Abstract
Background:
Though many women report sexual arousal difficulties, the mechanisms driving these difficulties are unclear. Sexual response relies on a host of psychophysiological processes that have bidirectional relationships with inflammation. Additionally, chronic inflammation may impair genital blood flow, which in turn may impact sexual arousal. C-reactive protein (CRP) is an acute-phase marker of inflammation produced in response to cytokine signaling throughout the body, which makes it a useful marker of systemic inflammation.
Aim:
The present study examined interactions between inflammation and women’s sexual arousal.
Methods:
CRP, self-reported frequency of partnered sexual activity, and subjective and vaginal arousal were assessed in 91 healthy, pre-menopausal women. Data were collected during a single laboratory session.
Main Outcome Measures:
Subjective sexual arousal and vaginal pulse amplitude (a measure of vaginal arousal) were the main outcome measures.
Results:
Change in subjective sexual arousal in response to a sexual film was unaffected by baseline CRP and sexual frequency. However, there were significant interactions between inflammation and sexual frequency in predicting vaginal arousal during the sexual film. Among women reporting more frequent sexual activity, higher CRP predicted lower magnitude arousal response and longer time to maximum vaginal arousal. Among women reporting less frequent sex, higher CRP predicted shorter time to maximum arousal and greater magnitude of arousal response. Controlling for cortisol strengthened the effects seen for time to maximum vaginal arousal but weakened those observed for percent change.
Conclusions:
Among healthy young women, higher CRP may be associated with vaginal arousal, but not subjective sexual arousal. Specifically, our results suggest that higher baseline CRP is associated with lower genital sexual arousal for women who have sex frequently, which is consistent with clinical evidence that elevated inflammation can be detrimental to sexual function.
Keywords: inflammation, sexual arousal, sexual function, women, C-reactive protein
Introduction
Low sexual arousal is among the most commonly reported sexual concerns among women* (McCabe et al., 2016). Despite apparent associations between women’s sexual functioning and general physical health (Di Stasi et al., 2019), existing research in this area has been largely descriptive rather than mechanistic. Inflammation underlies many chronic illnesses as well as aspects of sexual response, including motivation for sexual activity, cardiovascular function, and potentially genital arousal (Avitsur et al., 1997; Yao et al., 2012). The present study extended prior work showing inflammation as a mechanism by which the immune and reproductive systems reciprocally influence each other by examining interactions between inflammatory markers and women’s sexual arousal.
Broadly, inflammation is an adaptive response from a group of immune processes meant to protect the body from toxins and pathogens and stimulate tissue repair and rebuilding. Acute inflammation typically calls on stimulating factors (such as cytokines) that fight harmful agents and rapidly remove themselves once their job is done (Feghali & Wright, 1997; Murphy & Weaver, 2016).Though advantageous in the short-term, prolonged exposure to inflammatory agents (e.g., during chronic stress) can become harmful, particularly for cardiovascular (Ridker, 2001) and psychological health (Pariante, 2017).
C-reactive protein (CRP) may be a useful index of chronic inflammation relevant to the psychophysiological processes that support sexual arousal. CRP is an acute-phase protein that supports inflammatory processes (e.g., signaling to other immune agents to dispose of dead cells; Sproston & Ashworth, 2018). CRP is produced by the liver in response to signals from cytokines throughout the body, which makes CRP a useful measure of systemic inflammation, as it allows researchers to capture information across multiple cytokine systems (Ridker, 2001). CRP is also a downstream marker and thus particularly helpful for indexing chronic inflammation, as individual cytokines may act acutely and dissipate while CRP remains elevated (Pepys & Hirschfield, 2003). Usefully, salivary CRP is correlated with blood measures (Out et al., 2012) allowing for minimally invasive measurement of inflammation.
Clinically, CRP has been suggested as a prognostic for cardiovascular health (Ford et al., 2004) because increased inflammation contributes to arterial plaque buildup (Libby, 2012). Chronic inflammation restricts blood flow by reducing appropriate blood vessel dilation (Libby, 2012)– a phenomenon that notably interferes with genital blood flow. The link between erectile dysfunction and chronic inflammation is so well established that the American College of Cardiology recommends routine assessment of men’s sexual functioning to identify cardiovascular disease risk (Uddin et al., 2018) Accordingly, CRP levels correlate positively with the severity of penile arterial disease and erectile dysfunction, and men with erectile dysfunction have higher levels of CRP compared to men without such conditions (Bank et al., 2003). Elevated levels of CRP have also been found to predict erectile dysfunction in relatively healthy samples of men (Yao et al., 2012).
There is similar reason to suspect that CRP may capture aspects of inflammation relevant to women’s sexual functioning (Lorenz, 2019; Maseroli et al., 2018). Sexual arousal refers to the acute mental and physical state arising from sexual stimulation, which involves physiologic changes resulting from activation of the central nervous system (Georgiadis et al., 2012), autonomic nervous system (Ågmo, 2011), and endocrine system (Hamilton et al., 2008). These changes include increased heart rate, blood pressure, and vaginal engorgement and lubrication (Heiman & Pfaff, 2011). Conditions associated with chronic inflammation and poor cardiovascular functioning may lead to disrupted blood flow to the genitals, negatively impacting sexual arousal in a similar fashion to the poor blood flow noted in cardiovascular disease (Libby, 2012; Steinke, 2010). Such changes in the arousal process may or may not negatively impact sexual functioning through their effects on behavior and subjective sexual satisfaction or distress. Further support for a bidirectional relationship between women’s sexuality and immune function comes from reports that partnered sexual activity is associated with lower levels of inflammatory markers, particularly during fertile phases of the menstrual cycle (Lorenz & van Anders, 2014; Lorenz et al., 2015a, 2015b, 2017a; 2017b; 2018).
However, existing literature on immune activity and women’s sexual function presents several gaps. First, most investigations into the mechanisms linking the immune system and sexual response exclusively use male subjects (animals or humans) despite evidence that many of these mechanisms would also be relevant in females. Moreover, these studies have conflated experiences such as sexual arousal and orgasm (e.g., Haake et al., 2004). Similarly, studies that have investigated inflammation and sexual arousal have generally not distinguished subjective and genital arousal measures, despite evidence that these may be quite distinct in women (Rellini et al., 2005). While inflammation may also contribute to subjective arousal (e.g., via interactions with neural centers responsible for reward processing (Moriarity et al., 2020) as well as genital arousal, the mechanisms underlying these effects are likely very different. To that end, there is also limited work directly examining the effects of inflammation on vaginal sexual arousal in humans, although some work in animal models suggests that inflammatory responses predict poorer clitoral and vaginal blood flow (see Maseroli et al., 2018 for review). Finally, research on immune system activity and sexual response in women have almost exclusively focused on women with pre-existing conditions (e.g., Esposito et al., 2005; Maiorino et al., 2020), with little information from relatively healthy female populations.
What little work has investigated inflammation and women’s sexual response has not considered relevant covariates such as frequency of sexual activity or cortisol. Partnered sexual activity appears associated with and may mediate reproductively relevant immune trade-offs in women (Lorenz et al., 2015a, 2015b, 2017a, 2017b). For example, while women reporting high frequencies of partnered sexual activity show significant decreases in immunoglobulin A (a mucosal antibody) as well as interleukin-6 and interferon-γ (pro-inflammatory cytokines) from menses to ovulation, women reporting low frequencies of sexual activity show the opposite pattern (i.e., increased production of IgA, IL-6, and IFNγ at ovulation; (Lorenz et al., 2014, 2015a, 2015b, 2017a, 2017b). These findings suggest that frequency of partnered sexual activity is a critical variable to consider in understanding interactions between women’s immune function and their sexual response. Similarly, few studies in this area have accounted for the role that cortisol plays in regulating women’s inflammatory and sexual responses, despite evidence that cortisol may both directly impact women’s sexual arousal (Exton et al., 2000; Hamilton et al., 2008; Alley et al., 2020) and regulate acute inflammatory processes (Fantidis, 2010; Yeager et al., 2011).
The present analysis adds to this literature investigating the link between inflammation and sexual response in healthy women by examining CRP, subjective sexual arousal, and objective measures of physiological sexual response. Participants provided subjective ratings of sexual arousal before and after watching a sexually explicit film, during which genital blood flow was recorded with vaginal photoplethysmography. We hypothesized that baseline inflammation would predict state changes in both subjective sexual response and physiological arousal. Given that sexual activity has been suggested to mediate reproductive immune system tradeoffs in healthy women, we further hypothesized that relationships between baseline CRP and sexual arousal would be more pronounced among women reporting greater frequency of sexual activity.
Methods
Data for the present study came from a study conducted across two sites in the Midwestern US (n = 51) and the Southeastern US (n = 40) using similar methods and materials. Procedures were approved by the Institutional Review Boards at Indiana University and the University of North Carolina at Charlotte and all participants provided written informed consent.
Participants
Participants were healthy, premenopausal females (n = 91) recruited via physical and online community boards within their respective communities, social media advertisements, and online course-credit pools from two universities. Eligibility screens were conducted over the phone or online. Participants had to report some history of experience with visual sexual stimuli and specifically, ability to become aroused while viewing heterosexual sexual stimuli. Participants also had to endorse sexual activity with a partner within the past month. Additionally, participants were excluded based upon chronic health conditions, recreational drug use within the past month, and use of medications known to impact either sexual or immune function (e.g., psychotropics, antibiotics). Sample demographics are presented in Table 1. A portion of our sample was using hormonal contraception at the time of their study session (n = 22).
Table 1.
Demographic characteristics of the study sample.
| Mean (M) | St. Deviation (SD) | |
| Continuous variables | ||
|
| ||
| Age in years | 23.33 | 5.09 |
|
| ||
| Body mass index (BMI) | 25.95 | 6.91 |
|
| ||
| Lifetime number of vaginal sex partners | 5.12 | 3.54 |
| Number (N) | Percent (%) | |
| Race/ethnicity | ||
|
| ||
| European American/White | 41 | 45.1 |
|
| ||
| African American/Black | 17 | 18.7 |
|
| ||
| Asian/Pacific Islander | 12 | 13.2 |
|
| ||
| Latinx/Hispanic | 2 | 2.2 |
|
| ||
| Middle Eastern | 2 | 2.2 |
|
| ||
| Multiracial/Other | 8 | 8.8 |
| Sexual orientation | ||
|
| ||
| Heterosexual (0 – 12.5) | 32 | 35.2 |
|
| ||
| Mostly heterosexual (12.6 – 37.5) | 34 | 37.4 |
|
| ||
| Bisexual (37.6 – 62.5) | 14 | 15.4 |
|
| ||
| Mostly homosexual (62.6 – 87.5) | 4 | 4.4 |
|
| ||
| Homosexual (87.6 – 100) | 3 | 3.3 |
| Gender of current sexual partner | ||
|
| ||
| Male | 78 | 85.7 |
|
| ||
| Female | 7 | 7.7 |
| Typical partnered sexual frequency | ||
|
| ||
| Never/less than monthly | 2 | 2.2 |
|
| ||
| Less than weekly | 18 | 19.8 |
|
| ||
| 1–2 times per week | 34 | 37.4 |
|
| ||
| 3–4 times per week | 24 | 26.4 |
|
| ||
| Daily/near daily | 7 | 7.7 |
|
| ||
| More than daily | 2 | 2.2 |
Note: Participants reported their sexual orientation as a number between 0 and 100, where 0 was labeled “only heterosexual,” 25 labeled “mostly heterosexual,” 50 labeled “bisexual,” 75 labeled “mostly homosexual,” and 100 labeled “only homosexual.” For descriptive purposes, these numbers were converted to bins with value ranges displayed in the table above.
Though the number of participants included across analyses varied as a function of data completeness (with some participants missing data on individual variables, see below), linear multiple regression estimates in G*Power (Franz et al., 2007) indicated that 40 participants would be sufficient to capture small to medium effect sizes (f2 = 0.02 – 0.15) with 80% power. Incomplete data were mostly attributable to equipment malfunction (n = 7) during laboratory sessions and potential urinary tract infection (UTI; n = 8) as identified by urine test at the time of the study session. Notably, no missing data were due to participants withdrawing after providing baseline measures.
Procedure
Participants were asked to come to the laboratory session during their self-reported luteal phase of their menstrual cycle. To limit the influence of transient elevations in testosterone and cortisol on sexual arousal and biomarker data, all sessions were conducted in the afternoons and participants were instructed to refrain from sexual or other rigorous physical activity for 24 hours before the study session. Upon arrival, participants were greeted and led to a private room within the laboratory. The researcher outlined the study protocol in full, allowing time for questions before leaving participants to read over the informed consent document in private. Once participants agreed to take part in the study, they were escorted to the bathroom to complete a urine sample to test for pregnancy and UTI using commercially available testing strips. Because pregnancy and UTIs greatly influence hormonal and immune activation, if the test strip indicated possible pregnancy or UTI, participants were either asked to reschedule or compensated for their time and sent home.
Participants’ weight and height were recorded and then they were given privacy in the exam room to complete the saliva sample and first film scale. All participants were instructed on how to collect saliva via passive drool, and completion times were recorded. Participants were left alone in a private room and instructed via intercom to fit the vaginal photoplethysmograph (VPP, see below for details). After watching both the neutral and sexual stimulus videos, participants completed the film scale again and provided another saliva sample while they completed additional surveys. At the end of the study session, community participants were compensated either $20 in cash and/or given a $20 Amazon gift card while students were compensated with course credit for their time.
Instruments & Methods
Salivary CRP and cortisol.
Saliva samples were collected via passive drool into polypropylene tubes. Participants were asked to not eat, drink, or chew gum for an hour before their study session. All samples were frozen immediately after collection and stored at −80°C until day of assay. For the present analyses, saliva samples were assayed for CRP and cortisol using commercially available enzyme-linked immunosorbent assay (ELISA) kits. All assays were conducted according to manufacturer procedural recommendations (Salimetrics LLC). Inter-assay and intra-assay coefficients of variance were within acceptable ranges (CRP = 4.9 – 12.7%; cortisol = 3.07 – 11.6%). Serum CRP values of below 1mg/L are considered low and healthy, 1 – 3 mg/L moderately elevated, and >3mg/L significantly elevated (Pearson et al., 2003); the equivalent cutoffs in saliva have been validated at > 645pg/mL, 645 – 1629 pg/mL, and > 1629 pg/mL, respectively (Ouellet-Morin et al., 2011). Using these metrics, our sample included 85% low and 15% moderately elevated CRP values.
Participant characteristics.
In self-report measures, participants indicated their age and average frequency of vaginal, oral, and anal sex with a partner. For analyses, the maximum self-reported frequency across all three sex acts was categorized into one of six ordinal bins ranging from “never/less than monthly” to “more than daily.” Body mass index (BMI) was calculated from objective measures of height and weight taken during the laboratory session.
Audio-visual stimuli.
Segments from National Geographic and abbywinters.com (a women-oriented erotic film site) were chosen as the neutral and erotic videos, respectively. The neutral video (3 minutes) was presented first and included scenes of humans engaging with nature (e.g., walking through the woods, kayaking), followed by the erotic video (7 minutes), which included scenes of a heterosexual couple engaging in foreplay, oral sex, and vaginal intercourse, which is a well-validated stimuli structure for inducing sexual arousal (Velten et al., 2018).
Adapted Tape-Film Scale.
Before and after the video stimuli, participants completed a 40-item survey assessing a variety of subjective states (Heiman & Rowland, 1983; Heard-Davison et al., 2007). Items asked about emotional reactions (e.g., positive and negative affect) and physiological sensations (e.g., heart and breathing rates) on a 7-point scale ranging from “not at all” to “intensely.” For the purposes of this analysis, only changes in pre- to post-film sexual arousal was considered.
Genital sexual arousal.
Vaginal pulse amplitude (VPA) was used as a measure of vaginal arousal via VPP. The VPP indexes vaginal blood flow, which makes this measurement particularly useful for the purposes of characterizing the association between physical sexual arousal and inflammation. Further, the VPP is currently the gold-standard genital blood flow arousal measurement tool in women (Kukkonen, 2015). The VPP is a 4.75 cm x 1.25 cm plastic tube encasing with an orange-red spectrum light source (BIOPAC Systems Inc., Goleta, California). Signal was recorded at 200 Hz and band-pass filtered (0.5 – 30 Hz) for the duration of both neutral and erotic audio-visual stimuli. Data were collected with BioPac transducers and amplifiers, then processed with AcqKnowledge version 4.4 (BIOPAC Systems Inc.). VPA signal was reviewed and cleaned by trained research assistants according to standard protocols (Clephane & Lorenz, 2021) to remove movement artifacts prior to analyses.
Two primary metrics were computed for each participant for inclusion in analyses: time to maximum VPA signal, with smaller values indicating shorter latency to that individual participant’s peak blood flow during the erotic video, and percent change in VPA signal from the neutral to erotic videos, with greater values indicating a larger increase in blood flow. These metrics provide insight into both arousability and magnitude of arousal, respectively. Average latency to peak arousal was 4.364 minutes (SD = 1.683). Excluding outliers, the average percent change was 134.535% (SD = 104.927). However, the present analyses were conducting including all participants’ data, such that the average percent change was 179.592% (SD = 202.595) overall.
Statistics
Across all analyses, we statistically controlled for age and BMI as these covariates are known to interact with immune and/or sexual function (Roubenoff et al., 1998; Rowland et al., 2017; Rea et al., 2018; Madssen et al., 2019). In addition, given the possible anti-inflammatory influence of glucocorticoids, we tested models for robustness by conducting analyses with and without salivary cortisol as an additional predictor (controlling for time since waking). Lastly, preliminary analyses revealed high outlier values for CRP (n = 4) that were unresolved by log-transformations or winsorizing. Thus, following other studies of inflammation markers in healthy women (e.g., Mu et al., 2018), we binned the continuous CRP values into quintiles.
We first explored bivariate correlations between all predictors, covariates, and outcomes of interest. To test our hypotheses, we assessed if baseline CRP and sexual frequency interacted to predict responses to watching the sexual film. We conducted mixed linear models to assess change in subjective sexual arousal from pre- to post-film, entering time point as a repeated measure and specifying a diagonal covariance structure. Fit was determined by Akaike information criterion (AIC). To test genital responses to the film, we conducted sequential regressions with all covariates entered at the first step, then genital arousal measures (either time to maximum or percent change in VPA) and their interaction with sexual frequency in the second step. All analyses were performed in IBM SPSS Statistics 27, adopting an α threshold of 0.05 for determining statistical significance.
Results
Associations across demographics
Exploratory correlations between all model inputs are displayed in Table 2. Greater body mass index (BMI) was associated with older age (r = 0.310, p = 0.004), higher baseline CRP concentrations (r = 0.546, p < 0.001), greater percent change in VPA (r = 0.323, p = 0.010), and higher subjective arousal ratings after watching the sexual film (r = 0.215, p = 0.046). Other covariates (i.e., age, salivary cortisol, time since waking) were unrelated to inflammation, sexual frequency, and arousal. While baseline CRP was not correlated with subjective or physiological arousal, more frequent partnered sexual activity was related to longer latency to maximum VPA (r = 0.352, p = 0.004). Finally, there was partial evidence for correspondence between subjective and physiological arousal via a positive correlation between post-film subjective arousal and time to maximum VPA (r = 0.298, p = 0.017) but not percent change in VPA.
Table 2.
Correlations between all variables included in models.
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | — |
|||||||||
| 2. Body mass index (BMI) |
.310
**
|
— |
||||||||
| 3. Salivary cortisol | −.042 |
.277 |
— |
|||||||
| 4. Time since waking | .191 |
−.079 |
−.194 |
— |
||||||
| 5. C-reactive protein (CRP) quintile | −.130 |
.546
**
|
.027 |
−.137 |
— |
|||||
| 6. Typical partnered sexual frequency | .014 |
.158 |
.115 |
−.100 |
.024 |
— |
||||
| 7. Time to maximum VPA | .027 |
.121 |
−.306 |
.094 |
−.068 |
.352
**
|
— |
|||
| 8. Percent change in VPA | .169 |
.323
**
|
.046 |
.173 |
−.027 |
.007 |
.028 |
— |
||
| 9. Pre-film subjective arousal | −.169 |
−.002 |
−.053 |
.023 |
.032 |
−.060 |
.113 |
−.163 |
— |
|
| 10. Post-film subjective arousal | .010 | .215 * | .058 | −.043 | −.009 | .151 | .298 * | .036 | .014 | — |
correlation is significant at p < 0.05
correlation is significant at p < 0.01
Based upon recent literature (Juster et al., 2016; Mays et al., 2018), we explored links between sexual orientation, hormonal contraceptive use, and inflammation. Bisexual/lesbian women in our sample were more likely to have higher baseline CRP than heterosexual or mostly heterosexual women, and this difference was marginally significant (χ2 = 9.385, p = 0.052). Relative to women not taking hormonal contraceptives, those currently using hormonal contraceptives fell into lower CRP quintiles (χ2 = 9.471, p = 0.050). However, given that some CRP quintiles had as few as 0, 1, or 2 women reporting a same-sex orientation or hormonal contraceptive use, this study was not adequately powered to add these demographic characteristics into subsequent regression models.
Hypothesis testing: Does inflammation predict women’s subjective arousal?
We first examined change in self-reported sexual arousal during a sexual film (Table 3). There was a significant main effect of time on subjective sexual arousal measures (F1,97 = 123.812, p < 0.001), whereby ratings of arousal increased from pre- to post-film (estimated fixed effect = 3.230, standard error = 0.290). There were no significant main or interaction effects of baseline CRP and sexual frequency in predicting change in subjective sexual arousal. These results were unchanged when rerunning the model with cortisol and time since waking included as covariates, though overall model fit was improved (AIC of model without cortisol = 344.405; AIC of model with cortisol and time since waking = 155.464) and a small main effect emerged whereby women who had been awake for longer exhibited smaller increases or no change in subjective sexual arousal while watching the film (F1,38 = 4.452, p = 0.041, estimated fixed effect ≈ −0.001, standard error < 0.001).
Table 3.
Mixed linear model predicting change in subjective sexual arousal while watching a sexual film from inflammation, sexual frequency, and relevant covariates.
| Predictor | F | Estimate | SE | t | p |
|
| |||||
| Age | 2.270 | −0.031 | 0.020 | −1.507 | 0.135 |
|
| |||||
| BMI | 1.095 | 0.027 | 0.026 | 1.047 | 0.298 |
|
| |||||
| Time point | 123.812 ** | 3.230 | 0.290 | 11.127 | < 0.001 |
|
| |||||
| Salivary CRP | 1.688 | −0.149 | 0.114 | −1.299 | 0.197 |
|
| |||||
| Sexual frequency | 0.903 | −0.159 | 0.167 | −0.950 | 0.344 |
|
| |||||
| Time × CRP × frequency | 1.583 | 0.041 | 0.033 | 1.258 | 0.211 |
| The same mixed linear model including cortisol and time since waking. | |||||
| Predictor | F | Estimate | SE | t | p |
|
| |||||
| Age | 0.752 | −0.030 | 0.035 | −0.867 | 0.391 |
|
| |||||
| BMI | 0.042 | 0.007 | 0.035 | 0.205 | 0.838 |
|
| |||||
| Salivary cortisol | 1.217 | −1.492 | 1.353 | −1.103 | 0.277 |
|
| |||||
| Time since waking | 4.452 * | < −0.001 | < 0.001 | −2.110 | 0.041 |
|
| |||||
| Time point | 74.632 ** | 3.881 | 0.449 | 8.639 | < 0.001 |
|
| |||||
| Salivary CRP | 0.189 | 0.080 | 0.184 | 0.434 | 0.666 |
|
| |||||
| Sexual frequency | 0.001 | −0.008 | 0.253 | −0.031 | 0.975 |
|
| |||||
| Time × CRP × frequency | 0.212 | −0.025 | 0.054 | −0.460 | 0.648 |
significant at p < 0.05
significant at p < 0.01
Hypothesis testing: Does inflammation predict women’s genital arousability?
We next examined predictors of genital arousal, as measured by the time it took for participants to reach their maximum VPA signal (Table 4). The model that included just covariates (age and BMI) was not significant (F2,43 = 0.305, p = 0.740, AIC = 50.157, adjusted R2 = −0.033). However, model fit improved when adding inflammation and sexual frequency as predictors at the second step (F5,43 = 4.613, p = 0.002, AIC = 35.937, adjusted R2 = 0.296). There was a significant interaction between CRP and sexual frequency in predicting time to maximum VPA (standardized β = 1.813, t5,43 = 3.733, p = 0.001). Higher CRP predicted longer time to maximum VPA for women reporting frequent sexual activity but shorter time to maximum VPA among women reporting less frequent sex (Figure 1). There was also a significant negative main effect of CRP (standardized β = −1.283, t5,43 = −3.808, p < 0.001) as well as partnered sexual frequency (standardized β = −1.043, t5,43 = −2.678, p = 0.011), such that higher CRP and more frequent sexual activity each independently predicted shorter latencies to maximum VPA. All effects were weakened to non-significance by repeating the regressions with baseline cortisol and time since waking included as covariates (full model F6,27 = 1.762, p = 0.156, AIC = 30.724, adjusted R2 = 0.145), although cortisol exhibited an independent main effect (standardized β = −0.448, t6, 27 = −2.302, p = 0.032) such that women with higher cortisol had shorter latencies to maximum VPA.
Table 4.
Sequential regressions predicting time to maximum VPA while watching a sexual film from inflammation, sexual frequency, and relevant covariates.
| Step | F | adjusted R2 | Predictor | β | t | p |
|
| ||||||
| 1 | 0.305 | −0.033 | Age | −0.011 | −0.069 | 0.945 |
|
| ||||||
| BMI | 0.124 | 0.762 | 0.451 | |||
|
| ||||||
| 2 | 4.613 | 0.296 | Age | 0.117 | 0.762 | 0.451 |
|
| ||||||
| BMI | 0.308 | 1.735 | 0.091 | |||
|
| ||||||
| Salivary CRP | −1.283 ** | −3.808 | < 0.001 | |||
|
| ||||||
| Sexual frequency | −1.043 * | −2.678 | 0.011 | |||
|
| ||||||
| CRP × frequency | 1.813 ** | 3.733 | 0.001 | |||
| The same sequential regressions including cortisol and time since waking. | ||||||
| Step | F | adjusted R2 | Predictor | β | t | p |
|
| ||||||
| 1 | 0.985 | −0.002 | Age | −0.077 | −0.367 | 0.717 |
|
| ||||||
| BMI | 0.251 | 1.173 | 0.253 | |||
|
| ||||||
| Salivary cortisol | −0.368 | −1.795 | 0.086 | |||
|
| ||||||
| Time since waking | 0.057 | 0.284 | 0.779 | |||
|
| ||||||
| 2 | 1.762 | 0.145 | Age | −0.178 | −0.847 | 0.406 |
|
| ||||||
| BMI | 0.416 | 1.595 | 0.126 | |||
|
| ||||||
| Salivary cortisol | −0.448 * | −2.302 | 0.032 | |||
|
| ||||||
| Time since waking | 0.067 | 0.360 | 0.722 | |||
|
| ||||||
| Salivary CRP | −0.306 | −1.292 | 0.210 | |||
|
| ||||||
| Sexual frequency | 0.354 | 1.947 | 0.065 | |||
|
| ||||||
| CRP × frequency | — | — | — | |||
significant at p < 0.05
significant at p < 0.01
Figure 1. Time to maximum vaginal pulse amplitude (VPA) in minutes as predicted by C-reactive protein and sexual frequency.
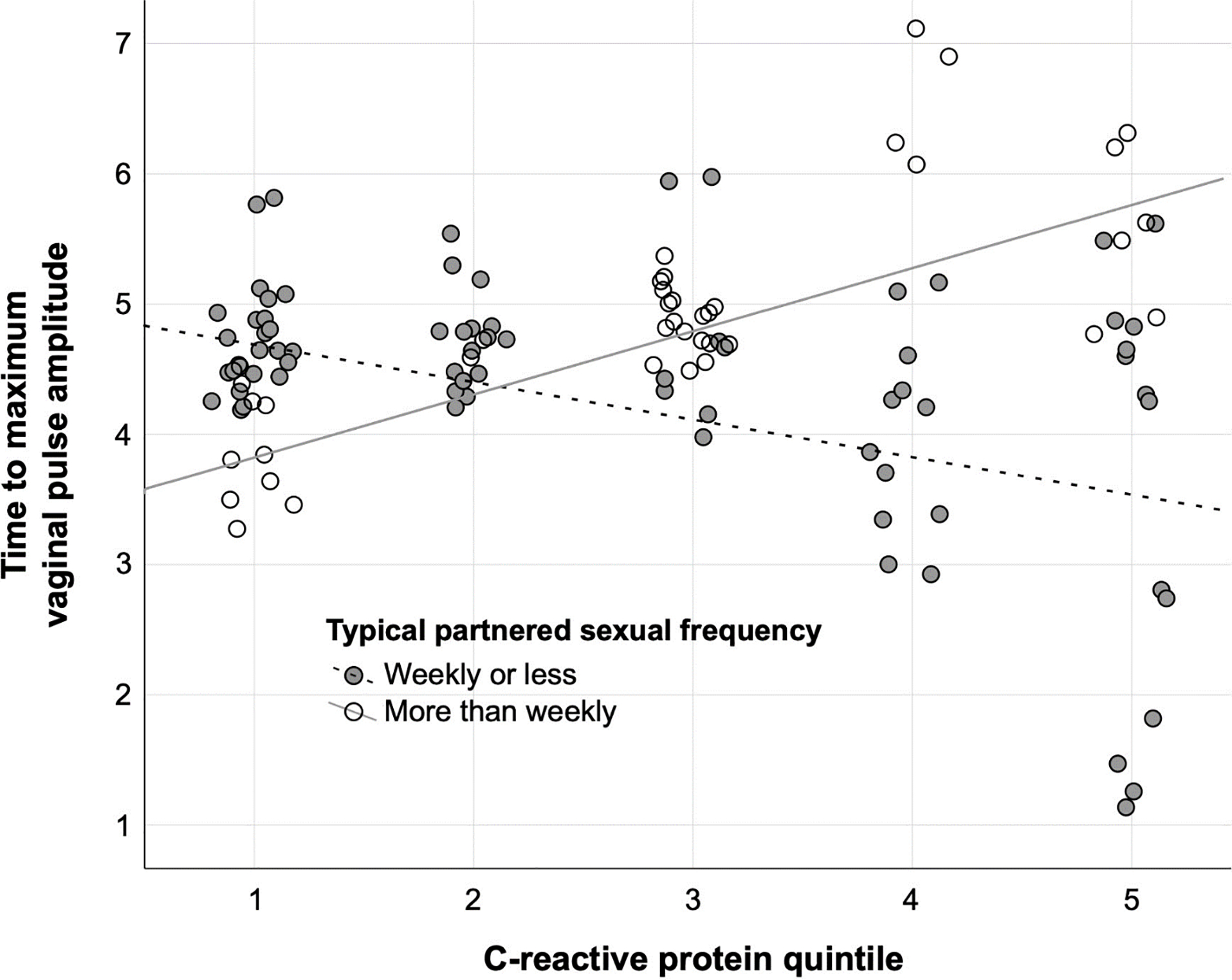
Note: In analyses, sexual frequency data consisted of six ordinal bins ranging from “never/less than monthly” to “more than daily.” For visualization purposes, sexual frequency is displayed as a binary between those reporting partnered activity at a frequency of weekly or less versus those reporting partnered activity more than once per week.
Hypothesis testing: Does inflammation predict the degree of women’s genital arousal?
Finally, we examined predictors of percent change in VPA from neutral to erotic stimuli (Table 5). As before, the model that included covariates alone was not significant (F2,42 = 2.456, p = 0.099, AIC = 456.773, adjusted R2 = 0.065). When adding CRP and sexual frequency, however, the model fit improved significantly (F5,42 = 5.731, p = 0.001, AIC = 443.092, adjusted R2 = 0.360). There was again a significant interaction between CRP and sexual frequency (standardized β = −1.969, t 5,42 = −4.204, p < 0.001). Higher CRP predicted less percent change in VPA for women reporting frequent sexual activity but greater percent change in VPA among women reporting less frequent sex (Figure 2). There were additional main effects of baseline CRP (standardized β = 0.883, t 5,42 = 2.717, p = 0.010) and sexual frequency (standardized β = 1.423, t5,42 = 3.786, p = 0.001), which both generally predicted greater magnitude of change in vaginal arousal. Effects were again weakened to non-significance by repeating the regressions while controlling for baseline cortisol and time since waking (full model F6,27 = 0.884, p = 0.524, AIC = 304.104, adjusted R2 = −0.026), neither of which independently predicted percent change in VPA.
Table 5.
Sequential regressions predicting percent change in VPA while watching a sexual film from inflammation, sexual frequency, and relevant covariates.
| Step | F | adjusted R2 | Predictor | β | t | p |
|
| ||||||
| 1 | 2.456 | 0.065 | Age | 0.076 | 0.486 | 0.630 |
|
| ||||||
| BMI | 0.299 | 1.906 | 0.064 | |||
|
| ||||||
| 2 | 5.731 | 0.360 | Age | −0.221 | −1.494 | 0.144 |
|
| ||||||
| BMI | 0.381 * | 2.220 | 0.033 | |||
|
| ||||||
| Salivary CRP | 0.883 * | 2.717 | 0.010 | |||
|
| ||||||
| Sexual frequency | 1.423 ** | 3.786 | 0.001 | |||
|
| ||||||
| CRP × frequency | −1.969 ** | −4.204 | < 0.001 | |||
| The same sequential regressions including cortisol and time since waking. | ||||||
| Step | F | adjusted R2 | Predictor | β | t | p |
|
| ||||||
| 1 | 0.972 | −0.004 | Age | 0.029 | 0.141 | 0.889 |
|
| ||||||
| BMI | 0.331 | 1.545 | 0.136 | |||
|
| ||||||
| Salivary cortisol | −0.007 | −0.032 | 0.975 | |||
|
| ||||||
| Time since waking | 0.192 | 0.957 | 0.349 | |||
|
| ||||||
| 2 | 0.884 | −0.026 | Age | −0.080 | −0.349 | 0.731 |
|
| ||||||
| BMI | 0.559 | 1.956 | 0.064 | |||
|
| ||||||
| Salivary cortisol | −0.064 | −0.301 | 0.766 | |||
|
| ||||||
| Time since waking | 0.172 | 0.841 | 0.410 | |||
|
| ||||||
| Salivary CRP | −0.316 | −1.217 | 0.237 | |||
|
| ||||||
| Sexual frequency | −0.048 | −0.243 | 0.811 | |||
|
| ||||||
| CRP × frequency | — | — | — | |||
significant at p < 0.05
significant at p < 0.01
Figure 2. Percent change in vaginal pulse amplitude (VPA) as predicted by C-reactive protein and frequency of partnered sexual activity.
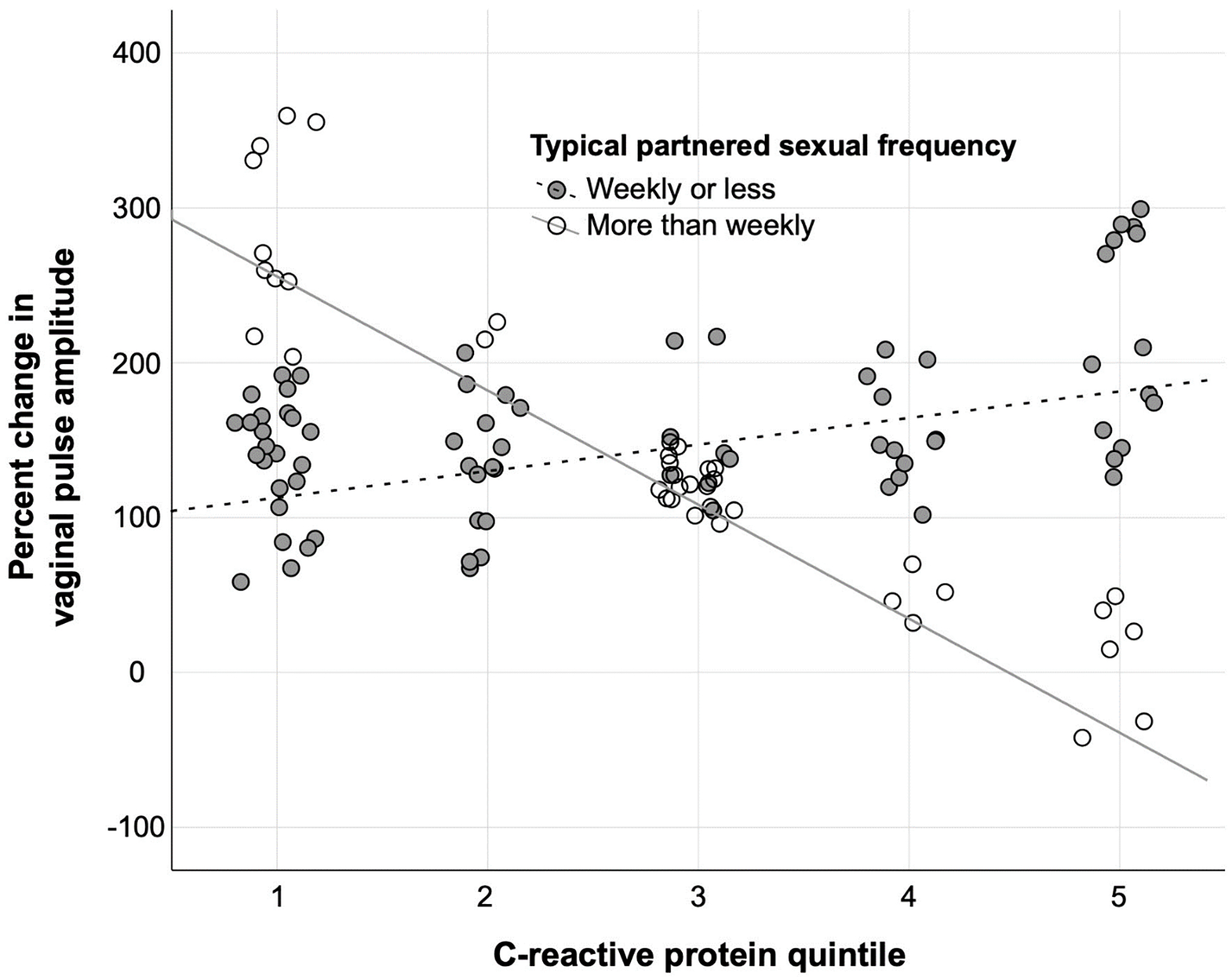
Note: In analyses, sexual frequency data consisted of six ordinal bins ranging from “never/less than monthly” to “more than daily.” For visualization purposes, sexual frequency is displayed as a binary between those reporting partnered activity at a frequency of weekly or less versus those reporting partnered activity more than once per week.
Discussion
In the present study, we explored the interactions between inflammation and subjective and vaginal sexual arousal among healthy women. Though neither inflammation nor sexual frequency were independently associated with change in subjective ratings of sexual arousal, individual differences in these predictors interacted to characterize changes in vaginal blood flow while watching a sexual film. Specifically, our results suggest that for women who have sex more regularly, higher baseline CRP is associated with lower genital sexual arousal (i.e., longer time to reach peak vaginal pulse with smaller overall percent change), consistent with clinical evidence that elevated inflammation can be detrimental to sexual function (Aydin et al., 2006; Timmer et al., 2007; Steinke, 2010; Lew-Starowicz & Rola, 2013). However, contrary to expectations, higher baseline CRP was associated with greater vaginal arousal (i.e., shorter time to maximum, greater percent change) among women who have sex less often. In interpreting these findings, it is important to note that these were healthy women with generally low to moderate levels of CRP in an absolute sense; thus, these data point to the subtle ways in which even moderate exposure to inflammation may interact with women’s sexual response.
That baseline CRP contributed to models of vaginal sexual arousal, but not subjective arousal, may reflect the broader literature on inflammation and chronic physical and mental health conditions. In prior research, associations between elevated levels of inflammatory markers and psychological outcomes have been most apparent among more severe mental health disorders such as treatment-resistant depression and PTSD (Kappelmann et al., 2018; Renna et al., 2018). Thus, we may not have observed significant effects of CRP on mental sexual arousal because of our relatively healthy sample; if so, we may expect that CRP would instead more strongly predict differences in subjective sexual functioning between women with and without mental health diagnoses. In contrast, while prolonged exposure to inflammatory cytokine signaling can lead to poor vascular health and overt disease (Dinh et al., 2014) Ridker, 2001; Pearson et al., 2003; Ford et al., 2004; Naugler & Karin, 2008), CRP may also index ongoing acute responses to even low-level tissue stress and/or injury in individuals without chronic health conditions (Kushner et al., 2006). It is thus possible that due to this study’s recruitment of healthy women, CRP demonstrated associations with physical sexual arousal more so than subjective report.
CRP’s role in even minor injury repair could help to explain differences observed between women reporting more regular or infrequent sexual activity in terms of changes to tissue of the vaginal canal. In healthy women who are frequently sexually active, CRP concentrations could reflect the body’s process of tending to micro-tears in vaginal tissue resulting from sexual acts. For example, intensive exercise causes a spike in immediate inflammatory agents (e.g., interluekin-6) that typically lessens within a day or two of recovery (Milias et al., 2005). However, prolonged or repeated exercise-induced muscle injuries have been found to cause a five-day increase in circulating CRP levels (Neubauer et al., 2008). Frequent sexual activity (e.g., several times per week) could thus mimic frequent exercise; if so, micro-tears may be less likely to be adequately repaired before the next sexual encounter among frequently sexually active women, leading to elevated baseline CRP and impairments in genital blood flow. However, minimal research has been invested in vaginal tissue recovery and subsequent inflammation, and thus it could be beneficial to further illuminate the links between specific tissue stress and prolonged inflammatory responses.
Though we predicted that inflammation would generally disrupt sexual arousal, higher baseline CRP concentrations were associated with shorter latencies to maximum vaginal arousal and greater percent change among women reporting less frequent sexual activity. CRP’s role in tissue repair could also explain this unexpected trend. Less frequent sexual activity might pose lower risk of vaginal micro-tears and/or allow sufficient time between sexual encounters for them to heal, resulting in less need for CRP to perform tissue repairing functions. Under these circumstances, lower CRP concentrations might be expected at baseline, such that the higher CRP levels observed among some less sexually active women in this laboratory study might reflect other aspects of physical or psychological functioning. Evidence suggests that there is an optimal balance of autonomic, neural, and psychological processes necessary for sexual arousal to occur (Georgiadis et al., 2012), such that a moderate level of excitatory activation (e.g., sympathetic nervous system response, anxiety) could potentially facilitate sexual arousal (Bradford & Meston, 2006; Lorenz et al., 2012) among women with low inflammation levels at baseline.
The specific mechanisms through which inflammation impacts or is impacted by sexual frequency and arousal, as distinct from other aspects of sexual function, have yet to be clarified. Existing animal literature suggests that inflammatory markers are associated with lower interest in sexual behavior for female rats (though not their male counterparts; (Yirmiya et al., 1995; Avitsur et al., 1997). The presence of inflammation may thus cue “sickness behaviors” (e.g. fatigue, withdrawal), whereby motivation for social activity is reduced to free up physiological resources for fighting off infection (Lorenz, 2019).
In this context, it is important to differentiate sexual desire and sexual arousal as discrete, albeit interconnected, psychophysiological processes (Georgiadis et al., 2012). Sexual desire may be thought of as a subjective state reflecting interest in or openness to sexual activity (Spector et al., 1996). Though sexual desire need not be present for sexual arousal to occur (Toates, 2009), it may promote approach-oriented behaviors from which sexual arousal may follow. Inflammatory cytokines may modulate neurotransmitter systems that are implicated in sexual reward and motivation (e.g., dopaminergic mesolimbic pathway; Felger & Miller, 2012; Rothaug et al., 2016). Dampening of sexual desire due to inflammation could thus have indirect consequences for sexual arousal and well-being more broadly. Conversely, arousal can precede desire (Laan & Both, 2008). Thus, poor sexual arousal may decrease experiences of sexual desire. It is also important to note that previous research has demonstrated only modest concordance between subjective and physiological sexual arousal in women under a variety of laboratory conditions (Chivers & Brotto, 2017). Such poor coupling may help to explain the lack of relationship between sexual desire and physiological factors examined in our study.
Limitations and Clinical Implications
These results are presented with several limitations. This was an exploratory, cross-sectional study, and thus neither directionality nor causality can be inferred from the effects reported. It is likely that the association between inflammation and women’s sexual response is bidirectional and multicausal. For example, recent research has suggested that androgens have play immunomodulatory role and promote anti-inflammatory properties in the vagina (Maseroli et al., 2020), which could be a useful piece of the psycho-physio-immuno-endocrine puzzle and necessitates further investigation.
As these were healthy participants with generally low to moderate levels of CRP, it is likely that we did not capture the effects of high levels of inflammation that would be observed in a clinical population. In addition, we were interested in recruiting sexually active participants free of chronic health conditions, heavy substance use, and medications that might substantially impact immune function or sexual response. Also of note, we did not assess participant’s experiences of social stress, which may impact both CRP and sexual functioning (Lorenz, 2019). Assessing stress related to discrimination may be relevant for understanding how links between inflammation and sexual function differ across sexuality-diverse populations. Tentative relationships between CRP, sexual orientation, minority stress, discrimination, and hormonal contraception observed in this sample and others (Juster et al., 2016; Mays et al., 2018) could be worthwhile to investigate in a larger sample.
It is worth noting that salivary CRP is a measurement of systemic inflammation, rather than inflammation in the local vaginal epithelium. Further research is needed to distinguish the local vs. systemic effects of inflammation, and how these may influence different aspects of arousal (subjective vs. genital). Moreover, further specificity in our understanding of how inflammatory mediators (such as pro-inflammatory cytokines) influence specific pathophysiologic processes underlying poor genital blood flow may reveal underlying pathways to target clinically.
We did not measure markers of individual differences in genital vascularization, which may be impacted by chronic inflammation. Indeed, several studies have suggested that the poorer sexual function often reported in populations with metabolic syndromes may be driven by vasculogenic effects associated with chronic inflammation (Maseroli et al., 2018). That said, the primary focus of the present study was on the effects of inflammation on the physiologic processes underpinning vaginal arousal (i.e., blood flow), which can function independently from vascularization (e.g., adequate vascularization with poor blood flow). Relatedly, other measures of genital arousal may reveal different pathways by which inflammation influences sexual function. For example, the impacts of inflammation on clitoral blood flow may be more likely to contribute to orgasm dysfunction than the same effects on vaginal arousal.
Although our methods for measuring sexual arousal in a laboratory setting using visual sexual stimuli offered a high degree of control and precision in our measurements, there are important aspects of partnered sexual activity that may not have been captured. It is also possible that continuous measurement of subjective arousal, as opposed to retrospective reporting, would have allowed us to examine relationships more closely between sexual arousal and responsive desire (Rellini, McCall, Randall, & Meston, 2005). Future replication could focus on investigating inflammation markers from the vaginal epithelium and broadening the scope of women studied, the kinds of sexual partnerships represented, and the ways in which sexual desire and arousal are measured.
Based on prior work documenting significant interactions between sexual activity levels and menstrual phase in predicting women’s inflammation levels, we scheduled all participant sessions to take place 21–25 days after the self-reported start of each woman’s menstrual cycle in order to approximate the luteal phase. It is likely that associations between inflammation and sexual response may differ at other points of the cycle. In particular, prior work has suggested a curvilinear pattern of CRP across the menstrual cycle, with lowest levels around ovulation and peaks in the late luteal phase (Schisterman et al., 2014). Moreover, this pattern differs in women with different levels of sexual activity, with greater variability across the menstrual cycle at higher sexual frequencies (Lorenz et al., 2015a; Lorenz et al., 2017a, 2017b). Thus, it is reasonable to expect that the association between CRP and sexual arousal would be smaller at ovulation than the luteal phase data reported here; however, this speculation must be tested directly.
Controlling for cortisol did not meaningfully change results with respect to subjective sexual arousal and weakened predictions of vaginal response. These results suggest that glucocorticoids might more strongly interact with variation in physiological rather than psychological state. However, cortisol has been reported to relate to self-reported sexual functioning over longer durations (e.g., past month; Hamilton et al., 2008), such that associations between cortisol, inflammation, and sexual function might be better clarified outside of the laboratory context. Similarly, recent studies have suggested that women diagnosed with low sexual desire differ from their healthy counterparts on both transient measures of cortisol (e.g., cortisol awakening response, single time-point measurements like the one used in the present study) as well as more global measures of HPA axis functioning (e.g., diurnal cortisol slope; Basson et al., 2019; O’Loughlin et al., 2020). Just as our decision to study healthy women may have obscured associations between CRP and subjective sexual arousal that might otherwise be observable among women with significant mental health complaints, future investigations of cortisol’s role in psycho-immunological interactions might be aided by including women with sexual disorders.
Our findings have implications for clinical interventions aimed at improving sexual function. The apparent link between inflammation and physiological sexual arousal underscores the importance of a multidisciplinary approach to the assessment and treatment of sexual dysfunction, particularly for women with health conditions characterized by chronically high levels of inflammation. Behavioral interventions designed to lower levels of inflammation in the body through healthy lifestyle changes, such as regular exercise and a balanced diet, have been shown to improve sexual dysfunction in men (e.g., Khoo et al., 2011). Such interventions likely would have a positive impact on women’s sexual functioning through similar mechanisms. One study found that the Mediterranean diet reduced CRP and improved overall sexual functioning in women with metabolic syndrome (Esposito et al., 2007). Additional research on women’s sexual function and immune activity may eventually enable physicians to make more accurate predictions about the sexual side effects expected from medical treatments, such as estrogen replacement therapy, which has been shown to influence levels of inflammation in the body (Vehkavaara et al., 2001).
Conclusions
Our findings suggest that higher levels of CRP may predict vaginal sexual arousal, but not subjective sexual arousal; moreover, these associations appear to differ as a function of frequency of partnered sexual activity. These findings may be impactful for those who have chronic illnesses associated with higher baseline CRP levels, though replications in both healthy and clinical populations are needed. It is encouraging that relatively simple interventions, such as changes in diet, can help alleviate chronic inflammation and subsequent symptoms. However, more research is required to fully examine the association between inflammatory processes and female sexual function.
Highlights:
Among healthy young women, higher levels of CRP may be associated with vaginal sexual arousal, but not subjective sexual arousal.
Frequency of sexual activity may be an important characterizing factor in the interaction of CRP and vaginal arousal in healthy women
Higher baseline CRP was found to be associated with lower genital sexual arousal in women who engage in sexual activity more than once a week
Higher baseline CRP was found to be associated with greater genital sexual arousal in women who engage in sexual activity less than once a week
Acknowledgements:
This study was supported by the National Institute of Child Health and Human Development [grant number T32 HD049336] and by internal funds from the Department of Psychological Science at the University of North Carolina at Charlotte (UNCC). Ms. Wilson is supported by the National Science Foundation (NSF) Graduate Research Fellowship Program [grand number 1342962]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, UNCC, or NSF.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
While we have made efforts to clarify when we are referencing issues related to either physical sex (e.g., genitals) or gender, this is not always possible as the primary literature is based almost exclusively on cisgender women and does not usually distinguish these factors.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agmo A (2011). On the intricate relationship between sexual motivation and arousal. Hormones and Behavior, 59(5), 681–688. [DOI] [PubMed] [Google Scholar]
- Alley J, Diamond LM, Lipschitz DL, & Grewen K (2020). Women’s Cortisol Stress Responsivity, Sexual Arousability, and Sexual History. Archives of Sexual Behavior, 49(5), 1489–1503. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Pollak Y, & Yirmiya R (1997). Different receptor mechanisms mediate the effects of endotoxin and interleukin-1 on female sexual behavior. Brain Research, 773(1–2), 149–161. [DOI] [PubMed] [Google Scholar]
- Aydin G, Başar MM, Keleş I, Ergün G, Orkun S, & Batislam E (2006). Relationship between sexual dysfunction and psychiatric status in premenopausal women with fibromyalgia. Urology, 67(1), 156–161. [DOI] [PubMed] [Google Scholar]
- Bank AJ, Billups KL, Kaiser DR, Kelly AS, Wetterling RA, Tsai MY, & Hanson N (2003). Relation of C-reactive protein and other cardiovascular risk factors to penile vascular disease in men with erectile dysfunction. International Journal of Impotence Research, 15(4), 231–236. [DOI] [PubMed] [Google Scholar]
- Basson R, O’Loughlin JI, Weinberg J, Young AH, Bodnar T, & Brotto LA (2019). Dehydroepiandrosterone and cortisol as markers of HPA axis dysregulation in women with low sexual desire. Psychoneuroendocrinology, 104, 259–268. PubMed. 10.1016/j.psyneuen.2019.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford A, & Meston CM (2006). The impact of anxiety on sexual arousal in women. Behaviour Research and Therapy, 44(8), 1067–1077. 10.1016/j.brat.2005.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clephane K, & Lorenz T (2021). Vaginal Pulse Amplitude Data Cleaning Guide. 10.17605/OSF.IO/T67QN [DOI] [Google Scholar]
- Di Stasi V, Verde N, Maseroli E, Scavello I, Cipriani S, Todisco T, Maggi M, & Vignozzi L (2019). Female Sexual Dysfunction as a Warning Sign of Chronic Disease Development. Current Sexual Health Reports, 11(4), 307–319. 10.1007/s11930-019-00229-4 [DOI] [Google Scholar]
- Dinh QN, Drummond GR, Sobey CG, & Chrissobolis S (2014). Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. BioMed Research International, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito K, Ciotola M, Giugliano F, Schisano B, Autorino R, Iuliano S, Vietri MT, Cioffi M, De Sio M, & Giugliano D (2007). Mediterranean diet improves sexual function in women with the metabolic syndrome. International Journal of Impotence Research, 19(5), 486–491. [DOI] [PubMed] [Google Scholar]
- Esposito K, Ciotola M, Marfella R, Di Tommaso D, Cobellis L, Giugliano D, Ciotola M, Marfella R, Tommaso DD, Cobellis L, & Giugliano D (2005). The metabolic syndrome: A cause of sexual dysfunction in women. International Journal of Impotence Research, 17(3), 224–226. 10.1038/sj.ijir.3901310 [DOI] [PubMed] [Google Scholar]
- Exton NG, Truong TC, Exton MS, Wingenfeld SA, Leygraf N, Saller B, Hartmann U, & Schedlowski M (2000). Neuroendocrine response to film-induced sexual arousal in men and women. Psychoneuroendocrinology, 25(2), 187–199. [DOI] [PubMed] [Google Scholar]
- Fantidis P (2010). The role of the stress-related anti-inflammatory hormones ACTH and cortisol in atherosclerosis. Current Vascular Pharmacology, 8(4), 517–525. [DOI] [PubMed] [Google Scholar]
- Feghali CA, & Wright TM (1997). Cytokines in acute and chronic inflammation. Front Biosci, 2(1), d12–d26. [DOI] [PubMed] [Google Scholar]
- Felger JC, & Miller AH (2012). Cytokine effects on the basal ganglia and dopamine function: The subcortical source of inflammatory malaise. Frontiers in Neuroendocrinology, 33(3), 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ES, Giles WH, Mokdad AH, & Myers GL (2004). Distribution and Correlates of C-Reactive Protein Concentrations among Adult US Women. Clinical Chemistry, 50(3), 574–581. 10.1373/clinchem.2003.027359 [DOI] [PubMed] [Google Scholar]
- Franz F, Edgar E, Albert-Georg L, & Axel B (2007). G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. [DOI] [PubMed] [Google Scholar]
- Georgiadis JR, Kringelbach ML, & Pfaus JG (2012). Sex for fun: A synthesis of human and animal neurobiology. Nature Reviews Urology, 9(9), 486. [DOI] [PubMed] [Google Scholar]
- Haake P, Krueger TH, Goebel MU, Heberling KM, Hartmann U, & Schedlowski M (2004). Effects of sexual arousal on lymphocyte subset circulation and cytokine production in man. Neuroimmunomodulation, 11(5), 293–298. [DOI] [PubMed] [Google Scholar]
- Hamilton LD, Rellini AH, & Meston CM (2008). Cortisol, sexual arousal, and affect in response to sexual stimuli. The Journal of Sexual Medicine, 5(9), 2111–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard-Davison A, Heiman JR, & Kuffel S (2007). Genital and subjective measurement of the time course effects of an acute dose of testosterone vs. Placebo in postmenopausal women. The Journal of Sexual Medicine, 4(1), 209–217. [DOI] [PubMed] [Google Scholar]
- Heiman JR, & Pfaff D (2011). Sexual arousal and related concepts: An introduction. [DOI] [PubMed]
- Heiman JR, & Rowland DL (1983). Effects of instructions on affective and physiological response in sexually-dysfunctional and normal men. J. Psychosom. Res, 27, 105–116. [DOI] [PubMed] [Google Scholar]
- Juster R-P, Almeida D, Cardoso C, Raymond C, Johnson PJ, Pfaus JG, Mendrek A, Duchesne A, Pruessner JC, & Lupien SJ (2016). Gonads and strife: Sex hormones vary according to sexual orientation for women and stress indices for both sexes. Psychoneuroendocrinology, 72, 119–130. 10.1016/j.psyneuen.2016.06.011 [DOI] [PubMed] [Google Scholar]
- Kappelmann N, Lewis G, Dantzer R, Jones PB, & Khandaker GM (2018). Antidepressant activity of anti-cytokine treatment: A systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Molecular Psychiatry, 23(2), 335–343. 10.1038/mp.2016.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo J, Piantadosi C, Duncan R, Worthley SG, Jenkins A, Noakes M, Worthley MI, Lange K, & Wittert GA (2011). Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. The Journal of Sexual Medicine, 8(10), 2868–2875. [DOI] [PubMed] [Google Scholar]
- Kukkonen TM (2015). Devices and methods to measure female sexual arousal. Sexual Medicine Reviews, 3(4), 225–244. [DOI] [PubMed] [Google Scholar]
- Kushner I, Rzewnicki D, & Samols D (2006). What Does Minor Elevation of C-Reactive Protein Signify? The American Journal of Medicine, 119(2), 166.e17–166.e28. 10.1016/j.amjmed.2005.06.057 [DOI] [PubMed] [Google Scholar]
- Laan E, & Both S (2008). What Makes Women Experience Desire? Feminism & Psychology, 18(4), 505–514. 10.1177/0959353508095533 [DOI] [Google Scholar]
- Lew-Starowicz M, & Rola R (2013). Prevalence of sexual dysfunctions among women with multiple sclerosis. Sexuality and Disability, 31(2), 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P (2012). Inflammation in Atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology, 32(9), 2045–2051. 10.1161/ATVBAHA.108.179705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz TA, Harte CB, Hamilton LD, & Meston CM (2012). Evidence for a curvilinear relationship between sympathetic nervous system activation and women’s physiological sexual arousal. Psychophysiology, 49(1), 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz TK (2019). Interactions Between Inflammation and Female Sexual Desire and Arousal Function. Current Sexual Health Reports, 11(4), 287–299. 10.1007/s11930-019-00218-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz TK, Demas GE, & Heiman JR (2017a). Partnered sexual activity moderates menstrual cycle–related changes in inflammation markers in healthy women: An exploratory observational study. Fertility and Sterility, 107(3), 763–773.e3. 10.1016/j.fertnstert.2016.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz TK, Heiman JR, & Demas GE (2015a). Sexual activity modulates shifts in TH1/TH2 cytokine profile across the menstrual cycle: An observational study. Fertility and Sterility, 104(6), 1513–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz TK, Heiman JR, & Demas GE (2017b). Testosterone and immune-reproductive tradeoffs in healthy women. Hormones and Behavior, 88, 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz TK, Heiman JR, & Demas GE (2018). Interactions Among Sexual Activity, Menstrual Cycle Phase, and Immune Function in Healthy Women. The Journal of Sex Research, 55(9), 1087–1095. 10.1080/00224499.2017.1394961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz TK, Worthman CM, & Vitzthum VJ (2015b). Links among inflammation, sexual activity and ovulationEvolutionary trade-offs and clinical implications. Evolution, Medicine, and Public Health, 2015(1), 304–324. 10.1093/emph/eov029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz T, & van Anders S (2014). Interactions of Sexual Activity, Gender, and Depression with Immunity. The Journal of Sexual Medicine, 11(4), 966–979. 10.1111/jsm.12111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madssen E, Skaug E-A, Wisløff U, Ellingsen Ø, & Videm V (2019). Inflammation is strongly associated with cardiorespiratory fitness, sex, BMI, and the metabolic syndrome in a self-reported healthy population: HUNT3 fitness study. Mayo Clinic Proceedings, 94(5), 803–810. [DOI] [PubMed] [Google Scholar]
- Maiorino MI, Bellastella G, & Esposito K (2020). Diabetes and Sexual Disorders. In Bonora E & DeFronzo RA (Eds.), Diabetes Complications, Comorbidities and Related Disorders (pp. 473–494). Springer International Publishing. 10.1007/978-3-030-36694-0_16 [DOI] [Google Scholar]
- Maseroli E, Cellai I, Filippi S, Comeglio P, Cipriani S, Rastrelli G, Rosi M, Sorbi F, Fambrini M, Petraglia F, Amoriello R, Ballerini C, Lombardelli L, Piccinni M-P, Sarchielli E, Guarnieri G, Morelli A, Maggi M, & Vignozzi L (2020). Anti-inflammatory effects of androgens in the human vagina. Journal of Molecular Endocrinology, 65(3), 109–124. 10.1530/JME-20-0147 [DOI] [PubMed] [Google Scholar]
- Maseroli E, Scavello I, & Vignozzi L (2018). Cardiometabolic Risk and Female Sexuality—Part I. Risk Factors and Potential Pathophysiological Underpinnings for Female Vasculogenic Sexual Dysfunction Syndromes. Sexual Medicine Reviews, 6(4), 508–524. 10.1016/j.sxmr.2018.02.009 [DOI] [PubMed] [Google Scholar]
- Mays VM, Juster R-P, Williamson TJ, Seeman TE, & Cochran SD (2018). Chronic physiologic effects of stress among lesbian, gay, and bisexual adults: Results from the National Health and Nutrition Examination Survey. Psychosomatic Medicine, 80(6), 551–563. 10.1097/PSY.0000000000000600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe MP, Sharlip ID, Lewis R, Atalla E, Balon R, Fisher AD, Laumann E, Lee SW, & Segraves RT (2016). Incidence and prevalence of sexual dysfunction in women and men: A consensus statement from the Fourth International Consultation on Sexual Medicine 2015. The Journal of Sexual Medicine, 13(2), 144–152. [DOI] [PubMed] [Google Scholar]
- Milias GA, Nomikos T, Fragopoulou E, Athanasopoulos S, & Antonopoulou S (2005). Effects of eccentric exercise-induced muscle injury on blood levels of platelet activating factor (PAF) and other inflammatory markers. European Journal of Applied Physiology, 95(5), 504–513. 10.1007/s00421-005-0031-6 [DOI] [PubMed] [Google Scholar]
- Moriarity DP, Ng T, Titone MK, Chat IK-Y, Nusslock R, Miller GE, & Alloy LB (2020). Reward Responsiveness and Ruminative Styles Interact to Predict Inflammation and Mood Symptomatology. Behavior Therapy, 51(5), 829–842. 10.1016/j.beth.2019.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu F, Harris HR, Rich-Edwards JW, Hankinson SE, Rimm EB, Spiegelman D, & Missmer SA (2018). A Prospective Study of Inflammatory Markers and Risk of Endometriosis. American Journal of Epidemiology, 187(3), 515–522. 10.1093/aje/kwx272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, & Weaver C (2016). Janeway’s Immunobiology. Garland Science. [Google Scholar]
- Naugler WE, & Karin M (2008). The wolf in sheep’s clothing: The role of interleukin-6 in immunity, inflammation and cancer. Trends in Molecular Medicine, 14(3), 109–119. 10.1016/j.molmed.2007.12.007 [DOI] [PubMed] [Google Scholar]
- Neubauer O, König D, & Wagner K-H (2008). Recovery after an Ironman triathlon: Sustained inflammatory responses and muscular stress. European Journal of Applied Physiology, 104(3), 417–426. 10.1007/s00421-008-0787-6 [DOI] [PubMed] [Google Scholar]
- O’Loughlin JI, Rellini AH, & Brotto LA (2020). How does childhood trauma impact women’s sexual desire? Role of depression, stress, and cortisol. The Journal of Sex Research, 57(7), 836–847. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I, Danese A, Williams B, & Arseneault L (2011). Validation of a high-sensitivity assay for C-reactive protein in human saliva. Brain, Behavior, and Immunity, 25(4), 640–646. 10.1016/j.bbi.2010.12.020 [DOI] [PubMed] [Google Scholar]
- Out D, Hall RJ, Granger DA, Page GG, & Woods SJ (2012). Assessing salivary C-reactive protein: Longitudinal associations with systemic inflammation and cardiovascular disease risk in women exposed to intimate partner violence. Brain, Behavior, and Immunity, 26(4), 543–551. 10.1016/j.bbi.2012.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM (2017). Why are depressed patients inflamed? A reflection on 20 years of research on depression, glucocorticoid resistance and inflammation. European Neuropsychopharmacology, 27(6), 554–559. 10.1016/j.euroneuro.2017.04.001 [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon III RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, & Myers GL (2003). Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation, 107(3), 499–511. [DOI] [PubMed] [Google Scholar]
- Pepys MB, & Hirschfield GM (2003). C-reactive protein: A critical update. The Journal of Clinical Investigation, 111(12), 1805–1812. 10.1172/JCI18921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, & Ross OA (2018). Age and age-related diseases: Role of inflammation triggers and cytokines. Front Immunol. 2018; 9: 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rellini AH, McCall KM, Randall PK, & Meston CM (2005). The relationship between women’s subjective and physiological sexual arousal. Psychophysiology, 42(1), 116–124. 10.1111/j.1469-8986.2005.00259.x [DOI] [PubMed] [Google Scholar]
- Renna ME, O’Toole MS, Spaeth PE, Lekander M, & Mennin DS (2018). The association between anxiety, traumatic stress, and obsessive–compulsive disorders and chronic inflammation: A systematic review and meta-analysis. Depression and Anxiety, 35(11), 1081–1094. 10.1002/da.22790 [DOI] [PubMed] [Google Scholar]
- Ridker PM (2001). High-Sensitivity C-Reactive Protein. Circulation, 103(13), 1813–1818. 10.1161/01.CIR.103.13.1813 [DOI] [PubMed] [Google Scholar]
- Rothaug M, Becker-Pauly C, & Rose-John S (2016). The role of interleukin-6 signaling in nervous tissue. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1863(6), 1218–1227. [DOI] [PubMed] [Google Scholar]
- Roubenoff R, Harris TB, Abad LW, Wilson PW, Dallal GE, & Dinarello CA (1998). Monocyte cytokine production in an elderly population: Effect of age and inflammation. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 53(1), M20–M26. [DOI] [PubMed] [Google Scholar]
- Rowland DL, McNabney SM, & Mann AR (2017). Sexual function, obesity, and weight loss in men and women. Sexual Medicine Reviews, 5(3), 323–338. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Mumford SL, & Sjaarda LA (2014). Failure to Consider the Menstrual Cycle Phase May Cause Misinterpretation of Clinical and Research Findings of Cardiometabolic Biomarkers in Premenopausal Women. Epidemiologic Reviews, 36(1), 71–82. 10.1093/epirev/mxt007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector IP, Carey MP, & Steinberg L (1996). The Sexual Desire Inventory: Development, factor structure, and evidence of reliability. Journal of Sex & Marital Therapy, 22(3), 175–190. [DOI] [PubMed] [Google Scholar]
- Sproston NR, & Ashworth JJ (2018). Role of C-Reactive Protein at Sites of Inflammation and Infection. Frontiers in Immunology, 9. 10.3389/fimmu.2018.00754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinke EE (2010). Sexual dysfunction in women with cardiovascular disease: What do we know? Journal of Cardiovascular Nursing, 25(2), 151–158. [DOI] [PubMed] [Google Scholar]
- Timmer A, Bauer A, Dignass A, & Rogler G (2007). Sexual function in persons with inflammatory bowel disease: A survey with matched controls. Clinical Gastroenterology and Hepatology, 5(1), 87–94. [DOI] [PubMed] [Google Scholar]
- Toates F (2009). An integrative theoretical framework for understanding sexual motivation, arousal, and behavior. Journal of Sex Research, 46(2–3), 168–193. [DOI] [PubMed] [Google Scholar]
- Uddin SI, Mirbolouk M, Dardari Z, Feldman DI, Cainzos-Achirica M, DeFilippis AP, Greenland P, Blankstein R, Billups KL, & Miner MM (2018). Erectile dysfunction as an independent predictor of future cardiovascular events: The multi-ethnic study of atherosclerosis. Circulation, 138(5), 540–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehkavaara S, Silveira A, Hakala-Ala-Pietilä T, Virkamäki A, Hovatta O, Hamsten A, Taskinen M-R, & Yki-Järvinen H (2001). Effects of Oral and Transdermal Estrogen Replacement Therapy on Markers of Coagulation, Fibrinolysis, Inflammation and Serum Lipids and Lipoproteins in Postmenopausal Women. Thrombosis and Haemostasis, 85(4), 619–625. 10.1055/s-0037-1615643 [DOI] [PubMed] [Google Scholar]
- Velten J, Chivers ML, & Brotto LA (2018). Does repeated testing impact concordance between genital and self-reported sexual arousal in women? Archives of Sexual Behavior, 47(3), 651–660. [DOI] [PubMed] [Google Scholar]
- Yao F, Huang Y, Zhang Y, Dong Y, Ma H, Deng C, Lin H, Liu D, & Lu K (2012). Subclinical endothelial dysfunction and low-grade inflammation play roles in the development of erectile dysfunction in young men with low risk of coronary heart disease. International Journal of Andrology, 35(5), 653–659. [DOI] [PubMed] [Google Scholar]
- Yeager MP, Pioli PA, & Guyre PM (2011). Cortisol exerts bi-phasic regulation of inflammation in humans. Dose-Response, 9(3), dose-response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Avitsur R, Donchin O, & Cohen E (1995). Interleukin-1 inhibits sexual behavior in female but not in male rats. Brain, Behavior, and Immunity, 9(3), 220–233. [DOI] [PubMed] [Google Scholar]


