Abstract
Purpose
Heightened regulations have decreased opioid prescribing across the United States, yet little is known about trends in opioid access among patients dying of cancer.
Methods
Among 270,632 Medicare fee-for-service decedents with poor prognosis cancers, we used part D data to examine trends from 2007-2017 in opioid prescription fills and opioid potency (morphine milligram equivalents per day [MMED]) near the end of life (EOL), defined as the 30 days before death or hospice enrollment. We used administrative claims to evaluate trends in pain-related emergency department (ED) visits near EOL.
Results
Between 2007-2017, the proportion of decedents with poor prognosis cancers receiving ≥1 opioid prescription near EOL declined 15.5%, from 42.0% (95%CI, 41.4%-42.7%) to 35.5% (95%CI, 34.9%-36.0%) and the proportion receiving ≥1 long-acting opioid prescription declined 36.5%, from 18.1% (95%CI, 17.6%-18.6%) to 11.5% (95%CI, 11.1%-11.9%). Among decedents receiving opioids near EOL, the mean daily dose fell 24.5%, from 85.6 MMED (95%CI, 82.9-88.3) to 64.6 (95%CI, 62.7-66.6) MMED. Overall, the total amount of opioids prescribed per decedent near EOL (averaged across those who did and did not receive an opioid) fell 38.0%, from 1075 MMEs per decedent (95%CI, 1042-1109) to 666 MMEs per decedent (95%CI, 646-686). Simultaneously, the proportion of patients with pain-related ED visits increased 50.8%, from 13.2% (95%CI, 12.7%-13.6%) to 19.9% (95%CI, 19.4%-20.4%). Sensitivity analyses demonstrated similar declines in opioid utilization in the 60 and 90 days before death or hospice, and suggested that trends in opioid access were not confounded by secular trends in hospice utilization.
Conclusion:
Opioid use among patients dying from cancer has declined substantially from 2007 to 2017. Rising pain-related ED visits suggests that EOL cancer pain management may be worsening.
BACKGROUND
The U.S. is experiencing a crisis of opioid use disorder. Although the sources of this crisis are complex, an important contributing factor was the significant liberalization of opioid prescribing during the late 1990s and early 2000s.1–4 In response, policymakers, healthcare organizations, and insurers enacted numerous regulations to curb inappropriate prescribing.5–9 Examples include the widespread implementation of prescription drug monitoring programs (PDMPs),10 state and insurance-mandated limits on the dose or quantity of opioid prescriptions,7,9 and the 2016 Centers for Disease Control (CDC) guidelines on opioid use for chronic pain.5 As of 2017 these efforts helped achieve a 30% reduction in per capita opioid prescribing from its peak in 2010-2012.2,4,11,12 Unfortunately, these prescribing reductions have not curbed overdose deaths, which have risen exponentially due to heroin and synthetic opioid overdoses.
Restrictions on opioid prescribing may also have unintended consequences for patients with pain from advanced, incurable cancers.13–15 Over three quarters of patients with advanced malignancies experience pain, with the highest symptom burdens occurring near the end of life (EOL).16,17 Opioids are the cornerstone of managing moderate-to-severe cancer pain, and are effective when used at appropriate doses.18,19 Unfortunately, 30-40% of patients with cancer pain receive analgesics that are insufficient for their pain severity.20–24 Opioid regulations may therefore exacerbate the problem of cancer pain undertreatment.
Opioid prescribing by oncologists is falling at rates similar to generalists,25,26 even though cancer patients are not the intended target of opioid regulations. However, it is unknown whether these trends in prescribing have translated into reduced utilization among patients dying from cancer, versus patients with early-stage cancers or cancer survivors—for whom the long-term risks of opioids are more relevant. To answer this question, we examined national trends in opioid use among Medicare beneficiaries with poor prognosis cancers near EOL and trends in pain-related emergency department (ED) visits as a potential indicator of undertreated pain.
METHODS
Data/study population:
Using the Centers for Medicare & Medicaid Services (CMS) administrative data for a 20% random sample of beneficiaries, decedents with poor prognosis cancers were identified from January 1, 2007 through December 31, 2017—years spanning the initial recognition of the opioid crisis,27,28 ensuing legislative reforms,6,8 and declines in population-based opioid prescribing.4 We focused on decedents aged ≥66 years who were continuously enrolled in fee-for-service Medicare Parts A, B, and D for ≥12 months before death. To examine those who likely died from cancer, instead of dying with cancer or a history of cancer, we identified decedents with ≥1 inpatient or ≥2 outpatient evaluation and management visits with an International Classification of Diseases Ninth (ICD-9) or Tenth (ICD-10) Revision code for a poor prognosis cancer, adapted from a prior list of relevant diagnosis codes29 to also include the ten most common causes of cancer death reported by the American Cancer Society30 and the National Vital Statistics System,31 and supplemented by ICD-9/10 codes for highly lethal rare cancers (e.g. gallbladder cancer, acute myeloid leukemia). Concurrent non-lymphatic metastatic codes were required for solid tumors frequently diagnosed at early stages (e.g., breast, prostate, colorectal). The Harvard Medical School institutional review board approved the study.
Outcomes:
We identified all outpatient opioid prescriptions filled ≤30 days before death or hospice enrollment (for hospice enrollees), referred to hereafter as “near EOL” using Medicare Part D claims. The hospice period was excluded because hospice patients receive opioids within an EOL “comfort pack”—making it difficult to ascertain whether they are prescribed for pain or symptoms of active dying. Moreover, symptom medications are paid by the hospice benefit, not Medicare Part D. Opioid claims were identified from a comprehensive list of opioid National Drug Codes (NDC) from the CDC32 and supplemented by Red Book Online, excluding addiction treatments (e.g., buprenorphine), cough suppressants (e.g., guiafenesin-codeine), and parenteral opioids. Opioid potency was determined by multiplying the total dose of each prescription filled in the last 30 days by standard conversion factors,32 summed across all of a patient’s prescriptions, and averaging to obtain a daily dose in morphine milligram equivalents per day (MMED). Given their distinct roles in cancer pain management, prescription fills were also examined separately for strong short-acting opioids (e.g., immediate release morphine, hydrocodone, oxycodone, hydromorphone), weak short-acting opioids (e.g., tramadol, codeine), and long-acting opioids (e.g., extended-release morphine, methadone, transdermal fentanyl). The average number of opioid prescriptions filled per decedent near EOL was calculated annually. Annual trends at the prescription level were also examined, including the mean days-supply and mean daily dose per prescription—calculated overall, and by medication type.
To examine potential consequences of poorly controlled pain, trends in overall and pain-related ED visits near EOL were examined. Visits were considered pain-related if a relevant ICD-9 or ICD-10 code [based upon pain diagnosis codes from the CMS OP-35 measure]33 was present in the first four positions of the ED claim (Appendix Table 1). ED visits for malignancy-associated pain (338.3 or G89.3) were examined separately as a sensitivity analysis. ED visits for nausea or vomiting were examined as a control condition, using diagnosis codes from the CMS OP-35 measure.
Patient Characteristics:
The Medicare Beneficiary Summary File was used to identify age at death, documented sex, race/ethnicity (White, Black, other), region (Northeast, South, Southwest, West), and median household income at the ZIP code level. Prior diagnoses of 14 coexisting medical conditions possibly associated with receipt of an opioid prescription or ED utilization (using the Chronic Conditions Data Warehouse) were examined.
Statistical Analysis:
Descriptive statistics characterized annual trends in the proportion of decedents filling ≥1 opioid prescription near EOL (overall and by opioid type), the proportion having ≥1 ED visit near EOL (overall, for pain and for nausea/vomiting), opioid potency among decedents filling ≥1 prescription, and the average total dose of opioids filled per decedent near EOL—averaged across those who did and did not fill an opioid. Bivariate linear probability models calculated absolute annual declines in EOL opioid access, using separate regression coefficients for 2007-2011 and 2012-2017 because of natural breakpoints in the data and because population-based opioid prescribing began declining in 2012.4 We then tested the statistical difference between these coefficients. Linear regression models examined annual trends in prescription-level outcomes including the number of opioid prescriptions filled per decedent near EOL, and the mean days-supply and mean daily dose per prescription.
We conducted multiple sensitivity analyses. First, we repeated analyses to assess opioid prescription fills in the 60 days and 90 days before death or hospice enrollment. To ensure that our main findings were not an artifact of secular trends in hospice utilization, we examined trends in EOL opioid access separately for decedents who ultimately enrolled in hospice, and those who did not. We also examined trends in opioid utilization in the 30 days prior to death, without censoring the hospice period. K.G. performed analyses using STATA software, version 16.1 and SAS software, version 9.4.
RESULTS
The cohort included 270,632 patients with poor-prognosis cancers who died between 2007 and 2017 (Figure 1). Decedents’ mean age was 77.3 [7.0] years, 51.8% were men, and 84.8% were White, 9.2% were Black, and 6.0% were of other races (Table 1). The most common cancer types were lung, colorectal, pancreatic, prostate, and breast. Overall, 166,962 patients (61.7%) enrolled on hospice before death, increasing from 57.1% in 2007 to 66.2% in 2017 (Ptrend<.001). The mean hospice length of stay increased slightly from 14.9 days to 15.2 days between 2007 and 2017 (Ptrend=.012). The mean days in a hospital or skilled nursing facility near EOL was 5.0 days, and stable over the study period (Ptrend=.60).
Figure 1: CONSORT Diagram of Study Cohort: Medicare Decedents with Poor Prognosis Cancers (2007-2017).
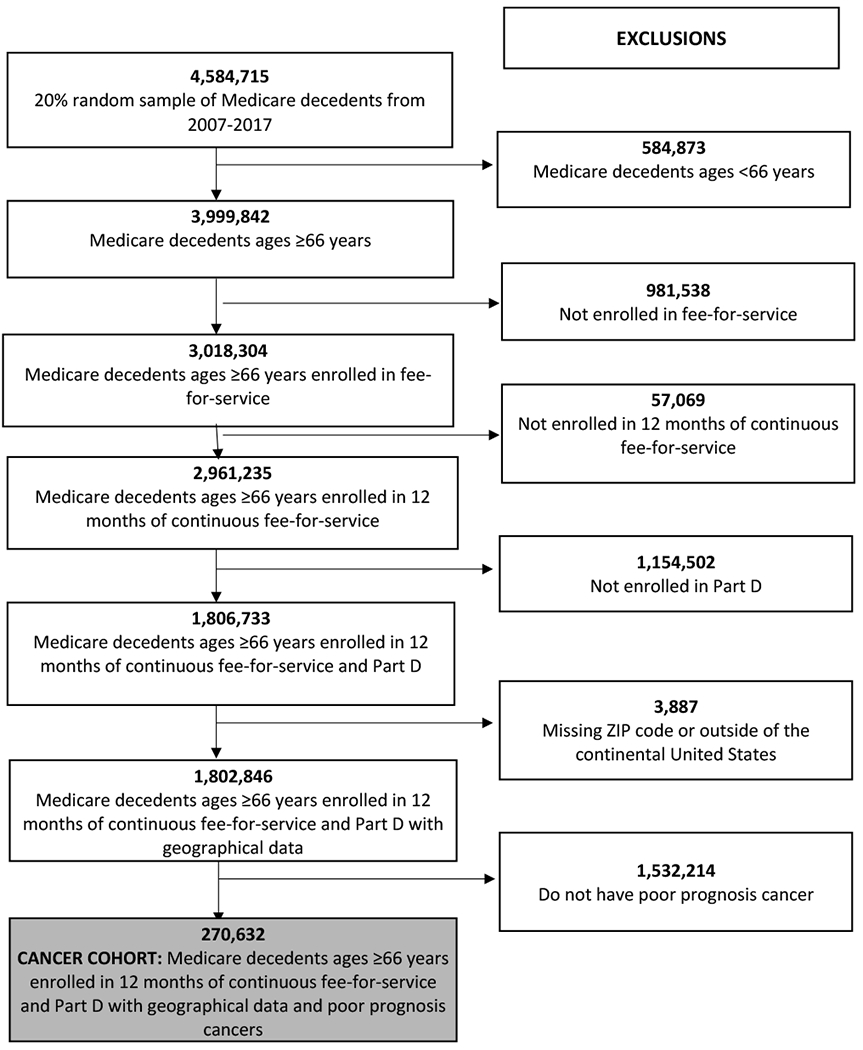
The study cohort was derived from administrative data from the Centers for Medicare and Medicaid Services 20% random sample of beneficiaries. Our final cohort included decedents aged >66 years with poor prognosis cancers who died between January 1, 2007 and December 31, 2017, with continuous fee-for-service Medicare part A, B, and D coverage ≥ 12 months before death. Patients living outside the US, or missing geographical data were excluded.
Table 1:
Patient Characteristics
| Characteristic | Overall population N=270,632 (%) |
2007 N=22,003 (%) |
2012 N= 23,620(%) |
2017 N= 27,345(%) |
|---|---|---|---|---|
| Sex | ||||
| Female | 140,113 (51.8) | 11,949 (54.3) | 12,226 (51.8) | 13,592 (49.7) |
| Race/ethnicity | ||||
| White | 229,383 (84.8) | 18,492 (84.0) | 19,969 (84.5) | 23,369 (85.5) |
| Black | 24,921 (9.2) | 2,202 (10.0) | 2,197 (9.3) | 2,344 (8.6) |
| Other | 16,330 (6.0) | 1,310 (6.0) | 1,454 (6.2) | 1,632 (6.0) |
| Age, years | ||||
| 66-74 | 105,769 (39.1) | 8,606 (39.1) | 9,216 (39.0) | 10,823 (39.6) |
| 75-84 | 111,343 (41.1) | 9,468 (43.0) | 9,595 (40.6) | 10,959 (40.1) |
| 85+ | 53,520 (19.8) | 3,929 (17.9) | 4,809 (20.4) | 5,563(20.3) |
| Cancer diagnosis | ||||
| Lung | 92,472 (34.2) | 7,950 (36.1) | 8,270 (35.0) | 8,546 (31.3) |
| Gastrointestinal | ||||
| Colorectal or anal* | 22,677 (8.4) | 2,098 (9.5) | 1,965 (8.3) | 1,988 (7.3) |
| Pancreas | 22,003 (8.1) | 1,670 (7.6) | 1.971 (8.3) | 2,582 (9.4) |
| Esophagogastric | 14,050 (5.2) | 1,175 (5.3) | 1,218 (5.2) | 1,414 (5.2) |
| Liver, gallbladder, biliary | 12,646 (4.7) | 965 (4.4) | 1,227 (5.2) | 1,607 (5.9) |
| Genitourinary | ||||
| Prostate* | 17,943 (6.6) | 1,402 (6.4) | 1,503 (6.4) | 1,827 (6.7) |
| Bladder* | 7,034 (2.6) | 482 (2.2) | 570 (2.4) | 749 (2.7) |
| Kidney* | 6,370 (2.4) | 470 (2.1) | 552 (2.3) | 660 (2.4) |
| Hematologic | ||||
| Non-Hodgkin lymphomas | 14,560 (5.4) | 1,311 (6.0) | 1,259 (5.3) | 1,363 (5.0) |
| Acute leukemias | 9.992 (3.7) | 532 (2.4) | 741 (3.1) | 1,716 (6.3) |
| Breast* | 17,915 (6.6) | 1,484 (6.7) | 1,568 (6.6) | 1,909 (7.0) |
| Gynecologic | ||||
| Ovarian* | 7,039 (2.6) | 584 (2.7) | 577 (2.4) | 721 (2.6) |
| Uterine* | 3,347 (1.2) | 236 (1.1) | 330 (1.4) | 285 (1.0) |
| Brain | 7,629 (2.8) | 624 (2.8) | 689 (2.9) | 768 (2.8) |
| Melanoma* | 4,303 (1.6) | 276 (1.3) | 391 (1.7) | 435 (1.6) |
| Other | 10,654 (3.9) | 745 (3.4) | 789 (3.3) | 775 (2.8) |
| Presence of chronic illness | ||||
| Acute myocardial infarction | 24,053 (8.9) | 1,727 (7.8) | 2,150 (9.1) | 2,657 (9.7) |
| Ischemic Heart Disease | 177,108 (65.4) | 13,848 (62.9) | 15,618 (66.1) | 18,112 (66.2) |
| Heart Failure | 130,973 (48.4) | 10,980 (49.9) | 11,461 (48.5) | 12,920 (47.2) |
| Atrial fibrillation | 71,290 (26.3) | 5,338 (24.3) | 6,204 (26.3) | 7,659 (28.0) |
| Stroke / Transient Ischemic Attack | 64,074 (23.7) | 5,017 (22.8) | 5,587 (23.7) | 6,576 24.0) |
| Chronic Obstructive Pulmonary Disease | 145,034 (53.6) | 11,899 (54.1) | 12,954 (54.8) | 14,057 (51.4) |
| Chronic kidney disease | 144,009 (53.2) | 8,778 (39.9) | 12,358 (52.3) | 17,727 (64.8) |
| Rheumatoid Arthritis/Osteoarthritis | 162,656 (60.1) | 11,520 (52.4) | 14,139 (59.9) | 17,845 (65.3) |
| Hip/Pelvic Fracture | 19,444 (7.2) | 1,568 (7.1) | 1,675 (7.1) | 1,917 (7.0) |
| Depression | 113,479 (41.9) | 7,700 (35.0) | 10,081 (42.7) | 12,851 (47.0) |
| Alzheimer or other dementias | 63,591 (23.5) | 4,681 (21.3) | 5,411 (22.9) | 7,579 (27.7) |
| Region | ||||
| Northeast | 55,673 (20.6) | 4,329 (19.7) | 4,920 (20.8) | 5,791 (21.2) |
| West | 44,494 (16.4) | 3,485 (15.8) | 3,952 (16.7) | 4,683 (17.1) |
| Mid-West | 66,596 (24.6) | 5,557 (25.3) | 5,875 (24.9) | 6,637 (24.3) |
| South | 103,869 (38.4) | 8,633 (39.2) | 8,873 (37.6) | 10,234 (37.4) |
| Healthcare utilization near end-of-life | ||||
| Hospice enrollment (%) | 166,953 (61.7) | 12,570 (57.1) | 14,439 (61.1) | 18,100 (66.2) |
| Hospice length of stay [mean (SD)] | 15.3 (10.6) | 14.9 (10.2) | 15.4 (10.6) | 15.2 (10.7) |
| Days in a facility in the 30d before death or hospice [mean (SD)] | 5.0 (7.4) | 5.2 (7.6) | 4.8 (7.2) | 5.7 (8.4) |
Diagnosis required a concurrent, non-lymphatic metastatic code for inclusion in the cohort
Between 2007 and 2017 the proportion of patients with poor prognosis cancer filling ≥1 opioid prescription near EOL fell from 42.0% (95%CI: 41.4%, 42.7%) to 35.5% (95%CI: 34.9%, 36.0%) (Figure 2A, Table S1)—declining faster from 2012-2017 (−1.1 percentage points per year; 95%CI: −1.4, −0.9) than 2007-2011 (−0.2 percentage points per year; 95%CI: −0.4, −0.1; P<0.001). The proportion filling long-acting opioids near EOL fell from 18.1% (95%CI: 17.6%, 18.6%) to 11.5% (95%CI: 11.1%, 11.9%)—also declining faster from 2012-2017 (−0.8 percentage points per year; 95%CI: −0.9, −0.7) than 2007-2011 (−0.5 percentage points per year; 95%CI: −0.6, −0.4; P=.001). The proportion filling strong, short-acting opioids near EOL fell from 31.7% (95%CI: 31.1%, 32.3%) to 28.5% (95%CI: 28.0%, 29.0)—being initially stable from 2007-2011 (0.3 percentage points per year; 95%CI: −0.2, 0.9), and then declining 1.0 percentage points per year (95% CI:−1.2, −0.8; p<0.001) beginning in 2012. The proportion filling weak short-acting opioids near EOL fell from 8.4% (95%CI:8.1%, 8.8%) to 6.5% (95%CI: 6.2%, 6.8%)—declining 0.6 percentage points per year between 2007-2011 (95%CI: −1.0, −0.2), and then stabilizing after 2012 (0.1 percentage points per year; 95%CI: −0.0, 0.2; p=0.02).
Figure 2. Annual trends from 2007-2017 in opioid prescription fills, opioid potency, and the total dose of opioids filled by decedents with poor prognosis cancers near the end of life (EOL).
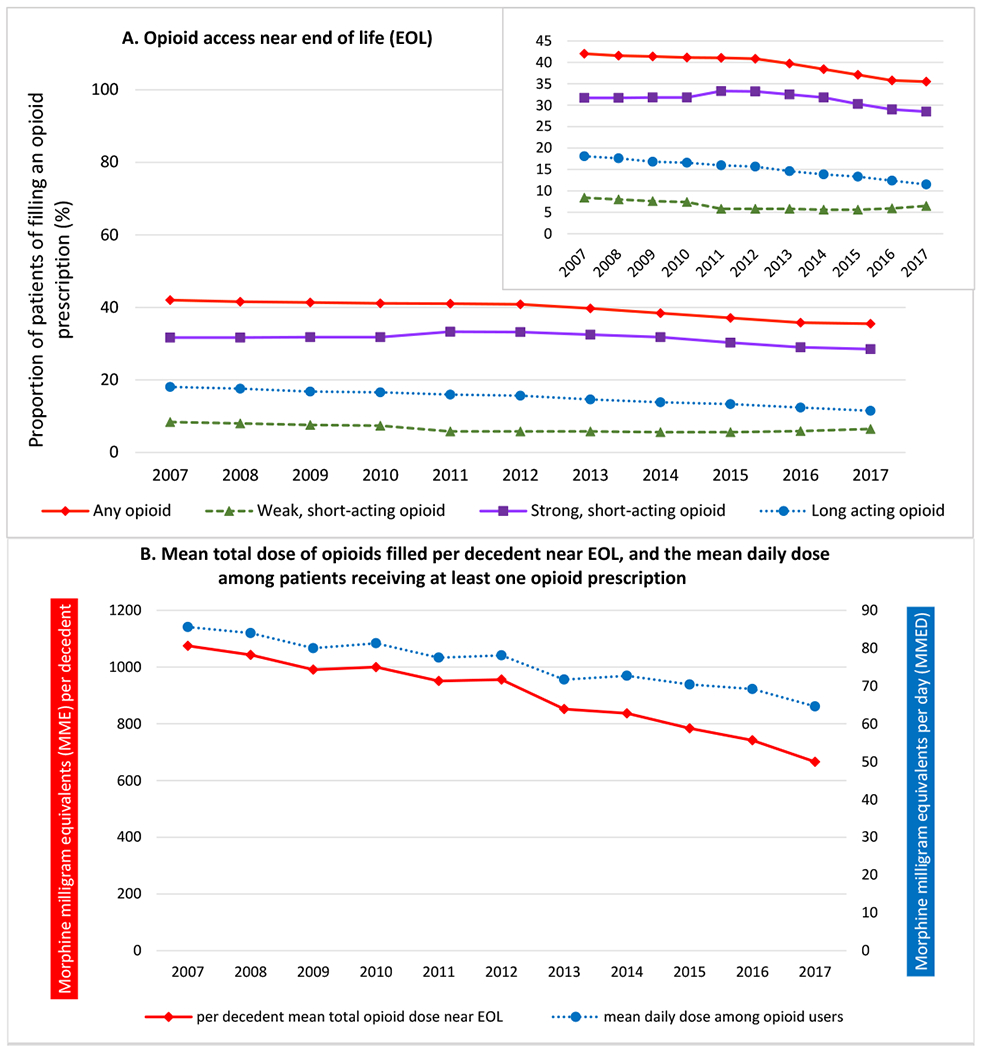
Panel 2A presents the proportion of patients filling any opioid prescription, including weak short-acting opioids, strong short-acting opioids, and long-acting opioids in the 30 days before death or hospice enrollment. The inset shows the same data, on an enlarged y axis. Panel 2B: The red line presents the mean total dose of opioids (in morphine milligram equivalents) provided to patients with poor prognosis cancers near EOL. This was calculated by summing the morphine equivalent dose of all opioid prescriptions filled by decedents near EOL in a given year, and dividing it by the number of decedents that year. The blue line presents the population mean daily opioid dose in morphine milligram equivalents per day (MMEDs) received by patients who filled ≥1 opioid prescription near EOL. Near EOL is defined as the last 30 days before death or hospice enrollment
Among patients filling ≥1 opioid near EOL, the population mean daily dose fell 24.5%, from 85.6 MMED (95%CI, 82.9 to 88.3) to 64.6 MMED (95%CI, 62.7 to 66.6) between 2007-2017 (Figure 2B, Table S2). Overall, the total dose of opioids filled by poor prognosis cancer decedents near EOL (averaged across those who did and did not receive an opioid) fell 38.0%, from 1075 MMEs (95%CI: 1042, 1109) to 666 MMEs (95%CI: 646, 686) per decedent.
As shown in Figure 3A, between 2007-2017 the number of opioid prescriptions filled per decedent near EOL fell from 0.887 to 0.584—reflecting an annual decline of 4.1% (95%CI: −4.8%, −3.4%). The number of long-acting opioid prescriptions filled per decedent fell by half, from 0.28 to 0.14—reflecting an annual decline of 6.3% (95%CI: −6.7%, −5.8%) (Figure 3B). The number of strong short-acting opioid prescriptions filled per decedent declined 1.3% annually (95%CI: −1.9%, −0.7%) and the number of weak short-acting opioid prescriptions declined 0.5% annually (95%CI: −0.6%, −0.3%). The mean daily dose per prescription fell 2.2% annually (95%CI: −2.4%, −2.0%) for long-acting opioids, 1.9% annually (95%CI: −2.1%, −1.7%) for strong short-acting opioids, and 7.8% annually (95%CI: −7.9%, −7.7%) for weak short-acting opioids. In contrast, the mean days-supply per prescription rose modestly across all opioid types.
Figure 3. 2007-2017 changes in the number of opioid prescriptions filled per poor prognosis cancer decedent near EOL, and the mean days-supply and mean daily dose per prescription.
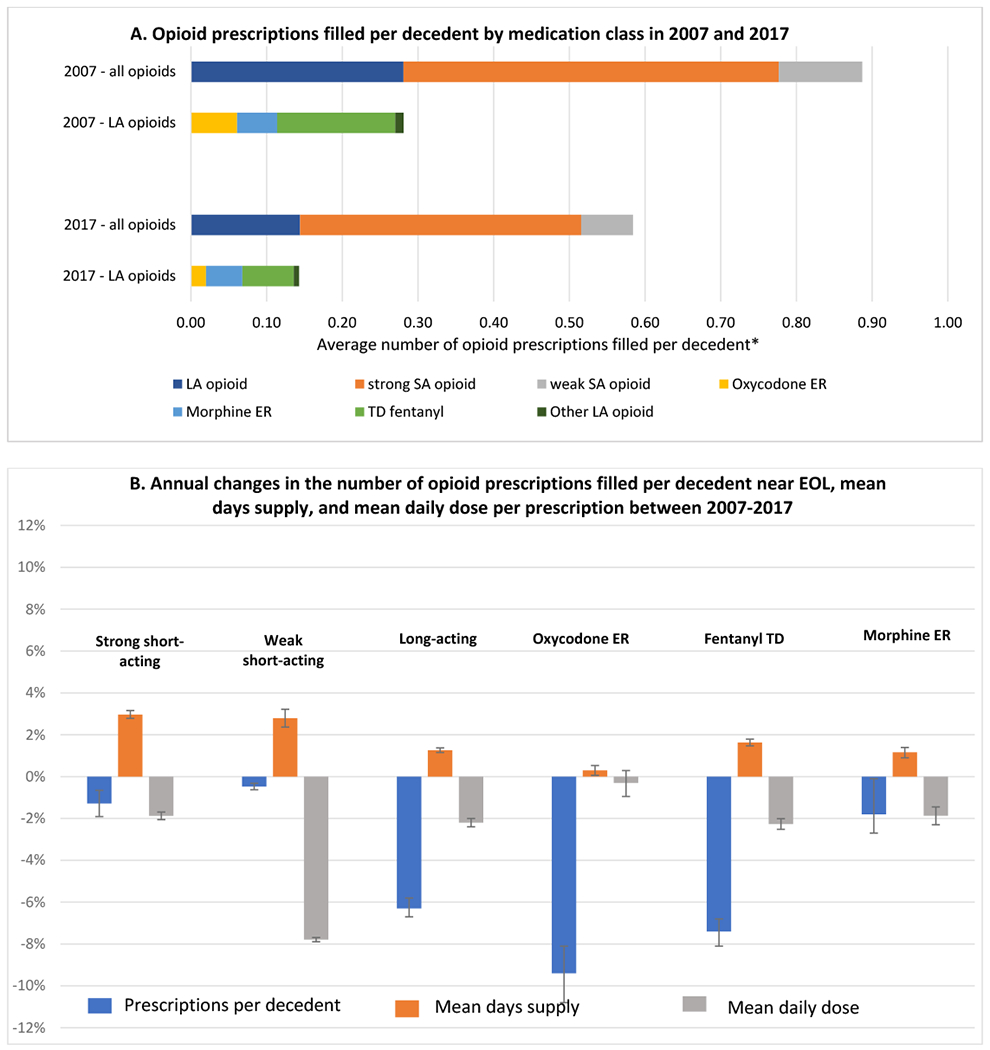
Panel 3A. The first two columns show the distribution of 25,006 opioid prescriptions filled by 22,003 patients near the EOL in 2007; the last two columns show the distribution of 22,974 opioid prescriptions filled by 27,345 patients near the EOL in 2017. *The x-axis represents the average number of opioid prescriptions filled per decedent (number of prescriptions filled by patients with poor prognosis cancers in the last 30 days, divided by the number of decedents with poor prognosis cancers in that year. Panel 3B. Blue bars represent the unadjusted annual change in rate in the number of opioid prescriptions filled per decedent; orange bars represent the unadjusted annual growth rate in the mean days-supply per prescription; grey bars represent the unadjusted annual change in rate in the mean daily dose per prescription, all calculated from 2007-2017. Error bars represent 95% confidence intervals derived from linear regression models.
Sensitivity analyses demonstrated that between 2007-2017 there were also meaningful declines in opioid utilization in the 60 and 90 days before death or hospice enrollment (Tables S3–5). Suggesting that our main findings were not attributable to secular trends in hospice utilization, stratified analyses demonstrated declines in EOL opioid utilization among poor prognosis cancer decedents who enrolled in hospice, and those who did not (Table S6). Moreover, when the EOL period was defined as the 30 days before death without censoring the hospice period, declines in EOL opioid utilization were similar to our primary analyses (Tables S7–8).
Rates of pain-related ED visits were explored as a potential consequence of undertreated pain. As shown in Figure 4 and Table S9, between 2007-2017, the proportion of patients with ≥1 pain-related ED visit near EOL increased 50.8%, from 13.2% (95%CI: 12.7%, 13.6%) to 19.9% (95%CI: 19.4%, 20.4%); the proportion with ≥1 ED visit with a code for malignancy-associated pain doubled, from 1.2% (95%CI: 1.1%, 1.3%) to 2.4% (95%CI: 2.2%, 2.5%). In contrast, the proportion of patients with ≥1 ED visit for nausea/vomiting did not change statistically over the study period (Ptrend=0.168), and the proportion with any ED visit near EOL increased 16%, from 55.6% (95%CI: 54.9%, 56.2%) to 64.5% (95%CI: 63.9%, 65.0%).
Figure 4. Annual trends in the proportion of patients with poor prognosis cancers receiving care in an emergency department (ED) near the EOL overall, and for pain.
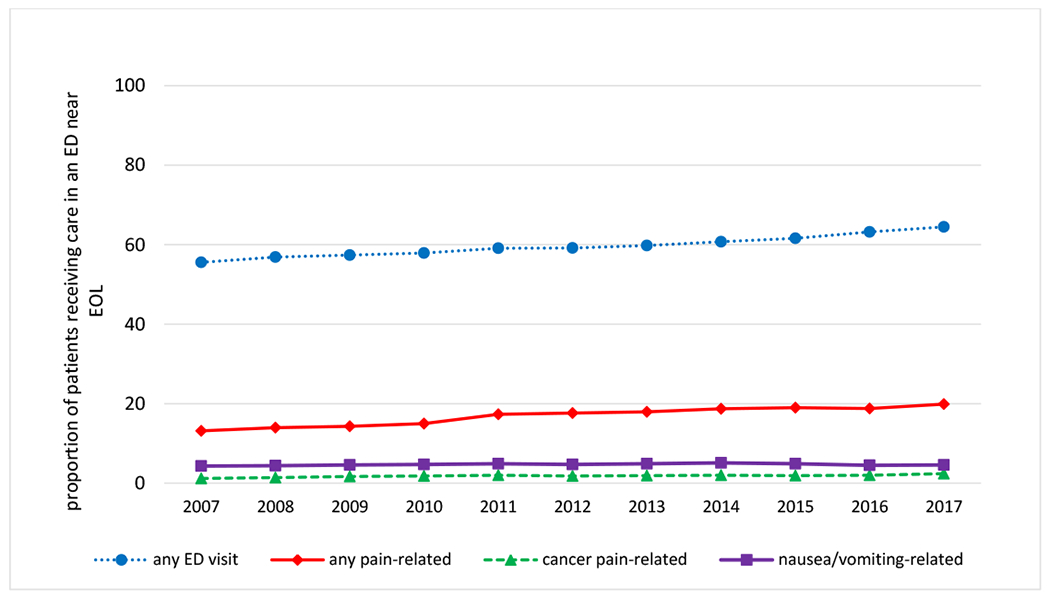
The blue line presents annual trends in the proportion of patients having ≥ 1 ED visit near EOL; the red line presents trends in the proportion of patients having ≥1 ED visit for pain near EOL using the CMS OP-35 definition; the green line presents trends in the proportion of patients having ≥1 ED visit with an ICD-9 or ICD-10 code for malignancy associated pain near EOL; the purple line presents trends in the proportion of patients having ≥1 ED visit for nausea or vomiting near EOL using the CMS OP-35 definition Outcomes were examined in the last 30d before death or hospice enrollment.
DISCUSSION
In this large representative cohort of Medicare decedents with poor prognosis cancers, we found that access to opioids near EOL decreased substantially between 2007 and 2017. The total amount of opioids prescribed per decedent fell by nearly 40% in relative terms. Moreover, the proportion of patients receiving any opioid near EOL decreased by 15.5%, and the proportion receiving long-acting opioids decreased by 36.5%. Declines in EOL opioid access were accompanied by a 50% rise in pain-related ED visits, suggesting that pain management may be worsening for patients dying of cancer.
This study provides the most direct evidence to date that advanced cancer patients have experienced reduced access to prescription opioids in the wake of the opioid crisis. Advocacy organizations have lobbied strongly to protect cancer patients from heightened opioid regulations34 based largely upon concerns of experts,13 non-peer-reviewed opinion surveys,35,36 and anecdotal evidence that regulations were becoming obstacles to care.15 Two recent analyses of 2013-2017 Medicare prescriber data found that oncologists’ opioid prescribing fell by approximately 21%, similar to that observed among generalists.25,26 These studies were unable to determine if these reductions affected patients with advanced-stage cancer, or patients with early-stage cancers or survivors—for whom a shift away from opioid analgesics might be appropriate.26,37 Our study clarifies these findings by demonstrating that patients dying of cancer have experienced notable reductions in opioid access near EOL. Our study also demonstrates that opioid prescribing has fallen in numerous ways, including: the number of prescriptions, use of long-acting opioids, and the potency of prescriptions.
Interestingly, the trends in opioid access observed here do not entirely mirror those described in the general population. Per-capita opioid prescribing in the US rose until 2010, and only in 2012 did it decline consistently.4,12,38 In contrast, EOL opioid utilization among cancer decedents was slowly declining from the beginning of the study period and accelerated after 2012. These trends may point to differing factors driving opioid utilization in the general population versus patients dying of cancer. It is thought that the increase in population-based opioid prescribing in the early 2000’s was driven both by the rising incidence of new opioid prescriptions and by the rising prevalence of people on chronic, long-term opioid therapy—many of whom required escalating doses over time.4,39,40 In contrast, EOL opioid utilization is by definition time-limited, and should be less affected by long-term opioid use. EOL opioid use among cancer decedents may therefore have been more sensitive to regulatory pressures and declined earlier than the general population.
The specific mechanisms for reduced EOL opioid access are less certain and likely multi-factorial. State- and insurance-based opioid regulations expanded rapidly over the study period,6–8,41,42 which may have disincentivized prescribing or prevented patients from filling prescriptions. A notable example was the expansion of electronic prescription drug monitoring programs (PDMPs) that began in the early 2000’s.43 Electronic PDMPs have now been implemented in every state but Missouri,8 and have been shown to reduce prescribing of Schedule II opioids10—even among oncologists.44 Simultaneously, Medicare Part D plans increasingly adopted opioid coverage restrictions,9 which reduce long-acting opioid prescribing, in particular.45 Although coverage restrictions can usually be overridden by a cancer diagnosis, patients may be left without pain medication or must pay out-of-pocket while prior authorizations are processed. More recently, there has been a proliferation of state- and pharmacy-mandated limits on the duration and doses of opioid prescriptions.7,46,47 The impact of this evolving regulatory landscape on cancer patients requires monitoring. Non-policy factors may also have contributed to opioid declines. Clinicians may have become more reluctant to prescribe opioids as their risks were increasingly recognized and prescribing became more onerous.48 Moreover, patients may have become more reluctant to accept opioids as these analgesics became increasingly stigmatized.49,50 Further research is required to identify the main drivers of declining EOL opioid utilization and to identify practical policy solutions.
The most substantial reductions in opioid prescribing observed were for long-acting medications, particularly extended-release oxycodone and transdermal fentanyl. Long-acting opioids play a critical role in managing severe or persistent pain related to advanced malignancies because they prevent severe pain that occurs when short-acting opioids are used only “on demand.” Yet, long-acting opioids have long been recognized for their abuse potential51 and have therefore been more tightly regulated and more highly stigmatized than other opioids.5,9,45 This may have led to earlier and steeper declines in prescribing of long-acting versus short-acting opioids. Strong, short-acting opioid prescribing was relatively stable during the first half of the study, and then declined modestly beginning in 2012—driven primarily by downtrends in morphine and hydrocodone use (Table S10). We observed a more precipitous decline in hydrocodone prescribing beginning in 2014 when the DEA rescheduled it from Schedule III to the more restrictive Schedule II.52 The proportion of patients receiving weak short-acting opioids declined during the half of the study, and then stabilized after 2011. This early decline was largely attributable to the withdrawal of propoxyphene-containing products from the US market in response to a 2010 FDA warning for cardiotoxicity.53 Weak opioids hold a controversial place in cancer pain management and have been proven inferior to low-dose morphine for treating moderate cancer pain.54,55 It is therefore problematic to observe weak opioid use persist, while prescribing of strong short-acting opioids and long-acting opioids continue to decline.
We were unable to determine whether the declines in EOL opioid prescribing directly harmed patients; however, the observed rise in pain-related ED visits raises this troubling possibility. Alternatively, these trends in pain-related ED visits could reflect secular shifts in providers’ coding practices. Unfortunately, we were unable to test these hypotheses because Medicare claims do not provide a valid way to ascertain patients’ pain levels. Nevertheless, it seems likely that reduced opioid access could exacerbate the problem of cancer pain undertreatment21 and threaten decades of progress in EOL cancer care.56
This study has several limitations. First, it did not examine opioid use among patients receiving hospice services, although sensitivity analyses suggest this did not bias the primary findings. Second, it could not determine whether patients used the opioid prescriptions filled; however, having opioids available is arguably just as relevant. Third, claims may not accurately characterize whether an ED visit was truly precipitated by pain and our assessment likely underestimates the true prevalence of pain among patients receiving care the ED. Finally, the study focused on older Medicare beneficiaries and may represent a conservative estimate of the reductions in opioid access near EOL. Future studies should examine opioid access in other populations.
In summary, during the years spanning heightened opioid regulations there have been striking reductions in opioid access among older patients dying from cancer. Future research is needed to understand the mechanisms for these declines, populations that may have been disproportionately affected (i.e. racial minorities), and how opioid prescribing may have changed across other phases of cancer care and for cancer patients with commercial or Medicaid insurance. Finally, policy solutions are needed to mitigate the burden of opioid regulations on patients with terminal cancer.
Supplementary Material
Key objective:
To determine trends in opioid utilization among patients with poor prognosis cancers near the end-of-life (EOL).
Knowledge generated:
Among 270,632 Medicare decedents with poor prognosis cancers who died between 2007-2017, we observed a 34% decline in opioid prescription fills per decedent, a 50% decline in long-acting opioid prescription fills per decedent, and a 38% decline in the total dose of opioids filled per decedent near the EOL. Over the same period, the proportion of decedents undergoing one or more pain-related emergency department visit near EOL increased by 51%.
Relevance:
Patients with terminal cancer have experienced substantial declines in opioid access and have increasingly received treatment for pain through the emergency department. More research is needed to determine the causes of these trends, and to advocate for sensible policies that balance the needs of advanced cancer populations with broader societal concerns about opioid misuse disorder and opioid safety.
Source of funding:
This study was funded by AHRQ U19HS024072
REFERENCES
- 1.Barnett ML, Olenski AR, Jena AB: Opioid-Prescribing Patterns of Emergency Physicians and Risk of Long-Term Use. N Engl J Med 376:663–673, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dart RC, Surratt HL, Cicero TJ, et al. : Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med 372:241–8, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Bohnert AS, Valenstein M, Bair MJ, et al. : Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA 305:1315–21, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Guy GP Jr., Zhang K, Bohm MK, et al. : Vital Signs: Changes in Opioid Prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep 66:697–704, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowell D, Haegerich TM, Chou R: CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. JAMA 315:1624–45, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meara E, Horwitz JR, Powell W, et al. : State Legal Restrictions and Prescription-Opioid Use among Disabled Adults. N Engl J Med 375:44–53, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis CS, Lieberman AJ, Hernandez-Delgado H, et al. : Laws limiting the prescribing or dispensing of opioids for acute pain in the United States: A national systematic legal review. Drug Alcohol Depend 194:166–172, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Parker AM, Strunk D, Fiellin DA: State Responses to the Opioid Crisis. J Law Med Ethics 46:367–381, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Samuels EA, Ross JS, Dhruva SS: Medicare Formulary Coverage Restrictions for Prescription Opioids, 2006 to 2015. Ann Intern Med 167:895–896, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Bao Y, Pan Y, Taylor A, et al. : Prescription Drug Monitoring Programs Are Associated With Sustained Reductions In Opioid Prescribing By Physicians. Health Aff (Millwood) 35:1045–51, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu W, Chernew ME, Sherry TB, et al. : Initial Opioid Prescriptions among U.S. Commercially Insured Patients, 2012-2017. N Engl J Med 380:1043–1052, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guy GP Jr., Zhang K, Schieber LZ, et al. : County-Level Opioid Prescribing in the United States, 2015 and 2017. JAMA Intern Med 179:574–576, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page R, Blanchard E: Opioids and Cancer Pain: Patients’ Needs and Access Challenges. J Oncol Pract 15:229–231, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Paice JA: Cancer pain management and the opioid crisis in America: How to preserve hard-earned gains in improving the quality of cancer pain management. Cancer 124:2491–2497, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Rubin R: Limits on Opioid Prescribing Leave Patients With Chronic Pain Vulnerable. JAMA 321:2059–2062, 2019 [DOI] [PubMed] [Google Scholar]
- 16.van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, et al. : Update on Prevalence of Pain in Patients With Cancer: Systematic Review and Meta-Analysis. J Pain Symptom Manage 51:1070–1090 e9, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Cleeland CS, Gonin R, Baez L, et al. : Pain and treatment of pain in minority patients with cancer. The Eastern Cooperative Oncology Group Minority Outpatient Pain Study. Ann Intern Med 127:813–6, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Caraceni A, Hanks G, Kaasa S, et al. : Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 13:e58–68, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Dalal S, Bruera E: Access to opioid analgesics and pain relief for patients with cancer. Nat Rev Clin Oncol 10:108–16, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Breivik H, Cherny N, Collett B, et al. : Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol 20:1420–33, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Deandrea S, Montanari M, Moja L, et al. : Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol 19:1985–91, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon JH, Hui D, Chisholm G, et al. : Experience of barriers to pain management in patients receiving outpatient palliative care. J Palliat Med 16:908–14, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cleeland CS, Gonin R, Hatfield AK, et al. : Pain and its treatment in outpatients with metastatic cancer. N Engl J Med 330:592–6, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Fisch MJ, Lee JW, Weiss M, et al. : Prospective, observational study of pain and analgesic prescribing in medical oncology outpatients with breast, colorectal, lung, or prostate cancer. J Clin Oncol 30:1980–8, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal A, Roberts A, Dusetzina SB, et al. : Changes in Opioid Prescribing Patterns Among Generalists and Oncologists for Medicare Part D Beneficiaries From 2013 to 2017. JAMA Oncol, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jairam V, Yang DX, Pasha S, et al. : Temporal Trends in Opioid Prescribing Patterns Among Oncologists in the Medicare Population. J Natl Cancer Inst 113:274–281, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall AJ, Logan JE, Toblin RL, et al. : Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA 300:2613–20, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Paulozzi LJ, Ryan GW: Opioid analgesics and rates of fatal drug poisoning in the United States. Am J Prev Med 31:506–11, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Obermeyer Z, Powers BW, Makar M, et al. : Physician Characteristics Strongly Predict Patient Enrollment In Hospice. Health Aff (Millwood) 34:993–1000, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2019. CA Cancer J Clin 69:7–34, 2019 [DOI] [PubMed] [Google Scholar]
- 31.National Vital Statistics System, Centrs for Disease Control and Prevention. https://www.cdc.gov/nchf/nvss/index.htm.Date of Access April 7, 2021
- 32.Centers for Disease Control and Prevention. Data Resources: Analyzing Prescription Data and Morphine Milligram Equivalents, 2018. https://www.cdc.gov/drogoverdose/resources/data.html.date accessed April 7, 2021.
- 33.Admissions and Emergency Department Visits for Patients Receiving Outpatient Chemotherapy Measure Technical Report, Mathmatica Policy Research, 2016 [Google Scholar]
- 34.ASCO Policy Statement on Opioid Therapy: Protecting Access to Treatment for Cancer-Related Pain, American Society of Clinical Oncology, 2016 [Google Scholar]
- 35.Opioids and cancer pain: patient needs and access challenges, State of Cancer Care in America, American Society of Clinical Oncology, 2017 [Google Scholar]
- 36.American Cancer Society Cancer Action Network, Patient Quality of Life Coalition: Key Findings Summary: Opioid Access Research Project, June2018
- 37.Enzinger AC, Wright AA: Reduced opioid prescribing by oncologists: progress made, or ground lost? J Natl Cancer Inst, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schieber LZ, Guy GP Jr., Seth P, et al. : Trends and Patterns of Geographic Variation in Opioid Prescribing Practices by State, United States, 2006-2017. JAMA Netw Open 2:e190665, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boudreau D, Von Korff M, Rutter CM, et al. : Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf 18:1166–75, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Von Korff M, Saunders K, Thomas Ray G, et al. : De facto long-term opioid therapy for noncancer pain. Clin J Pain 24:521–7, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis CS, Carr D: Physician continuing education to reduce opioid misuse, abuse, and overdose: Many opportunities, few requirements. Drug Alcohol Depend 163:100–7, 2016 [DOI] [PubMed] [Google Scholar]
- 42.Garcia MC, Dodek AB, Kowalski T, et al. : Declines in Opioid Prescribing After a Private Insurer Policy Change - Massachusetts, 2011-2015. MMWR Morb Mortal Wkly Rep 65:1125–1131, 2016 [DOI] [PubMed] [Google Scholar]
- 43.Clark T, Eadie J, Kreiner P, et al. : Prescription Drug Monitoring Programs: An Assessment of the Evidence for Best Practices. Philadelphia, Heller School for Social Policy and Management, Brandeis University, 2012 [Google Scholar]
- 44.Graetz I, Yarbrough CR, Hu X, et al. : Association of Mandatory-Access Prescription Drug Monitoring Programs With Opioid Prescriptions Among Medicare Patients Treated by a Medical or Hematologic Oncologist. JAMA Oncol, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnett ML, Olenski AR, Thygeson NM, et al. : A Health Plan’s Formulary Led To Reduced Use Of Extended-Release Opioids But Did Not Lower Overall Opioid Use. Health Aff (Millwood) 37:1509–1516, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.AMA says Walmart “refusal to fill” policy interferes with the practice of medicine, California Medical Association, 2019. https://www.cmadocs.org/newsroom/news/view/articled/28201.Date of Access, May 19, 2021
- 47.CVS Health Response to the Nation’s Opioid Crisis, 2017. https://cvshealth.com/thought-leadership/cvs-health-enterprise-response-opioid-epidemic/cvs-health-responds-to-nations-opioid-crisis
- 48.Barnett ML: Opioid Prescribing in the Midst of Crisis - Myths and Realities. N Engl J Med 382:1086–1088, 2020 [DOI] [PubMed] [Google Scholar]
- 49.Azizoddin DR, Knoerl R, Adam R, et al. : Cancer pain self-management in the context of a national opioid epidemic: Experiences of patients with advanced cancer using opioids. Cancer, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bulls HW, Hoogland AI, Craig D, et al. : Cancer and Opioids: Patient Experiences With Stigma (COPES)-A Pilot Study. J Pain Symptom Manage 57:816–819, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shah A, Hayes CJ, Martin BC: Characteristics of Initial Prescription Episodes and Likelihood of Long-Term Opioid Use - United States, 2006-2015. MMWR Morb Mortal Wkly Rep 66:265–269, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drug Enforcement Administration DoJ: Schedules of controlled substances: rescheduling of hydrocodone combination products from schedule III to schedule II. Final rule. Fed Regist 79:49661–82, 2014 [PubMed] [Google Scholar]
- 53.Hayes CJ, Hudson TJ, Phillips MM, et al. : The influence of propoxyphene withdrawal on opioid use in veterans. Pharmacoepidemiol Drug Saf 24:1180–8, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bandieri E, Romero M, Ripamonti CI, et al. : Randomized Trial of Low-Dose Morphine Versus Weak Opioids in Moderate Cancer Pain. J Clin Oncol 34:436–42, 2016 [DOI] [PubMed] [Google Scholar]
- 55.Marinangeli F, Ciccozzi A, Leonardis M, et al. : Use of strong opioids in advanced cancer pain: a randomized trial. J Pain Symptom Manage 27:409–16, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Greco MT, Roberto A, Corli O, et al. : Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol 32:4149–54, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


