Abstract
The COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a global crisis with unprecedented challenges for public health. Vaccinations against SARS-CoV-2 have slowed the incidence of new infections and reduced disease severity. As the time-of-day of vaccination has been reported to influence host immune responses to multiple pathogens, we quantified the influence of SARS-CoV-2 vaccination time, vaccine type, participant age, sex, and days post-vaccination on anti-Spike antibody responses in healthcare workers. The magnitude of the anti-Spike antibody response associated with the time-of-day of vaccination, vaccine type, participant age, sex, and days post vaccination. These results may be relevant for optimizing SARS-CoV-2 vaccine efficacy.
The circadian clock is an endogenous 24 hour clock that regulates many aspects of physiology, including the response to infectious disease and vaccination (Allada and Bass, 2021). A recent report demonstrated significant daytime variation in multiple immune parameters in >300,000 participants in the UK Biobank, highlighting the diurnal nature of innate and adaptive immune responses (Wyse et al., 2021). Human lung diseases frequently show time-of-day variation in symptom severity and respiratory function and the circadian transcriptional activator BMAL1 has been shown to regulate respiratory inflammation (Ehlers et al., 2018; Ince et al., 2019). Influenza A virus infection of circadian-arrhythmic mice is associated with elevated inflammatory responses and a higher viral burden (Edgar et al., 2016; Sengupta et al., 2019). The time-of-day of influenza vaccination in elderly men affected antibody responses with higher titres noted in the morning (Phillips et al., 2008; Long et al., 2016). An additional influenza vaccination study reported that the time of sample collection rather than vaccination had a more significant effect on antibody responses (Kurupati et al., 2017). We and others have proposed a role for circadian signalling in regulating SARS-CoV-2 host immune responses and COVID-19 severity (Ray and Reddy, 2020; Maidstone et al., 2021; Sengupta et al., 2021). Clearly, it is important to assess whether the time of SARS-CoV-2 vaccination impacts host antibody responses.
In the UK, healthcare workers were identified as a priority group to receive SARS-CoV-2 vaccine starting in December 2020. At this time, the Alpha B.1.1.7 variant was the dominant circulating strain. As part of this initiative, data were collected on all asymptomatic staff members (Eyre et al., 2021; Lumley et al., 2021) in keeping with enhanced hospital infection prevention and control guidelines issued by the UK Department of Health and Social Care. Anonymised data were obtained from the Infections in Oxfordshire Research Database with Research Ethics Committee approvals (19/SC/0403, ECC5-017(A)/2009). Peripheral blood samples were collected during Dec 2020–Feb 2021 and were tested for anti-Spike (Abbott IgG assay) (Ainsworth et al., 2020) and anti-nucleocapsid (Abbott SARS-CoV-2 IgG anti-nucleocapsid assay) antibody levels. We analysed anti-Spike responses during the 2–10 weeks after vaccination. In this data set, 2190 people contributed one blood sample, 549 contributed two samples and 45 three or more samples (total of 3425 samples). Participants with evidence of prior SARS-CoV-2 infection (PCR for viral RNA or anti-nucleocapsid antibody), samples with anti-Spike responses <50 AU, and samples obtained after second vaccination were excluded.
Data from 2784 participants (Table 1A) were analyzed using linear mixed modelling to investigate the effects of time of vaccination on anti-Spike antibody levels. Variation between participants was modelled with fixed factors of time-of-day of vaccination (Time 1, 07:00–10:59; Time 2, 11:00–14:59; Time 3, 15:00–21:59) (Supplemental Figure 1), vaccine type (Pfizer, mRNA bnt162b2 or AstraZeneca, Adenoviral AZD1222), age group (16–29, 30–39, 40–49 or 50–74 years), sex, and the number of days post-vaccination. A B-spline transformation of days post-vaccination was used to model the non-linear pattern of anti-Spike responses (log10 transformed) (Supplemental Figure 2). This analysis allowed us to estimate the average anti-Spike levels in each participant group at 2 and 6 weeks post-vaccination (Figure 1).
Table 1A.
Participant numbers
| Pfizer mRNA (Time 1/Time 2/Time 3) |
AstraZeneca Adenoviral (Time 1/Time 2/ Time 3) |
|||
|---|---|---|---|---|
| Age (years) | Female | Male | Female | Male |
| 16–29 | 90/143/163 | 18/26/26 | 39/54/53 | 11/12/10 |
| 30–39 | 100/146/149 | 30/46/40 | 38/44/34 | 10/7/8 |
| 40–49 | 120/160/170 | 17/36/42 | 43/56/43 | 8/11/8 |
| 50–74 | 127/152/199 | 24/26/38 | 68/52/59 | 7/4/7 |
Figure 1:
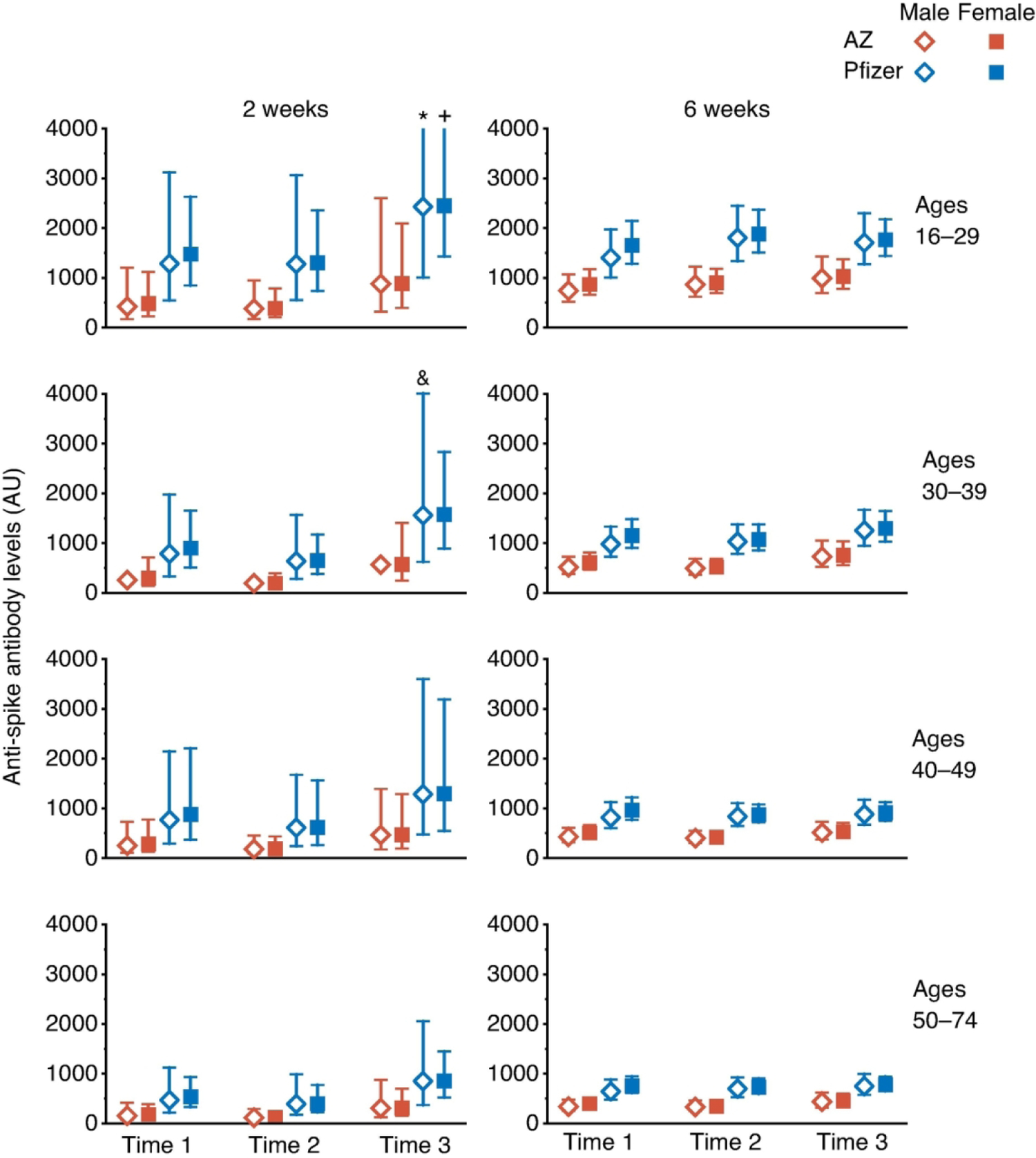
Estimated Anti-Spike antibody levels at 2 and 6 weeks after first SARS-CoV-2 vaccination, partitioned by age, sex, and time-of-day of vaccination (Time 1, 07:00–10:59; Time 2, 11:00–14:59; Time 3, 15:00–20:59). Mean value (symbol) with 95% confidence values (vertical line). Three confidence intervals extend beyond the Y-axis limits (* = 4275, + = 5996 and & = 4028).
Using a linear mixed-model approach, we found that anti-Spike responses were higher in those who were vaccinated later in the day (p=0.013), in those who received the Pfizer mRNA vaccine (p<0.0001), in women (p=0.013) and in younger participants (p<0.0001) (Table 1B). We observed significant interactions between days post-vaccination and vaccine type (p<0.0001) and age (p=0.032), but not with vaccine time (p=0.238). Analysing the data using two time intervals (before or after 1 pm) gave similar results. We did not observe a significant effect of time of day of sample collection (using the same time intervals as for vaccination times) (p=0.097), and this parameter was not included in the final model; results from the model including sample times are shown in Supplemental Table 1. Sixty seven samples gave values beneath the cut-off (<50) in the anti-Spike assay and were classified as “non-responders”, we found no significant association with the time-of-day of vaccination for these samples (linear mixed-effects logistic regression, p=0.23).
Table 1B.
Type III tests of fixed effects from mixed effects model
| Effect | Num DF | F Value† | Probability |
|---|---|---|---|
| Main Effects | |||
| Vaccination Time (Time 2, Time 3 vs. Time 1) ‡ |
2 | 4.33 | 0.0133 |
| Vaccine type (AstraZeneca vs. Pfizer) |
1 | 148.31 | <0.0001 |
| Age (30–39, 40–49, 50–74 vs.16–29) |
3 | 51.15 | <0.0001 |
| Sex (Female vs. Male) |
1 | 6.16 | 0.0131 |
| Days post-vaccination | 6 | 18.78 | <0.0001 |
| Interaction terms | |||
| Days*Vaccination_Time | 12 | 1.26 | 0.2380 |
| Days*Vaccine type | 6 | 7.24 | <0.0001 |
| Days*Age | 18 | 1.70 | 0.0319 |
| Days*sex | 6 | 1.03 | 0.4010 |
| Vaccination_Time*Vaccine type | 2 | 1.22 | 0.2945 |
| Vaccination_Time*Age | 6 | 0.71 | 0.6446 |
| Vaccination_Time*Sex | 2 | 0.44 | 0.6412 |
Details of the linear mixed modeling are: Time of vaccination (Time 1, 07:00–10:59; Time 2, 11:00–14:59; Time 3, 15:00–21:59), vaccine type (Pfizer mRNA or AstraZeneca Adenovirus), age groups (from Table 1A), sex, and days postvaccination were treated as fixed factors. A B-spline transformation of days postvaccination was used to model the non-linear pattern of anti-Spike antibody responses (log10 transformed) post vaccination.
DF= Degrees of Freedom.
For all F tests the denominator DF was 3359.
For each F test, the fixed effect referent is the last term shown, the F and P values are the Type III tests of overall fixed effects.
Our analysis of 2784 healthcare workers reveals a significant effect of the time of vaccination on anti-Spike antibody levels following the administration of two alternative SARS-CoV-2 vaccines (mRNA or Adenovirus based). A recent report studying a small cohort of healthcare workers immunised with an inactivated SARS-CoV-2 vaccine in the morning (09:00–11:00,n=33) or afternoon (15:00–17:00, n=30) showed increased B-cell responses and anti-Spike antibodies in participants vaccinated in the morning (Zhang et al., 2021). This contrasts with our observations and may reflect the use of an inactivated whole virus immunogen that will likely induce polytypic responses to a range of SARS-CoV-2 encoded proteins. Our observation contrasts with earlier studies in elderly men that reported higher anti-influenza titers in the morning (Phillips et al., 2008; Long et al., 2016). This may reflect differences between the cohorts studied, particularly with regard to immune status; we studied seronegative participants whereas responses to influenza vaccination will involve the stimulation of memory responses. Sample collection time in this study showed no significant association with anti-Spike levels, in contrast to previous reports (Kurupati et al., 2017; McNaughton et al., 2021). These data highlight the importance of recording the time of vaccination in clinical and research studies, and highlight the importance of considering time-of-day factors in future study designs that may reduce inter-individual variance and the number of participants needed to obtain statistical significance.
Additional studies are warranted to evaluate the circadian regulation of natural and vaccine-induced SARS-CoV-2 immunity. McNaughton and colleagues reported a diurnal variation in SARS-CoV-2 PCR test results, showing a 2-fold variation in Ct values implying higher viral RNA levels in the afternoon (McNaughton et al., 2021). These data are consistent with our recent study showing a role for the circadian component BMAL1 in regulating SARS-CoV-2 replication (Zhuang et al., 2021) that could influence the induction of host innate and adaptive responses.
It is worth noting that, despite the significant differences in anti-Spike levels detected in participants receiving Pfizer mRNA or AstraZeneca Adenoviral vaccines, both show comparable efficacies highlighting the robust nature of the host antibody response. Limitations of this retrospective observational study include: (i) relatively few participants had more than one anti-Spike antibody measurement, limiting our ability to study both longitudinal immune responses and the effect of time-of-day of sample collection; (ii) the health profiles of our healthcare workers may differ from the general population and no information was available on their medical or medication history, except that they had no prior infection with SARS-CoV-2 and were seronegative; (iii) there was limited serological sampling following second vaccination, precluding the analysis of time-of-day effects following a two-dose schedule; (iv) the extent to which anti-Spike levels are a correlate of clinical efficacy is not known; (v) the sleep and shift-work patterns of the participants, that are known to influence vaccine responses (Spiegel et al., 2002; Lange et al., 2003; Prather et al., 2021), were not available; and (vi) our cohort does not include children or high-risk groups, such as the elderly or immunocompromised. We recommend future studies address these limitations when documenting natural and vaccine-induced SARS-CoV-2 immune responses.
Supplementary Material
Acknowledgements:
We thank Helene Borrmann, Xiaodong Zhuang and Tanya Wilson for early discussions on this project; Tess Lambe and Merryn Voysey for their constructive comments on the paper. We also thank all OUH staff who participated in the staff testing program and the staff and medical students who ran the program. This work uses data provided by healthcare workers and collected by the UK’s National Health Service as part of their care and support. We thank all the people of Oxfordshire who contributed to the Infections in Oxfordshire Research Database. Research Database Team: L Butcher, H Boseley, C Crichton, O Freeman, J Gearing (community), R Harrington, M Landray, A Pal, TEA Peto, TP Quan, J Robinson (community), J Sellors, B Shine, AS Walker, D Waller. Patient and Public Panel: G Blower, C Mancey, P McLoughlin and B Nichols.
Support:
EBK is funded by NIH K24-HL105664, P01-AG009975 and R01-HL128538. JAM is funded by a Wellcome Investigator Award 200838/Z/16/Z, UK Medical Research Council project grant MR/R022011/1 and Chinese Academy of Medical Sciences Innovation Fund for Medical Science, China (grant number: 2018-I2M-2-002). WW is funded by NCATS Harvard Clinical and Translational Science Center grant 5UL1TR002541-02. DWE is a Robertson Foundation Fellow. DCA is funded by the NIHR Oxford Biomedical Research Centre. The report presents independent research. The views expressed in this publication are those of the authors and not necessarily those of the NHS, NIHR, or the UK Department of Health. PCM is funded by a Wellcome intermediate fellowship grant Ref 110110/Z/15/Z.
Disclosures:
WW has a consultancy for the National Sleep Foundation. PB has no relevant disclosures. DWE declares lecture fees from Gilead, outside the submitted work. EBK received travel support from Gordon Research Conference, Sleep Research Society, Santa Fe institute, DGSM (German Sleep Society); consultancy for Circadian Therapeutics, National Sleep Foundation, Puerto Rico Science Technology Trust, Sanofi-Genzyme; partner owns Chronsulting. JAM has no relevant disclosures.
References
- Ainsworth M, Andersson M, Auckland K, Baillie JK, Barnes E, Beer S, Beveridge A, Bibi S, Blackwell L, Borak M, Bown A, Brooks T, Burgess-Brown NA, Camara S, Catton M, Chau KK, Christott T, Clutterbuck E, Coker J, Cornall RJ, Cox S, Crawford-Jones D, Crook DW, D’Arcangelo S, Dejnirattsai W, Dequaire JMM, Dimitriadis S, Dingle KE, Doherty G, Dold C, Dong T, Dunachie SJ, Ebner D, Emmenegger M, Espinosa A, Eyre DW, Fairhead R, Fassih S, Feehily C, Felle S, Fernandez-Cid A, Fernandez Mendoza M, Foord TH, Fordwoh T, Fox McKee D, Frater J, Gallardo Sanchez V, Gent N, Georgiou D, Groves CJ, Hallis B, Hammond PM, Hatch SB, Harvala HJ, Hill J, Hoosdally SJ, Horsington B, Howarth A, James T, Jeffery K, Jones E, Justice A, Karpe F, Kavanagh J, Kim DS, Kirton R, Klenerman P, Knight JC, Koukouflis L, Kwok A, Leuschner U, Levin R, Linder A, Lockett T, Lumley SF, Marinou S, Marsden BD, Martinez J, Martins Ferreira L, Mason L, Matthews PC, Mentzer AJ, Mobbs A, Mongkolsapaya J, Morrow J, Mukhopadhyay SMM, Neville MJ, Oakley S, Oliveira M, Otter A, Paddon K, Pascoe J, Peng Y, Perez E, Perumal PK, Peto TEA, Pickford H, Ploeg RJ, Pollard AJ, Richardson A, Ritter TG, Roberts DJ, Rodger G, Rollier CS, Rowe C, Rudkin JK, Screaton G, Semple MG, Sienkiewicz A, Silva-Reyes L, Skelly DT, Sobrino Diaz A, Stafford L, Stockdale L, Stoesser N, Street T, Stuart DI, Sweed A, Taylor A, Thraves H, Tsang HP, Verheul MK, Vipond R, Walker TM, Wareing S, Warren Y, Wells C, Wilson C, Withycombe K, and Young RK (2020) Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. The Lancet Infectious Diseases 20:1390–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allada R, and Bass J (2021) Circadian Mechanisms in Medicine. N Engl J Med 384:550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RS, Stangherlin A, Nagy AD, Nicoll MP, Efstathiou S, O’Neill JS, and Reddy AB (2016) Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Proceedings of the National Academy of Sciences of the United States of America 113:10085–10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers A, Xie W, Agapov E, Brown S, Steinberg D, Tidwell R, Sajol G, Schutz R, Weaver R, Yu H, Castro M, Bacharier LB, Wang X, Holtzman MJ, and Haspel JA (2018) BMAL1 links the circadian clock to viral airway pathology and asthma phenotypes. Mucosal Immunol 11:97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre DW, Lumley SF, Wei J, Cox S, James T, Justice A, Jesuthasan G, O’Donnell D, Howarth A, Hatch SB, Marsden BD, Jones EY, Stuart DI, Ebner D, Hoosdally S, Crook DW, Peto TEA, Walker TM, Stoesser NE, Matthews PC, Pouwels KB, Walker AS, and Jeffery K (2021) Quantitative SARS-CoV-2 anti-spike responses to Pfizer-BioNTech and Oxford-AstraZeneca vaccines by previous infection status. Clin Microbiol Infect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince LM, Zhang Z, Beesley S, Vonslow RM, Saer BR, Matthews LC, Begley N, Gibbs JE, Ray DW, and Loudon ASI (2019) Circadian variation in pulmonary inflammatory responses is independent of rhythmic glucocorticoid signaling in airway epithelial cells. FASEB J 33:126–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurupati RK, Kossenkoff A, Kannan S, Haut LH, Doyle S, Yin X, Schmader KE, Liu Q, Showe L, and Ertl HCJ (2017) The effect of timing of influenza vaccination and sample collection on antibody titers and responses in the aged. Vaccine 35:3700–3708. [DOI] [PubMed] [Google Scholar]
- Lange T, Perras B, Fehm HL, and Born J (2003) Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom Med 65:831–835. [DOI] [PubMed] [Google Scholar]
- Long JE, Drayson MT, Taylor AE, Toellner KM, Lord JM, and Phillips AC (2016) Morning vaccination enhances antibody response over afternoon vaccination: A cluster-randomised trial. Vaccine 34:2679–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley SF, Wei J, O’Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB, Marsden BD, Cox S, James T, Peck LJ, Ritter TG, de Toledo Z, Cornall RJ, Jones EY, Stuart DI, Screaton G, Ebner D, Hoosdally S, Crook DW, Conlon CP, Pouwels KB, Walker AS, Peto TEA, Walker TM, Jeffery K, Eyre DW, and Oxford University Hospitals Staff Testing G (2021) The duration, dynamics and determinants of SARS-CoV-2 antibody responses in individual healthcare workers. Clin Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidstone R, Anderson SG, Ray DW, Rutter MK, Durrington HJ, and Blaikley JF (2021) Shift work is associated with positive COVID-19 status in hospitalised patients. Thorax 76:601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton CD, Adams NM, Johnson CH, Ward MJ, and Lasko TA (2021) Diurnal variation in SARS-CoV-2 PCR test results: Test accuracy may vary by time of day. MedRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AC, Gallagher S, Carroll D, and Drayson M (2008) Preliminary evidence that morning vaccination is associated with an enhanced antibody response in men. Psychophysiology 45:663–666. [DOI] [PubMed] [Google Scholar]
- Prather AA, Pressman SD, Miller GE, and Cohen S (2021) Temporal Links Between Self-Reported Sleep and Antibody Responses to the Influenza Vaccine. Int J Behav Med 28:151–158. [DOI] [PubMed] [Google Scholar]
- Ray S, and Reddy AB (2020) COVID-19 management in light of the circadian clock. Nat Rev Mol Cell Biol 21:494–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Ince L, Sartor F, Borrmann H, Zhuang X, Naik A, Curtis A, and McKeating JA (2021) Clocks, Viruses, and Immunity: Lessons for the COVID-19 Pandemic. J Biol Rhythms 36:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Tang SY, Devine JC, Anderson ST, Nayak S, Zhang SL, Valenzuela A, Fisher DG, Grant GR, Lopez CB, and FitzGerald GA (2019) Circadian control of lung inflammation in influenza infection. Nature communications 10:4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel K, Sheridan JF, and Van Cauter E (2002) Effect of sleep deprivation on response to immunization. JAMA 288:1471–1472. [DOI] [PubMed] [Google Scholar]
- Wyse C, O’Malley G, Coogan AN, McConkey S, and Smith DJ (2021) Seasonal and daytime variation in multiple immune parameters in humans: Evidence from 329,261 participants of the UK Biobank cohort. iScience 24:102255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Liu Y, Liu D, Zeng Q, Li L, Zhou Q, Li M, Mei J, Yang N, Mo S, Liu Q, Liu M, Peng S, and Xiao H (2021) Time of day influences immune response to an inactivated vaccine against SARS-CoV-2. Cell Res. 31(11):1215–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Tsukuda S, Wrensch F, Wing PA, Schilling M, Harris JM, Borrmann H, Morgan SB, Cane JL, Mailly L, Thakur N, Conceicao C, Sanghani H, Heydmann L, Bach C, Ashton A, Walsh S, Tan TK, Schimanski L, Huang KA, Schuster C, Watashi K, Hinks TS, Jagannath A, Vausdevan SR, Bailey D, Baumert TF, and McKeating JA (2021) The circadian clock component BMAL1 regulates SARS-CoV-2 entry and replication in lung epithelial cells. iScience in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


