Abstract
Introduction
Although IgA nephropathy (IgAN) is the most common recurrent glomerulonephritis encountered in the kidney allograft, the clinical and immunogenetic characteristics remain poorly understood. We sought to study determinants and prognosis of recurrent IgAN with special focus on HLA antigens.
Materials and Methods
Between 2005 and 2019, we identified 282 transplanted patients with failure secondary to IgAN from two North American and one European Medical Centers, including 80 with recurrent IgAN and 202 without recurrence. Prevalence of HLA antigens was compared to external healthy controls of European ancestry (n=15,740). Graft survival was assessed by Kaplan-Meier method and log rank test. Cox proportional hazards were used for multivariable analyses.
Results:
Compared to external controls of European ancestry, kidney transplant recipients of European ancestry with kidney failure secondary to IgAN had higher frequency of HLA-DQ5 (42% vs. 30%, OR=1.68, P=0.002) and lower frequency of HLA-DR15 (15% vs. 28%, OR=0.46, P<0.001) and HLA-DQ6 (32% vs. 45%, OR=0.59, P=0.003); however, the frequency of these HLA antigens were similar in recurrent versus non-recurring IgAN. Younger recipient age at transplantation was an independent predictor of recurrence. HLA-matching was an independent predictor for recurrent IgAN only in recipients of living-related but not deceased or living unrelated transplants. Recurrent IgAN was an independent predictor of allograft failure, along with acute rejection. In patients with recurrent IgAN, serum creatinine at biopsy, degree of proteinuria, and concurrent acute rejection were associated with inferior allograft survival.
Discussion/ Conclusion:
Recurrent IgAN negatively affects allograft survival. Younger recipient age at transplantation is an independent predictor of recurrent IgAN, while the presence of HLA antigens associated with IgAN in the native kidney and HLA-matching in recipients of deceased or living unrelated transplants are not.
Keywords: IgA nephropathy, Kidney transplantation, Recurrent disease, HLA-matching, Pathology
Introduction
Observational studies have suggested an inherited component of IgAN by reporting large pedigrees with multiple affected individuals [1]. Moreover, several studies have shown associations of IgAN with certain HLA alleles, including positive correlations with HLA-B35 and DQ5 and negative correlations with HLA-DR3, DR15, DQ6, and DQ2 [2-5].
Our understanding of the determinants of recurrence and prognosis of IgAN in the kidney allograft is limited [6, 7]. Some [8-10], but not all [11], reports have shown that recurrent IgAN is associated with guarded outcome. A few studies have suggested that younger recipient age [8, 10-15], steroid-free regimen [16-18], allograft from living-related donor [8, 14, 19] and donor-recipient HLA-matching [10, 20, 21] may predict recurrent disease. The latter findings propose that mismatching donor-recipient pairs may reduce recurrent IgAN and potentially improve allograft survival.
In this study, we report on a large cohort (n=282) of kidney transplant patients with kidney failure secondary to IgAN from two medical centers in the USA [Columbia University Irving Medical Center (CUIMC) and Oregon Health & Science University (OHSU)] and one European medical center [Centro Hospitalar e Universitário de Coimbra (HUC)], including 80 patients with recurrent IgAN and 202 patients without evidence of recurrence. We analyzed predictors of recurrent IgAN and allograft failure in kidney transplant recipients with native kidney failure secondary to IgAN with particular focus on the impact of HLA-matching and HLA antigens (B35, DQ5, DR3, DR15, DQ6, and DQ2) in the donor or recipient.
Materials and Methods:
Study Population and Data Collection:
A retrospective review to identify kidney transplant recipients with recurrent and non-recurring IgAN was carried-out at three medical centers (CUIMC, OHSU, and HUC) with approval of each center’s Institutional Review Board. Pathology dataset was used to identify patients with native kidney failure secondary to IgAN who underwent kidney transplantation and had allograft biopsies between 2005-2019, including recurrent and non-recurring IgAN.
(1) Recurrent IgAN was defined as kidney allograft biopsy with positive IgA-dominant immunofluorescence staining. Positive IgA staining was defined as strong IgA staining (>1 on a scale from 0-3 or ≥2 on a scale from 0-4). To avoid misclassifying patients as recurrent IgAN due to non-definitive IgA staining, patients with biopsies showing weaker IgA staining were excluded from the study. According to these criteria, 80 patients were classified with recurrent IgAN (44 from CUIMC, 29 from OHSU, and 7 from HUC).
(2) Non-recurring IgAN was defined as all patients with IgAN in the native kidney who did not develop recurrent IgAN either on for-cause or protocol allograft biopsy. We identified 202 recipients with non-recurring IgAN [CUIMC (n=128), OHSU (n=45), and HUC (n=29)]. Demographic and clinicopathological data were extracted. Data collected at OHSU and HUC were de-identified and shared with CUIMC for analyses.
Renal allograft biopsies were performed for clinical indications at all centers, or per-protocol in all OHSU patients (at 3 and 12 months after transplantation) and HUC patients (at 1 month after transplantation); CUIMC performed protocol biopsies in patients with pre-transplant circulating donor-specific antibodies or positive flow cross-match at 1 week, 2 weeks, 1 month, 3 months, 6 months, 1 year, 2 years, 3 years, and 5 years after transplantation.
The primary outcome was recurrence of IgAN in the allograft. A secondary outcome of allograft failure was defined as the initiation of renal replacement therapy or re-transplantation. Steroid-free regimens were defined as steroid wean immediately post-transplantation. Patients with disease recurrence or rejection requiring steroid treatment were considered steroid-free, unless they were on steroids prior to the event. In six patients where only spot urine protein was available at the time of biopsy, quantification of proteinuria of <20 mg/dL (trace), 30 mg/dL (1+), 100 mg/dL (2+), 300 mg/dL (3+) or >1,000 mg/dL (4+) was estimated for statistical purposes as 0.3 g/g, 0.5 g/g, 1 g/g, 2 g/g, and 3 g/g, respectively [22]. The enrolled individuals were censored at loss of follow-up or allograft failure.
HLA Typing
Serologic and/or molecular typing for HLA-A, -B, -DR, and -DQ was performed for both donors and recipients. The number of HLA matches between donor and recipient HLA antigens (A, B, and DR: scale 0 to 6) was recorded. Attention was directed to the presence of HLA antigens highlighted in the literature as having potential association with IgAN, namely B35, DQ5, DR3, DR15, DQ6, and DQ2 in donors and recipients.
Pathologic Evaluation:
Allograft biopsies were stained with hematoxylin and eosin, periodic acid Schiff, Masson trichrome, and Jones methenamine silver. Immunofluorescence staining for IgG, IgM, IgA, C3, C1q, albumin, fibrin, kappa, lambda, and C4d was performed. Allograft biopsies were evaluated for MEST-C histologic scores using modified Oxford criteria [23]. Acute rejection was defined by the presence of either acute T cell mediated rejection (grade IA or higher) or antibody mediated rejection, according to Banff criteria [24-26].
External controls:
To explore the association of HLA antigens with IgAN, we used HLA typing of 15,740 US residents of European descent with data available from the National Marrow Donor Program [27], who were designated as “external controls”. In these controls, the prevalence of specific HLA serotype with an allelic frequency p was determined by summing up homozygote (p2) and heterozygote [2p(1-p)] frequencies as calculated under the assumption of Hardy-Weinberg equilibrium [p2 + 2p(1-p) + (1-p)2].
Statistical Analysis:
Statistical analyses were performed using Prism 5 2007 (Graphpad Inc, San Diego, CA), SPSS Statistics, Version 26.0 (IBM, Armonk, NY), and R v3.6.3. Continuous data were presented as median and interquartile range (IQR; 25th and 75th percentile). Continuous variables were compared using the Mann-Whitney test while categorical variables were compared using Fisher’s exact or Chi-square tests as appropriate. Kaplan-Meier methodology and log rank test were utilized to assess allograft survival. Cox proportional hazards models were constructed to account for confounders. All factors that demonstrated a suggestive association with the outcome (P value <0.1) at the univariable analysis were included in the multivariable cox proportional hazards models.
Recruitment site and year of transplantation were additionally included in multivariable analyses to account for differences in the medical approaches over time and among centers. Of note, the variable “year of transplant” was only included to adjust for confounders since it may not be a clinically meaningful predictor per se given the unique design of this study [pathology database was utilized to retrospectively select patients and controls with allograft biopsies within a specific period of time (2005-2019). Given the relatively late occurrence of recurrent IgAN, patients with biopsies showing recurrent IgAN are expected to have older transplant date in non-time-to-event analyses. In contrast, when time-to-event analyses is performed, it is not completely surprising to observe opposite results (association with more recent transplant date) given the effects of a few recent and early events whereas events from older transplant year would only include late events (events occurring before 2005 would not be captured)].
A forward stepwise selection method was applied on factors with P value <0.1 at the univariable analysis to avoid overfitting in the multivariable analysis of allograft failure in the small subgroup of patients with recurrent IgAN (n=80). P values <0.05 were considered statistically significant. Since 6 HLA antigens have been studied, a Bonferroni-corrected significance cutoff of 0.008 was used for assessment of these antigens. Individuals with missing information on a tested predictor were excluded from the corresponding univariable time-to-event analysis; individuals with missing data in one or more predictors at the multivariable analyses were also excluded from the latter analyses.
Results:
Demographic, clinical, and pathological features of patients with kidney failure secondary to IgAN
Our cohort included 282 patients with kidney failure secondary to IgAN who underwent kidney transplantation. The majority of the recipients were first-time kidney transplant recipients (89%) who were maintained on both tacrolimus and mycophenolate mofetil (82%). The median age at transplantation was 42 years. The cohort included 31% women, 61% recipients of European ancestry, 47% recipients of allografts from deceased donors, and a median of 2 donor HLA antigens matched with the recipients. 50% of patients received induction therapy with thymoglobulin and 41% received steroid-free regimen (Table 1).
Table 1:
Demographics and clinical characteristics for patients with kidney failure secondary to IgAN
| Total (n=282) |
Recurrent IgAN (n=80) |
Non-recurring IgAN (n=202) |
P values (recurrent vs. not) |
|
|---|---|---|---|---|
| Age at transplant (yrs) | 42 (33, 50) | 36 (28, 43) | 45 (36, 54) | <0.001 |
| < 35 yrs | 82/282 (29%) | 36/80 (45%) | 46/202 (23%) | <0.001 |
| 35-45 yrs | 87/282 (31%) | 27/80 (34%) | 60/202 (30%) | 0.57 |
| >45 yrs | 113/282 (40%) | 17/80 (21%) | 96/202 (47%) | <0.001 |
| Female sex | 87/282 (31%) | 28/80 (35%) | 59/202 (29%) | 0.39 |
| Recipient Ancestry 1 | ||||
| -European | 169/279 (61%) | 54/79 (68%) | 115/200 (58%) | 0.10 |
| -Hispanic/Latinx | 43/279 (15%) | 10/79 (13%) | 33/200 (16%) | 0.85 |
| -East Asian | 38/279 (14%) | 7/79 (9%) | 31/200 (15%) | 0.18 |
| -Black | 14/279 (5%) | 4/79 (5%) | 10/200 (5%) | 1.0 |
| -Other | 15/279 (5%) | 4/79 (5%) | 11/200 (6%) | 1.0 |
| Allograft Source | ||||
| -Living Related | 97/282 (34%) | 34/80 (43%) | 63/202 (31%) | 0.10 |
| -Living Unrelated | 54/282 (19%) | 14/80 (17%) | 40/202 (20%) | 0.74 |
| -Deceased Donor | 131/282 (47%) | 32/80 (40%) | 99/202 (49%) | 0.19 |
| Year of transplantation | 2011 (2006, 2015) | 2008 (2003, 2011) | 2012 (2008, 2015) | <0.001 |
| Donor Ancestry 2 | ||||
| -European | 172/237 (73%) | 45/62 (73%) | 127/175 (73%) | 1.0 |
| -Hispanic/ Latinx | 32/237 (13%) | 8/62 (13%) | 24/175 (14%) | 1.0 |
| -East Asian | 7/237 (3%) | 3/62 (5%) | 4/175 (2%) | 0.38 |
| -Black | 17/237 (7%) | 4/62 (6%) | 13/175 (7%) | 1.0 |
| -Other | 9/237 (4%) | 2/62 (3%) | 7/175 (4%) | 1.0 |
| Induction therapy 3 | ||||
| -Thymoglobulin | 135/267 (50%) | 27/69 (39%) | 108/198 (55%) | 0.04 |
| - IL2R Inhibitors | 76/267 (29%) | 25/69 (36%) | 51/198 (26%) | 0.12 |
| -Alemtuzumab | 37/267 (14%) | 9/69 (13%) | 28/198 (14%) | 1.0 |
| No induction | 14/267 (5%) | 5/69 (7%) | 9/198 (4%) | 0.36 |
| Other induction | 5/267 (2%) | 3/69 (5%) | 2/198 (1%) | 0.11 |
| Steroid-free regimen | 116/282 (41%) | 28/80 (35%) | 88/202 (44%) | 0.23 |
| #HLA Matches (0-6) | 2 (1, 3) | 3 (1, 4) | 2 (1, 3) | <0.001 |
| Recipient HLA antigens 4 | ||||
| - B35 | 72/282 (26%) | 24/80 (30%) | 47/202 (23%) | 0.29 |
| - DQ5 | 99/250 (40%) | 26/71 (37%) | 73/179 (41%) | 0.57 |
| - DR3 | 37/282 (13%) | 12/80 (15%) | 25/202 (12%) | 0.56 |
| - DR15 | 51/282 (18%) | 14/80 (18%) | 37/202 (18%) | 1.00 |
| - DQ6 | 76/250 (30%) | 20/71 (28%) | 56/179 (31%) | 0.65 |
| - DQ2 | 77/250 (31%) | 23/71 (32%) | 54/179 (30%) | 0.76 |
| Donor HLA antigens 5 | ||||
| - B35 | 59/282 (21%) | 17/80 (21%) | 42/202 (21%) | 1.0 |
| - DQ5 | 86/248 (35%) | 25/66 (38%) | 61/182 (34%) | 0.55 |
| - DR3 | 51/282 (18%) | 16/80 (20%) | 35/202 (17%) | 0.61 |
| - DR15 | 60/282 (21%) | 15/80 (19%) | 45/202 (22%) | 0.63 |
| - DQ6 | 96/248 (39%) | 25/66 (38%) | 71/182 (39%) | 1.0 |
| - DQ2 | 90/248 (36%) | 20/66 (30%) | 70/182 (38%) | 0.30 |
| Pre-transplant DSA | 15/276 (5%) | 4/76 (5%) | 11/200 (5.5%) | 0.9 |
Abbreviations: DSA: donor-specific autoantibodies
HLA match is calculated based on A, B, and DR antigens
Information on recipient ancestry was not available for 3 patients (1 recurrent and 2 non-recurring)
Information on donor ancestry was not available for 45 patients (18 recurrent and 27 non-recurring)
Information on induction therapy was not available for 15 patients (11 recurrent and 4 non-recurring)
Recipient HLA-DQ typing was not available for 32 patients (9 recurrent and 23 non-recurring)
Donor HLA-DQ typing was not available for 34 patients (14 recurrent and 20 non-recurring)
There were 80 patients with recurrent IgAN and 202 patients with IgAN without recurrence. Recurrent IgAN occurred at a median of 43 months (IQR: 13, 111 months) post-transplantation and these patients were followed for a median of 92 months (IQR: 60, 147 months) after transplantation while patients without IgAN recurrence were followed for a median of 74 months (IQR: 36, 123 months) post-transplantation. Compared to non-recurring IgAN, patients with recurrent IgAN were younger (36 vs. 45 years at transplantation, P<0.001), had greater donor-recipient HLA-matching scores (median 3 vs. 2, P<0.001), were transplanted earlier (median year of transplantation 2008 vs. 2012, P<0.001), and were less likely to receive induction with thymoglobulin (39% vs. 55%, P=0.04) (Table 1).
Recipients with kidney failure secondary to IgAN are associated with high frequency of HLA-DQ5 but low frequency of HLA-DR15 and HLA-DQ6.
We compared the frequencies of HLA-B35, HLA-DQ5, HLA-DR3, HLA-DR15, HLA-DQ6, and HLA-DQ2 antigens in our recipients of European ancestry with kidney failure secondary to IgAN (n=169/279) [including patients with recurrent (n=54) and non-recurring IgAN (n=115)], to a group of external healthy controls of European descent from the U.S. National Marrow Donor Program (n=15,740) [27]. Kidney transplant recipients with renal failure secondary to IgAN had higher frequency of HLA-DQ5 [OR=1.68 (1.21 – 2.33), P=0.002] and lower frequency of both HLA-DR15 [OR=0.46 (0.31 – 0.71), P<0.001] and HLA-DQ6 [OR=0.59 (0.42 – 0.84), P=0.003] (shown in Fig. 1). The latter two (DR15 and DQ6) are in linkage disequilibrium to form a common HLA haplotype. Notably, recipients with recurrent and non-recurring IgAN had similar frequency of these HLA antigens (shown in Fig. 1).
Fig. 1. Prevalence of specific HLA antigens in US controls of European ancestry and transplant recipients of European ancestry who had kidney failure secondary to IgAN in the native kidney, including these with recurrent and non-recurring disease.
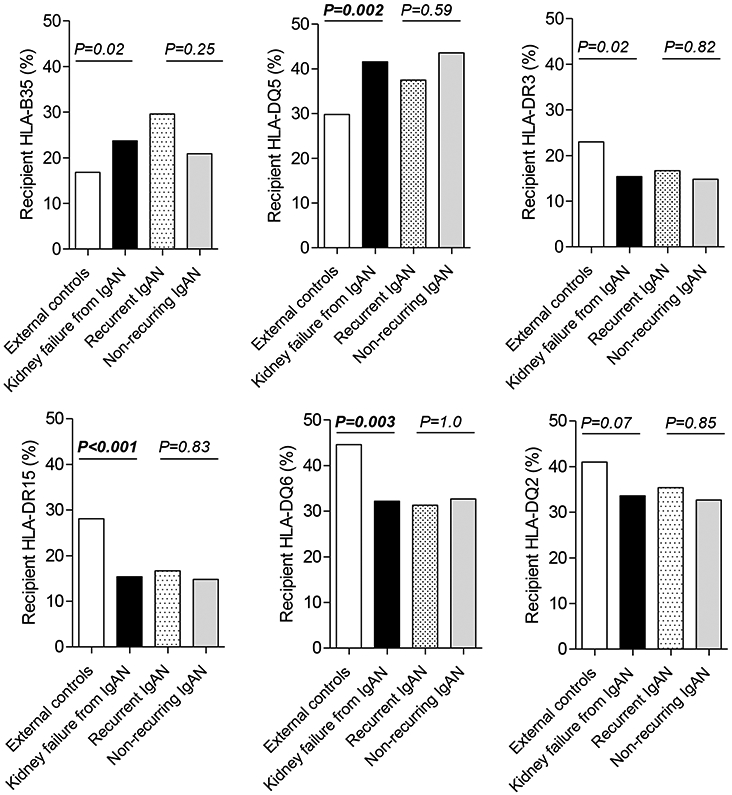
Patients of European ancestry with kidney failure secondary to IgAN (n=169) [combining 54 recurrent IgAN and 115 non-recurring IgAN] were compared to controls from European ancestry (n=15,740) from US donors of stem cell transplantation. DQ typing was only available for 149 patients with native kidney failure secondary to IgAN, including 48 recurrent IgAN and 101 non-recurring disease. Another comparison was performed between recurrent and non-recurring IgAN.
Since 6 HLA antigens were compared, Bonferroni-corrected cutoff of 0.008 was considered significant.
Of note, DR15 and DQ6 are in linkage disequilibrium to form a common haplotype.
Younger recipient age at transplantation is associated with recurrent IgAN
Transplant patients with kidney failure secondary to IgAN (n=282) were assessed for variables predictive of recurrent disease (Table 2). Univariable time-to-event analyses showed that younger recipient age at transplantation and donor-recipient HLA-matching were risk factors for recurrence. On multivariable analysis, only younger recipient age [adjusted HR (aHR)=0.96 per-year, P=0.001] and more recent year of transplantation (aHR=1.08, P=0.006) were significantly associated with IgAN recurrence (Table 2).
Table 2:
Univariable and multivariable analyses of the associations with recurrent disease in patients with kidney failure secondary to IgAN
| Variables | Univariable (n=282) | Multivariable (n=267), N events=69 | |||
|---|---|---|---|---|---|
| N events | HR (95% CI) | P value | aHR (95% CI) | P value | |
| Recipient age at transplant (per each year) | 80 | 0.96 (0.94 – 0.98) | <0.001 | 0.96 (0.94 – 0.98) | 0.001 |
| Recipient female gender | 80 | 1.16 (0.73 – 1.85) | 0.52 | ||
| Recipient European ancestry | 79 | 1.39 (0.86 – 2.25) | 0.18 | ||
| Donor European ancestry | 62 | 1.09 (0.62 – 1.93) | 0.77 | ||
| Allograft source | 80 | ||||
| Deceased donor | Ref | – | |||
| Living related | 1.29 (0.80 – 2.11) | 0.3 | |||
| Living unrelated | 1.16 (0.62 – 2.19) | 0.64 | |||
| Medical Sites | 80 | ||||
| CUIMC | Ref | – | Ref | – | |
| OHSU | 1.36 (0.83 – 2.22) | 0.23 | 1.06 (0.56 – 2.02) | 0.86 | |
| HUC | 0.70 (0.31 – 1.55) | 0.37 | 0.59 (0.25 – 1.37) | 0.22 | |
| Year of transplantation | 80 | 1.05 (1.00 – 1.08) | 0.06 | 1.08 (1.02 – 1.14) | 0.006 |
| # HLA matches (per antigen: 0-6) | 80 | 1.15 (1.02 – 1.30) | 0.02 | 1.12 (0.98 – 1.29) | 0.11 |
| Induction with Thymoglobulin 1 | 69 | 0.65 (0.40 – 1.06) | 0.08 | 0.65 (0.36 – 1.15) | 0.14 |
| Steroid-free regimens | 80 | 0.77 (0.48 – 1.24) | 0.28 | ||
| Acute rejection * | 80 | 0.90 (0.55 – 1.46) | 0.66 | ||
| Recipient HLA-B35 | 80 | 1.40 (0.87 – 2.27) | 0.17 | ||
| Recipient HLA-DQ5 | 71 | 1.00 (0.61 – 1.64) | 0.99 | ||
| Recipient HLA-DR3 (DR17 or 18) | 80 | 1.20 (0.65 – 2.23) | 0.56 | ||
| Recipient HLA-DR15 | 80 | 0.99 (0.56 – 1.78) | 0.98 | ||
| Recipient HLA-DQ6 | 71 | 1.04 (0.62 – 1.75) | 0.89 | ||
| Recipient HLA-DQ2 | 71 | 1.09 (0.66 – 1.80) | 0.74 | ||
| Donor HLA-B35 | 80 | 1.09 (0.63 – 1.89) | 0.77 | ||
| Donor HLA-DQ5 | 66 | 1.24 (0.75 – 2.04) | 0.41 | ||
| Donor HLA-DR3 (DR17 or 18) | 80 | 1.38 (0.80 – 2.40) | 0.25 | ||
| Donor HLA-DR15 | 80 | 0.77 (0.44 – 1.36) | 0.37 | ||
| Donor HLA-DQ6 | 66 | 0.88 (0.54 – 1.46) | 0.63 | ||
| Donor HLA-DQ2 | 66 | 0.90 (0.53 – 1.53) | 0.7 | ||
| Pre-transplant DSA | 76 | 1.25 (0.46-3.43) | 0.7 | ||
Abbreviations: CUIMC, Columbia University Irving Medical center; DSA, donor-specific antibodies; OHSU, Oregon Health & Science University; HUC, Hospitais da Universidade de Coimbra
Acute rejection is defined as any episode of acute rejection that occurred before recurrence of disease in recurrent IgAN or any time before the end of follow-up in non-recurring controls.
Even when induction therapy with Thymoglobulin was not included in the main multivariable analysis (information on induction therapy was lacking 15 patients, including 11 with recurrent IgAN), the new multivariable analysis (n=282) revealed similarly that only younger age at transplant [0.96 (0.94 – 0.98), P<0.001) and year of transplantation [1.10 (1.04 – 1.15), P<0.001) remained independent predictors for IgAN but not HLA-matches [1.13 (0.99 – 1.29), P=0.06] or medical sites [OHSU: 1.38 (0.83 – 2.29), P=0.21, HUC: 0.65 (0.29– 1.45), P=0.29]
HLA-matching can predict IgAN recurrence only in recipients of living-related kidney allograft
The degree of donor-recipient HLA-matching was significantly higher in recurrent IgAN compared with non-recurring IgAN in recipients of kidneys from living-related donors [recurrent IgAN: 4 (3, 6) vs. non-recurring IgAN: 3 (2, 4), P=0.002] whereas this difference was not significant in recipients of kidney allograft from living-unrelated or deceased donors [recurrent IgAN: 2 (1, 4), non-recurring IgAN: 1 (1, 2), P=0.1].
In recipients of living-related renal transplant, the number of HLA matches (aHR=1.40, P=0.01), younger age at transplant (aHR=0.95, P=0.006), and a more recent year of transplant (aHR=1.16, P=0.004) were independent risk factors for IgAN recurrence (Supplemental Table 1). In recipients of living-unrelated or deceased donor transplants, younger age at transplant (aHR=0.97, P=0.03) was significant predictor for recurrent IgAN while induction therapy with thymoglobulin (aHR=0.46, P=0.05) exerted a protective effects against IgAN recurrence (Supplemental Table 2). In this subgroup of recipients, the number of HLA matches was not associated with the recurrent IgAN (P=0.9).
Recurrent IgAN is independently associated with inferior allograft survival
Recurrent IgAN was significantly associated with inferior post-transplant allograft survival [HR=1.94 (1.13 – 3.31, P=0.016] (Fig. 2), where the survival curves began showing clear separation after 70 months of transplantation. Subsequently, variables associated with allograft survival were analyzed in all transplant patients with kidney failure secondary to IgAN. By univariable Cox regression analysis, living donor allografts, HLA-matching, and steroid-free regimens were associated with superior allograft survival, while recurrent IgAN, acute rejection episodes, and more recent transplantation, were associated with inferior allograft survival (Table 3).
Fig. 2. Allograft survival in recurrent and non-recurring IgAN.
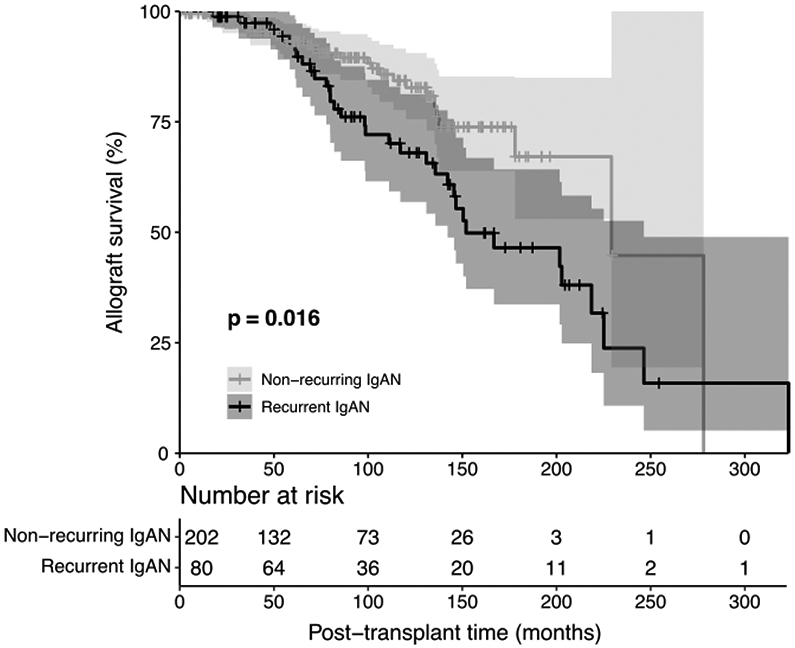
Kaplan–Meier curves for cumulative kidney allograft survival from time of transplant in patients with recurrent vs. non-recurring IgAN. Shaded areas around the curves represent the 95% confidence interval.
Table 3:
Univariable and multivariable analyses of the associations with allograft failure in transplant patients with kidney failure secondary to IgAN
| Variables | Univariable (n=282) | Multivariable (n=282), N events=60 |
|||
|---|---|---|---|---|---|
| N events | HR (95% CI) | P value | HR (95% CI) | P value | |
| Recipient age at transplant (per each year) | 60 | 0.98 (0.96 – 1.00) | 0.09 | 0.97 (0.94 – 0.99) | 0.02 |
| Recipient female gender | 60 | 0.88 (0.50 – 1.54) | 0.66 | ||
| Recipient European ancestry | 60 | 0.91 (0.54 – 1.56) | 0.74 | ||
| Donor Recipient European ancestry | 41 | 2.07 (0.87 – 4.94) | 0.1 | ||
| Allograft from living donor | 60 | 0.56 (0.34 – 0.95) | 0.03 | 0.36 (0.19 – 0.67) | 0.001 |
| Medical Sites | 60 | ||||
| CUIMC | Ref | – | Ref | – | |
| OHSU | 1.70 (0.96 – 3.01) | 0.07 | 0.89 (0.41 – 1.92) | 0.77 | |
| HUC | 0.95 (0.39 – 2.29) | 0.9 | 0.39 (0.14 – 1.11) | 0.08 | |
| Year of transplantation | 60 | 1.07 (1.01 – 1.15) | 0.03 | 1.16 (1.08 – 1.25) | <0.001 |
| Recurrence of IgA nephropathy | 60 | 1.89 (1.12 – 3.19) | 0.02 | 2.45 (1.38 – 4.36) | 0.002 |
| # HLA Matches (per antigen: 0-6) | 60 | 0.86 (0.74 – 0.99) | 0.04 | 0.87 (0.73 – 1.04) | 0.13 |
| Induction with Thymoglobulin | 56 | 0.91 (0.52 – 1.59) | 0.73 | ||
| Steroid-free regimens | 60 | 0.54 (0.29 – 0.98) | 0.04 | 0.59 (0.26 – 1.30) | 0.19 |
| Acute rejection | 60 | 2.46(1.46 – 4.16) | 0.001 | 2.68 (1.51 – 4.76) | 0.001 |
| Recipient HLA-B35 | 60 | 1.29 (0.74 – 2.24) | 0.37 | ||
| Recipient HLA-DQ5 | 57 | 1.07 (0.62 – 1.84) | 0.82 | ||
| Recipient HLA-DR3 (DR17 or 18) | 60 | 1.05 (0.49 – 2.22) | 0.91 | ||
| Recipient HLA-DR15 | 60 | 1.90 (1.08 – 3.35) | 0.02 | 1.90 (1.04 – 3.47) | 0.04 |
| Recipient HLA-DQ6 | 57 | 1.44 (0.83 – 2.51) | 0.2 | ||
| Recipient HLA-DQ2 | 57 | 0.63 (0.33 – 1.20) | 0.16 | ||
| Donor HLA-B35 | 60 | 0.97 (0.51 – 1.83) | 0.92 | ||
| Donor HLA-DQ5 | 57 | 1.07 (0.59 – 1.91) | 0.83 | ||
| Donor HLA-DR3 (DR17 or 18) | 60 | 1.06 (0.53 – 2.10) | 0.88 | ||
| Donor HLA-DR15 | 60 | 1.23 (0.71 – 2.30) | 0.42 | ||
| Donor HLA-DQ6 | 57 | 1.47 (0.84 – 2.56) | 0.18 | ||
| Donor HLA-DQ2 | 57 | 1.01 (0.55 – 1.86) | 0.97 | ||
| Pre-transplant DSA | 55 | 1.02 (0.25-4.22) | 0.8 | ||
Rejection is defined as any episode of acute rejection encountered during follow-up period
Abbreviations: CUIMC, Columbia University Irving Medical center; DSA, donor-specific antibodies; OHSU, Oregon Health & Science University; HUC, Hospitais da Universidade de Coimbra
By multivariable Cox proportional hazards regression analysis, recurrent IgAN (aHR=2.45, P=0.002), acute rejection episodes (aHR=2.68, P=0.001), and younger recipient age at transplant (aHR=0.97, P=0.02) were associated with shorter allograft survival while receiving an allograft from a living-donor (aHR=0.36, P=0.001) was associated with superior allograft survival (Table 3). There was a trend toward worse allograft survival in recipients with HLA-DR15 that did not reach statistical significance by Bonferroni-corrected cutoff (Table 3). Given the design of the study, it was not surprising that more recent transplants were associated with worse survival in this cohort. Again, while the latter variable is probably not a meaningful predictor, it was used mainly to account to for confounders.
To confirm the importance of recurrence IgAN in graft failure, we used IgAN recurrence as a time-varying covariate in the multivariable Cox proportional hazards model for time from transplant to allograft failure. The latter confirmed the independent association between IgAN recurrence and graft failure (data not shown).
Characteristics, treatment, and outcome of recurrent IgAN
Demographic, clinical, and pathologic characteristics of patients with recurrent IgAN are presented in Table 1 and Supplemental Table 3. At the time of diagnosis, recipients had median post-transplant interval of 43 months, median serum creatinine of 1.9 mg/dl, and median proteinuria of 0.5 g/g. Seven IgAN recurrence events were detected on protocol biopsies, the remaining 73 were detected on biopsies performed for clinical reasons. Histologically, concurrent acute rejection was present in 16 of 80 (20%) of index biopsies, including 11 T cell mediated rejection, 2 antibody-mediated rejection, and 3 mixed rejection. Diffuse mesangial proliferation, endocapillary proliferation, and crescents were seen in 46%, 33%, and 16% of index biopsies, respectively, while segmental sclerosis and >25% tubulointerstitial scarring were present in 51% and 48% of index biopsies, respectively. Median combined MEST-C score was 2 (IQR: 1, 3) (Supplemental Table 3).
Data on treatment of recurrent IgAN were available for 71 (88%) index biopsies. Treatment was variable and was initiated for recurrent IgAN and, to a lesser extent, for other concurrent conditions, such as acute rejection episodes (Supplemental Table 4). Thirty two (45%) of these patients were managed without adjustment of immunosuppression. Corticosteroids were used in 32 (45%) patients, while thymoglobulin and rituximab were administered to 3 (4%) and 2 (3%) patients, respectively. Mycophenolate mofetil dose was increased in one patient and conversion from tacrolimus to belatacept was initiated in one subject given the presence of significant arteriolar hyalinosis.
We investigated variables associated with post-biopsy allograft failure in the 80 patients with recurrent IgAN (Table 4). On univariable analysis, longer post-transplant interval, serum creatinine values, degree of proteinuria, concurrent acute rejection, and histologic scores for each of mesangial proliferation, segmental sclerosis, tubulointerstitial scarring, as well as combined MEST-C scores in index allograft biopsies were all associated with inferior allograft survival (Table 4). Using Cox multivariable analysis that included the 6 variables with P<0.1 in univariate analyses (combined MEST-C scores rather than individual scores was used as a histologic variable), serum creatinine at biopsy, degree of proteinuria, and concurrent acute rejection were predictors of inferior allograft survival, while HLA-matching represented a significant predictor of superior allograft survival (Table 4). The same 4 variables remained independent predictors of allograft survival when forward stepwise analysis was performed (data presented in the legend of Table 4), supporting their role in determining allograft outcome in patients with recurrent IgAN.
Table 4:
Univariable and multivariable analyses of the associations with post-biopsy allograft failure in patients with recurrent IgAN
| Variables | Univariable (n=80) | Multivariable (n=67), N events=33 * |
|||
|---|---|---|---|---|---|
| N events | HR (95% CI) | P value | HR (95% CI) | P value | |
| Recipient age at biopsy (per each year) | 33 | 1.01 (0.98 – 1.04) | 0.55 | ||
| Recipient female gender | 33 | 0.71 (0.33 – 1.54) | 0.39 | ||
| Recipient European ancestry 1 | 33 | 0.79 (0.37 – 1.70) | 0.55 | ||
| Donor European ancestry 2 | 23 | 0.99 (0.36 – 2.75) | 0.99 | ||
| Allograft from living donor | 33 | 0.62 (0.31 – 1.25) | 0.18 | ||
| Induction with Thymoglobulin 3 | 30 | 1.16 (0.55 – 2.45) | 0.7 | ||
| Steroid-free regimens | 33 | 0.68 (0.31 – 1.47) | 0.33 | ||
| Post-transplant interval (per month) | 33 | 1.01 (1.01 – 1.01) | <0.001 | 1.01 (0.99 – 1.01) | 0.14 |
| Serum creatinine at biopsy (mg/dL) 4 | 33 | 1.94 (1.40 – 2.69) | <0.001 | 2.81 (1.69 – 4.68) | <0.001 |
| Proteinuria (g/g vs. mg/dL)5 | 33 | 1.15 (1.07 – 1.24) | <0.001 | 1.20 (1.07 – 1.34) | 0.002 |
| Concurrent acute rejection | 33 | 3.21 (1.54 – 6.70) | 0.002 | 3.51 (1.11 – 11.0) | 0.03 |
| # of HLA Matches (per antigen: 0-6) | 33 | 0.82 (0.67 – 1.01) | 0.06 | 0.68 (0.50 – 0.91) | 0.009 |
| Protocol biopsy | 33 | 0.4 (0.05 – 2.49) | 0.3 | ||
| Mesangial score (M: 0-1)6 | 33 | 2.14 (1.05 – 4.34) | 0.04 | ||
| Endocapillary proliferation score (E: 0-1)6 | 33 | 1.50 (0.74 – 3.05) | 0.26 | ||
| Cellular or fibrocellular crescent score (C0-2) 6 | 33 | 1.71 (0.73 – 3.98) | 0.22 | ||
| Segmental sclerosis score (S: 0-1) 5 | 33 | 2.79 (1.32 – 5.93) | 0.007 | ||
| Tubular atrophy/interstitial fibrosis score (T: 0-2) 6 | 33 | 2.56 (1.64 – 4.00) | <0.001 | ||
| Combined MEST-C score (0-7)5 | 33 | 1.51 (1.23 – 1.85) | <0.001 | 1.20 (0.86 – 1.66) | 0.28 |
To avoid potential overfitting in multivariable analysis, we also used a forward and backward stepwise multivariable analysis that included variables with P<0.1. Only combined MEST-C score rather than individual components were entered in multivariable analysis. Forward stepwise analyses showed that each of serum creatinine [3.09 (1.88 – 5.10), P<0.001], proteinuria [1.26 (1.14 – 1.38), P<0.001], concurrent acute rejection [6.67 (2.54 – 17.6), P<0.001], and # HLA matches [0.73 (0.55 – 0.97), P=0.03] were associated with inferior allograft survival.
Information on recipient ancestry was not available for 1 patient
Information on donor ancestry was not available for 18 patients
Information on induction therapy was not available for 11 patients
Information on serum creatinine was not available for 4 patients
Information on proteinuria was not available for 11 patients
Histologic Oxford scores could not be assessed in 1 patient
Discussion /Conclusion
In contrast to IgAN in the native kidney, there are limited data on the prognosis and risk factors for recurrent IgAN in the kidney allograft. The available reports are largely restricted to small single center studies [7, 8, 10-13, 28] or rely on data from registries [20, 29-31], where the information is incomplete and subject to bias. While prior studies have identified potential risk factors for recurrent IgAN, the results varied amongst studies. Furthermore, despite the described association of some HLA antigens with IgAN in the native kidney [2-5], an evaluation of donor and recipient HLA antigens in recurrent and non-recurring IgAN in the renal allograft has not been performed to date. To our knowledge, the current report represents one of the largest non-registry study to assess predictors for recurrent IgAN, and is the first to analyze the association of HLA antigens in donors and recipients with recurrence and prognosis of IgAN.
Some [8-10], but not all [11], studies have shown detrimental impact of IgAN on allograft survival. In the current study, recurrent IgAN emerged as independent predictor of inferior allograft survival, together with acute rejection. When predictors of recurrent IgAN were examined in multivariable analysis, younger recipient age at transplantation, which is a non-modifiable extra-renal variable related to the recipient, was significantly associated with recurrent IgAN. Prior studies have shown an association between younger recipient age and recurrent IgAN [10-15]. Notably, younger recipient age is not universally associated with recurrent glomerulonephritis. In contrast, older recipient age is an independent predictor for recurrent membranous nephropathy post-transplantation [32].
Data on genetic predisposing factors for recurrent IgAN are very limited. Despite the suggested association of HLA-B35, HLA-DQ5, HLA-DR3, HLA-DR15, HLA-DQ6 and HLA-DQ2 with IgAN in the native kidney [2-5], these antigens have not been studied systematically in the context of recurrent IgAN. Compared to the general population of US residents of European descent [27], transplant recipients of European ancestry who had kidney failure secondary to IgAN showed higher frequencies of HLA-DQ5 and lower frequencies of HLA-DR15 and HLA-DQ6 (Fig. 1) supporting a predisposing role of DQ5 for IgAN in the native kidney and a protective role of DR15-DQ6 haplotype. In contrast, the lack of difference in frequencies of such HLA antigens in the recipients or the donors between recurrent and non-recurring IgAN argue against an important role of these specific antigens in recurrence of IgAN and do not support donor selection based on presence/absence of any of these HLA antigens.
Previous studies have suggested that HLA-mismatch may reduce the risk of recurrent IgAN [10, 20, 21]. Our findings do not support an approach that encourages HLA-mismatching in patients with IgAN undergoing renal transplantation for two reasons. First, while donor-recipient pairs with recurrent IgAN were more HLA-matched than their counterparts without recurring disease, HLA-matching was not independently associated with protection from recurrence in time-to-event analyses, except in the subgroup of patients who received kidney allograft from living-related donors. Second, HLA-mismatch may increase susceptibility to alloimmunity, which can negatively affect allograft survival. Indeed, more rigorous HLA-matching remained an independent predictor for superior post-biopsy allograft survival in patients with recurring IgAN (Table 4). However, the association between HLA-matching in recipients of a kidney from living-related donors and IgAN recurrence suggest that familiar risk of recurrent IgAN may be mostly carried in genetic regions within the HLA region. This finding may help in guiding future studies to explore genomic donor-recipient matching to reduce recurrent IgAN.
Although some prior studies suggested a benefit of steroid maintenance in preventing recurrent IgAN [16-18], others have not [8, 12]. Our study did not show any benefit of steroids maintenance on recurrent disease or allograft survival. Varying immunosuppressive regimens and definitions of steroid-free regimens may account for some of the different results. Prospective trials will be needed to address this issue with certainty.
A few studies have shown that allografts from living-related donors [8, 14, 19] are associated with an increased risk for recurrent IgAN while others [8, 10, 12, 13], including our study, do not support an independent association. The lack of correlation between acute rejection and recurrent IgAN is an interesting finding that argues against such association, especially in a cohort where most allograft biopsies were performed for allograft dysfunction.
Regarding the prognosis of patients with recurrent IgAN, serum creatinine, proteinuria and concurrent acute rejection were all significant predictors for allograft failure. Whereas a previous report, focusing on allograft survival in patients with post-transplant IgAN, revealed a significant negative impact of MEST-C scores on allograft survival [33], our study, albeit smaller, failed to demonstrate such an association on multivariable analyses. However, the aforementioned study combined both de novo and recurrent IgAN and did include proteinuria or serum creatinine at biopsy together with histologic score in the same multivariable analysis.
Unique strengths of the study includes its relatively large sample size with prolonged follow-up, and exclusion of de uncertain cases with low IgA staining intensity to include pure cases of recurrent IgAN. However, the findings in this study need to be interpreted in light of several limitations, including the retrospective nature of the data, center-based bias, and incomplete data. Other limitations include case ascertainment due to inability to precisely exclude potential cases with subclinical recurrence of IgAN that did not reach threshold needed to trigger follow-up allograft biopsies and shorter follow-up for non-recurring IgAN compared with recurrent IgAN. To overcome some of these biases, recurrence of IgAN was treated as a time-dependent variable and year of transplantation and medical sites were included in multivariable analyses. Additionally, the median follow-up time in patients with no evidence of recurrent IgAN is longer than the median time of recurrence (median 74 vs. 43 months after transplantation, respectively). Finally, while using pathology dataset may be regarded as a limitation, it should be stressed that the search included post-reperfusion biopsies (performed routinely for CUIMC patients) and protocol biopsies (performed routinely for OHSU and HUC patients). This would have assured inclusion of all non-recurring patients with native kidney failure secondary to IgAN who underwent kidney transplant at CUIMC, OHSU, and HUC.
In conclusion, recurrent IgAN is an important predictor of long-term allograft survival. Younger recipient age at transplantation is independently associated with recurrent IgAN. While HLA-matching may play a role in recurrent IgAN, it may only be important in the setting of living-related transplantation. Whereas our results support the predisposing role of HLA-DQ5 and the protective role of HLA-DR15 and HLA-DQ6 in IgAN in the native kidney, the presence of any of these antigens in the recipients or in the donors did not appear to affect recurrent disease post-transplantation.
Supplementary Material
Acknowledgments:
The work was presented in part as oral presentation at the ASN 2019 Kidney Week (Washington, DC). IB is supported from 2020 Mendez National Institute of Transplantation Foundation Research Grant. KK is supported by R01-DK105124 from the NIH/NIDDK.
Abbreviations
- CUIMC
Columbia University Irving Medical Center
- HLA
human leukocyte antigen
- HUC
Hospitalar da Universitário de Coimbra
- IgAN
IgA nephropathy
- MEST-C score
Oxford histologic scores for IgA nephropathy
- OHSU
Oregon Health & Science University
Footnotes
Statement of Ethics:
This study is in compliance with the guidelines for human subjects and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The study was approved by each site’s Institutional Review Board (Columbia University [Protocol Number AAAO2107], Oregon Health & Science University [STUDY00017467], Centro Hospitalar e Universitario de Coimbra [CHUC 167-20]) and granted waiver of informed consent.
Disclosure:
The authors declare no conflicts of interest
Data Availability Statement:
All data generated or analyzed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author.
References:
- 1.Julian BA, Quiggins PA, Thompson JS, Woodford SY, Gleason K, Wyatt RJ. Familial IgA nephropathy. Evidence of an inherited mechanism of disease. N Engl J Med. 1985;312:202–208. [DOI] [PubMed] [Google Scholar]
- 2.Doxiadis II, De Lange P, De Vries E, Persijn GG, Claas FH. Protective and susceptible HLA polymorphisms in IgA nephropathy patients with end-stage renal failure. Tissue Antigens. 2001;57:344–347. [DOI] [PubMed] [Google Scholar]
- 3.Feehally J, Farrall M, Boland A, et al. HLA has strongest association with IgA nephropathy in genome-wide analysis. J Am Soc Nephrol. 2010;21:1791–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiryluk K, Li Y, Sanna-Cherchi S, et al. Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet. 2012;8:e1002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiryluk K, Li Y, Scolari F, et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46:1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moroni G, Belingheri M, Frontini G, Tamborini F, Messa P. Immunoglobulin A Nephropathy. Recurrence After Renal Transplantation. Front Immunol. 2019; 10:1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berthoux F, Suzuki H, Mohey H, et al. Prognostic Value of Serum Biomarkers of Autoimmunity for Recurrence of IgA Nephropathy after Kidney Transplantation. J Am Soc Nephrol. 2017;28:1943–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nijim S, Vujjini V, Alasfar S, et al. Recurrent IgA Nephropathy After Kidney Transplantation. Transplant Proc. 2016;48:2689–2694. [DOI] [PubMed] [Google Scholar]
- 9.Moroni G, Longhi S, Quaglini S, et al. The long-term outcome of renal transplantation of IgA nephropathy and the impact of recurrence on graft survival. Nephrol Dial Transplant. 2013;28:1305–1314. [DOI] [PubMed] [Google Scholar]
- 10.Rodas LM, Ruiz-Ortiz E, Garcia-Herrera A, et al. IgA Nephropathy Recurrence after Kidney Transplantation: Role of Recipient Age and Human Leukocyte Antigen-B Mismatch. Am J Nephrol. 2020;51:357–365. [DOI] [PubMed] [Google Scholar]
- 11.Ponticelli C, Traversi L, Feliciani A, Cesana BM, Banfi G, Tarantino A. Kidney transplantation in patients with IgA mesangial glomerulonephritis. Kidney Int. 2001;60:1948–1954. [DOI] [PubMed] [Google Scholar]
- 12.Ahn S, Min SI, Min SK, et al. Different Recurrence Rates Between Pediatric and Adult Renal Transplant for Immunoglobulin A Nephropathy: Predictors of Posttransplant Recurrence. Exp Clin Transplant. 2015;13:227–232. [PubMed] [Google Scholar]
- 13.Cazorla-Lopez JM, Wu J, Villanego-Fernandez F, et al. IgA Nephropathy After Renal Transplant: Recurrences and De Novo Cases. Transplant Proc. 2020;52:515–518. [DOI] [PubMed] [Google Scholar]
- 14.Han SS, Huh W, Park SK, et al. Impact of recurrent disease and chronic allograft nephropathy on the long-term allograft outcome in patients with IgA nephropathy. Transpl Int. 2010;23:169–175. [DOI] [PubMed] [Google Scholar]
- 15.Sato K, Ishida H, Uchida K, Nitta K, Tanabe K. Risk factors for recurrence of immunoglobulin a nephropathy after renal transplantation: single center study. Ther Apher Dial. 2013;17:213–220. [DOI] [PubMed] [Google Scholar]
- 16.Von Visger JR, Gunay Y, Andreoni KA, et al. The risk of recurrent IgA nephropathy in a steroid-free protocol and other modifying immunosuppression. Clin Transplant. 2014;28:845–854. [DOI] [PubMed] [Google Scholar]
- 17.Di Vico MC, Messina M, Fop F, Barreca A, Segoloni GP, Biancone L. Recurrent IgA nephropathy after renal transplantation and steroid withdrawal. Clin Transplant. 2018;32:e13207. [DOI] [PubMed] [Google Scholar]
- 18.Clayton P, McDonald S, Chadban S. Steroids and recurrent IgA nephropathy after kidney transplantation. Am J Transplant. 2011;11:1645–1649. [DOI] [PubMed] [Google Scholar]
- 19.Andresdottir MB, Hoitsma AJ, Assmann KJ, Wetzels JF. Favorable outcome of renal transplantation in patients with IgA nephropathy. Clin Nephrol. 2001;56:279–288. [PubMed] [Google Scholar]
- 20.Andresdottir MB, Haasnoot GW, Doxiadis II, Persijn GG, Claas FH. Exclusive characteristics of graft survival and risk factors in recipients with immunoglobulin A nephropathy: a retrospective analysis of registry data. Transplantation. 2005;80:1012–1018. [DOI] [PubMed] [Google Scholar]
- 21.McDonald SP, Russ GR. Recurrence of IgA nephropathy among renal allograft recipients from living donors is greater among those with zero HLA mismatches. Transplantation. 2006;82:759–762. [DOI] [PubMed] [Google Scholar]
- 22.Proteinuria measurement and urine-based markers for preeclampsia diagnosis Technology Opportunity Assessment 2014.
- 23.Trimarchi H, Barratt J, Cattran DC, et al. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91:1014–1021. [DOI] [PubMed] [Google Scholar]
- 24.Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708–714. [DOI] [PubMed] [Google Scholar]
- 25.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. [DOI] [PubMed] [Google Scholar]
- 26.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–760. [DOI] [PubMed] [Google Scholar]
- 27.Maiers M, Gragert L, Klitz W. High-resolution HLA alleles and haplotypes in the United States population. Hum Immunol. 2007;68:779–788. [DOI] [PubMed] [Google Scholar]
- 28.Avasare RS, Rosenstiel PE, Zaky ZS, et al. Predicting Post-Transplant Recurrence of IgA Nephropathy: The Importance of Crescents. Am J Nephrol. 2017;45:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang SH, Kennard AL, Walters GD. Recurrent glomerulonephritis following renal transplantation and impact on graft survival. BMC Nephrol. 2018;19:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen PJ, Chadban SJ, Craig JC, et al. Recurrent glomerulonephritis after kidney transplantation: risk factors and allograft outcomes. Kidney Int. 2017;92:461–469. [DOI] [PubMed] [Google Scholar]
- 31.Kennard AL, Jiang SH, Walters GD. Increased glomerulonephritis recurrence after living related donation. BMC Nephrol. 2017; 18:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batal I, Vasilescu ER, Dadhania DM, et al. Association of HLA Typing and Alloimmunity With Posttransplantation Membranous Nephropathy: A Multicenter Case Series. Am J Kidney Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park S, Go H, Baek CH, et al. Clinical importance of the updated Oxford classification in allograft IgA nephropathy. Am J Transplant. 2019;19:2855–2864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author.


