Abstract
Stable mixed donor/host chimerism has been reliably established in dogs given a sublethal dose of 2 Gy total body irradiation (TBI) before and immunosuppression with mycophenolate mofetil (MMF) or rapamycin combined with cyclosporine (CSP) after marrow transplantation from DLA-identical littermates (HCT). When TBI was reduced to 1 Gy, only transient engraftment was observed. Here we asked whether stable engraftment after 1 Gy TBI could be accomplished by reducing host vs. donor immune responsiveness through preceding CD154 blockade and infusion of donor peripheral blood mononuclear cells (PBMC). The anti-human CD154 antibody, 5c8, cross-reacted with canine lymphocytes and blocked allo-immune responses in vitro. Based on pharmacokinetic studies, six dogs received a single i.v. injection of 5 mg/kg anti-CD154 antibody (day -5) followed one day later by donor PBMC. On day 0, dogs were given 1 Gy TBI and DLA-identical marrow grafts. Postgrafting immunosuppression consisted of MMF and CSP. All six dogs showed initial engraftment, which was sustained in three of the six for >26 weeks, while three dogs rejected their grafts after 9, 22, and 24 weeks, respectively, and survived with autologous recovery. Graft survival was significantly improved over that among 11 historical controls conditioned with 1 Gy TBI and given either MMF or rapamycin with CSP after HCT, all of which rejected their grafts between 3 and 12 weeks (P = 0.03). Preceding donor PBMC infusion and CD154 blockade improved survival of DLA-identical marrow grafts after 1 Gy TBI.
Keywords: anti-CD154 antibody, costimulatory blockade, marrow transplantation, dogs, nonmyeloablative conditioning
INTRODUCTION
Sustained engraftment of DLA-identical littermate marrow was the rule in dogs conditioned with a non-myeloablative dose of 2 Gy total body irradiation (TBI) and given short courses of postgrafting immunosuppression with either mycophenolate mofetil (MMF) or rapamycin along with cyclosporine (CSP) [1,2]. However uniform graft rejections were seen when TBI conditioning was decreased to 1 Gy. In contrast, a majority of dogs showed sustained engraftment when 1 Gy TBI was preceded by intravenous injections of both peripheral blood mononuclear cells (PBMC) from the marrow donor and the T-cell costimulatory blocker CTLA4-Ig [3]. Here we used 1 Gy TBI conditioning to evaluate whether blockade of another costimulatory pathway, the interaction between CD40 and CD154, was equally effective in enhancing marrow engraftment. Blockade of this pathway has been successful in murine models of hematopoietic stem cell transplantation and different animal models of solid organ transplantation [4-6].
MATERIALS AND METHODS
Dogs
Litters of beagles, mini-mongrel, basenji and golden retriever crossbreeds, either raised at the Fred-Hutchinson Cancer Research Center (FHCRC) or purchased from commercial kennels, were assessed for disease and enrolled in a veterinary preventive medicine program against worms, distemper, parvovirus, adenovirus type 2, parainfluenza virus, corona virus, rabies and canine papilloma virus. Dogs were 6–24 months old and weighed 8.1–15.1 (median 9.6) kg. The study was approved by the Institutional Animal Care and Use Committee at the FHCRC, which has been accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International. DLA-identical littermates were selected as donor-recipient pairs on the basis of matching for highly polymorphic microsatellite markers within DLA class I and class II regions [7], which was confirmed by DLA-DRB1 sequencing [8].
Monoclonal antibody 5c8
The hybridoma cell line obtained from American Type Culture Collection (ATCC [Manassas, VA]) produced the mouse anti-human CD154 monoclonal antibody (mAb), 5c8 [9]. Hybridoma cells were cultivated according to the guidelines of the provider. The mAb was purified from supernatant and checked for endotoxin by the FHCRC biologics production facility. Endotoxin-free antibody was diluted in phosphate buffered saline (PBS) without calcium and magnesium at a concentration of 1.4-1.5 mg/mL and immediately frozen at −70°C until use.
Cross reactivity with canine CD154
Cross reactivity of the mAb 5c8 was determined by flow cytometry using canine lymphocytes stimulated with 4α-Phorbol 12-myristate 13-acetate (PMA) and Ionomycin.
For these studies, canine PBMC were separated from 20–30 mL of blood using Ficoll gradient centrifugation (specific density 1.074 g/dL). The cells were washed, counted, and resuspended in RPMI medium supplemented with 10% heat-inactivated canine serum, 2mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 μM β-Mercaptoethanol at a concentration of 4 × 106 cells per mL. One mL of cell suspension per well was plated on a 24-well plate. Ionomycin and PMA were added to the lymphocyte culture at a final concentration of 5 μM and 100 μg/mL, respectively. Medium and supplements were obtained from Gibco Invitrogen Inc. (Carlsbad, CA) and Sigma-Aldrich Inc. (St. Louis, MO). The PBMC were cultivated for 4 hours and 30 min, harvested, washed, and resuspended in PBS with 2% goat serum and 0.2% sodium azide. Cells were counted, the concentration adjusted to 1 × 106/mL, and centrifuged at 800 rpm for 10 minutes. After removal of the supernatant, the cells were resuspended in 100 μL of mAb solution (concentration 10 μg/mL), incubated on ice for 30 minutes, and then washed with 1 mL of PBS +2% goat serum and 0.2% sodium azide. After centrifugation, the supernatant was removed, and the cells resuspended in FITC-labeled goat anti-mouse secondary antibody solution (dilution 1:100) (Jackson ImmunoResearch Laboratories INC West Grove, PA), incubated in the dark at 4°C for 30 minutes, washed, and resuspended in 1 mL of PBS with 2% goat serum and 0.2% sodium azide. The cells were analyzed using a FACS-Calibur machine and Cell Quest ProTM software from Becton Dickinson and Co. (Franklin Lakes, NJ). Corresponding isotype controls were obtained from Dako North America Inc. (Carpinteria, CA).
Mixed leukocyte reactions (MLR)
MLR were done as described [10]. Purified anti-CD154 mAb 5c8 was added to the medium in a concentration of 10μg/mL for initial testing and in doses increasing from 1 μg/mL to 50 μg/mL to determine dose effects. CTLA4-Ig (courtesy of Richard Boismenu, Ph.D., Repligen Corporation, Waltham, MA) was used as a positive control and the irrelevant mouse antibody 31A as negative control.
Pharmacokinetic studies
Three dogs received single i.v. doses of anti-CD154 mAb 5c8 of 1, 5 and 10 mg/kg respectively. The dogs had not been previously exposed to mouse immunoglobulin. After injection, serial blood samples were taken at 10 and 30 minutes; 1, 2, 4, 6 and 9 hours; and 1, 2, 3, 4, 5, 6, 7, 8,10, and 13 days. The blood was centrifuged, and the serum immediately stored at −70°C.
Antibody concentrations and immune responses
The serum concentrations of the mAb 5c8 were measured using an enzyme-linked immunoabsorbent assay (ELISA). Briefly, anti-mouse IgG precoated 96-well plates (R&D Biosystems Minneapolis, MN) were blocked with 5% chicken serum and 0.5% Tween 20 containing PBS and incubated with canine serum from treated dogs (dilution: sample dependent 1:25000 to 1:100). Bound mAb 5c8 was detected by horseradish peroxidase-conjugated goat anti-mouse IgG (H+L, Pierce, Rockford, IL) as the secondary antibody. The color reagent was 2,2′azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) (Sigma, St. Louis, MO). Plates were read with a Vmax microtiter plate reader (Molecular Devices, Menlo Park, CA) at 405 nm. Standard curves were established with known concentrations of mAb 5c8; sera obtained from dogs before mAb infusion served as controls. A similar procedure was used to detect canine anti-5c8 antibodies, except plates were coated with mAb 5c8 solution, and bound antibodies were detected by horseradish peroxidase-conjugated goat anti-dog IgM (μ-chain) and goat anti-dog IgG (H+L-chain) (Immunology Consultants Laboratory, Newberg, OR) to distinguish between the two isotypes. Active pooled normal dog serum served as negative control. The canine serum samples were diluted 1:100.
HCT
On day -6 and -5 before marrow transplantation (day 0), recipients were administered 0.5 mg/kg Ketorolac i.v. as prophylaxis against thrombosis, which has been described with anti-CD154 mAb 5c8 treatment [11,12]. On day -5, the animals received 5mg/kg anti-CD154 mAb 5c8 i.v. On day -4, 1 × 107 donor PBMC/kg were administered; half of the cells were infused i.v. and the other half subcutaneously for optimal antigen presentation.
On day 0, recipients were given 1 Gy TBI and donor marrow as described [2,13]. Marrow grafts contained between 2.26 and 8.08 (median 4.22) × 108 nucleated cells/kg. This is comparable to the cell doses used in previous studies, with a median of 4.0 × 108 total nucleated cells/kg in the study with CSP and MMF [1], and 3.8 × 108 cells/kg in the study with CSP and sirolimus, respectively [2]. Dogs were given standard postgrafting care. Immunosuppression consisted of CSP, 15 mg/kg twice a day orally from day -1 to day 35, and MMF, 10 mg/kg twice a day s.c. from day 0 to day 27. CSP levels were measured once weekly, and results were used for CSP dose adjustments; MMF doses were adjusted according to clinical toxicity, which was mainly gastrointestinal.
Assessment of chimerism
Hematopoietic engraftment was assessed by documentation of donor dinucleotide and tetranucleotide variable number tandem repeat (VNTR) polymorphisms in granulocyte and mononuclear cells from peripheral blood using a previously described polymerase chain reaction (PCR)-based assay [14]. Samples were drawn weekly for the first 12 weeks and then every 2 weeks until 6 months after transplantation. PCR products were analyzed by gel electrophoresis, and percentages of donor chimerism in the recipients were determined using Image-quant™ (AMPL Software Pty Ltd, Turramurra, NSW, Australia) software after autoradiography.
Antibody concentrations and immune responses
On days -5, -4, 0, and then weekly for the first 7–8 weeks after transplantation, serum samples were obtained to determine mAb 5c8 serum concentrations and the dogs' immune responses against the antibody.
Statistical analysis
Responses between anti-CD154-treated MLR cultures and controls were compared with a paired Student's t-test. The durations of engraftment among current dogs were compared with those in historical controls (Table1) [2] using the log-rank test. Associations between transplanted marrow cell doses, donor chimerism levels at week 6, and duration of donor chimerism were evaluated with the Spearman rank correlation coefficient. All reported P-values were two-sided, and those <0.05 were considered significant.
Table 1.
Results in dogs given DLA-identical littermate marrow grafts after conditioning.*
| TBI dose [Gy] |
Pre- transplantation Immuno- modulation |
Post- transplantation Immuno- suppression |
Sustained Engraftment (%) |
Median time of Rejection [weeks] |
P-value | References |
|---|---|---|---|---|---|---|
| 2 | None | MMF+CSP | 11/12 (92) | 12 | 0.0373 | [1] |
| 2 | None | SRL+CSP | 6/7 (86) | 11 | [2] | |
| 1 | None | MMF+CSP | 0/6 (0) | 10 | 0.0014 | [1] |
| 1 | None | SRL+CSP | 0/5 (0) | 9 | [2] | |
| 1 | ATG | MMF+CSP | 1/5 (20) | 9.5 | 0.145 | [13] |
| 1 | MTX+Donor PBMC |
MMF+CSP | 2/6 (33) | 8 | 0.315 | [25] |
| 1 | CTLA4-Ig+ Donor PBMC |
MMF+CSP | 4/6 (66) | 14 | 0.721 | [3] |
Conditioning consisted of either 1 or 2 Gy total body irradiation (TBI), and post-grafting immunosuppression with cyclosporine (CSP) combined with mycophenolate mofetil (MMF) or sirolimus (SRL). The p-values refer to comparisons of current results with those in previous studies using the log-rank test.
RESULTS
Flow cytometry, MLR, pharmacokinetic studies, and dog anti mAb 5c8 responses
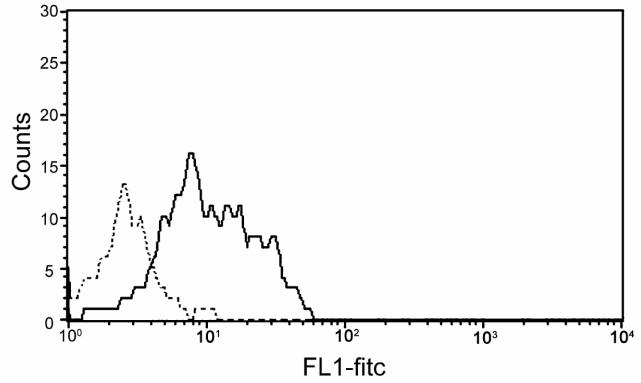
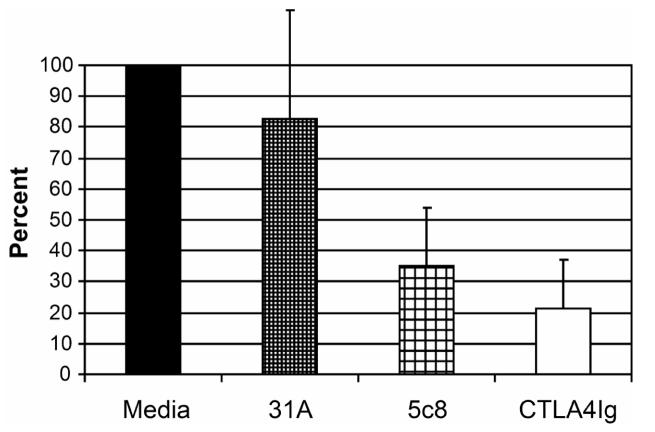
Figure 1 illustrates cross reactivity of the anti-CD154 mAb 5c8 with activated canine lymphocytes compared to a murine isotype control antibody. Flow cytometry results indicate nearly a log increase in intensity of binding of mAb 5c8 over the negative control. MLR studies used cells from DLA-mismatched dogs after addition of irrelevant antibody 31A, anti-CD154 mAb 5c8, or CTLA4-Ig at concentrations of 10 µg/mL medium, each (Figure 2). Anti-CD154 mAb 5c8 reduced the 3H-thymidine uptake to 35% compared to medium alone (P<0.0001). CTLA4-Ig reduced the MLR response to 20% (P<0.0001). The difference in MLR reactivities between mAb 5c8 and CTLA4-Ig was significant (P=0.04). Increasing the mAb 5c8 concentration beyond 10 μg/mL did not further decrease 3H-thymidine uptake, while 5 μg/mL mAb 5c8 also led to significant MLR suppression (P=0.002; data not shown). A concentration of 1 μg/mL did not significantly decrease the 3H-thymidine uptake.
Figure 1.
Distribution of PMA and ionomycin activated canine lymphocytes stained with FITC-labeled anti-CD154 antibody 5c8 (black line) and a mouse isotype control (dotted line). Events are gated for living lymphocytes (vital stain: propidium iodide).
Figure 2.
MLR results. Shown are averages of six experiments with each individual experiment done in triplicate. Anti-CD154 antibody 5c8, irrelevant mAb 31A (negative control) and CTLA4-Ig (positive control) were added at concentrations of 10 µg/ml, each. The maximum 3H-thymidine uptake of the medium control in each experiment was set at 100%. Error bars represent 1 standard deviation. P-values were calculated using the paired Student T-test. Medium vs. irrelevant mAb 31A: P = 0.14; medium vs. 5c8: P < 0.0001; medium vs. CTLA4Ig: P < 0.0001; mAb 5c8 vs. CTLA4Ig: P = 0.04
Ten minutes after each injection of mAb 5c8, serum concentrations reached maximum levels of 9.7 μg/mL (dog G397, 1 mg/kg dose), 84.6 μg/mL (dog G229, 5 mg/kg dose), and 137.4 μg/mL (dog G389, 10 mg/kg dose), respectively (Figure 3A). The serum levels declined rapidly within 24 hours, likely reflecting distribution of the antibody. Serum levels at 24 hours were 4.7 μg/mL, 54.9 μg/mL, and 76.8 μg/mL, respectively. Over the ensuing days, serum levels gradually continued to decline, followed by rapid clearing of the mAb between days 8 and 10. No side effects were seen from the mAb injections.
Figure 3.
- Serum concentrations of anti-CD154 mAb 5c8 in 3 dogs given 1, 5 and 10 mg mAb/kg, respectively. Concentrations were calculated against a standard of purified mAb 5c8.
- Antibody responses to anti- CD154 mAb 5c8 in 3 dogs given 1, 5 and 10 mg mAb/kg, respectively. IgG and IgM responses were assessed by iso-type specific secondary antibodies.
Anti-mAb5c8 antibodies began to appear between days 5 and 8 after injection, and their titers increased as mAb 5c8 was cleared from the circulation (Figure 3B). The immune responses were predominantly of the IgG isotype with lesser contributions of IgM antibodies. Parallel ELISA testing on mAb 5c8 and mouse IgG2a coated plates suggested that two animals had either pure (dog G397) or predominantly (dog G389) anti-idiotype IgG-responses, while one animal had an anti-mouse isotype response (dog G229).
Hematopoietic cell transplantation
The six recipient dogs' characteristics and the outcome of HCT are summarized in Table 2. Figure 4 summarizes serum concentrations of mAb 5c8 (Figure 4A) and canine antibody responses against mAb 5c8 (Figure 4B) in the transplanted dogs. The former ranged from 45 to 81 (median 59) μg/mL on day -4 and from 24 to 48 (median 37) μg on day 0. The antibody clearances were considerably slower than those seen in the pharmacokinetic studies (days 35-52). Four dogs developed IgG anti-5c8 antibodies. In 3 of the 4 dogs, antibodies became detectable on the day of transplantation with a second peak after cessation of the post-grafting immunosuppression. In one dog, anti-mAb 5c8 antibodies appeared beyond day 35. There were no correlations between antibody responses and duration of hematopoietic engraftment.
Table 2.
HCT outcomes among 6 dogs given 5mg/kg anti-CD154 mAb 5c8, and donor PBMC before 1 Gy TBI conditioning and DLA-identical marrow grafts*
| Dog ID No. |
Nucleated marrow cells [×108/kg] |
Toxicities | Maximum donor chimerism [%] |
Cumulative CSP/MMF dosing [% of intended dose] |
Rejection (week) |
Outcome | |
|---|---|---|---|---|---|---|---|
| Mononuclear cells |
Granulocytes | ||||||
| G487 | 3.32 | GI | 9.4 | 10.8 | 100/79 | Yes (22) | Alive, autologous recovery |
| G496 | 7.84 | GI, infection allergic reaction to mAb 5c8 |
71.0 | 82.8 | 100/64 | No | Alive, mixed chimerism |
| G521 | 8.08 | ∅ | 51.2 | 73.0 | 100/100 | No | Alive, mixed chimerism |
| G536 | 4.22 | GI | 24.5 | 33.9 | 100/75 | Yes (24) | Alive, autologous recovery |
| G577 | 2.26 | GI, intussusception | 10.2 | 28.9 | 89/79 | Yes (9) | Alive, autologous recovery |
| G579 | 3.59 | GI, allergic reaction to mAb 5c8 | 31.9 | 66.6 | 100/74 | No | Alive, mixed chimerism |
MMF+CSP were administered after grafting. GI: gastrointestinal toxicities usually manifested as diarrhea, nausea or anorexia.
Figure 4.
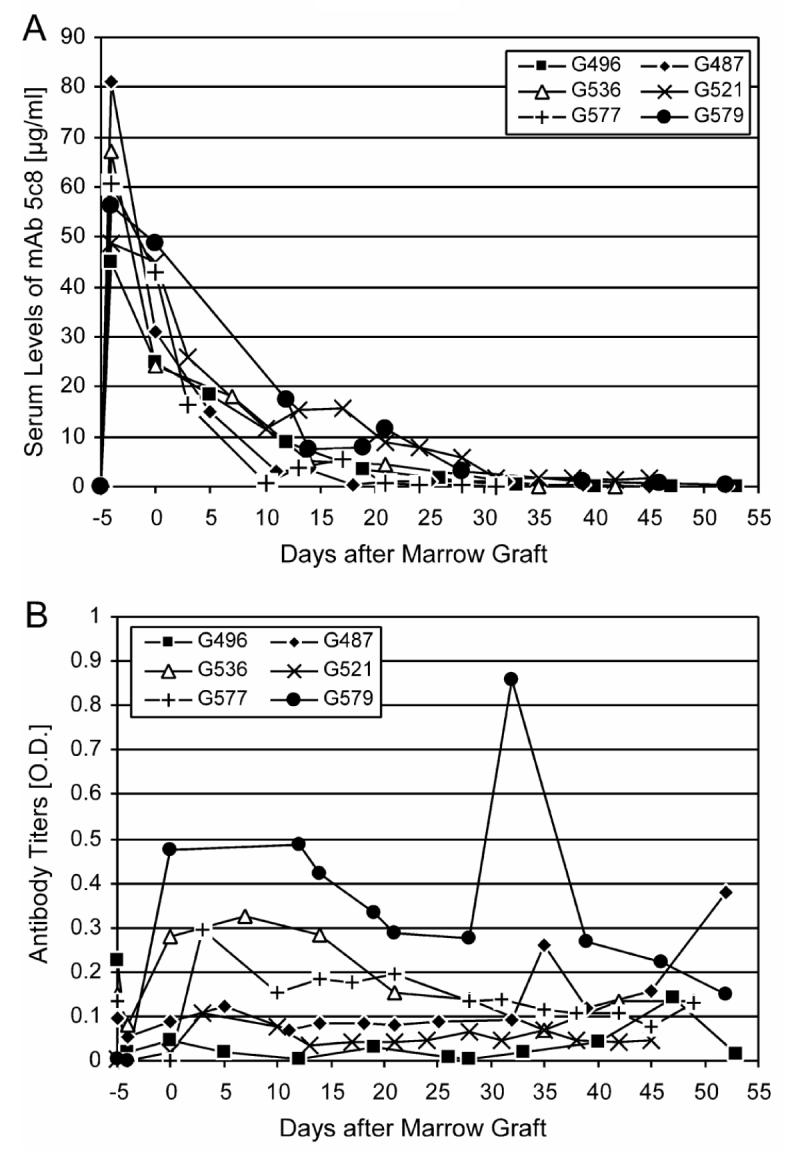
- Serum concentrations of mAb 5c8 in the 6 transplanted dogs. Concentrations were calculated against a standard of purified mAb 5c8.
- IgG antibody responses against mAb 5c8. IgG responses were assessed by iso-type specific secondary antibodies.
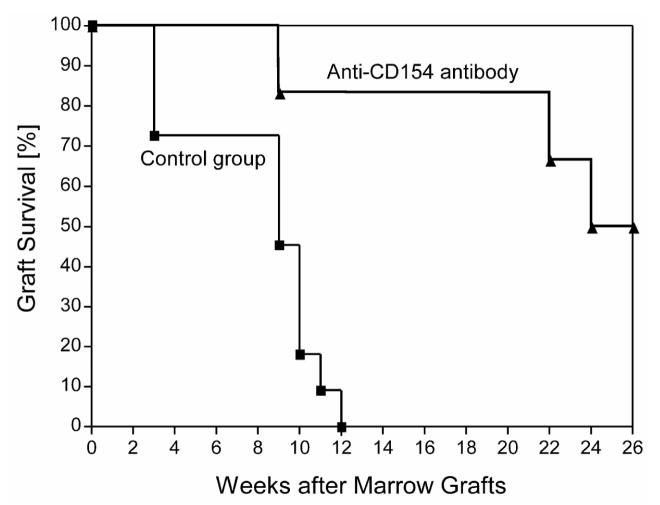
All six dogs showed prompt initial marrow engraftment. Both neutrophil and platelet counts showed nadirs at around day 20 after HCT before eventual blood count recoveries occurred (Figure 5A). Lymphocyte counts remained <1000/μL between days 3 and 40 and then recovered. Peak donor chimerism levels ranged between 10.8 and 82.8 (median 50.5)% for granulocytes (weeks 7 to 26; Figure 5B, Table 2) and between 9.4 and 71.0 (median 28)% for mononuclear cells (weeks 4 to 26; Figure 5C, Table 2), respectively. Three of the 6 dogs rejected their grafts at weeks 9, 22, and 24, respectively. The remaining three were stable mixed donor/host chimeras for >26 weeks of follow up. The median durations of engraftment among the 6 dogs were significantly longer than those among 11 historical controls [1,2] not given pretransplantation treatment (p=0.0014; log-rank test; Table 1; Figure 6). However, durations of engraftment were shorter than those achieved with 2Gy TBI (Table1). There were no statistically significant differences between current results and those of three regimens that employed treatment before TBI with either ATG, donor PBMC and methotrexate (MTX), or donor PBMC and CTLA4-Ig (Table1). The cumulative rate of engraftment of 50% was significantly higher than the 0% rate among historical controls without pretransplantation treatment (p=0.03 Fisher's exact test, Table 1). High granulocyte chimerism levels at week 6 correlated significantly with sustained engraftment (Spearman rank correlation coefficient 0.8783; P=0.033).
Figure 5.
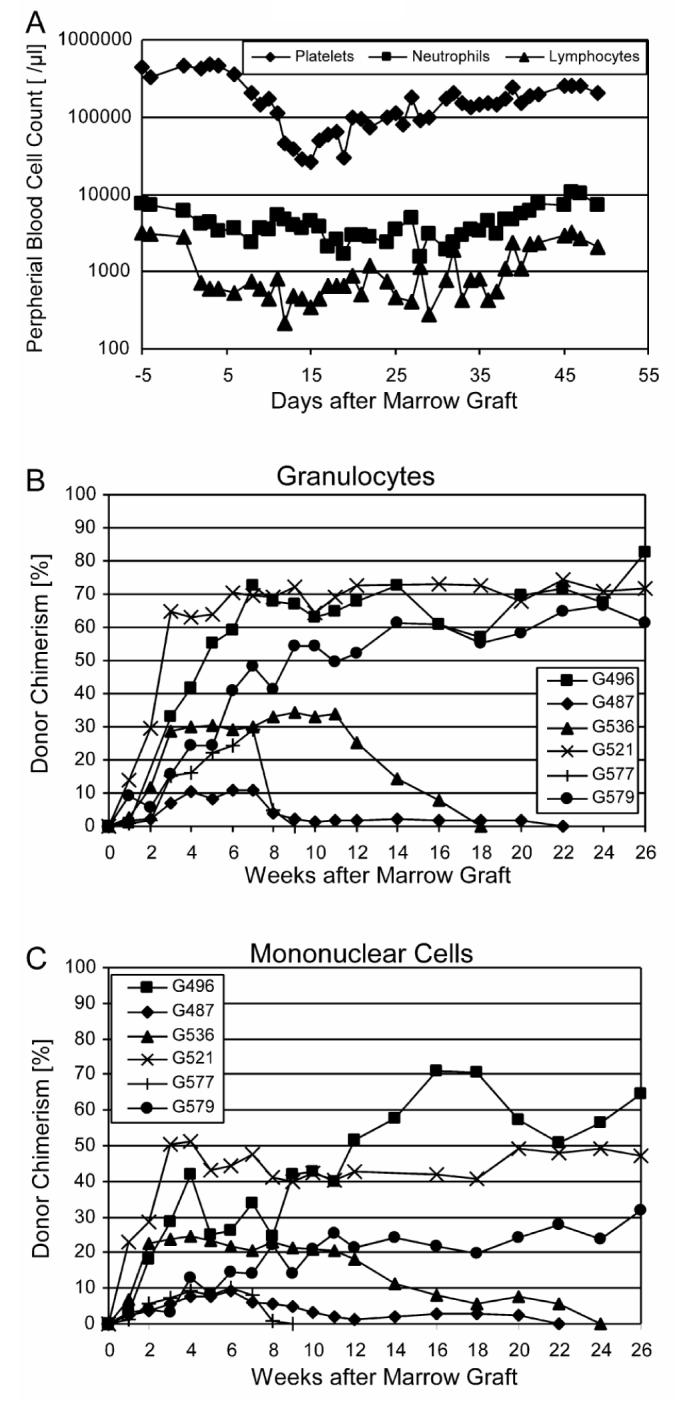
- Median neutrophil, lymphocyte and platelet counts.
- Donor granulocyte chimerism levels.
- Donor mononuclear cell chimerism levels.
Figure 6.
Kaplan-Meyer estimates of graft survival among six dogs pretreated with anti-CD154 mAb 5c8 and donor PBMC and 11 control dogs that did not receive pretransplantation treatment. All dogs were conditioned with 1Gy TBI, received marrow grafts from DLA identical littermates, and were given MMF or rapamycin for 28 days and CSP for 35 days after transplantation.
The hematological and non-hematological toxicities were mild and comparable to those in previous studies using 1 Gy TBI and postgrafting MMF and CSP [1,3,13]. The first dog showed allergic reactions to the mAb infusion, which resolved with diphenylhydramine and hydration. Therefore, subsequent dogs received diphenylhydramine, 2.5mg/kg i.v. 5 minutes before mAb infusion, as prophylaxis against allergic reactions. One dog (G577) developed an intussusception that was likely related to CSP and required surgical correction and suspension of post-grafting immunosuppression for 24 hours. Graft-versus host disease was not observed in any of the dogs.
DISCUSSION
The current study showed that pretreatment of recipients with the anti-CD154 mAb 5c8 and donor PBMC led to prolonged engraftment of DLA-identical marrow in dogs conditioned with 1 Gy TBI and treated with short courses of MMF and CSP after transplantation. However, sustained engraftment was seen only in half of the recipients. Results were similar to those seen in mice [4,5] where uniform engraftment depended both on the strain combinations used and total marrow cell doses injected. A dose of 1 × 108 marrow cells (∼4 × 109 cells/kg) resulted in 100% engraftment even in the most resistant mouse strain combination, while injection of 0.5 × 108 marrow cells led to chimerism in only 68% of mice [4]. Comparable marrow cell doses could not be achieved in dogs. Of note, the two dogs with the highest marrow cell doses (7.84 and 8.08 × 108cells/kg, respectively) were among the three, which showed stable engraftment for more than 26 weeks.
In studies of marrow transplantation in mice [4,5] and solid organ transplantation in non-human primates [15,16], higher total doses of anti-CD154 mAb were administered than in the current study. Only limited pharmacokinetic data for anti-CD154 mAb have been reported in the literature [17,18]. Two studies examined the pharmacokinetics of humanized anti-CD154 mAbs (5c8 [17] and IDEC-131 [17,18], respectively) in monkeys. No antibody responses against these mAbs were detected in the primates. In both studies, the suppression of T-cell dependent B-cell responses against soluble antigens (tetanus-toxoid [17] or ovalbumin [17,18]) were used to determine the in-vivo efficacy of the antibodies. In both studies, single doses of 5 mg/kg anti-CD154 mAb were effective in suppressing the antibody responses against the foreign antigens, although the humanized mAb 5c8 did not result in a complete suppression, even at a dose of 20 mg mAb/kg. In the present study, complete MLR suppression was not observed, even with high doses of mAb 5c8. We chose the smallest antibody dose for in vivo studies that achieved serum antibody levels above 10 μg/mL for at least five days. This was realizable with 5mg mAb /kg i.v. as a single dose since the serum concentration of mAb 5c8 even five days after injection was 39 μg/mL.
All dogs in the pharmacokinetic studies and 4 of the 6 dogs in the transplantation studies developed IgG antibody responses against mAb 5c8 beginning as early as 5 days after injection. The emerging canine antibodies likely determined the tempo of clearance of mAb 5c8 from the circulation. The development of IgG-responses without preceding IgM responses was surprising, since dogs had no known previous exposures to mouse immunoglobulins. In 2 of 3 dogs analyzed, anti-idiotype or idiotype-like antibodies were found. Unlike mice, dogs were not maintained in pathogen-free conditions that might have resulted in more educated immune systems [19,20].
The results achieved with CD154 blockade in dogs were not significantly different than those previously seen with B7-CD28 blockade using CTLA4-Ig [3], since both studies failed to accomplish uniform sustained engraftment. Given the increasing number of newly discovered T-cell regulatory and costimulatory molecules [21], it seemed likely that the blockade of only one system might not be sufficient to overcome host resistance after a low or very low intensity conditioning regimen. Perhaps combining anti-CD154 mAb and CTLA4-Ig might be synergistic or additive and lead to more uniform sustained engraftment, as has been described in murine models of marrow and islet transplantation [22-24].
In conclusion, blocking the CD40-CD154 costimulatory signal was feasible and partially successful in establishing sustained allogenic canine marrow grafts after conditioning with only 1 Gy TBI. Canine specific molecules and combinations of different costimulatory blockers might be required for more uniform success.
ACKNOWLEDGMENTS
The authors are grateful to Drs. Roland Buelow and Elizabeth Squires, SangStat Medical Corporation, Fremont, CA, for the gifts of oral cyclosporine, and Dr. Sabine Hadulco, Roche Bioscience, Nutley, NJ, for the gift of mycophenolate mofetil. The authors would like to thank Michele Spector DVM; Alix Joslyn, and the technicians in the canine facilities of the Fred Hutchinson Cancer Research Center; Drs. Baron, Bethge, Burroughs, Diaconescu, Georges, Kiem, Nash, Mielcarek, and Sorror, who participated in the weekend treatments; Serina Gisburne, Sam Shin and Patrice Stroup for DLA typing, Erlinda Santos for assistance with the MLR; and the technicians of the hematology and pathology laboratories of Seattle Cancer Care Alliance.
Footnotes
Supported in part by grants nos. CA78902, CA15704 and AI067770 awarded by the National Institutes of Health, Department of Health and Human Services, Bethesda, MD. Additional support was provided by the Laura Landro Salomon Endowment Fund (R.S.), the Jose Carreras International Leukemia Foundation (R.S.), the Lupin Foundation (R.S.), and grant no. BMBFLPD 9901/8-63 of Deutsche Akademie der Naturforscher Leopoldina, Halle a.d. Saale, Germany (C.J.).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–3054. [PubMed] [Google Scholar]
- 2.Hogan WJ, Little M-T, Zellmer E, et al. Postgrafting immunosuppression with sirolimus and cyclosporine facilitates stable mixed hematopoietic chimerism in dogs given sublethal total body irradiation before marrow transplantation from DLA-identical littermates. Biol Blood Marrow Transplant. 2003;9:489–495. doi: 10.1016/s1083-8791(03)00148-4. [DOI] [PubMed] [Google Scholar]
- 3.Storb R, Yu C, Zaucha JM, et al. Stable mixed hematopoietic chimerism in dogs given donor antigen, CTLA4Ig, and 100 cGy total body irradiation before and pharmacologic immunosuppression after marrow transplant. Blood. 1999;94:2523–2529. [PubMed] [Google Scholar]
- 4.Seung E, Mordes JP, Rossini AA, Greiner DL. Hematopoietic chimerism and central tolerance created by peripheral-tolerance induction without myeloablative conditioning. J Clin Invest. 2003;112:795–808. doi: 10.1172/JCI18599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan Y, Luo B, Sozen H, et al. Blockade of the CD40/CD154 pathway enhances T-cell-depleted allogeneic bone marrow engraftment under nonmyeloablative and irradiation-free conditioning therapy. Transplantation. 2003;76:216–224. doi: 10.1097/01.TP.0000069602.30162.A1. [DOI] [PubMed] [Google Scholar]
- 6.Elster EA, Hale DA, Mannon RB, Cendales LC, Swanson SJ, Kirk AD. The road to tolerance: renal transplant tolerance induction in nonhuman primate studies and clinical trials (Review) Transpl Immunol. 2004;13:87–99. doi: 10.1016/j.trim.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Wagner JL, Burnett RC, DeRose SA, Francisco LV, Storb R, Ostrander EA. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation. 1996;62:876–877. doi: 10.1097/00007890-199609270-00032. [DOI] [PubMed] [Google Scholar]
- 8.Wagner JL, Works JD, Storb R. DLA-DRB1 and DLA-DQB1 histocompatibility typing by PCR-SSCP and sequencing (Brief Communication) Tissue Antigens. 1998;52:397–401. doi: 10.1111/j.1399-0039.1998.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 9.Lederman S, Yellin MJ, Krichevsky A, Belko J, Lee JJ, Chess L. Identification of a novel surface protein on activated CD4+ T cells that induces contact-dependent B cell differentiation (help) J Exp Med. 1992;175:1091–1101. doi: 10.1084/jem.175.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raff RF, Deeg HJ, Farewell VT, DeRose S, Storb R. The canine major histocompatibility complex. Population study of DLA-D alleles using a panel of homozygous typing cells. Tissue Antigens. 1983;21:360–373. [PubMed] [Google Scholar]
- 11.Koyama I, Kawai T, Andrews D, et al. Thrombophilia associated with anti-CD154 monoclonal antibody treatment and its prophylaxis in nonhuman primates. Transplantation. 2004;77:460–462. doi: 10.1097/01.TP.0000110291.29370.C0. [DOI] [PubMed] [Google Scholar]
- 12.Buhler L, Alwayn IP, Appel JZ, III, Robson SC, Cooper DK. Anti-CD154 monoclonal antibody and thromboembolism. Transplantation. 2001;71:491. doi: 10.1097/00007890-200102150-00028. [DOI] [PubMed] [Google Scholar]
- 13.Diaconescu R, Little M-T, Leisenring W, et al. What role is there for antithymocyte globulin in allogeneic nonmyeloablative canine hematopoietic cell transplantation? Biol Blood Marrow Transplant. 2005;11:335–344. doi: 10.1016/j.bbmt.2005.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu C, Ostrander E, Bryant E, Burnett R, Storb R. Use of (CA)n polymorphisms to determine the origin of blood cells after allogeneic canine marrow grafting. Transplantation. 1994;58:701–706. [PubMed] [Google Scholar]
- 15.Elster EA, Xu H, Tadaki DK, et al. Treatment with the humanized CD154-specific monoclonal antibody, hu5C8, prevents acute rejection of primary skin allografts in nonhuman primates. Transplantation. 2001;72:1473–1478. doi: 10.1097/00007890-200111150-00001. [DOI] [PubMed] [Google Scholar]
- 16.Kirk AD, Harlan DM, Armstrong NN, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci USA. 1997;94:8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gobburu JV, Tenhoor C, Rogge MC, et al. Pharmacokinetics/dynamics of 5c8, a monoclonal antibody to CD154 (CD40 ligand) suppression of an immune response in monkeys. J Pharmacol Exp Ther. 1998;286:925–930. [PubMed] [Google Scholar]
- 18.Brams P, Black A, Padlan EA, et al. A humanized anti-human CD154 monoclonal antibody blocks CD154-CD40 mediated human B cell activation. International Immunopharmacology. 2001;1:277–294. doi: 10.1016/s1567-5769(00)00020-5. [DOI] [PubMed] [Google Scholar]
- 19.Adams AB, Pearson TC, Larsen CP. Heterologous immunity: an overlooked barrier to tolerance (Review) Immunol Rev. 2003;196:147–160. doi: 10.1046/j.1600-065x.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 20.Adams AB, Williams MA, Jones TR, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarkson MR, Sayegh MH. T-cell costimulatory pathways in allograft rejection and tolerance (Review) Transplantation. 2005;80:555–563. doi: 10.1097/01.tp.0000168432.60022.99. [DOI] [PubMed] [Google Scholar]
- 22.Wekerle T, Kurtz J, Ito H, et al. Allogeneic bone marrow transplantation with costimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nat Med. 2000;6:464–469. doi: 10.1038/74731. [DOI] [PubMed] [Google Scholar]
- 23.Wekerle T, Sayegh MH, Ito H, et al. Anti-CD154 or CTLA4Ig obviates the need for thymic irradiation in a non-myeloablative conditioning regimen for the induction of mixed hematopoietic chimerism and tolerance. Transplantation. 1999;68:1348–1355. doi: 10.1097/00007890-199911150-00022. [DOI] [PubMed] [Google Scholar]
- 24.Jin YZ, Xie SS. Bicistronic adenovirus-mediated gene transfer of CTLA4Ig gene and CD40Ig gene result in indefinite survival of islet xenograft. Transplant Proc. 2003;35:3165–3166. doi: 10.1016/j.transproceed.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 25.Jochum C, Beste M, Zellmer E, Graves SG, Storb R. Donor-specific mononuclear cell transfusion and methotrexate as pretransplant treatment in dogs given DLA-identical marrow grafts after nonmyeloablative conditioning (Letter to the Editor) Biol Blood Marrow Transplant. 2006;12:885–886. doi: 10.1016/j.bbmt.2006.04.002. [DOI] [PubMed] [Google Scholar]