (17.4) | 9.5 (12.7) | 8.6 (13.4) | 4.3 (8.5) | 5.0 (10.2) | 8.8 (15.5) | 5.5 (12.9) | 11.1 (15.5) |
Means (standard deviation) or percentage for maximum observations available for analysis. Sample sizes may vary depending on availability of genotype and covariate information. Cohorts are ordered by estimate of sugar-sweetened beverage intake. Cohort study abbreviations: The Raine Study (Raine Study), Atherosclerosis Risk in Communities Study (ARIC), Framingham Heart Study (FHS), Netherlands Epidemiology in Obesity Study (NEO), The Fenland Study (Fenland), Young Finns Study (YFS), Women’s Genome Health Study (WGHS), Women’s Health Initiative (WHI), Multi-Ethnic Study of Atherosclerosis (MESA), Cardiovascular Health Study (CHS), and the Rotterdam Study (RS).
CHARGE, Cohorts for Heart and Aging Research in Genomic Epidemiology; HDL-C, high-density lipoprotein cholesterol concentrations; n, total sample size; SSB, sugar-sweetened beverages; TG, triglyceride concentrations.
Difference Test Interactions between SSB Consumption and SNPs on HDL-C and TG in CHARGE Cohorts
We identified 55 SNPs that displayed a significant (pDiff <0.0001) or suggestive (pDiff <0.005) difference in estimated effect by category of SSB consumption on HDL-C concentrations in either of the two covariate models in the meta-analysis of the CHARGE cohorts. Among these 55 top SNPs, four distinct signals for HDL-C concentrations were observed when applying the difference test interaction. Two distinct SNPs in moderate LD (linkage disequilibrium) with one another [rs35709627 and rs71556729; R2 = 0.55 (Figure II in the Supplemental Material)] and in low LD with the top SNP identified in the overall analysis for HDL-C concentrations (R2<0.3) displayed a statistically significant difference in effect by category of SSB intake on HDL-C concentrations in fully adjusted models (Model 2; pDiff<0.0001) (Table 2 and Figures III and IV in the Supplemental Material). In model 2, each additional minor allele at rs35709627 [β (SE): 2.72 (0.72), p=0.0002] and rs71556729 [β (SE): 3.89 (1.04), p=0.0002] was associated with higher mean concentrations of HDL-C concentrations among the highest SSB consumers (> 1 serving/day), but was not associated with mean HDL-C concentrations among the lowest SSB consumers (<1 serving/month; p>0.05). The effect sizes of these SNPs among the highest SSB consumers were consistent across all the cohorts. There was no heterogeneity (I2=0%) observed for the top 4 distinct signals (statistically significant and suggestive) among the highest SSB consumers (>1 serving/day), which could be due to low power to detect heterogeneity given the smaller sample size available among the highest SSB consumers (maximum n=4,033).
Table 2.
Top SNPs in meta-analysis of difference test (pDiff<0.005) and cross-product (pinteract<0.005) interactions between SSB consumption and SNPs on HDL-C and TG concentrations in CHARGE consortium cohorts*
| SNP | Location (Hg19) | Alleles (E/A)† | Minor Allele Frequency | Model‡ | SSB Intake Category | n | Effect Size (SE)§ | P-value | Direction‖ | I2 | p # |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| HDL-C (mg/dl) | |||||||||||
|
| |||||||||||
| Difference Test | p Diff | ||||||||||
|
| |||||||||||
| rs35709627†† | 72999171 | A/G | 0.05 | Model 1 | <1 serving/month | 24,389 | −0.01 (0.04) | 0.86 | +−++−−+++?? | 23% | 1.98E-05** |
| >1 serving/day | 4,033 | 3.23 (0.77) | 2.94E-05 | +?+?+?+?+?? | 0% | ||||||
| Model 2 | <1 serving/month | 23,801 | 0.006 (0.04) | 0.86 | +−++−−+++?? | 30% | 0.0001 | ||||
| >1 serving/day | 3,955 | 2.72 (0.72) | 0.0002 | +?+?+?+?+?? | 0% | ||||||
|
| |||||||||||
| rs71556729†† | 72989516 | T/C | 0.05 | Model 1 | <1 serving/month | 23,974 | 0.02 (0.06) | 0.77 | +?++−+++−?? | 0% | 4.78E-05** |
| >1 serving/day | 3,359 | 4.47 (1.10) | 5.02E-05 | ??+?+?+?+?? | 0% | ||||||
| Model 2 | <1 serving/month | 22,835 | 0.01 (0.05) | 0.83 | +?++−+−?−?? | 0% | 0.0001 | ||||
| >1 serving/day | 3,299 | 3.89 (1.04) | 0.0002 | ??+?+?+?+?? | 0% | ||||||
|
| |||||||||||
| rs71556736 | 73034929 | T/C | 0.13 | Model 1 | <1 serving/month | 24,389 | −0.0005 (0.02) | 0.98 | +−+++−−++?− | 60% | 0.0003 |
| >1 serving/day | 4,033 | 1.65 (0.47) | 0.0004 | +?+?+?+?+?? | 0% | ||||||
| Model 2 | <1 serving/month | 23,801 | 0.007 (0.02) | 0.69 | +−++++−++?? | 67% | 0.002 | ||||
| >1 serving/day | 3,955 | 1.34 (0.43) | 0.002 | +?+?+?+?+?? | 0% | ||||||
|
| |||||||||||
| rs73137017 | 72974413 | G/A | 0.04 | Model 1 | <1 serving/month | 24,020 | −0.05 (0.06) | 0.46 | −+−−+−++−?? | 0% | 0.002 |
| >1 serving/day | 3,933 | −3.13 (0.99) | 0.002 | −?−?−?−?−?? | 0% | ||||||
| Model 2 | <1 serving/month | 23,437 | −0.008 (0.05) | 0.88 | ++−−+−++−?? | 0% | |||||
| >1 serving/day | 3,855 | −2.64 (0.91) | 0.004 | −?−?−?−?−?? | 0% | 0.003 | |||||
|
| |||||||||||
| Cross-Product Interaction Test | p interact | ||||||||||
|
| |||||||||||
| rs71556729 | 72989516 | T/C | 0.03 | Model 1 | - | 55,418 | 0.66 (0.21) | - | +++++?+−+−− | 0% | 0.001 |
| Model 2 | - | 53,394 | 0.68 (0.20) | - | ++−++?+++?− | 26% | 0.0007 | ||||
|
| |||||||||||
| rs79578725 | 73002455 | A/G | 0.05 | Model 1 | - | 53,662 | −0.51 (0.18) | - | +?−+−?−−−−− | 0% | 0.005 |
| Model 2 | - | 52,328 | −0.18 (0.17) | - | +?++−?−−−−− | 0% | 0.28 | ||||
|
| |||||||||||
| TG (ln-mg/dl) | |||||||||||
|
| |||||||||||
| Difference Test | p Diff | ||||||||||
|
| |||||||||||
| rs799157 | 73020301 | T/C | 0.05 | Model 1 | <1 serving/month | 23,974 | 0.02 (0.01) | 0.11 | +?++++−++?? | 59% | 0.005 |
| >1 serving/day | 4,033 | 0.11 (0.03) | 0.002 | +?+?+?+?+?? | 0% | ||||||
|
| |||||||||||
| Model 2 | <1 serving/month | 23,403 | 0.02 (0.01) | 0.17 | +?++−−?+? | 68% | 0.008 | ||||
| >1 serving/day | 3,955 | 0.09 (0.03) | 0.004 | +?+?++?+? | 0% | ||||||
|
| |||||||||||
| Cross-Product Interaction Test | p interact | ||||||||||
|
| |||||||||||
| rs55673514 | 73021456 | G/A | 0.04 | Model 1 | - | 57,977 | 0.02 (0.01) | - | −+++++++?++ | 17% | 0.04 |
| Model 2 | - | 56,578 | 0.02 (0.01) | - | −+++++++++? | 0% | 0.005 | ||||
Top signals represent suggestive interactions pDiff<0.005 or pinteract<0.005c
Alleles presented as effect (E)/alternative (A) alleles
Model 1 adjusted for age (years), sex (male/female), total energy intake (kcal/day) field center (CHS, FHS, YFS, Fenland, RS, MESA), and accounted for family or population structure where applicable (FHS, YFS, Fenland, NEO, MESA, WGHS, Raine Study, MESA); Model 2 adjusted for Model 1 covariates plus cohort-specific definition of education, smoking, physical activity, alcohol intake, and body mass index (kg/m2).
For the difference test, β (SE) represents the direction and magnitude of the difference in the outcome trait with each additional effect allele among categories of SSB consumption. For the cross-product interaction test, β (SE) represents the direction and magnitude of the difference in the outcome trait with each additional effect allele, per each increase in category of SSB intake (<1 serving/month, 1-4 servings/month, 1-2 servings/week, 3-7 servings/week, >1 serving/day).
Order of cohorts for regression coefficient directions: Framingham Heart Study, Young Finns Study, Fenland Study, Cardiovascular Health Study, Netherlands Epidemiology in Obesity Study, Rotterdam Study, Women’s Genome Health Study, Women’s Health Initiative, Atherosclerosis Risk in Communities Sutyd, The Raine Study, Multi-Ethnic Study of Atherosclerosis (+, positive effect size; -, negative effect size; ?, SNP not available in cohort).
P represents pDiff for the difference test for the highest and lowest category of SSB intake (<1 serving/month vs. >1 serving/day). P represents pinteract for the cross-product interaction regression coefficient of additive SSBxSNP categories.
Linkage disequilibrium (R2) between rs13240662 and rs71556729=0.55 in European ancestry groups of Phase 3 (Version 5) of the 1000 Genomes Project.
Indicates a statistically significant interaction based on Bonferonni-corrected pDiff or pinteract<0.0001
CHARGE, Cohorts for Heart and Aging Research in Genetic Epidemiology; HDL-C, high-density lipoprotein cholesterol concentrations; SE, standard error; SNP, single nucleotide polymorphism; SSB, sugar-sweetened beverages; TG, triglyceride concentrations.
No statistically significant differences in effect by category of SSB intake on TG concentrations were observed when applying the difference test (pDiff >0.0001 for all SNPs). One SNP (rs799157) in moderate LD with a top SNP identified in the overall analysis for TG concentrations (Table X in the Supplemental Material; R2 with rs42124=0.44) displayed a suggestive difference in effect by category of SSB intake on TG concentrations in minimally adjusted models (Model 1; pDiff =0.005) (Table 2). Each additional minor allele at rs799157 was associated with higher mean TG concentrations among the highest SSB consumers (> 1 serving/day) [β (SE): 0.11 (0.03) ln-mg/dl, p=0.002], but this association was attenuated among the lowest SSB consumers [β (SE): 0.01 (0.01) ln-mg/dl, p=0.11] (Figure V in the Supplemental Material). The direction of the effect size of this SNP among the highest SSB consumers was consistent across all the cohorts in which these SNPs were available, and heterogeneity was low among the highest SSB consumers (I2 = 0%).
Cross-Product Interactions between SSB Consumption and SNPs on HDL-C and TG in CHARGE Cohorts
No statistically significant cross-product interactions between SNPs and SSB consumption on HDL-C or TG concentrations were observed (pinteraction>0.0001), while some tests were suggestive (pinteraction<0.005) (Table 2). Three SNPs displayed a suggestive interaction with SSB consumption on HDL-C concentrations in either covariate model, and the clumping identified two distinct signals (rs71556729 and rs79578725). One SNP (rs55673514) displayed a suggestive interaction with SSB on TG concentrations in Model 2. Forest plots for top distinct signals in SSBxSNP interaction analyses on lipid traits are presented in Figures VI and VII in the Supplemental Material.
Interactions between SSB Consumption and SNPs on Lipid Traits in the UKB and Meta-Analysis with CHARGE Cohort Results
General characteristics and mean dietary intakes for the 59,220 UKB participants are shown in Table VI in the Supplemental Material. Two out of five top signals for HDL-C (rs35709627 and rs71556729) and one out of two top signals for TG in the CHARGE consortium were replicated among the UKB participants (Table VII in the Supplemental Material). In a meta-analysis of the top results from the CHARGE consortium and data from the UKB, three out of the five top SNPs for HDL-C and one out of the two top SNPs for TG concentrations displayed statistically significant interactions (Table 3). The top SNP for HDL-C concentrations was located at rs71556729 (Figure 1A). In fully adjusted models, the association between the minor allele at rs71556729 with HDL-C concentrations was observed only among the highest SSB consumers [β (95% CI): 2.12 (1.16, 3.07) mg/dl, p<0.0001], and not the lowest SSB consumers (p=0.81; pDiff <0.0001). Similarly, two SNPs in low to moderate LD with rs71556729 (TBL2-rs35709627: R2 with rs71556729=0.55; rs71556736: R2 with rs71556729=0.19) displayed similar statistically significant differences in effect by category of SSB intake (pDiff <0.0001). The SNP at rs55673514 displayed a suggestive interaction with TG concentrations in the CHARGE meta-analysis and was statistically significant after including data from the UKB (Figure 1B, pDiff <0.0005). The association of the minor allele at rs55673514 with TG concentrations was observed only among the highest SSB consumers [β (95% CI): 0.06 (0.02, 0.09) ln-mg/dl, p=0.001], and not the lowest SSB consumers (p=0.84). The SNP at rs55673514 is not in appreciable LD with any of the top SNPs in the overall analysis for TG concentrations (R2<0.1). A heatmap of LD among top SNPs in overall and interaction analyses is provided in Figure II in the Supplemental Material. Sensitivity analyses examining the influence of adjustment for other dietary factors and fasting hours among UKB participants yielded similar results for the top SNPs identified in the meta-analysis (Supplemental Results in the Supplemental Material).
Table 3.
Fixed-effects meta-analysis of top candidate SNPs for difference test interactions between SSB consumption and SNPs on HDL-C and TG concentrations in CHARGE consortium cohorts and UKB*
| SNP | Location (Hg19) | Alleles (E/A)† | MAF | SSB Intake Category | n | Effect Size (SE) | P-value | Direction‡ | I2 | p Diff |
|---|---|---|---|---|---|---|---|---|---|---|
| HDL-C (mg/dl) | ||||||||||
|
| ||||||||||
| rs71556729§ | 72989516 | T/C | 0.05 | Low | 68,701 | 0.01 (0.05) | 0.81 | ++ | 0 % | 1.5E-06‖ |
| High | 15,227 | 2.06 (0.44) | 3.48E-06 | ++ | 74 % | |||||
|
| ||||||||||
| rs35709627§ | 72999171 | A/G | 0.05 | Low | 69,667 | 0.01 (0.04) | 0.74 | ++ | 0 % | 1.0E-05‖ |
| High | 15,883 | 1.37 (0.32) | 2.15E-05 | ++ | 87 % | |||||
|
| ||||||||||
| rs71556736 | 73034929 | T/C | 0.13 | Low | 69.667 | 0.02 (0.02) | 0.33 | ++ | 93 % | 2.5E-05‖ |
| High | 15,882 | 0.84 (0.20) | 3.27E-05 | ++ | 42 % | |||||
|
| ||||||||||
| rs73137017 | 72974413 | G/A | 0.04 | Low | 69,303 | 0.01 (0.05) | 0.82 | ++ | 0 % | 0.04 |
| High | 15,783 | 0.73 (0.37) | 0.05 | ++ | 81 % | |||||
|
| ||||||||||
| rs79578725 | 73002455 | A/G | 0.05 | Low | 68,929 | −0.02 (0.04) | 0.64 | −− | 21 % | 0.55 |
| High | 15,783 | −0.22 (0.36) | 0.53 | −− | 0 % | |||||
|
| ||||||||||
| TG (ln-mg/dl) | ||||||||||
|
| ||||||||||
| rs55673514 | 73021456 | G/A | 0.04 | Low | 69,096 | −0.002 (0.01) | 0.84 | +− | 29 % | 0.0005‖ |
| High | 15,395 | −0.06 (0.02) | 0.001 | −− | 0 % | |||||
|
| ||||||||||
| rs799157 | 73020301 | T/C | 0.05 | Low | 70,235 | 0.03 (0.01) | 2.55E-07 | ++ | 59 % | 0.05 |
| High | 16,006 | 0.06 (0.02) | 0.0002 | ++ | 19 % | |||||
Top candidates represent statistically significant or suggestive interactions (pDiff<0.005 or pinteract<0.005) in CHARGE cohort meta-analysis. Models adjusted for age, sex, total energy intake, field center and accounted for family or population structure where applicable plus education, smoking, physical activity, alcohol intake, and body mass index (kg/m2). For the difference test, interaction coefficients are shown as β (SE), where β represents the direction and magnitude of change in the outcome trait with each additional effect allele among participants with low (CHARGE:<1 serving/month; UKB: non-consumers) or high (CHARGE: >1 serving/day; UKB: consumers) SSB consumption.
Alleles presented as effect (E)/alternative (A) alleles
Order of cohorts for regression coefficient directions: CHARGE cohorts, UKB (+, positive effect size; −, negative effect size).
Linkage disequilibrium (R2) between rs13240662 and rs71556729=0.55 in European ancestry groups of Phase 3 (Version 5) of the 1000 Genomes Project.
Indicates a statistically significant interaction based on Bonferroni-corrected pDiff<0.01 (0.05/5 top signals) for HDL-C and pDiff<0.025 (0.05/2 top signals) for TG concentrations
CHARGE, Cohorts for Heart and Aging Research in Genetic Epidemiology; HDL-C, high-density lipoprotein cholesterol; MAF, minor allele frequency; SNP, single nucleotide polymorphism; SSB, sugar-sweetened beverages; TG, triglyceride; and UKB, UK Biobank.
Figure 1.
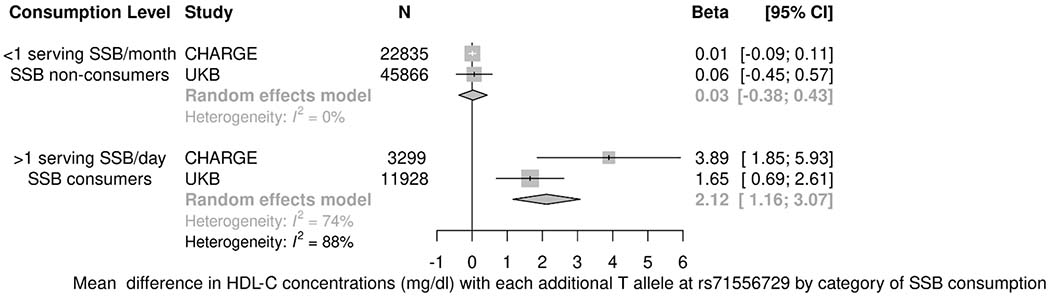
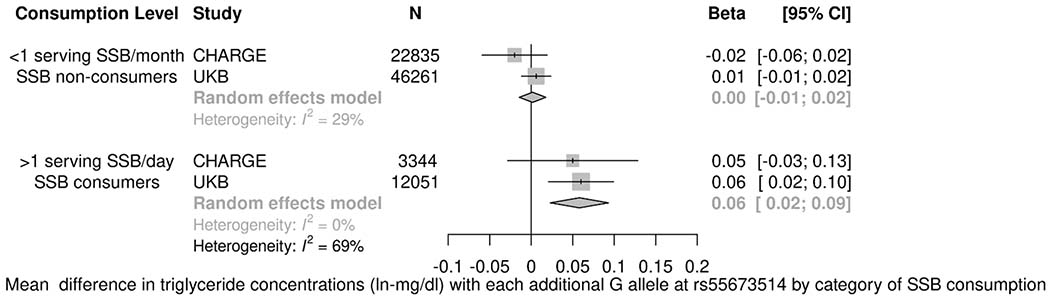
Associations between top candidate SNPs and HDL-C and TG concentrations stratified by category of SSB intake in a meta-analysis of the CHARGE cohorts and the UKB. A) In a random effects meta-analysis of the CHARGE cohorts and the UKB, the association of the minor allele at rs71556729 with HDL-C concentrations was observed only among the highest SSB consumers [β (95% CI): 2.12 (1.16, 3.07) mg/dl, p<0.0001], and not the lowest SSB consumers (p=0.81; pDiff<0.0001); B) In a random effects meta-analysis of the CHARGE cohorts and the UKB, the association of the minor allele at rs55673514 with TG concentrations was observed only among the highest SSB consumers [β (95% CI): 0.06 (0.02, 0.09) ln-mg/dl, p=0.001], and not the lowest SSB consumers (p=0.84; pDiff<0.0005); Linear regression models represent associations between each additional effect allele and HDL-C (mg/dl) or TG (ln-mg/dl) concentrations among SSB consumption categories accounting for family, population structure, and/or field center (where applicable) and adjusting for age, sex, total energy intake, education, smoking, physical activity, alcohol intake, and body mass index. Intake categories are different for the highest SSB consumers (CHARGE: >1 serving/day; UKB: SSB consumers) and lowest SSB consumers (CHARGE: <1 serving/month; UKB: SSB non-consumers) in the two samples. CI, confidence interval; CHARGE, Cohorts for Heart and Aging Research in Genetic Epidemiology; HDL-C, high-density lipoprotein cholesterol concentrations; I2, percentage of variance in a meta-analysis that is attributable to study heterogeneity; SSB, sugar-sweetened beverage consumption; TG, triglyceride concentrations; UKB, UK Biobank.
Discussion
In this study, including up to 86,241 participants for whom genetic and SSB consumption data were available, we identified novel interactions between genetic variants at the CHREBP locus and SSB consumption on HDL-C and TG concentrations. Our data suggest that the magnitude of the inverse association between SSB consumption and HDL-C concentrations is lower among individuals harboring genetic variants at rs71556729, rs35709627, and/or rs71556736 and the positive association between SSB consumption and TG concentrations is exacerbated among individuals harboring genetic variants at rs55673514. In the CHARGE cohorts, we also observed a consistent inverse association between SSB consumption on fasting HDL-C and positive association on TG concentrations. We also replicated previously observed main associations between SNPs in the CHREBP locus and HDL-C and TG concentrations.
Our study provides evidence that SSB consumption may modify the association of genetic variants in the CHREBP locus with HDL-C and TG concentrations. Participants with the minor allele at rs71556729, rs35709627, and/or rs71556736 and high SSB consumption had higher mean HDL-C concentrations than those with the major allele who also had high SSB consumption. This suggests that participants with the minor allele at rs71556729 (MAF [minor allele frequency] = 0.05), rs35709627 (MAF = 0.05), and/or rs71556736 (MAF = 0.13) may be protected against SSB-induced reductions in HDL-C concentrations. The region containing these SNPs is enriched for enhancer histone marks and these SNPs lie within putative regulatory motifs for transcription factors that could potentially regulate ChREBP expression and function in an SSB dependent manner.33 Similarly, rs55673514, which associates with TG only among the highest SSB consumers, lies within a region enriched for enhancer histone marks in several tissues, including liver.33 Given the strong inverse relationship between HDL-C and TG concentrations, additional investigation into how these SNPs may independently influence HDL-C or TG concentrations could provide new insights into the distinct mechanisms contributing to plasma HDL-C and TG concentrations. Additional discussion of main associations between SNPs and SSB on TG and HDL-C in the CHARGE cohorts is provided in the Supplemental Discussion in the Supplemental Material.
The rs71556729 interaction was a top signal when testing for interactions using the difference test and the cross-product interaction test on HDL-C concentrations in the CHARGE cohorts. However, when applying the cross-product interaction test, the interaction appeared less significant than the result from the difference test. This may be due to heterogeneity in the association between rs71556729 and HDL-C concentrations resulting from increased misclassification of SSB consumption among those reporting low (1-4 servings/month) to moderate (1-2 and 3-7 servings/week) SSB consumption (Figure IV in the Supplemental Material). These results suggest that the difference test may be a useful method for identifying gene-diet interactions in observational studies, and this could be due to a reduction in misclassification of SSB intake and the potential to detect non-linear interaction effects. However, we do not comprehensively compare the difference test to the cross-product interaction test. Future methodological studies comparing the usefulness of these two methods with varying degrees of misclassification and types of exposures may be useful to inform future gene-diet interaction studies.
There is a limited body of evidence describing how genes implicated in various diseases may interact with SSB consumption to modify cardiometabolic health and noncommunicable disease risk.34 One large prospective cohort study among Swedish adults examined whether genetic risk for dyslipidemia (using a weighted genetic risk score) interacted with SSB consumption to influence plasma lipid concentrations, but no significant interactions were observed.35 Although genetic risk scores can be useful for translation, as previously shown for the interaction between SSB consumption and genetic risk for obesity,36 a weakness of genetic risk scores is that aggregation of multiple SNPs from across the genome does not allow inclusion of potential interacting SNPs that may not be associated with the outcome in overall analyses. In addition, interaction effects of SNPs may be mitigated by the null interaction effects of other SNPs included in the genetic risk score. The candidate gene approach in the current study allows for the potential to generate hypotheses of the mechanisms underlying the interaction that could be tested using animal and human models in future studies.
No previous studies have examined the interaction between SNPs in the CHREBP region and SSB consumption on lipid concentrations. We previously investigated how selected SNPs in the ChREBP-FGF21 pathway interacted with SSB consumption to influence fasting insulin and glucose measures among 34,748 adults from CHARGE cohorts, but we did not identify a significant cross-product interaction that was consistent among the discovery and replication phases of that study.37 In the current study, we applied a comprehensive approach that tested a wide range of SNPs in the CHREBP region that were not necessarily identified in GWAS. Given that our suggestive interaction results do not include any SNPs that were statistically significant in the overall SNP analyses, our data indicate that there may be additional SNPs not identified in GWAS contributing to the heritability of HDL-C and TG concentrations, but their contribution is influenced by SSB consumption. Similar to previous GWAS for body mass index that have identified new loci when adjusting for environmental factors38,39, we provide an additional example of how missing genetic heritability may be revealed when accounting for environmental factors, such as SSB consumption in the current study.
The strengths of our study include the large sample size attained through meta-analysis of multiple independent cohorts, the ability to standardize the analyses conducted in all cohorts through a collaborative approach, the use of an external cohort to validate findings, and the use of multiple methods to screen for potential interactions between SSB consumption and over 1,606 SNPs in the CHREBP region on HDL-C and TG concentrations. The analytic approach revealed novel SNPs that may contribute to unexplained heritability of HDL-C and TG concentrations. Limitations of this study include its observational design that constrain our ability to infer causality, the sample of European-descent adults that limits generalizability, the use of self-reported dietary data from food frequency questionnaires and 24-hour recall that may lead to misclassification of food and nutrient intakes, and the possibility of residual confounding, even after controlling for potential dietary and lifestyle factors that co-vary with SSB intake. Our focus on the comparison of the highest SSB consumers to the lowest SSB consumers helps minimize this potential misclassification by focusing on extreme consumption patterns. Misclassification in the UKB is likely given that a snapshot of intake on a single day cannot provide a reliable estimate of usual SSB consumption. However, this misclassification is likely non-differential by genotype, which would only result in attenuation of our results. Additionally, while our definition of SSB did consider a range of SSB, it was not comprehensive. For example, it did not include commonly consumed beverages, such as sweetened tea or coffee, and we included several types of SSB in the same exposure definition (colas and fruit drinks). The blood collection among UKB participants was conducted after less than the recommended 8 hours of fasting prior to measurement of lipids. We adjusted for fasting hours to help account for this variability and conducted a sensitivity analysis to examine the top interactions observed by fasting hours. The LD-based method used to estimate the number of independent tests in the region may be overly conservative, which could potentially lead to inflation of type II error rate. Thus, we additionally present suggestive results that did not reach statistical significance. Given these weaknesses, results from this study should be used to inform future studies with larger samples sizes or detailed experimental studies. Minority populations are disproportionality burdened by dyslipidemia and have higher SSB intake,40,41 and thus more studies in these populations may help reduce health inequality and disparity.
In conclusion, our findings suggest that the minor alleles of three SNPs in the CHREBP region (rs71556729, rs35709627, and rs71556736) may be protective against SSB-induced low HDL-C concentrations and the minor allele at rs55673514 may exacerbate positive associations between SSB consumption and TG concentrations. Several of the top SNPs identified in the interaction analyses were not top SNPs identified in the overall analyses, providing evidence that some genetic associations may be revealed only when conditioned on environmental factors, such as the range of SSB consumption in the current study. As larger datasets with genetics, diet, and lipids data become available, additional suggestive interactions between SSB consumption and SNPs within the CHREBP region on HDL-C and TG concentrations observed here may warrant further investigation.
Supplementary Material
Acknowledgments:
Preliminary results were presented as abstracts at the annual meeting for the American Society for Nutrition 2020. Please see Table I in the Supplemental Material for cohort-specific acknowledgements.
The authors’ responsibilities were as follows: DEH, GMP, MG, HSD, AHL, CES, JD, MAH, and NMM designed the study. DEH, GMP, MG, RNL, NT, DOM-K, KN, JSV, LS, YM-R, LWM, WHO, CEP, FRR, MAI, AGU, TV, BMP, DM, JIR, KDT, TL, OTR, KAL, NGF, NJW, JL, RM, SSR, JEM, SM, PMR, JBM, DIC, AHL, CES, JD, MAH, and NMM played a role in acquisition of the data and critical editing of the manuscript; DEH, GMP, MG, FI, TMB, ANP, CAW, RLG, JMW, NP, KLY, MG, ACW, KVEB, JL, MK, JCK-dJ, MG, and NT conducted statistical analyses; DEH, GMP, MG, HSD, JM, AHL, CES, JD, MAH, and NMM interpreted the data; DEH, GMP, MG, HSD, JM, KAL, AHL, CES, JD, MAH, and NMM contributed to writing of the manuscript; all authors read and approved the final version of the manuscript. DEH and NMM are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
Sources of Funding:
This work is supported by National Institutes of Health (NIH) 5T32HL069772-15 and NIH 2T32CA009001-39 (Haslam), American Heart Association 16CSA28590003 (Haslam, McKeown, and Herman), NIH R01 DK100425 (Herman), R01 DK121710 (McKeown, Herman, Smith, and Dupuis), K08 HL112845 (Smith), USDA ARS agreement No. 58-1950-4-003 (McKeown) and 588-1950-9-001 (Lichtenstein). Infrastructure for the CHARGE Consortium is supported in part by the National Heart, Lung, and Blood Institute grant HL105756. Please see Table I in the Supplemental Material for funding sources associated with investigators and infrastructure of individual CHARGE cohorts.
Disclosures:
SM received institutional research grant support from Atherotech Diagnostics for research outside the current work, served as a consultant (modest) to Quest Diagnostics and Pfizer outside the current work. The other authors report no conflicts.
Nonstandard Abbreviations and Acronyms
- HDL-C
high-density lipoprotein cholesterol
- TG
triglyceride
- T2D
type 2 diabetes
- CVD
cardiovascular disease
- GWAS
genome-wide association studies
- ChREBP
Carbohydrate Responsive Element Binding Protein
- SSB
sugar-sweetened beverages
- SNPs
single nucleotide polymorphisms
- CHARGE
Cohorts for Heart and Aging Research in Genetic Epidemiology
- UKB
UK Biobank
References:
- 1.Wu L, Parhofer KG. Diabetic dyslipidemia. Metabolism. 2014;63:1469–1479. [DOI] [PubMed] [Google Scholar]
- 2.Rader DJ, Hovingh GK. HDL and cardiovascular disease. The Lancet. 2014;384:618–625. [DOI] [PubMed] [Google Scholar]
- 3.Navar AM. The Evolving Story of Triglycerides and Coronary Heart Disease Risk. JAMA. 2019;321:347–349. [DOI] [PubMed] [Google Scholar]
- 4.Goff David C, Lloyd-Jones Donald M, Bennett Glen, Coady Sean, D’Agostino Ralph B., Gibbons Raymond, Greenland Philip, Lackland Daniel T., Levy Daniel, O’Donnell Christopher J., et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk. Circulation. 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 5.Grundy Scott M, Stone Neil J, Bailey Alison L, Beam Craig, Birtcher Kim K., Blumenthal Roger S., Braun Lynne T., de Ferranti Sarah, Faiella-Tommasino Joseph, Forman Daniel E., et al. 2018AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 0:CIR.0000000000000625. [Google Scholar]
- 6.Heller DA, de Faire U, Pedersen NL, Dahlén G, McClearn GE. Genetic and environmental influences on serum lipid levels in twins. N Engl J Med. 1993;328:1150–1156. [DOI] [PubMed] [Google Scholar]
- 7.Abney M, McPeek MS, Ober C. Broad and Narrow Heritabilities of Quantitative Traits in a Founder Population. Am J Hum Genet. 2001;68:1302–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kathiresan S, Melander O, Guiducci C, Surti A, Burtt NP, Rieder MJ, Cooper GM, Roos C, Voight BF, Havulinna AS, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peloso GM, Demissie S, Collins D, Mirel DB, Gabriel SB, Cupples LA, Robins SJ, Schaefer EJ, Brousseau ME. Common genetic variation in multiple metabolic pathways influences susceptibility to low HDL-cholesterol and coronary heart disease. J Lipid Res. 2010;51:3524–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45:1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klarin D, Damrauer SM, Cho K, Sun YV, Teslovich TM, Honerlaw J, Gagnon DR, DuVall SL, Li J, Peloso GM, et al. Genetics of Blood Lipids Among ~300,000 Multi-Ethnic Participants of the Million Veteran Program. Nat Genet. 2018;50:1514–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 2006;4:107–110. [DOI] [PubMed] [Google Scholar]
- 14.Fisher ffolliott M, Kim M, Doridot L, Cunniff JC, Parker TS, Levine DM, Hellerstein MK, Hudgins LC, Maratos-Flier E, Herman MA. A critical role for ChREBP-mediated FGF21 secretion in hepatic fructose metabolism. Mol Metab. 2017;6:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kooner JS, Chambers JC, Aguilar-Salinas CA, Hinds DA, Hyde CL, Warnes GR, Gómez Pérez FJ, Frazer KA, Elliott P, Scott J, et al. Genome-wide scan identifies variation in MLXIPL associated with plasma triglycerides. Nat Genet. 2008;40:149–151. [DOI] [PubMed] [Google Scholar]
- 16.Chasman DI, Paré G, Mora S, Hopewell JC, Peloso G, Clarke R, Cupples LA, Hamsten A, Kathiresan S, Mälarstig A, et al. Forty-Three Loci Associated with Plasma Lipoprotein Size, Concentration, and Cholesterol Content in Genome-Wide Analysis. PLOS Genet. 2009;5:e1000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postic C, Dentin R, Denechaud P-D, Girard J. ChREBP, a transcriptional regulator of glucose and lipid metabolism. Annu Rev Nutr. 2007;27:179–192. [DOI] [PubMed] [Google Scholar]
- 18.Erion DM, Popov V, Hsiao JJ, Vatner D, Mitchell K, Yonemitsu S, Nagai Y, Kahn M, Gillum MP, Dong J, et al. The Role of the Carbohydrate Response Element-Binding Protein in Male Fructose-Fed Rats. Endocrinology. 2013;154:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim M, Astapova II, Flier SN, Hannou SA, Doridot L, Sargsyan A, Kou HH, Fowler AJ, Liang G, Herman MA. Intestinal, but not hepatic, ChREBP is required for fructose tolerance. JCI Insight [Internet] [cited 2018 Jun 8];2. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5752301/ [DOI] [PMC free article] [PubMed]
- 20.Linden AG, Li S, Choi HY, Fang F, Fukasawa M, Uyeda K, Hammer RE, Horton JD, Engelking LJ, Liang G. Interplay between ChREBP and SREBP-1c coordinates postprandial glycolysis and lipogenesis in livers of mice. J Lipid Res. 2018;59:475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Koning L, Malik VS, Kellogg MD, Rimm EB, Willett WC, Hu FB. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation. 2012;125:1735–1741, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welsh JA, Sharma A, Abramson JL, Vaccarino V, Gillespie C, Vos MB. Caloric Sweetener Consumption and Dyslipidemia Among US Adults. JAMA. 2010;303:1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haslam Danielle E, Peloso Gina M, Herman Mark A, Dupuis Josée, Lichtenstein Alice H., Smith Caren E., McKeown Nicola M. Beverage Consumption and Longitudinal Changes in Lipoprotein Concentrations and Incident Dyslipidemia in US Adults: The Framingham Heart Study. J Am Heart Assoc. 2020;9:e014083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men123. Am J Clin Nutr. 2011;93:1321–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu FB, Malik VS. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: Epidemiologic evidence. Physiol Behav. 2010;100:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imamura F, O’Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN, Forouhi NG. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. The BMJ. 2015;351:h2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Popkin BM, Hawkes C. Sweetening of the global diet, particularly beverages: patterns, trends, and policy responses. Lancet Diabetes Endocrinol. 2016;4:174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh GM, Micha R, Khatibzadeh S, Lim S, Ezzati M, Mozaffarian D. Estimated Global, Regional, and National Disease Burdens Related to Sugar-Sweetened Beverage Consumption in 2010. Circulation. 2015;132:639–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai H, Much AA, Maor E, Asher E, Younis A, Xu Y, Lu Y, Liu X, Shu J, Bragazzi NL. Global, regional, and national burden of ischaemic heart disease and its attributable risk factors, 1990–2017: results from the Global Burden of Disease Study 2017. Eur Heart J Qual Care Clin Outcomes. 2020October5: qcaa076, 10.1093/ehjqcco/qcaa076. [DOI] [PMC free article] [PubMed]
- 31.Psaty BM, O’Donnell CJ, Gudnason V, Lunetta KL, Folsom AR, Rotter JI, Uitterlinden AG, Harris TB, Witteman JCM, Boerwinkle E. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: Design of prospective meta-analyses of genome-wide association studies from five cohorts. Circ Cardiovasc Genet. 2009;2:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLOS Med. 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kheradpour P, Kellis M. Systematic discovery and characterization of regulatory motifs in ENCODE TF binding experiments. Nucleic Acids Res. 2014;42:2976–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haslam DE, McKeown NM, Herman MA, Lichtenstein AH, Dashti HS. Interactions between Genetics and Sugar-Sweetened Beverage Consumption on Health Outcomes: A Review of Gene–Diet Interaction Studies. Front Endocrinol [Internet]. 2018. [cited 2018 Jan 8];8. Available from: https://www.frontiersin.org/articles/10.3389/fendo.2017.00368/full?&utm_source=Email_to_authors_&utm_medium=Email&utm_content=T1_11.5e1_author&utm_campaign=Email_publication&field=&journalName=Frontiers_in_Endocrinology&id=320441 [DOI] [PMC free article] [PubMed]
- 35.Sonestedt E, Hellstrand S, Drake I, Schulz C-A, Ericson U, Hlebowicz J, Persson MM, Gullberg B, Hedblad B, Engström G, et al. Diet Quality and Change in Blood Lipids during 16 Years of Follow-up and Their Interaction with Genetic Risk for Dyslipidemia. Nutrients [Internet]. 2016. [cited 2019 Feb 1];8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4882687/ [DOI] [PMC free article] [PubMed]
- 36.Qi Q, Chu AY, Kang JH, Jensen MK, Curhan GC, Pasquale LR, Ridker PM, Hunter DJ, Willett WC, Rimm EB, et al. Sugar-Sweetened Beverages and Genetic Risk of Obesity. N Engl J Med. 2012;367:1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKeown NM, Dashti HS, Ma J, Haslam DE, Jong JCK, Smith CE, Tanaka T, Graff M, Lemaitre RN, Rybin D, et al. Sugar-sweetened beverage intake associations with fasting glucose and insulin concentrations are not modified by selected genetic variants in a ChREBP-FGF21 pathway: a meta-analysis. Diabetologia. 2018;61:317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Justice AE, Winkler TW, Feitosa MF, Graff M, Fisher VA, Young K, Barata L, Deng X, Czajkowski J, Hadley D, et al. Genome-wide meta-analysis of 241,258 adults accounting for smoking behaviour identifies novel loci for obesity traits. Nat Commun [Internet]. 2017. [cited 2021 Feb 8];8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5414044/ [DOI] [PMC free article] [PubMed]
- 39.Graff M, Scott RA, Justice AE, Young KL, Feitosa MF, Barata L, Winkler TW, Chu AY, Mahajan A, Hadley D, et al. Genome-wide physical activity interactions in adiposity ― A meta-analysis of 200,452 adults. PLoS Genet [Internet]. 2017. [cited 2021 Feb 8];13. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5407576/ [DOI] [PMC free article] [PubMed]
- 40.Pu J, Romanelli R, Zhao B, Azar KMJ, Hastings KG, Nimbal V, Fortmann SP, Palaniappan LP. Dyslipidemia in Special Ethnic Populations. Cardiol Clin. 2015;33:325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han E, Powell LM. CONSUMPTION PATTERNS OF SUGAR SWEETENED BEVERAGES IN THE UNITED STATES. J Acad Nutr Diet. 2013;113:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becker BJ, Wu M-J. The Synthesis of Regression Slopes in Meta-Analysis. Stat Sci. 2007;22:414–429. [Google Scholar]
- 43.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 44.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 45.Yang J, Ferreira T, Morris AP, Medland SE, Genetic Investigation of ANthropometric Traits (GIANT) Consortium, DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium, Madden PAF, Heath AC, Martin NG, Montgomery GW, et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat Genet. 2012;44:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winkler TW, Justice AE, Cupples LA, Kronenberg F, Kutalik Z, Heid IM, Consortium the G. Approaches to detect genetic effects that differ between two strata in genome-wide meta-analyses: Recommendations based on a systematic evaluation. PLOS ONE. 2017;12:e0181038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voorman A, Lumley T, McKnight B, Rice K. Behavior of QQ-Plots and Genomic Control in Studies of Gene-Environment Interaction. PLoS ONE [Internet]. 2011. [cited 2017 Oct 23];6. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3093379/ [DOI] [PMC free article] [PubMed]
- 49.Gao X Multiple testing corrections for imputed SNPs. Genet Epidemiol. 2011;35:154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.1000 Genomes Project Consortium. A global reference for human genetic variation. Nature. 2015;526:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am J Hum Genet. 2007;81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou W, Nielsen JB, Fritsche LG, Dey R, Gabrielsen ME, Wolford BN, LeFaive J, VandeHaar P, Gagliano SA, Gifford A, et al. Efficiently controlling for case-control imbalance and sample relatedness in large-scale genetic association studies. Nat Genet. 2018;50:1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hert KA, Fisk II PS, Rhee YS, Brunt AR. Decreased consumption of sugar-sweetened beverages improved selected biomarkers of chronic disease risk among US adults: 1999 to 2010. Nutr Res. 2014;34:58–65. [DOI] [PubMed] [Google Scholar]
- 54.Duffey KJ, Gordon-Larsen P, Steffen LM, Jacobs DR, Popkin BM. Drinking caloric beverages increases the risk of adverse cardiometabolic outcomes in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2010;92:954–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D’Agostino RB, Gaziano JM, Vasan RS. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116:480–488. [DOI] [PubMed] [Google Scholar]
- 56.Yu Z, Ley SH, Sun Q, Hu FB, Malik VS. Cross-sectional association between sugar-sweetened beverage intake and cardiometabolic biomarkers in US women. Br J Nutr. 2018;119:570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morenga LAT, Howatson AJ, Jones RM, Mann J. Dietary sugars and cardiometabolic risk: systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am J Clin Nutr. 2014;100:65–79. [DOI] [PubMed] [Google Scholar]
- 58.Talmud PJ, Drenos F, Shah S, Shah T, Palmen J, Verzilli C, Gaunt TR, Pallas J, Lovering R, Li K, et al. Gene-centric Association Signals for Lipids and Apolipoproteins Identified via the HumanCVD BeadChip. Am J Hum Genet. 2009;85:628–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 60.The cardiovascular health study: Design and rationale. Ann Epidemiol. 1991;1:263–276. [DOI] [PubMed] [Google Scholar]
- 61.Dawber TR, Kannel WB, Lyell LP. An Approach to Longitudinal Studies in a Community: The Framingham Study. Ann N Y Acad Sci. 1963;107:539–556. [DOI] [PubMed] [Google Scholar]
- 62.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–525. [DOI] [PubMed] [Google Scholar]
- 63.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D’Agostino RB, Fox CS, Larson MG, Murabito JM, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: Design, Recruitment, and Initial Examination. Am J Epidemiol. 2007;165:1328–1335. [DOI] [PubMed] [Google Scholar]
- 64.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Kronmal R, Liu K, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 65.Mutsert R de, Heijer M den, Rabelink TJ, Smit JWA, Romijn JA, Jukema JW, Roos A de, Cobbaert CM, Kloppenburg M, Cessie S le, et al. The Netherlands Epidemiology of Obesity (NEO) study: study design and data collection. Eur J Epidemiol. 2013;28:513–523. [DOI] [PubMed] [Google Scholar]
- 66.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLOS Med. 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Newnham JP, Evans SF, Michael CA, Stanley FJ, Landau LI. Effects of frequent ultrasound during pregnancy: a randomised controlled trial. Lancet Lond Engl. 1993;342:887–891. [DOI] [PubMed] [Google Scholar]
- 68.Ikram MA, Brusselle G, Ghanbari M, Goedegebure A, Ikram MK, Kavousi M, Kieboom BCT, Klaver CCW, de Knegt RJ, Luik AI, et al. Objectives, design and main findings until 2020 from the Rotterdam Study. Eur J Epidemiol. 2020;35:483–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ridker PM, Chasman DI, Zee RYL, Parker A, Rose L, Cook NR, Buring JE. Rationale, Design, and Methodology of the Women’s Genome Health Study: A Genome-Wide Association Study of More Than 25 000 Initially Healthy American Women. Clin Chem. 2008;54:249–255. [DOI] [PubMed] [Google Scholar]
- 70.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 71.Raitakari OT, Juonala M, Rönnemaa T, Keltikangas-Järvinen L, Räsänen L, Pietikäinen M, Hutri-Kähönen N, Taittonen L, Jokinen E, Marniemi J, et al. Cohort profile: the cardiovascular risk in Young Finns Study. Int J Epidemiol. 2008;37:1220–1226. [DOI] [PubMed] [Google Scholar]
- 72.Stevens J, Metcalf PA, Dennis BH, Tell GS, Shimakawa T, Folsom AR. Reliability of a food frequency questionnaire by ethnicity, gender, age and education. Nutr Res. 1996;16:735–745. [Google Scholar]
- 73.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 74.Kumanyika S, Tell GS, Fried L, Martel JK, Chinchilli VM. Picture-Sort Method for Administering a Food Frequency Questionnaire to Older Adults. J Am Diet Assoc. 1996;96:137–144. [DOI] [PubMed] [Google Scholar]
- 75.Bingham SA, Gill C, Welch A, Day K, Cassidy A, Khaw KT, Sneyd MJ, Key TJ, Roe L, Day NE. Comparison of dietary assessment methods in nutritional epidemiology: weighed records v. 24 h recalls, food-frequency questionnaires and estimated-diet records. Br J Nutr. 1994;72:619–643. [DOI] [PubMed] [Google Scholar]
- 76.Bingham SA, Gill C, Welch A, Cassidy A, Runswick SA, Oakes S, Lubin R, Thurnham DI, Key TJ, Roe L, et al. Validation of dietary assessment methods in the UK arm of EPIC using weighed records, and 24-hour urinary nitrogen and potassium and serum vitamin C and carotenoids as biomarkers. Int J Epidemiol. 1997;26Suppl 1:S137–151. [DOI] [PubMed] [Google Scholar]
- 77.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126; discussion 1127-1136. [DOI] [PubMed] [Google Scholar]
- 78.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, Hemphill S, Tsaroucha G, Rushing J, Levin S. Validity and reproducibility of a food frequency interview in a Multi-Cultural Epidemiology Study. Ann Epidemiol. 1999;9:314–324. [DOI] [PubMed] [Google Scholar]
- 79.Verkleij-Hagoort AC, de Vries JHM, Stegers MPG, Lindemans J, Ursem NTC, Steegers-Theunissen RPM. Validation of the assessment of folate and vitamin B 12 intake in women of reproductive age: the method of triads. Eur J Clin Nutr. 2007;61:610–615. [DOI] [PubMed] [Google Scholar]
- 80.Voortman T, Kiefte-de Jong JC, Ikram MA, Stricker BH, van Rooij FJA, Lahousse L, Tiemeier H, Brusselle GG, Franco OH, Schoufour JD. Adherence to the 2015 Dutch dietary guidelines and risk of non-communicable diseases and mortality in the Rotterdam Study. Eur J Epidemiol. 2017;32:993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klipstein-Grobusch K, den Breeijen J, Goldbohm RA, Geleijnse JM, Hofman A, Grobbee DE, Witteman JCM. Dietary assessment in the elderly: validation of a semiquantitative food frequency questionnaire. Eur J Clin Nutr. 1998;52:588–596. [DOI] [PubMed] [Google Scholar]
- 82.Goldbohm RA, van den Brandt PA, Brants HA, van’t Veer P, Al M, Sturmans F, Hermus RJ. Validation of a dietary questionnaire used in a large-scale prospective cohort study on diet and cancer. Eur J Clin Nutr. 1994;48:253–265. [PubMed] [Google Scholar]
- 83.Feunekes GI, Van Staveren WA, De Vries JH, Burema J, Hautvast JG. Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol. Am J Clin Nutr. 1993;58:489–496. [DOI] [PubMed] [Google Scholar]
- 84.Paalanen L, Männistö S, Virtanen MJ, Knekt P, Räsänen L, Montonen J, Pietinen P. Validity of a food frequency questionnaire varied by age and body mass index. J Clin Epidemiol. 2006;59:994–1001. [DOI] [PubMed] [Google Scholar]
- 85.Liu B, Young H, Crowe FL, Benson VS, Spencer EA, Key TJ, Appleby PN, Beral V. Development and evaluation of the Oxford WebQ, a low-cost, web-based method for assessment of previous 24 h dietary intakes in large-scale prospective studies. Public Health Nutr. 2011;14:1998–2005. [DOI] [PubMed] [Google Scholar]
- 86.McCance RA, Widdowson EM, Institute of Food Research (Great Britain), Public Health England, & Royal Society of Chemistry (Great Britain), 2015. McCance and Widdowson’s the composition of foods [Google Scholar]. [Google Scholar]
- 87.Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O’Connell J, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brage S, Westgate K, Franks PW, Stegle O, Wright A, Ekelund U, Wareham NJ. Estimation of Free-Living Energy Expenditure by Heart Rate and Movement Sensing: A Doubly-Labelled Water Study. PLoS ONE [Internet]. 2015. [cited 2020 Oct 28];10. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4562631/ [DOI] [PMC free article] [PubMed]
- 89.Kannel WB, Sorlie P. Some Health Benefits of Physical Activity: The Framingham Study. Arch Intern Med. 1979;139:857–861. [PubMed] [Google Scholar]
- 90.Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 2015;31:3555–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


