Abstract
Background:
Sleep disturbances are prevalent in women living with HIV (WLWH) and can affect mental health and overall quality of life. We examined the prevalence and predictors of poor sleep quality in a U.S. cohort of WLWH and HIV-uninfected controls and the relationship between sleep quality and mental health symptom burden stratified by HIV disease status (viremic WLWH, aviremic WLWH, HIV-uninfected).
Methods:
Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI) in 1,583 (400 viremic WLWH, 723 aviremic WLWH, and 460 HIV-uninfected) Women’s Interagency HIV Study (WIHS) participants. Depressive and anxiety symptoms were concurrently assessed using the Center for Epidemiological Studies-Depression (CES-D) scale and General Anxiety Disorder (GAD-7) scale. Associations between poor sleep quality (global PSQI >5) and both high depressive (CES-D ≥16) and anxiety (GAD-7 ≥10) symptoms were each assessed by HIV disease status using multivariable logistic regression models.
Results:
Prevalence of poor sleep quality in the overall sample was 52%, differed by HIV disease status (p=0.045), and was significantly associated with high depressive and anxiety symptoms in 1) viremic WLWH, 2) aviremic WLWH, and 3) HIV-uninfected women [CES-D: 1) adjusted odds ratio (aOR)=7.50; 95% CI: 4.10-13.7, 2) aOR=4.54; 95% CI: 3.07-6.73, 3) aOR= 6.03; 95% CI: 3.50-10.4; GAD-7: 1) aOR=5.20; 95% CI: 2.60-10.4, 2) aOR=6.03; 95% CI: 3.67-9.91, 3) aOR=6.24; 95% CI: 3.11-12.6].
Conclusions:
Poor sleep quality is highly prevalent, as is mental health symptom burden, among WLWH and HIV-uninfected controls. Future longitudinal studies are necessary to clarify the directionality of the relationship.
Keywords: Sleep, minority women, HIV, HIV viremia, mental health
INTRODUCTION
Systematic reviews suggest that 30-90% of people living with HIV (PLWH) report disturbed sleep with women and individuals older than 40 years of age particularly affected.1,2 In addition to subjective measures of sleep quality, objectively measured shorter total sleep duration, poorer sleep continuity, and substantial night-to-night variability in sleep timing (indicative of circadian disruption), have been reported among PLWH.3–6 We previously reported that over 60% of women living with HIV (WLWH) and HIV-uninfected women in the Women’s Interagency HIV Study (WIHS) met criteria for insomnia.7
Life circumstances including unstable housing, high neighborhood disorder, trauma histories, social isolation, and polypharmacy put aging WLWH at greater risk for sleep disturbances.8–11 Compared to age-matched women from the general population, WLWH are more likely to have disordered breathing due to high rates of obesity, smoking, and a higher burden of pulmonary disease, all contributors to disrupted sleep.12,13 Additionally, adverse environmental factors are more common among aging WLWH and often include significant co-occurring everyday life stressors, HIV-related stigma and discrimination, low levels of physical activity, inadequate micronutrient intake, too much light/noise at night and too little daytime light exposure.14–18 These may all disrupt the sleep/wake cycle directly and indirectly through a number of pathways including neurohormonal dysregulation of melatonin, serotonin, cortisol, and catecholamines17,18 and vitamin D deficiency.16 Furthermore, with a decline in estrogen throughout the menopausal transition, vasomotor symptoms and increasing mood/anxiety symptoms interfere with various aspects of sleep.19
Sleep disturbances have emerged as a potential cause– and consequence– of higher rates of mental health disorders in samples of men living with HIV, however relatively little is known about the relationship among WLWH.20,21 Mood and anxiety disorders are prevalent among WLWH; we reported a 12-month prevalence of 22% mood and 45% anxiety disorders in the WIHS.22 Understanding the co-occurrence of these mental health problems with poor sleep quality in the context of HIV infection is a first step for mitigating untoward effects.22,23 Although aging WLWH are heavily impacted by mental health disorders and sleep problems particularly as they transition through menopause, sleep disorders remain infrequently diagnosed, leading to missed opportunities to improve health and quality of life.1,24–26
There is relatively limited information on sleep among WLWH and specifically in relation to mental health. Therefore, our primary aim was to determine the prevalence and predictors of poor sleep quality and their relationship with mental health symptom burden in the WIHS. As a secondary aim, we sought to determine whether these sleep/mental health relationships differed when stratified by HIV disease status (viremic WLWH, aviremic WLWH, and HIV-uninfected).
METHODS
Study Population
WIHS, now the Multicenter AIDS Cohort Study (MACS)/WIHS Combined Cohort Study, is an ongoing, multicenter longitudinal cohort study that includes WLWH and HIV-uninfected women in the United States. Enrollment occurred in 1994/95, 2001/02, and 2011/12 at sites in Bronx and Brooklyn, NY, Los Angeles and San Francisco, CA, Washington D.C., and Chicago, IL27,28 and during 2013-2015 at Southern sites in Chapel Hill, NC, Miami, FL, Atlanta, GA, Birmingham, AL, and Jackson, MS.29 Briefly, women were seen semiannually for a targeted clinical assessment with specimen collection and a comprehensive structured interview to collect sociodemographic, behavioral, and health history data. All study participants provided written informed consent after review and approval by each site’s Institutional Review Board. WIHS study protocols, recruitment procedures, and cohort characteristics have been previously described.27–29 WIHS interviewers added the Pittsburgh Sleep Quality Index (PSQI) questionnaire for those who agreed (78%) during a single WIHS visit in 2018 when sociodemographic, behavioral, and clinical data for this nested cross-sectional analysis was collected.
Sleep Quality
Global sleep quality during the prior month was measured using the validated PSQI, in which 18 of the 19 items in the self-report questionnaire are scored to generate seven sleep components: perceived sleep quality, sleep latency (time to fall asleep), sleep duration (hours of sleep per night), sleep efficiency (calculated percent of time asleep divided by time spent in bed), sleep disturbances (number of reasons for trouble sleeping), use of sleep medications (prescribed or over the counter), and daytime dysfunction (functional impairment due to loss of sleep). The generated components are ordinal variables scored 0 to 3 per scoring guidelines; higher scores represent worse sleep outcomes.30 The seven components are summed to produce a global PSQI score ranging from 0 to 21; higher scores indicate worse sleep quality. The global PSQI score was analyzed continuously and as a binary variable with a score greater than five indicating poor sleep quality. A global PSQI>5 demonstrates a sensitivity of 89.6% and specificity of 86.5% in distinguishing poor versus better sleep quality.30
Anxiety and Depressive Symptom Burden
The Center for Epidemiological Studies-Depression (CES-D) scale assesses depressive symptom severity in the past week using a 20-item questionnaire in which each response receives a score of 0 to 3. The items were summed for a CES-D total score ranging from 0 to 60, with higher scores representing higher depressive symptom burden.31 The WIHS has demonstrated that the CES-D is a reliable assessment of the burden of depressive symptoms among WLWH.32 Anxiety symptoms were measured using the 7-item self-report General Anxiety Disorder (GAD-7) scale. Users are asked how often anxiety symptoms have bothered them in the past two weeks with each of the 7 items receiving a score from 0 to 3. The 7 items are summed for a total GAD-7 score from 0 to 21. The higher the score, the more frequently they experienced symptoms.33
Both questionnaires were scored and analyzed as continuous and as binary variables using cut-points indicative of high vs. low symptom burden (CES-D ≥16; GAD-7 ≥10).31,33 An analysis in the WIHS showed that the CES-D standard cut-point of 16, when compared to a higher cut-point and with somatic items excluded, produced similar associations with antiretroviral therapy use and demographic characteristics.34 We examined the CES-D total score with and without the sleep disturbance item (“my sleep was restless”) due to concerns about collinearity. Inclusion of this item did not change results; therefore, the item was included in the total CES-D score. A GAD-7≥10 demonstrated optimal sensitivity (89%) and specificity (82%) in the general population.33
Covariates
Covariates were selected a priori based on published literature and likely confounding; these included: age in years, race/ethnicity (non-Hispanic Black, Hispanic, other, non-Hispanic White), highest level of education (<high school, high school, >high school), annual household income (≤$18,000, >$18,000), employment, housing stability, partner status, cigarette (current, former/never), hazardous alcohol (>7 drinks/week), non-injection drug use (current, former/never), and injection drug use (ever, never), self-reported menopausal status (menopausal, not menopausal, n/a due to hysterectomy), body mass index (measured BMI; kg/m2), and WIHS site. Additional variables of interest for WLWH included CD4+ T lymphocyte count (CD4 cells/mm3), HIV viral load (copies/ml) and as detectable (>20 copies) versus undetectable (≤20 copies), and antiretroviral adherence reported (≥95% of the time).
Statistical Methods
Descriptive statistics were generated for demographic, behavioral, and clinical characteristics in the sample overall. The continuous global PSQI score and each of the seven components as categorical measures were compared by HIV disease status (HIV-uninfected, aviremic WLWH, and viremic WLWH) using Anova/t-tests (mean and SD) for continuous and Chi-square tests for categorical variables. Cronbach alpha was run to test the internal reliability of the PSQI (alpha=0.74).
Participant characteristics by sleep quality (global PSQI>5 vs. ≤5) were compared with Chi-square tests for categorical variables and Wilcoxon rank-sum and t-tests for continuous variables. Odds ratios and 95% confidence intervals were calculated in bivariate analyses to identify predictors of poor sleep quality. Multivariable logistic regression models predicted odds of poor sleep, adjusting for age in years, race, education, income, and study site for the overall sample. The relationship between poor sleep quality and mental health symptom burden (high burden: CES-D ≥16 and GAD-7 ≥10) was also examined in unadjusted and adjusted analyses for the overall sample and stratified by HIV disease status. Additional covariates were included if they were of a priori interest or significant in unadjusted analyses at p<0.05. Final multivariable models retained all statistically significant variables as well as those identified from previous literature (including HIV disease status in the overall model, age, race, education, employment, non-injection drug use, injection drug use, BMI, smoking, hazardous alcohol use, menopausal status, and WIHS site). All analyses were performed using SAS software, version 9.4 (SAS Institute, Inc. Cary, NC). A p value <0.05 was considered statistically significant.
RESULTS
Sleep and participant characteristics
A total of 1,583 participants (1,123 WLWH/460 HIV-uninfected women) were included in the current analysis. Study characteristics are presented in Table 1. Median age was 51 years old (IQR: 44 – 57 years). The majority of participants were Black (62%), had a high school education or less (63%), were unemployed (60%), had an income ≤$18,000/year (62%), and were obese (BMI >30, 56%). Nearly half were menopausal (43%), 38% were current smokers, and 26% were current non-injection drug users. Among WLWH, median CD4 count was 680 cells/mm3 and although 85% reported ≥95% antiretroviral adherence, 36% had detectable HIV viral loads (>20 copies/ml).
Table 1.
Demographic, behavioral, and clinical characteristics among Women’s Interagency HIV Study participants. (n=1583)
| Variables | Overall |
|---|---|
| n (%) | |
| HIV serostatus | |
| HIV seropositive | 1123 (70.9) |
| HIV seronegative | 460 (29.1) |
| Age at visit, mean (SD) | 50.8 (9.3) |
| Race/ethnicity | |
| Non-Hispanic Black | 985 (62.2) |
| Hispanic | 207 (13.1) |
| Non-Hispanic other | 249 (15.7) |
| Non-Hispanic White | 142 (9.0) |
| Education | |
| <High school | 487 (30.8) |
| High school | 516 (32.6) |
| >High school | 579 (36.6) |
| Income, ≤$18,000/year | 958 (61.5) |
| Unemployed | 949 (60.0) |
| Unstable housing | 264 (16.7) |
| Partner status, no partner | 1128 (72.0) |
| Cigarette smoker, current | 596 (37.7) |
| Hazardous alcohol, >7 drink/wk | 131 (8.3) |
| Non-injection druga, current | 418 (26.4) |
| Injection drugb, ever | 251 (15.9) |
| Menopausal status | |
| Menopausal | 679 (42.9) |
| Not menopausal | 651 (41.1) |
| N/A due to hysterectomy | 253 (16.0) |
| Body mass index (BMI), mean (SD) | 32.7 (9.1) |
| Underweight | 26 (1.6) |
| Normal | 263 (16.8) |
| Overweight | 400 (25.5) |
| Obese | 880 (56.1) |
| Depressive symptoms (CES-D), median (IQR) | 8 (3-18) |
| Anxiety symptoms (GAD-7), median (IQR) | 2 (0-7) |
| HIV positive participants (n=1123) | |
| CD4 count (cells/mm3), median (IQR) | 680 (473 – 918) |
| CD4 count below 350 | 157 (14.0) |
| Detected viral load | 400 (35.7) |
| Viral load (copies/mL), median (IQR) | 98 (36 – 1115) |
| cART adherence, ≥95% | 902 (85.4) |
Due to missing data, counts may not add up to total n=1583. Data presented as counts (frequency), unless otherwise noted.
Non-injected recreational drugs use since last visit. Includes crack, cocaine, and/or heroin use, any marijuana use, hallucinogens, club drugs and methamphetamines.
Ever reported use
The global PSQI score for our cohort ranged from 0 to 20; mean was 6.4 and median was 6 (Figure 1). The PSQI had an overall Cronbach’s alpha of 0.74 indicating adequate internal reliability. There were no significant differences in global PSQI score by HIV serostatus (WLWH mean=6.45 vs. HIV-uninfected mean=6.43), however global PSQI score differed significantly when stratified by viremia status (viremic WLWH mean=6.90 vs. aviremic WLWH mean=6.20; p=0.009). Among the PSQI components, sleep disturbances significantly differed by HIV disease status (p=0.010), a higher proportion of viremic WLWH (32%) reported more frequent sleep disturbances than aviremic WLWH (25%) and HIV-uninfected women (28%). Aviremic WLWH were more likely to report “very good sleep” (34%) than HIV-uninfected women (26%) and viremic WLWH (28%) (p=0.022). Viremic WLWH (17%) reported more daytime dysfunction than aviremic WLWH (11%) and HIV-uninfected (10%) women. The other PSQI components did not significantly differ by HIV disease status. (Data not shown)
Figure 1.
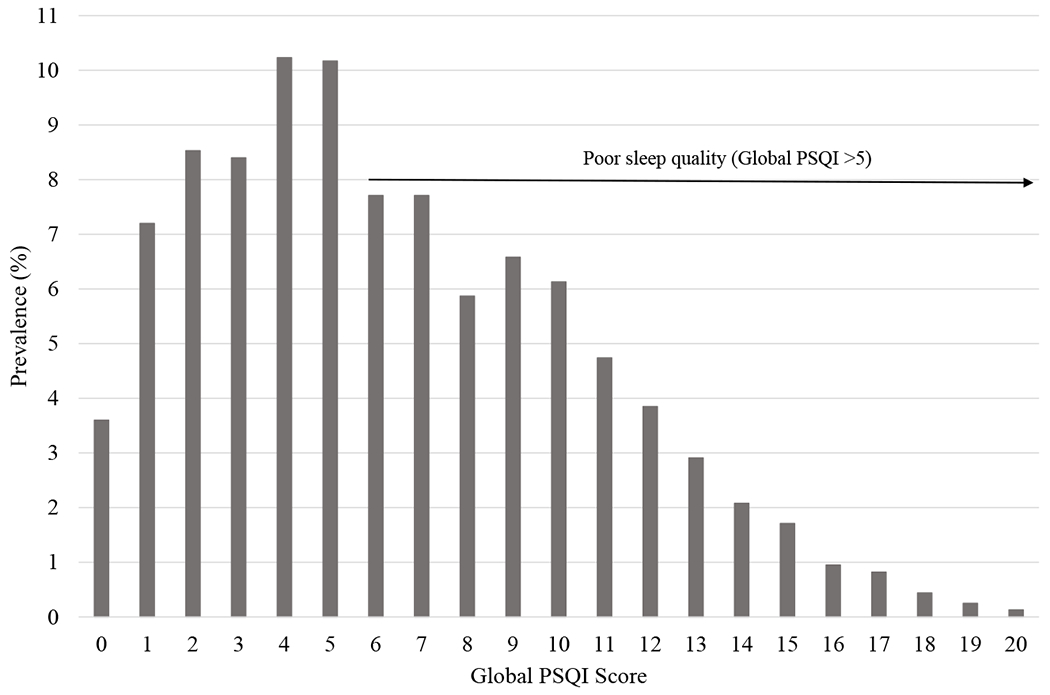
Distribution of global PSQI score among WIHS participants (n=1583)
Predictors of poor sleep
Participant characteristics stratified by sleep quality (poor sleep: PSQI>5) are shown in Table 2; poor sleep quality was found in 821 (52%) participants, which differed significantly by HIV disease status (56.5% viremic WLWH vs. 48.8% aviremic WLWH vs. 52.6% HIV-uninfected; p=0.045). Compared to participants with better sleep quality, those with poor sleep quality were older (p=0.0004), had lower household income (≤$18,000/year; p=0.002), were unemployed (p<0.0001), and more likely to be unstably housed (p=0.015). Those with poor sleep quality were also more likely to report current smoking, current non-injection drug use, and history of injection drug use (all p<0.0001). Depressive (CES-D) and anxiety symptoms (GAD-7) were significantly associated with poor sleep quality (both p<0.0001).
Table 2.
Factors associated with poor sleep in Women’s Interagency HIV Study participants. (n=1583)
| Variables | Poor sleep, PSQI >5 (n=821) | Better sleep, PSQI ≤5 (n=762) | Unadjusted | Adjustedb | ||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| n (%) | n (%) | OR (95% CI) | p valuea | OR (95% CI) | p value | |
| HIV disease status | ||||||
| Viremic WLWH | 226 (27.5) | 174 (22.8) | 1.36 (1.07-1.74) | 0.045 | 1.26 (0.96-1.64) | 0.199 |
| Aviremic WLWH | 353 (43.0) | 370 (48.6) | Ref. | Ref. | ||
| HIV-uninfected | 242 (29.5) | 218 (28.6) | 1.16 (0.92-1.47) | 1.18 (0.92-1.51) | ||
| Age at visit, mean (SD) | 51.6 (9.0) | 49.9 (9.5) | 1.02 (1.01-1.03) | 0.0004 | 1.02 (1.01-1.03) | 0.003 |
| Race/ethnicity | ||||||
| Non-Hispanic Black | 482 (58.7) | 503 (66.0) | 0.47 (0.33-0.69) | 0.0008 | 0.56 (0.38-0.83) | 0.036 |
| Hispanic | 110 (13.4) | 97 (12.7) | 0.56 (0.36-0.87) | 0.61 (0.37-0.99) | ||
| Non-Hispanic other | 134 (16.3) | 115 (15.1) | 0.58 (0.38-0.89) | 0.66 (0.42-1.04) | ||
| Non-Hispanic White | 95 (11.6) | 47 (6.2) | Ref. | Ref. | ||
| Education | ||||||
| <High school | 268 (32.6) | 219 (28.8) | 1.13 (0.89-1.44) | 0.146 | 1.07 (0.82-1.40) | 0.140 |
| High school | 252 (30.7) | 264 (34.7) | 0.88 (0.70-1.12) | 0.83 (0.64-1.08) | ||
| >High school | 301 (36.7) | 278 (36.5) | Ref. | Ref. | ||
| Income, ≤$18,000/year | 527 (65.2) | 431 (57.5) | 1.38 (1.13-1.70) | 0.002 | 1.33 (1.06-1.67) | 0.015 |
| Unemployed | 555 (67.6) | 394 (51.7) | 1.95 (1.59-2.39) | <0.0001 | 1.82 (1.41-2.34) | <0.0001 |
| Unstable housing | 155 (18.9) | 109 (14.3) | 1.39 (1.07-1.82) | 0.015 | 1.31 (0.98-1.76) | 0.067 |
| Partner status, no partner | 598 (73.6) | 530 (70.4) | 1.17 (0.94-1.46) | 0.163 | 1.14 (0.90-1.44) | 0.275 |
| Cigarette smoker, current | 353 (43.0) | 243 (31.9) | 1.61 (1.31-1.98) | <0.0001 | 1.49 (1.19-1.86) | 0.0005 |
| Hazardous alcohol, >7 drink/wk | 82 (10.0) | 49 (6.4) | 1.61 (1.12-2.33) | 0.010 | 1.51 (1.03-2.23) | 0.037 |
| Non-injection drugc, current | 263 (32.0) | 155 (20.3) | 1.85 (1.47-2.32) | <0.0001 | 1.70 (1.33-2.17) | <0.0001 |
| Injection drugd, ever | 166 (20.2) | 85 (11.2) | 2.02 (1.52-2.68) | <0.0001 | 1.47 (1.07-2.01) | 0.016 |
| Menopausal status | ||||||
| Menopausal | 374 (45.6) | 305 (40.0) | 1.48 (1.19-1.84) | <0.0001 | 1.30 (0.95-1.78) | 0.003 |
| Not menopausal | 295 (35.9) | 356 (46.7) | Ref. | Ref. | ||
| N/A due to hysterectomy | 152 (18.5) | 101 (13.3) | 1.82 (1.35-2.44) | 1.83 (1.29-2.60) | ||
| BMI, mean (SD) | 32.8 (9.2) | 32.5 (9.0) | -- | 0.580 | -- | |
| Underweight | 15 (1.9) | 11 (1.5) | 1.35 (0.60-3.06) | 0.799 | 1.22 (0.52-2.83) | 0.346 |
| Normal | 132 (16.2) | 131 (17.3) | Ref. | Ref. | ||
| Overweight | 213 (26.2) | 187 (24.7) | 1.13 (0.83-1.54) | 1.35 (0.97-1.88) | ||
| Obese | 453 (55.7) | 427 (56.5) | 1.05 (0.80-1.39) | 1.26 (0.94-1.70) | ||
| CES-D, median (IQR) | 14 (6-24) | 4 (1-10) | 1.10 (1.09-1.12) | <0.0001 | 1.10 (1.09-1.11) | <0.0001 |
| GAD-7, median (IQR) | 6 (2-11) | 0 (0-3) | 1.22 (1.19-1.25) | <0.0001 | 1.21 (1.17-1.24) | <0.0001 |
| HIV positive participants (n=1123) | ||||||
| CD4 count (cells/mm3), median (IQR) | 681 (460 – 918) | 680 (488 – 918) | -- | 0.947 | -- | |
| CD4 count below 350 | 79 (13.6) | 78 (14.4) | 0.94 (0.67-1.31) | 0.709 | 0.93 (0.65-1.33) | 0.686 |
| Viral load (copies/mL), median (IQR) | 108.5 (39 – 1120) | 79 (35 – 849) | -- | 0.237 | -- | |
| cART adherence, ≥95% | 454 (83.9) | 448 (87.0) | 0.78 (0.55-1.10) | 0.158 | 0.72 (0.50-1.04) | 0.077 |
Due to missing data, counts may not add up to total n=1583. Data presented as counts (frequency), unless otherwise noted.
p-values calculated from chi-square tests for categorical variables, Wilcoxon (median), or t-tests (mean)
Adjusted for age in years, race, education, income, and WIHS site
Includes crack, cocaine, and/or heroin use, any marijuana use, hallucinogens, club drugs and methamphetamines.
Ever reported use
In multivariable models, higher age, lower household income, unemployment, current cigarette smoking, hazardous alcohol use (>7 drinks/week), current non-injection drug use, and injection drug use ever were significantly associated with poor sleep quality (all p<0.05). Depressive and anxiety symptoms remained significantly associated with poor sleep quality after adjusting for age, race, education, income, and WIHS site (p <0.0001). (Table 2)
Associations of poor sleep and mental health symptom burden
Prevalence of poor sleep quality by mental health symptom burden (high vs. low burden) is presented in Table 3. In unadjusted analyses, when compared to better sleep, participants with poor sleep quality had increased odds of depressive (OR=5.92; 95% CI: 4.60-7.63, p=<0.0001) and anxiety symptoms (OR=6.31; 95% CI: 4.57-8.71, p=<0.0001). In multivariable models, poor sleep remained significantly associated with high depressive (adjusted OR [aOR]=5.06; 95% CI: 3.87-6.63, p=<0.0001) and anxiety symptoms (aOR=5.70; 95% CI: 4.05-8.02, p=<0.0001) after adjusting for HIV disease status, age, race, education, employment, BMI, smoking, alcohol use, any drug use, menopausal status, and WIHS site.
Table 3.
Prevalence and association of sleep quality as measured by the Pittsburgh Sleep Quality Index by mental health symptom burden. (n=1583)
| Measures | Poor sleep PSQI >5 (n=821) | Better sleep PSQI ≤5 (n=762) | p value | Unadjusted OR (95% CI) | Adjusteda OR (95% CI) |
|---|---|---|---|---|---|
|
| |||||
| n (%) | n (%) | ||||
| High depressive symptoms | |||||
| Yes, CES-D ≥16 | 383 (46.7) | 98 (12.9) | <0.0001 | 5.92 (4.60-7.63)* | 5.06 (3.87-6.63)* |
| No, CES-D <16 | 438 (53.3) | 664 (87.1) | Ref. | Ref. | |
| High anxiety symptoms | |||||
| Yes, GAD-7 ≥10 | 252 (30.7) | 50 (6.6) | <0.0001 | 6.31 (4.57-8.71)* | 5.70 (4.05-8.02)* |
| No, GAD-7 <10 | 569 (69.3) | 712 (93.4) | Ref. | Ref. | |
Significant at p<0.0001
Adjusted for HIV disease status, age in years, race, education, employment, BMI, smoking, drinking, non-injection and injection drug use, menopausal status, and WIHS site
When stratified by HIV disease status, those with poor sleep quality had significantly higher mental health symptom burden across all groups (all p<0.0001). This association remained significant for all three HIV disease status groups even after adjusting for age, race, education, employment, BMI, smoking, alcohol use, drug use, menopausal status, and WIHS site: 1) viremic WLWH, 2) aviremic WLWH, and 3) HIV-uninfected respectively [CES-D: 1) aOR=7.50; 95% CI: 4.10-13.7, 2) aOR=4.54; 95% CI: 3.07-6.73, 3) aOR= 6.03; 95% CI: 3.50-10.4; GAD-7: 1) aOR=5.20; 95% CI: 2.60-10.4, 2) aOR=6.03; 95% CI: 3.67-9.91, 3) aOR=6.24; 95% CI: 3.11-12.6]. (Table 4)
Table 4.
Association of sleep quality as measured by the Pittsburgh Sleep Quality Index by mental health symptom burden, stratified by HIV disease status.
| Viremic WLWH (n=400) | Aviremic WLWH (n=723) | HIV-Uninfected (n=460) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Measures | Unadjusted OR (95% CI) | Adjusteda OR (95% CI) | Unadjusted OR (95% CI) | Adjusteda OR (95% CI) | Unadjusted OR (95% CI) | Adjusteda OR (95% CI) |
| High depressive symptoms | ||||||
| Yes, CES-D ≥16 | 7.29 (4.34-12.2)* | 7.50 (4.10-13.7)* | 4.90 (3.43-7.02)* | 4.54 (3.07-6.73)* | 6.83 (4.14-11.3)* | 6.03 (3.50-10.4)* |
| No, CES-D <16 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| High anxiety symptoms | ||||||
| Yes, GAD-7 ≥10 | 6.03 (3.21-11.3)* | 5.20 (2.60-10.4)* | 5.91 (3.74-9.35)* | 6.03 (3.67-9.91)* | 7.51 (3.85-14.6)* | 6.24 (3.11-12.6)* |
| No, GAD-7 <10 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
Significant at p<0.0001
Adjusted for age in years, race, education, employment, BMI, smoking, drinking, non-injection and injection drug use, menopausal status, and WIHS site
DISCUSSION
This study demonstrated that poor sleep quality was common among WLWH and HIV-uninfected women enrolled in the WIHS, and significantly differed by HIV viremia status. While the prevalence of poor sleep was similar in aviremic WLWH and HIV-uninfected women, we found worse sleep quality in WLWH who had not achieved viral control. Additional factors significantly associated with poor sleep quality included older age, low income, unemployment, smoking, hazardous alcohol use, non-injection and past injection drug use. These findings suggest that poor sleep is likely exacerbating the burden of comorbidities in this population.
This study corroborated the high prevalence of poor sleep found in previous studies and expanded these findings by exploring validated measures of mental health symptom burden in a large cohort of women. Significant associations between sleep quality and depressive and anxiety symptoms were found in multivariable models and remained significant when stratified by HIV disease status. In a cohort of French HIV-infected adults (n=1,354), similar proportions of poor sleep quality (global PSQI score >5: 47%) and depressive symptoms (20%) were observed.35 While predominantly males, they found poor sleep quality more frequently in females. Similar to our study, the odds of sleep disturbances among participants with depression was 4.6 times that of those without depression.
Qiu et al evaluated sleep quality in a cohort of HIV-uninfected pregnant U.S. women (n=1,488).36 Like our study, they found that poor sleep measured by the PSQI was associated with 6.5 and 3.6 increased odds of depression and anxiety. Qiu et al used a different scale to measure anxiety, which could explain the differences in the magnitude of the odds of association.36
In our prior study among 1,682 WIHS participants, Jean-Louis et al. assessed prevalence and predictors of insomnia symptoms and found that WLWH were more likely to report insomnia symptoms than HIV-uninfected women and depression was a significant predictor of insomnia symptoms. Unlike the current study, insomnia symptoms were used as a measure of sleep disturbances and defined as a report of difficulty initiating or maintaining sleep or early morning waking three or more times per week in the past two weeks. This study, while using the same cohort, included measures collected 12 years prior to the current study, and as a result sociodemographic changes due to increasing age and time period are expected and were observed. The cohort also expanded during the past 12 years. Two additional enrollment waves occurred, one in 2011/12 and another targeting women at new Southern sites in 2013-15, resulting in increased representativeness of the cohort.7
Given the high co-occurrence of poor sleep and mental health symptoms found in this study, both should be assessed and effectively treated to prevent and/or mitigate effects of the other. We found the PSQI components had adequate internal reliability (Cronbach α=0.74); therefore, in the context of clinical assessment, sleep can reliably be evaluated using the PSQI. The opportunity for patient-provider discussion about problematic sleep during the clinical encounter could enhance understanding of and treatment for sleep but also co-occurring mental health problems in this population. Most importantly, given the high co-occurrence of poor sleep and poor mental health, it is possible that effective treatment of either condition may help resolve or prevent the other and lead to higher quality of life. We also acknowledge that research suggests that neighborhood environment and life stressors beyond one’s control could negatively influence sleep and mental health and these are not easily modifiable at the individual level. Finding ways to promote and support policy level changes and empower community members could prove useful in the context of neighborhood disorder from an ecological perspective.
We also found that the association between sleep quality and mental health symptom burden was significant for all HIV disease status groups. Although WIHS studies a decade earlier found that depression was associated with reduced ART adherence and greater viral load,37 it is possible that in the current era, poor sleep quality and mental health symptom burden are attributable to additional factors beyond ART nonadherence and lack of viral control.
We found that sleep quality was worse among viremic WLWH, a similar finding to Balthazar et al., who found that higher sleep efficiency was associated with lower HIV-RNA levels.38 Like our study, they did not find an association with lower CD4 count and greater sleep disturbances despite prior evidence,39 suggesting that HIV viral control may play a more important role in sleep quality than CD4 count. Viremic and aviremic WLWH in our study did differ significantly by smoking, drinking, and substance use (current and prior) which were adjusted for in analyses. Possibly, there is residual confounding that includes risk behaviors that contribute to HIV viremia independent of ART adherence and these behaviors may negatively influence sleep quality.
Markers of inflammation/immune activation have been found to be associated with both depressive symptoms40,41 and sleep disturbance42,43 in the general population and in PLWH. It is possible that disturbed sleep and poor viral control have a bidirectional association through other pathways including activation of the kynurenine pathway by indoleamine 2,3 dioxygenase (IDO) enzyme responsible for tryptophan catabolism. Tryptophan is both a precursor of melatonin, a sleep and circadian regulator, and important in depression.44 In a study conducted by Cho et al. among currently, previously, and never depressed participants, they found an association between sleep disturbances (PSQI) and kynurenine metabolism in depressed participants only.45 Inflammation/immune activation may be an additional pathway by which poor sleep and depressive symptoms interact. In PLWH, evidence suggests that the HIV Tat protein can trigger IDO activity and subsequently, the kynurenine pathway.46 This may be a mechanism linking poor sleep and high depressive symptom burden and the stronger association among viremic WLWH. Our cross-sectional study is unable to determine the complex relationships between inflammation/immune activation, sleep quality and depressive symptoms, so future studies are needed.
While this cross-sectional study highlighted a significant association between sleep quality and mental health symptoms, we could not determine the directionality of this relationship. In a prospective study of men who have sex with men, self-reported sleep disturbance was associated with a significant increase in depression six months later in men with HIV, but not in men without HIV, suggesting that sleep disturbances may lead to depressive symptoms, particularly among people living with HIV.20 Future steps for the current cohort include repeat administrations of the PSQI for longitudinal analyses to delineate the directionality of the relationship between sleep and mental health symptom burden including stratification by HIV disease status.
Strengths and Limitations
Other studies have looked at the association between sleep quality and mental health burden in PLWH, but our study is unique because of its size, the presence of a HIV-uninfected control group, the use of a large number of relevant covariates, and the wide geographic and racial/ethnic representation of WLWH in the US. Nesting this study within a large longitudinal cohort lays the foundation for future longitudinal quality of sleep studies to assess causality in the association of sleep quality and mental health symptom burden.
This study had several limitations. We assessed the associations between the PSQI and mental health symptom burden cross-sectionally, and therefore directionality cannot be established. Using self-reported sleep quality with the well-validated PSQI allowed us to assess sleep quality in a large number of women, however self-reported data have limitations compared to objective measures of sleep quality, including social desirability and recall biases and/or the participant’s perception of sleep need. Other disordered sleep may also be missed or underrepresented by the PSQI. Our mental health instruments were also subjective measures; however, they are widely accepted in the literature. It is also plausible that the PSQI differentially captures psychological aspects of sleep. Similarly, some of the emotional symptoms identified by the CES-D and/or GAD-7 could be manifestations of daytime dysfunction due to sleep disturbances.
CONCLUSIONS
Poor sleep is highly prevalent, associated with mental health symptom burden, and likely under-appreciated in the context of care in aging WLWH. Sleep quality and mental health should be priority components of the clinical management of chronic HIV. Providers should be aware of the high frequencies of these symptoms, particularly as PLWH age and risk of aging-related comorbidities increases. Routine and concurrent assessment and effective treatment of mental health and sleep disorders, as well as maintained viral suppression, have the potential to profoundly impact the overall quality of life in WLWH.
ACKNOWLEDGEMENTS:
The authors gratefully acknowledge the contributions of the study participants and dedication of the staff at the MWCCS sites.
Source of Funding:
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS), now the MACS/WIHS Combined Cohort Study (MWCCS). Data for this manuscript were analyzed by the Chicago Cook County site U01-HL146245 (French, A. Cohen, M.) with additional support provided by R-01 HL142116-01 (French, A. Burgess, H.). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; UAB-MS CRS (Mirjam-Colette Kempf, Jodie Dionne-Odom, and Deborah Konkle-Parker), U01-HL146192; UNC CRS (Adaora Adimora), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute on Minority Health and Health Disparities (NIMHD), and in coordination and alignment with the research priorities of the National Institutes of Health, Office of AIDS Research (OAR).
Footnotes
Conflicts of Interest: None of the authors have conflicts of interest with regard to the contents of this manuscript.
REFERENCES
- 1.Gutierrez J, Tedaldi EM, Armon C, Patel V, Hart R, Buchacz K. Sleep disturbances in HIV-infected patients associated with depression and high risk of obstructive sleep apnea. SAGE Open Med. 2019;7:2050312119842268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu J, Wu H, Lu C, Guo L, Li P. Self-reported sleep disturbances in HIV-infected people: a meta-analysis of prevalence and moderators. Sleep Med. 2015;16(8):901–907. [DOI] [PubMed] [Google Scholar]
- 3.Gamaldo CE, Gamaldo A, Creighton J, et al. Evaluating sleep and cognition in HIV. J Acquir Immune Defic Syndr. 2013;63(5):609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee KA, Gay C, Portillo CJ, et al. Types of sleep problems in adults living with HIV/AIDS. J Clin Sleep Med. 2012;8(1):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taibi DM, Price C, Voss J. A pilot study of sleep quality and rest-activity patterns in persons living with HIV. J Assoc Nurses AIDS Care. 2013;24(5):411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoon IY, Kripke DF, Elliott JA, Youngstedt SD, Rex KM, Hauger RL. Age-related changes of circadian rhythms and sleep-wake cycles. J Am Geriatr Soc. 2003;51(8):1085–1091. [DOI] [PubMed] [Google Scholar]
- 7.Jean-Louis G, Weber KM, Aouizerat BE, et al. Insomnia symptoms and HIV infection among participants in the Women’s Interagency HIV Study. Sleep. 2012;35(1):131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babson KA, Feldner MT. Temporal relations between sleep problems and both traumatic event exposure and PTSD: a critical review of the empirical literature. J Anxiety Disord. 2010;24(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Wang R, Zee P, et al. Racial/Ethnic Differences in Sleep Disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep. 2015;38(6):877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho HJ, Seeman TE, Kiefe CI, Lauderdale DS, Irwin MR. Sleep disturbance and longitudinal risk of inflammation: Moderating influences of social integration and social isolation in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Brain Behav Immun. 2015;46:319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dale S, Cohen M, Weber K, Cruise R, Kelso G, Brody L. Abuse and resilience in relation to HAART medication adherence and HIV viral load among women with HIV in the United States. AIDS Patient Care STDS. 2014;28(3):136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzpatrick ME, Kunisaki KM, Morris A. Pulmonary disease in HIV-infected adults in the era of antiretroviral therapy. AIDS. 2018;32(3):277–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53(11):1120–1126. [DOI] [PubMed] [Google Scholar]
- 14.Crockett KB, Kalichman SC, Kalichman MO, Cruess DG, Katner HP. Experiences of HIV-related discrimination and consequences for internalised stigma, depression and alcohol use. Psychol Health. 2019;34(7):796–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fekete EM, Seay J, Antoni MH, et al. Oxytocin, social support, and sleep quality in low-income minority women living with HIV. Behav Sleep Med. 2014;12(3):207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao Q, Kou T, Zhuang B, Ren Y, Dong X, Wang Q. The Association between Vitamin D Deficiency and Sleep Disorders: A Systematic Review and Meta-Analysis. Nutrients. 2018;10(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waller KL, Mortensen EL, Avlund K, et al. Melatonin and cortisol profiles in late midlife and their association with age-related changes in cognition. Nat Sci Sleep. 2016;8:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright KP, Lowry CA, Lebourgeois MK. Circadian and wakefulness-sleep modulation of cognition in humans. Front Mol Neurosci. 2012;5:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Han Y, Cho HH, Kim MR. Sleep Disorders and Menopause. J Menopausal Med. 2019;25(2):83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irwin MR, Archer G, Olmstead R, et al. Increased risk of depression in non-depressed HIV infected men with sleep disturbance: Prospective findings from the Multicenter AIDS Cohort Study. EBioMedicine. 2018;36:454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers BG, Lee JS, Bainter SA, Bedoya CA, Pinkston M, Safren SA. A multilevel examination of sleep, depression, and quality of life in people living with HIV/AIDS. J Health Psychol. 2020;25(10-11):1556–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook JA, Burke-Miller JK, Steigman PJ, et al. Prevalence, Comorbidity, and Correlates of Psychiatric and Substance Use Disorders and Associations with HIV Risk Behaviors in a Multisite Cohort of Women Living with HIV. AIDS Behav. 2018;22(10):3141–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Todd JV, Cole SR, Pence BW, et al. Effects of Antiretroviral Therapy and Depressive Symptoms on All-Cause Mortality Among HIV-Infected Women. Am J Epidemiol. 2017;185(10):869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris JL, Rohay J, Chasens ER. Sex Differences in the Psychometric Properties of the Pittsburgh Sleep Quality Index. J Womens Health (Larchmt). 2018;27(3):278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stone KL, Xiao Q. Impact of Poor Sleep on Physical and Mental Health in Older Women. Sleep Med Clin. 2018;13(3):457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Travaglini LE, Himelhoch SS, Fang LJ. HIV Stigma and Its Relation to Mental, Physical and Social Health Among Black Women Living with HIV/AIDS. AIDS Behav. 2018;22(12):3783–3794. [DOI] [PubMed] [Google Scholar]
- 27.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9(2):117–125. [PubMed] [Google Scholar]
- 28.Bacon MC, von Wyl V, Alden C, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12(9):1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adimora AA, Ramirez C, Benning L, et al. Cohort Profile: The Women’s Interagency HIV Study (WIHS). Int J Epidemiol. 2018;47(2):393–394i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 31.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied psychological measurement. 1977;1(3):385–401. [Google Scholar]
- 32.Adams LM, Wilson TE, Merenstein D, et al. Using the Center for Epidemiologic Studies Depression Scale to assess depression in women with HIV and women at risk for HIV: Are somatic items invariant? Psychol Assess. 2018;30(1):97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. [DOI] [PubMed] [Google Scholar]
- 34.Cook JA, Cohen MH, Burke J, et al. Effects of depressive symptoms and mental health quality of life on use of highly active antiretroviral therapy among HIV-seropositive women. J Acquir Immune Defic Syndr. 2002;30(4):401–409. [DOI] [PubMed] [Google Scholar]
- 35.Allavena C, Guimard T, Billaud E, et al. Prevalence and Risk Factors of Sleep Disturbance in a Large HIV-Infected Adult Population. AIDS Behav. 2016;20(2):339–344. [DOI] [PubMed] [Google Scholar]
- 36.Qiu C, Gelaye B, Zhong QY, Enquobahrie DA, Frederick IO, Williams MA. Construct validity and factor structure of the Pittsburgh Sleep Quality Index among pregnant women in a Pacific-Northwest cohort. Sleep Breath. 2016;20(1):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cook JA, Grey D, Burke J, et al. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. Am J Public Health. 2004;94(7):1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balthazar MS, Webel A, Gary F, Burant CJ, Totten VY, Voss JG. Sleep and immune function among people living with human immunodeficiency virus (HIV). AIDS Care. 2020: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seay JS, McIntosh R, Fekete EM, et al. Self-reported sleep disturbance is associated with lower CD4 count and 24-h urinary dopamine levels in ethnic minority women living with HIV. Psychoneuroendocrinology. 2013;38(11):2647–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu H, Surkan PJ, Irwin MR, et al. Inflammation and Risk of Depression in HIV: Prospective Findings From the Multicenter AIDS Cohort Study. Am J Epidemiol. 2019;188(11):1994–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Penninx BW, Kritchevsky SB, Yaffe K, et al. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatry. 2003;54(5):566–572. [DOI] [PubMed] [Google Scholar]
- 42.Huang Y, Jiang Y, Zhu M. The Relationship Between Global Sleep Score And Inflammatory Markers In Obese Adults From The United States. Nat Sci Sleep. 2019;11:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Irwin MR, Olmstead R, Carroll JE. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol Psychiatry. 2016;80(1):40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis I, Liu A. What is the tryptophan kynurenine pathway and why is it important to neurotherapy? Expert Rev Neurother. 2015;15(7):719–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho HJ, Savitz J, Dantzer R, Teague TK, Drevets WC, Irwin MR. Sleep disturbance and kynurenine metabolism in depression. J Psychosom Res. 2017;99:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samikkannu T, Saiyed ZM, Rao KV, et al. Differential regulation of indoleamine-2,3-dioxygenase (IDO) by HIV type 1 clade B and C Tat protein. AIDS Res Hum Retroviruses. 2009;25(3):329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]


