Abstract
In research, policy, and practice, continuous variables are often categorized. Statisticians have generally advised against categorization for many reasons, such as loss of information and precision as well as distortion of estimated statistics. Here we consider a different kind of problem with categorization: the idea that, for a given continuous variable, there is a unique set of cut points that is the objectively correct or best categorization. We show that this is unlikely to be the case because categorized variables typically exist in webs of statistical relationships with other variables. The choice of cut points for a categorized variable can influence the values of many statistics relating that variable to others. We explore the substantive trade-offs that can arise between different possible cut points to categorize a continuous variable, making it difficult to say that any particular categorization is objectively best. Limitations of different approaches to selecting cut points are discussed. Contextual trade-offs may often be an argument against categorization. At the very least, such trade-offs mean that research inferences, or decisions about policy or practice, that involve categorized variables should be framed and acted upon with flexibility and humility.
Keywords: statistics, translational medical science, translational medical research, data analysis, statistical data interpretation
Lay summary:
In research, policy, and practice, continuous variables are often turned into categorical variables using cut points that define the boundaries between categories. This involves choices about how many categories to create and what cut point values to use. Here, I show that different choices about which cut points to use can lead to different sets of trade-offs across multiple statistical relationships between the categorized variable and other variables. These trade-offs mean that no single categorization is objectively best or correct. This context is critical when deciding whether and how to categorize a continuous variable.
Precis:
In practical settings, the choice of cut points to categorize a continuous variable is likely to entail trade-offs across multiple statistical relationships between the categorized variable and other variables. These trade-offs mean that no single categorization is objectively best or correct.
Introduction
Why is diabetes defined as a fasting plasma glucose concentration of at least 126 mg/dL, rather than a value higher or lower1? Why are body mass index cut points of 18.5, 25.0 and 30.0 kg/m2 commonly used to define weight categories of underweight, normal weight, overweight, and obese2? These are questions about categorizing phenomena that are inherently continuous.
Statisticians have generally advised against categorization for many reasons, including loss of information3–11, statistical power9, 10, 12–18, and efficiency7, 13, 19; unrealistic assumptions of constant within-category risks3, 7, 12 and about the nature of dose-response relationships5, 12, 20; biased estimates3, 7, 9, 11–13; the incomplete control of confounding by adjustment for categorized confounders7, 9, 13, 21; exacerbation of problems due to measurement error in the original continuous variable7, 22–25; unrealistic exaggeration of differences between individuals with values just above and just below a cut point15, 18; and a diminished ability to discern or estimate non-linear relationships16, 18. Opinions diverge on when it remains acceptable to categorize. Some believe it is almost never appropriate, at least not until all data collection and analyses are complete and one is ready to interpret15. Others suggest it may be fine in particular circumstances, such as discussions between clinicians and patients about patient health goals, specific fields requiring quick decisions like emergency medicine26, or statistical situations like estimating values from a cumulative distribution function when the true and assumed models are highly different27.
Here, I want to talk about a different kind of problem with categorization: the idea that, for a given continuous variable, there is a unique set of cut points that is the objectively correct or best choice. This is not necessarily a matter of whether to categorize, but of comparing different possible categorizations to each other. The problem arises because most biological, medical, public health, and social science variables exist in webs of statistical relationships with other variables. As we will see, the selection of cut points to categorize one continuous variable can, by itself, simultaneously influence the values of many statistical relationships between that variable and others. When changing the cut points leads to changes in the values of multiple statistical relationships, trade-offs are likely to emerge that make it difficult to say that any particular choice of cut points is objectively best. These trade-offs can then have implications for policy, practice, and scientific inference.
Impact of Cut Point Selection on Analytic Results
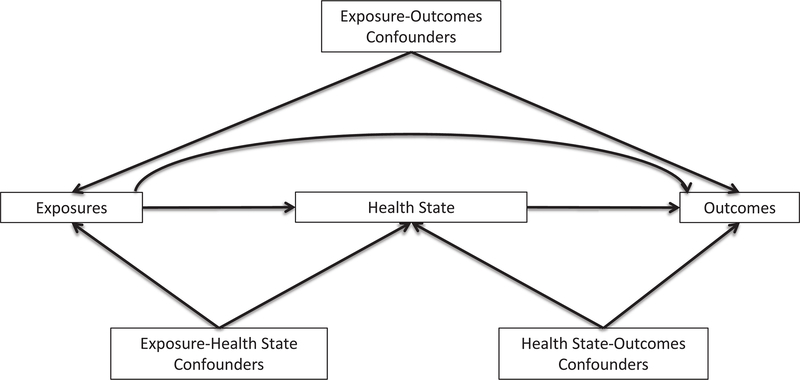
Let’s explore how the choice of cut points can influence the values of statistical relationships between the categorized variable and other variables. For simplicity, most of the examples use dichotomization so that only a single cut point is involved, but the upshot extends to ordinal variables with more than two categories and therefore multiple cut points. To illustrate the context of different variables, Figure 1 depicts a generic health process with hypothetical relationships between population exposures, the distribution of a health state, and outcomes. The left half of the figure—relationships between the exposures and health state—is the etiologic portion of the process, whereas the right half—relationships between the health state and outcomes—is the survivorship portion. Within each of the etiologic and survivorship settings, we may be interested in differing mixtures of causal and predictive analyses.
Figure 1.
A simple process of health or disease
Measures of Association
We begin purely in terms of association. While the following example uses two common types of relative measures of association—odds ratios and hazard ratios—the implications extend to other types of relative measures (e.g. risk ratios) as well as absolute measures (e.g. risk differences).
In an analysis of endometrial cancer patients28, the exposure of immediate concern was body mass index (specifically obesity), the disease state was endometrial tumor expression of the estrogen receptor (ER) biomarker, and the outcome was mortality, of which two kinds were evaluated: 1) time from endometrial cancer diagnosis to all-cause mortality and 2) time from diagnosis to endometrial cancer-specific mortality. ER, measured as the continuous percentage of tumor cells that expressed the marker, was dichotomized as high expression, i.e. above the cut point, or low expression, i.e. below the cut point. Varying the ER cut point changed which tumors were considered to have high expression.
For ER cut points ranging from 0% to 50% in increments of 10%, Table 1 presents estimates for three different associations: between obesity and dichotomous tumor ER status (odds ratios), between ER status and time to all-cause mortality (hazard ratios), and between ER status and time to endometrial cancer-specific mortality (hazard ratios). ER status was the dependent variable in the association with obesity and an independent variable in the associations with mortality. Each estimate represented a result from a different model. From row to row within each type of association—for example, comparing all the obesity-ER models to each other—the only change was the cut point to dichotomize ER. Everything else remained the same, including, as appropriate, the set of participants in the model, the coding of obesity and other variables, and the observed time from diagnosis to mortality.
Table 1.
Associations of endometrial tumor estrogen receptor expression with obesity and with mortality outcomes
| ER Cut Point (%) | Obesity/ER Association | ER/All-Cause Mortality Association | ER/Cancer-Specific Mortality Association | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | CLR | HR | 95% CI | CLR | HR | 95% CI | CLR | |
|
| |||||||||
| 0 | 2.83 | 1.26, 6.37 | 5.06 | 0.62 | 0.29, 1.30 | 4.48 | 0.32 | 0.13, 0.83 | 6.38 |
| 10 | 2.92 | 1.34, 6.33 | 4.72 | 0.61 | 0.30, 1.22 | 4.07 | 0.27 | 0.11, 0.65 | 5.91 |
| 20 | 2.40 | 1.22, 4.74 | 3.89 | 0.55 | 0.30, 1.03 | 3.43 | 0.29 | 0.12, 0.69 | 5.75 |
| 30 | 1.54 | 0.86, 2.75 | 3.20 | 0.55 | 0.31, 0.97 | 3.13 | 0.23 | 0.10, 0.51 | 5.10 |
| 40 | 1.35 | 0.78, 2.36 | 3.03 | 0.55 | 0.31, 0.96 | 3.10 | 0.21 | 0.09, 0.48 | 5.33 |
| 50 | 1.10 | 0.65, 1.87 | 2.88 | 0.59 | 0.34, 1.02 | 3.00 | 0.20 | 0.09, 0.47 | 5.22 |
ER expression measured as continuous percent positive tumor cells (0–100%), then dichotomized at a given cut point (ER+ vs. ER−). ER+ was defined as expression at or above the cut point, except for a cut point of 0% where ER+ was only those above the cut point. Dichotomous ER status was the dependent variable in obesity-ER models and an independent variable in ER-mortality models. The obesity variable was coded as body mass index at or above 30 versus below 30.
Table adapted with permission from Tables 2 and 4 in Busch EL, Crous-Bou M, Prescott J, et al. Endometrial Cancer Risk Factors, Hormone Receptors, and Mortality Prediction. Cancer Epidemiol Biomarkers Prev. 2017;26: 727–735.
CI=confidence interval, CLR=confidence limit ratio (upper limit/lower limit), ER=estrogen receptor, HR=hazard ratio, OR=odds ratio
Inspecting Table 1, we find several consequences of choosing one cut point over other possibilities. First, for each of the three associations, the magnitude and precision of the estimate changed as the cut point changed. For a particular association, one cut point may yield the estimate of greatest magnitude (point estimate furthest from the null value of 1.00) and another yield the best precision (smallest confidence limit ratio). For the obesity-ER association, the greatest magnitude was at an ER cut point of 10% and the most precise estimate was at 50%.
Second, when comparing associations, the cut point with the greatest magnitude for one association may not have the greatest magnitude for other associations. In Table 1, the ER cut point yielding the greatest magnitude was 10% for the association with obesity; a tie between 20%, 30%, and 40% for the association with all-cause mortality; and 50% for the association with endometrial cancer-specific mortality.
Third, while the choice of cut point can influence the numerical values of estimates of association, it can also impact our inferences. Suppose we interpreted the results strictly by statistical significance. Making 10% the cut point would lead to the conclusions that ER expression was associated with obesity and was not associated with all-cause mortality. Had 30% been chosen, we would have reached the opposite conclusions.
Examining multiple cut points revealed a series of trade-offs between them, and this despite confining our attention to one exposure, one measure of the disease state, and two outcomes. The story becomes vastly more complicated when we remember that each node in Figure 1 could include any number of variables and that many processes require more complicated figures with more nodes. The cut points chosen for a variable could potentially affect the magnitude and precision of its association with every variable at every node.
When cut points are identified using a particular dataset to select based on largest effect sizes or smallest p-values, they may not replicate in other datasets3, 29. But even if the final choice of cut points replicates successfully, we would still have to contend with trade-offs such as those shown above.
Other Statistics
Trade-offs between cut point values extend to statistics other than measures of association. Sensitivity and specificity are well-known examples. Table 2 presents a variety of prediction statistics that can vary with the choice of cut points30. The c-index, a measure of discrimination, is the censored-outcomes analog of the area under the receiver operating characteristic (ROC) curve31. The rest of the table consists of various measures from the risk reclassification framework for prediction models31–33. The risk reclassification calibration statistic is a measure of model calibration (larger p-values indicate better calibration), while the event and non-event Net Reclassification Indices (NRI) and Integrated Discrimination Improvement (IDI) are measures of improvement in model discrimination (larger values indicate greater improvement).
Table 2.
Prediction of all-cause mortality after adding E-cadherin measurements to standard diagnostic tests of cancer cell detachment from colorectal primary tumors
| E-cadherin Variable Added to Standard Tests | ||||
|---|---|---|---|---|
| Dichotomous E-cadherin Cut Point | ||||
| Continuous | 0.52 | 0.60 | 0.85 | |
|
| ||||
| C-Index, % (95% CI) | 66 (58, 72) | 51 (41, 59) | 54 (45, 62) | 56 (48, 63) |
| Reclassification Metric | ||||
| Number (%) moved to higher risk category | 47 (25) | 11 (6) | 27 (14) | 41 (22) |
| Number (%) moved to lower risk category | 55 (29) | 93 (49) | 83 (44) | 70 (37) |
| Total number (%) reclassified | 102 (54) | 104 (55) | 110 (59) | 111 (59) |
| Reclassification Calibration Statistic P-value | 0.1 | 0.1 | 0.1 | 0.2 |
| Event Net Reclassification Index, % (95% CI) | 14 (−11, 30) | −22 (−38, −7) | −7 (−23, 10) | 3 (−15, 21) |
| Non-Event Net Reclassification Index, % (95% CI) | 13 (3, 35) | 54 (44, 63) | 41 (29, 52) | 24 (12, 37) |
| Integrated Discrimination Improvement, % (95% CI) | 3.4 (1.9, 5.6) | 4.3 (2.2, 6.8) | 3.4 (1.8, 5.3) | 3.7 (1.7, 5.9) |
E-cadherin measured on a continuous average intensity scale of 0–3, then modeled as either continuous or dichotomized at a given cut point. Each C-index value is for a Cox model of all-cause mortality based on standard diagnostic tests of cancer cell detachment (lymph node evaluation and radiologic imaging) plus the respective E-cadherin variable. Reclassification metrics compare a Cox model of standard diagnostic tests estimating all-cause mortality to a Cox model of standard diagnostic tests plus the respective E-cadherin variable.
Table modified with permission from Table 4 in Busch EL, Don PK, Chu H, et al. Diagnostic accuracy and prediction increment of markers of epithelial-mesenchymal transition to assess cancer cell detachment from primary tumors. BMC Cancer. 2018;18: 82. Published under a CC BY 4.0 license.
As with measures of association, for each statistic, the magnitude can change depending on whether a variable is modeled as continuous or categorized, and if categorized, the magnitude also depends on cut point selection. Comparing the three dichotomization cut points in Table 2 to each other, once again we find trade-offs. A cut point of 0.85 yields the best calibration. In terms of discrimination, 0.85 is best according to the c-index and event NRI, while 0.52 is best according to the non-event NRI and IDI. Furthermore, the event NRI and non-event NRI are best considered together as a pair of values without being collapsed into a single summary measure32. The table illustrates different trade-offs between the event/non-event NRI “pairs”. Which pair should be considered best depends on substantive considerations of the cost of misclassifying a true event compared to misclassifying a true non-event. Similar to association measures, none of these findings would necessarily replicate in other datasets, but if they did, the trade-offs would remain.
Weighing costs also applies to predictive calibration in the form of the relative costs of overprediction versus underprediction. Consider prostate cancer screening using the continuous biomarker prostate-specific antigen. The consequences of underdiagnosing potentially lethal tumors (death) may be more serious than those of overdiagnosing indolent tumors (unnecessary surgeries sometimes leading to complications such as impotence or incontinence), but the costs of overdiagnosis are not negligible and more patients are likely to be overdiagnosed than underdiagnosed34. The trade-off between overdiagnosis and underdiagnosis depends on the cut point defining a positive test. Creating this trade-off by categorizing occurs regardless of whether categorization is done directly on the biomarker measurements or is delayed until after calculating the probability of cancer based on the biomarker and any other predictors. Miscalibration in either direction has costs, and there can be a further trade-off between calibration and discrimination35–37.
Summarizing and extending these observations, Table 3 lists examples of statistical quantities that can be sensitive to the selection of cut point values. The list is not exhaustive.
Table 3.
Quantities sensitive to choices of variable category cut point values
| Number and proportion of units within the category |
| Measures of association (relative and absolute)* |
| Model fit statistics (e.g. AIC, BIC) |
| P-values |
| Hypothesis test statistics |
| Correlations* |
| Splines |
| Sensitivity* |
| Specificity* |
| Positive Predictive Value* |
| Negative Predictive Value* |
| C-statistic, i.e. area under ROC curve* |
| C-index |
| Predicted probability of an outcome |
| Event Net Reclassification Index* |
| Non-Event Net Reclassification Index* |
| Integrated Discrimination Improvement* |
| Reclassification Calibration Statistic* |
| Number and proportion of units reclassified across outcome risk categories |
The list in this table is not exhaustive.
Both magnitude and precision of the measure sensitive to cut point selection.
AIC=Akaike Information Criterion, BIC=Bayesian Information Criterion, ROC=Receiver Operating Characteristic
Limitations of Cut Point Selection Approaches
The choice of cut points might affect the values of many different quantities, but aren’t there several ways to objectively identify the best possible choice? Many statistical tools are thought of this way, but none of them deserves that much deference. We will consider seven approaches to cut point selection: quantiles, model fit statistics, magnitude of effect, statistical significance, mean squared error, ROC curves, and machine learning. An exhaustive, theoretically rigorous discussion will not be attempted here. Instead, practical problems will be illustrated that cast doubt on the notion that any of these tools invariably selects the best or correct cut points in real-world applications.
Perhaps the most common way of setting category cut points is to use quantiles of the distribution, such as the median or quartiles. This approach has at least two problems38. First, since quantile cut points derive from the distribution irrespective of how the categorized variable relates to any other variable, the cut points may not correspond to physically, biologically, or socially important thresholds within the distribution. Second, the observed distribution of the variable could vary from study to study, so that the values defining the quantile cut points in one study may not match their counterparts in another study. This last point means that studies ostensibly categorizing the variable in the same way—say, into quartiles—might not be genuinely comparable. Using a larger number of categories, such as creating a four- or five-category variable rather than dichotomizing, may soften, but not eliminate, the drawbacks of categorization4, 21, 25, 29.
The use of magnitudes of association, statistical significance, model fit, or mean squared error as cut point selection tools is premised on the idea of choosing cut points that, in a certain dataset, provide an extreme (“optimal”) value of a particular statistic: largest magnitude of association or smallest p-value, model fit statistic, or mean-squared error. Such an approach can be biased in the sense that selection is based on a value in a particular direction; in addition, the chosen cut points may not replicate using the same procedures in other datasets3, 7, 9, 11–13, 29. The mean square error has another problem in that it is heavily influenced by outliers, as squaring the terms weights large errors more than small errors39. It is worth noting that statistical significance thresholds such as alpha=0.05 are themselves cut points on a continuum.
The limitations of choosing cut points based on extreme statistical values are illustrated by a study of a candidate biomarker to identify colorectal cancer patients at risk for distant spread of disease, the major cause of cancer-related death40. Prior literature had suggested that at least 25% of the patients should be flagged as high risk41. The biomarker analysis examined multiple cut points to dichotomize patients into high-risk and low-risk groups based on tumor expression of the biomarker. For the association between dichotomous marker status and time to mortality, the best model fit and the largest magnitude of association each corresponded to a cut point that would have flagged fewer than 10% of patients as high risk, much lower than the target proportion. In this case, picking a cut point based on model fit or magnitude of association would lead to a clinical disaster.
The ROC curve is used for research questions about prediction rather than causality. Researchers commonly generate an ROC curve and select the variable cut point corresponding to the most upper-left point of the curve42. However, this choice tells us nothing about model calibration35, absolute levels of risk43, or the proportion of subjects at a given level of risk43. It also assumes that false-negative and false-positive results are of equal consequence, which is unlikely in many applications.
One could also select cut points using various machine learning methods, but their results can still create substantive trade-offs. Machine learning does not erase the biases, errors, or limitations of the data used to develop, test, or validate the model. In addition, categorization algorithms usually have methodologic trade-offs embedded in them because they must account for two competing considerations: first, finding intervals of the continuous variable that are uniform in terms of some attribute of interest (information quality), and second, maintaining enough sample size within each interval to ensure quality estimation (statistical quality)44. Some machine learning methods focus on information quality, others statistical quality, and still others make different intermediate trade-offs between information and statistical quality44. Many categorization algorithms have been developed, such as entropy-based, chi-square-based, Gini, and Fusinter criteria; Bayesian scores; description length; overall percent accuracy in classification; and effect strength for sensitivity44–46. In practice, the best one would normally do is to run multiple learning algorithms on the best available data and hope to obtain similar results. But to claim that machine learning has delivered an inarguably best or correct categorization of a variable requires clearing a high bar. One would need to arrive at the same categorization from each of an exhaustive set of algorithms run in multiple large, unbiased, error-free real-world datasets and repeat this for every relationship of the categorized variable to every other variable in its context.
This brings us to a problem with most approaches to cut point selection. They typically involve choosing a statistic, identifying the cut point values that optimize the statistic—often for the relationship of the categorized variable to just one other variable—and then treating that set of cut points as “objectively” best simply for that reason. Nothing guarantees that the categorization that optimizes any particular statistic will optimize every statistic relating the categorized variable to every other variable in its context, raising the question of why the chosen statistic is the one necessary and sufficient arbiter of the best categorization. Given that the choice of cut points may influence an entire web of statistical relationships between the categorized variable and other variables, a better approach would be to use statistics like those discussed in this section as one of several contributions to evaluating cut points, but not definitive by themselves.
Why the Search for Objectively Best Cut Points May Often Be Pointless
What would make a particular categorization the objectively correct or best choice for a given continuous variable? It would have to optimize every statistical property of every substantively relevant statistical relationship to every other variable in every study of every process in which the variable is involved. Anything less would mean that another choice is better in some way, and then we are dealing with trade-offs and judgment calls.
To illustrate, let’s return to the study of endometrial tumor ER expression. The disease process involves more variables than those mentioned earlier. Besides body mass index, additional exposures include age, hormone therapy use, smoking, parity, oral contraceptive use, and genetic factors. Patient outcomes beyond mortality could be disease progression, response to therapy, recurrence, and return to normal levels of circulating monitoring biomarkers. An objectively best cut point to dichotomize tumor ER status would yield the greatest magnitude and best precision of the associations of ER with each exposure and outcome of interest. That same cut point would also yield the best model fit, minimize the loss function, and be suggested by the dose-response curve for the relationship of the variable with each of the other variables in the process, and so far this might only account for causal analyses. If there are important prediction questions, the cut point would need to optimize every measure of discrimination, calibration, classification accuracy, and improvement in decision making for any prediction models of disease etiology and survivorship. These do not exhaust the statistical requirements that the cut point would need to satisfy to be considered objectively best, and it would also need to outperform every other possible cut point value on all statistical measures in every replication study.
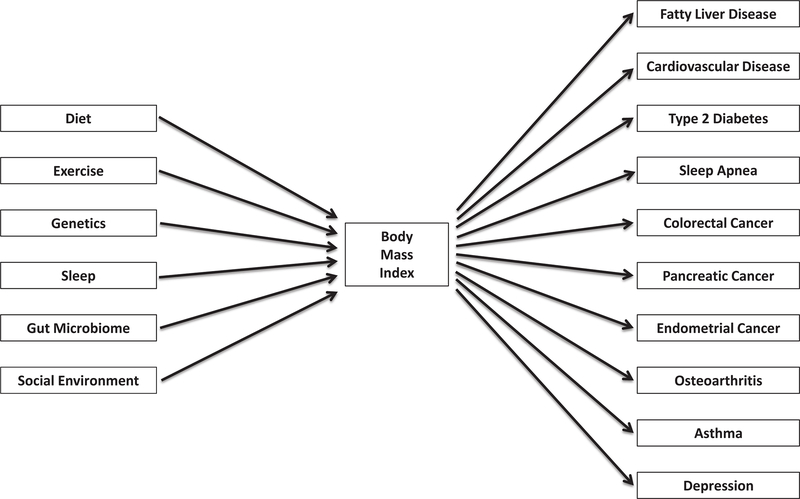
This is easier to see with a variable like ER that is being dichotomized and sits in the middle of a diagram like Figure 1, but the same requirements apply to variables at any diagram node or with multiple cut points. Body mass index, an exposure in the context of endometrial cancer, is commonly categorized into four bins with cut points (in kg/m2) at 18.5, 25.0 and 30.02. The World Health Organization has suggested that relationships between body mass index and a range of health outcomes might vary across different populations47. But even if the stated four-category scheme optimized every statistical relationship between body mass index and other variables for every population in the context of endometrial cancer, for 18.5, 25.0, and 30.0 to be the objectively best set of cut points would require that they also optimize the relationship of body mass index to every other variable upstream and downstream from it in any context. Figure 2 suggests the implausibility that any single categorization of body mass index would optimize every statistical aspect of every causal or predictive relationship in the figure.
Figure 2.
A partial context of body mass index: a sampling of upstream and downstream variables
While perhaps possible in theory, in practice it is extremely unlikely that any particular categorization of a continuous variable can be proven to be objectively best, with no quantitative arguments in favor of any other possibility.
Conclusions
The cut points used to categorize continuous variables can influence our conclusions about causal and predictive relationships, and about whether and how to act for public health, medical, economic, or social purposes. The mathematical sharpness of cut points can obscure a real-life fuzziness, as the choice of cut points for one variable at any part of a process can lead to a complicated cascade of statistical consequences that reverberate throughout that process and other processes involving the variable. Demonstrating the contextual trade-offs entailed by a categorization would be a useful contribution whenever categorization is warranted. The fact that there are likely no objectively correct or best cut points to categorize any particular continuous variable may often amount to an argument against categorization. At the very least, it means that inferences, or decisions about policy or practice, that involve categorized variables should be framed and acted upon with flexibility and humility.
Acknowledgements:
I thank Jennifer Atlas, Justin Busch, Jonathan Epstein, and Charles Poole for helpful feedback on earlier drafts of the manuscript. Thanks to Marta Crous-Bou for assistance with Figure 1. This work was supported by the National Cancer Institute (grant number 5T32CA009001). The sponsor had no role in the conception, composition, submission, or any other aspect of the work.
Financial Support: This work was supported by the National Cancer Institute (grant number 5T32CA009001). The sponsor had no role in the conception, composition, submission, or any other aspect of the work.
Footnotes
Conflicts of Interest: The author declares no competing interests.
References
- 1.Vijan S Type 2 Diabetes. Ann Intern Med. 2019;171: Itc65–itc80. [DOI] [PubMed] [Google Scholar]
- 2.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63: 2985–3023. [DOI] [PubMed] [Google Scholar]
- 3.Altman DG. Problems in dichotomizing continuous variables. Am J Epidemiol. 1994;139: 442–445. [DOI] [PubMed] [Google Scholar]
- 4.Cox DR. Note on Grouping. Journal of the American Statistical Association. 1957;52: 543–547. [Google Scholar]
- 5.Greenland S Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6: 356–365. [DOI] [PubMed] [Google Scholar]
- 6.Kuss O The danger of dichotomizing continuous variables: A visualization. Teaching Statistics. 2013;35: 78–79. [Google Scholar]
- 7.Naggara O, Raymond J, Guilbert F, Roy D, Weill A, Altman DG. Analysis by categorizing or dichotomizing continuous variables is inadvisable: an example from the natural history of unruptured aneurysms. AJNR Am J Neuroradiol. 2011;32: 437–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owen SV, Froman RD. Why carve up your continuous data? Res Nurs Health. 2005;28: 496–503. [DOI] [PubMed] [Google Scholar]
- 9.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25: 127–141. [DOI] [PubMed] [Google Scholar]
- 10.Spruijt B, Vergouwe Y, Nijman RG, Thompson M, Oostenbrink R. Vital signs should be maintained as continuous variables when predicting bacterial infections in febrile children. J Clin Epidemiol. 2013;66: 453–457. [DOI] [PubMed] [Google Scholar]
- 11.van Walraven C, Hart RG. Leave ‘em alone - why continuous variables should be analyzed as such. Neuroepidemiology. 2008;30: 138–139. [DOI] [PubMed] [Google Scholar]
- 12.Bennette C, Vickers A. Against quantiles: categorization of continuous variables in epidemiologic research, and its discontents. BMC Med Res Methodol. 2012;12: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Cohen P, Chen S. Biased odds ratios from dichotomization of age. Stat Med. 2007;26: 3487–3497. [DOI] [PubMed] [Google Scholar]
- 14.Cohen J The Cost of Dichotomization. Applied Psychological Measurement. 1983;7: 249–253. [Google Scholar]
- 15.Dinero TE. Seven reasons why you should not categorize continuous data. J Health Soc Policy. 1996;8: 63–72. [DOI] [PubMed] [Google Scholar]
- 16.Ensor J, Burke DL, Snell KIE, Hemming K, Riley RD. Simulation-based power calculations for planning a two-stage individual participant data meta-analysis. BMC Med Res Methodol. 2018;18: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenland S Avoiding power loss associated with categorization and ordinal scores in dose-response and trend analysis. Epidemiology. 1995;6: 450–454. [DOI] [PubMed] [Google Scholar]
- 18.Riley RD, Debray TPA, Fisher D, et al. Individual participant data meta-analysis to examine interactions between treatment effect and participant-level covariates: Statistical recommendations for conduct and planning. Stat Med. 2020;39: 2115–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Senn S, Julious S. Measurement in clinical trials: a neglected issue for statisticians? Stat Med. 2009;28: 3189–3209. [DOI] [PubMed] [Google Scholar]
- 20.Greenland S Problems in the average-risk interpretation of categorical dose-response analyses. Epidemiology. 1995;6: 563–565. [DOI] [PubMed] [Google Scholar]
- 21.Groenwold RH, Klungel OH, Altman DG, van der Graaf Y, Hoes AW, Moons KG. Adjustment for continuous confounders: an example of how to prevent residual confounding. Cmaj. 2013;185: 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flegal KM, Keyl PM, Nieto FJ. Differential misclassification arising from nondifferential errors in exposure measurement. Am J Epidemiol. 1991;134: 1233–1244. [DOI] [PubMed] [Google Scholar]
- 23.Senn S Individual response to treatment: is it a valid assumption? Bmj. 2004;329: 966–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Smeden M, Lash TL, Groenwold RHH. Reflection on modern methods: five myths about measurement error in epidemiological research. Int J Epidemiol. 2020;49: 338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wacholder S, Dosemeci M, Lubin JH. Blind assignment of exposure does not always prevent differential misclassification. Am J Epidemiol. 1991;134: 433–437. [DOI] [PubMed] [Google Scholar]
- 26.Wynants L, van Smeden M, McLernon DJ, Timmerman D, Steyerberg EW, Van Calster B. Three myths about risk thresholds for prediction models. BMC Med. 2019;17: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fedorov V, Mannino F, Zhang R. Consequences of dichotomization. Pharm Stat. 2009;8: 50–61. [DOI] [PubMed] [Google Scholar]
- 28.Busch EL, Crous-Bou M, Prescott J, et al. Endometrial Cancer Risk Factors, Hormone Receptors, and Mortality Prediction. Cancer Epidemiol Biomarkers Prev. 2017;26: 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst. 1994;86: 829–835. [DOI] [PubMed] [Google Scholar]
- 30.Busch EL, Don PK, Chu H, et al. Diagnostic accuracy and prediction increment of markers of epithelial-mesenchymal transition to assess cancer cell detachment from primary tumors. BMC Cancer. 2018;18: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med. 2009;150: 795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerr KF, Wang Z, Janes H, McClelland RL, Psaty BM, Pepe MS. Net reclassification indices for evaluating risk prediction instruments: a critical review. Epidemiology. 2014;25: 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pencina MJ, D’Agostino RB Sr., D’Agostino RB Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27: 157–172; discussion 207–112. [DOI] [PubMed] [Google Scholar]
- 34.Heijnsdijk EA, Wever EM, Auvinen A, et al. Quality-of-life effects of prostate-specific antigen screening. N Engl J Med. 2012;367: 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115: 928–935. [DOI] [PubMed] [Google Scholar]
- 36.Diamond GA. What price perfection? Calibration and discrimination of clinical prediction models. J Clin Epidemiol. 1992;45: 85–89. [DOI] [PubMed] [Google Scholar]
- 37.Gail MH, Pfeiffer RM. On criteria for evaluating models of absolute risk. Biostatistics. 2005;6: 227–239. [DOI] [PubMed] [Google Scholar]
- 38.Rothman KJ. Six persistent research misconceptions. J Gen Intern Med. 2014;29: 1060–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bermejo S, Cabestany J. Oriented principal component analysis for large margin classifiers. Neural Netw. 2001;14: 1447–1461. [DOI] [PubMed] [Google Scholar]
- 40.Busch EL, Keku TO, Richardson DB, et al. Evaluating markers of epithelial-mesenchymal transition to identify cancer patients at risk for metastatic disease. Clin Exp Metastasis. 2016;33: 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young PE, Womeldorph CM, Johnson EK, et al. Early detection of colorectal cancer recurrence in patients undergoing surgery with curative intent: current status and challenges. J Cancer. 2014;5: 262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akobeng AK. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. 2007;96: 644–647. [DOI] [PubMed] [Google Scholar]
- 43.Janes H, Pepe MS, Gu W. Assessing the value of risk predictions by using risk stratification tables. Ann Intern Med. 2008;149: 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boullé M MODL: A Bayes optimal discretization method for continuous attributes. Machine learning. 2006;65: 131–165. [Google Scholar]
- 45.Linden A, Yarnold PR. Using machine learning to assess covariate balance in matching studies. J Eval Clin Pract. 2016;22: 844–850. [DOI] [PubMed] [Google Scholar]
- 46.Lustgarten JL, Gopalakrishnan V, Grover H, Visweswaran S. Improving classification performance with discretization on biomedical datasets. AMIA Annu Symp Proc. 2008;2008: 445–449. [PMC free article] [PubMed] [Google Scholar]
- 47.WHO. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363: 157–163. [DOI] [PubMed] [Google Scholar]