Abstract
OBJECTIVE:
SARS-CoV-2 infection is a major cause of morbidity and mortality, often as a result of Acute Respiratory Distress Syndrome (ARDS). Respiratory failure is characterized by a hyperinflammatory immune response, lung vascular injury and edema formation. The potential for immunomodulatory therapy to prevent lung vascular injury and edema formation is not well understood.
APPROACH AND RESULTS:
We show that SARS-CoV-2 infection in humanized K18-hACE-2 mice activated inflammatory NLRP3-Caspase-1 pyroptotic signaling in lungs, release of IL-1β and downregulation of the lung endothelial adherens junction protein VE-cadherin. Primary human lung microvascular endothelial cells (hLMVECs) were susceptible to SARS-CoV-2 infection and displayed pyroptosis-like injury. We observed profound lung vascular injury post-SARS-CoV-2 infection and resultant protein-rich lung edema formation. Selective blockade of IL-1 receptor signaling by the IL-1 receptor antagonist (IL-1RA) Anakinra prevented downregulation of VE-cadherin as well as accompanying lung vascular hyperpermeability. IL-1RA also significantly increased survival.
CONCLUSIONS:
These results provide insights into the central role of NLRP3-Caspase-1 pyroptotic innate immune signaling and loss of lung endothelial adherens junctions in the mechanism of ARDS induced by SARS-CoV-2. Our data show that treatment with IL-1 receptor antagonist during activation of inflammasome provides the ideal scenario for preventing lung vascular injury and respiratory failure in COVID-19.
Keywords: SARS-CoV-2, lung endothelium, vascular injury, IL-1β, IL-1 receptor antagonist (IL-1RA), ACE2 receptor, humanized mice, Pulmonary biology, vascular biology, inflammation
INTRODUCTION
The devastating COVID-19 pandemic is mediated by the SARS-CoV-2 virus which enters host cells via direct binding of the SARS-CoV-2 spike (S)-protein to the ACE-2 receptor and TMPRSS2 membrane protease that are primarily expressed in type II lung alveolar epithelial cells1. Although most COVID-19 patients have mild or moderate course of the disease, up to 5~10% patients progress to Acute Respiratory Distress Syndrome (ARDS)2, characterized by maladaptive hyperinflammation, excessive influx of immune cells such as neutrophils in lungs, intractable hypoxemia due to lung endothelial hyperpermeability and resultant fulminant protein-rich alveolar pulmonary edema, defective alveolar gas exchange, and respiratory failure3,4. Analyses of post-mortem lungs from COVID-19 patients as well as animal models show severe lung endothelial injury following SARS-CoV-2 infection5–7. Much of the focus on SARS-CoV-2 induced lung endothelial dysfunction has been on the coagulopathy5–7 and less is known about the pathogenic role of the lung endothelium in promoting immune activation and lung edemagenesis.
Hyperinflammation and “cytokine storm” are key pathogenic features underlying severe COVID-19 mediating ARDS and respiratory failure8–12. While more than 90% of COVID-19 patients exhibit mild to moderate symptoms because their host defense responses efficiently to eliminate the virus after which inflammation subsides, a subset develop an exaggerated feed-forward inflammatory response in which immune cells release excessive cytokines and chemokines, resulting in damage of lungs and other tissues10–12. Upregulation of NLRP3 inflammasome which activates the inflammatory caspase-1 required for the cleavage and release of the pro-inflammatory cytokine IL-1β has recently been identified as a critical prognostic marker of poor COVID-19 outcome in patients13,14 and mouse and cell culture studies15. SARS-CoV-2 infection also induces inflammasome activation and pyroptosis in human primary monocytes16. The importance of inflammasome activation for COVID-19 severity is likely a reflection of the central pathogenic role of the NLRP3 inflammasome and IL-1β generation in ARDS. In ARDS induced by endotoxemia or bacterial sepsis, IL-1β disrupted lung endothelial barrier by downregulating the transcription factor CREB and its target VE-cadherin, the primary adhesive protein mediating homotypic endothelial cell interaction in microvessels at adherens junctions17. In addition, IL-1β-induced recruitment of neutrophils through enhanced migration across the leaky endothelial barrier, contributing to the hyperinflammation state17. The lung endothelium is not only a target of IL-1β released by immune cells but endothelial cells themselves can release IL-1β while undergoing pyroptosis, a form of inflammatory cell death18.
The rapid development of COVID-19 vaccines with efficacy rates of 70%−95% has been highly successful in reducing COVID-19 transmission and prevalence; however, vaccine hesitancy, limited vaccine accessibility in poor countries, loss of vaccine efficacy, and emergence of novel SARS-CoV-2 variants of concern showing greater transmissibility 19–22 underscore the urgent need for other therapeutic approaches for severe COVID-19. Therapies targeting severe COVID-19 can be broadly divided into two categories: antivirals which prevent cell entry or replication of SARS-CoV-2 or immunomodulatory agents which arrest the vicious cycle of hyperinflammation2,23. While broad immunomodulators such as dexamethasone show success in patients with severe COVID-19 requiring mechanical ventilation24, these immunosuppressive approaches also raise concerns by compromising host-defense responses required to eliminate SARS-CoV-225,26. We posited in light of the critical role of IL-1β in disrupting lung endothelial barrier function during ARDS17, a therapy targeting SARS-CoV-2 activated inflammasome-Caspase-1/11-IL-1β signaling might avert lung vascular endothelial injury and edema formation and subsequent respiratory failure. Anakinra, a minimally modified IL-1 receptor antagonist (IL-1RA) which has been FDA approved for patients with autoimmune disease27 may block IL-1β signaling and prevent the lung complications of SARS-CoV-2. Here, we employed the humanized K18-hACE-2 mouse model in which lung alveolar type II epithelium expressed human ACE-2 and hence could be infected by SARS-CoV-228–31.
MATERIALS AND METHODS
The data that support the findings of this study will be available from the corresponding author upon request.
Mice
Hemizygous K18-hACE c57BL/6J mice (strain#034860: B6.Cg-Tg(K18-ACE2)2Prlmn/J) were purchased from The Jackson Laboratory. Mice of different ages and both sexes were intranasally inoculated with either lethal dose (1 × 105 p.f.u.), sublethal dose (2 × 104 p.f.u.) of SARS-CoV-2 or mock-infected with PBS as controls. Virus inoculations were performed under anesthesia that was induced and maintained with ketamine hydrochloride and xylazine, and all efforts were made to minimize animal suffering. Biosafety level 3 (BSL-3) experiments employed live SARS-CoV-2 were performed by personnel equipped with powered air-purifying respirators in strict compliance with the National Institutes of Health guidelines and approved by the University of Illinois Animal Care & Use Committee.
Genotyping of mice was performed by PCR using tail DNA. All experimental mice were 2~3 months old. For the number of animals needed to achieve statistically significant results, we conducted the a priori power analysis. We calculated power and sample sizes according to data from small pilot experiments, variations within each group of data, and variance similarities between the groups that were statistically compared. Animals with sex- and age-matched littermates were randomly included in experiments. No animals were excluded attributed to illness after experiments. Animal experiments were carried out in a blinded fashion.
Cells and viruses
2019n-CoV/USA_WA1/2019 isolate of SARS-CoV-2 (NR-52281) was obtained from BEI Resources, NIAID, NIH. Vero E6 (CRL-1586; American Type Culture Collection) cells cultured at 37 °C in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 10 mM HEPES (pH 7.3), 1 mM sodium pyruvate, 1× non-essential amino acids and penicillin–streptomycin. Infectious stocks were grown by inoculating Vero E6 cells and collecting supernatant upon observation of cytopathic effect; debris were removed by centrifugation and passage through a 0.22-μm filter. Supernatant was then aliquoted and stored at −80 °C. Titers of viral stocks (5× 106 p.f.u.) were determined by plaque assays. Primary human lung microvascular endothelial cells (hLMVECs) from Lonza were cultured in EBM-2 supplemented with 10% endotoxin-free fetal bovine serum (Omega Scientific). Human lung carcinoma epithelial cells (A549) with hACE-2 stably expressed (NR-53821) were obtained from BEI Resources. Virus propagation experiments were carried out under BSL-3 containment with approval of the University of Illinois, College of Medicine Institutional Biosafety Committee.
Antibodies and reagents
We purchased antibodies against NLRP3 (AdipoGen, AG-20B-0014-C100), Caspase-1 (AdipoGen, AG-20B-0042-C100), caspase-11 (Novus Biologicals, NB-120–10454), IL-1β (R&D Systems, AF-401-NA), caspase-4 (Santa Cruz Biotechnology Inc., sc-56056), caspase-5 (Santa Cruz Biotechnology Inc., sc-393346), CREB (Cell Signaling, 48H2), VE-cadherin (Santa Cruz Biotechnology, sc-6458), and β-actin (Sigma, A-5316). Polyclonal anti-SARS-CoV-2 spike glycoprotein was obtained from BEI Resources. IL-1R antagonist Anakinra (IL-1RA, #407616) was obtained from Calbiochem; Anakirna (C759H1186N208O232S10.) is a recombinant biopharmaceutical and slightly modified version of the human interleukin 1 receptor antagonist protein-IL-1RA. Albumin (catalog A7906), O-Dianisidine dyhydrochloride (catalog D3252), and protease inhibitor cocktail (catalog P8340) were from Millipore Sigma. The CytoTox 96® Non-Radioactive Cytotoxicity Assay kit (G1780) were purchased from Promega. Enhanced chemiluminescence (ECL) Western blotting Detection Reagents and nitrocellulose membranes (Hybond-ECL) were from Amersham Biosciences Corp. Lipofectamine® 3000 transfection reagents were from Invitrogen.
mRNA expression by RT-PCR and quantitative real-time PCR
One-step RT-PCR amplification was performed using the SuperScript 1-step RT-PCR system with Platinum Taq DNA polymerase (Invitrogen). One microgram total RNA isolated from hACE-2-stabled expressed A549, A549 and primary human lung microvascular endothelial cells (hLMVECs) was used as a template for subsequent 1-step RT-PCR. One-step RT-PCR products were analyzed by electrophoresis on 1.2% agarose gels containing ethidium bromide. For quantitative real-time PCR, total RNA (1 μg) was reverse transcribed with Superscript III (Invitrogen) using random primers. Synthesized cDNA samples were amplified in the ABI PRISM 7000 Sequence Detection System (Applied Biosystems) thermocycler using SYBR Green JumpStart Taq ReadyMix (MilliporeSigma). The results of relative expression were normalized to GAPDH mRNA levels in each sample. Results are expressed as mean plus or minus SEM.
Western blotting
Lungs of mice post SARS-CoV-2 infection and shACE-2 V2.4 treatment were surgically removed and washed in cold PBS and homogenized on ice in lysis buffer (50 mM HEPES pH 7.4, 50 mM NaCl, 1% Triton X-100, 5 mM EDTA, 1 mM DTT, 1 mM PMSF, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 10 mM sodium pyrophosphate, 50 mM sodium fluoride, and 1 mM sodium orthovanadate) for 1 hour. Samples were centrifuged at 12000 g for 10 minutes. Supernatants were collected for measurement of protein concentrations by BCA methods. Samples were mixed with sample buffer and boiled in a heat blocker at 90 °C for 10 minutes to ensure inactivation of the remaining live virus before moving out of BSL-3 facility. Boiled samples were separated by SDS-PAGE, transferred to nitrocellulose membranes, and incubated overnight with the indicated antibodies. After incubation with secondary antibodies, proteins were detected by enhanced chemiluminescence. Quantification of band intensities by densitometry was carried out using ImageJ software.
Histology
Mouse lungs were collected in a hood within animal BSL-3 facility. Lung tissues post PBS reperfusion were grossly examined and then fixed in 10% formalin solution, and paraffin sections (5 μm in thickness) were prepared routinely. Haematoxylin and eosin (H&E) and the modified Masson’s trichrome stains were used to identify histopathological changes. Quantification of lung fibrosis was assessed according to Ashcroft method of analysis with a standardized modification as described previously32–34. The histopathology of the lung tissue was evaluated by light microscopy. Aperio brightfield 20x whole slide scans were performed. Infiltration of activated polymorphonuclear neutrophils (PMNs) into the lung post SARS-CoV-2 infection was calculated by a computer-based stereological method previously described35,36.
Myeloperoxidase (MPO) assay
Lung tissues isolated from mice post SARS-CoV-2 infection and drug treatment were homogenized in cold lysis buffer (50 mM HEPES [pH 7.4], 50 mM NaCl, 1% Triton X-100, 5 mM EDTA, 1 mM DTT, 10 mM sodium pyrophosphate, 50 mM sodium fluoride, and 1 mM sodium orthovanadate) with freshly added proteinase inhibitors. Samples were centrifuged at 13,000 × g for 10 minutes at 4 °C to remove insoluble material. Protein concentration (about 1–2 μg/μl) was determined by the standard Bio-Rad BCA method. The supernatants (Protein Samples) were collected for a direct MPO assay. MPO reaction was performed according to Kit’s instructions. Reaction was stopped by adding 100 μl of stop buffer (2 M HCL or 1 M H2SO4) for 5 minutes. Absorption was measured at 460 nm to estimate MPO activity. Data were calculated as A460/min/g protein.
Lung vascular endothelial permeability measurements
Evans blue–albumin pulmonary transvascular flux measurements were performed to measure lung vascular leakage. Briefly, 200 μL Evans blue–albumin (1% Evans blue dye [EBD] and 4% albumin in PBS) was injected into anesthetized mice and allowed to circulate in the blood vessels for 30 minutes. Mice were scarified and lungs were perfused by 5 mL PBS. Lung tissues were then excised, weighed, homogenized in 1 mL PBS and extracted overnight in 2 mL formamide at 60°C. Samples were centrifuged at 10000g for 5 minutes. Evans blue concentration in lung homogenate supernatants was quantified by the spectrophotometric method at absorbance of 620 nm. Tissue EBD content (μg EBD/g fresh lung tissue) was calculated by comparing tissue supernatant A620 readings with an EBD standard curve. Concentration of Evans blue dye was determined in micrograms per gram of wet lung tissue. The ratio of wet lung to dry lung weight for edema measurement was calculated.
Evaluation of endothelial pyroptosis
Release of lactate dehydrogenase (LDH) from pyroptotic cells was measured by supplying lactate, NAD+ and INT as substrates in the presence of diaphorase. LDH release method with a combination of inflammatory caspase-1 or 11 activation have been used for evaluation of pyroptotic cell death18,37,38. Briefly, 80% confluent hLMVECs were seeded in 24-well cell culture plate. Cells culturing in serum free EBM-2 medium were infected with increasing titers of SARS-CoV-2 for 1 day. The supernatants were collected and centrifuged (500 ×g, 5 minutes). Then 50 μl of the supernatant from each sample was transferred to a new 96-well plate and mixed with 50 μl the CytoTox 96® Reagent for 30 minutes at room temperature. Stop Solution (50μl) was added to each well of the 96-well plate. Serum-free medium was used as the 0% control and lysates of the untreated cell were used as the 100% maximal release. The absorbance was measured at 490 nm within 1 hour after adding the Stop Solution on a spectrophotometric microplate reader.
Statistical analysis.
Data were analyzed by two-tailed unpaired Student’s t test for comparisons of two groups or one-way ANOVA of the repeated experiments followed by the Tukey’s post hoc pairwise multiple comparisons when appropriate with Prism 9 (GraphPad Software Inc.). P value of <0.05 was considered significant. For all bar graphs, mean ± S.E.M. is plotted unless otherwise indicated. Normality and variance were not tested to determine whether the applied parametric test was appropriate.
RESULTS
SARS-CoV-2 infection induces NLRP3-Caspase-1 inflammatory signaling, IL-1β release, VE-cadherin downregulation, and PMN infiltration in K18-hACE-2 mice
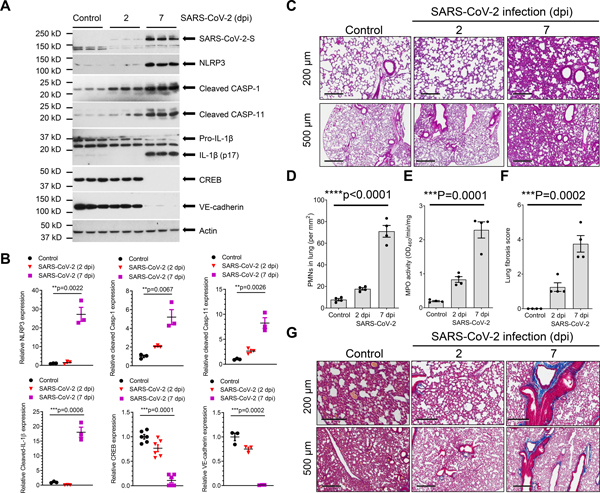
We first investigated changes in NLRP3 inflammasome and expression of VE-cadherin in humanized K18-hACE-2 mice challenged with SARS-CoV-2; the epithelial cell cytokeratin-18 (K18) promoter in these mice induces expression of hACE-2 and thus epithelial cells can be infected by SARS-CoV-228–31. In these mice, non-epithelial cells express murine ACE-2 and therefore they are not infected by the virus. Lung SARS-CoV-2 infection and replication were evaluated by immunoblotting for the spike protein in total lung tissue. We found that intranasal inoculation with SARS-CoV-2 (strain 2019n-CoV/USA_WA1/2020) upregulated NLRP3, inflammatory caspase-1 and −11, and induced maturation of IL-1β, while concomitantly downregulating endothelial adherens junction VE-cadherin expression (Figures 1A and 1B). A previous study identified the critical role of transcription factor CREB in regulating the expression of VE-cadherin17. The post-SARS-CoV-2 lungs also showed increased polymorphonuclear neutrophil (PMN) infiltration into lung (Figures 1C–1E). At 7 dpi, we observed marked deposition of collagen fibers (Figures 1F and 1G) suggesting onset of lung fibrosis. These findings show that even though SARS-CoV-2 can only infect lung epithelial cells in K18-h-ACE-2 mice, it also activates NLRP3 inflammatory signaling resulting in the release of cytokine IL-1β, which may suppress VE-cadherin in neighboring non-infected endothelial cells, mirroring what has been reported for VE-cadherin downregulation in bacterial infections17.
Figure 1. SARS-CoV-2 infection activates lung NLRP3-Caspase-1 inflammatory signaling and lung inflammation in K18-ACE-2 mice.
K18-hACE-2 humanized mice (2-months old) were inoculated with SARS-CoV-2 (1× 105 p.f.u.) for 2 and 7 days. (A) Expression of NLRP3 inflammasome, cleavage of Caspase-1/11, IL-1β maturation and CREB and VE-cadherin expression in the lung following SARS-CoV-2 infection at Day 7 as assessed by immunoblotting with quantification in (B). two-tailed unpaired t test. (C) Lung histopathology in K18-hACE2 mice post-inoculation. H&E-staining showed inflammatory infiltrates composed of lymphocytes and neutrophils. Representative images with lower-power magnification (scale bars, 500 μm) and higher-power magnifications (scale bars, 200 μm) from two independent experiments are shown. (D) Morphometric quantification of neutrophil infiltration in lungs (n= 4). ****P<0.0001, two-tailed unpaired t test. (E) Analysis of neutrophil infiltration by measurement of lung tissue myeloperoxidase (MPO) activity (n= 4). two-tailed unpaired t test. PMN infiltration primarily occurred between Days 2 and 7 (D and E). (f) Lung fibrosis was half-quantified using the Ashcroft method of analysis (f). Results are shown as mean ± SEM. ***P< 0.001, two-tailed unpaired t test. (G) Lung collagen deposition evaluated by Masson Trichrome stain. Collagen fibers are evident on Day 7. Representative images are selected from two independent experiments (n = 4 mice per group).
SARS-CoV-2 infection induces lung vascular hyperpermeability and edema formation
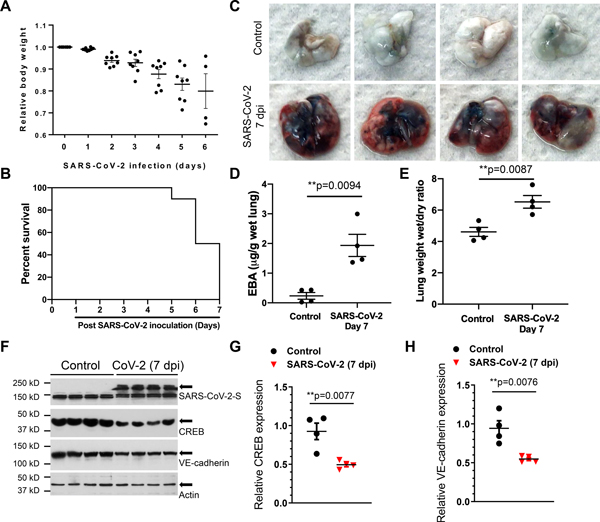
Following SARS-CoV-2 infection (1×105 p.f.u.), K18-hACE2 mice showed progressive body-weight loss and high mortality by Day 7 (Figures 2A and 2B). As IL-1β-induced downregulation of VE-cadherin expression mediates lung vascular injury in bacterial sepsis via suppression of the transcription factor CREB17, we addressed the possibility that SARS-CoV-2 infection may also lead to lung hyperpermeability of the endothelium via disruption of the lung endothelial barrier due to downregulation of VE-cadherin expression. We observed that K18-hACE-2 mice challenged with SARS-CoV-2 showed marked increases in lung vascular permeability and severe pulmonary edema that are the central features of ARDS (Figures 2C–2E). Furthermore, SARS-CoV-2 infection at 7 dpi reduced both CREB and VE-cadherin expression (Figures 2F–2H) consistent the described role of SARS-CoV-2 infection impairing adherens junctions.
Figure 2. SARS-CoV-2 infection induces lung vascular hyperpermeability and reduces CREB and VE-cadherin expression in K18-ACE-2 mice.
K18-hACE-2 mice (2-month-old) were infected with SARS-CoV-2 (1× 105 p.f.u.) for the indicated days. (A) Mouse body weight was measured post SARS-CoV-2 inoculation. (B) Survival of mice post SARS-CoV-2 infection for the indicated days was monitored and is presented as a Kaplan-Meier plot. (C) Lung vascular leak assessed by Evans Blue Albumin (EBA) dye infusion is shown in representative images from two independent experiments. (D) Quantification of lung vascular permeability (n= 4). Extracted dye contents were quantified by measuring absorption at 620 nm. (E) Lung edema formation by the ratios of the wet lung to dry lung weight was assessed (n= 4). Two-tailed unpaired t test. (F-H) CREB and VE-cadherin were assessed by immunoblotting. Quantification of protein expression was analyzed by ImageJ. Results are shown as mean ± SEM. **P< 0.01, Two-tailed unpaired t test (n=4).
SARS-CoV-2 infection in human microvascular endothelial cells induces pyroptotic cell death coincident with activation of NLRP3-Caspase-1 signaling
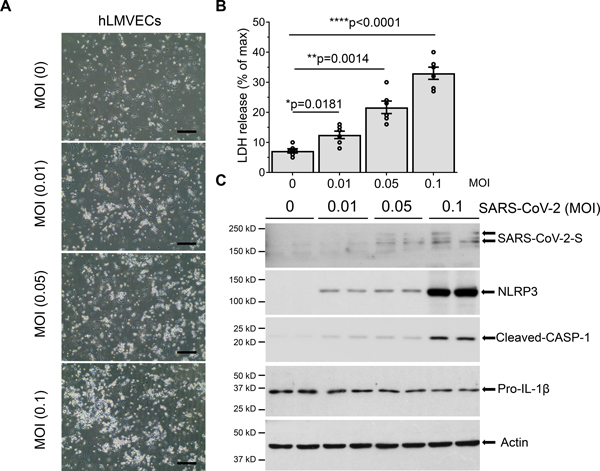
Human pathology and autopsy studies showed that lung vascular endothelium is a primary site of injury in SARS-CoV-2 infection5,6,39–41. To address mechanisms of endothelial injury, we determined endogenous mRNA expression of the SARS-CoV-2 receptor-ACE-2 and the associated main protease, TMPRSS2, in human lung microvascular endothelial cells (hLMVECs). mRNA expression of ACE-2 and TMPRSS2 were evident in both A549 ATII epithelial cells and hLMVECs (Figure S1 in Data Supplement), suggesting that ATII epithelial cells and lung microvascular endothelial cells exhibit similar susceptibility to SARS-CoV-2 infection. hLMVECs exposed to increasing titers of SARS-CoV-2 infection for 1 day displayed dose dependent increase in cell death (Figures 3A and 3B). The observed cell death of hLMVECs at 1dpi was quantified by LDH release and was consistent with pyroptotic cell death, as evident by the activation of inflammatory NLRP3-Caspase-1 signaling and maturation of IL-1β (Figure 3C). We also observed that at high titers of SARS-CoV-2, hLMVECs became susceptible to infection as seen in the endothelial expression of the SARS-CoV-2 spike protein (Figure 3C). Our data thus support a key role of lung vascular endothelium in responding to SARS-CoV-2 infection.
Figure 3. SARS-CoV-2 infection induces hLMVECs pyroptosis coincident with activation of inflammatory NLPR3-Caspase-1 signaling and IL-1β cleavage.
hLMVECs were infected with increasing titers of SARS-CoV-2 (MOI: 0.01, 0.1 and 0.5) for 1 day. (A) Phase-contrast micrographs of hLMVECs post SARS-CoV-2 infection for 1day. Scale bars: 200μm. (B) Cytotoxicity activity of SARS-CoV-2 infection was analyzed by LDH release. n=6/group, *P< 0.05, **P< 0.01, ****P< 0.0001, Two-tailed unpaired t test. (C) Western Blot was performed with the indicated antibodies. Activation of inflammatory NLRP3-Caspase-1 pyroptotic signaling and IL-1β cleavage were shown in the blots.
Blockade of IL-1 receptor prevents SARS-CoV-2-induced lung endothelial barrier injury
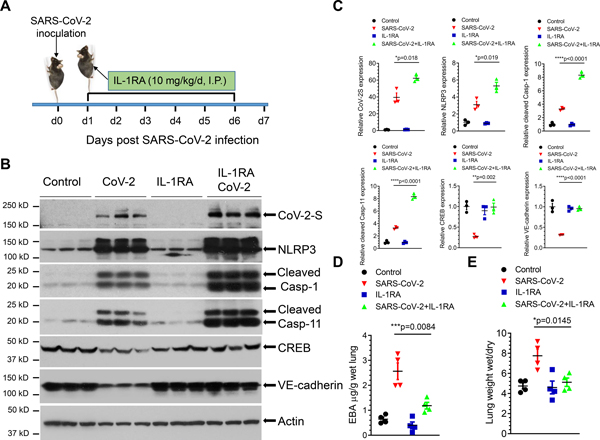
Lung vascular hyperpermeability is the primary cause of protein-rich edema formation in lungs leading to ARDS, and ultimately to death due to defective gas exchange in the fluid-filled alveoli 3. To address whether IL-1β-induced downregulation of VE-cadherin could underpin lung vascular hyperpermeability observed in SARS-CoV-2 infection in mice, we investigated the therapeutic potential of the IL-1 receptor antagonist (IL-1RA) Anakinra27. K18-hACE2 mice received Anakinra (10 mg/kg/d, I.P.) 24 hours after the sublethal dose of SARS-CoV-2 (2×104 p.f.u.) and thereafter daily injections (Figure 4A). We observed that IL-1RA abrogated the downregulation of CREB and VE-cadherin expression even though blockade of IL-1R signaling by IL-1RA did not prevent the upstream activation of NLRP3-Caspase-1 inflammasome pathway and the lung became infected with SARS-CoV-2 (Figures 4B and 4C). IL-1R blockade prevented lung vascular leakage (Figure 4D) and edema formation (Figure 4E) indicating the primary role of IL-1 in mediating lung vascular endothelial injury and edema. Together these data show that IL-1 receptor antagonism acts downstream of NLRP3 inflammasome and restores expression of lung endothelial adherens junction protein VE-cadherin, and thereby prevents lung vascular hyperpermeability in SARS-CoV-2 infection.
Figure 4. IL-1 receptor blockade by IL-1 receptor antagonist Anakinra prevents SARS-CoV-2-induced lung vascular hyperpermeability and edema.
K18-hACE-2 mice (2-month-old) were infected with a sublethal dose of SARS-CoV-2 (2×104 p.f.u.) for 7 days. (A) Mice were administered IL-1RA Anakinra (10 mg/kg/d) or vehicle by I.P. injection at 24 hours post infection and every day thereafter as shown. (B and C) Lung lysates were assessed for the expression of SARS-CoV-2 spike protein, NLRP3, cleaved caspase-1 and caspase-11, CREB, and VE-cadherin by immunoblotting. IL-RA did not prevent the infection-induced expression of the viral spike protein or inflammasome activation but restored the expression of CREB and VE-cadherin. Protein expression levels from blots are quantified in (C). Two-tailed unpaired t test (n=3). *P< 0.05, **P< 0.01 ****P< 0.0001. (D) Lung vascular permeability was determined by lung transvascular albumin flux measurements using Evans Blue Albumin (EBA). (E) Lung edema was determined by ratio of wet-to-dry lung weights (n= 4 in each group). Results are shown as mean ± SEM. *P< 0.05. ***P< 0.001.
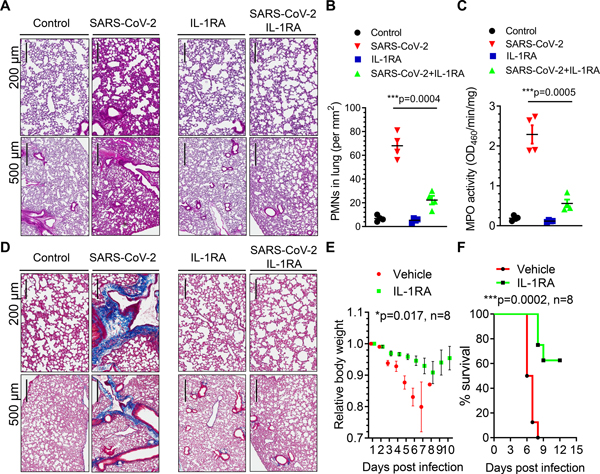
IL-1 receptor antagonism mitigates lung neutrophil infiltration, lung fibrosis and mortality in mice induced by high titers of SARS-CoV-2
We next investigated the effects of IL-1RA in mice challenged with a lethal dose of SARS-CoV-2 (1×105 p.f.u.). K18-hACE-2 mice received IL-1RA (10 mg/kg/d) or vehicle by I.P. injection at 24 hours post-SARS-CoV-2 and daily injections thereafter. We observed that IL-1RA therapy prevented neutrophil infiltration in infected lungs (Figures 5A–5C). Interestingly, lung collagen fiber deposition at 7dpi was also significantly reduced by IL-1RA (Figures 5D and S2 in the Data Supplement). The severe and progressive loss of body weight was suppressed with IL-1RA treatment (Figure 5E). Strikingly 90% of the mice in the SARS-CoV-2 infected group died at 7 dpi whereas 60% mice treated with IL-1RA were still alive at 12 dpi (Figure 5F). Thus, IL-1RA Anakinra markedly reduced mortality in a lethal model of SARS-CoV-2 infection.
Figure 5. IL-1 receptor blockade mitigates SARS-CoV-2-induced ARDS, fibrosis, and mortality in K18-ACE-2 mice.
K18-hACE-2 mice (2-month-old) were infected with a lethal dose of SARS-CoV-2 (1×105 p.f.u.). Mice also received the IL-1 receptor antagonist (IL-1RA) Anakinra (10 mg/kg/d) or vehicle by I.P. injection at 24 hours post infection and daily thereafter. (A) Lung histopathology in K18-hACE2 mice post-inoculation. H&E-stained sections (scale bars, 200 μm and 500 μm) showed inflammatory infiltrates composed of lymphocytes and PMNs. Representative images from two independent experiments are shown. (B) PMN numbers in lung were morphometrically quantified (n= 4). Two-tailed unpaired t test (n=4). ***P< 0.001. (C) Lung PMN infiltration determined by measurement of lung tissue MPO activity (n= 4). Two-tailed unpaired t test (n=4). ***P< 0.001. (D) Lung collagen deposition (blue) post SARS-CoV-2 infection and the effects of IL-1RA treatment evaluated by Masson Trichrome staining. Representative images from two independent experiments are shown (scale bars, 200 μm and 500 μm). (E) Body weight of mice was monitored post SARS-CoV-2 inoculation. IL-1RA treatment prevented weight loss. Two-tailed unpaired t test (n=8). *P< 0.05. (f) Survival of mice post-SARS-CoV-2 infection presented as a Kaplan-Meier plot. IL-RA treatment markedly improved survival. ***P< 0.001. n=8.
DISCUSSION
Although several animal models have been developed to investigate the susceptibility to SARS-CoV-2 infection, few of them have recapitulated the severe disease seen in humans who have been hospitalized29,42. The development of countermeasures that reduce COVID-19 morbidity and mortality is a priority for the global research community, and animal models are particularly urgent for this effort. Hamsters, ferrets and non-human primates develop mild to moderate viral disease and recover spontaneously29,43,44. Conventional laboratory strains of mice cannot be infected efficiently with SARS-CoV-2 because hACE-2, but not mouse ACE-2, supports SARS-CoV-2 binding45,46. Multiple strategies for introducing hACE-2 into mice have been developed including: (1) transient introduction of hACE-2 via adenoviral or adenoviral-associated vectors47,48; (2) expression of hACE-2 as a transgene driven by heterologous gene promoters28,49,50; or (3) expression of hACE-2 by the mouse ACE-2 promoter 51,52. While these animals all support SARS-CoV-2 infection, only the models with hACE-2 expression driven by the HFH4 promoter50 and the epithelial cell cytokeratin-18 (K18) promoter develop severe disease and show high mortality28,31,53,54. Rathnasinghe et al 55 recently demonstrated that the K18-hACE2 model are more stringent for testing vaccines and antivirals than the adenovirus delivery system. In the current study, we employed K18-hACE-2 mice to investigate the role of inflammasome activation in triggering lung vascular leakage and address the role of IL-1RA in ameliorating SARS-CoV-2-induced lung vascular permeability, lung edema formation, and mortality. Our findings do not rule out the need for additional animal models such as non-human primates to better understand the mechanisms of the devastating systemic inflammatory syndrome and inflammatory lung injury but provide proof of concept in a highly reproducible mouse model of Covid 19 induced lung injury and failure.
Multiple small clinical trials have investigated the role of immunomodulation in COVID-19 patients because the hyperinflammatory state is a key pathogenic factor of disease initiation and progression. Recently, two major trials involving the interleukin-6 antagonists tocilizumab and sarilumab were conducted in COVID-19 patients. However, the conclusions were contradictory 56–58. In the REMAP-CAP trial, critically ill COVID-19 patients receiving the interleukin-6 receptor antagonists tocilizumab and sarilumab showed improved survival 56. However, in non-critically ill hospitalized patients 57 tocilizumab did not improve survival. These findings suggest that selection of the target patient population may be critical for the success of immunomodulatory therapy in COVID-19 patients.
In the present study, we first focused on IL-1 receptor antagonism instead of IL-6 antagonism because IL-1β signaling initiates the breakdown of the lung vascular barrier in ARDS 17. We found that the K18-hACE-2 mouse accurately modeled severe lung hyperpermeability following SARS-CoV-2 infection, similar to advanced COVID-19 patients. Furthermore, we observed that Anakinra treatment restored the expression of the endothelial adherens junction protein VE-cadherin, prevented lung hyperpermeability, and reduced mortality. Results from clinical trials with Anakinra in small number of COVID-19 patients have been equivocal 59–65. In a study early blockade of IL-1β signaling by IL-1RA exhibited significant survival benefit in severe hyper-inflammatory respiratory failure in COVID-19 patients 59. In another trial, high dosages of IL-1RA also showed clinical improvements in patients 60. In an open-label trial of Anakinra in 120 patients, Anakinra decreased risk of progression to severe respiratory failure by 70% 64. However, in mild-moderate COVID-19 disease, Anakinra treatment did not improve of outcomes 63. Our results in a controlled experimental model of SARS-CoV-2 infection and lung injury may provide insights into the variable clinical findings and identify patients most likely to benefit from IL-1RA treatment. We found that IL-1RA reversed the downregulation of VE-cadherin in lungs, thus restoring lung endothelial barrier function, preventing lung edema and improving survival.
IL-1β is a key mediator released by activation of inflammasome NLRP3, a cytoplasmic protein complex mediating the production of mature pro-inflammatory cytokine IL-1β via the inflammatory caspase-166. This complex includes polymerization of ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) and recruitment and activation of caspase-166. While our previous study showed that IL-1β release followed the activation of NLRP3 inflammasome in bacterial ARDS and induced lung vascular hyper-permeability17,67, the present study shows a SARS-CoV-2 infection induces similar changes. IL-1R antagonism prevented the IL-1β-mediated decrease in expression of the endothelial junctional protein VE-cadherin, which maintains the integrity of endothelial adherens junctions68. The excessive generation of pro-inflammatory cytokines such as IL-1β during the “cytokine storm” described in severe SARS-CoV-2 infection 10–12 likely leads to widespread VE-cadherin downregulation in lungs, and thus explains the high incidence of pulmonary edema and respiratory failure in severe COVID-19 patients. The lung endothelium is especially vulnerable to inflammatory activation because it expresses higher levels of inflammatory genes than endothelial cells of other vascular beds 69 and the lung endothelium also known to serve as a source of IL-1β in ARDS 18.
Increasing evidence suggests that that vascular endothelium is an important target tissue in response to SARS-CoV-2 infection5,39,70–73. An highly debated issue is whether SARS-CoV-2 directly infects and impairs functions of the endothelium. Some previous reports74–76 showed some human endothelial cells have the lower or nondetectable expression of ACE-2 and TMPRSS2 and less susceptible to SARS-CoV-2 infection. However, none of these results were directly obtained from the primary human lung microvascular endothelial cells (hLMVECs), which were used in our current study. Consistent with our observations, Caccuri, et al73 recently demonstrated a direct role of SARS-CoV-2-infection of hLMVECs, inducing vascular dysfunction during the early phases of infection by employing an immunofluorescence assay and in situ RNA-hybridization as well as proteome analysis. The present study provides direct evidence that hLMVECs are susceptible to SARS-CoV-2 infection and there is comparable mRNA expression of ACE-2 and TMPRSS2 in hLMVECs and AT-II epithelial cell line-A549 as evaluated by one step-RT-PCR and Realtime PCR. Further, we show that SARS-CoV-2 infection in hLMVECs induced activation of NLRP3-Caspase-1-IL-1β inflammatory signaling pathway and pyroptotic cell death in a dose-dependent manner. SARS-CoV-2 infection and entry to cells were also evident by the detection of SARS-CoV-2 Spike protein in endothelial cell lysates. It has been recently shown that tissue-specific heterogeneity and plasticity of the endothelium is maintained during systemic in vivo inflammatory injury69. It is possible that tissue-specific endothelial cultures display variable susceptibility to SARS-CoV-2 infection for their different expression abundance of SARS-CoV-2 receptors. Results from some groups concerning the infectability of human endothelial cultures might thus be due to age of cells or using endothelial derived from different organs which may lack the capacity of viral uptake because of low or nonexistent expression of ACE-2 and TMPRSS2.
The present findings suggest a path forward for clinical trials deploying IL-1 receptor antagonism in COVID-19 by selecting the target patient population most likely to benefit from this therapy. NLRP3 inflammasome activation in circulating peripheral blood mononuclear cells mirrors the activation seen in the lung itself 13. Our results suggest that patients showing evidence of NLRP3 activation are likely to benefit from treatment with IL-1 receptor antagonism. A parallel approach would be patient selection based on evidence of endothelial injury, such as through measurement of circulating endothelial microparticles 18. By introducing such an approach, it would be possible to maximize the benefits of immunomodulation through blockade of IL-1 receptor blockade.
Supplementary Material
HIGHLIGHTS.
Infection with SARS-CoV-2 in the transgenic K18-hACE2 mouse model results in NLRP3-Caspase 1 inflammasome activation and IL-1β release in lungs
SARS-CoV-2 infection leads to downregulation of lung endothelial VE-cadherin and severe lung edema
Initiation of treatment with the IL-1-receptor antagonist Anakinra after SARS-CoV-2 infection prevents VE-cadherin downregulation and edema formation
Treatment with Anakinra after SARS-CoV-2 infection reduces lung fibrosis and mortality
Acknowledgments:
We thank Dr. Maria Swerdlov at the Histology Tissue Core for her support.
Sources of funding:
The work was supported in part by NIH grants P01-HL60678, T32-HL007829, R01-HL154538, R01-HL149300, R01-HL118068, R01-HL157489 and R01-HL152515 as well as by intramural funds of the University of Illinois, College of Medicine.
ABBREVIATIONS
- ARDS
Acute Respiratory Distress Syndrome
- hLMVEC
human lung microvascular endothelial cells
- IL-1RA
IL-1 receptor antagonist
- ACE-2
Angiotensin-converting enzyme 2
- TMPRSS2
Transmembrane serine protease 2
- PMNs
Polymorphonuclear neutrophils
- ATII
Alveolar epithelial type II cells
Footnotes
Disclosures:
S.X., L.Z., J.R and A.B.M. are co-inventors on a patent application describing ACE-2 peptides which prevent entry of coronaviruses into cells. S.X. is now employed by Merck Research Laboratories. The work in this manuscript does not involve the use of ACE-2 peptides and does not involve the use of products developed by Merck Research Laboratories.
REFERENCES
- 1.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383(25):2451–2460. [DOI] [PubMed] [Google Scholar]
- 3.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson BT, Chambers RC, Liu KD. Acute Respiratory Distress Syndrome. N Engl J Med. 2017;377(6):562–572. [DOI] [PubMed] [Google Scholar]
- 5.Goshua G, Pine AB, Meizlish ML, Chang CH, Zhang H, Bahel P, Baluha A, Bar N, Bona RD, Burns AJ, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575–e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aid M, Busman-Sahay K, Vidal SJ, Maliga Z, Bondoc S, Starke C, Terry M, Jacobson CA, Wrijil L, Ducat S, et al. Vascular Disease and Thrombosis in SARS-CoV-2-Infected Rhesus Macaques. Cell. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rendeiro AF, Ravichandran H, Bram Y, Chandar V, Kim J, Meydan C, Park J, Foox J, Hether T, Warren S, et al. The spatial landscape of lung pathology during COVID-19 progression. Nature. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, Samir P, Zheng M, Sundaram B, Banoth B, Malireddi RKS, et al. Synergism of TNF-alpha and IFN-gamma Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell. 2021;184(1):149–168 e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren X, Wen W, Fan X, Hou W, Su B, Cai P, Li J, Liu Y, Tang F, Zhang F, et al. COVID-19 immune features revealed by a large-scale single-cell transcriptome atlas. Cell. 2021;184(7):1895–1913 e1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med. 2020;383(23):2255–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigues TS, de Sa KSG, Ishimoto AY, Becerra A, Oliveira S, Almeida L, Goncalves AV, Perucello DB, Andrade WA, Castro R, et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med. 2021;218(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toldo S, Bussani R, Nuzzi V, Bonaventura A, Mauro AG, Cannata A, Pillappa R, Sinagra G, Nana-Sinkam P, Sime P, et al. Inflammasome formation in the lungs of patients with fatal COVID-19. Inflamm Res. 2021;70(1):7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan P, Shen M, Yu Z, Ge W, Chen K, Tian M, Xiao F, Wang Z, Wang J, Jia Y, et al. SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat Commun. 2021;12(1):4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira AC, Soares VC, de Azevedo-Quintanilha IG, Dias S, Fintelman-Rodrigues N, Sacramento CQ, Mattos M, de Freitas CS, Temerozo JR, Teixeira L, et al. SARS-CoV-2 engages inflammasome and pyroptosis in human primary monocytes. Cell Death Discov. 2021;7(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiong S, Hong Z, Huang LS, Tsukasaki Y, Nepal S, Di A, Zhong M, Wu W, Ye Z, Gao X, et al. IL-1beta suppression of VE-cadherin transcription underlies sepsis-induced inflammatory lung injury. J Clin Invest. 2020;130(7):3684–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng KT, Xiong S, Ye Z, Hong Z, Di A, Tsang KM, Gao X, An S, Mittal M, Vogel SM, et al. Caspase-11-mediated endothelial pyroptosis underlies endotoxemia-induced lung injury. J Clin Invest. 2017;127(11):4124–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang P, Nair MS, Liu L, Iketani S, Luo Y, Guo Y, Wang M, Yu J, Zhang B, Kwong PD, et al. Antibody Resistance of SARS-CoV-2 Variants B.1.351 and B.1.1.7. Nature. 2021. [DOI] [PubMed] [Google Scholar]
- 20.Planas D, Bruel T, Grzelak L, Guivel-Benhassine F, Staropoli I, Porrot F, Planchais C, Buchrieser J, Rajah MM, Bishop E, et al. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat Med. 2021. [DOI] [PubMed] [Google Scholar]
- 21.Davies NG, Jarvis CI, Group CC-W, Edmunds WJ, Jewell NP, Diaz-Ordaz K, Keogh RH. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng X, Garcia-Knight MA, Khalid MM, Servellita V, Wang C, Morris MK, Sotomayor-González A, Glasner DR, Reyes KR, Gliwa AS, et al. Transmission, infectivity, and neutralization of a spike L452R SARS-CoV-2 variant. Cell. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, Dorgham K, Philippot Q, Rosain J, Beziat V, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, Ogishi M, Sabli IKD, Hodeib S, Korol C, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370(6515). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winkler ES, Bailey AL, Kafai NM, Nair S, McCune BT, Yu J, Fox JM, Chen RE, Earnest JT, Keeler SP, et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol. 2020;21(11):1327–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia H, Yue X, Lazartigues E. ACE2 mouse models: a toolbox for cardiovascular and pulmonary research. Nat Commun. 2020;11(1):5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yinda CK, Port JR, Bushmaker T, Offei Owusu I, Purushotham JN, Avanzato VA, Fischer RJ, Schulz JE, Holbrook MG, Hebner MJ, et al. K18-hACE2 mice develop respiratory disease resembling severe COVID-19. PLoS Pathog. 2021;17(1):e1009195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oladunni FS, Park JG, Pino PA, Gonzalez O, Akhter A, Allue-Guardia A, Olmo-Fontanez A, Gautam S, Garcia-Vilanova A, Ye C, et al. Lethality of SARS-CoV-2 infection in K18 human angiotensin-converting enzyme 2 transgenic mice. Nat Commun. 2020;11(1):6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41(4):467–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hubner RH, Gitter W, El Mokhtari NE, Mathiak M, Both M, Bolte H, Freitag-Wolf S, Bewig B. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques. 2008;44(4):507–511, 514–507. [DOI] [PubMed] [Google Scholar]
- 34.O’Hare M, Amarnani D, Whitmore HAB, An M, Marino C, Ramos L, Delgado-Tirado S, Hu X, Chmielewska N, Chandrahas A, et al. Targeting Runt-Related Transcription Factor 1 Prevents Pulmonary Fibrosis and Reduces Expression of Severe Acute Respiratory Syndrome Coronavirus 2 Host Mediators. Am J Pathol. 2021;191(7):1193–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao X, Xu N, Sekosan M, Mehta D, Ma SY, Rahman A, Malik AB. Differential role of CD18 integrins in mediating lung neutrophil sequestration and increased microvascular permeability induced by Escherichia coli in mice. J Immunol. 2001;167(5):2895–2901. [DOI] [PubMed] [Google Scholar]
- 36.Gao XP, Standiford TJ, Rahman A, Newstead M, Holland SM, Dinauer MC, Liu QH, Malik AB. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by Gram-negative sepsis: studies in p47phox−/− and gp91phox−/− mice. J Immunol. 2002;168(8):3974–3982. [DOI] [PubMed] [Google Scholar]
- 37.Rayamajhi M, Zhang Y, Miao EA. Detection of pyroptosis by measuring released lactate dehydrogenase activity. Methods Mol Biol. 2013;1040:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia C, Zhang J, Chen H, Zhuge Y, Chen H, Qian F, Zhou K, Niu C, Wang F, Qiu H, et al. Endothelial cell pyroptosis plays an important role in Kawasaki disease via HMGB1/RAGE/cathespin B signaling pathway and NLRP3 inflammasome activation. Cell Death Dis. 2019;10(10):778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gavriilaki E, Anyfanti P, Gavriilaki M, Lazaridis A, Douma S, Gkaliagkousi E. Endothelial Dysfunction in COVID-19: Lessons Learned from Coronaviruses. Curr Hypertens Rep. 2020;22(9):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iba T, Connors JM, Levy JH. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rockx B, Kuiken T, Herfst S, Bestebroer T, Lamers MM, Oude Munnink BB, de Meulder D, van Amerongen G, van den Brand J, Okba NMA, et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science. 2020;368(6494):1012–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cleary SJ, Pitchford SC, Amison RT, Carrington R, Robaina Cabrera CL, Magnen M, Looney MR, Gray E, Page CP. Animal models of mechanisms of SARS-CoV-2 infection and COVID-19 pathology. Br J Pharmacol. 2020;177(21):4851–4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim YI, Kim SG, Kim SM, Kim EH, Park SJ, Yu KM, Chang JH, Kim EJ, Lee S, Casel MAB, et al. Infection and Rapid Transmission of SARS-CoV-2 in Ferrets. Cell Host Microbe. 2020;27(5):704–709 e702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5(4):562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020;94(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Israelow B, Song E, Mao T, Lu P, Meir A, Liu F, Alfajaro MM, Wei J, Dong H, Homer RJ, et al. Mouse model of SARS-CoV-2 reveals inflammatory role of type I interferon signaling. J Exp Med. 2020;217(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hassan AO, Case JB, Winkler ES, Thackray LB, Kafai NM, Bailey AL, McCune BT, Fox JM, Chen RE, Alsoussi WB, et al. A SARS-CoV-2 Infection Model in Mice Demonstrates Protection by Neutralizing Antibodies. Cell. 2020;182(3):744–753 e744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCray PB Jr., Pewe L, Wohlford-Lenane C, Hickey M, Manzel L, Shi L, Netland J, Jia HP, Halabi C, Sigmund CD, et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol. 2007;81(2):813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang RD, Liu MQ, Chen Y, Shan C, Zhou YW, Shen XR, Li Q, Zhang L, Zhu Y, Si HR, et al. Pathogenesis of SARS-CoV-2 in Transgenic Mice Expressing Human Angiotensin-Converting Enzyme 2. Cell. 2020;182(1):50–58 e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bao L, Deng W, Huang B, Gao H, Liu J, Ren L, Wei Q, Yu P, Xu Y, Qi F, et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020;583(7818):830–833. [DOI] [PubMed] [Google Scholar]
- 52.Sun SH, Chen Q, Gu HJ, Yang G, Wang YX, Huang XY, Liu SS, Zhang NN, Li XF, Xiong R, et al. A Mouse Model of SARS-CoV-2 Infection and Pathogenesis. Cell Host Microbe. 2020;28(1):124–133 e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenfeld R, Noy-Porat T, Mechaly A, Makdasi E, Levy Y, Alcalay R, Falach R, Aftalion M, Epstein E, Gur D, et al. Post-exposure protection of SARS-CoV-2 lethal infected K18-hACE2 transgenic mice by neutralizing human monoclonal antibody. Nat Commun. 2021;12(1):944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng M, Karki R, Williams EP, Yang D, Fitzpatrick E, Vogel P, Jonsson CB, Kanneganti TD. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat Immunol. 2021;22(7):829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rathnasinghe R, Strohmeier S, Amanat F, Gillespie VL, Krammer F, Garcia-Sastre A, Coughlan L, Schotsaert M, Uccellini MB. Comparison of transgenic and adenovirus hACE2 mouse models for SARS-CoV-2 infection. Emerg Microbes Infect. 2020;9(1):2433–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Investigators R-C, Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, Annane D, Beane A, van Bentum-Puijk W, et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med. 2021;384(16):1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, Criner GJ, Kaplan-Lewis E, Baden R, Pandit L, et al. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N Engl J Med. 2021;384(1):20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rubin EJ, Longo DL, Baden LR. Interleukin-6 Receptor Inhibition in Covid-19 - Cooling the Inflammatory Soup. N Engl J Med. 2021;384(16):1564–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cauchois R, Koubi M, Delarbre D, Manet C, Carvelli J, Blasco VB, Jean R, Fouche L, Bornet C, Pauly V, et al. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc Natl Acad Sci U S A. 2020;117(32):18951–18953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, Oltolini C, Castiglioni B, Tassan Din C, Boffini N, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2(6):e325–e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huet T, Beaussier H, Voisin O, Jouveshomme S, Dauriat G, Lazareth I, Sacco E, Naccache JM, Bezie Y, Laplanche S, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393–e400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kooistra EJ, Waalders NJB, Grondman I, Janssen NAF, de Nooijer AH, Netea MG, van de Veerdonk FL, Ewalds E, van der Hoeven JG, Kox M, et al. Anakinra treatment in critically ill COVID-19 patients: a prospective cohort study. Crit Care. 2020;24(1):688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.group C-C. Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet Respir Med. 2021;9(3):295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kyriazopoulou E, Panagopoulos P, Metallidis S, Dalekos GN, Poulakou G, Gatselis N, Karakike E, Saridaki M, Loli G, Stefos A, et al. An open label trial of anakinra to prevent respiratory failure in COVID-19. Elife. 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pasin L, Cavalli G, Navalesi P, Sella N, Landoni G, Yavorovskiy AG, Likhvantsev VV, Zangrillo A, Dagna L, Monti G. Anakinra for patients with COVID-19: a meta-analysis of non-randomized cohort studies. Eur J Intern Med. 2021;86:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16(7):407–420. [DOI] [PubMed] [Google Scholar]
- 67.Di A, Xiong S, Ye Z, Malireddi RKS, Kometani S, Zhong M, Mittal M, Hong Z, Kanneganti TD, Rehman J, et al. The TWIK2 Potassium Efflux Channel in Macrophages Mediates NLRP3 Inflammasome-Induced Inflammation. Immunity. 2018;49(1):56–65 e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell. 2013;26(5):441–454. [DOI] [PubMed] [Google Scholar]
- 69.Jambusaria A, Hong Z, Zhang L, Srivastava S, Jana A, Toth PT, Dai Y, Malik AB, Rehman J. Endothelial heterogeneity across distinct vascular beds during homeostasis and inflammation. Elife. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qin Z, Liu F, Blair R, Wang C, Yang H, Mudd J, Currey JM, Iwanaga N, He J, Mi R, et al. Endothelial cell infection and dysfunction, immune activation in severe COVID-19. Theranostics. 2021;11(16):8076–8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thacker VV, Sharma K, Dhar N, Mancini GF, Sordet-Dessimoz J, McKinney JD. Rapid endotheliitis and vascular damage characterize SARS-CoV-2 infection in a human lung-on-chip model. EMBO Rep. 2021;22(6):e52744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maccio U, Zinkernagel AS, Shambat SM, Zeng X, Cathomas G, Ruschitzka F, Schuepbach RA, Moch H, Varga Z. SARS-CoV-2 leads to a small vessel endotheliitis in the heart. EBioMedicine. 2021;63:103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Caccuri F, Bugatti A, Zani A, De Palma A, Di Silvestre D, Manocha E, Filippini F, Messali S, Chiodelli P, Campisi G, et al. SARS-CoV-2 Infection Remodels the Phenotype and Promotes Angiogenesis of Primary Human Lung Endothelial Cells. Microorganisms. 2021;9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goldsmith CS, Miller SE, Martines RB, Bullock HA, Zaki SR. Electron microscopy of SARS-CoV-2: a challenging task. Lancet. 2020;395(10238):e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hikmet F, Mear L, Edvinsson A, Micke P, Uhlen M, Lindskog C. The protein expression profile of ACE2 in human tissues. Mol Syst Biol. 2020;16(7):e9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nascimento Conde J, Schutt WR, Gorbunova EE, Mackow ER. Recombinant ACE2 Expression Is Required for SARS-CoV-2 To Infect Primary Human Endothelial Cells and Induce Inflammatory and Procoagulative Responses. mBio. 2020;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.