Abstract
The naked mole-rat (Heterocephalus glaber) has fascinated zoologists for at least half a century. It has also generated considerable biomedical interest not only because of its extraordinary longevity, but also because of unusual protective features (e.g. its tolerance of variable oxygen availability), which may be pertinent to several human disease states, including ischemia/reperfusion injury and neurodegeneration. A recent article entitled ‘Surprisingly long survival of premature conclusions about naked mole-rat biology’ described 28 “myths” which, those authors claimed, are a “perpetuation of beautiful, but falsified, hypotheses” and impede our understanding of this enigmatic mammal. Here, we re-examine each of these “myths” based on evidence published in the scientific literature. Following Braude et al., we argue that these “myths” fall into four main categories: (1) “myths” that would be better described as oversimplifications, some of which persist solely in the popular press; (2) “myths” that are based on incomplete understanding, where more evidence is clearly needed; (3) “myths” where the accumulation of evidence over the years has led to a revision in interpretation, but where there is no significant disagreement among scientists currently working in the field; (4) “myths” where there is a genuine difference in opinion among active researchers, based on alternative interpretations of the available evidence. The term “myth” is particularly inappropriate when applied to competing, evidence-based hypotheses, which form part of the normal evolution of scientific knowledge. Here, we provide a comprehensive critical review of naked mole-rat biology and attempt to clarify some of these misconceptions.
Keywords: naked mole-rat, longevity, hypoxia, cancer, nociception, eusociality, cancer, thermoregulation, ageing, ecology
I. INTRODUCTION
Although naked mole-rats (Heterocephalus glaber) were first described more than 150 years ago (Rüppell, 1842) it is only over the last 40 years that scientists have begun to dig deeply into the many facets of their unusual biology (Buffenstein, Park & Holmes, 2021). The naked mole-rat has not only garnered attention for being a eusocial mammal (Holmes & Goldman, 2021; Jarvis, 1981), highly specialised for life underground through sensory (Vice et al., 2021; Lewin et al., 2021) and ecophysiological adaptations (Buffenstein & Craft, 2021), but it has also been used in biomedical studies. These include research on pain (Eigenbrod et al., 2019; Park et al., 2008) hypoxia tolerance (Larson et al., 2014; Ivy et al., 2020; Park et al., 2021; Park et al., 2017), cardiac function (Grimes et al., 2014b), cancer (Seluanov et al., 2018; Shepard & Kissil, 2020; Miyawaki et al., 2016; Delaney, Imai & Buffenstein, 2021; Tian et al., 2013; Taylor, Milone & Rodriguez, 2017), neuropeptidergic control of reproduction and behaviour (Coen et al., 2021), and ageing (Buffenstein, 2005; Ruby, Smith & Buffenstein, 2018; Lewis & Buffenstein, 2016). Collectively, these studies constitute a new paradigm recognising the naked mole-rat as an important non-traditional biomedical model for understanding how evolutionary processes optimise physiological mechanisms for survival under adverse conditions encountered in challenging habitats. Investigating these evolved systems in eusocial naked mole-rats, which are adapted to crowded, hot, arid, and resource-restricted niches in East Africa, also allows us to understand better mechanistic aspects of several human diseases that share features in common with subterranean ecophysiological stressors (Buffenstein & Ruby, 2021).
A recent review of naked mole-rat biology (Braude et al., 2021) – referred to as ‘Braude et al.’ herein – identified 28 “myths” (see Table 1) relating to naked mole-rats, and concluded that many are a “perpetuation of beautiful, but falsified, hypotheses”, or speculation “perpetuated as fact” (Braude et al., 2021, p. 377). In effect, they challenge numerous aspects of the naked mole-rat paradigm.
Table 1.
A summary of the 28 ostensible “myths” of Braude et al. (2021) regarding naked mole-rat biology and our categorisation based upon available evidence. The origin of each myth is classified as (1) myths that are in headline form and hence represent oversimplifications or are of unknown provenance; (2) suppositions requiring more evidence; (3) former conclusions that have been revised in the scientific literature; and (4) differing interpretations of available evidence.
| Myth | Response | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Myth 1: naked mole-rats are hairless | Naked mole-rats do have a few hairs on their body, but they lack a fur coat. The sparse hairs are specialised sensory structures, similar to facial vibrissae. These hairs are nevertheless fundamentally different from pelage (hair or fur). | x | |||
| Myth 2: naked mole-rats are strictly subterranean and never go above ground | Naked mole-rats have been found above ground, although this is a very rare occurrence, and the cause and purpose of these rare events is unknown. There is no clear evidence that they forage, find mates, or disperse above ground. Rather, their rare appearances on the surface may be due to very rare dispersals or colony displacement from disturbed, breached, or flooded burrows. | x | x | x | |
| Myth 3: naked mole-rats have unusually long burrows | Irrespective of whether naked mole-rats in future studies remain the record holder for the longest burrow system among the social mole-rats, naked mole-rats have exceptionally long burrows for their body size and colony biomass. | x | |||
| Myth 4: naked mole-rats are the only poikilothermic mammals | This myth is predicated on two words - “only” and “poikilothermic”. Some other mole-rat species also show pronounced thermolability. Body temperature (Tb) of naked mole-rats, when given sufficient time to attain thermal equilibrium, closely tracks that of ambient temperature (Ta), being only slightly warmer than Ta. They nevertheless can and do employ endothermy, however this is ineffective at maintaining body temperature outside of a very narrow range of Ta. | x | x | ||
| Myth 5: naked mole-rats have uniquely low thyroid hormone levels | “Unique” is a perilous word, but naked mole-rats certainly have low free thyroxine (T4) levels. However, to date, neither triiodothyronine (T3) level nor the T4:T3 ratio have been reported in the peer-reviewed literature. | x | x | ||
| Myth 6: naked mole-rat burrows are hypoxic and hypercapnic | Measuring underground atmospheres without altering their composition is difficult. To date, oxygen and carbon dioxide levels have not been measured in nest chambers. Burrow composition, organisation, and animal density would suggest that naked mole-rats encounter a range of atmospheric conditions, including hypoxia and hypercapnia, in their daily routine. Also, numerous physiological traits demonstrate that naked mole-rats are extremely well-adapted to both hypoxic and hypercapnic conditions. | x | x | ||
| Myth 7: naked mole-rats are blind | The naked mole-rat visual system is degenerate, with significant atrophy of the structures required for visual image formation. Their visual cortex is primarily responsive to somatosensory inputs. However, some ancillary structures remain intact, and while they can detect changes in luminance, they cannot see images. | x | |||
| Myth 8: naked mole-rats have degenerated hearing |
Compared with surface-dwelling mammals, naked mole-rats have very restricted high-frequency hearing, high auditory thresholds, and very poor sound-localisation abilities. Questions remain about how their low-frequency hearing compares with that of related species. | x | x | ||
| Myth 9: naked mole-rats are the most vocal rodents because they live in large groups | Current evidence does not permit group size to be discounted as an important contributor to vocal complexity; it is likely to be one of several key evolutionary drivers that shaped the extensive range of the naked mole-rat’s vocal repertoire. | x | |||
| Myth 10: naked mole-rats feel no pain | This claim has no basis in the scientific literature. Naked mole-rats do respond to certain noxious stimuli (e.g. heat and mustard oil), but lack responses to others, such as acid and capsaicin. | x | |||
| Myth 11: naked mole-rats are the only eusocial mammals | The definition of the term ‘eusocial’ is controversial and has been extensively debated. Both naked and Damaraland mole-rats appear distinct from other species in that they have very high levels of skew in lifetime reproductive success, but solid evidence for task specialisation among workers is lacking. | x | x | x | x |
| Myth 12: colonies have castes of breeders and non-breeders, involving frequent workers, infrequent workers, non-workers, and dispersers | There is no disagreement about distinct reproductive division of labour, accompanied by some morphological specialisation in breeding queen naked mole-rats. However, there is no conclusive evidence of distinct worker and non-worker castes among non-breeding animals. | x | x | x | x |
| Myth 13: colonies have up to three male breeders (pashas) | Whilst copulation does not guarantee paternity, up to three male consorts have been reported, with evidence from both behavioural and genetic studies. It is not known if there could be more than three breeding males within a colony. | x | x | ||
| Myth 14: colonies have a single queen | Dual queening is well documented, but it is a very rare event both in captivity and in the wild. To date, there has been no recorded incidence of three or more reproductive females within a colony, and most colonies only have a single queen. | x | |||
| Myth 15: not all females can become queens | When isolated from a colony, most new pairs will breed. However, not all females appear capable of attaining and holding the dominant position of queen within a colony. | x | x | ||
| Myth 16: queens suppress workers with pheromones | Initial suggestion that pheromones “may” be involved in suppression was revised in the 1990s as behavioural contact between queen and non-breeders was recognised as key to reproductive suppression. To date, there is no conclusive evidence to suggest a role for pheromones in reproductive suppression. | x | x | ||
| Myth 17: queens shove workers to get them to work | Since the original paper proposing this, this idea has been debunked in multiple studies. | x | |||
| Myth 18: naked mole-rats never leave their natal colonies | Genetic evidence supports outbreeding as vital to the evolutionary ecology of the naked mole-rat. Some dispersal from the natal nest is thus required. | x | x | ||
| Myth 19: naked mole-rats are inbred | While facultative inbreeding does occur in captivity, it is rare in other social mole-rats. While early studies in the 1980s of a single population suggested extreme inbreeding, more recent studies reveal considerable heterozygosity in both wild and captive populations. | x | |||
| Myth 20: the GH/IGF axis is impaired in naked mole-rats | Substantial data support the premise that the GH/IGF axis is indeed dampened in this species. | x | x | x | |
| Myth 21: naked mole-rats are long-lived because they have low oxidative stress and damage | Although naked mole-rats have high levels of oxidative tissue damage, this manifests early in captive life and does not accumulate with age. This suggests that naked mole-rats can efficiently neutralise or repair oxidative damage. Production of reactive oxygen species and antioxidant expression vary among tissues. | x | x | x | |
| Myth 22: naked mole-rat cells do not display cellular senescence | While equivocal findings have been reported, it appears that naked mole-rat cells can undergo stress and oncogene-induced senescence in vitro. | x | |||
| Myth 23: naked mole-rats are immune to disease | Compared to other rodents in managed care, naked mole-rats have fewer documented diseases including age-related dysfunction (e.g. neurodegeneration, heart and kidney disease) and bacterial infections. Rare, lethal viral infections have been reported. | x | x | ||
| Myth 24: naked mole-rats do not get tumours or cancer | Although several cases of cancer have been identified in this species, the incidence of spontaneous neoplasia in naked mole-rats relative to other similarly sized mammals and even humans is extremely low. | x | |||
| Myth 25: naked mole-rats have extremely large hyaluronan | Naked mole-rat hyaluronan is larger than hyaluronan from several other mammals examined and has unusual material properties. Results may differ among research groups due to hyaluronan molecular weight being affected by the isolation procedure. | x | x | x | |
| Myth 26: naked mole-rat cells have early contact inhibition that prevents cancer |
Naked mole-rat cell growth is highly dependent upon tissue culture conditions, which can impede cell proliferation prior to confluence and exhibit contact inhibition properties. Under certain conditions (e.g. daily medium changes), cells grow to high density and show no signs of contact inhibition. | x | x | ||
| Myth 27: naked mole-rats are non-aging | Demographic data show no increased mortality risk as animals increase in age. Also, many physiological phenotypes show negligible age-related changes. These data support the premise of a non-ageing mammal and/or suggest that morbidities are likely compressed into the final years of life. | x | x | ||
| Myth 28: naked mole-rats are the single member of a taxonomic family | Whether the naked mole-rat is a single member of the family Heterocephalidae or a member of the family Bathyergidae that also includes other sub-Saharan mole-rats is controversial. The Sub-Saharan African mole-rats nevertheless appear to be a monophyletic group regardless of whether they are considered a single family or a superfamily. | x | x |
In response, we here re-examine each of these “myths”. We provide a comprehensive critical review and clarifying compilation of our current knowledge about naked mole-rat biology. We re-examine the context of evidence and illuminate certain experimental details that we believe may have been misinterpreted. We argue that many of the so-called “myths” are in fact supported by the available evidence, while others are based on over-simplifications. As correctly pointed out by Braude et al., some incorrect ideas may indeed be propagated in the popular press, although this is generally not the case in the scientific literature. Nascent hypotheses are continuously refined as new data are acquired; such is the nature of the ‘re-’ in ‘research’. There are also ongoing differences in interpretation of data, which are inevitable in an emerging but rapidly growing field. We will demonstrate that, although more work is certainly needed in many areas, we have a much more solid understanding of naked mole-rat biology than implied by Braude et al. (2021).
II. ECOPHYSIOLOGY AND ENVIRONMENT
(1). Myth 1: naked mole-rats are hairless
Both the common name and the scientific name of the naked mole-rat (H. glaber) reflect the fact that these animals, unlike most other mammals, lack an insulatory pelage made of fine, densely packed hairs (Cui et al., 2020). As such, naked mole-rats are ‘naked’ in much the same way as Desmond Morris saw us, in his book The Naked Ape (Morris, 1967).
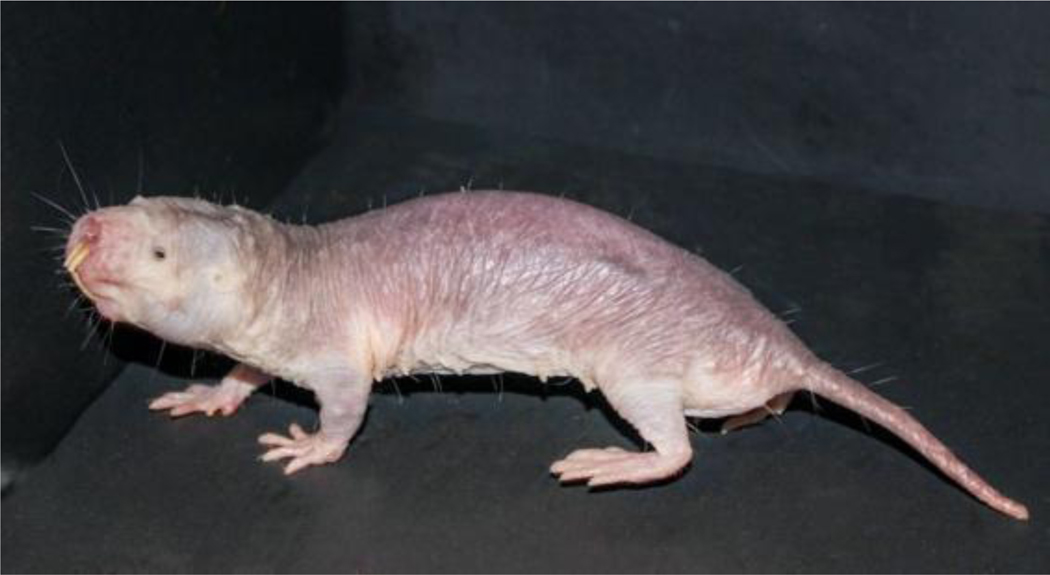
Braude et al. are correct in stating that naked mole-rats are not strictly hairless. They possess carefully arranged grids of sparse, whisker-like guard hairs or vibrissae that criss-cross their body or surround their mouth and ears (Fig. 1). These vibrissae do not have a thermoregulatory role, but rather are specialised, highly innervated sensory tactile organs (Crish et al., 2003). Indeed, Lewin et al. (2021) made direct recordings from sensory afferents innervating these hairs and showed that they have the unusual property of signalling the direction of hair deflection. Sensory afferents innervating these hairs could provide the animal with information about the direction and speed of their movement in narrow tunnels (Lewin et al., 2021). Braude et al. refer to two papers loosely describing naked mole-rats as hairless, but such claims are certainly not widespread in the scientific literature (Daly & Buffenstein, 1998; Crish et al., 2003; Park et al., 2003; Lewin et al., 2021).
Fig. 1.
Sparsely distributed sensory vibrissae, although distributed all over the body, are very different to the fine, dense hair that constitutes the fur of most mammals. Photograph credit: Megan Smith.
(2). Myth 2: naked mole-rats are strictly subterranean and never go above ground
Naked mole-rats have been found in pit-traps or wandering on the surface during fieldwork, revealing that they do occasionally go above ground. As such, we agree that the statement in the subtitle above is untrue. Braude et al. go on to conclude that “while there is no direct evidence of above-ground foraging, naked mole-rats do disperse above ground” (Braude et al., 2021, p. 378). Biological dispersal refers to the movement of individuals from their birth or breeding site to another breeding site. Rare instances of above-ground dispersal were originally proposed by O’Riain, Jarvis & Faulkes (1996) following the discovery of dispersive morphs in captive colonies. However, given their poor eyesight, limited thermoregulatory abilities, very fine, permeable skin, and their inability to jump or hop out of reach of a predator, it is unlikely that these rodents would survive for long above ground in the arid conditions of East Africa. Wandering on the surface to find a mate, pairing, and thereafter excavating a new burrow system, or going in search of a neighbouring open burrow, would be a highly risky and life-threatening strategy. Braude et al. themselves report that they caught only nine mole-rats in pit-traps over the duration of their three-year study. This supports the premise that this is a very rare occurrence (Braude et al., 2020), and may possibly have been due to burrow damage during fieldwork. If animals indeed venture above ground for the purpose of dispersal, this has not yet been proved.
In order better to understand why naked mole-rats might occasionally be found on the surface, we can look at other bathyergids. In over 10 years of fieldwork during both summer and winter seasons, neither Damaraland (Fukomys damarensis) nor common (Cryptomys hottentotus) mole-rats were ever found above ground (N.C. Bennett & J.U.M. Jarvis, personal observations). However, one study reported that numerous Damaraland mole-rats were found in a 2 m deep concrete-lined water channel in Otjiwarongo, Namibia (Hazell et al., 2000). Closer inspection revealed that the concrete edges of the channel formed a barrier to burrowing, thus forcing mole-rats onto the surface where they subsequently fell into the channel. The only mole-rat that appears to be regularly found on the surface is the solitary Cape dune mole-rat (Bathyergus suillus). It is typically found after the long winter rains in a hypothermic state, suggesting that burrow flooding was a causative factor (Bennett & Faulkes, 2000). We therefore believe that there is no strong evidence that either the naked mole-rat or its larger, better-insulated relatives commonly move above ground to disperse or forage.
(3). Myth 3: naked mole-rats have unusually long burrows
The social mole-rats tend to live in burrow systems that may often span several hundred metres or more in total length (Spinks, Bennett & Jarvis, 2000; Bennett, 1988; Herbst & Bennett, 2006; Thomas et al., 2012; Thomas, Swanepoel & Bennett, 2016; Šklíba et al., 2012). Brett reports two naked mole-rat burrows that were meticulously mapped having total tunnel lengths of 3027 m (Kamboyo 1) and 595 m (Lerata 1) (Brett, 1986, 1991). For Kamboyo 1, Brett gives an evidence-based argument (see Brett, 1986) that this is an underestimate and that it was actually more likely to be 3.6–4.0 km. Even at the conservatively reported 3027 m, Kamboyo 1 is the largest mole-rat burrow mapped to date, contradicting what has been claimed for Fukomys mechowii: “Both uncovered burrow systems were very large (total lengths, 2245 m and 743 m), making them the largest burrow systems ever mapped” (Šumbera et al., 2012). Both studies used similar techniques involving radio-tracking and excavation. Šumbera et al. (2012) compare the longer 2245 m F. mechowii burrow to the shorter “Lerata” naked mole-rat burrow of Brett’s dissertation, but overlook the larger “Kamboyo 1” burrow that was described in detail in the same paragraph (Brett, 1986). Furthermore, two of the F. mechowii burrows in Šumbera et al. (2012) were connected through a single blocked tunnel. It is difficult to ascertain if the excavated tunnel was created by one or more groups, potentially biasing those results. Braude et al. attempt to qualify the species comparison by saying that the F. mechowii burrow is the “largest burrow systems ever mapped in relation to biomass” (Braude et al., 2021, p. 380), yet they do not provide the biomass value. Since Šumbera et al. (2012) reported that not all animals were caught and no breeding male was found, it is impossible to determine the biomass of that colony and so a direct comparison with naked mole-rat biomass and burrow length cannot be made. More data are needed before comparisons of average tunnel length and colony biomass can be made between bathyergid species. Since H. glaber is less than half the body mass of F. mechowii, it would be true to say that both in absolute terms and relative to individual animal size, naked mole-rats have the longest tunnel systems recorded to date.
(4). Myth 4: naked mole-rats are the only poikilothermic mammals
All thermoregulatory studies of naked mole-rats agree that they are thermally labile, unable precisely to regulate body temperature (Tb) when isolated from the colony (Withers & Jarvis, 1980; Goldman et al., 1999; McNab, 1966; Buffenstein & Yahav, 1991). The controversy associated with this “myth” revolves around the use of the word “only” and the Greek thermoregulatory terminology used to describe this highly variable Tb. We agree that “only” would be incorrect, as later thermoregulatory studies have revealed that other species of mole-rats when isolated from their colonies also show pronounced thermolability (Bennett, Jarvis & Cotterili, 1993).
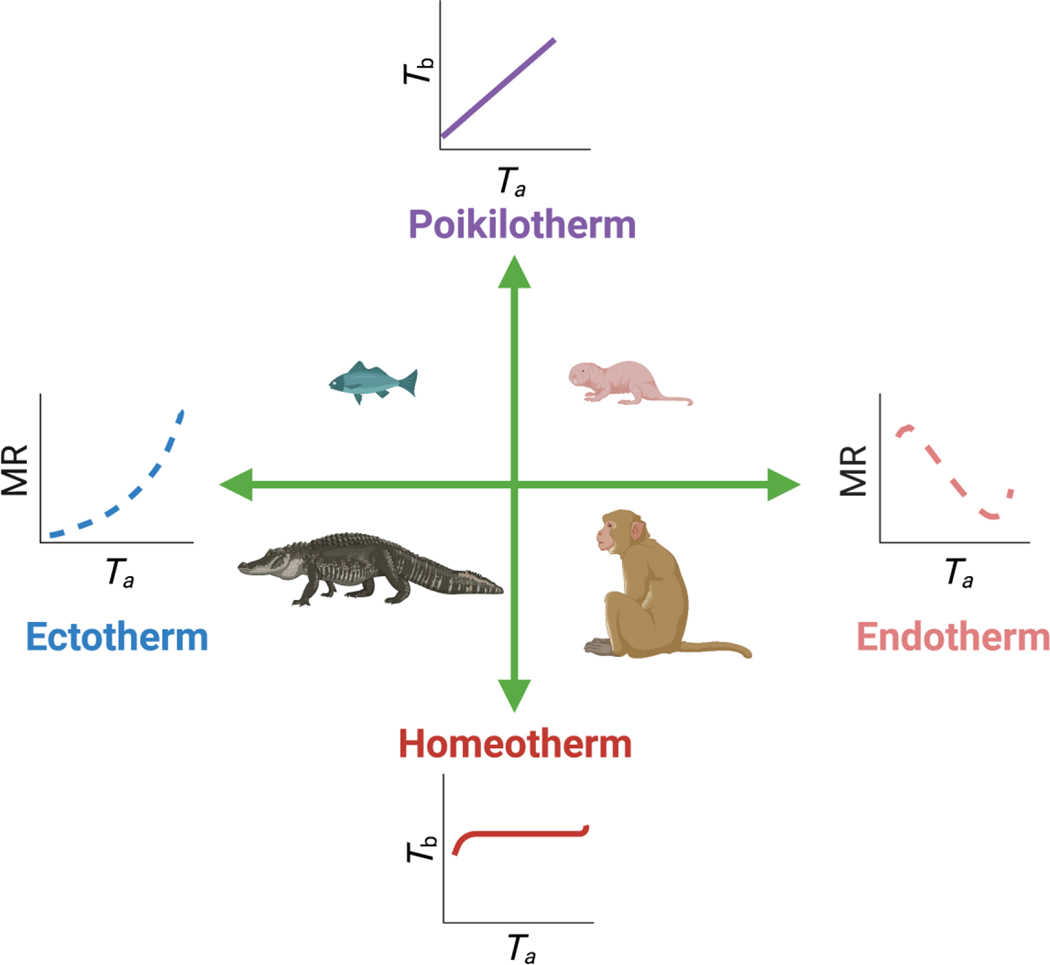
Buffenstein & Yahav (1991) considered the observed naked mole-rat thermoregulatory profile of Tb closely tracking ambient temperature (Ta) (when animals are housed individually in metabolic cages with dried incoming air) to be poikilothermic. The International Union of Physiological Sciences (IUPS) Thermal Commission define poikilothermy as the “large variability of Tb as a function of ambient conditions in organisms without effective autonomic temperature regulation” (IUPS, 2001, p. 263). Braude et al. appear to consider the terms endothermy and poikilothermy as antonyms; however, the antonym of poikilothermy is actually homeothermy, a state where “Tb is maintained within a very narrow range” (<2 °C) (IUPS, 2001, p. 257) whereas endothermy refers to the endogenous mechanisms employed in adaptive heat generation for thermoregulatory purposes (see Fig. 2). Naked mole-rats employ both the ectothermic mechanisms of huddling and basking (under heat lamps or on heating pads) and endothermic heat-generating mechanisms, utilising their considerable depots of brown adipose tissue (BAT) (Oiwa et al., 2020; Daly, Williams & Buffenstein, 1997) for non-shivering thermogenesis (Hislop & Buffenstein, 1994). As such it is undisputed that they employ endothermy. However, this mode of thermoregulation is not very effective in maintaining Tb of most animals when isolated from the colony, although it is generally adequate in larger breeding females (Oiwa et al., 2020; Urison & Buffenstein, 1994). Endothermy may be sufficient to regulate their low Tb (32–34°C) over the narrow range of Tas found in their hot, humid, equatorial burrows and under their hot, humid captive housing conditions. These environmental conditions reduce both the thermal gradient and vapour pressure gradient needed for heat loss. Group housing also reduces the surface area exposed for all avenues of heat exchange (Yahav & Buffenstein, 1991).
Fig. 2.
Thermoregulatory terms explained. Here, antonyms are shown opposite to each other. ‘Homeotherm’ refers to the maintenance of a core body temperature (Tb) within a limited range (<2 °C), whereas ‘poikilotherm’ refers to the thermal state whereby animals show large variability in Tb such that Tb is primarily dependent upon ambient temperature (Ta). ‘Ectothermy’ and ‘endothermy’ refer to the two main mechanisms involved in heat environment, whereas ‘endothermy’ refers to endogenous heat production. Using this cartoon, one can deduce that a crocodile, given its large thermal inertia, is able to maintain homeothermy using ectothermic mechanisms, i.e. shuttling from cooler to warmer areas to maintain body temperature, whereas a monkey primarily uses facultative thermogenesis to maintain a constant body temperature. By contrast, despite employing endothermy, given their large surface area to volume ratio and high rates of heat loss the naked mole-rat cannot regulate Tb effectively outside of a very narrow range of environmental Ta. MR, metabolic rate. Image modified from (Hislop & Buffenstein, 1994). Drawn with biorender.com
Braude et al. state that naked mole-rats should rather be “considered endothermic and partially homeothermic, although with a limited ability to maintain a stable Tb, but not poikilothermic” (Braude et al., 2021, p. 380). In support of their premise that they are “partially homeothermic”, they mention that “skin temperature immediately upon capture was shown to vary mostly within the species thermoneutral zone (TNZ) despite a large temperature range inside the burrows (Holtze et al., 2018)” (Braude et al., 2021, p. 380). This statement is hard to interpret: the TNZ refers to a range of Tas in which facultative thermogenesis is not employed, and not Tb. Further, they mention that burrow temperatures are highly variable and imply that burrow Tas extend beyond the critical limits of the TNZ. Perhaps what the authors were meaning to say is that skin temperature immediately upon capture correlated with that of Tb within the TNZ. Given that warm mole-rat burrows usually have a high relative humidity, thus reducing evaporative cooling at the skin–air interface, and the burrow–skin temperature gradient is also small reducing heat loss by other means (e.g. convection), it is highly likely that animals immediately upon capture would have a skin temperature that approximates core Tb, and that it is less variable than if measured experimentally in metabolic chambers with fast-flowing, dry air. However, this observation cannot be interpreted to mean these animals are “partially homeothermic”. Further compounding this interpretation is the fact that skin temperature (especially that of hands and feet) of most mammals is considerably more variable and generally lower than core Tb.
Braude et al. emphasise that the differential between Tb and Ta varies among studies, ranging between ∼1 and 5 °C (Withers & Jarvis, 1980; McNab, 1966; Buffenstein & Yahav, 1991). That this temperature differential differs among studies is in keeping with differences in experimental protocols; it can be influenced by (a) prior acclimation conditions, (b) the provision and type of nesting material, (c) the flow rate and dryness of air entering the metabolic chamber, (d) the time in the metabolic chamber and if this is sufficient to allow acclimation, and (e) activity levels of the animals in question. For example, the larger differential observed by Withers & Jarvis (1980) reflects that in their study, animals were housed with considerable insulatory nesting material. Furthermore, when cooler temperatures were tested, the experimental duration was shortened, or animals were housed in groups and allowed to huddle, reducing the surface area for heat exchange. Nevertheless, all these studies report pronounced thermolability at Ta below the TNZ.
Further support of the use of the term ‘poikilothermy’ comes from studies in which animals were subjected to prolonged exposure (>1 year) to standard room temperature (23–25 °C). Despite an increase in BAT and free thyroxine (T4), and a considerable time to acclimate to room temperature, the Tb (25–28 °C) of these naked mole-rats was more than 6 °C below the Tb of naked mole-rats when either caught in the wild (32–34 °C) or housed at >27 °C (Tb 32–35 °C) in captivity (Woodley & Buffenstein, 2002; Buffenstein et al., 2001). Not only can naked mole-rats tolerate prolonged periods with such a low Tb, they can even successfully reproduce when their Tb is maintained at such low levels, although interbirth intervals, like that of poikilothermic viviparous reptiles, are extended and fewer and larger pups are born (Woodley & Buffenstein, 2001).
Collectively, the tolerance of a low Tb, as well as their thermoregulatory profile shows that naked mole-rats are indeed closer on the thermoregulatory spectrum to poikilotherms than to homeotherms (for an in-depth review of this topic, see Willmer, Stone & Johnston, 2000). We do agree, however, that in their natural hot and humid milieu, living in large groups they would be able to regulate Tb better and could thus be regarded as stenotherms: animals able to regulate Tb over a very narrow range of Tas. The choice of terminology is clearly an area where there is a difference in opinion among scientists, but the possession of distinct poikilothermic traits is certainly not a “myth”.
(5). Myth 5: naked mole-rats have uniquely low thyroid hormone levels
Thyroid hormone is found in two principal forms: T4 (thyroxine) is the main circulating form, while T3 (triiodothyronine) is more biologically active and is synthesised from T4, by the enzyme iodothyronine deiodinase. Low free T4 levels have been reported in naked mole-rats (Buffenstein et al., 2001; Buffenstein & Pinto, 2009) and Ansell’s mole-rat (Henning et al., 2014), although it is not known if this a shared trait in all mole-rat species. To date, there are no peer-reviewed published studies on T3 in naked mole-rats. Rather, Braude et al. refer to a “news clip” in Science Daily and use this source to state that naked mole-rat T3 profiles are not low and that naked mole-rats have a similarly low T4:T3 ratio to what they observe in F. anselli. This news clip referred to a student interview concerning an upcoming conference poster presentation. That study included preliminary data, which upon repetition of the experiments could not be replicated – a finding attributed to problems encountered with the assay methods used. Given the lack of published data on T3 levels in naked mole-rats, it is premature to conclude that they have a low T4:T3 ratio. Braude et al. go on to state that the resulting T4:T3 ratio, shared by naked mole-rats and F. anselli, is “unique among mammals” (Anderson, Nixon & Akasha, 1988), suggesting that the African mole-rats “have a completely different thyroid hormone physiology” (Braude et al., 2021, p. 380). The statement that either species has a “completely different thyroid hormone physiology” is unfounded. Indeed, it remains to be proved that the T4:T3 ratio observed in captive F. anselli is even typical for Ansell’s mole-rat because, as evident in humans and other mammals, a diet deficient in iodine could explain a low T4:T3 ratio (Kabadi, 1982; Hulbert, 2000). Since iodine is an essential component of thyroid hormone synthesis, less T4 would be made and most of the T4 synthesised would then be converted into the active hormone T3. Measurements of thyroid stimulating hormone or experimental dietary interventions are needed to address this observation.
In summary, captive naked mole-rats do have low thyroid hormone (T4) levels, but they are not unique, sharing this phenotype with F. anselli. However, there is no evidence to suggest that either species has a “completely different thyroid physiology” to other mammals.
(6). Myth 6: naked mole-rat burrows are hypoxic and hypercapnic
The gaseous composition within an underground burrow system may vary considerably. This depends upon many abiotic and biotic factors, including soil depth, compaction and porosity, temperature, moisture, the number of burrow entrances, microorganisms within the soil, and the number of burrow inhabitants, to mention just a few (Buffenstein, 1996). A rarity among mammals, eusocial naked mole-rats live in large family groups consisting of as many as 295 individuals (Brett, 1991). Notably, naked mole-rats tend to collect in large numbers in a single basketball-sized chamber (the nest), possibly to maintain social coherence, defend the matriarch, rest, share resources, and/or keep warm. This behaviour is routinely observed in captivity (Zions et al., 2020; Smith & Buffenstein, 2021). It is reasonable to theorise that so many animals respiring in a confined space would markedly alter underground atmospheric conditions, creating variable hypoxic and hypercapnic conditions (Brett, 1991). Unfortunately, there is very limited published data on the underground climate within the naked mole-rat burrow system (Bennett, Jarvis & Davies, 1988; Holtze et al., 2018), primarily because precise measurements of air composition within burrows, and particularly nests, where large numbers of animals congregate for prolonged periods of time, have proved technically difficult to attain. For example, as outlined in Braude et al., McNab (1966) was unable to obtain accurate microclimate measurements within the naked mole-rat burrow. Subsequent citations of McNab’s work have presumed that the low O2 and high CO2 concentration reported therein for other predominantly solitary fossorial mammals are likely to be similar or exacerbated in eusocial naked mole-rat burrows. A recent study by Holtze et al. (2018) reported only slight variation in ambient O2 and CO2 levels compared to above-ground air sampled directly outside the burrow. However, these measurements were conducted in recently excavated burrows and may not reflect atmospheric conditions in older or deeper burrows, let alone fully occupied nests. Furthermore, these measurements were taken in December – the dry season in Ethiopia – when soils are more permeable to gases than when moist (Buffenstein, 1996; Arieli & Nevo, 1991) and may have resulted in less-extreme conditions than those routinely encountered by naked mole-rats in other seasons. Furthermore, according to Table 1 of Holtze et al. (2018), the number of animals residing in the burrows during measurements ranged from 0 to 3 with a mean of 0.8 across 20 measurements, suggesting that many measurements were made in unoccupied portions of burrows. Absence of respiring individuals likely contributed to their inability to detect environmental hypoxia or hypercapnia. As such, their report may underestimate the true gaseous conditions in occupied naked mole-rat nests.
Although there is sparse information regarding the gaseous composition of mole-rat burrows in nature, a growing body of research has catalogued a remarkable array of adaptations to hypoxic and hypercapnic conditions in naked mole-rats. Astoundingly, these animals can survive 80% CO2 for 5 h, and 18 min in 0% O2, more than 20 times longer than a mouse (Park et al., 2017). Adaptations supporting these capabilities encompass multiple aspects of physiology and biochemistry, suggesting that naked mole-rats have evolved mechanisms to cope with environmental hypoxia and hypercapnia. For example, naked mole-rats have gain-of-function mutations in their hypoxia-inducible factor pathway (Kim et al., 2011), consistent with mutations in most other species adapted to hypoxia across millions of years (Pamenter et al., 2020). They also have smaller brain volumes (Orr et al., 2016), fewer neurons (Herculano-Houzel et al., 2011), lower cardiac function (Grimes et al., 2014b) and lower basal metabolic rates (McNab, 1979), relative to body size and decrease oxygen requirements still further through cessation of thermogenesis (Kirby, Fairman & Pamenter, 2018), lowering heart rate (Pamenter et al., 2019) and metabolic rate (Pamenter, Dzal & Milsom, 2015). This ‘idling on low’ and ability to dial this down even further when acutely exposed to low O2 levels are characteristic of hypoxia-tolerant species.
To survive elevated CO2 levels, naked mole-rats counteract tissue acidosis (Park et al., 2017), show a lack of hypercapnia-triggered increases in ventilation or behaviour (Clayson, Devereaux & Pamenter, 2020), have diminished substance P signalling under hypercapnia, and a decreased CO2 sensitivity of neuronal gap junctions (de Wolf, Cook & Dale, 2017). Correspondingly related to elevated CO2 levels, naked mole-rats show a loss of sensitivity to noxious acidic stimuli via a mutation in the voltage-gated sodium channel NaV1.7 (Smith et al., 2011) which is also found in many hibernating species (Liu et al., 2014), and a novel mutation in the gene encoding the neuronal potassium cotransporter KCC2 (Zions et al., 2020). This energy-saving KCC2 variant makes naked mole-rats dependent on higher CO2 levels to avoid seizures that would otherwise arise due to loss of brain inhibition.
Such remarkable tolerances to hypoxia and hypercapnia, and the multi-faceted nature of the systemic adaptations that support them, suggest that these environmental stressors have applied evolutionary pressure to naked mole-rats. While it is outdated to think that naked mole-rats live in a steady state of hypoxia and hypercapnia, we do believe that the burrow/nest system means these animals likely experience bouts of hypoxia/hypercapnia interspersed with periods of relative normoxia/normocapnia. The statement in the subtitle above, then, likely represents an oversimplification rather than a “myth”.
III. SENSORY ECOLOGY
(1). Myth 7: naked mole-rats are blind
Braude et al. concluded that the naked mole-rat is not blind since they react to bright light flashes and summarised anatomical, physiological, and behavioural studies on naked mole-rats spanning the past 18 years. These studies reveal an exceptionally thin optic nerve, an underdeveloped lateral geniculate nucleus, a visual cortex predominantly receiving input from tactile stimuli, a very disorganised retina and the prevalence of cataracts even in very young animals (Mills & Catania, 2004; Hetling et al., 2005; Catania & Remple, 2002; Xiao, Levitt & Buffenstein, 2006; Crish, Dengler‐Crish & Catania, 2006). While naked mole-rats may be able to detect changes in luminance, they cannot form visual images (Hetling et al., 2005; Nikitina et al., 2004), and have no collicular or cortical response to visual stimuli (Catania & Remple, 2002). Although their poor vision would far exceed the criteria for ‘legal blindness’ in humans and they can accurately be considered functionally blind, we are not aware of any widespread “myth” in the scientific literature that naked mole-rats are literally blind, and unable to distinguish light and dark.
(2). Myth 8: naked mole-rats have degenerated hearing
The behavioural audiogram of the naked mole-rat obtained by Heffner & Heffner (1993) shows a low-frequency hearing limit of 65 Hz, a high-frequency limit of 12.8 kHz [both at 60 dB sound pressure level (SPL)], and a lowest threshold of 35 dB SPL at 4 kHz. These observations were characterised in the title of that paper as “degenerate hearing and sound localization”. The controversy noted by Braude et al. centres on what is meant by “degenerate”. The Oxford English Dictionary (2021; online) defines this word as “Having lost the qualities proper to the race or kind; having declined from a higher to a lower type; hence, declined in character or qualities; debased, degraded”. Having ‘degenerate hearing’ would therefore mean that naked mole-rats had poorer hearing than an ancestral species from which they evolved. In order to establish whether this is so, careful comparisons must be made.
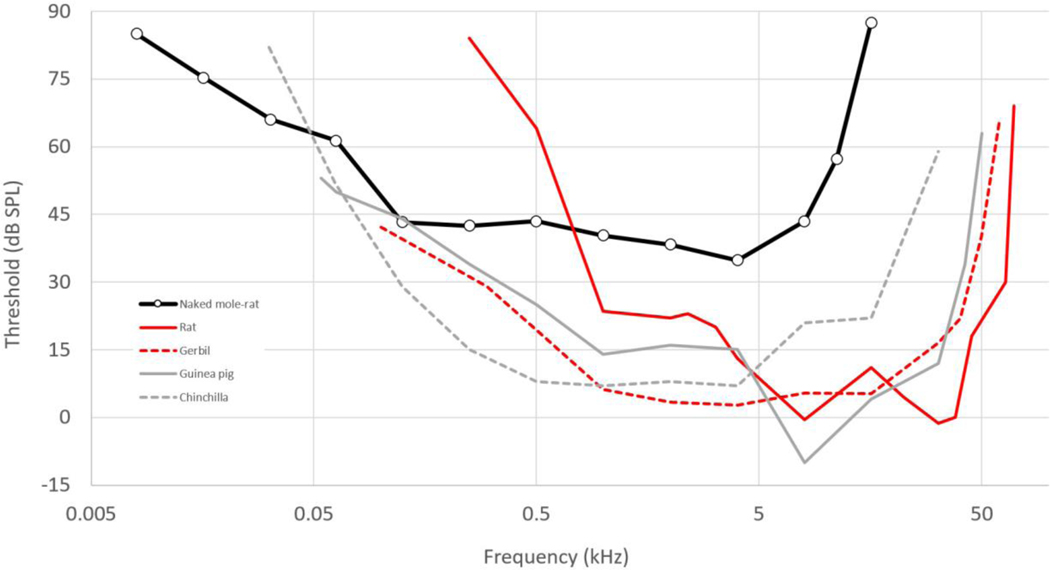
Braude et al. compared the hearing of Heterocephalus with that of the rat. The hearing range of the hooded Norway rat (Rattus norvegicus) at 60 dB SPL extends from 530 Hz to 68 kHz, with lowest thresholds just below 0 dB SPL at 8 and 32 kHz (Heffner et al., 1994). The naked mole-rat’s thresholds are lower than those of the rat at frequencies of 500 Hz and below (Fig. 3), which might be interpreted as a potentially adaptive, low-frequency shift in hearing range. Okanoya et al. (2018), on the other hand, used the gerbil (Meriones unguiculatus) instead of the rat for comparison. Gerbil hearing sensitivity at all frequencies tested (above 100 Hz) is better than that of the naked mole-rat, and it extends to over 50 kHz at 60 dB SPL (Ryan, 1976). The gerbil’s lowest threshold is 30 dB lower than that of the naked mole-rat, at the same best frequency of 4 kHz. Its excellent low-frequency hearing is associated with an expanded middle ear cavity (Ravicz & Rosowski, 1997), a feature commonly found in small mammals from arid regions (Mason, 2016a,b). The middle ear cavity of the naked mole-rat, however, is much less voluminous (Mason, Cornwall & Smith, 2016).
Fig. 3.
The behavioural audiogram of the naked mole-rat in comparison with those of four other rodent species (see text for references). Rats and gerbils are murids; the other species are ctenohystricans.
The problem with both of the above comparisons, however, is that rats and gerbils come from a different rodent clade to Heterocephalus. Guinea pigs and chinchillas, which are much closer relatives, have excellent low-frequency hearing (Fig. 3), more similar to the gerbil than the rat (Heffner, Heffner & Masterton, 1971; Heffner & Heffner, 1991). Both guinea pigs and chinchillas are substantially larger than naked mole-rats; ideally, the hearing of the naked mole-rat should be compared to that of a close relative (i.e. a ctenohystrican rodent) of more similar size. Compared to mammals in general, however, there is no doubt that the high-frequency hearing limit is significantly lower than expected in the naked mole-rat (Heffner, Koay & Heffner, 2001); the threshold at the best frequency is much increased and sound localisation abilities are poorer than in any surface-dwelling mammal (Heffner & Heffner, 1993). This supports the claim that these aspects of hearing are indeed ‘degenerate’, according to the above definition.
Several aspects of naked mole-rat ear morphology are indicative of reduced functionality and may contribute to these limitations (Mason et al., 2016). Recent work showed that these animals have missing or abnormally organised hair bundles on their cochlear outer hair cells (Pyott et al., 2020). The reduced hearing sensitivity of the naked mole-rat may result from the lack of cochlear amplification, which was also demonstrated by Pyott et al. (2020).
Alterations in hearing arise evolutionarily from changes in selection. Reduced high-frequency hearing and sound localisation could in principle arise from loss of selective pressure in the underground acoustic environment. Alternatively, these changes could come about through shifts in selective pressures. Evidence for this has been found at the gene and codon level, for hair bundle proteins involved in cochlear function (Pyott et al., 2020). In arguing that the hearing of naked mole-rats is adapted to their environment, Braude et al. emphasise that their hearing range encompasses the airborne sound frequencies of a few hundred Hertz which propagate best in tunnels (Heth, Frankenberg & Nevo, 1986; Lange et al., 2007; Okanoya et al., 2018). The high thresholds even at these low frequencies have been explained by invoking the so-called ‘stethoscope effect’, whereby certain frequencies may even be amplified (Lange et al., 2007). Based on the transmission of sound in pipes of similar diameter to naked mole-rat tunnels, Okanoya et al. (2018) concluded that the mole-rat should be able to hear conspecific calls over distances of some metres.
In conclusion, the hearing of the naked mole-rat is indeed ‘degenerate’, in that several aspects of its audition have clearly deteriorated from what would reasonably be assumed to be the condition of its terrestrial ancestors. Hence, this is not a “myth”. That is not to say, however, that there are no selective pressures acting on its auditory system. Future studies, concentrating on comparisons with close relatives of Heterocephalus, are needed to establish whether the residual low-frequency hearing of this animal has actually improved and, if so, whether this was driven by demands imposed by its underground niche, and/or the need for intraspecific communication.
(3). Myth 9: naked mole-rats are the most vocal rodents because they live in large groups
“Most vocal” could mean many things, but Braude et al. focus on the extent of the vocal repertoire. In the studies referenced in their paper (Bednářová et al., 2013; Knotkova et al., 2009; Dvořáková, Hrouzková & Šumbera, 2016; Vanden Hole et al., 2014; Pepper, Braude & Lacey, 1991), observational classification by the experimenter was the primary method for determining vocalisation types, making it difficult to standardise comparisons across species and to ensure that inter- and intra-individual variability were appropriately weighted. Vocal complexity should not be strictly defined by the number of vocalisations within an animal’s vocal repertoire: information content contained within each vocalisation must also be weighted (Freeberg, Dunbar & Ord, 2012). Recent work (Barker et al., 2021b), using supervised machine learning-driven classification, demonstrated that complex information can be encoded in a single naked mole-rat vocalisation, and Yosida & Okanoya (2009) demonstrated that when two animals meet head to head in a tunnel, social status affects the number of soft chirps emitted. These studies suggest that the vocal richness of the naked mole-rat extends beyond their number of vocalisations to their ability to modify vocal cues to communicate multiple types of social information. We agree with Braude et al. that previous studies likely underestimated the number of vocalisations used by all members of the African mole-rat family (summarised in Dvořáková et al., 2016). A recent comprehensive review (Barker et al., 2021a) identifies several additional naked mole-rat vocalisations too, expanding the reported repertoire from 17 to 25. Taken together, the current data suggest that the naked mole-rat is one of the most vocally complex rodent species, with a repertoire that even rivals that of primates (see McComb & Semple, 2005). We would not, however, seek to defend the contention that this rodent is necessarily the “most vocal” since this is impossible to define; comparing counts of vocalisation types in a limited number of the more than 4000 species of rodents is specious.
The second part of “Myth 9” refers to the driving force behind this vocal complexity. More robust comparative studies across the other African mole-rats (Bathyergidae) are clearly needed before definitive conclusions about the interplay between social complexity (including group size) and vocal complexity can be made. As such, it is too soon to discount group size as an important contributor to vocal complexity within the Bathyergidae family, but group size is likely to be one of several key evolutionary drivers.
(4). Myth 10: naked mole-rats feel no pain
This is certainly a “myth” circulating in the non-scientific press, but this headline statement has not, it seems, been claimed in scientific articles. Indeed, perhaps the first comprehensive assessment of nociception in this species (Park et al., 2008), is entitled, ‘Selective inflammatory pain insensitivity in the African naked mole-rat (Heterocephalus glaber)’, the key word being ‘selective’. Normal nocifensive responses were reported for noxious heat and mechanical stimuli. Moreover, several detailed analyses from gene to behaviour have mechanistically explained the unusual nocifensive behaviour of this species. For example, Park et al. (2008) showed that naked mole-rats show no nocifensive response to acid, which was later shown likely to result from a genetic variation in a gene encoding NaV1.7 (Smith et al., 2011). The gene variant in question renders the NaV1.7 channel more susceptible to inhibition by acid, thus shutting down action potential firing in nociceptor fibres. The same variant was found in the Cape mole-rat (Georychus capensis) the only one of seven bathyergid species tested that was also insensitive to acid-induced pain (Eigenbrod et al., 2019).
Braude et al. comment that, “contrary to Browe et al., 2020; Griffin, 2008, naked mole-rats can sense pain via Aδ fibres, and respond normally to tissue damage (S. Braude, T.B. Hildebrandt & S. Holtze, personal observations; Browe et al., 2020; Griffin, 2008)” (Braude et al., 2021, p. 382). This is misleading because Browe et al. (2020) do note that, “our behavioural data may reflect, to some extent, activity in neurons other than purinergic C-fibers, namely A-delta fibers” (p. 11); moreover, the article from Griffin is a mini-review that provides no discussion of fibre types, Aδ or otherwise. Furthermore, Park et al. (2008) provide a thorough electrophysiological characterisation of Aδ fibres in the naked mole-rat compared to the mouse, finding a similar proportion of mechanoreceptors (D-hairs) and Aδ-mechanonociceptors, as well as a similar range of von Frey thresholds in both groups (see also Poulson et al., 2020). Thus, to our knowledge, there is no published literature arguing against a role for Aδ fibres in naked mole-rat nociception. To clarify these issues better, we refer readers to a recent and extensive review of published data on naked mole-rat nociception (Smith, Park & Lewin, 2020).
IV. SOCIAL BEHAVIOUR AND REPRODUCTION
(1). Myth 11: naked mole-rats are the only eusocial mammals
This “myth” encompasses two claims, firstly that naked mole-rats are eusocial, and secondly that no other mammals are. There has been much controversy about the use of the term ‘eusocial’ in the mole-rat literature. Originally, the term ‘eusocial’ was coined to include organisms that fitted three criteria: a reproductive division of labour, an overlap of generations, and cooperative care of the young (Michener, 1969). Two species of mole-rats that were extensively studied in the 1980s appeared to meet these criteria, the naked mole-rat and the Damaraland mole-rat (Jarvis, 1981; Bennett & Jarvis, 1988). However, in the 1990s it became apparent that the criteria set to define eusociality in the social insects, including morphological castes, task specialisation for work-related behaviours and even ‘overlapping generations’, did not necessarily apply to mole-rats (Boomsma & Gawne, 2018; Crespi & Yanega, 1995). Lacking the formation of specialised castes prior to sexual maturity, naked mole-rats would be excluded from their very stringent definitions (Crespi & Yanega, 1995). As a consequence, for vertebrate social species the assessment of skew in lifetime reproductive success of an individual was used to distinguish eusocial organisms from those that are social. Laboratory-based studies revealed that fewer than 1% of individual naked mole-rats were able to reproduce (Jarvis, 1991), whereas long-term field studies on marked populations of Damaraland mole-rats estimated that between 10 and 15% of individuals reproduced during their lifetime (Jarvis & Bennett, 1993). This is in marked contrast to the social insects – bees and termites – where a negligible number of individuals actually reproduce. These differences in reproductive skew were attributed to divergent developmental pathways, noting that complete metamorphosis in bees and ants allowed for greater specialisation for helping or breeding and hence higher levels of skew (Beekman, Peeters & O’Riain, 2006). In the 1990s and early 2000s, morphological and physiological castes were proposed for both breeding female naked mole-rats and Damaraland mole-rats and for non-breeding Damaraland mole-rats (O’Riain et al., 2000; Young & Bennett, 2010; Scantlebury et al., 2006). However, disagreements about the use of the terms ‘eusocial’, ‘caste’, ‘worker’ and ‘non-worker’ have continued until today (see Zöttl et al., 2016; Thorley et al., 2018; Gilbert, Rossiter & Faulkes, 2020). While there may not be task specialisation in Damaraland mole-rats, it does appear that animals change in the frequency of working behaviours with age (Thorley et al., 2018). Whether the naked mole-rat can be regarded as the only ‘eusocial’ mammal therefore depends on the preferred definition of that term. Indeed, Jarvis & Bennett (1993) have suggested that the Damaraland mole-rat has also been considered eusocial. If eusociality is taken to encompass a multifactorial spectrum, naked mole-rats would certainly be on it. Other social bathyergid species would be on that same spectrum too, although their smaller colony sizes and smaller skew in lifetime reproductive success would attest to a lower degree of eusociality.
(2). Myth 12: colonies have castes of breeders and non-breeders, involving frequent workers, infrequent workers, non-workers, and dispersers
As pointed out by Braude et al., there is a distinct reproductive division of labour with some morphological specialisation in breeding queen naked mole-rats (O’Riain et al., 2000), so it is puzzling that this forms part of the above “myth”. Queens become irreversibly morphologically distinct from female non-breeders (O’Riain et al., 2000), but no such morphological distinction is apparent amongst the rest of the colony members, as discussed in both earlier (Jarvis, O’Riain & McDaid, 1991) and more recent research (Gilbert et al., 2020). However, the degree to which behavioural specialisation reflects subcastes among non-breeding animals is still an area of active research and debate. The notion of frequent and infrequent worker castes mentioned in Jarvis’ seminal paper (Jarvis, 1981) had already been questioned and modified as long ago as 1991, where evidence of age and body mass polyethisms were presented for some colonies (Lacey & Sherman, 1991; Faulkes & Abbott, 1991; Jarvis et al., 1991). All specialisations observed are behavioural and varied along a continuum, e.g. defence and work-related activities (O’Riain & Faulkes, 2008; O’Riain & Jarvis, 1997). Recent, more extensive studies also report that tasks such as pup care behaviour, working behaviour, colony defence, and dispersal-like behaviour are unevenly distributed among non-breeding mole-rats and somewhat stable among individuals across time (Toor et al., 2020; Mooney et al., 2015). Positive relationships between age and colony defence, and negative relationships between age and pup carrying, were also reported (Mooney et al., 2015). Gilbert et al. (2020) further highlight the complexity and variability in worker behaviour allocation. With regard to a “disperser caste”, distinctive disperser males have been shown to exist in laboratory colonies (O’Riain, Jarvis & Faulkes, 1996) and in the field (Braude, 2000). A recent follow-up study on laboratory colonies replicated some components of the putative disperser subcaste, with a subset of larger/heavier non-breeders showing motivation to leave the colony (Toor et al., 2020). Here, females were also noted to leave the colony in equal numbers, similar to what was reported in wild naked mole-rats (Braude, 2000). It is still unclear to what extent naked mole-rat subordinates show behavioural specialisation and if the increase in body fat that characterises the dispersive morph phenotype is simply a consequence of advancing age and preparing to disperse (Siegmann et al., 2021). These types of questions will only be resolved with considerably more field data examining life-history traits and cooperative behaviours in their natural milieu. Moreover, it will be essential to determine the ontogeny and perpetuation of individual differences in the diverse behaviours of non-breeders fully to answer the question of whether true non-breeder sub-castes can be considered to exist.
(3). Myth 13: colonies have up to three male breeders (pashas)
The “myth” regarding multi-male mating by specific “breeders” that form a long-term bond with the queen is obscure. As Braude et al. correctly state, copulation itself does not guarantee paternity, and relatively few parentage studies utilising genetic markers have been conducted in naked mole-rats. However, in a study examining two different litters within a colony, unequivocal genetic evidence revealed at least two males fathering the offspring within these litters, while a third putative breeding male could not be definitively excluded as a father (Faulkes et al., 1997). Clearly, there may be more than one breeding male in a colony, and generally the same few males are observed mating with the queen. However, there is no evidence to say that three is the upper limit, and hence this is not a “myth”.
(4). Myth 14: colonies have a single queen
Evidence of dual queening has been reported many times; see Faulkes & Bennett (2009) for an extensive review on this topic. Jarvis (1991) in the book The Biology of the Naked Mole-rat provides percentages of single and dual queens in her large collection of naked mole-rat colonies. However, in her 1980 collecting field trip in Kenya, all the colonies Jarvis trapped only had one breeding female. Similarly, over more than 25 years of fieldwork, only two instances of plural breeding were reported in 23 monitored colonies (Braude, 1991). In one of those colonies, the dual queens were both present a year later. The Buffenstein group has seen plural breeding occur seven times over a 10-year period. All these instances of plural breeding occurred within only three out of 326 colonies (comprising 7,542 individual mole-rats; R. Buffenstein, personal observations). Similarly, the Holmes group has observed dual breeding in only two out of over 100 colonies (M. Holmes, personal observations). To date, in more than 40 years of maintaining naked mole-rats in multiple laboratories, no one has reported any colony with three or more reproductive females. In all other social mole-rats there are no multiple queens on record, but the colonies are much smaller (Bennett & Faulkes, 2000). Dual queening is clearly a relatively rare event both in captivity and in the wild, supporting the premise that most animals within a colony are reproductively suppressed. “Myth 14” therefore represents an oversimplification rather than a complete falsehood.
(5). Myth 15: not all females can become queens
Some individual females of any mammalian species will fail to breed successfully, so it must be trivially true that not all female naked mole-rats can become queens. What this “myth” really focuses on is whether there is a particular physiological condition which precludes breeding in some Heterocephalus females, which might serve some adaptive purpose. Beekman et al. (2006) classified all social vertebrates, including naked mole-rats, as totipotent, i.e. having the potential at birth to become either a breeder or non-breeder. Despite this, there is substantial variability in reproductive maturation of non-breeding females. Some high-ranking, non-breeding females that are contenders for the queen position may activate while within the colony and usurp the queen by killing her. If subordinate females, 6 months of age or older, are removed from the colony and paired many, but not necessarily all, transition to breeders; some inevitably fail to reproduce. The Buffenstein, Holmes, Park and Smith groups commonly have a 50% pairing success if we randomly pair up individuals. Success can get closer to 100% if we carefully choose the females to remove from colonies for pairing. In this regard, we check if the female’ vagina becomes perforate when the queen is gravid, and screen for aggressive behaviour (Smith & Buffenstein, 2021). Some pairs successfully reproduce within three months after being paired. Given their long gestation, this implies that they come into oestrus within a week of being removed from the queen, but others take considerably longer (we have had a pair produce their first litter 2.5 years after pairing), and some pairs may never breed. Understanding the factors contributing to this variability is a focus of current research. Clearly, it could never be proved that all naked mole-rat females could potentially become queens. Given our current knowledge, “not all females can become queens” is not so much a “myth” as a working hypothesis.
(6). Myth 16: queens suppress workers with pheromones
This “myth” is better described as a rejected hypothesis. As Braude et al. correctly point out, Jarvis (1981) in the concluding comments of her seminal paper suggests “pheromones may be involved in caste determination” (p. 573). Given the wealth of literature on rodent pheromones at the time this paper was published, and the fact that naked mole-rats use a communal toilet chamber, a role for urinary primer pheromones emitted by the queen in suppressing reproduction in the non-breeders was a reasonable hypothesis to propose. Experimental investigation of the role of chemosignals in reproductive suppression in the late 1980s and early 1990s found no evidence for primer pheromones blocking reproduction, but instead implicated direct behavioural contact with the breeding queen (Faulkes & Abbott, 1993; Smith, Faulkes & Abbott, 1997). The Faulkes, Abbott & Jarvis (1990) paper referred to by Braude et al. to support the notion of social control of reproduction is not the correct citation in the context of whether or not pheromones are involved in reproductive suppression; this paper describes the physiology of suppression. With the exception of a BBC documentary (‘Supersense’) broadcast in 1989 that ignored the prevailing evidence and suggested a role for pheromones, pheromonal suppression of reproduction in naked mole-rats has to our knowledge never been “mythologised”.
One recent paper of relevance to this discussion is that of Watarai et al. (2018). Faeces from the pregnant queen contained abundant oestrogen; non-breeders fed this faeces increased their urinary oestrogen concentration, and their excreted faeces also contained abundant oestrogen. The authors proposed that faecal steroid consumption influences helping behaviour, which Braude et al. discount as a form of pheromonal transfer. However, faecal steroids fit the definition of pheromones in that they are transferred to conspecifics via the external environment, inducing a specific reaction. Transfer of oestrogen in this manner might in principle have a similar reproduction-suppressing effect to its use in the combined contraceptive pill, although there is no direct evidence for this at present. Braude et al. state that “there is no evidence that naked mole-rat non-breeders ever consume queen faeces either in the wild or the laboratory setting” (Braude et al., 2021, p. 384). However, both the queen and her subordinates practice coprophagy, eating faeces previously dropped by other colony members, and are commonly observed ‘begging’ for faeces from other members of their colony, which are then voided upon demand (Smith & Buffenstein, 2021). Although additional experimentation is needed, it is not impossible to believe that faeces of breeding females might be consumed by non-breeders, and that this could potentially contribute to reproductive suppression.
(7). Myth 17: queens shove workers to get them to work
Only one study proposed this (Reeve, 1992). While there are several citations of this paper to support the notion that subordinates in cooperative species are coerced into carrying out burrow maintenance and foraging activities, several subsequent studies have failed to support this idea (Clarke & Faulkes, 2001; Jacobs & Jarvis, 1996). Therefore, this bullying-to-work for the colony could be considered a “myth”.
(8). Myth 18: naked mole-rats never leave their natal colonies
This “myth” was clearly debunked in the references Braude et al. cited in support of mole-rats not being strictly subterranean (Myth 2). While most naked mole-rats seldom leave the colony of their birth, dispersive morphs with distinct features have been reported (O’Riain et al., 1996) and dispersal has been discussed in some detail in a study on their ecology (Brett, 1991) as well as throughout the seminal book The Biology of the Naked Mole-rat (Sherman, Jarvis & Alexander, 1991). Indeed, while direct evidence has yet to be acquired, all of the recent behavioural and genetic work supports the hypothesis that dispersal is an integral component of the ecology and evolution of this species (Braude, 2000; Ingram et al., 2015; Toor et al., 2020). Although more data are needed as to how and when dispersal occurs, and by which animals, there is no “myth” in this regard.
(9). Myth 19: naked mole-rats are inbred
It is undisputed that naked mole-rats are facultative inbreeders, a trait that sets them apart from other African mole-rats. However, the initial evidence supporting extreme inbreeding has long been dismissed as more data, using more sophisticated techniques, have become available (Ingram et al., 2015; Chau et al., 2018). As Braude et al. point out, “consistent with Burda (1995), Clarke & Faulkes (1999) found that queens prefer to mate with unrelated males, and Ciszek (2000) demonstrated that naked mole-rats avoid mating with siblings” (Braude et al., 2021, p. 384). The Burda paper referred to in this quotation is only of indirect relevance as it does not refer to naked mole-rats but rather to “common mole-rats”, most likely a species of Fukomys.
V. DEVELOPMENT, LONGEVITY, AGEING AND SENESCENCE
(1). Myth 20: the GH/IGF axis is impaired in naked mole-rats
In mammals, systemic insulin-like growth factor (IGF1) levels are controlled by growth hormone (GH) as part of the somatotropic hormone axis. This hormone system regulates postnatal organismal growth, body composition and metabolism (Yakar, Werner & Rosen, 2018). Although direct functional characterisation of this ancestral hormone system in naked mole-rats has not yet been investigated, the somatotropic endocrine axis undoubtedly operates in this species and there is a great deal of indirect evidence (see below) suggesting that it is downregulated. Mole-rats have exceptionally slow growth rates both in utero and postnatally (Buffenstein et al., 2020). Their gestation is threefold longer than that of wild-type mice and they exhibit postnatal somatic growth that continues for at least 18-months (O’Riain & Jarvis, 1998). Naked mole-rats share several other characteristics with GH/IGF1 mutant mice (Zhou et al., 1997). These include (a) their small size, relative to other sub-Saharan mole-rats (Bennett & Faulkes, 2000), (b) sharply reduced rates of tumorigenesis (Junnila et al., 2013; Seluanov et al., 2018), (c) increased insulin sensitivity (Junnila et al., 2013; Kramer & Buffenstein, 2004) coupled, remarkably, with high fat/lean mass ratios (O’Connor et al., 2002; Berryman et al., 2004), (d) resistance of isolated fibroblasts to physical and chemical stressors (Lewis et al., 2012; Salmon et al., 2005), which likely stems from increased activity of nuclear factor erythroid 2-related factor 2 (NRF2) in both dwarf mice and naked mole-rats (Lewis et al., 2015; Leiser & Miller, 2010), (e) a circulating metabolomic signature reminiscent of fasting or hibernating mammals (Lewis, Rubinstein & Buffenstein, 2018), and (f) slow age-related declines of youthful phenotypes (Masternak et al., 2018; Edrey et al., 2012; Buffenstein, 2008; Buffenstein et al., 2020).
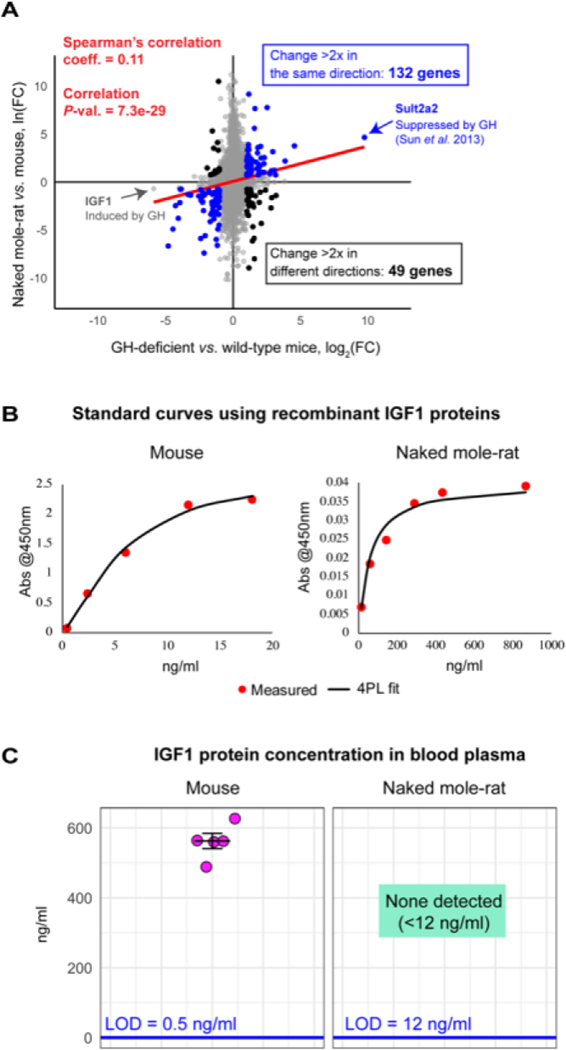
There is also a transcriptional signature of GH deficiency in the naked mole-rat liver. The Buffenstein and Kenyon groups found that transcriptomes of naked mole-rats and dwarf mice resemble each other when comparing naked mole-rat liver RNA sequencing (RNAseq) data to the published liver microarray data from GH-deficient mice (Sun et al., 2013). There was a significant overall correlation between the two gene expression profiles (Fig. 4A; red line) and a significant overlap between the top differentially expressed genes (Fisher test P-value = 4.7e-09, comparing top 5% of effect sizes). Gene set enrichment analysis (GSEA) indicates patterns of transcript expression in naked mole-rats that are consistent with pathways that are perturbed in GH-deficient mice, including upregulation of the tricarboxylic acid (TCA) cycle and respiratory electron transport, and down-regulation of protein translation, mechanistic target of rapamycin (mTOR) signalling, and unfolded protein response.
Fig. 4.
(A) Liver gene-expression profiles of growth hormone (GH)-deficient (Ghrh–/–) mice and naked mole-rats. Gene fold-changes (FCs) were calculated relative to wild-type mice for each organism, using ortholog conversion for naked mole-rat genes (reciprocal best BLAST hits). Spearman’s rank correlation coefficient for all genes on the plot and the correlation P-value are shown in red. Of the genes that change substantially in both organisms (>twofold), those that change concordantly (blue) outnumber those that change non-concordantly (black). Genes of special interest are labelled – insulin-like growth factor 1 (IGF1; induced by GH) is down-regulated in both organisms and sulfotransferase family 2A (Sult2a2) (repressed by GH) is upregulated in both organisms. Data for Ghrh–/– mice was obtained from GSE51108. Data for naked mole-rats were generated by N. Rubinstein and R.B. and analysed by K.P. (B) Standard curves for commercial IGF1 enzyme-linked immunosorbent assay (ELISA) (ALPCO 22-IG1MS-E01) generated using recombinant mouse and naked mole-rat IGF1 proteins and four-parameter logistic regression (4PL). (C) Concentration of IGF1 in blood plasma of young adult animals, measured using standard curves in B. Mice were 3.5 months old and naked mole-rats were 2–2.3 years old; three females and two males were used in both groups. LOD, limit of detection.
Increased hepatic expression of IGF1 receptor (IGF1R) in mole-rats (Fang et al., 2014) was proposed as a key argument against decreased IGF1 signalling in naked mole-rats by Braude et al. However, IGF1R expression in the mammalian liver (regardless of species) is very low and variable (Wu et al., 2009; Uhlon, Fagerberg & Hallstrom, 2015). Indeed, an earlier naked mole-rat study (Kim et al., 2011), reported decreased IGF1R levels in mole-rats relative to mice, and in our own (Buffenstein and Kenyon groups) data we do not detect any IGF1R expression in the livers of either species. A more sensitive and widely accepted index of GH/IGF1 axis activity is the abundance of hepatic IGF1: the liver is the main source of circulating IGF1, as GH binding to the GH receptor on hepatocytes stimulates IGF1 transcription. Consistent with reduced activity of this hormone axis, we and others (Fang et al., 2014; Kim et al., 2011) report low levels of IGF1 messenger RNA (mRNA) in the naked mole-rat liver (Fig. 4A). Circulating IGF1 protein levels of naked mole-rats and mice were also measured using commercial enzyme-linked immunosorbent assay (ELISA). To account for differences in antibody affinities to proteins from different species, separate standard curves using recombinant mouse or recombinant naked mole-rat IGF1 protein were generated (Fig. 4B), and limits of IGF1 detection were calculated: 0.5 ng/ml for the mouse and 12 ng/ml for the naked mole-rat protein. Average IGF1 concentration in mouse plasma was 560 ng/ml, consistent with published values, but no IGF1 protein was detected in naked mole-rat plasma (Fig. 4C). While post-translational modifications could account for lack of detection, these results are also consistent with previously published low circulating IGF1 levels (<12 ng/ml) in naked mole-rats (Buffenstein, 2005).
Cultured naked mole-rat fibroblasts secrete functional pregnancy associated plasma protein-A (PAPP-A) protease, and thereby can cleave IGF1 binding proteins and liberate IGF1 in local tissue microenvironments (Brohus et al., 2015). Therefore, it is unlikely that any differences in the activity of the GH/IGF1 axis between mice and naked mole-rats result from differential regulation of IGF1 bioavailability. Instead, this likely arises upstream, at the level of GH production (Fig. 4). In addition, both Fang et al. (2014) and Brohus et al. (2015) reported that transcript levels of IGF2 are retained at high levels throughout adult life in naked mole-rats (Kim et al., 2011; Brohus et al., 2015).
Although additional studies are needed to prove conclusively that the GH/IGF axis is “impaired” in naked mole-rats, current studies strongly suggest that this endocrine pathway is diminished in this species and that this “myth” is likely to be true. This is an important and pertinent finding, as inhibition of GH/IGF1 signalling has been shown to extend lifespan in several other species (Kenyon, 2010).
(2). Myth 21: naked mole-rats are long-lived because they have low oxidative stress and damage
The oxidative stress theory of ageing states that there is an imbalance between the production of reactive oxygen species (ROS) and their neutralisation by antioxidants, leading to an accumulation of oxidative damage with age that contributes to the physiological decline and loss of dynamic homeostasis during ageing (Harman, 2006). Braude et al. suggest that naked mole-rat longevity does not align with the oxidative stress theory of ageing because naked mole-rats paradoxically exhibit high levels of oxidative stress and damage, evident even at young ages (Andziak et al., 2006; Perez et al., 2009; Edrey et al., 2013). These high levels of damage are consistent with similar levels of ROS production observed in the heart mitochondria of mice and mole-rats (Lambert et al., 2007; Munro et al., 2019), and equivocal findings regarding antioxidant defences that appear to be antioxidant, tissue and cell-site specific (Munro et al., 2019; Andziak, O’Connor & Buffenstein, 2005; Viltard et al., 2019). Collectively, these findings appear to refute the concept that naked mole-rats encounter low levels of oxidative stress.
However, the basic interpretation of the oxidative stress theory of ageing put forth by Braude et al. that “oxidative stress and damage inevitably result in premature ageing and age-related diseases” (Braude et al., 2021, p. 386) does not take into account the evolution of this theory over the 65 years since it was first proposed (Harman, 1956). For example, how oxidative damage accumulates over time may be more closely related to ageing than a static point comparison or comparisons between species. In this regard, naked mole-rats align rather well with this ageing theory. In contrast to the steady rise in oxidative damage in mice as they age, while young naked mole-rats exhibit higher levels of oxidative damage than seen in young mice, there is relatively little accumulation of oxidative damage in naked mole-rat tissues throughout the life course (Andziak & Buffenstein, 2006; Perez et al., 2009).
In a similar regard, the molecular and cellular responses to oxidative stress/damage may prove more important to the regulation of ageing than the damage moieties themselves. Naked mole-rat cells, in culture, are exceedingly resistant to multiple forms of stressors, including agents capable of generating oxidative stress (Salmon et al., 2008; Lewis et al., 2012). This is attributed to mechanisms in place that can repair or remove macromolecules or cells that have been irreversibly damaged. Numerous upregulated cytoprotective pathways, e.g. signalling from NRF2 and p53 (Lewis et al., 2015, 2010), upregulated DNA repair pathways (MacRae et al., 2015), and mechanisms involved in tolerating damage to proteins (De Waal et al., 2013; Perez et al., 2009) and lipids (Hulbert, Faulks & Buffenstein, 2006; Mitchell, Buffenstein & Hulbert, 2007), likely play a pivotal role in preventing age-associated accrual of oxidative damage. These data collectively suggest that there is, in fact, some truth to this “myth”.
(3). Myth 22: naked mole-rat cells do not display cellular senescence
Cellular senescence is a protective mechanism to prevent the proliferation of cells that may be damaged or compromised by short telomeres. It has commonly been used as a sign of ageing and cancer resistance. Cellular senescence was first described by Leonard Hayflick in the 1960s, where he and colleagues observed that human cells undergo between 40 and 60 replications before they stop proliferating as a result of “replicative senescence” (Hayflick, 1965). Several studies regarding telomere length and shortening have been reported in naked mole-rats, with somewhat conflicting results. Multiple groups have reported that, compared to similarly sized but shorter-lived mice, naked mole-rats have relatively short telomeres and low levels of telomerase (Gomes et al., 2011; Seluanov et al., 2007). However, additional reports present evidence that naked mole-rat telomeres do not shorten substantially with age, which they do in mice (Adwan Shekhidem et al., 2019; Leonida et al., 2020). More studies are needed to link these phenomena conclusively in the naked mole-rat.
The measurement of actual senescence, both in vitro and in vivo, has proved to be extremely challenging in naked mole-rats. Biochemical measures of senescence include β-galactosidase staining, molecular markers like p16, and inflammatory senescence-associated secretory phenotypes (SASPs). However, measurement of these markers and how they change with age or cellular replication in naked mole-rats has proved problematic and remains largely unexplored. There is one study using β-galactosidase staining, which showed that senescence plays a role in naked mole-rat neonatal development, as well as RAS-oncogene mediated and γ-irradiation responses (Zhao et al., 2018). Additionally, Zhao et al. (2018) showed that after γ-irradiation, SASP pathways are enriched in both naked mole-rat and mouse skin fibroblasts suggesting that senescence can be induced in naked mole-rat cells under certain conditions. Thus, given our current knowledge, we agree that Myth 22 is indeed a “myth”, or at least an oversimplification. However, more data are needed to assess the precise roles of senescence in replication, cancer, and ageing in the naked mole-rat.
(4). Myth 23: naked mole-rats are immune to disease
Delaney et al. (2021) extensively catalogued diseases observed in captive naked mole-rat colonies. Based upon fairly large numbers of post-mortem analyses it is clear that, relative to other mammals, naked mole-rats exhibit a low incidence of non-infectious chronic diseases or age-related degenerative diseases, such as osteoporosis, cardiovascular disease, neurodegeneration and cancer (Delaney et al., 2016a, 2013, 2021; Ward, Cartoceti & Delaney, 2020; Buffenstein et al., 2012; Edrey et al., 2011). Similarly, Braude et al. report a low incidence of non-infectious and infectious diseases both from the literature and from a decade of observations at the Leibniz Zoological Gardens. However, incidence of specific diseases may vary among institutions: for example, chronic renal disease is rarely identified post-mortem at the National Zoo colonies (Washington DC), but it is more prevalent in post-mortems carried out at zoological gardens in Illinois (Delaney, Kinsel & Treuting, 2016b). This may reflect differences in husbandry, housing, or possibly genetic influences.
Adult individuals both in the wild (Hill et al., 1957) and in captivity also exhibit a very low incidence of infectious diseases (Delaney et al., 2021). However, an acute death syndrome has been anecdotally reported from multiple institutions where several animals within a particular colony that previously looked healthy are found dead with no signs of illness or lesions, suggestive of an acute contagious infection. When infections, specifically viral aetiologies, are found in captive colonies, these are often fatal. Both the Jarvis (University of Cape Town) and Sherman (University of Cornell) laboratories lost colonies to viruses, with the Jarvis group reporting that death was due to a coronavirus. This acute infection killed almost 50% of colony members (Ross‐Gillespie, O’Riain & Keller, 2007). Moreover, experimental infection with a recombinant herpes simplex virus type 1, which is avirulent to mice, caused 100% mortality when administered to naked mole-rats (Artwohl et al., 2009). These findings are further supported by studies that their immune cell populations lack natural killer cells, the cells responsible for immune surveillance and eradication of virally infected cells (Hilton et al., 2019). In conclusion, although naked mole-rats do appear relatively resistant to many types of chronic disease, it is clearly a “myth” that they are fully immune to disease. This is not a contention that we are aware of in the scientific literature, however.
(5). Myth 24: naked mole-rats do not get tumours or cancer
In the first large-scale report on lack of cancer prevalence, it was not claimed that naked mole-rats “do not get tumors or cancer”, but rather that, “our unusual mortality pattern may reflect the low susceptibility of these animals to cancer” (Buffenstein, 2008) (p. 441). Over time we will acquire a more accurate assessment of the actual degree of cancer resistance. In a comparison of incidence with other wild rodents, the cancer rates in wild animals are difficult to measure accurately and cancer prevalence in wild-caught house mice is influenced by environmental factors (Gardner et al., 1973; Harper, Leathers & Austad, 2006). However, naked mole-rats appear highly resistant compared to other laboratory rodents. By studying them, significant insights into tumorigenesis, mechanisms of resistance, prevention and treatment strategies may arise. It will also be important to elucidate in future studies how naked mole-rat individuals respond to several types of experimental carcinogenesis induction.
(6). Myth 25: naked mole-rats have extremely large hyaluronan
Hyaluronan is ubiquitously found in the extracellular matrix of cells and most tissues throughout the bodies of mammals (Fraser, Laurent & Laurent, 1997). Hyaluronan increases the hydration and viscoelastic properties of the surrounding extracellular matrices and spaces due to its high net-negative charge (Engstrom-Laurent, 1997). Abundant high molecular weight hyaluronan was found both in the culture media conditioned by naked mole-rat fibroblasts and in naked mole-rat tissues (Tian et al., 2013; Taguchi et al., 2020; Takasugi et al., 2020). Hyaluronan was detected by multiple methods including Alcian blue staining, including all necessary controls such as treatment with specific hyaluronan-degrading enzymes (Tian et al., 2013; Taguchi et al., 2020; Takasugi et al., 2020). Thus, we reject the criticism by Braude et al. that Alcian blue staining performed in this way has no specificity.
Hyaluronan size is determined by the balance between synthesis and degradation. Braude et al. argue that it is unclear how the two mutations in the conserved catalytic core of the naked mole-rat hyaluronan synthase 2 (HAS2) enzymes could lead to higher molecular weight, as a variety of sizes was produced when naked mole-rat HAS2 was expressed in cancer cells. This is not surprising at all, as different cancer cell lines differ greatly in expression levels of the hyaluronan-degrading enzymes, hyaluronidases.
How high is ‘high’ molecular weight? It was originally observed that naked mole-rat hyaluronan had a very high molecular weight (6–12 MDa) (Tian et al., 2013). However, determining the exact size of large hyaluronan molecules is complicated being potentially affected by the isolation procedure. The largest available hyaluronan size markers typically end at 6 MDa. A recent analysis has demonstrated that naked mole-rat hyaluronan has a high average molecular weight, but not greater than 2.5 MDa, whether from tissue or cell supernatant (Del Marmol et al., 2021).
What is important is that hyaluronan in the naked mole-rat may contribute to the unique physiology of this species (Takasugi et al., 2020; Gorbunova, Takasugi & Seluanov, 2020). Indeed, it has been shown using atomic force microscopy that hyaluronan extracted from naked mole-rat tissues forms a range of assemblies (corresponding to a distribution of molecular weights), including supercoiled structures that are absent from hyaluronan extracted from mouse tissue, as well as an ability spontaneously to form gels. These properties likely contribute to the elasticity of naked mole-rat skin and may have the potential to function as a barrier to tumour invasion (Kulaberoglu et al., 2019).
Tian et al. (2013) reported the first evidence that hyaluronan contributes to cancer resistance in the naked mole-rat. In regard to this resistance of naked mole-rat cells to oncogenic transformation, Braude et al. state that “a recent study that failed to reproduce these findings” (Braude et al., 2021, p. 388), but fail to cite the reply to Hadi et al. (2020) published in the same issue, which demonstrated that the discrepant observations between Tian et al. (2013) and Hadi et al. (2020) originate from the different expression levels of oncogenic HRas (HRas G12V). When oncogenic Harvey Rat sarcoma virus (HRas) is driven by a long terminal repeat (LTR) promoter to express at a moderate level, Tian et al. (2013) and Liang et al. (2010) independently reported that naked mole-rat fibroblasts are more resistant to oncogenic transformation than mouse cells. However, when HRas G12V is expressed at a higher level, naked mole-rat cells generate tumours in xenografts (Zhao et al., 2020). The original studies did not claim that naked mole-rat cells can never be transformed by oncogenes, but rather, as is stated in the title of the paper by Liang et al. (2010), they are more resistant to transformation than those of mice. Overall, given the susceptibility of naked mole-rat cells to transformation, it is important to identify the mechanisms responsible for naked mole-rat tumour resistance, including any potential role for their hyaluronan in creating a cancer-resistant tissue microenvironment.
(7). Myth 26: naked mole-rat cells have early contact inhibition that prevents cancer
Primary cultures of dermal fibroblasts from adult naked mole-rats are relatively difficult to establish under the standard conditions routinely used for humans and mice, and these cells grow more slowly than those of embryos or neonates. The growth of naked mole-rat fibroblasts is particularly sensitive to culture conditions: if conditions are suboptimal, the proliferation of these cells slows, or stops completely. We have found that even ostensibly minor changes in our cell culture maintenance protocols are sufficient to impact proliferation. Similarly, unlike the remaining viable fibroblasts of mice which may continue to proliferate even when most of the mouse fibroblasts are killed following toxin exposure, naked mole-rat fibroblasts readily stop proliferating when subject to toxin exposure without any obvious signs of senescence (Lewis et al., 2012). This stress-induced growth arrest likely reflects an important role in the cancer resistance of the naked mole-rat.
Some authors have observed that naked mole-rat fibroblasts secrete abundant hyaluronan into the culture medium which increases its viscosity and arrests cell proliferation before cells reach confluence via cluster of differentiation 44 (CD44) receptor signalling (Seluanov et al., 2009; Tian et al., 2013). It takes 4–5 days for sufficient hyaluronan to accumulate to trigger cell growth arrest. Frequent medium changes remove this hyaluronan and result in confluent cell culture with naked mole-rat cells attaining higher densities than observed for mouse cells, suggesting that contact inhibition is not a cell autonomous process (Lewis et al., 2012; Narayan et al., 2021; Liang et al., 2010). Similarly, some authors have observed that degradation of hyaluronan by bacterial hyaluronidase generates a confluent cell culture (Tian et al., 2013), whereas others have observed no change in cell confluence when hyaluronidase is added to cell cultures (Del Marmol et al., 2021). Considering the abundant hyaluronan present in the microenvironment of naked mole-rat tissues, the potential role of hyaluronan signalling in naked mole-rat cell culture may contribute to the cancer resistance of this species.
The differences in culture conditions for naked mole-rat cells used by different laboratories resulted in lively debate. However, attempts have been made to understand why the data are at variance (Seluanov et al., 2009; Narayan et al., 2021; Lewis et al., 2012; Liang et al., 2010). Having built upon the knowledge gleaned from these divergent findings, we now have a far better understanding of how conditions affect naked mole-rat cell growth and susceptibility to cell cycle arrest. Braude et al. refer to unpublished data from their own group (T. B. Hildebrandt, N. Kichler, S. Holtze & M. Vyssokikh, in preparation) to dismiss this “myth”. Without any relevant information on their growth conditions, it is impossible to evaluate whether their statement contradicts or supports the model of naked mole-rat growth described in this section.
(8). Myth 27: naked mole-rats are non-ageing
As adult organisms age, their risk of dying increases exponentially. This concept of age-dependent mortality, first proposed by the actuarial scientist Benjamin Gompertz, is widely regarded as a fundamental law of mortality (Gompertz, 1825) and a prominent metric in the comparative biology of ageing (Sacher, 1977; Kirkwood, 2015). The Gompertz–Makeham laws provide a context for observing the rate at which a species ages, measured in terms of the doubling rate of mortal hazard across a population (therefore referred to as ‘demographic’ ageing). Ruby et al. (2018) analysed a large compendium of lifespan data (>3200 animals) in order to estimate the rate of demographic ageing for naked mole-rats and found that the risk of dying did not increase at all with age, even at ages more than 25 times the age of sexual maturity. Dammann et al. (2019) previously wrote a ‘comment’ to that publication, expressing concern about the potential of missing data from the distant past to confound the conclusion. An additional new analysis that specifically addressed the concerns of Dammann et al. (2019) was undertaken using left-censorship as specified by Kaplan & Meir (1958) for the analyses of incomplete demographic data (Ruby, Smith & Buffenstein, 2019). That new analysis yielded the same conclusion as the original paper: a lack of demographic ageing in naked mole-rats across decades of life (Ruby et al., 2019).
Furthermore, we disagree that these findings are undermined by earlier incidence reports of reduced activity or other physiological or morphological changes in some older animals (Edrey et al., 2011; Lewis & Buffenstein, 2016; Delaney et al., 2021). For example, these studies have reported that skin colour becomes more uniform and paler in older subordinates as well as in breeders at any age. While this may be akin to hair greying without associated pathology, there may be other ecophysiological explanations for why this occurs. The paper ‘Successful ageing and sustained good health in the naked mole-rat’, reports on the observed signs of physiological declines occurring wherever these are evident, even if these incidences, like that of cancer, are rare (Edrey et al., 2011). However, this and other studies also report that the great majority of animals remain active, continue to breed well into their third decade, show no menopause, unchanged cardiac function, body composition and metabolic profiles and indeed retain numerous paedomorphic traits well into adulthood (Buffenstein, 2008; O’Connor et al., 2002; Grimes et al., 2014a; Buffenstein & Craft, 2021; Buffenstein et al., 2020; Lewis & Buffenstein, 2016; Edrey et al., 2012). The health deteriorations that we have observed in naked mole-rats (e.g. cataracts and osteoarthritis) appear to change neither in severity nor frequency as age increases: for example, cataracts have been observed in juveniles (<3 months) and adults in every decade of life (Edrey et al., 2011; M.A. Hanes & R. Buffenstein, personal communication). From an examination of data covering the first three decades of life, death and disease both appear to be stochastic and independent of chronological age, consistently supporting the classification of the naked mole-rat, at least until their third decade of life, as a “non-ageing” mammal (Ruby et al., 2018). As such, based upon Gompertzian laws as well as age-related phenotypes, that naked mole-rats are non-ageing mammals is not a “myth”.
VI. TAXONOMY
(1). Myth 28: naked mole-rats are the single member of a taxonomic family
Braude et al. state “The taxonomic status of Heterocephalus is not so much a myth as a scientific error” (Braude et al., 2021, p. 389), referring to the contention by Patterson & Upham (2014) that the differences between Heterocephalus and the other bathyergids warrant its placement within its own family, Heterocephalidae. Braude et al. dispute some of the evidence used by Patterson & Upham (2014), and argue that Heterocephalus should be retained in the Bathyergidae. A recent review of the phylogeny and biogeography of the Bathyergidae reveals that the species richness of the Bathyergidae needs to be revisited in a systematic and comprehensive manner (Visser, Bennett & van Vuuren, 2019). We therefore feel that it is premature to regard Heterocephalus as a member of a separate family to the Bathyergidae. However, while we acknowledge that the placement of familial designations is to some extent subjective, we dispute the contention that the taxonomic proposal of Patterson & Upham (2014) represents either a “myth” or a “scientific error”. Biosystematic revisions, based on differing combinations of characteristics (e.g. genes, fossil records, and morphological traits), as well as emerging sophisticated analytical techniques, lead to competing and evolving hypotheses that should converge towards the true solution over time.
VII. CONCLUSIONS
(1) We would not deny that there is, and will continue to be, misinformation about the naked mole-rat circulating in the popular press and electronic media. However, we feel that many of the “myths” identified by Braude et al. (2021) serve to create misunderstanding where little exists. We hope to have demonstrated herein that these do not, in fact, represent widespread and unsubstantiated beliefs among the scientific community (see Table 1 for a summary). We have identified several common themes which unite several of these ostensible “myths”: (a) some “myths”, as presented in headline form, are better described as oversimplifications. For example, we agree that the naked mole-rat is not completely hairless, nor completely blind, and it is likely not to be continuously exposed to hypoxic and hypercapnic conditions (myths 1, 6, 7) – but there is an element of truth to all of these statements. (b) Other so-called “myths” may or may not have an element of truth, but we simply do not have enough information at the present time to be sure (e.g. myths 5, 9, 15, 28). Those myth-statements could represent the “premature conclusions” referred to by Braude et al., but it is not clear that anyone in the field believes that these cases are, indeed, closed. (c) Alleged “myths” based on conclusions of earlier studies which have subsequently been revised in line with new evidence (e.g. myths 16, 17, 19, 21). (d) Aspects of naked mole-rat biology that are currently interpreted differently by different research groups, perhaps because of semantic disagreements (e.g. myths 4, 8, 11, 12). Many of the 28 “myths” combine one or more of these common themes.
(2) The addition of exclusive terms such as ‘strictly’, ‘uniquely’, ‘the only’, ‘the most’ or ‘never’ contributes to some of the other myth-statements being false or at least questionable, sometimes because of similarities between naked mole-rats and other sub-Saharan mole-rats. Braude et al. have helped to bring prominence to studies of other African mole-rats, which is important in serving to put some of the naked mole-rat data into a wider context, but similarities with close relatives do not negate the interest value and importance of the naked mole-rat as a model species.
(3) Unfortunately, it is inevitable that researchers will find and cite some papers while missing others, so there will always be some perpetuation of outdated or incorrect ideas. This is the case in any scientific field, of course. Competing hypotheses and interpretations are also inevitable, and indeed are a healthy part of the scientific endeavour. Fortunately, in the fullness of time, science is self-correcting – as Thomas Kuhn (quoting Francis Bacon) stated, “Truth emerges more readily from error than from confusion” (Kuhn, 1962, p. 38). Although we respectfully disagree with many of the contentions of Braude et al. (2021), and we have attempted to show why, we encourage debate and welcome challenging articles that serve to drive reconsideration and discussion. We do, however, reject their labelling of evidence-based hypotheses, even if outdated, as “myths”, and we feel that this is particularly inappropriate when it comes to competing theories where there is disagreement amongst scientists. We hope that readers of the Braude et al. paper have not been misled into thinking that naked mole-rat research involves any more confusion and error than any other active area of research in biology, and that research in this burgeoning field would be wasted effort. To the contrary, we firmly believe that there is much to be learnt from this non-conventional laboratory species.
(4) Decades of research by many groups across the world, with very different areas of expertise, has contributed to a great deal now being known about the naked mole-rat. Some of this is proving to be directly pertinent to understanding and mitigating human disease. This knowledge base will continue to be refined and built upon. No doubt, some of our current understanding will need to be revised in the light of evidence yet to be uncovered, and although some of the current debates will be settled, new ones will surely arise. Every new publication helps us better to understand the unusual biology of this curious little mammal.
VIII. ACKNOWLEDGEMENTS
Many of the authors of this paper are supported by grants from a variety of sources, including the NIH, NSF, and ERC. R.B. gratefully acknowledges financial support from Calico Life Sciences, LLC and E.St.J.S.) acknowledges funding from the Dunhill Medical Trust (RPGF2002\188). This publication would not have been possible without the excellent care of our naked mole-rat colonies.
VIII. REFERENCES
- Adwan Shekhidem H, Sharvit L, Leman E, Manov I, Roichman A, Holtze S, M Huffman D, Y Cohen H, Bernd Hildebrandt T & Shams I (2019). Telomeres and longevity: A cause or an effect? International Journal of Molecular Sciences 20(13), 3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R, Nixon D & Akasha M (1988). Total and free thyroxine and triiodothyronine in blood serum of mammals. Comparative Biochemistry and Physiology. Part A Comparative Physiology 89(3), 401–404. [DOI] [PubMed] [Google Scholar]
- Andziak B & Buffenstein R (2006). Disparate patterns of age-related changes in lipid peroxidation in long-lived naked mole-rats and shorter-lived mice. Aging Cell 5(6), 525–532. [DOI] [PubMed] [Google Scholar]
- Andziak B, O’Connor TP & Buffenstein R (2005). Antioxidants do not explain the disparate longevity between mice and the longest-living rodent, the naked mole-rat. Mechanisms of Ageing and Development 126(11), 1206–1212. [DOI] [PubMed] [Google Scholar]
- Andziak B, O’Connor TP, Qi W, DeWaal EM, Pierce A, Chaudhuri AR, Van Remmen H & Buffenstein R (2006). High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell 5(6), 463–471. [DOI] [PubMed] [Google Scholar]
- Arieli R & Nevo E (1991). Hypoxic survival differs between two mole rat species (Spalax ehrenbergi) of humid and arid habitats. Comparative Biochemistry and Physiology Part A Comparative Physiology 100(3), 543–545. [DOI] [PubMed] [Google Scholar]
- Artwohl J, Ball-Kell S, Valyi-Nagy T, Wilson SP, Lu Y & Park TJ (2009). Extreme susceptibility of african naked mole rats (Heterocephalus glaber) to experimental infection with herpes simplex virus type 1. Comparative Medicine 59(1), 83–90. [PMC free article] [PubMed] [Google Scholar]
- Barker A, Koch U, Lewin G & Pyott S (2021a). Hearing and vocalizations in the naked mole-rat. In The Extraordinary Biology of the Naked Mole-Rat. (eds Buffenstein R, Park T and Holmes M). Springer, New York. [DOI] [PubMed] [Google Scholar]
- Barker AJ, Veviurko G, Bennett NC, Hart DW, Mograby L & Lewin GR (2021b). Cultural transmission of vocal dialect in the naked mole-rat. Science 371(6528), 503–507. [DOI] [PubMed] [Google Scholar]
- Bednářová R, Hrouzková-Knotková E, Burda H, Sedláček F & Šumbera R (2013). Vocalizations of the giant mole-rat (Fukomys mechowii), a subterranean rodent with the richest vocal repertoire. Bioacoustics 22(2), 87–107. [Google Scholar]
- Beekman M, Peeters C & O’Riain MJ (2006). Developmental divergence: neglected variable in understanding the evolution of reproductive skew in social animals. Behavioral Ecology 17(4), 622–627. [Google Scholar]
- Bennett N, Jarvis J & Cotterili F (1993). Poikilothermic traits and thermoregulation in the Afrotropical social subterranean Mashona mole-rat (Cryptomys hottentotus darlingi)(Rodentia: Bathyergidae). Journal of Zoology 231(2), 179–186. [Google Scholar]
- Bennett N, Jarvis J & Davies K (1988). Daily and seasonal temperatures in the burrows of African rodent moles. African Zoology 23(3), 189–195. [Google Scholar]
- Bennett NC (1988). The Trend Towards Sociality in Three Species of Southern African Mole-Rats (Bathyergidae): Causes and Consequences. Ph.D. Dissertation. University of Cape Town, Cape Town. [Google Scholar]
- Bennett NC & Faulkes CG (2000). African Mole-Rats: Ecology and Eusociality. Cambridge University Press, Cambridge. [Google Scholar]
- Bennett NC & Jarvis JU (1988). The social structure and reproductive biology of colonies of the mole-rat, Cryptomys damarensis (Rodentia, Bathyergidae). Journal of Mammalogy 69(2), 293–302. [Google Scholar]
- Berryman DE, List EO, Coschigano KT, Behar K, Kim JK & Kopchick JJ (2004). Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Hormone & IGF Research 14(4), 309–318. [DOI] [PubMed] [Google Scholar]
- Boomsma JJ & Gawne R (2018). Superorganismality and caste differentiation as points of no return: how the major evolutionary transitions were lost in translation. Biological Reviews of the Cambridge Philosophical Society 93(1), 28–54. [DOI] [PubMed] [Google Scholar]
- Braude S (1991). The Behavior and Demographics of the Naked Mole-Rat, Heterocephalus glaber, Ph.D. Dissertation, University of Michigan, Ann Arbor. [Google Scholar]
- Braude S (2000). Dispersal and new colony formation in wild naked mole-rats: evidence against inbreeding as the system of mating. Behavioral Ecology 11(1), 7–12. [Google Scholar]
- Braude S, Holtze S, Begall S, Brenmoehl J, Burda H, Dammann P, Del Marmol D, Gorshkova E, Henning Y & Hoeflich A (2021). Surprisingly long survival of premature conclusions about naked mole-rat biology. Biological Reviews of the Cambridge Philosophical Society 96, 376–393. [DOI] [PubMed] [Google Scholar]
- Braude S, Holtze S, Hildebrandt T & Koch R (2020). Naked mole-rats do not disperse or deliver pups in correlation with moon phase. African Journal of Ecology 58(3), 592–595. [Google Scholar]
- Brett RA (1986). The Ecology and Behavior of the Naked Mole-Rat (Heterocephalus glaber, Ruppell) (Rodentia, Bathyergidae) Ph.D. Dissertation University of London, London. [Google Scholar]
- Brett RA (1991). The ecology of naked mole-rat colonies: burrowing, food and limiting factors. In The biology of the Naked Mole-Rat. (eds Jarvis JUM, Sherman PW, Alexander RD), pp. 137–184. Princeton University Press, Princeton. [Google Scholar]
- Brohus M, Gorbunova V, Faulkes CG, Overgaard MT & Conover CA (2015). The insulin-like growth factor system in the long-lived naked mole-rat. PLoS One 10(12), e0145587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browe BM, Olsen AR, Ramirez C, Rickman RH, Smith ESJ & Park TJ (2020). The naked mole-rat has a functional purinergic pain pathway despite having a non-functional peptidergic pain pathway. Neurobiology of Pain, 8, 100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffenstein R (1996). Ecophysiological responses to a subterranean habitat; A Bathyergid perspective. Mammalia 60(4), 591–605. [Google Scholar]
- Buffenstein R (2005). The naked mole-rat: a new long-living model for human aging research. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences 60(11), 1369–1377. [DOI] [PubMed] [Google Scholar]
- Buffenstein R (2008). Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. Journal of Comparative Physiology B 178(4), 439–445. [DOI] [PubMed] [Google Scholar]
- Buffenstein R & Craft W (2021). The idiosyncratic physiological traits of the naked mole-rat; a resilient animal model of aging, longevity, and healthspan. In The Extraordinary Biology of the Naked Mole-Rat. (eds Buffenstein R, Park T and Holmes M). Springer, New York. [DOI] [PubMed] [Google Scholar]
- Buffenstein R, Lewis KN, Gibney PA, Narayan V, Grimes KM, Smith M, Lin TD & Brown-Borg HM (2020). Probing pedomorphy and prolonged lifespan in naked mole-rats and dwarf mice. Physiology 35(2), 96–111. [DOI] [PubMed] [Google Scholar]
- Buffenstein R, Park T, Hanes M & Artwohl JE (2012). Naked mole-rat. In The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. (eds Suckow MA,Stevens KA, and Wilson RP) pp. 1055–1074. Elsevier, Amsterdam. [Google Scholar]
- Buffenstein R, Park TJ & Holmes MM (2021). The Extraordinary Biology of the Naked Mole-Rat. Springer Nature, New York. [Google Scholar]
- Buffenstein R & Pinto M (2009). Endocrine function in naturally long-living small mammals. Molecular and Cellular Endocrinology 299(1), 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffenstein R & Ruby JG (2021). Opportunities for new insight into aging from the naked mole-rat and other non-traditional models. Nature Aging 1, 3–4. [DOI] [PubMed] [Google Scholar]
- Buffenstein R, Woodley R, Thomadakis C, Daly TJ & Gray DA (2001). Cold-induced changes in thyroid function in a poikilothermic mammal, the naked mole-rat. American Journal of Physiology- Regulatory Integrative and Comparative Physiology 280(1), R149–155. [DOI] [PubMed] [Google Scholar]
- Buffenstein R & Yahav S (1991). Is the naked mole-rat, Heterocephalus glaber, a poikilothermic or poorly thermoregulating endothermic mammal? Journal of Thermal Biology 16, 227–232. [Google Scholar]
- Burda H (1995). Individual recognition and incest avoidance in eusocial common mole-rats rather than reproductive suppression by parents. Experientia 51(4), 411–413. [DOI] [PubMed] [Google Scholar]
- Catania KC & Remple MS (2002). Somatosensory cortex dominated by the representation of teeth in the naked mole-rat brain. Proceedings of the National Academy of Sciences USA 99(8), 5692–5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau LM, Groh AM, Anderson EC, Alcala MO, Mendelson JR III, Slade SB, Kerns K, Sarro S, Lusardi C & Goodisman MA (2018). Genetic diversity and sex ratio of naked mole rat, Heterocephalus glaber, zoo populations. Zoo Biology 37(3), 171–182. [DOI] [PubMed] [Google Scholar]
- Ciszek D (2000). New colony formation in the “highly inbred” eusocial naked mole-rat: outbreeding is preferred. Behavioral Ecology 11(1), 1–6. [Google Scholar]
- Clarke F & Faulkes C (2001). Intracolony aggression in the eusocial naked mole-rat, Heterocephalus glaber. Animal Behaviour 61(2), 311–324. [Google Scholar]
- Clarke FM & Faulkes CG (1999). Kin discrimination and female mate choice in the naked mole-rat Heterocephalus glaber. Proceedings of the Royal Society B: Biological Sciences 266(1432), 1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayson MS, Devereaux ME & Pamenter ME (2020). Neurokinin-1 receptor activation is sufficient to restore the hypercapnic ventilatory response in the Substance P-deficient naked mole-rat. American Journal of Physiology–Regulatory, Integrative and Comparative Physiology 318(4), R712–R721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen C, Bennett N, Holmes M & Faulkes C (2021). Neuropeptidergic and neuroendocrine systems underlying eusociality and the concomitant social regulation of reproduction in naked mole-rats: a comparative approach. In The Extraordinary Biology of the Naked Mole-rat. (eds Buffenstein R, Park T and Holmes M). Springer, New York. [DOI] [PubMed] [Google Scholar]
- Crespi BJ & Yanega D (1995). The definition of eusociality. Behavioral Ecology 6(1), 109–115. [Google Scholar]
- Crish SD, Dengler-Crish CM & Catania KC (2006). Central visual system of the naked mole-rat (Heterocephalus glaber). The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology 288(2), 205–212. [DOI] [PubMed] [Google Scholar]
- Crish SD, Rice FL, Park TJ & Comer CM (2003). Somatosensory organization and behavior in naked mole rats: vibrissae-like body hairs comprise a sensory array that mediates orientation to tactile stimuli. Brain Behavior and Ecology 62, 141–151. [DOI] [PubMed] [Google Scholar]
- Cui LY, Liu W, Xu YC, Yang SH & Dahmer TD (2020). A new method to estimate hair density of small mammal pelage. Journal of Mammalogy 101(4), 1205–1212. [Google Scholar]
- Daly TJ & Buffenstein R (1998). Skin morphology and its role in thermoregulation in mole-rats, Heterocephalus glaber and Cryptomys hottentotus . Journal of Anatomy 193(4), 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly TJ, Williams LA & Buffenstein R (1997). Catecholaminergic innervation of interscapular brown adipose tissue in the naked mole-rat (Heterocephalus glaber). Journal of Anatomy 190(3), 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammann P, Scherag A, Zak N, Szafranski K, Holtze S, Begall S, Burda H, Kestler HA, Hildebrandt T & Platzer M (2019). Comment on ‘Naked mole-rat mortality rates defy Gompertzian laws by not increasing with age’. Elife 8, e45415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waal EM, Liang H, Pierce A, Hamilton RT, Buffenstein R & Chaudhuri AR (2013). Elevated protein carbonylation and oxidative stress do not affect protein structure and function in the long-living naked-mole rat: a proteomic approach. Biochemical Biophysical Research Communication 434(4), 815–819. [DOI] [PubMed] [Google Scholar]
- de Wolf E, Cook J & Dale N (2017). Evolutionary adaptation of the sensitivity of connexin26 hemichannels to CO2. Proceedings of the Royal Society B: Biological Sciences 284(1848), 20162723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Marmol D, Holtze S, Kichler N, Sahm A, Bihin B, Bourguignon V, Dogne S, Szafranski K, Hildebrandt TB & Flamion B (2021). Abundance and size of hyaluronan in naked mole-rat tissues and plasma. Scientific Reports 11(1), 7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney M, Imai D & Buffenstein R (2021). Spontaneous disease and pathology of naked mole-rats In The Extraordinary Biology of the Naked Mole-rat. (eds Buffenstein R, Park T and Holmes M). Springer, New York. [DOI] [PubMed] [Google Scholar]
- Delaney M, Ward J, Walsh TF, Chinnadurai S, Kerns K, Kinsel M & Treuting P (2016a). Initial case reports of cancer in naked mole-rats (Heterocephalus glaber). Veterinary Pathology 53(3), 691–696. [DOI] [PubMed] [Google Scholar]
- Delaney MA, Kinsel MJ & Treuting PM (2016b). Renal pathology in a nontraditional aging model: the naked mole-rat (Heterocephalus glaber). Veterinary Pathology 53(2), 493–503. [DOI] [PubMed] [Google Scholar]
- Delaney MA, Nagy L, Kinsel MJ & Treuting PM (2013). Spontaneous histologic lesions of the adult naked mole rat (Heterocephalus glaber): a retrospective survey of lesions in a zoo population. Veterinary Pathology 50(4), 607–621. [DOI] [PubMed] [Google Scholar]
- Dvořáková V, Hrouzková E & Šumbera R (2016). Vocal repertoire of the social Mashona mole-rat (Fukomys darlingi) and how it compares with other mole-rats. Bioacoustics 25(3), 253–266. [Google Scholar]
- Edrey YH, Casper D, Huchon D, Mele J, Gelfond JA, Kristan DM, Nevo E & Buffenstein R (2012). Sustained high levels of neuregulin-1 in the longest-lived rodents; a key determinant of rodent longevity. Aging Cell 11(2), 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edrey YH, Hanes M, Pinto M, Mele J & Buffenstein R (2011). Successful aging and sustained good health in the naked mole rat: a long-lived mammalian model for biogerontology and biomedical research. Institute for Laboratory Animal Research Journal 52(1), 41–53. [DOI] [PubMed] [Google Scholar]
- Edrey YH, Medina DX, Gaczynska M, Osmulski PA, Oddo S, Caccamo A & Buffenstein R (2013). Amyloid beta and the longest-lived rodent: the naked mole-rat as a model for natural protection from Alzheimer’s disease. Neurobiology of Aging 34(10), 2352–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenbrod O, Debus KY, Reznick J, Bennett NC, Sánchez-Carranza O, Omerbašić D, Hart DW, Barker AJ, Zhong W & Lutermann H (2019). Rapid molecular evolution of pain insensitivity in multiple African rodents. Science 364(6443), 852–859. [DOI] [PubMed] [Google Scholar]
- Engstrom-Laurent A (1997). Hyaluronan in joint disease. Journal of Internal Medicine 242(1), 57–60. [DOI] [PubMed] [Google Scholar]
- Fang X, Seim I, Huang Z, Gerashchenko MV, Xiong Z, Turanov AA, Zhu Y, Lobanov AV, Fan D & Yim SH (2014). Adaptations to a subterranean environment and longevity revealed by the analysis of mole rat genomes. Cell Reports 8(5), 1354–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkes C & Abbott D (1991). Social control of reproduction in breeding and non-breeding male naked mole-rats (Heterocephalus glaber). Reproduction 93(2), 427–435. [DOI] [PubMed] [Google Scholar]
- Faulkes C & Abbott D (1993). Evidence that primer pheromones do not cause social suppression of reproduction in male and female naked mole-rats (Heterocephalus glaber). Reproduction 99(1), 225–230. [DOI] [PubMed] [Google Scholar]
- Faulkes CG, Abbott DH & Jarvis JU (1990). Social suppression of ovarian cyclicity in captive and wild colonies of naked mole-rats, Heterocephalus glaber. Journal of Reproduction and Fertility 88(2), 559–568. [DOI] [PubMed] [Google Scholar]
- Faulkes CG, Abbott DH, O’Brien HP, Lau L, Roy MR, Wayne RK & Bruford MW (1997). Micro- and macrogeographical genetic structure of colonies of naked mole-rats Heterocephalus glaber. Molecular Ecology 6(7), 615–628. [DOI] [PubMed] [Google Scholar]
- Faulkes CG & Bennett NC (2009). Reproductive skew in African mole-rats: behavioural and physiological mechanisms to maintain high skew. In Reproductive Skew in Vertebrates: Proximate and Ultimate Causes, (eds Hager R and Jones CB) pp. 369–396. Cambridge University Press, Cambridge. [Google Scholar]
- Fraser JR, Laurent TC & Laurent UB (1997). Hyaluronan: its nature, distribution, functions and turnover. Journal of Internal Medicine 242(1), 27–33. [DOI] [PubMed] [Google Scholar]
- Freeberg TM, Dunbar RI & Ord TJ (2012). Social complexity as a proximate and ultimate factor in communicative complexity. Philosophical Transactions of The Royal Society of London B, Biological Sciences 367(1597), 1785–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MB, Henderson BE, Estes JD, Menck H, Parker JC & Huebner RJ (1973). Unusually high incidence of spontaneous lymphomas in wild house mice. Journal of the National Cancer Institute 50(6), 1571–1579. [DOI] [PubMed] [Google Scholar]
- Gilbert JD, Rossiter SJ & Faulkes CG (2020). The relationship between individual phenotype and the division of labour in naked mole-rats: it’s complicated. PeerJ 8, e9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman B. d., Goldman SL, Lanz T, Magaurin A & Maurice A (1999). Factors influencing metabolic rate in naked mole-rats (Heterocephalus glaber). Physiology & Behavior 66, 447–459. [DOI] [PubMed] [Google Scholar]
- Gomes NM, Ryder OA, Houck ML, Charter SJ, Walker W, Forsyth NR, Austad SN, Venditti C, Pagel M & Shay JW (2011). Comparative biology of mammalian telomeres: hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell 10(5), 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompertz B (1825). XXIV. On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. In a letter to Francis Baily, Esq.. Philosophical Transactions of the Royal Society of London 115(1825), 513–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbunova V, Takasugi M & Seluanov A (2020). Hyaluronan goes to great length. Cell Stress 4(9), 227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin AS (2008). Naked mole-rat. Current Biology 18(18), R844–R845. [DOI] [PubMed] [Google Scholar]
- Grimes KM, Reddy AK, Lindsey ML & Buffenstein R (2014a). And the beat goes on: maintained cardiovascular function during aging in the longest-lived rodent, the naked mole-rat. American Journal of Physiology– Heart and Cirulatory Physiology 307(3), H284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes KM, Voorhees A, Chiao YA, Han HC, Lindsey ML & Buffenstein R (2014b). Cardiac function of the naked mole-rat: ecophysiological responses to working underground. American Journal of Physiology– Heart and Cirulatory Physiology 306(5), H730–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadi F, Kulaberoglu Y, Lazarus KA, Bach K, Ugur R, Beattie P, Smith ESJ & Khaled WT (2020). Transformation of naked mole-rat cells. Nature 583(7814), E1–E7. [DOI] [PubMed] [Google Scholar]
- Harman D (1956). Aging: a theory based on free radical and radiation chemistry. Journal of Gerontology 11(3), 298–300. [DOI] [PubMed] [Google Scholar]
- Harman D (2006). Free radical theory of aging: an update increasing the functional life span. Understanding and Modulating Aging 1067, 10–21. [DOI] [PubMed] [Google Scholar]
- Harper JM, Leathers CW & Austad SN (2006). Does caloric restriction extend life in wild mice? Aging Cell 5(6), 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L (1965). The limited in vitro lifetime of human diploid cell strains. Experimental Cell Research 37(3), 614–636. [DOI] [PubMed] [Google Scholar]
- Hazell R, Bennett N, Jarvis J & Griffin M (2000). Adult dispersal in the co‐operatively breeding Damaraland mole-rat (Cryptomys damarensis): a case study from the Waterberg region of Namibia. Journal of Zoology 252(1), 19–25. [Google Scholar]
- Heffner HE, Heffner RS, Contos C & Ott T (1994). Audiogram of the hooded Norway rat. Hearing Research 73(2), 244–248. [DOI] [PubMed] [Google Scholar]
- Heffner R, Heffner H & Masterton B (1971). Behavioral measurements of absolute and frequency-difference thresholds in guinea pig. Journal of the Acoustical Society of America 49(6), 1888–1895. [DOI] [PubMed] [Google Scholar]
- Heffner R, Koay G & Heffner H (2001). Audiograms of five species of rodents: implications for the evolution of hearing and the perception of pitch. Hearing Research 157(1–2), 138–152. [DOI] [PubMed] [Google Scholar]
- Heffner RS & Heffner HE (1991). Behavioral hearing range of the chinchilla. Hearing Research 52(1), 13–16. [DOI] [PubMed] [Google Scholar]
- Heffner RS & Heffner HE (1993). Degenerate hearing and sound localization in naked mole rats (Heterocephalus glaber), with an overview of central auditory structures. Journal of Comparative Neurology 331, 418–433. [DOI] [PubMed] [Google Scholar]
- Henning Y, Vole C, Begall S, Bens M, Broecker-Preuss M, Sahm A, Szafranski K, Burda H & Dammann P (2014). Unusual ratio between free thyroxine and free triiodothyronine in a long-lived mole-rat species with bimodal ageing. PLoS One 9(11), e113698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst M & Bennett N (2006). Burrow architecture and burrowing dynamics of the endangered Namaqua dune mole rat (Bathyergus janetta)(Rodentia: Bathyergidae). Journal of Zoology 270(3), 420–428. [Google Scholar]
- Herculano–Houzel S, Ribeiro P, Campos L, Da Silva AV, Torres LB, Catania KC & Kaas JH (2011). Updated neuronal scaling rules for the brains of Glires (rodents/lagomorphs). Brain, Behavior and Evolution 78(4), 302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heth G, Frankenberg E & Nevo E (1986). Adaptive optimal sound for vocal communication in tunnels of a subterranean mammal (Spalax ehrenbergi). Experientia 42(11–12), 1287–1289. [DOI] [PubMed] [Google Scholar]
- Hetling JR, Baig-Silva MS, Comer CM, Pardue MT, Samaan DY, Qtaishat NM, Pepperberg DR & Park TJ (2005). Features of visual function in the naked mole-rat, Heterocephalus glaber. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural and Behavioural Physiology 191(4), 317–330. [DOI] [PubMed] [Google Scholar]
- Hill W, Porter A, Bloom R, Seago J & Southwick M (1957). Field and laboratory studies on the naked mole rat, Heterocephalus glaber. Proceedings of the Zoological Society of London, 128, 455–514. [Google Scholar]
- Hilton HG, Rubinstein ND, Janki P, Ireland AT, Bernstein N, Fong NL, Wright KM, Smith M, Finkle D & Martin-McNulty B (2019). Single-cell transcriptomics of the naked mole-rat reveals unexpected features of mammalian immunity. PLoS Biology 17(11), e3000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hislop MS & Buffenstein R (1994). Noradrenaline induces nonshivering thermogenesis in both the naked mole-rat (Heterocephalus glaber) and the Damara mole-rat (Cryptomys damarensis) despite very different modes of thermoregulation. Journal of Thermal Biology 19, 25–32. [Google Scholar]
- Holmes M & Goldman B (2021). Social behavior in naked mole-rats: individual differences in phenotype and proximate mechanisms of mammalian eusociality. In The Extraordinary Biology of the Naked Mole-rat. (eds Buffenstein R, Park T and Holmes M). Springer, New York. [DOI] [PubMed] [Google Scholar]
- Holtze S, Braude S, Lemma A, Koch R, Morhart M, Szafranski K, Platzer M, Alemayehu F, Goeritz F & Hildebrandt TB (2018). The microenvironment of naked mole-rat burrows in East Africa. African Journal of Ecology 56(2), 279–289. [Google Scholar]
- Hulbert AJ (2000). Thyroid hormones and their effects: a new perspective. Biological Reviews of the Cambridge Philosophical Society 5(4), 519–631. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Faulks SC & Buffenstein R (2006). Oxidation-resistant membrane phospholipids can explain longevity differences among the longest-living rodents and similarly-sized mice. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences 61(10), 1009–1018. [DOI] [PubMed] [Google Scholar]
- Ingram CM, Troendle NJ, Gill CA, Braude S & Honeycutt RL (2015). Challenging the inbreeding hypothesis in a eusocial mammal: population genetics of the naked mole-rat, Heterocephalus glaber. Molecular Ecology 24(19), 4848–4865. [DOI] [PubMed] [Google Scholar]
- IUPS (2001). IUPS Thermal Comission glossary of terms for thermal physiology. Third Edition. The Japanese Journal of Physiology 51, 245–280. [Google Scholar]
- Ivy CM, Sprenger RJ, Bennett NC, van Jaarsveld B, Hart DW, Kirby AM, Yaghoubi D, Storey KB, Milsom WK & Pamenter ME (2020). The hypoxia tolerance of eight related African mole-rat species rivals that of naked mole-rats, despite divergent ventilatory and metabolic strategies in severe hypoxia. Acta Physiologica 228(4), e13436. [DOI] [PubMed] [Google Scholar]
- Jacobs DS & Jarvis J (1996). No evidence for the work-conflict hypothesis in the eusocial naked mole-rat (Heterocephalus glaber). Behavioral Ecology and Sociobiology 39(6), 401–409. [Google Scholar]
- Jarvis J (1991). Reproduction of naked mole-rats. In The Biology of the Naked Mole-rat. (eds Sherman PW, Jarvis JUM and Alexander RD), pp. 384–425. Princeton University Press, Princeton. [Google Scholar]
- Jarvis J & Bennett N (1993). Eusociality has evolved independently in two genera of bathyergid mole-rat but occurs in no other subterranean mammal. Behavioral Ecology and Sociobiology 33(4), 253–260. [Google Scholar]
- Jarvis J, O’Riain M & McDaid E (1991). Growth and factors affecting body size in naked mole-rats. In The Biology of the Naked Mole-rat. (eds Sherman PW, Jarvis JUM and Alexander RD), pp. 358–383. Princeton University Press, Princeton. [Google Scholar]
- Jarvis JU (1981). Eusociality in a mammal: cooperative breeding in naked mole-rat colonies. Science 212(4494), 571–573. [DOI] [PubMed] [Google Scholar]
- Junnila RK, List EO, Berryman DE, Murrey JW & Kopchick JJ (2013). The GH/IGF-1 axis in ageing and longevity. Nature Reviews, Endocrinology 9(6), 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabadi UM (1982). Laboratory tests for evaluating thyroid therapy. American Family Physician 26(3), 183–188. [PubMed] [Google Scholar]
- Kaplan EL & Meier P (1958). Nonparametric estimation from incomplete observations. Journal of the American Statistical Association 53, 457–481. [Google Scholar]
- Kenyon CJ (2010). The genetics of ageing. Nature 464(7288), 504–512. [DOI] [PubMed] [Google Scholar]
- Kim EB, Fang X, Fushan AA, Huang Z, Lobanov AV, Han L, Marino SM, Sun X, Turanov AA, Yang P, Yim SH, Zhao X, Kasaikina MV, Stoletzki N, Peng C, et al. (2011). Genome sequencing reveals insights into physiology and longevity of the naked mole rat. Nature 479(7372), 223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby AM, Fairman GD & Pamenter ME (2018). Atypical behavioural, metabolic and thermoregulatory responses to hypoxia in the naked mole rat (Heterocephalus glaber). Journal of Zoology 305(2), 106–115. [Google Scholar]
- Kirkwood TB (2015). Deciphering death: a commentary on Gompertz (1825) ‘On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies’. Philosophical Transactions of the Royal Society B: Biological Sciences 370(1666), 20140379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knotkova E, Veitl S, Šumbera R, Sedláček F & Burda H (2009). Vocalisations of the silvery mole-rat: comparison of vocal repertoires in subterranean rodents with different social systems. Bioacoustics 18(3), 241–257. [Google Scholar]
- Kramer B & Buffenstein R (2004). The pancreas of the naked mole-rat (Heterocephalus glaber): an ultrastructural and immunocytochemical study of the endocrine component of thermoneutral and cold acclimated animals. General and Comparative Endocrinology 139(3), 206–214. [DOI] [PubMed] [Google Scholar]
- Kuhn TS (1962). The Structure of Scientific Revolutions. University of Chicago Press, Chicago. [Google Scholar]
- Kulaberoglu Y, Bhushan B, Hadi F, Chakrabarti S, Khaled WT, Rankin KS, Smith ESJ & Frankel D (2019). The material properties of naked mole-rat hyaluronan. Scientific Reports 9(1), 6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey E & Sherman P (1991).Social organizationof naked mole-rat colonies: evidence for divisions of labor. In The Biology of the Naked Mole-rat. (eds Sherman PW, Jarvis JUM and Alexander RD), pp. 275–336. Princeton University Press, Princeton. [Google Scholar]
- Lambert AJ, Boysen HM, Buckingham JA, Yang T, Podlutsky A, Austad SN, Kunz TH, Buffenstein R & Brand MD (2007). Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum lifespan in vertebrate homeotherms. Aging Cell 6(5), 607–618. [DOI] [PubMed] [Google Scholar]
- Lange S, Burda H, Wegner RE, Dammann P, Begall S & Kawalika M (2007). Living in a “stethoscope”: burrow-acoustics promote auditory specializations in subterranean rodents. Naturwissenschaften 94(2), 134–138. [DOI] [PubMed] [Google Scholar]
- Larson J, Drew KL, Folkow LP, Milton SL & Park TJ (2014). No oxygen? No problem! Intrinsic brain tolerance to hypoxia in vertebrates. Journal of Experimental Biology 217(7), 1024–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser SF & Miller RA (2010). Nrf2 signaling, a mechanism for cellular stress resistance in long-lived mice. Molecular and Cellular Biology 30(3), 871–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonida SR, Bennett NC, Leitch AR & Faulkes CG (2020). Patterns of telomere length with age in African mole-rats: New insights from quantitative fluorescence in situ hybridisation (qFISH). PeerJ 8, e10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin G, Smith E, Reznick J, Debus K, Barker A & Park T (2021). The somatosensory world of the African naked mole-rat. In The Extraordinary Biology of the Naked Mole-rat. (eds Buffenstein R, Park T and Holmes M). Springer, New York. [DOI] [PubMed] [Google Scholar]
- Lewis KN & Buffenstein R (2016). The naked mole-rat: a resilient rodent model of aging, longevity and healthspan. In Handbook of the Biology of Aging. (eds Kaeberlein MR and Martin G). pp. 179–204. Academic Press, London. [Google Scholar]
- Lewis KN, Mele J, Hayes JD & Buffenstein R (2010). Nrf2, a guardian of healthspan and gatekeeper of species longevity. Integrative and Comparative Biology 50(5), 829–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KN, Mele J, Hornsby PJ & Buffenstein R (2012). Stress resistance in the naked mole-rat: the bare essentials – a mini-review. Gerontology 58(5), 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KN, Rubinstein ND & Buffenstein R (2018). A window into extreme longevity; the circulating metabolomic signature of the naked mole-rat, a mammal that shows negligible senescence. Geroscience 40(2), 105–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KN, Wason E, Edrey YH, Kristan DM, Nevo E & Buffenstein R (2015). Regulation of Nrf2 signaling and longevity in naturally long-lived rodents. Proceedings of the National Academy of Sciences USA 112(12), 3722–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Mele J, Wu Y, Buffenstein R & Hornsby PJ (2010). Resistance to experimental tumorigenesis in cells of a long-lived mammal, the naked mole-rat (Heterocephalus glaber). Aging Cell 9(4), 626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang W, Zhang T-Z, Li G-H, He K, Huang J-F, Jiang X-L, Murphy RW & Shi P (2014). Repeated functional convergent effects of NaV1. 7 on acid insensitivity in hibernating mammals. Proceedings of the Royal Society B: Biological Sciences 281(1776), 20132950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae SL, Zhang Q, Lemetre C, Seim I, Calder RB, Hoeijmakers J, Suh Y, Gladyshev VN, Seluanov A, Gorbunova V, Vijg J & Zhang ZD (2015). Comparative analysis of genome maintenance genes in naked mole rat, mouse, and human. Aging Cell 14(2), 288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MJ (2016a). Structure and function of the mammalian middle ear. I: Large middle ears in small desert mammals. Journal of Anatomy 228(2), 284–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MJ (2016b). Structure and function of the mammalian middle ear. II: Inferring function from structure. Journal of Anatomy 228(2), 300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MJ, Cornwall HL & Smith ES (2016). Ear structures of the naked mole-rat, Heterocephalus glaber, and its relatives (Rodentia: Bathyergidae). PLoS One 11(12), e0167079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak MM, Darcy J, Victoria B & Bartke A (2018). Dwarf mice and aging. Progress in Molecular Biology and Translational Science. 155, 69–83. [DOI] [PubMed] [Google Scholar]
- McComb K & Semple S (2005). Coevolution of vocal communication and sociality in primates. Biology Letters 1(4), 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab BK (1966). The metabolism of fossorial rodents: a study of convergence. Ecology 47(5), 712–733. [Google Scholar]
- McNab BK (1979). The influence of body size on the energetics and distribution of fossorial and burrowing mammals. Ecology 60(5), 1010–1021. [Google Scholar]
- Michener CD (1969). Comparative social behavior of bees. Annual Review of Entomology 14(1), 299–342. [Google Scholar]
- Mills SL & Catania KC (2004). Identification of retinal neurons in a regressive rodent eye (the naked mole-rat). Visual Neuroscience 21(2), 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell TW, Buffenstein R & Hulbert AJ (2007). Membrane phospholipid composition may contribute to exceptional longevity of the naked mole-rat (Heterocephalus glaber): a comparative study using shotgun lipidomics. Experimental Gerontology 42(11), 1053–1062. [DOI] [PubMed] [Google Scholar]
- Miyawaki S, Kawamura Y, Oiwa Y, Shimizu A, Hachiya T, Bono H, Koya I, Okada Y, Kimura T & Tsuchiya Y (2016). Tumour resistance in induced pluripotent stem cells derived from naked mole-rats. Nature Communications 7(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney SJ, Coen CW, Holmes MM & Beery AK (2015). Region-specific associations between sex, social status, and oxytocin receptor density in the brains of eusocial rodents. Neuroscience 303, 261–269. [DOI] [PubMed] [Google Scholar]
- Morris D (1967). The Naked Ape: A Zoologist’s Study of the Human Animal. Random House, New York. [Google Scholar]
- Munro D, Baldy C, Pamenter ME & Treberg JR (2019). The exceptional longevity of the naked mole-rat may be explained by mitochondrial antioxidant defenses. Aging Cell 18(3), e12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan V, McMahon M, O’Brien J, McAllister F & Buffenstein R (2021). Insights into the molecular basis of genome stability and pristine proteostasis in naked mole-rats. In The Extraordinary Biology of the Naked Mole-rat. (eds Buffenstein R, Park T and Holmes M). Springer, New York. [DOI] [PubMed] [Google Scholar]
- Nikitina NV, Maughan-Brown B, O’Riain MJ & Kidson SH (2004). Postnatal development of the eye in the naked mole rat (Heterocephalus glaber). Anatomical Record Part A Discoveries in Molecular, Cellular and Evolutionary Biology 277(2), 317–337. [DOI] [PubMed] [Google Scholar]
- O’Connor TP, Lee A, Jarvis JU & Buffenstein R (2002). Prolonged longevity in naked mole-rats: age-related changes in metabolism, body composition and gastrointestinal function. Comparative Biochemistry and Physiolog,y Part A Molecular and Integrative Physiology 133(3), 835–842. [DOI] [PubMed] [Google Scholar]
- O’Riain M & Jarvis J (1998). The dynamics of growth in naked mole-rats: the effects of litter order and changes in social structure. Journal of Zoology 246(1), 49–60. [Google Scholar]
- O’Riain M, Jarvis J, Alexander R, Buffenstein R & Peeters C (2000). Morphological castes in a vertebrate. Proceedings of the National Academy of Sciences USA 97(24), 13194–13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Riain MJ & Faulkes CG (2008). African mole-rats: eusociality, relatedness and ecological constraints. In Ecology of Social Evolution. (eds Korb J and Heinze J) pp. 207–223. Springer-Verlag, Berlin. [Google Scholar]
- O’Riain MJ, Jarvis JU & Faulkes CG (1996). A dispersive morph in the naked mole-rat. Nature 380(6575), 619–621. [DOI] [PubMed] [Google Scholar]
- O’Riain M & Jarvis J (1997). Colony member recognition and xenophobia in the naked mole-rat. Animal Behaviour 53(3), 487–498. [Google Scholar]
- Oiwa Y, Oka K, Yasui H, Higashikawa K, Bono H, Kawamura Y, Miyawaki S, Watarai A, Kikusui T & Shimizu A (2020). Characterization of brown adipose tissue thermogenesis in the naked mole-rat (Heterocephalus glaber), a poikilothermic mammal. Scientific Reports 10, 19488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okanoya K, Yosida S, Barone CM, Applegate DT, Brittan-Powell EF, Dooling RJ & Park TJ (2018). Auditory-vocal coupling in the naked mole-rat, a mammal with poor auditory thresholds. Journal of Comparative Physiology A 204, 905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr ME, Garbarino VR, Salinas A & Buffenstein R (2016). Extended postnatal brain development in the longest-lived rodent: prolonged maintenance of neotenous traits in the naked mole-rat brain. Frontiers in Neuroscience 10, 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamenter ME, Dzal YA, &s Milsom WK (2015). Adenosine receptors mediate the hypoxic ventilatory response but not the hypoxic metabolic response in the naked mole rat during acute hypoxia. Proceedings of the Royal Society B: Biological Sciences. 282(1800), 20141722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamenter ME, Dzal YA, Thompson WA & Milsom WK (2019). Do naked mole rats accumulate a metabolic acidosis or an oxygen debt in severe hypoxia? Journal of Experimental Biology 222(3), jeb191197. [DOI] [PubMed] [Google Scholar]
- Pamenter ME, Hall JE, Tanabe Y & Simonson TS (2020). Cross-species insights into genomic adaptations to hypoxia. Frontiers in Genetics 11, 743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park T, Smith E, Reznick J, Bennett N, Applegate D, Larson J & Lewin G (2021). African naked mole-rats demonstrate extreme tolerance to hypoxia and hypercapnia. In The Extraordinary Biology of the Naked Mole-rat. (eds Buffenstein R, Park T and Holmes M). Springer, New York. [DOI] [PubMed] [Google Scholar]
- Park TJ, Comer C, Carol A, Lu Y, Hong HS & Rice FL (2003). Somatosensory organization and behavior in naked mole-rats: II. Peripheral structures, innervation, and selective lack of neuropeptides associated with thermoregulation and pain. Journal of Comparative Neurology 465(1), 104–120. [DOI] [PubMed] [Google Scholar]
- Park TJ, Lu Y, Juttner R, Smith ES, Hu J, Brand A, Wetzel C, Milenkovic N, Erdmann B, Heppenstall PA, Laurito CE, Wilson SP & Lewin GR (2008). Selective inflammatory pain insensitivity in the African naked mole-rat (Heterocephalus glaber). PLoS Biol 6(1), e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Reznick J, Peterson BL, Blass G, Omerbasic D, Bennett NC, Kuich P, Zasada C, Browe BM, Hamann W, Applegate DT, Radke MH, Kosten T, Lutermann H, Gavaghan V, et al. (2017). Fructose-driven glycolysis supports anoxia resistance in the naked mole-rat. Science 356(6335), 307–311. [DOI] [PubMed] [Google Scholar]
- Patterson BD & Upham NS (2014). A newly recognized family from the Horn of Africa, the Heterocephalidae (Rodentia: Ctenohystrica). Zoological Journal of the Linnean Society. 172(4), 942–963. [Google Scholar]
- Pepper J, Braude S & Lacey E (1991). Vocalizations of the naked mole-rat. In The Biology of the Naked Mole-rat. (eds Sherman PW, Jarvis JUM and Alexander RD), pp. 243–274. Princeton University Press, Princeton. [Google Scholar]
- Perez VI, Buffenstein R, Masamsetti V, Leonard S, Salmon AB, Mele J, Andziak B, Yang T, Edrey Y, Friguet B, Ward W, Richardson A & Chaudhuri A (2009). Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proceedings of the National Academy of Sciences USA 106(9), 3059–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulson SJ, Aldarraji A, Arain II, Dziekonski N, Motlana K, Riley R, Holmes MM & Martin LJ (2020). Naked mole-rats lack cold sensitivity before and after nerve injury. Molecular Pain 16, 1744806920955103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyott SJ, van Tuinen M, Screven LA, Schrode KM, Bai J-P, Barone CM, Price SD, Lysakowski A, Sanderford M & Kumar S (2020). Functional, morphological, and evolutionary characterization of hearing in subterranean, eusocial African mole-rats. Current Biology 30(22), 4329–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravicz ME & Rosowski JJ (1997). Sound-power collection by the auditory periphery of the Mongolian gerbil Meriones unguiculatus: III. Effect of variations in middle-ear volume. The Journal of the Acoustical Society of America 101(4), 2135–2147. [DOI] [PubMed] [Google Scholar]
- Reeve HK (1992). Queen activation of lazy workers in colonies of the eusocial naked mole-rat. Nature 358(6382), 147–149. [DOI] [PubMed] [Google Scholar]
- Ross-Gillespie A, O’Riain MJ & Keller LF (2007). Viral epizootic reveals inbreeding depression in a habitually inbreeding mammal. Evolution 61(9), 2268–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby JG, Smith M & Buffenstein R (2018). Naked mole-rat mortality rates defy Gompertzian laws by not increasing with age. Elife 7, e31157, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby JG, Smith M & Buffenstein R (2019). Response to comment on ‘Naked mole-rat mortality rates defy Gompertzian laws by not increasing with age’. Elife 8, e47047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüppell E (1842). Säugethiere aus der Ordnung der Nager: beobachtet im nördöstlichen Africa. Museum Senckenbergianum: Abhandlungen aus dem Gebiete der Beschreibenden Naturgeschichte. 3, 99–101. [Google Scholar]
- Ryan A (1976). Hearing sensitivity of the mongolian gerbil, Meriones unguiculatis. Journal of the Acoustical Society of America 59(5), 1222–1226. [DOI] [PubMed] [Google Scholar]
- Sacher G (1977). Life table modification and life prolongation. In Handbook of the Biology of Aging. (eds Finch CE and Hayflick L), pp. 582–638. Van Nostrand Reinhold Company, New York. [Google Scholar]
- Salmon AB, Akha AAS, Buffenstein R & Miller RA (2008). Fibroblasts from naked mole-rats are resistant to multiple forms of cell injury, but sensitive to peroxide, ultraviolet light, and endoplasmic reticulum stress. Journals of Gerontology Series a–Biological Sciences and Medical Sciences 63(3), 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K & Miller RA (2005). Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. American Journal of Physiology– Endocrinology and Metabolism 289(1), E23–29. [DOI] [PubMed] [Google Scholar]
- Scantlebury M, Speakman JR, Oosthuizen MK, Roper TJ & Bennett NC (2006). Energetics reveals physiologically distinct castes in a eusocial mammal. Nature 440(7085), 795–797. [DOI] [PubMed] [Google Scholar]
- Seluanov A, Chen Z, Hine C, Sasahara TH, Ribeiro AA, Catania KC, Presgraves DC & Gorbunova V (2007). Telomerase activity coevolves with body mass not lifespan. Aging Cell 6(1), 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seluanov A, Gladyshev VN, Vijg J & Gorbunova V (2018). Mechanisms of cancer resistance in long-lived mammals. Nature Review Cancer 18(7), 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seluanov A, Hine C, Azpurua J, Feigenson M, Bozzella M, Mao Z, Catania KC & Gorbunova V (2009). Hypersensitivity to contact inhibition provides a clue to cancer resistance of naked mole-rat. Proceedings of the National Academy of Sciences USA 106(46), 19352–19357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard A & Kissil JL (2020). The use of non-traditional models in the study of cancer resistance—the case of the naked mole rat. Oncogene, 1–15. [DOI] [PubMed] [Google Scholar]
- Sherman PW, Jarvis JUM & Alexander RD (1991). The Biology of the Naked Mole-rat. Princeton University Press, Princeton. [Google Scholar]
- Siegmann S, Feitsch R, Hart DW, Bennett NC, Penn DJ & Zöttl M (2021). Naked mole-rats (Heterocephalus glaber) do not specialise on cooperative tasks. BioRxiv, 2021.03.22.436002. [Google Scholar]
- Šklíba J, Mazoch V, Patzenhauerová H, Hrouzková E, Lövy M, Kott O & Šumbera R (2012). A maze-lover’s dream: burrow architecture, natural history and habitat characteristics of Ansell’s mole-rat (Fukomys anselli). Mammalian Biology 77(6), 420–427. [Google Scholar]
- Smith ES, Omerbasic D, Lechner SG, Anirudhan G, Lapatsina L & Lewin GR (2011). The molecular basis of acid insensitivity in the African naked mole-rat. Science 334(6062), 1557–1560. [DOI] [PubMed] [Google Scholar]
- Smith ESJ, Park TJ & Lewin GR (2020). Independent evolution of pain insensitivity in African mole-rats: origins and mechanisms. Journal of Comparative Physiology A, 206, 313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M & Buffenstein R (2021). Managed care of naked mole-rats In The Extraordinary Biology of the Naked Mole-rat. (eds Buffenstein R, Park TJ and Holmes MM). Springer, New York. [Google Scholar]
- Smith T, Faulkes C & Abbott D (1997). Combined olfactory contact with the parent colony and direct contact with nonbreeding animals does not maintain suppression of ovulation in female naked mole-rats (Heterocephalus glaber). Hormones and Behavior 31(3), 277–288. [DOI] [PubMed] [Google Scholar]
- Spinks AC, Bennett NC & Jarvis JU (2000). A comparison of the ecology of two populations of the common mole-rat, Cryptomys hottentotus hottentotus: the effect of aridity on food, foraging and body mass. Oecologia 125(3), 341–349. [DOI] [PubMed] [Google Scholar]
- Šumbera R, Mazoch V, Patzenhauerová H, Lövy M, Šklíba J, Bryja J & Burda H (2012). Burrow architecture, family composition and habitat characteristics of the largest social African mole-rat: the giant mole-rat constructs really giant burrow systems. Acta Theriologica 57(2), 121–130. [Google Scholar]
- Sun LY, Spong A, Swindell WR, Fang Y, Hill C, Huber JA, Boehm JD, Westbrook R, Salvatori R & Bartke A (2013). Growth hormone-releasing hormone disruption extends lifespan and regulates response to caloric restriction in mice. Elife 2, e01098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi T, Kotelsky A, Takasugi M, Chang M, Ke Z, Betancourt M, Buckley MR, Zuscik M, Seluanov A & Gorbunova V (2020). Naked mole-rats are extremely resistant to post-traumatic osteoarthritis. Aging Cell 19(11), e13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi M, Firsanov D, Tombline G, Ning H, Ablaeva J, Seluanov A & Gorbunova V (2020). Naked mole-rat very-high-molecular-mass hyaluronan exhibits superior cytoprotective properties. Nature Communications 11(1), 2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KR, Milone NA & Rodriguez CE (2017). Four cases of spontaneous neoplasia in the naked mole-rat (Heterocephalus glaber), a putative cancer-resistant species. Journal of Gerontology Series A, Biological Sciences and Medical Sciences 72(1), 38–43. [DOI] [PubMed] [Google Scholar]
- Thomas HG, Bateman PW, Scantlebury M & Bennett NC (2012). Season but not sex influences burrow length and complexity in the non-sexually dimorphic solitary Cape mole-rat (Rodentia: Bathyergidae). Journal of Zoology 288(3), 214–221. [Google Scholar]
- Thomas HG, Swanepoel D & Bennett NC (2016). Burrow architecture of the Damaraland mole-rat (Fukomys damarensis) from South Africa. African Zoology 51(1), 29–36. [Google Scholar]
- Thorley J, Katlein N, Goddard K, Zöttl M & Clutton-Brock T (2018). Reproduction triggers adaptive increases in body size in female mole-rats. Proceedings of the Royal Society B: Biological Sciences 285(1880), 20180897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Azpurua J, Hine C, Vaidya A, Myakishev-Rempel M, Ablaeva J, Mao Z, Nevo E, Gorbunova V & Seluanov A (2013). High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 499(7458), 346–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toor I, Edwards PD, Kaka N, Whitney R, Ziolkowski J, Monks DA & Holmes MM (2020). Aggression and motivation to disperse in eusocial naked mole-rats, Heterocephalus glaber. Animal Behaviour 168, 45–58. [Google Scholar]
- Uhlon M, Fagerberg L & Hallstrom B (2015). Proteomics. Tissue-based map of the human proteome. Science 347(6220), 1260419. [DOI] [PubMed] [Google Scholar]
- Urison NT & Buffenstein R (1994). Shifts in thermoregulatory patterns with pregnancy in the poikilothermic mammal—the naked mole-rat (Heterocephalus glaber). Journal of Thermal Biology 19(6), 365–371. [Google Scholar]
- Vanden Hole C, Van Daele PA, Desmet N, Devos P & Adriaens D (2014). Does sociality imply a complex vocal communication system? A case study for Fukomys micklemi (Bathyergidae, Rodentia). Bioacoustics 23(2), 143–160. [Google Scholar]
- Vice E, Lagestee S, Browe B, Deb D, Smith E & Park T (2021). Sensory Systems of the African Naked Mole-rat. In The Extraordinary Biology of the Naked Mole-rat. (eds Buffenstein R, Park T and Holmes M). Springer, New York. [DOI] [PubMed] [Google Scholar]
- Viltard M, Durand S, Perez-Lanzon M, Aprahamian F, Lefevre D, Leroy C, Madeo F, Kroemer G & Friedlander G (2019). The metabolomic signature of extreme longevity: naked mole rats versus mice. Aging 11(14), 4783–4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser JH, Bennett NC & van Vuuren BJ (2019). Phylogeny and biogeography of the African Bathyergidae: a review of patterns and processes. PeerJ 7, e7730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J, Cartoceti A & Delaney M (2020). Brains lesions in aging zoo-housed naked mole-rats (Heterocephalus glaber). Veterinary Pathology. 58 (1), 142–146. [DOI] [PubMed] [Google Scholar]
- Watarai A, Arai N, Miyawaki S, Okano H, Miura K, Mogi K & Kikusui T (2018). Responses to pup vocalizations in subordinate naked mole-rats are induced by estradiol ingested through coprophagy of queen’s feces. Proceedings of the National Academy of Sciences USA 115(37), 9264–9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmer P, Stone G & Johnston I (2000). Environmental Physiology of Animals. Blackwell Science, Oxford. [Google Scholar]
- Withers PC & Jarvis JUM (1980). The effect of huddling on thermoregulation and oxygen consumption for the naked mole-rat. Comparative Biochemistry and Physiology 66, 215–219. [Google Scholar]
- Woodley R & Buffenstein R (2001). Reproductive function of naked mole-rats is extremely sensitive to ambient temperature. American Zoologist 41(6), 1629–1629. [Google Scholar]
- Woodley R & Buffenstein R (2002). Thermogenic changes with chronic cold exposure in the naked mole-rat (Heterocephalus glaber). Comparative Biochemistry and Physiology –Part A Molecular and Integrative Physiology 133(3), 827–834. [DOI] [PubMed] [Google Scholar]
- Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, Hodge CL, Haase J, Janes J & Huss JW (2009). BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biology 10(11), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Levitt JB & Buffenstein R (2006). The use of a novel and simple method of revealing neural fibers to show the regression of the lateral geniculate nucleus in the naked mole-rat (Heterocephalus glaber). Brain Research 1077(1), 81–89. [DOI] [PubMed] [Google Scholar]
- Yahav S & Buffenstein R (1991). Huddling behavior facilitates homeothermy in the naked mole rat Heterocephalus glaber. Physiological Zoology 64(3), 871–884. [Google Scholar]
- Yakar S, Werner H & Rosen CJ (2018). Insulin-like growth factors: actions on the skeleton. Journal of Molecular Endocrinology 61(1), T115–T137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosida S & Okanoya K (2009). Naked mole-rat is sensitive to social hierarchy encoded in antiphonal vocalization. Ethology 115(9), 823–831. [Google Scholar]
- Young AJ & Bennett NC (2010). Morphological divergence of breeders and helpers in wild Damaraland mole-rat societies. Evolution 64(11), 3190–3197. [DOI] [PubMed] [Google Scholar]
- Zhao J, Tian X, Zhu Y, Zhang Z, Rydkina E, Yuan Y, Zhang H, Roy B, Cornwell A, Nevo E, Shang X, Huang R, Kristiansen K, Seluanov A, Fang X & Gorbunova V (2020). Reply to: ‘Transformation of naked mole-rat cells’. Nature 583(7814), E8–E13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Tyshkovskiy A, Muñoz-Espín D, Tian X, Serrano M, De Magalhaes JP, Nevo E, Gladyshev VN, Seluanov A & Gorbunova V (2018). Naked mole rats can undergo developmental, oncogene-induced and DNA damage-induced cellular senescence. Proceedings of the National Academy of Sciences USA 115(8), 1801–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K & Wagner TE (1997). A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proceedings of the National Academy of Sciences USA 94(24), 13215–13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zions M, Meehan EF, Kress ME, Thevalingam D, Jenkins EC, Kaila K, Puskarjov M & McCloskey DP (2020). Nest carbon dioxide masks GABA-dependent seizure susceptibility in the naked mole-rat. Current Biology 30, 2068–2077. [DOI] [PubMed] [Google Scholar]
- Zöttl M, Vullioud P, Mendonça R, Ticó MT, Gaynor D, Mitchell A & Clutton-Brock T (2016). Differences in cooperative behavior among Damaraland mole rats are consequences of an age-related polyethism. Proceedings of the National Academy of Sciences USA 113(37), 10382–10387. [DOI] [PMC free article] [PubMed] [Google Scholar]