Abstract
Purpose –
we examined patients in a large clinical registry to assess factors associated with laser trabeculoplasty (LTP) response durations.
Methods –
this is a retrospective cohort study with LTP patients in the Intelligent Research in Sight Registry. Data were extracted if the eye had a LTP procedure code and a glaucoma diagnosis. In responders (≥20% intraocular pressure [IOP] reduction), any post-LTP IOP that was above 80% of baseline was considered a failure event. Eyes were censored if IOP-lowering medication/procedure was added/performed, or if the eye reached the end of follow-up. First eye of bilaterally treated patients were included.
Results –
79,332 patients/eyes were included; 53.2% female; mean age 71.5 years; 64.5% white; 71.2% primary open angle glaucoma. Mean baseline IOP was 21.6 +/− 5.3 mmHg (2.1 +/− 1.5 medications). Eyes with higher baseline IOP had longer survival (>24 mmHg median 349 days; 18–24 mmHg median 309 days; <18 mmHg median 256 days, p < 0.001for all comparisons). Overall failure at 0, 6, 12, 18 and 24 months were 0.2%, 6.1%, 16.8%, 29.1% and 40.8%. Angle recession and uveitis increased the risk of failure (hazard ratios 1.69 and 1.80, respectively). Eyes without medications at baseline remained medication-free for a median of 197 days (interquartile range 106, 395 days).
Conclusions –
angle recession and uveitis increase the risk of LTP failure. LPT may be effective in prolonging medication-free IOP-control in some patients.
Keywords: IRIS Registry®), uveitis, angle recession, laser trabeculoplasty
Précis –
In eyes with trabeculoplasty response, those with lower baseline pressure, angle recession or uveitis had shorter survival. Eyes without medications before treatment remained medication-free for a median of 197 days.
Introduction
Laser trabeculoplasty (LTP) is one of the most frequently performed ophthalmic procedures and comprised approximately 40% of all glaucoma interventions in 2014 1. Recently, several studies have demonstrate adequate efficacy and safety of LTP as initial treatment in primary open angle glaucoma (POAG) 2–4, while others suggest cost-saving implications of LTP either as initial or adjunctive therapies 5,6. Prior studies of modestly-sized cohorts have shown LTP efficacy to be maintained in about 80% of patients after 2 years 7, with younger age and higher baseline intraocular pressure (IOP) associated with failure.8 As the utilization of LTP is likely to increase, the characterization of its treatment effect duration is a priority 1,9, and analysis of a larger cohort is needed to assess these potential predictive factors.
The Intelligent Research in Sight (IRIS®) Registry is an electronic health record-based clinical data registry that includes data submitted by more than 2,300 ophthalmology practices in the United States. In 2016, the registry contained approximately 17.4 million unique patient entries that captured fields including patient demographics, payer types, social history, ocular examination laterality and values, diagnoses, procedures and medications 10. Recently, analyses of the IRIS® Registry have provided “real-world” clinical insight to several important ophthalmologic diagnoses and treatments including: the prevalence and treatment patterns of myopic choroidal neovascularization, the incidence of post-cataract surgery endophthalmitis, and outcomes of age-related macular degeneration treatment, macular hole surgery, and strabismus surgery. 11–17 In this study, we performed an analysis of a large cohort of eyes that initially responded to LTP 18 using the IRIS® Registry to assess potential predictive factors of LTP treatment survival.
Methods
The study protocol has been reviewed and was exempted by the Institutional Review Board of the University of Miami Miller School of Medicine as it did not meet the criteria of research involving human subjects as defined by the Department of Health and Human Services and Food and Drug Administration regulations.
Data Source
The IRIS® Registry data acquisition have been described elsewhere (https://www.aao.org/iris-registry/about) 10. Study eyes met the following inclusion and exclusion criteria:
Inclusion:
1) Current Procedural Terminology (CPT) code for LTP (65855); 2) All entries between January 1, 2013 and August 31, 2019; 3) Eyes with a glaucoma or glaucoma suspect diagnosis (Supplemental Table 1) 18 not excluded below.
Exclusion:
1) Entries without LTP laterality (coded as “unspecified”) in a patient with two sighted eyes; 2) LTP eye that had angle-closure International Classification of Diseases codes (9th and 10th editions; ICD-9/10): 365.2X, H40.2X; 3) Eyes with no light perception; 4) Eyes without visual acuity and/or pretreatment baseline IOP measurements (defined below) prior to LTP; and 5) Eyes that have reached an “exclusion event,” as defined below.
All data referred to below were for the study eyes, except as noted. The inclusion criteria did not distinguish between selective and argon trabeculoplasty, as the two procedures share the same CPT code. Separate from the glaucoma or glaucoma suspect diagnosis, the presence of “angle recession” (ICD-9 364.77, ICD-10 H21.55X) and/or “uveitis” (ICD-9 364.XX, ICD-10 H20.XX, H30.XX, H44.XX) codes designated the eyes as having “angle recession” and/or “uveitis,” respectively. If neither code was present, since “glaucoma secondary to eye inflammation (H40.43XS),” “glaucoma secondary to ocular inflammation (365.62),” and “glaucoma secondary to drugs (H40.6XX)” were nonspecific and many instances of “glaucoma secondary to drugs” may be due to steroid use to treat ocular inflammation, these diagnoses were grouped as “glaucoma due to inflammation/drugs.” Similarly, since “glaucoma associated with ocular trauma (365.65),” “glaucoma secondary to eye trauma (H40.3),” and “glaucoma secondary to other eye disorders (H40.5XX) may in fact be part of the same continuum (e.g., iris sphincter tear without angle recession), these diagnoses were grouped as “glaucoma due to trauma/other eye disorders.” The lens status of the study eyes were inferred from diagnostic codes. If codes for “pseudophakia,” “aphakia” or “cataract” were present, the eyes were labeled as such. Otherwise, the lens status was categorized as “unknown.” If the glaucoma diagnosis code had specified mild, moderate, severe or indeterminate, the eye was designated as such. If the glaucoma diagnosis code had severity available but no severity information was coded, the glaucoma severity for the eye was designated as “missing/unspecified.” Otherwise, the glaucoma severity was “not applicable.” An eye was considered to have prior glaucoma procedure if, within the IRIS® Registry study period and prior to LTP treatment date, the treated eye had undergone glaucoma procedure (CPT 658XX, 661XX, 665XX, 666XX, 667XX). The number of medications refer to the number of topical or systemic IOP-lowering agents, with fixed-dosed combination medications counted based on their constituent agents. Medications recorded in the IRIS® Registry database are not eye-specific, and every glaucoma medication for a patient was attributed to the study eye. Presence of diabetes/hypertension were based on diagnostic codes and/or presence of medications commonly used to treat diabetes/hypertension.
Defining treatment groups.
Each study eye was classified into one of two groups based on the sequence of LTP procedures. “Treatment” refers to the entirety of the management protocol; “procedure” refers to each individual LTP episode. 1) “Single LTP” was one LTP procedure without an additional LTP within 8 weeks. Treatment Date (TD) was the date of the procedure. 2) “Double LTP” was an initial LTP procedure followed by one or more additional LTP procedures within 8 weeks. Dates of the first and last procedures were recorded, as “early procedure date” (EPD) and “later procedure date” (LPD). LPD was designated as the TD. If more than one TD is available per eye during the IRIS® Registry study period, only the earliest TD was included in the analysis.
Defining IOP baseline and treatment responses.
Pre-treatment baseline IOP was defined as the average of the immediate two (or more if they were all on the same day) measurements prior to LTP TD (before the LTP procedure in “Single LTP,” before EPD in “Double LTP”). Following LTP TD, responders were those who were not censored within the first 8 weeks, but whose first day’s mean IOP measurement on or after 8 weeks post treatment (as long as this IOP measurement was within 6 months of LTP TD) was at or below 80% of the pre-treatment baseline IOP. An analysis of factors associated with responders versus nonresponderes was reported previously 18. Only responders were included in the present analysis.
Defining censoring event.
Censoring occurred on the date following LTP TD when: 1) IOP-lowering medication was added and/or 2) IOP-lowering procedure (CPT 658XX, 661XX, 665XX, 666XX, 667XX) was performed on the study eye (or if procedure laterality was unspecified) and/or 3) cataract surgery (CPT 668XX, 6698X) was performed on the study eye (or if the procedure laterality was unspecified) and/or 4) reaching the end of IRIS® Registry follow up. Since medication is not laterality specific, to censor whenever medication was added ensured a conservative assessment of LTP efficacy. If the IOP-lowering procedure or cataract surgery was coded as “unspecified” laterality, then censoring occurred.
Defining failure event.
Following LTP TD + 8 weeks, when the mean post-LTP IOP of any single day was above 80% of the pre-treatment baseline IOP, a failure event had occurred. If a failure event and censoring event occurred on the same date, the former was declared. The failure criterion was purposefully stringent in order to provide a conservative estimate of LTP response duration. While alternative proposed endpoints, such as obtaining two consecutive IOP measurements above 80% of pre-treatment baseline IOP (one index measurement followed by one confirmatory measurement) may more closely resemble real-world practice patterns, this approach risks overestimating response duration if medical treatment were added after the index measurement in such a way that was not captured by the IRIS® registry (e.g. using a medication previously prescribed only for the fellow eye in the study eye). In this context, “failure event” was considered for its technical meaning particular to this study and not equated as “treatment failure.”
Defining provider status –
the providers were examined and categorized based on number and types of procedures captured by the IRIS® Registry. Group 1 – glaucoma surgeon – at least 25 of trabeculectomy and/or tube shunts each year when there is IRIS® data for the provider (based on Association of University Professors of Ophthalmology/Fellowship Compliance Committee requirement for glaucoma fellowship training; https://aupofcc.org/system/files/resources/2017–08/glaucoma_guidelines.pdf, accessed May 25, 2021). Group 2 – other anterior segment surgeon – those who were not in Group 1 but had at least 85 cataract surgeries each year when there is IRIS® data for the provider. Group 3 – unknown – those who are not Group 1 nor Group 2. Provider LTP count per year was arbitrarily grouped into < 50, 50–99, 100–499, and > 500.
Statistical methods
Continuous data were summarized as mean +/− standard deviation (SD) and/or 5-number summary (5NS: minimum, 25th percentile, median, 75th percentile, and maximum), while categorical data were summarized with counts and/or percentages. Mean times were compared using analysis of variance (ANOVA). Univariable and multivariable Cox proportional hazards regression (proc PHREG) was used to estimate hazard ratios (HRs), and Kaplan-Meier survival analysis was used to produce survival curves. For patients who had bilateral LTP during the study period, the sample include only the first eye that received LTP or a randomly selected eye if a patient had a bilateral LTP on the same date. Each eye for all patients who had unilateral LTP was also included in the sample. All statistical analyses were performed using SAS (Cary, NC) version 9.4. The figures were produced with R version 4.0.2 (https://www.r-project.org/). A p-value of <0.050 was considered to be statistically significant.
Results
Responder baseline and demographics
The initial CPT code search yielded 668,128 eyes. After applying the exclusion criteria, 380,957 eyes were included. The main reasons of exclusion were: no pre-LTP IOP recorded (34.6%), no baseline visual acuity (24.6%), no laterality specified (16.6%), and no sufficient IOP measurements for baseline (12.0%). There were 117,477 eyes categorized as “response-unknown” (excluded from present analysis) and 166,332 categorized as “nonresponders” (excluded form present analysis) and 97,148 (36.9%) were categorized as “responders.”18 From the responder cohort, we included only the first treated eye (if both eyes were treated on different dates) or a randomly selected eye (if both eyes treated on the same date), as well as all unilaterally treated eyes, which resulted in the 79,332 patients/eyes included in the present analysis.
Among the responders, there was a slight female predominance (53.2%), with mean +/− SD age of 71.5 +/− 11.8 years. The majority were white (64.5%) or black (12.0%), and the most common glaucoma diagnoses were POAG (71.2%), glaucoma suspect (20.0%) and unspecified (7.8%). Baseline mean visual acuity was 0.23 +/− 0.29 logarithm of the minimal angle of resolution (logMAR, Snellen equivalence of approximately 20/34), mean IOP was 21.6 +/− 5.3 mmHg on 2.1 +/− 1.5 IOP-lowering medications (Table 1).
Table 1:
Baseline descriptive statistics of the 79, 332 IRIS® Registry LTP responder eyes
| Variable | n | % | |
|---|---|---|---|
| Age | 18–39 | 928 | 1.2% |
| 40–64 | 19,237 | 24.3% | |
| 65–79 | 37,715 | 47.5% | |
| 80+ | 21,090 | 26.6% | |
| Unknown/Missing | 362 | 0.5% | |
| Sex | Male | 36,883 | 46.5% |
| Female | 42,222 | 53.2% | |
| Unknown | 227 | 0.3% | |
| Race | Asian | 1530 | 1.9% |
| Black | 9487 | 12.0% | |
| Unknown | 5779 | 7.3% | |
| White | 51,143 | 64.5% | |
| Hispanic | 3810 | 4.8% | |
| Other/Multi-Racial | 7583 | 9.6% | |
| Insurance | Dual Medicaid & Medicare | 10,196 | 12.9% |
| Medicaid | 1761 | 2.2% | |
| Medicare Advantage | 9140 | 11.5% | |
| Medicare Fee-for-Service | 35,036 | 44.2% | |
| Military | 491 | 0.6% | |
| Other Government | 131 | 0.2% | |
| Private | 16,519 | 20.8% | |
| Unknown/No Payment Listed | 6058 | 7.6% | |
| Region | Midwest | 18,255 | 23.0% |
| Northeast | 14,153 | 17.8% | |
| South | 30,910 | 39.0% | |
| West | 14,258 | 18.0% | |
| Unknown | 1756 | 2.2% | |
| Diabetes | Yes | 14,546 | 18.3% |
| Hypertension | Yes | 3.1% | |
| LTP Type | Single | 77,354 | 97.5% |
| Double | 1978 | 2.5% | |
| Angle Recession | Yes | 46 | 0.1% |
| Uveitis | Yes | 552 | 0.7% |
| Prior Glaucoma Procedure | Yes | 1463 | 1.8% |
| Prior Lens Surgery | Yes | 4978 | 6.3% |
| Prior Intravitreal Injection/Surgery | Yes | 2254 | 2.8% |
| Provider Status | Group 1 | 16118 | 20.3% |
| Group 2 | 33,601 | 42.4% | |
| Group 3 | 28,406 | 35.8% | |
| No Provider Information | 1207 | 1.5% | |
| Provider LTP Count Per Year | 50 or fewer | 30,204 | 38.1% |
| 51–99 | 15,812 | 19.9% | |
| 100–499 | 30,175 | 38.0% | |
| 500 or more | 3141 | 4.0% | |
| Glaucoma Type | Glaucoma Suspect | 15,850 | 20.0% |
| POAG | 56,457 | 71.2% | |
| Trauma/Other Eye Disorders | 408 | 0.5% | |
| Inflammation/Drugs | 297 | 0.4% | |
| Other Glaucoma | 172 | 0.2% | |
| Unspecified Glaucoma | 6148 | 7.8% | |
| Severity | Mild | 13,442 | 16.9% |
| Moderate | 17,566 | 22.1% | |
| Severe | 11,434 | 14.4% | |
| Indeterminate | 2065 | 2.6% | |
| Missing/Unspecified | 12,655 | 16.0% | |
| Not Applicable | 22,170 | 28.0% | |
| Lens Status | Cataract | 25,505 | 32.2% |
| Pseudophakia | 4042 | 5.1% | |
| Aphakia | 219 | 0.3% | |
| Unknown | 49,566 | 62.5% | |
| Mean (SD) | min-max | ||
|
| |||
| Baseline Intraocular pressure (mmHg) | 21.6 (5.3) | 4–68 | |
| Baseline Visual Acuity (LogMAR) | 0.23 (0.3) | −0.12–2.00 | |
| Baseline Number of Glaucoma Medications * | 2.1 (1.5) | 0–7 | |
| Age (years) | 71.5 (11.8) | 18–99 | |
Note: only one eye per patient is included
fixed-dose combination medications were counted as the number of their constituents. IRIS® (Intelligent Research In Sight), LTP (laser trabeculoplasty), LogMAR (logarithm of minimum angle of resolution), min-max (minimum-maximum), POAG (primary open angle glaucoma), SD (standard deviation)
Follow-up and response duration
Median follow-up time (from LTP treatment date + 8 weeks, which was defined as time = 0 for the survival analysis) to the last date in the IRIS® Registry for 79,332 responder eyes was 245 days (5NS: 0, 125, 245, 460, 2315 days; mean of 354.5 +/− 346.6 days).
Overall, 20,423 eyes (25.7%) failed with a median follow-up of 385 days (5NS: 0, 192, 385, 726, 2315 days), while 58,909 (74.3%) were censored with a median follow-up of 217 days (5NS:was 1, 117, 217, 378, 2284 days); 6,625 (8.4%) were censored due to addition of IOP-lowering medications, 3,323 (4.2%) due to additional IOP-lowering procedures, 3,845 (4.8%) due to cataract surgery, 45,116 (56.9%) by reaching the end of IRIS® follow up. Eyes with higher baseline IOP had longer time to failure event compared to eyes with lower baseline IOP (>24 mmHg median 349 days; 18–24 mmHg median 309 days; <18 mmHg median 256 days, p < 0.001for all comparisons). Time to failure event in other subgroups are outlined in Table 2.
Table 2.
Time to failure or censoring event after treatment (days between treatment date and when failure or censor events occurred)
| Mean (SD) | Median | Min-Max | Q1-Q3 | P value | |
|---|---|---|---|---|---|
| For all Eyes | |||||
| Overall | 410.50 (346.62) | 301 | 56–2371 | 181–516 | |
| IOP Categories | <.0001* | ||||
| For baseline IOP < 18 mmHg | 323.80 (250.91) | 256 | 56–2300 | 168–385 | |
| For baseline IOP 18–24 mmHg | 412.08 (338.72) | 309 | 56–2371 | 186–520 | |
| For baseline IOP > 24 mmHg | 480.41 (410.38) | 349 | 56–2360 | 182–644 | |
| Glaucoma Categories | |||||
| Glaucoma Suspect | 464.47 (391.58) | 332 | 56–2371 | 189–608 | |
| POAG | 388.88 (321.21) | 294 | 56–2360 | 178–488 | |
| Trauma | 416.63 (380.65) | 279 | 56–2070 | 149–552 | |
| Inflammation/Drugs | 379.78 (352.02) | 260 | 57–2220 | 158–476 | |
| Other Glaucoma | 390.20 (356.65) | 263.5 | 57–2321 | 175–447.5 | |
| Unspecified Glaucoma | 471.58 (415.76) | 320 | 56–2334 | 188–609 | |
| Angle Recession Status | |||||
| No | 410.53 (346.63) | 301 | 56–2371 | 181–516 | |
| Yes | 362.26 (328.79) | 287.5 | 62–1932 | 153–428 | |
| Uveitis Status | |||||
| No | 411.08 (347.07) | 302 | 56–2371 | 181–517 | |
| Yes | 327.44 (261.10) | 254 | 57–1932 | 144–406 | |
| For eyes in the analysis that reached fail event | |||||
| Overall | 574.85 (453.85) | 441 | 56–2371 | 248–782 | |
| IOP Categories | <.0001* | ||||
| For baseline IOP < 18 mmHg | 431.79 (360.93) | 331 | 56–2300 | 163–567 | |
| For baseline IOP 18–24 mmHg | 563.23 (441.94) | 433 | 56–2371 | 248–758 | |
| For baseline IOP > 24 mmHg | 650.35 (488.54) | 511 | 56–2360 | 291–901 | |
| Glaucoma Categories | |||||
| Glaucoma Suspect | 675.27 (501.94) | 533 | 56–2371 | 279–1008 | |
| POAG | 527.15 (414.69) | 414 | 56–2360 | 238–699 | |
| Trauma | 633.21 (492.04) | 522 | 56–2070 | 215–896 | |
| Inflammation/Drugs | 643.95 (466.90) | 518 | 71–2220 | 324–871 | |
| Other Glaucoma | 603.64 (528.48) | 352 | 57–2321 | 251–830 | |
| Unspecified Glaucoma | 835.20 (599.96) | 744 | 56–2334 | 275–1296 | |
| Angle Recession Status | |||||
| No | 574.99 (453.84) | 441 | 56–2371 | 248–782 | |
| Yes | 411.76 (451.93) | 322 | 66–1932 | 171–390 | |
| Uveitis Status | |||||
| No | 575.58 (454.66) | 441 | 56–2371 | 248–783 | |
| Yes | 489.35 (336.88) | 385.5 | 60–1932 | 244–703 | |
| For eyes in the analysis that did not reach failure event | |||||
| Overall | 353.52 (278.88) | 273 | 57–2340 | 173–434 | |
| IOP Categories | <.0001* | ||||
| For baseline IOP < 18 mmHg | 302.42 (216.49) | 248 | 57–1943 | 168–357 | |
| For baseline IOP 18–24 mmHg | 360.98 (277.77) | 281 | 57–2296 | 180–446 | |
| For baseline IOP > 24 mmHg | 390.86 (328.88) | 290 | 57–2340 | 160–508 | |
| Glaucoma Categories | |||||
| Glaucoma Suspect | 385.09 (305.26) | 293 | 57–2227 | 179–490 | |
| POAG | 339.53 (263.39) | 266 | 57–2340 | 170–414 | |
| Trauma | 328.51 (281.24) | 245.5 | 58–1840 | 133–409 | |
| Inflammation/Drugs | 272.11 (214.96) | 208 | 57–1185 | 125–341 | |
| Other Glaucoma | 327.62 (258.80) | 252 | 57–1604 | 168–385 | |
| Unspecified Glaucoma | 401.37 (326.08) | 297 | 57–2212 | 183–510 | |
| Angle Recession Status | |||||
| No | 353.53 (278.90) | 273 | 57–2340 | 173–434 | |
| Yes | 333.24 (233.82) | 259 | 62–959 | 153–428 | |
| Uveitis Status | |||||
| No | 354.17 (279.32) | 273 | 57–2340 | 173–434 | |
| Yes | 254.16 (174.99) | 208 | 57–992 | 126–329 |
Statistically significant; IOP (intraocular pressure), Min-Max (minimum to maximum range); POAG (primary open angle glaucoma); Q1-Q3 (first and third quartiles); SD (standard deviation). All P values are from analysis of variance.
Responder survival analysis
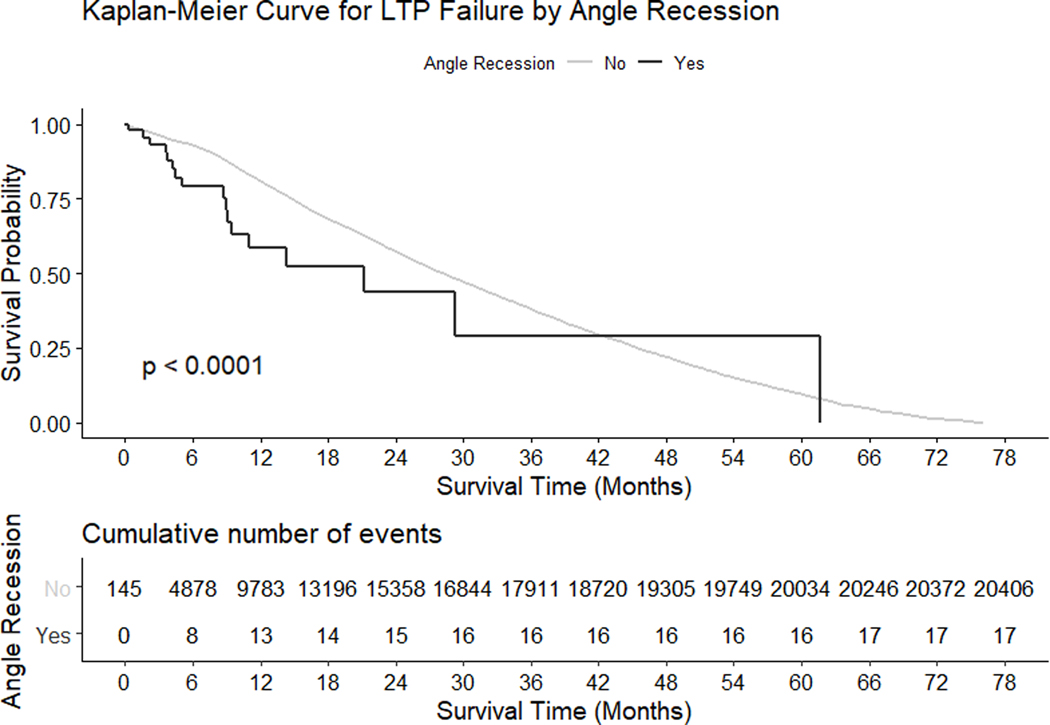
The proportions that reached failure event for the overall cohort at 0, 6, 12, 18 and 24 months following LTP were 0.2%, 6.1%, 16.8%, 29.1% and 40.8%. The proportion of eyes with angle recession and eyes with uveitis that failed at various time points are outlined in Table 3. In univariable analyses (Supplemental Table 2) of the 79,332 responders, angle recession (HR 1.69, 95% confidence interval [CI] 1.05 to 2.73, p = 0.0299) and uveitis (HR 1.80, 95% CI 1.55 to 2.09, p <0.0001) significantly increased the risk of failure (Figures 1 and 2). In multivariable analyses (Table 4), the effects of uveitis remained significant.
Table 3.
Proportion of failure for overall cohort, cohort with angle recession and cohort with uveitis at various timepoints following laser trabeculoplasty treatment.
| Time point following LTP treatment | ||||||
|---|---|---|---|---|---|---|
| 0 month* | 6 months | 12 months | 18 months | 24 months | ||
| Cohort | Overall | 0.2% | 6.1% | 16.8% | 29.1% | 40.8% |
| Angle recession | 0% | 20.9% | 41.6% | 47.5% | 55.2% | |
| Uveitis | 0% | 9.8% | 31.5% | 48.3% | 67.7% | |
Treatment response is assessed after treatment date (TD) + 8 weeks. Any responders who reached failure between TD+8 weeks and TD+12 weeks is considered to have failed after “0 month.” LTP (laser trabeculoplasty).
Figure 1.
Kaplan-Meier survival curves following laser trabeculoplasty (LTP) treatment with and without angle recession. Cumulative number of failure events are shown at the bottom. P-values are from log rank tests.
Figure 2.
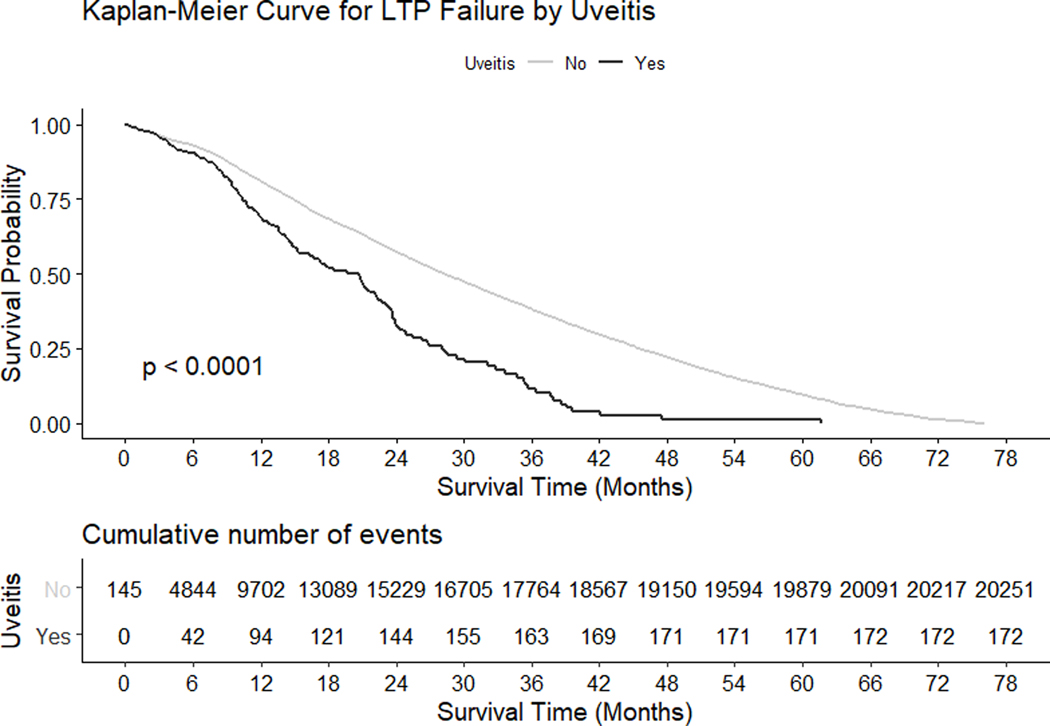
Kaplan-Meier survival curves following laser trabeculoplasty (LTP) treatment with and without uveitis. Cumulative number of failure events are shown at the bottom. P-values are from log rank tests.
Table 4.
Multivariable Survival Analysis of Laser Trabeculoplasty Outcomes
| Parameters | Hazard Ratio | 95% Hazard Ratio Confidence Limits | P value | Comments | ||
|---|---|---|---|---|---|---|
| Age | 18–39 | 1.37 | 1.22 | 1.53 | <.0001* | Age: per patient eye, 5 levels: 18–39, 40–64, 65–79 (reference), 80+ |
| 40–64 | 0.96 | 0.93 | 1.00 | 0.0738 | ||
| 80+ | 1.44 | 1.39 | 1.49 | <.0001* | ||
| Unknown | 1.60 | 1.33 | 1.93 | <.0001* | ||
| Gender | Female | 0.93 | 0.90 | 0.95 | <.0001* | Gender: per patient, 3 levels: male (reference), Female, Unspecified |
| Unspecified | 1.19 | 0.93 | 1.48 | 0.1345 | ||
| Region | Midwest | 0.92 | 0.89 | 0.95 | <.0001* | Region: per patient eye, provider region, 5 levels: Midwest, Northeast, West, South (reference), Unknown |
| Northeast | 0.91 | 0.88 | 0.95 | <.0001* | ||
| West | 0.94 | 0.90 | 0.98 | 0.0046* | ||
| Unknown | 1.22 | 1.05 | 1.42 | 0.0103* | ||
| Race | Asian | 1.15 | 1.04 | 1.28 | 0.0092* | Race: per patient, 6 levels: White (reference), Asian, Black, Hispanic, Multi-Race/Other, Unknown |
| Black | 1.10 | 1.05 | 1.15 | <.0001* | ||
| Hispanic | 0.91 | 0.85 | 0.98 | 0.0088* | ||
| Multi/Other | 0.97 | 0.92 | 1.02 | 0.2722 | ||
| Unknown | 1.54 | 1.46 | 1.62 | <.0001* | ||
| Insurance type | Dual Medicare and Medicaid | 0.72 | 0.68 | 0.76 | <.0001* | Insurance: per patient, 8 levels: Dual Medicare/Medicaid, Medicaid, Medicare Advantage, Medicare Fee for Service, Military, Other Government, Unknown, Private (reference) |
| Medicaid | 1.01 | 0.92 | 1.11 | 0.8087 | ||
| Medicare Adv | 0.82 | 0.77 | 0.86 | <.0001* | ||
| Medicare FFS | 0.77 | 0.74 | 0.80 | <.0001* | ||
| Military | 1.28 | 1.10 | 1.49 | 0.0016* | ||
| Other Government | 1.31 | 0.96 | 1.79 | 0.0864 | ||
| Unknown | 1.13 | 1.07 | 1.19 | <.0001* | ||
| Number of glaucoma medications | 0.96 | 0.95 | 0.97 | <.0001* | ||
| Mean baseline IOP | 1.01 | 1.01 | 1.01 | <.0001* | ||
| Mean baseline VA | 1.18 | 1.13 | 1.24 | <.0001* | ||
| Provider status | Group 2 | 1.10 | 1.05 | 1.14 | <.0001* | Provider Status: per patient eye, 3 levels: Group 1 (glaucoma surgeon) (reference), Group 2 (other anterior segment surgeon), or Group 3 (unknown) |
| Group 3 | 1.25 | 1.20 | 1.31 | <.0001* | ||
| No provider information | 0.72 | 0.60 | 0.88 | 0.001* | ||
| Prior glaucoma procedures | yes | 1.03 | 0.92 | 1.14 | 0.6224 | |
| Prior lens surgery | yes | 1.28 | 1.21 | 1.36 | <.0001* | |
| Prior intravitreal injections | yes | 1.05 | 0.96 | 1.16 | 0.2983 | |
| Diabetes | yes | 1.07 | 1.03 | 1.11 | 0.0003* | |
| Hypertension | yes | 0.93 | 0.85 | 1.01 | 0.0765 | |
| Angle recession | yes | 0.91 | 0.55 | 1.50 | 0.7104 | |
| Uveitis | yes | 1.30 | 1.10 | 1.52 | 0.0009* | |
| Provider LTP count in preceding year | 51–99 | 1.10 | 1.06 | 1.15 | <.0001* | Number of LTP performed by provider in the year preceding treatment date, per patient eye, 4 levels: <50 (reference), 50–99, 100–499, 500+ |
| 100–499 | 1.21 | 1.17 | 1.25 | <.0001* | ||
| 500+ | 1.24 | 1.14 | 1.34 | <.0001* | ||
| Glaucoma type | Inflammation/Drugs | 1.62 | 1.30 | 2.01 | <.0001* | Glaucoma Type: per patient eye, 6 levels: Suspect (reference), POAG, Trauma, Inflammation, Other, Unspecified |
| POAG | 1.71 | 1.64 | 1.79 | <.0001* | ||
| Trauma/Other Eye Disorders | 1.59 | 1.32 | 1.93 | <.0001* | ||
| Other Glaucoma | 1.10 | 0.80 | 1.50 | 0.5712 | ||
| Unspecified Glaucoma | 0.66 | 0.61 | 0.70 | <.0001* | ||
| Glaucoma severity | Moderate | 0.91 | 0.88 | 0.95 | <.0001* | Glaucoma Severity: per patient eye, stage closest to LTP date, 6 levels: Mild (reference), Moderate, Severe, Indeterminate, Unspecified/Missing, Not Applicable |
| Severe | 0.89 | 0.85 | 0.94 | <.0001* | ||
| Indeterminate | 1.06 | 0.98 | 1.16 | 0.1557 | ||
| Missing/Unspecified | 0.37 | 0.35 | 0.39 | <.0001* | ||
| Lens status | Aphakia | 0.76 | 0.59 | 0.98 | 0.0339 | Lens Status: 4 levels: Cataract (reference), Pseudophakia, Aphakia, Unknown |
| Pseudophakia | 0.85 | 0.80 | 0.90 | <.0001* | ||
| Unknown | 0.57 | 0.55 | 0.59 | <.0001* | ||
| LTP treatment group | Double | 1.09 | 1.00 | 1.20 | 0.0589 | LTP treatment in one session (reference) or in two or more session (“double”) |
Statistically significant; Adv (Advantage); FFS (Fee for Service); IOP (intraocular pressure); LTP (laser trabeculoplasty); POAG (primary open angle glaucoma); VA (visual acuity). All P values are from a multivariable Cox proportional hazards regression.
For eyes that were not on any IOP-lowering medications at the time of LTP (n = 1488), they remained medication-free for a median of 197 days (5NS: 57, 106, 197, 395, 2211 days; mean of 317.6 +/− 311.6 days).
Discussion
LTP has been shown to be safe and efficacious as initial glaucoma therapy 4,19, while several studies that compared LTP to medication as initial treatment showed comparable efficacies 2,3,20. The ability to identify factors associated with different response durations in LTP responders is crucial in planning follow-up and setting treatment expectations.
Our findings of 0.2% failure at 6 months, 6.1 % failure at 12 months and 40.8% failure at 24 months are better than previously published literature, which ranged between 25% to 33% failure by 6 months and 47% to 73% failure by 24 months 21–26. This may be due either the IRIS® Registry cohort being older and/or our study defining a technical failure event separate from “treatment failure.” Khawaja et al analyzed a large database of LTP patients and defined failure clinically based insufficient IOP reduction, IOP >21 mmHg or addition of IOP-lowering procedures and/or medications 24. In contrast, our survival analysis included only patients who had initially responded with adequate IOP reduction, such that the “nonresponders” that were excluded from our study would have been counted as “failures” by prior studies.18 Furthermore, since the goal of our study was to assess the longevity of the LTP treatment effect, rather than the ability of LTP to stave off the addition of medications (which can occur despite a 20% or more IOP reduction from LTP), the addition of IOP-lowering medications after LTP treatment was a censoring rather than a failure event. This strategy perhaps better reflects the therapeutic effect of LTP in the context of medication confounders, although it limits the prognostic value of LTP in delaying additional IOP-lowering medication and/or procedures.
The analysis of angle recession and uveitis failures following LTP treatment may imply different mechanisms of trabecular dysfunction in these diseases. At 6 months post-LTP, a larger proportion of angle recession eyes had failed compared to uveitic eyes (20.9% vs 9.8%, Table 3). However, by 18 months, the proportion of failures in both groups were comparable (47.5% angle recession vs 48.3% uveitic). The mechanism behind the earlier failure in eyes with angle recession compared to uveitis remains uncertain, and may be attributed to the different ways the trabecular tissues are affected in angle recession (irreversible metaplasia)27 versus uveitis (partially reversible trabecular dysfunction)28. Furthermore, as IOP fluctuations may be associated with uveitis flareup (not captured by the IRIS® Registry), the LTP failure rates may vary greatly in different types of uveitis. Due to the small proportion of eyes with either angle recession or uveitis in our cohort, the generalizability of these observations remain uncertain.
Previously, we have reported higher odds of favorable IOP responses to LTP treatment with high baseline IOP 18, and the current cohort with highest baseline IOP (>24 mmHg) had the longest time to failure. However, baseline IOP had only a modest effect on time-to-failure (HR of 1.01, 95% CI 1.01 to 1.01, p <0.0001) for eyes that initially responded to LTP. Thus, while the effect of high baseline IOP was significant, we did not feel it be strong enough to be clinically important. This finding is similar a previous multi-center retrospective study in which baseline IOP conferred a failure hazard ratio of 0.96, which is statistically, but not clinically, significant.24
In eyes without medications at baseline, following LTP, they remained medication-free for a median of 197 days (mean 317.6 days). When a medication is added, since laterality is not specified, it may or may not apply to the study eye. Thus, the duration reported here is a conservative estimate with the actual medication-free period to be possibly longer. The cost comparison of LTP versus topical prostaglandins in the United States favors LTP 29, and the IRIS® Registry data suggests that the relative longevity of the LTP treatment effect may have cost-saving implications when performed in medication-free eyes (a subset of which would be as initial therapy in eyes with newly diagnosed glaucoma), in addition to the benefits of the therapy not being compliance-dependent.
This study has several notable limitations. First, as with all studies involving very large sample sizes, many associations that are statistically significant may not necessarily be clinically significant. As there are no accepted consensus on the magnitude of HR that renders a finding clinically significant, we have decided to present the entire output of uni- and multivariable analyses (Supplemental Table 2, Table 4) such that the readers can determine for themselves the importance of each association, while limiting our discussion to a few findings we believed to be particularly relevant clinically. Second, this study is subjected to the limitations inherent to all retrospective cohort studies using large clinical database, namely observational data not subjected to the same rigorous validation as those produced by a clinical trial. Similarly, the variabilities inherent in ICD-9/ICD-10 coding in clinical practice may limit the Registry’s ability to provide precise glaucoma type, stage, and nomenclature beyond the large categories which we have utilized. However, the direct extraction of longitudinal clinical information from the electronic health records at a scale that would not be practical through other means makes IRIS® Registry a useful large-scale real-world database for assessing ophthalmology treatment outcomes and practice patterns. Multiple publications in high-impact journals using the IRIS® Registry and similar registries has established this mode of research as relevant and impactful 15–18,30. Nevertheless, clinicians should recognized the limitations of such registries, as information, selection and confounding biases are possible 31.
In conclusion, this analysis of 79,332 eyes that had undergone LTP in the IRIS® Registry and had initially responded to this treatment revealed a median duration of 385 days (mean 518.9 days) before reaching failure events, with 84.2% survival at 1 year and 59.2% survival at 2 years. Uveitis and angle recession significantly increased the risk of reaching failure events, while eyes with high baseline IOP (>24 mmHg) had the longest survival compared to eyes with lower baseline IOP. Eyes not receiving glaucoma medications at the time of LTP treatment remained medication-free for a median of 197 days (mean 317.6 days). Overall, this data supports offering LTP to medication-free eyes as a means of obviating medication burden in order to optimize medical resource utilization in glaucoma therapeutics. Future studies that analyze LTP practice patterns and implementation lag would facilitate resource optimization in glaucoma therapy.
Supplementary Material
Supplemental Table 1. Diagnosis codes included in the IRIS Registry Laser Trabeculoplasty Study
Supplemental Table 2. Univariable Survival Analysis of Laser Trabeculoplasty Outcomes.
Acknowledgments/Disclosures
Financial Disclosures: No financial disclosures for any of the authors.
Funding/Support: This work was supported the NIH Center Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant, The University of Miami Institute for Advanced Study of the Americas 2019 Pilot Grant and the 2019 American Glaucoma Society IRIS® Registry-AGS Research Initiative Grant.
Footnotes
Relevant Financial Interests: None
Prior Presentations: None
Conflict of interest: no conflicting relationship exists for any author.
References
- 1.Arora KS, Robin AL, Corcoran KJ, Corcoran SL, Ramulu PY. Use of Various Glaucoma Surgeries and Procedures in Medicare Beneficiaries from 1994 to 2012. Ophthalmology. 2015;122(8):1615–1624. [DOI] [PubMed] [Google Scholar]
- 2.Garg A, Vickerstaff V, Nathwani N, et al. Primary Selective Laser Trabeculoplasty for Open-Angle Glaucoma and Ocular Hypertension: Clinical Outcomes, Predictors of Success, and Safety from the Laser in Glaucoma and Ocular Hypertension Trial. Ophthalmology. 2019;126(9):1238–1248. [DOI] [PubMed] [Google Scholar]
- 3.Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet. 2019;393(10180):1505–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Realini T, Shillingford-Ricketts H, Burt D, Balasubramani GK. West Indies Glaucoma Laser Study (WIGLS): 1. 12-Month Efficacy of Selective Laser Trabeculoplasty in Afro-Caribbeans With Glaucoma. Am J Ophthalmol. 2017;184:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stein JD, Kim DD, Peck WW, Giannetti SM, Hutton DW. Cost-effectiveness of medications compared with laser trabeculoplasty in patients with newly diagnosed open-angle glaucoma. Arch Ophthalmol. 2012;130(4):497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantor LB, Katz LJ, Cheng JW, Chen E, Tong KB, Peabody JW. Economic evaluation of medication, laser trabeculoplasty and filtering surgeries in treating patients with glaucoma in the US. Curr Med Res Opin. 2008;24(10):2905–2918. [DOI] [PubMed] [Google Scholar]
- 7.Odberg T, Sandvik L. The medium and long-term efficacy of primary argon laser trabeculoplasty in avoiding topical medication in open angle glaucoma. Acta Ophthalmol Scand. 1999;77(2):176–181. [DOI] [PubMed] [Google Scholar]
- 8.Investigators A. The Advanced Glaucoma Intervention Study (AGIS): 11. Risk factors for failure of trabeculectomy and argon laser trabeculoplasty. Am J Ophthalmol. 2002;134(4):481–498. [DOI] [PubMed] [Google Scholar]
- 9.Stein JD, Zhao PY, Andrews C, Skuta GL. Comparison of Outcomes of Laser Trabeculoplasty Performed by Optometrists vs Ophthalmologists in Oklahoma. JAMA Ophthalmol. 2016;134(10):1095–1101. [DOI] [PubMed] [Google Scholar]
- 10.Chiang MF, Sommer A, Rich WL, Lum F, Parke DW 2nd, The 2016 American Academy of Ophthalmology IRIS((R)) Registry (Intelligent Research in Sight) Database: Characteristics and Methods. Ophthalmology. 2018;125(8):1143–1148. [DOI] [PubMed] [Google Scholar]
- 11.Willis J, Morse L, Vitale S, et al. Treatment Patterns for Myopic Choroidal Neovascularization in the United States: Analysis of the IRIS Registry. Ophthalmology. 2017;124(7):935–943. [DOI] [PubMed] [Google Scholar]
- 12.Rao P, Lum F, Wood K, et al. Real-World Vision in Age-Related Macular Degeneration Patients Treated with Single Anti-VEGF Drug Type for 1 Year in the IRIS Registry. Ophthalmology. 2018;125(4):522–528. [DOI] [PubMed] [Google Scholar]
- 13.Mehta H, Tufail A, Daien V, et al. Real-world outcomes in patients with neovascular age-related macular degeneration treated with intravitreal vascular endothelial growth factor inhibitors. Prog Retin Eye Res. 2018;65:127–146. [DOI] [PubMed] [Google Scholar]
- 14.Atchison EA, Wood KM, Mattox CG, Barry CN, Lum F, MacCumber MW. The Real-World Effect of Intravitreous Anti-Vascular Endothelial Growth Factor Drugs on Intraocular Pressure: An Analysis Using the IRIS Registry. Ophthalmology. 2018;125(5):676–682. [DOI] [PubMed] [Google Scholar]
- 15.Parke DW 3rd, Lum F. Return to the Operating Room after Macular Surgery: IRIS Registry Analysis. Ophthalmology. 2018;125(8):1273–1278. [DOI] [PubMed] [Google Scholar]
- 16.Repka MX, Lum F, Burugapalli B. Strabismus, Strabismus Surgery, and Reoperation Rate in the United States: Analysis from the IRIS Registry. Ophthalmology. 2018;125(10):1646–1653. [DOI] [PubMed] [Google Scholar]
- 17.Pershing S, Lum F, Hsu S, et al. Endophthalmitis after Cataract Surgery in the United States: A Report from the Intelligent Research in Sight Registry, 2013–2017. Ophthalmology. 2019. [DOI] [PubMed] [Google Scholar]
- 18.Chang TC, Parrish RK, Fujino D, Kelly SP, Vanner EA. Factors associated with favorable laser trabeculoplasty response: IRIS Registry Analysis. Am J Ophthalmol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Realini T. Selective laser trabeculoplasty for the management of open-angle glaucoma in St. Lucia. JAMA Ophthalmol 2013;131(3):321–327. [DOI] [PubMed] [Google Scholar]
- 20.The Glaucoma Laser Trial (GLT) and glaucoma laser trial follow-up study: 7. Results. Glaucoma Laser Trial Research Group. Am J Ophthalmol. 1995;120(6):718–731. [DOI] [PubMed] [Google Scholar]
- 21.Nagar M, Luhishi E, Shah N. Intraocular pressure control and fluctuation: the effect of treatment with selective laser trabeculoplasty. Br J Ophthalmol. 2009;93(4):497–501. [DOI] [PubMed] [Google Scholar]
- 22.Kent SS, Hutnik CM, Birt CM, et al. A randomized clinical trial of selective laser trabeculoplasty versus argon laser trabeculoplasty in patients with pseudoexfoliation. J Glaucoma. 2015;24(5):344–347. [DOI] [PubMed] [Google Scholar]
- 23.Martow E, Hutnik CM, Mao A. SLT and adjunctive medical therapy: a prediction rule analysis. J Glaucoma. 2011;20(4):266–270. [DOI] [PubMed] [Google Scholar]
- 24.Khawaja AP, Campbell JH, Kirby N, et al. Real-World Outcomes of Selective Laser Trabeculoplasty in the United Kingdom. Ophthalmology. 2020;127(6):748–757. [DOI] [PubMed] [Google Scholar]
- 25.Weinand FS, Althen F. Long-term clinical results of selective laser trabeculoplasty in the treatment of primary open angle glaucoma. Eur J Ophthalmol. 2006;16(1):100–104. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Birt CM. Argon versus selective laser trabeculoplasty in younger patients: 2-year results. J Glaucoma. 2012;21(2):112–115. [DOI] [PubMed] [Google Scholar]
- 27.Razeghinejad R, Lin MM, Lee D, Katz LJ, Myers JS. Pathophysiology and management of glaucoma and ocular hypertension related to trauma. Surv Ophthalmol. 2020;65(5):530–547. [DOI] [PubMed] [Google Scholar]
- 28.Munoz-Negrete FJ, Moreno-Montanes J, Hernandez-Martinez P, Rebolleda G. Current Approach in the Diagnosis and Management of Uveitic Glaucoma. Biomed Res Int. 2015;2015:742792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao PY, Rahmathullah R, Stagg BC, et al. A Worldwide Price Comparison of Glaucoma Medications, Laser Trabeculoplasty, and Trabeculectomy Surgery. JAMA Ophthalmol. 2018;136(11):1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khawaja AP, Campbell JH, Kirby N, et al. Real-World Outcomes of Selective Laser Trabeculoplasty in the United Kingdom. Ophthalmology. 2019. [DOI] [PubMed] [Google Scholar]
- 31.Nathan H, Pawlik TM. Limitations of claims and registry data in surgical oncology research. Ann Surg Oncol. 2008;15(2):415–423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Diagnosis codes included in the IRIS Registry Laser Trabeculoplasty Study
Supplemental Table 2. Univariable Survival Analysis of Laser Trabeculoplasty Outcomes.