Abstract
Objective:
To determine the temporal expression of ACE2, a receptor for SARS-COV-2, in dominant follicles across the periovulatory period in women and the regulatory mechanisms underlying ACE2 expression in human granulosa/lutein cells (hGLC).
Design:
Experimental prospective clinical study and laboratory-based investigation.
Setting:
University Medical Center and private IVF center.
Patients:
Thirty premenopausal women undergoing surgery for tubal ligation and 16 premenopausal women undergoing IVF.
Intervention (s):
Administration of hCG and harvesting of preovulatory/ovulatory follicles by timed laparoscopy and collection of granulosa/lutein cells and cumulus cells at the time of oocyte retrieval.
Main Outcome Measures:
Expression and localization of ACE2 in granulosa cells and dominant follicles collected across the periovulatory period of the menstrual cycle and in hGLC using qPCR, immunoblotting, and immunohistochemistry.
Results:
ACE2 expression (mRNA and protein) is up-regulated in human ovulatory follicles after hCG administration. ACE2 expression was higher in cumulus cells than granulosa cells. hCG increased the expression of ACE2 in primary hGLC cultures, but the increase was inhibited by both RU486 (an antagonist for progesterone receptor and glucocorticoid receptor) and CORT125281 (a selective glucocorticoid receptor), but not by AG1478 (an EGF-receptor tyrosine kinase inhibitor) or by dexamethasone.
Conclusions:
The hormone-regulated expression of ACE2 in granulosa cells suggests a potential role of ACE2 in the ovulatory process. These data also implicate the possible impact of COVID-19 in a vital cyclic event of ovarian function, thus women’s overall reproductive health. However, SAR-COV-2 infection in ovarian cells in vivo or in vitro has yet to be determined.
Keywords: ACE2, ovulation, ovary, follicle, humans
INTRODUCTION
Since the beginning of the COVID-19 pandemic, heightened attention has been given to angiotensin-converting enzyme 2 (ACE2) as it acts as an entry receptor for SARS-COV-2, a novel strain of the virus that causes COVID-19 (1). However, this role of ACE2 in the viral cellular entry is in contrast to its original reported function as a component of the renin-angiotensin system (RAS).
Initially, ACE2 was discovered as a zinc metalloprotease that is capable of catalyzing angiotensin I (Ang I) and angiotensin II (Ang II) to angiotensin-(1–9) and angiotensin-(1–7), respectively (2,3). ACE2 displays high homology to human angiotensin-converting enzyme (ACE)(2,4). ACE is well known for its ability to convert inactive angiotensin Ang I into the functionally active hormone Ang II (5). The best-elucidated actions of Ang II include vasoconstriction, stimulation of aldosterone and vasopressin secretion, vascular smooth muscle proliferation, and renal tubular sodium reuptake, thereby regulating body fluid volume, electrolyte balance, and arterial pressure (6,7). However, these effects of Ang II are tightly controlled by ACE2 whose role is to limit locally available Ang II concentration by rapidly hydrolyzing Ang II to Ang-(1–7)(8). Ang-(1–7) has also been shown to enhance vasodilation and exert anti-oxidative, anti-inflammatory, and pro-resolution effects, likely through the activation of the G protein-coupled receptor MAS1 (MAS1 Proto-Oncogene)(9). Therefore, ACE2 functions as a key counter-regulatory enzyme in the RAS. In addition to the role in the RAS, ACE2 can cleave other peptide substrates such as neurotensin, kinetensin, des-Arg bradykinin, and Apelins (−13, −17, and −36)(2,10).
Besides these enzymatic functions, ACE2 was identified as a binding site for human coronavirus HCoV-NL63 and the human severe acute respiratory syndrome coronaviruses, SARS-COV and SARS-COV-2 (11). The spike proteins on the surface of SARS coronaviruses bind to ACE2 on its target cells, initiating the virus-cell membrane fusion and ultimately resulting in viral replication inside the host cells (11).
ACE2 mRNA was detected in most human tissues examined, yet the detection of ACE2 protein was limited in several tissues, including the lung, kidney, heart, and intestine (12). Specifically, the abundant ACE2 expression was localized to lung alveolar epithelial cells, enterocytes of the small intestine, and vascular endothelial cells of the heart and kidney (13). The testis was also among the tissues that expressed high levels of ACE2 (mRNA and protein)(14). In contrast, little is known about the expression pattern of ACE2 in the human ovary. Even two public databases for protein expression profiles (Human protein atlas and GeneCards) contradict each other; the latter suggests the high expression of ACE2, whereas the former indicates little to no expression. Meanwhile, there were a limited number of studies documenting the expression of ACE2 in the ovary of animals (15,16). Pereira et al. have reported that ACE2 expression was increased in theca/interstitial layer of the immature rat ovary when administrated with eCG to stimulate follicle development (16). In the bovine ovary, the follicular levels of ACE2 mRNA were transiently downregulated during the ovulatory period (15). As a proxy indicator of ACE2 expression, Ang II and Ang (1–7) were reported to be present in the ovary of multiple species (15–18). Previous studies showed that Ang II and Ang-(1–7) stimulated steroid hormone production and enhanced ovulation and oocyte maturation in rats, rabbits, and cattle (17,19–22). Similarly, Ang-(1–7) was detected in the follicular fluid of women undergoing IVF procedure; this heptapeptide level was correlated clinically with the oocyte maturation rate (23).
The ovary undergoes constant remodeling and cyclic ovulation that requires precisely controlled angiogenesis and an acute inflammatory response {reviewed in (24)}. Given that ACE2 has been shown to play an important role in both angiogenesis and inflammatory responses, it is conceivable that this enzyme is expressed and involved in these processes in the human ovary. Moreover, accumulating evidence indicates that SARS-COV-2 can infect multiple organs including the ovary (25), but the long-term health impact of COVID-19 is yet to be determined. Therefore, it is critically important to assess the expression of ACE2 in the human ovary. In the present study, we sought to: 1) characterize the expression pattern of ACE2 (mRNA and protein) using dominant follicles collected before the LH surge and throughout the periovulatory period from naturally cycling women with ovulation induced by hCG and 2) dissect the cellular regulatory mechanism controlling ACE2 expression using primary human granulosa/lutein cells.
MATERIALS AND METHODS
Materials
Unless otherwise noted, all reagents were purchased from Sigma Chemical Co. or Thermo Fisher Scientific.
Human tissue collection
The protocol using human tissues was approved by the Human Ethics Committee of the Sahlgrenska Academy at the University of Gothenburg, and all patients had given their informed written consent before participating. Whole follicles were collected from patients across the periovulatory period as previously described (26). Women (age 30–38 yr) exhibiting regular menstrual cycles and had not taken hormonal contraceptives for at least 3 months prior to their enrollment in the study underwent laparoscopic sterilization. Women were monitored by transvaginal ultrasound for 2 to 3 menstrual cycles before surgery to ascertain cycle regularity and to monitor the growth of a dominant follicle during the follicular phase. These patients were divided into 4 groups: pre-, early-, late- and post-ovulatory phases. In the preovulatory group, surgery was performed when the follicle reached >14 mm and ≤17.5 mm in diameter prior to the endogenous LH surge. These patients were not given hCG. The remaining women were given recombinant hCG (Ovitrelle, 250 μg) and were divided into three groups; early-ovulatory (surgery between 12 to 18h post-hCG) and late-ovulatory (surgery between 18 to 34h post-hCG). To confirm that these patients followed a normal hormonal pattern before the LH surge or after hCG administration, blood samples were taken at surgery and measured for serum progesterone and estradiol (27). The whole intact follicle/early corpus luteum was removed using laparoscopic scissors and processed for either immunohistochemical or gene expression analysis. The follicle was bisected, and mural granulosa cells were gently scraped off from the interior of the follicle by small tissue forceps. For the gene expression study, the follicular fluid and cell suspension were combined and centrifuged at 500 × g to pellet and collect granulosa cells. For the immunohistochemistry study, dominant follicles were fixed as described below.
Human granulosa/lutein cell (hGLC) cultures
Human granulosa/lutein cells were obtained from aspirates of IVF patients. The collection protocol was approved by the Institutional Review Board of the University of Kentucky Office of Research Integrity. Ovarian hyper-stimulation was induced by the administration of recombinant human FSH in individualized doses to patients at the Bluegrass Fertility Center (Lexington, Kentucky). IVF patients were then given with hCG (10,000U) on Days 9 to 11 when one lead follicle reaches 18 mm or if there are 2 lead follicles of 16 mm or greater in mean diameter, and dominant follicles were aspirated 36 h later. The experiments with hGLC were carried out as described previously (26,28). Briefly, immediately after retrieval of cumulus oocyte complexes, the remaining cells in aspirates were subjected to percoll gradient centrifugations to remove red blood cells. The cells from patients aged 24–40 with non-ovarian etiologies (e.g., male factor and egg donor) were used. The isolated cells were first examined under the microscope for their morphology, counted, and resuspended with OptiMEM media supplemented with 10% fetal bovine serum and antibiotic-antimycotic and then seeded onto culture plates (2.5 × 105 cells/ml). The cells were acclimatized for 6 days, changing media every 24 h. At the end of acclimation, the hGLCs were treated with or without hCG (1 IU/ml) in the absence or presence of various reagents described in the results section and cultured in OptiMEM media supplemented with antibiotic-antimycotic cultured for stated hours.
Immunohistochemistry
Follicles were fixed in 4% formaldehyde, embedded in paraffin, and sectioned (7 μm). Immunostaining was conducted in the Markey Biospeciment Procurement and Translational Pathology Shared Resource Facility at the University of Kentucky as previously described (28). Briefly, heat-induced epitope retrieval was performed in a Biocare Medical Decloaking chamber utilizing Dako’s low pH Target Retrieval Solution. Primary antibody incubation was carried out at 4°C overnight for ACE2 (Sigma-Aldrich, HPA000288, 1:200 dilution) and for 2 h at room temperature for PECAM1 (Roche Diagnostics, JC70 monoclonal antibody, pre-dilute), respectively. Rabbit IgG was used in place of primary antibodies as a negative control. The antibody staining was detected using an appropriate Immpress alkaline phosphatase kit and Vector Red AP chromogen (Vector Laboratories).
Gene expression analysis
The levels of mRNA for ACE2 was measured using a methodology previously described (28). Briefly, total RNA was isolated from granulosa cells using an RNeasy mini kit (Qiagen). The synthesis of the first-strand cDNA was performed by reverse transcription of 500 ng total RNA using superscript III. The levels of mRNA for genes examined were measured by qPCR using Brilliant 3 Ultra-Fast SYBR green (Stratagene). Oligonucleotide primers corresponding to ACE2 were designed using Primer3 software (5’-GGTGGGAGATGAAGCGAGAG-3’, 5’-ACATGGAACAGAGATGCGGG-3’). The relative abundance of the target transcript was normalized to internal reference genes (GAPDH for in vivo sample and RNA18S5 for in vitro sample) as previously described (26,28) and calculated according to the 2−ΔΔCT method (29).
Western blot analysis
Nuclear extracts were isolated from cultured cells, denatured, run on a 10% polyacrylamide gel, and then transferred onto a nitrocellulose membrane as described previously (28). The membrane was incubated overnight at 4°C in 5% skim milk in Tris-buffered saline including 0.1% Tween-20 solution containing primary antibodies against ACE2 (Sigma-Aldrich, HPA000288, 1:100 dilution) and ACTB (Santa Cruz Biotechnology, sc-47778, 1:1,000 dilution). The blots were incubated with the respective secondary HRP-conjugated antibody for 1 h at room temperature. Peroxidase activity was visualized using the SuperSignal®West Pico Chemiluminescent Substrate (Pierce Chemical).
Statistical analyses
All data are presented as means ± SEM. Data were tested for homogeneity of variance by Levene’s test, and log transformations were performed as appropriate. Paired sample t-test or analysis of variance (ANOVA) were used to test differences in levels of mRNA for ACE2 between cell types or across the time of tissue collection, time of culture, or among treatments in vitro, as appropriate. If the test revealed significant effects, the means were compared by Duncan’s test, with p < 0.05 considered significant.
RESULTS
The expression of ACE2 in dominant follicles during the ovulatory period
To determine whether ACE2 is expressed and regulated in human preovulatory follicles during the ovulatory period, the levels of mRNA for ACE2 were assessed in granulosa cells isolated from dominant follicles collected during the pre-, early, late, and post-ovulatory period. The levels of mRNA for ACE2 were markedly increased in granulosa cells during both early and late ovulatory phases (> 40-fold) compared to those obtained before hCG administration (Fig. 1). After ovulation, the levels of ACE2 mRNA in granulosa cells of post-ovulatory follicles were similar to those of dominant follicles collected at the early and late ovulatory phases, but higher than those of dominant follicles collected before hCG administration (Fig.1A). Next, to determine whether ACE2 is also expressed in cumulus cells of ovulatory follicles, we collected granulosa cells and cumulus cells from women undergoing IVF procedures at the time of oocyte retrieval. As shown in Fig. 1B, we found the abundant expression of ACE2 in cumulus cells of ovulatory follicles. The levels of mRNA and protein for ACE2 were higher in cumulus cells compared to those in granulosa cells from all patients tested.
Figure 1. The levels of mRNA for ACE2 in granulosa cells and cumulus cells of periovulatory follicles.
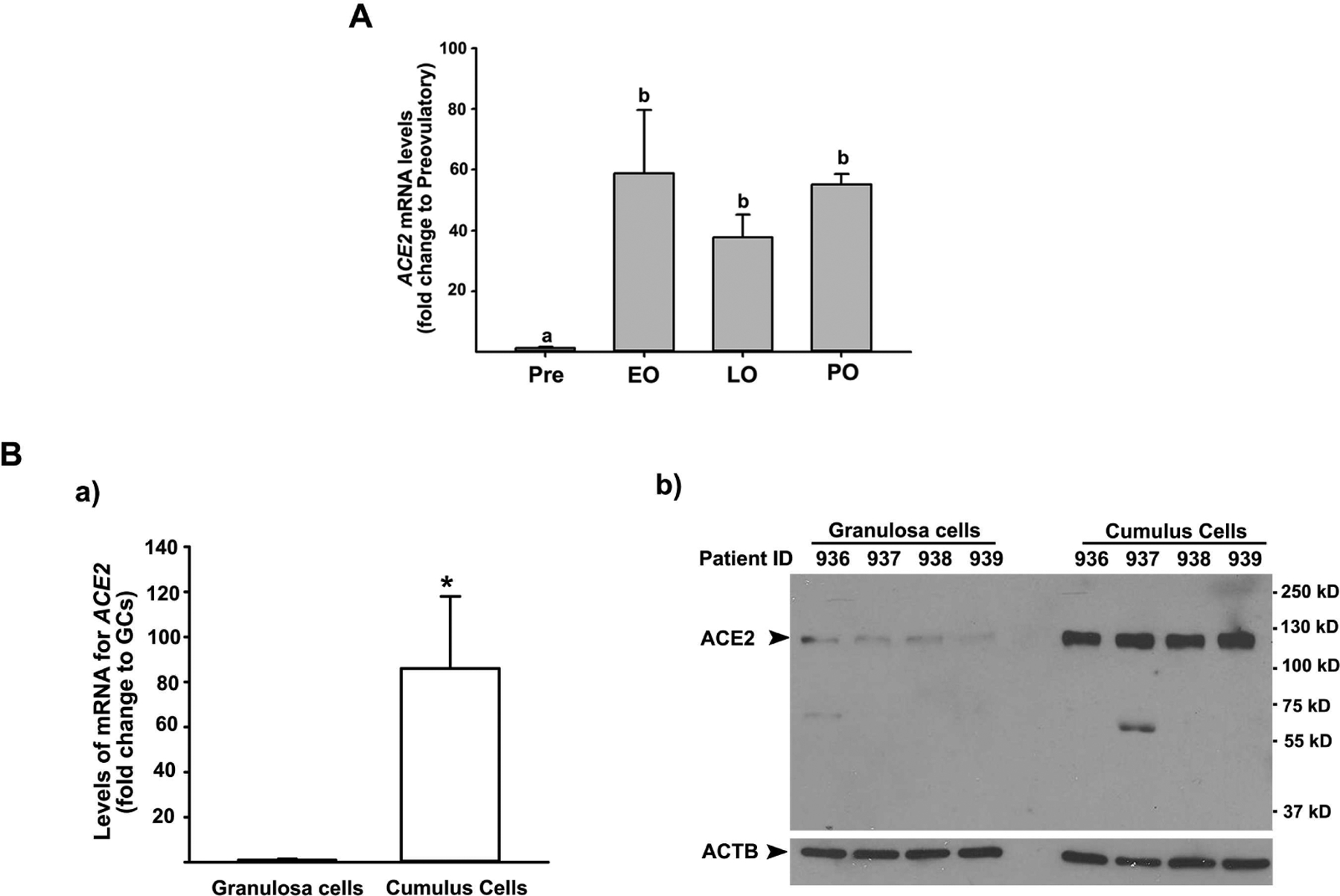
A) Dominant follicles were retrieved from the ovaries of women undergoing laparoscopic tubal sterilization before the LH surge or at various times after recombinant hCG administration and divided into four phases: pre- (Pre, n=6), early (EO, n=5), late (LO, n=6), and post- (PO, n=2) ovulatory phases. The levels of mRNA for ACE2 were measured using qPCR in granulosa cells isolated from a dominant follicle collected at Pre, EO, and LO and whole follicles retrieved at PO and normalized to the levels of GAPDH mRNA in each sample. The levels were presented as fold change to Pre values. Bars with no common superscripts are significantly different (p < 0.05). B) Cumulus cells and granulosa cells were collected at the time of oocyte retrieval from women undergoing a standardized IVF procedure. a) The levels of mRNA for ACE2 were measured by qPCR and normalized to the levels of RNA18S5 in each sample (n = 5 independent samples). * p < 0.05. b) A representative Western blot image detecting ACE2 protein. Samples loaded were from independent patients indicated. ACTB detection in each lane was used as a protein loading control.
To determine whether the increase in ACE2 mRNA levels translated to ACE2 protein and what types of cells expressed ACE2, the dominant follicles collected throughout the periovulatory phases were analyzed by immunohistochemistry analyses. The staining for ACE2 was negligible in granulosa cells and theca cell layer of dominant follicles obtained before the endogenous LH surge (Fig. 2A). In the dominant follicle obtained during the early ovulatory phase, positive staining for ACE2 became evident in granulosa cells and theca cell layer (Fig. 2B). During the late ovulatory period, intensive staining for ACE2 was localized to both granulosa cells and theca layer of dominant follicles (Fig. 2C&D). Interestingly, some cells in granulosa and thecal layer showed more robust staining (arrows) compared to the rest of cells in late ovulatory follicles. This concentrated immunopositive staining of ACE2 was more prominent in the late ovulatory follicle that displays the morphological change typical of follicles immediately prior to ovulation (e.g., dispersed granulosa cells, dissolution of basement membrane, and thinning of theca interna layer, Fig. 2D). Beside granulosa and theca cell layers, intense staining for ACE2 was detected throughout the stroma layer of late ovulatory follicles (Fig. 2D, wavy arrow). After ovulation, the staining for ACE2 was persistent in both granulosa- and theca-lutein cells of the post-ovulatory follicle as well as in the stroma layer, showing intense staining in some, but not all follicular cells (arrows and wavy arrows in Fig. 2E&F).
Figure 2. The expression pattern of ACE2 protein in periovulatory follicles.
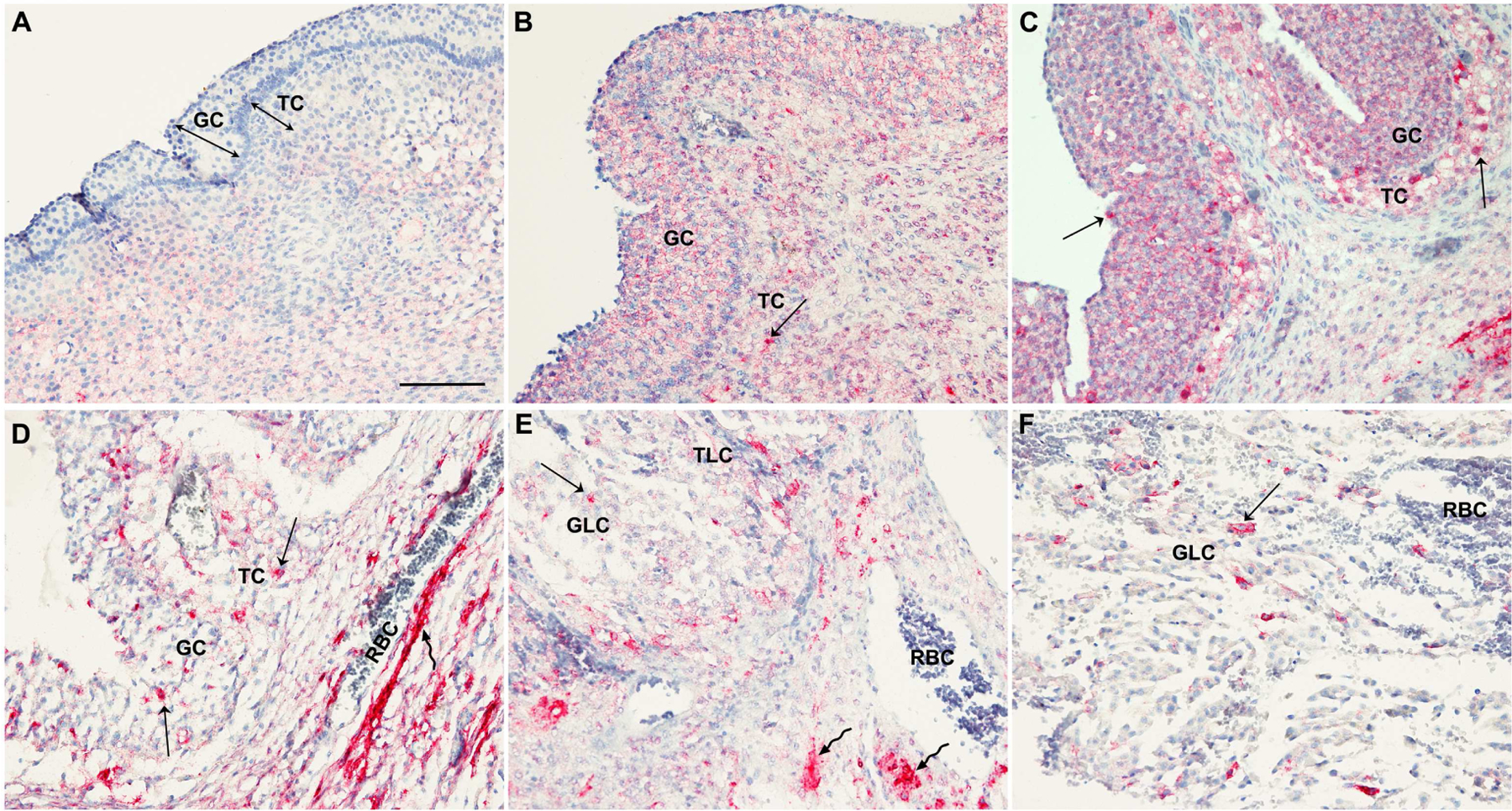
Dominant follicles were retrieved from the ovaries of women undergoing laparoscopic tubal sterilization before the LH surge or at various times after recombinant hCG administration. The paraffin embedded sections of dominant follicles obtained from various time points (A, preovulatory phase n=2; B, early ovulatory phase n=4; C & D, late ovulatory phase n=4; E, late ovulatory phase n=1) were subjected to immunohistochemical analyses to detect ACE2 protein. Sections in C & D are from different patients obtained from the late ovulatory period. F is an antrum region of the postovulatory follicle shown in the panel E. Pink/red staining indicates positive signals for ACE2. All sections were lightly stained with hematoxylin (blue staining) for nuclear counterstaining. Arrows point to granulosa and theca cells where the intense staining for ACE2 is detected. Wavy arrows point to ACE2 staining in the stroma layer. GC, granulosa cells; TC, theca cells; GLC, granulosa lutein cells; TLC, theca lutein cells; RBC, red blood cells. Scale bar in A; 100 μm
In the ovary, endothelial cells and leukocytes infiltrate into the granulosa and theca cell layer of ovulating follicles during follicle transformation into the corpus luteum (30). With the current finding that a subpopulation of cells in the granulosa and theca cell layer exhibited intense staining for ACE2, we questioned whether ACE2 was expressed in infiltrating endothelial cells and leukocytes. Therefore, the serial sections of late and post-ovulatory follicles used to detect ACE2 protein were evaluated for PECAM1 (Platelet And Endothelial Cell Adhesion Molecule 1) known to be expressed on the surface of endothelial cells, platelets, monocytes, neutrophils, and some types of T-cells (31). As expected, the immunopositive staining for PECAM1 was localized to endothelial cells lining various types of blood vessels and leukocytes inside blood vessels surrounding the ovulatory and postovulatory follicles (Fig. 3A & E). PECAM1 staining was also detected in endothelial cells located between the granulosa and theca interna layer throughout the late ovulatory follicle (Fig. 3B, arrows) and theca lutein layer of the post-ovulatory follicle (Fig. 3F, arrows). However, this endothelial cell and leukocyte expression of PECAM1 in the ovulatory and post-ovulatory follicles was not co-localized to the distribution of ACE2 (arrows in Fig. 3F and H point the same location).
Figure 3. The comparison of the expression pattern between PECAM1 and ACE2 in late ovulatory and post-ovulatory follicles.
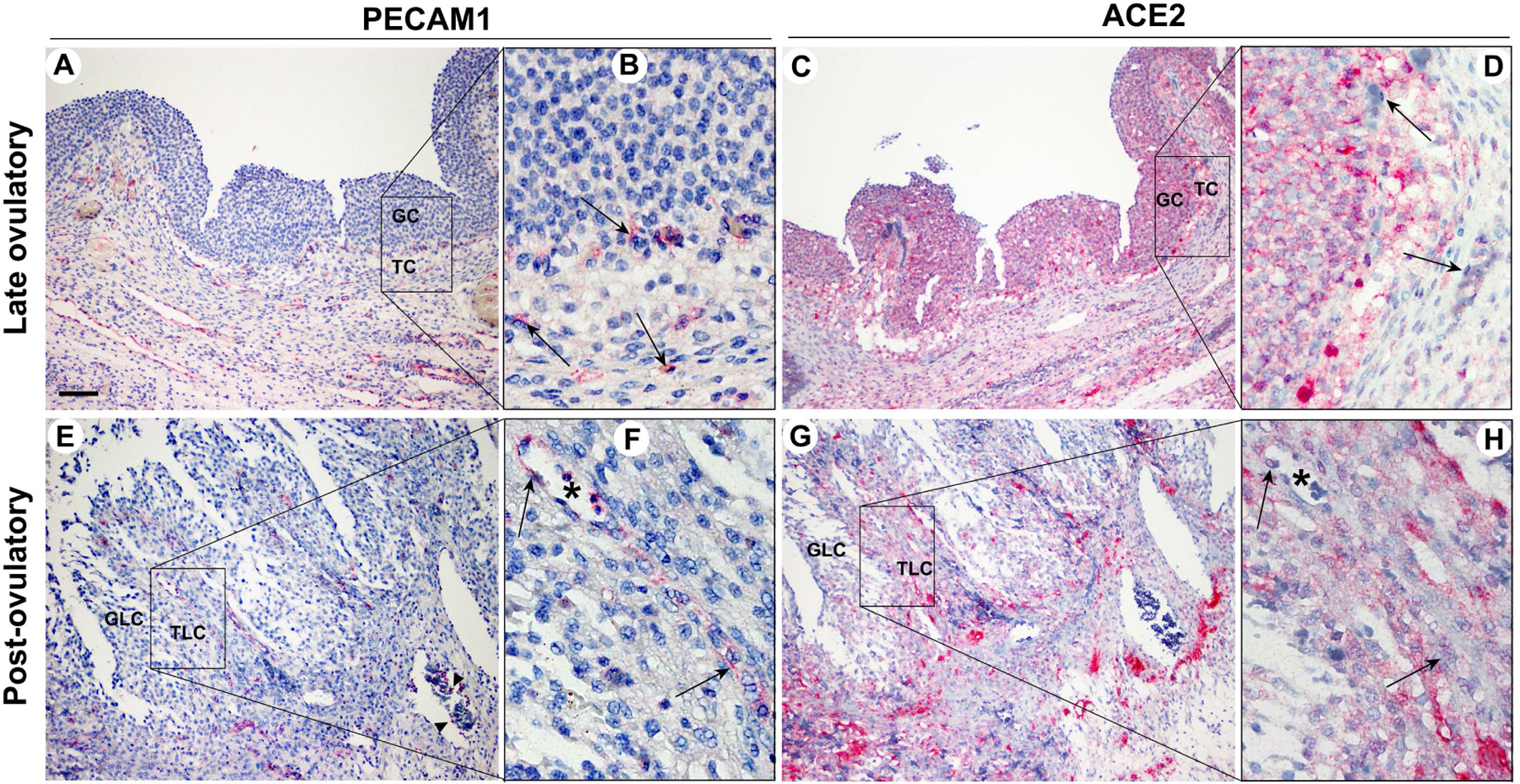
Dominant follicles were retrieved from the ovaries of women undergoing laparoscopic tubal sterilization. The serial sections of dominant follicles obtained during the late (A & B) and post (C & D) ovulatory phase were subjected to immunohistochemical analyses to detect PECAM1 and ACE2. Square bars in A, C, E, and G are amplified in B, D, F, and H, respectively. PECAM1 was used to detect endothelial cells; it also stained a subpopulation of leukocytes. Asterisks in F and H were used to locate the same structure in the serial section of the same post-ovulatory follicle. Pink/red staining indicates positive signals for PECAM1 and ACE2. All sections were lightly stained with hematoxylin (blue staining) for nuclear counterstaining. Arrows (B, D, F, H) and arrowheads (E) point to endothelia cells and leukocytes stained with PECAM1, respectively. GC, granulosa cells; TC, theca cells; GLC, granulosa lutein cells; TLC, theca lutein cells. Scale bar in A; 100 μm
Regulation of ACE2 expression in human granulosa cells
To investigate the cellular mechanisms underlying the marked up-regulation of ACE2 expression in periovulatory follicles after hCG administration in vivo, we utilized primary human granulosa/lutein cells (hGLC) that were acclimated in cultures for 6 days without any hormone treatments to regain hCG responsiveness. As shown in Fig. 4A, we found that hCG treatment increased the expression of ACE2, both at the levels of mRNA and protein compared to those in control samples, similar to those observed during the peri-ovulatory period of the menstrual cycle.
Figure 4. Regulation of ACE2 expression in human granulosa cells.
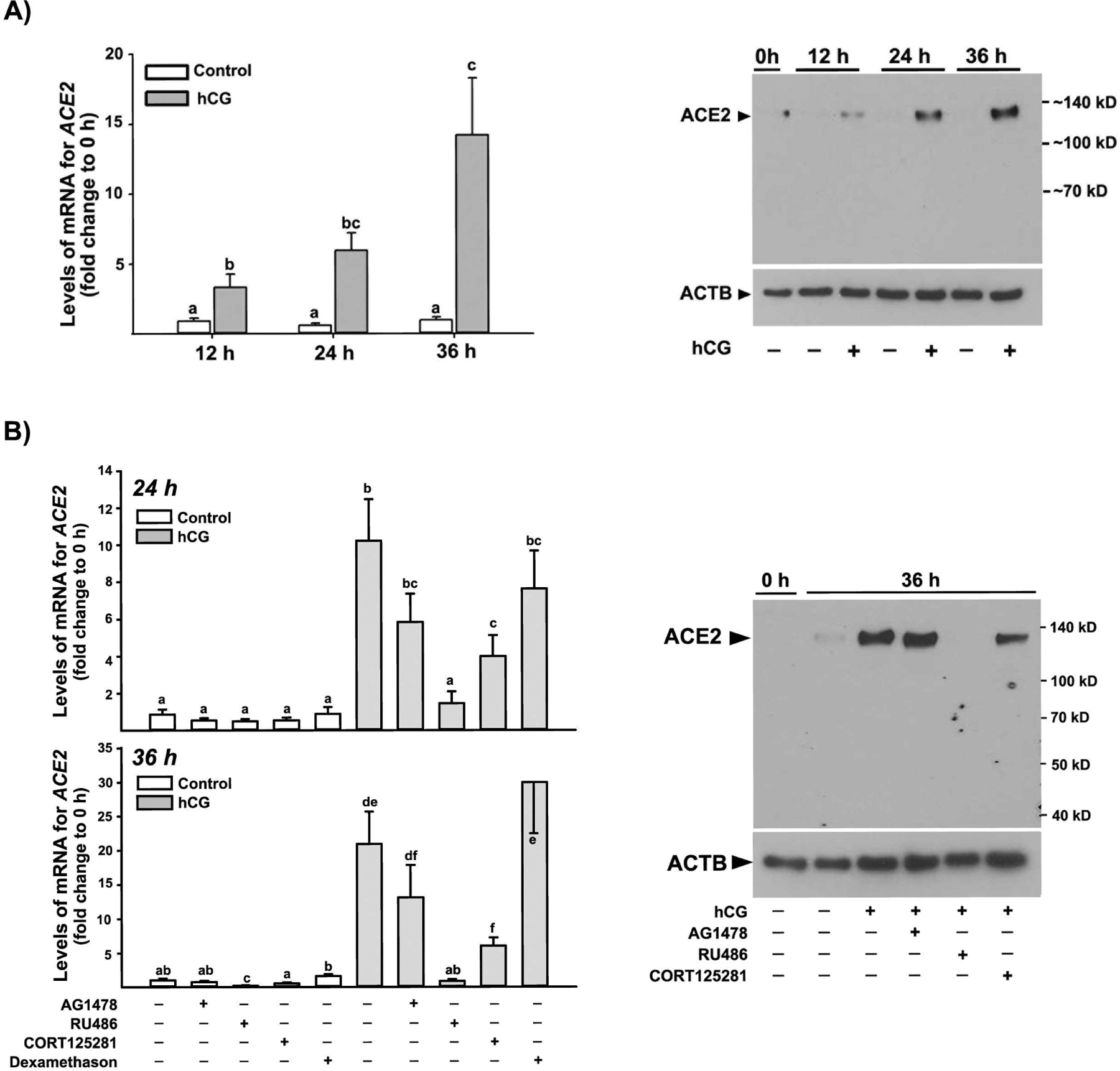
Human granulosa/lutein cells (hGLC) obtained from women undergoing a standardized IVF procedure were acclimated for 6 days. A) hGLCs were treated without (Control) or with hCG (1IU/ml) for 0, 12, 24, and 36 h. Right panel) the levels of mRNA for ACE2 were measured by qPCR and normalized to the levels of RNA18S5 in each sample (n ≥7 independent samples). Bars with no common superscripts are significantly different (p < 0.05). Left panel) a representative Western blot image detecting ACE2 protein. ACTB detection in each lane was used as a protein loading control. The experiments were repeated 3 times, each with independent samples. B) The cells were treated without (Control) or with hCG in the presence or absence of AG1478 (10μM), RU486 (20 μM), CORT123567 (50 μM) or dexamethasone (20 μM) for 24 and 36 h. Right panel) the levels of mRNA for ACE2 were measured by qPCR and normalized to the levels of RNA18S5 in each sample (n = 7 independent patient samples). Bars with no common superscripts are significantly different (p < 0.05). Left panel) a representative Western blot image detecting ACE2 protein. ACTB detection in each lane was used as a protein loading control. The experiments were repeated three times, each with independent patient samples.
Next, to determine whether the hCG-induced increases in ACE2 expression were mediated by key ovulatory mediators such as the EGF-receptor signaling, P4/PGR, or glucocorticoids/NR3C1, the hGLCs were treated with or without hCG in the absence or presence of AG1478 (an EGF-receptor inhibitor), RU486 (a dual antagonist for progesterone receptor and glucocorticoid receptor), CORT123567 (a selective NR3C1 inhibitor), or dexamethasone (a synthetic glucocorticoid). As expected, hCG increased ACE2 expression, but the hCG-induced increases were completely abolished by RU486 at both 24 and 36 h (Fig. 4B). CORT125281 treatment also resulted in a partial reduction of hCG-induced ACE2 expression at both time points. In contrast, neither AG1478 nor dexamethasone had effects on basal and hCG-induced ACE2 expression.
DISCUSSION
In women throughout their reproductive age, the ovary undergoes a series of cyclic changes during the menstrual cycle, with the culmination being ovulation. The preovulatory LH surge initiates these cyclic changes by inducing the expression of specific genes. The encoded products of these ovulatory genes exert their biological actions to bring about necessary cellular and extracellular changes required for ovulation and corpus luteum (CL) formation. In the present study, we revealed that the expression of ACE2, an enzyme with carboxypeptidase activity and a primary receptor for SARS-COV-2, is rapidly and dramatically induced after hCG administration in dominant follicles obtained from naturally cycling women throughout the periovulatory period. Using the primary hGLC model that can recapitulate key ovulatory changes in gene expression, this study further provided experimental evidence that the ovulatory induction of ACE2 expression was mediated by hCG and hCG-induced steroid hormones, progesterone and glucocorticoid, in ovulatory follicles. Noteworthy is also our finding of the higher expression of ACE2 in cumulus cells than granulosa cells collected immediately prior to ovulation. Together, not only does this novel information suggest the potential involvement of ACE2 as a critical enzyme for the LH surge-induced cyclic events of ovulation, cumulus expansion, oocyte maturation, and luteal formation, but also implicate the possible impact of COVID-19 in vital cyclic ovarian functions, thus women’s overall reproductive health.
Our comprehensive in vivo study is novel in that these data are: 1) the first report documenting the ovulatory induction of ACE2 expression in naturally cycling women and 2) the only report showing the dramatic and rapid up-regulation after ovulatory induction among any species studied so far. In cattle, ACE2 mRNA levels were initially down-regulated in granulosa cells after the LH surge induction, but then increased back to the preovulatory level at 24 h after GnRH injection, while no changes were observed in thecal levels of ACE2 mRNA throughout the periovulatory period (15). No significant changes in ACE2 expression pattern was observed in preovulatory follicles obtained before and throughout the ovulatory period in the non-human primate (Supplementary Fig. 1)(32). In the mouse ovary, ACE2 mRNA profile showed low abundance without a clear pattern throughout follicular development and the ovulatory period (http://okdb.appliedbioinfo.net, Supplementary Fig. 2). Therefore, our data indicate that the dramatic up-regulation of ACE2 expression is specific to human ovulatory follicles and further points to the differences in specific mechanisms underlying the ovulatory process among different species. Of note, in the present study, hCG (rhCG for in vivo and urinary hCG for in vitro studies) was given to patients to mimic the endogenous LH surge. This was to control exactly when the ovulatory trigger was initiated in individual patients. Therefore, it remains to be determined whether the endogenous LH surge and hCG administration would exert the same effect on ACE2 expression.
Another intriguing finding is the unique localization pattern for ACE2 protein observed in human ovulatory follicles. The initial induction of ACE2 staining was localized evenly throughout the granulosa and theca cell layer during the early ovulatory phase, and then, the staining became more intense and progressively more sporadic among cells when the dominant follicle progressed toward ovulation and transformed into the CL. The uneven, localized staining of ACE2 did not appear to be in non-follicular cells such as endothelial cells or leukocytes when compared to PECAM1 staining pattern in serial sections of the same follicle. Consistent with this finding, qPCR analysis of ACE2 mRNA levels showed little to undetectable levels of ACE2 mRNA in leukocytes compared to granulosa cells (Supplementary Fig.3). One possible explanation for this unique localization pattern might be that ACE2 exists in two different forms: the membrane-bound form and secreted form (33). During the early ovulatory phase when the level of ACE2 mRNA was rapidly up-regulated, ACE2 would have been processed and expressed as a single-pass type I membrane protein. Then, with the progression toward the late and post-ovulatory phases, the enzymatically active extracellular domain of ACE2 could be cleaved, released, and aggregated onto specific cells or the area where the substrates for ACE2 are present. This possibility needs to be explored in future studies.
To explore the cellular mechanism underlying ovulatory ACE2 expression, we have utilized granulosa cells pooled from multiple follicles of each patient obtained during IVF oocyte retrieval. Despite the potential limitations of pooling granulosa cells that may have come from different development stages of follicles, these cells (hGLC) increased the expression of genes involved in the ovulatory process, including PGR and EGF-like peptides and the production of progesterone and prostaglandins in response to hCG (28). Similarly, consistent with the in vivo expression pattern, hCG stimulated ACE2 expression in hGLC in vitro. Furthermore, this induction was completely inhibited by RU486, a dual antagonist for PGR and NR3C1 and partially by CORT125281, a selective antagonist for NR3C1. Our pilot study showed that hCG increased NR3C1 expression and cortisol production in hGLCs (Supplementary Fig. 4). These data, taken together, indicate that the up-regulated expression of ACE2 was mediated by the actions of progesterone and glucocorticoids in granulosa cells of the ovulatory follicle. Similar to our findings from granulosa cells, a recent study by Chadchan et al. reported that progesterone promoted ACE2 expression in the endometrial stroma of both humans and mice (34). Meanwhile, the existing data on the regulation of ACE2 expression by glucocorticoids or its receptor appear to be dependent on cell types and models tested (35,36). Therefore, our findings of the regulatory mechanisms involved in ACE2 expression provide new insights into the ovulatory process and valuable data that may be useful in understanding SARS-COV-2 infection in reproductive-age women.
In normal physiology, ACE2 is best known for its ability to hydrolyze Ang II to Ang (1–7) (8,9). Therefore, the marked increases in ACE2 expression could mean the shift in the balance between Ang II and Ang-(1–7), reducing Ang II levels while increasing Ang-(1–7). Both Ang II and Ang-(1–7) are present in the follicular fluid collected at the time of IVF in women (18). Studies using animal models have shown that Ang II and Ang-(1–7) stimulated steroid production, regulated blood flow, and promoted oocyte maturation and ovulation (20,37–39). Therefore, the current findings showing rapid induction of ACE2 expression in human ovulatory follicles and high expression of ACE2 in cumulus cells support the notion that Ang II/ACE2/Ang-(1–7) is likely involved in cumulus expansion, oocyte maturation, and the ovulatory process in the human ovary.
With the current global challenge with COVID-19, our findings take on another level of significance since ACE2 serves as a primary entry receptor for SARS-CO-2. A recent study has reported ACE2 expression in oocytes, cumulus, and granulosa cells collected from patients at the time of IVF procedure (40). In that study, the expression of TMPRSS2, CD147, and CTSL was also documented in these ovarian cells as factors involved in SARS-COV-2 infection (40). Therefore, our current findings showing the rapid and dramatic induction of ACE2 expression, together with the findings from the previous study (40), offer a compelling possibility that the ovary could be a target of SARS-COV-2, particularly vulnerable during the periovulatory period. In the scenario of SARS-COV-2 infection in the ovary, this virus could elicit negative impacts on female fertility, not only by blocking the physiological action of ACE2 necessary for oocyte maturation, ovulation, and CL formation but also destroying a mature oocyte and ovulatory follicle. In support of these possibilities, Orvieto et al. (41) reported that the proportion of top-quality embryos was significantly reduced in couples undergoing consecutive IVF cycles after recovering from COVID-19 infection compared to that obtained before the infection. Therefore, it will be vital to give adequate attention to the reproductive health to women who had COVID-19 and appropriate precautions and screening to women seeking IVF treatments. Once more data and information becomes available, it will be of great interest to assess the impact of COVID-19 on female fertility.
Supplementary Material
Supplementary Figure 1) ACE2 mRNA profile extracted from GEODatasets (Accession: GDS3863). Microarray analysis of ovulatory, luteinizing follicles from a primate model of controlled ovulation at 0, 12, 24, 36 hr after exposure to an ovulatory (exogenous hCG) stimulus during the menstrual cycle (PMID: 21036944). A bar graph above was generated by imputing the term “ACE2” (https://www.ncbi.nlm.nih.gov/geoprofiles?term=GDS3863[ACCN]+ACE2). A red bar represents the expression measurement from the value of each sample.
Supplementary Figure 2) The profile of Ace2 mRNA during the follicular development and ovulatory period in the mouse ovary. The profile of mRNA for Ace2 depicted in the Ovarian Kaleidoscope Database (http://okdb.appliedbioinfo.net) was redrawn. This dataset was based on microarray data using immature mice that were injected with HMG (FSH+LH activity) and 48 h later with hCG to induce ovulation. Ovaries were collected before and every 2 hours after HMG and every 1 h after hCG administration.
Supplementary Figure 3) Comparison of levels of mRNA for ACE2 in granulosa cells (GC) vs. leukocytes (Leuko). Follicular aspirates collected at the time of oocyte retrieval from IVF patients were incubated in red blood cell lysis buffer and filtered through a 40-μm cell strainer. The flow-through cells were mixed and incubated with a human CD45 microbead (Miltenyi Biotec) to magnetically label leukocytes and then subjected to a Magnetic-activated cell sorting (MACS) column placed in an AutoMAC Pro Separator (Miltenyi Biotec). Negative (GCs) and positive (Leuko) fractions were separately collected and used to isolate to total RNA. Experiments were performed from 3 individual patient samples. *, p < 0.05
Supplementary Figure 4) Effects of hCG on NR3C1 expression and cortisol production in hGLCs. Granulosa/lutein cells obtained from IVF patients were cultured for 6 d and then treated with or without hCG (1 IU/ml) for 36 h. (A) NR3C1 was detected by Western blots using whole-cell extracts. The membrane was re-probed with a monoclonal antibody against ACTB to assess protein loading in each lane. (B) Cortisol levels in culture media were measured by a cortisol ELISA kit (R&D systems) after performing extraction procedures described by Yong et. al (PMID: 11134135)(n=4 individual patient samples; *, p ≤ 0.05).
ACKNOWLEDGEMENT
We thank Drs. Patrick Hannon and Ketan Shrestha for critical reading of the manuscript. This research was supported by P01HD71875 (MJ, and TEC), R03HD095098 (MJ), and R01HD096077 (MJ) and the BTPSRF of the University of Kentucky Markey Cancer Center (P30CA177558).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have nothing to disclose.
REFERENCES
- 1.Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020; 367:1444–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000; 87:E1–9 [DOI] [PubMed] [Google Scholar]
- 3.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, et al. Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002; 277:14838–14843 [DOI] [PubMed] [Google Scholar]
- 4.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000; 275:33238–33243 [DOI] [PubMed] [Google Scholar]
- 5.Soubrier F, Hubert C, Testut P, Nadaud S, Alhenc-Gelas F, Corvol P. Molecular biology of the angiotensin I converting enzyme: I. Biochemistry and structure of the gene. J Hypertens 1993; 11:471–476 [DOI] [PubMed] [Google Scholar]
- 6.Brewster UC, Perazella MA. The renin-angiotensin-aldosterone system and the kidney: effects on kidney disease. Am J Med. 2004; 116:263–272 [DOI] [PubMed] [Google Scholar]
- 7.Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010; 2:247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos RA, Ferreira AJ, Verano-Braga T, Bader M. Angiotensin-converting enzyme 2, angiotensin-(1–7) and Mas: new players of the renin-angiotensin system. J Endocrinol. 2013; 216:R1–r17 [DOI] [PubMed] [Google Scholar]
- 9.Santos RAS, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, et al. The ACE2/Angiotensin-(1–7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1–7). Physiol Rev. 2018; 98:505–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, McKinnie SM, Farhan M, Paul M, McDonald T, McLean B, et al. Angiotensin-Converting Enzyme 2 Metabolizes and Partially Inactivates Pyr-Apelin-13 and Apelin-17: Physiological Effects in the Cardiovascular System. Hypertension. 2016; 68:365–377 [DOI] [PubMed] [Google Scholar]
- 11.Li W, Zhang C, Sui J, Kuhn JH, Moore MJ, Luo S, et al. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005; 24:1634–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Wang Y, Luo W, Huang L, Xiao J, Li F, et al. A comprehensive investigation of the mRNA and protein level of ACE2, the putative receptor of SARS-CoV-2, in human tissues and blood cells. Int J Med Sci. 2020; 17:1522–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004; 203:631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douglas GC, O’Bryan MK, Hedger MP, Lee DK, Yarski MA, Smith AI, et al. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology 2004; 145:4703–4711 [DOI] [PubMed] [Google Scholar]
- 15.Tonellotto dos Santos J, Ferreira R, Gasperin BG, Siqueira LC, de Oliveira JF, Santos RA, et al. Molecular characterization and regulation of the angiotensin-converting enzyme type 2/angiotensin-(1–7)/MAS receptor axis during the ovulation process in cattle. J Renin Angiotnsin Aldosterone Syst. 2012; 13:91–98 [DOI] [PubMed] [Google Scholar]
- 16.Pereira VM, Reis FM, Santos RA, Cassali GD, Santos SH, Honorato-Sampaio K, et al. Gonadotropin stimulation increases the expression of angiotensin-(1–7) and MAS receptor in the rat ovary. Reprod Sci. 2009; 16:1165–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viana GE, Pereira VM, Honorato-Sampaio K, Oliveira CA, Santos RA, Reis AM. Angiotensin-(1–7) induces ovulation and steroidogenesis in perfused rabbit ovaries. Exp Physiol. 2011; 96:957–965 [DOI] [PubMed] [Google Scholar]
- 18.Reis FM, Bouissou DR, Pereira VM, Camargos AF, dos Reis AM, Santos RA. Angiotensin-(1–7), its receptor Mas, and the angiotensin-converting enzyme type 2 are expressed in the human ovary. Fertil Steril 2011; 95:176–181 [DOI] [PubMed] [Google Scholar]
- 19.Costa AP, Fagundes-Moura CR, Pereira VM, Silva LF, Vieira MA, Santos RA, et al. Angiotensin-(1–7): a novel peptide in the ovary. Endocrinology 2003; 144:1942–1948 [DOI] [PubMed] [Google Scholar]
- 20.Honorato-Sampaio K, Pereira VM, Santos RA, Reis AM. Evidence that angiotensin-(1–7) is an intermediate of gonadotrophin-induced oocyte maturation in the rat preovulatory follicle. Exp Physiol. 2012; 97:642–650 [DOI] [PubMed] [Google Scholar]
- 21.Ferreira R, Oliveira JF, Fernandes R, Moraes JF, Gonçalves PB. The role of angiotensin II in the early stages of bovine ovulation. Reproduction 2007; 134:713–719 [DOI] [PubMed] [Google Scholar]
- 22.Mikuni M, Brännström M, Hellberg P, Peterson CA, Pall M, Edwin SS, et al. Saralasin-induced inhibition of ovulation in the in vitro perfused rat ovary is not replicated by the angiotensin II type-2 receptor antagonist PD123319. Am J Obstet Gynecol. 1998; 179:35–40 [DOI] [PubMed] [Google Scholar]
- 23.Cavallo IK, Dela Cruz C, Oliveira ML, Del Puerto HL, Dias JA, Lobach VN, et al. Angiotensin-(1–7) in human follicular fluid correlates with oocyte maturation. Hum Reprod 2017; 32:1318–1324 [DOI] [PubMed] [Google Scholar]
- 24.Duffy DM, Ko C, Jo M, Brannstrom M, Curry TE. Ovulation: Parallels With Inflammatory Processes. Endocr Rev. 2019; 40:369–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bian X-W, Team TC-P. Autopsy of COVID-19 patients in China. Natl Sci Rev. 2020; 7:1414–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Alem L, Puttabyatappa M, Rosewell K, Brännström M, Akin J, Boldt J, et al. Chemokine Ligand 20: A Signal for Leukocyte Recruitment During Human Ovulation? Endocrinology 2015; 156:3358–3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lind AK, Dahm-Kähler P, Weijdegård B, Sundfeldt K, Brännström M. Gelatinases and their inhibitors during human ovulation: increased expression of tissue inhibitor of matrix mtalloproteinase-1. Mol Hum Reprod. 2006; 12(12):725–736 [DOI] [PubMed] [Google Scholar]
- 28.Choi Y, Wilson K, Hannon PR, Rosewell KL, Brännström M, Akin JW, et al. Coordinated Regulation Among Progesterone, Prostaglandins, and EGF-Like Factors in Human Ovulatory Follicles. J Clin Endocrinol Metab. 2017; 102:1971–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402–408 [DOI] [PubMed] [Google Scholar]
- 30.Trau HA, Davis JS, Duffy DM. Angiogenesis in the primate ovulatory follicle is stimulated by luteinizing hormone via prostaglandin E2. Biol Reprod. 2015; 92:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergom C, Paddock C, Gao C, Holyst T, Newman DK, Newman PJ. An alternatively spliced isoform of PECAM-1 is expressed at high levels in human and murine tissues, and suggests a novel role for the C-terminus of PECAM-1 in cytoprotective signaling. J Cell Sci. 2008; 121:1235–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu F, Stouffer RL, Müller J, Hennebold JD, Wright JW, Bahar A, et al. Dynamics of the transcriptome in the primate ovulatory follicle. Mol Human Reprod. 2011; 17:152–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grobe N, Di Fulvio M, Kashkari N, Chodavarapu H, Somineni HK, Singh R, et al. Functional and molecular evidence for expression of the renin angiotensin system and ADAM17-mediated ACE2 shedding in COS7 cells. Am J Physiol Cell Physiol. 2015; 308:C767–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chadchan SB, Popli P, Maurya VK, Kommagani R. The SARS-CoV-2 receptor, angiotensin-converting enzyme 2, is required for human endometrial stromal cell decidualization. Biol Reprod. 2021; 104:336–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiang Z, Liu J, Shi D, Chen W, Li J, Yan R, et al. Glucocorticoids improve severe or critical COVID-19 by activating ACE2 and reducing IL-6 levels. Int J Biol Sci 2020; 16:2382–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young MJ, Clyne CD, Chapman KE. Endocrine aspects of ACE2 regulation: RAAS, steroid hormones and SARS-CoV-2. J Endocrinol. 2020; 247:R45–r62 [DOI] [PubMed] [Google Scholar]
- 37.Domińska K Involvement of ACE2/Ang-(1–7)/MAS1 Axis in the Regulation of Ovarian Function in Mammals. Int J Mol Sci 2020; 21(13):4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonçalves PB, Ferreira R, Gasperin B, Oliveira JF. Role of angiotensin in ovarian follicular development and ovulation in mammals: a review of recent advances. Reproduction 2012; 143:11–20 [DOI] [PubMed] [Google Scholar]
- 39.Mitsube K, Mikuni M, Matousek M, Zackrisson U, Brännström M. Role of the angiotensin II system in regulation of ovulation and blood flow in the rat ovary. Reproduction 2003; 125:425–435 [DOI] [PubMed] [Google Scholar]
- 40.Rajput SK, Logsdon DM, Kile B, Engelhorn HJ, Goheen B, Khan S, et al. Human eggs, zygotes, and embryos express the receptor angiotensin 1-converting enzyme 2 and transmembrane serine protease 2 protein necessary for severe acute respiratory syndrome coronavirus 2 infection. F S Sci. 2021; 2:33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orvieto R, Segev-Zahav A, Aizer A. Does COVID-19 infection influence patients’ performance during IVF-ET cycle?: an observational study. Gynecol Endocrinol. 2021;11:1–3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1) ACE2 mRNA profile extracted from GEODatasets (Accession: GDS3863). Microarray analysis of ovulatory, luteinizing follicles from a primate model of controlled ovulation at 0, 12, 24, 36 hr after exposure to an ovulatory (exogenous hCG) stimulus during the menstrual cycle (PMID: 21036944). A bar graph above was generated by imputing the term “ACE2” (https://www.ncbi.nlm.nih.gov/geoprofiles?term=GDS3863[ACCN]+ACE2). A red bar represents the expression measurement from the value of each sample.
Supplementary Figure 2) The profile of Ace2 mRNA during the follicular development and ovulatory period in the mouse ovary. The profile of mRNA for Ace2 depicted in the Ovarian Kaleidoscope Database (http://okdb.appliedbioinfo.net) was redrawn. This dataset was based on microarray data using immature mice that were injected with HMG (FSH+LH activity) and 48 h later with hCG to induce ovulation. Ovaries were collected before and every 2 hours after HMG and every 1 h after hCG administration.
Supplementary Figure 3) Comparison of levels of mRNA for ACE2 in granulosa cells (GC) vs. leukocytes (Leuko). Follicular aspirates collected at the time of oocyte retrieval from IVF patients were incubated in red blood cell lysis buffer and filtered through a 40-μm cell strainer. The flow-through cells were mixed and incubated with a human CD45 microbead (Miltenyi Biotec) to magnetically label leukocytes and then subjected to a Magnetic-activated cell sorting (MACS) column placed in an AutoMAC Pro Separator (Miltenyi Biotec). Negative (GCs) and positive (Leuko) fractions were separately collected and used to isolate to total RNA. Experiments were performed from 3 individual patient samples. *, p < 0.05
Supplementary Figure 4) Effects of hCG on NR3C1 expression and cortisol production in hGLCs. Granulosa/lutein cells obtained from IVF patients were cultured for 6 d and then treated with or without hCG (1 IU/ml) for 36 h. (A) NR3C1 was detected by Western blots using whole-cell extracts. The membrane was re-probed with a monoclonal antibody against ACTB to assess protein loading in each lane. (B) Cortisol levels in culture media were measured by a cortisol ELISA kit (R&D systems) after performing extraction procedures described by Yong et. al (PMID: 11134135)(n=4 individual patient samples; *, p ≤ 0.05).


