Abstract
Vulvodynia is debilitating vulvar pain accompanied by dyspareunia (pain with sexual intercourse). Ehlers-Danlos syndromes (EDS) and hypermobility spectrum disorders (HSD) may represent a predisposing factor for vulvodynia given a high rate of dyspareunia in these conditions. We conducted an online survey of women with EDS or HSD to: assess rates of dyspareunia and estimate rates of vulvodynia, report rates of comorbid conditions common to EDS or HSD and vulvodynia, and examine rates of conditions contributing to dyspareunia in women with EDS or HSD. Women with EDS or HSD (N=1146) recruited via social media were 38.2±11.5 years old, primarily White (94.4%), and resided in the U.S. (78.5%). 63.7% of participants reported dyspareunia and 50% screened positive for vulvodynia. The rate of comorbid conditions common to EDS or HSD and vulvodynia were: irritable bowel syndrome, 6.5%; fibromyalgia, 40.0%; temporomandibular joint dysfunction, 56.4%; migraine, 6.7%; interstitial cystitis,1.7%; and mast cell activation syndrome, 10.2%. Participants reporting dyspareunia also reported ovarian cysts, fibroids, or abdominal or pelvic scars, 47.5%; endometriosis, 26.5%; and genital lacerations, 19.3%. Women with EDS or HSD may have a higher rate of vulvodynia (50.0%) than women in the U.S. population at large (8%) and should be assessed for dyspareunia and vulvodynia.
Keywords: Dyspareunia, Vulvodynia, Pelvic Pain, Ehlers-Danlos Syndromes, Social Media
Introduction
Pain is nearly universal in hypermobility spectrum disorders (HDS) (Castori & Hakim, 2017; Demes et al., 2020), is a diagnostic criterion of hypermobile Ehlers-Danlos syndrome (hEDS) (Malfait et al., 2017), and is common in adults with rarer types of EDS (Schubart et al., 2019; Voermans et al., 2010). Co-morbidities of EDS are equally common in HSD and hEDS (Copetti et al., 2019). Given the evolving use of the terms hEDS (Tinkle et al., 2017), HSD (Castori et al., 2017), and joint hypermobility syndrome (Grahame et al., 2000) an “old” term incorporated in HSD (Grahame et at., 2000), in this paper we used the acronym “hEDS/HSD” to identify the community of phenotypes belonging to these three, partially overlapping groups. In hEDS and presumably HSD, as an individual ages recurrent injuries accumulate resulting in chronic pain, with hypothesized central and/or peripheral nervous system sensitization (Castori, 2016; Sacheti et al., 1997). Pain is estimated to affect 90% (Voermans et al., 2010) of individuals with hEDS/HSD and has such a profound effect that even with pain management 87% (Voermans et al., 2010) report difficulties performing activities of daily living (Castori, 2016). Phenotypic dimensions of pain in EDS are heterogeneous and still incompletely understood.
Women with EDS were reported to have an alarming 77% rate of dyspareunia (pain with sexual intercourse) (Castori et al., 2012; Hugon-Rodin et al., 2016; Hurst et al., 2014) compared to 20% (Latthe et al., 2006; Seehusen et al., 2014) of women in the general global population. Vulvodynia, debilitating chronic vulvar pain accompanied by dyspareunia (Bornstein et al., 2016) affects up to 8% (Reed et al., 2012b), of women in the U.S. and has no identifiable underlying pathology. Desperate for pain relief women with vulvodynia may go as far as having a vulvectomy to relieve their pain, knowing it is possible that pain may still continue (Andrews, 2011; Goldstein et al., 2016; Tommola et al., 2010). Several EDS studies have categorized dyspareunia and vulvodynia as the same condition (Castori et al., 2013b; Castori et al., 2012; Chopra et al., 2017); however, more specifically, dyspareunia is a symptom of vulvodynia. No studies have examined vulvodynia in women with EDS or HSD (Castori et al., 2013b; Castori et al., 2012; Chopra et al., 2017; Gilliam et al., 2020; Hugon-Rodin et al., 2016; Hurst et al., 2014; McIntosh et al., 1995).
Due to the rare nature of EDS and the lack of healthcare specialists familiar with EDS and HSD, patients have used Facebook™ (Meta Platform Inc, Menlo Park, CA) to form support groups where tips and resources are shared. In these groups, we observed women complaining of dyspareunia. Pain from dyspareunia can decimate a person’s life, rendering them incapable of having sexual intercourse, shattering an intimate relationship (Schlaeger et al., 2019b). A review of the literature found five studies that reported the rate of dyspareunia in EDS and HSD but no studies examined the conditions that may contribute to dyspareunia in EDS and HSD (Castori et al., 2012; Chopra et al., 2017; Hugon-Rodin et al., 2016; Hurst et al., 2014; McIntosh et al., 1995). In the general population, vulvodynia; endometriosis; pelvic inflammatory disease; infection and neurological conditions affecting the genitals; ovarian cysts, fibroids, and scarring; previous injury to the genitals; laceration of the genitals; vaginal dryness; atrophic vaginitis; chemotherapy and radiation; and cancer have been found to be associated with dyspareunia (Alimi et al., 2018; American College of Obstetricians and Gynecologists & Committee on Gynecologic Practice, 2016; Seehusen et al., 2014; Sorensen et al., 2018). Separately, it is known that EDS, HSD, and vulvodynia are both associated with comorbid conditions such as irritable bowel syndrome (Maeland et al., 2011; Reed et al., 2012b; Vieira-Baptista et al., 2014), fibromyalgia (Reed et al., 2012a; Vieira-Baptista et al., 2014), temporomandibular joint dysfunction (Murray et al., 2013; Vieira-Baptista et al., 2014), interstitial cystitis (Castori et al., 2013a; Reed et al., 2012a; Vieira-Baptista et al., 2014), mast cell disorders (McDonald & Rapkin, 2012; Regauer et al., 2015; Seneviratne et al., 2017), and migraine (Hakim & Grahame, 2004; Puledda et al., 2015; Vieira-Baptista et al., 2014). The intersections of these comorbid conditions have never been reported in the EDS, HSD, or vulvodynia literature. The primary aim of this study was to determine the rate of dyspareunia and vulvodynia in women with EDS or HSD. The secondary aim was to report the rate of comorbid conditions that are common in both EDS, HSD, and vulvodynia. The third aim was to examine the rate of conditions known to contribute to dyspareunia in women with EDS or HSD.
Method and Materials
The study was a cross-sectional online self-reported survey that was completed from June to July of 2019. This study was approved by the University of Illinois Chicago Institutional Review Board.
A convenience sample of participants was recruited through social media and met the inclusion criteria of: (1) a self-reported diagnosis of EDS or HSD previously confirmed by a healthcare provider; (2) assigned to the female sex at birth and not had genital gender reassignment surgery; (3) 18 years of age or older; and (4) able to read English. Figure 1 illustrates participation in the study. A total sample of 1146 participants were used for all calculations. Fourteen participants skipped occasional questions regarding conditions associated with dyspareunia but were kept in the final count due to the small percentage of missing data.
Figure 1:
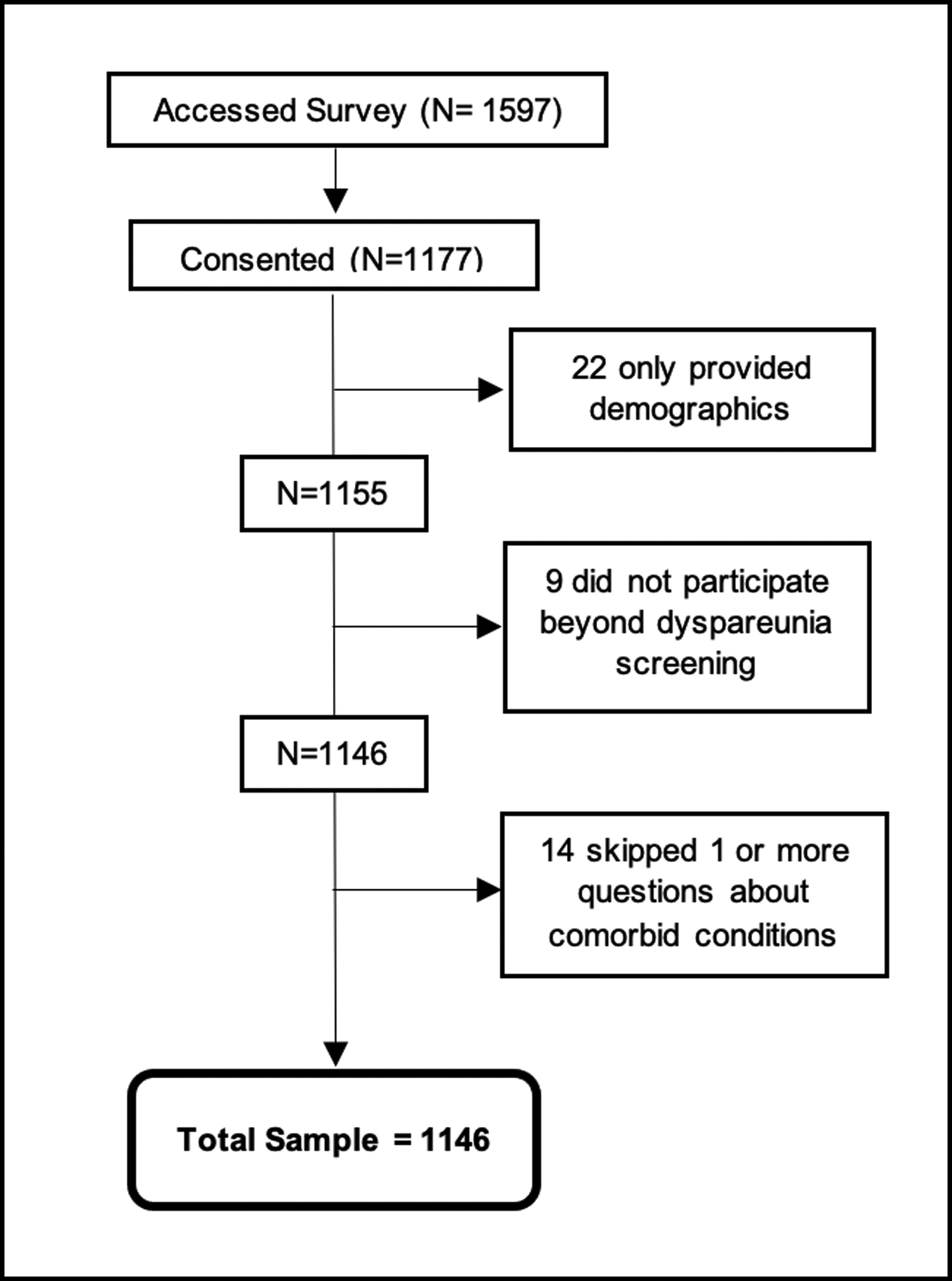
Participation flow chart
The survey was conducted using Qualtrics (Qualtrics®, Provo, UT) and was accessed via a link posted in EDS Facebook support groups and on Twitter (Twitter Inc, San Francisco, CA). Of the 171 EDS support groups approached on Facebook, 55 gave permission to post our survey link once. The survey was posted with the title Ehlers-Danlos Syndrome & Women’s Health Issues to prevent participants from ascertaining that the survey was focused on dyspareunia and vulvodynia which could skew participation. Furthermore, the survey description encouraged women with and without women’s health issues to participate to accurately reflect the presence of women’s health issues in women with EDS or HSD. The same study description and link was also posted on Twitter with the hashtag #EDS. Individuals that were interested in this survey selected the link via either Facebook or Twitter and were brought to Qualtrics where electronic consent was obtained. Qualtrics prevented participants from completing the survey more than once by limiting participation to one per Internet Protocol (IP) address. Qualtrics deidentified data available to researchers by removing the IP addresses.
This survey was developed based on a previously validated online survey used to evaluate dyspareunia and vulvodynia (Schlaeger et al., 2019a). For this study dyspareunia was defined as painful vaginal sexual intercourse; vulvodynia was defined as chronic vulvar pain accompanied by dyspareunia. The survey consisted of multiple choice and open-ended questions. Multiple choice questions asked about participant’s demographics, type of EDS, comorbid conditions associated with EDS, HSD, and vulvodynia, conditions associated with dyspareunia, and vulvodynia symptoms. Questions regarding conditions associated with dyspareunia had response options of yes/no/I’m not sure. All conditions included are shown in Figure 4 (Alimi et al., 2018; American College of Obstetricians and Gynecologists & Committee on Gynecologic Practice, 2016; Seehusen et al., 2014; Sorensen et al., 2018). Open ended questions were used to follow up on “I’m not sure” responses providing space for the participant to elaborate on their specific situation. Comorbid conditions shared by EDS or HSD and vulvodynia were selected from a list of choices with an additional write-in option to include conditions that were not listed. The presence of vulvodynia was determined in the sample using two methods: 1) Vulvodynia Screening Criteria (Figure 2) and 2) participant’s self-report of a vulvodynia diagnosis. Both methods were used to ascertain the rate of vulvodynia in this often-underdiagnosed chronic pain syndrome. We developed the Vulvodynia Screening Criteria based on 4 vulvodynia symptoms including dyspareunia, found to be valid and reliable at diagnosing vulvodynia in two surveys (one online and one that used paper questionnaires) in lieu of a pelvic exam (Harlow et al., 2009; Reed et al., 2006). The Vulvodynia Screening Criteria developed for this study was used in a previous vulvodynia study (Schlaeger et al., 2019a) and was adapted from surveys by Reed et al. (2006) and Harlow et al. (2009). Reed et al.’s (2006) survey had a Cohen’s ĸ of 0.78 (95% CI 0.64 – 0.92) with accuracy of 96.4% for vulvodynia diagnoses, with the survey compared to an in-office visit. Reed et al.’s (2006) survey also had 94.1% accuracy in determining subjects without vulvodynia. Our survey ranged from 29 to 56 questions using branch logic (depending on reported symptomatology) and took an average of 10–20 minutes to complete, with only deidentified data collected.
Data were exported from Qualtrics into Microsoft Excel for cleaning and coding prior to analysis using Stata Software for Statistics 15 (StataCorp LLC, College Station Texas). Missing data were minimal (<3%) in the analysis sample, suggesting minimal bias to our results. Listwise deletion was used in cases with missing responses. Descriptive statistics were used to summarize and describe data results. Correlation tables were used to assess for multicollinearity. Logistic regression was used to describe the effect of demographics and EDS type on a participant’s odds of having dyspareunia and vulvodynia. Logistic regression was also used to describe the effect of comorbid conditions in common between EDS (or HSD) and vulvodynia on a participant’s odds of having vulvodynia (Table 2). The α level was set at < 0.05. “I’m not sure” follow-up questions were assessed by a vulvodynia expert (JS) and were left as “I’m not sure” or changed to “Yes” or “No” based on the response.
Results
The survey was accessed by 1597 potential participants with 1177 women consenting to participate. Of the 1177 participants 1146 completed the survey with < 3% (n=14) skipping occasional questions regarding vulvodynia symptoms and conditions known to be associated with dyspareunia. Participants had a mean age of 38.2 years (±11.5 years SD), were predominantly White, from the U.S, and most had a diagnosis of hEDS/HSD. Of the 14 EDS types 8 were represented; with hEDS/HSD, classical EDS (cEDS), and vascular EDS (vEDS) being the most common types (Table 1). Eighty eight percent of participants resided in either the U.S. or England, and 12% in one of 27 other countries. Characteristics of the sample are summarized in Table 1.
Table 1:
Characteris of sample (N=1146).
| Demographics | N | % | Country | N | % | EDS Type | N | % |
|---|---|---|---|---|---|---|---|---|
| Age | U.S. | 900 | 78.5 | Hypermobile/hypermobility | ||||
| Mean | 1146 | 38.2±11.5 | England | 111 | 9.7 | spectrum disorders | 1046 | 91.3 |
| Range | 1146 | 18–77 | Canada | 46 | 4.0 | Classic | 50 | 4.4 |
| Menopausal | 217 | 18.9% | Australia | 23 | 2.0 | Not sure which type | 21 | 1.8 |
| Gender | Scotland | 8 | 0.7 | Vascular | 10 | 0.9 | ||
| Female | 1144 | 99.8 | Belgium | 6 | 0.5 | Classical-like | 9 | 0.8 |
| Transgender | 2 | 0.2 | Ireland | 6 | 0.5 | Kyphoscoliotic | 6 | 0.5 |
| Race a | Norway | 5 | 0.4 | Cardiac-Valvular | 2 | 0.2 | ||
| White | 1063 | 92.8 | South Africa | 5 | 0.4 | Arthrochalasia | 1 | 0.1 |
| Black or African American | 12 | 1.0 | Wales | 5 | 0.4 | Myopathic | 1 | 0.1 |
| American Indian or Alaskan Native | 17 | 1.5 | Netherlands | 4 | 0.3 | Brittle Cornea | 0 | 0 |
| Asian | 11 | 1.0 | New Zealand | 4 | 0.3 | Dermatosparaxis | 0 | 0 |
| Native Hawaiian or Pacific Islander | 3 | 0.3 | Sweden | 3 | 0.3 | Musculocontractural | 0 | 0 |
| Other | 47 | 4.1 | Denmark | 2 | 0.2 | Periodontal | 0 | 0 |
| Ethnicity (N=1138) | France | 2 | 0.2 | Spondylodysplastic | 0 | 0 | ||
| Hispanic/Latino | 41 | 3.6 | Germany | 2 | 0.2 | |||
| Not Hispanic/Latino | 1046 | 91.9 | Italy | 2 | 0.2 | |||
| Unknown or Not Reported | 51 | 4.5 | Jordan | 2 | 0.2 | |||
| Countries with 1 Participant | 10 | 1.0 |
Abbreviations: EDS, Ehlers-Danlos Syndromes
Participants selected all races they identified with
The rate of dyspareunia was determined by the response to the question “Do you have, or have you had pain with sexual intercourse?”. 63.7% (n=730) of all participants reported pain with intercourse and 3.6% (n=42) of all participants reported they were virgins or not sexually active and were unable to determine if they had dyspareunia. Of the 42 (3.6%) participants who reported they were virgins or not sexually active, 15 reported pain with tampon insertion. The rate of vulvodynia was determined using the Vulvodynia Screening Criteria (Figure 2) with 573 (50%) participants screening positive. The rate of each vulvodynia symptom is shown in Figure 3. Figure 4 shows the rate of each condition associated with dyspareunia. Vulvodynia, ovarian cysts, fibroids, abdominal and pelvic scars, and endometriosis were the most common conditions associated with dyspareunia in our sample.
Figure 2:
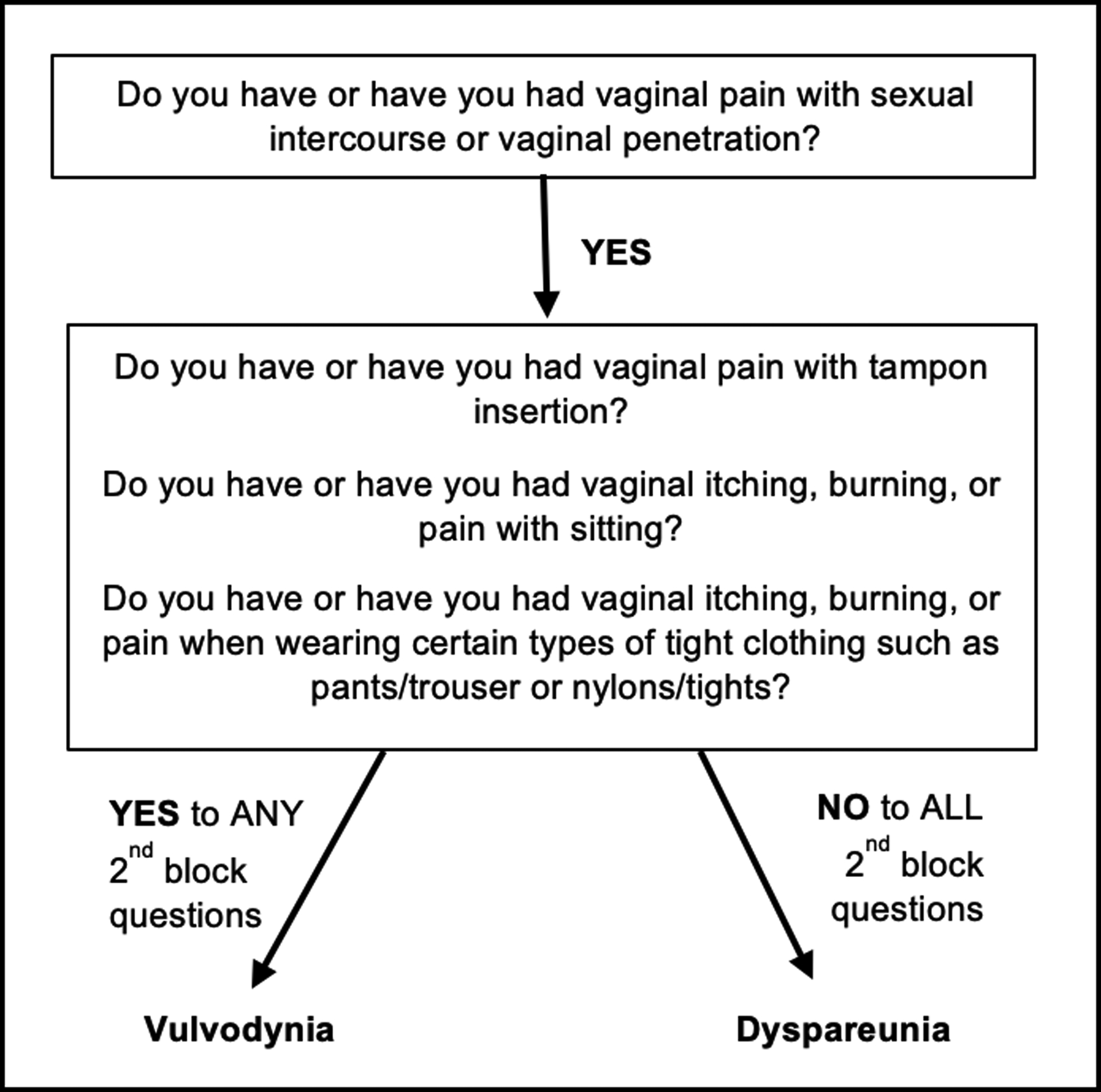
Vulvodynia screening criteria adapted from Reed et al. (Reed et al., 2006) & Harlow et al. (Harlow et al., 2009).
Figure 3:
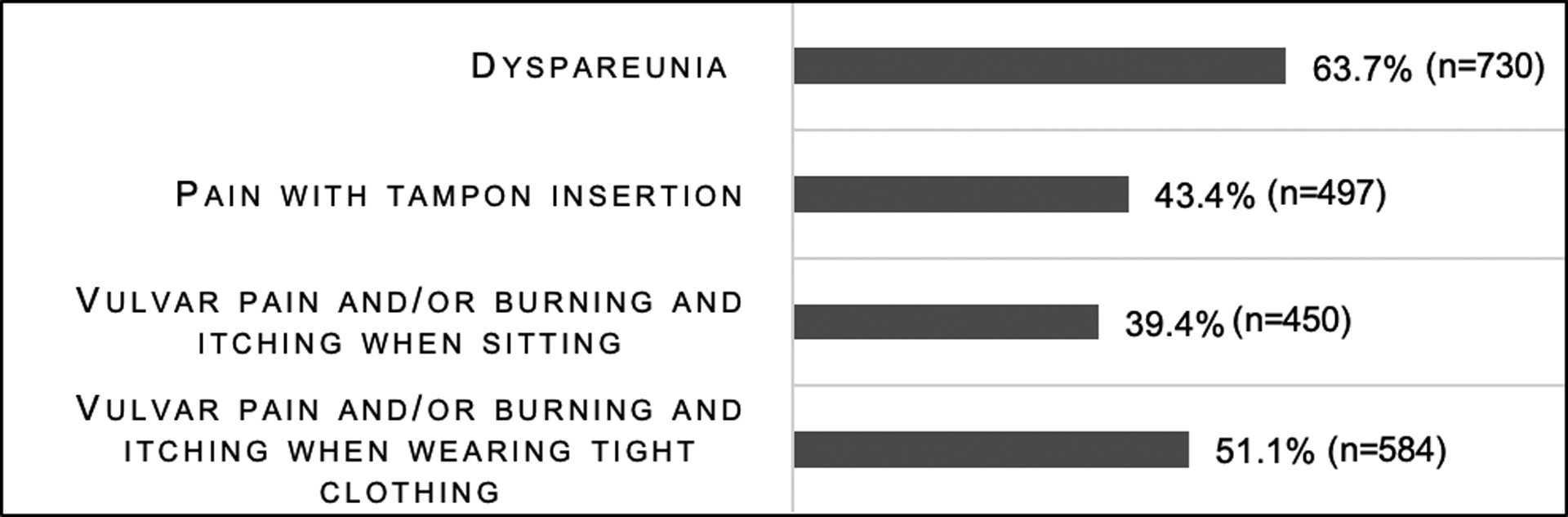
Vulvodynia symptoms in women with Ehlers-Danlos syndromes and hypermobility spectrum disorders.
Each question had a slightly different sample size due to missing data. Sample sizes range from 1142 to 1155.
Figure 4:
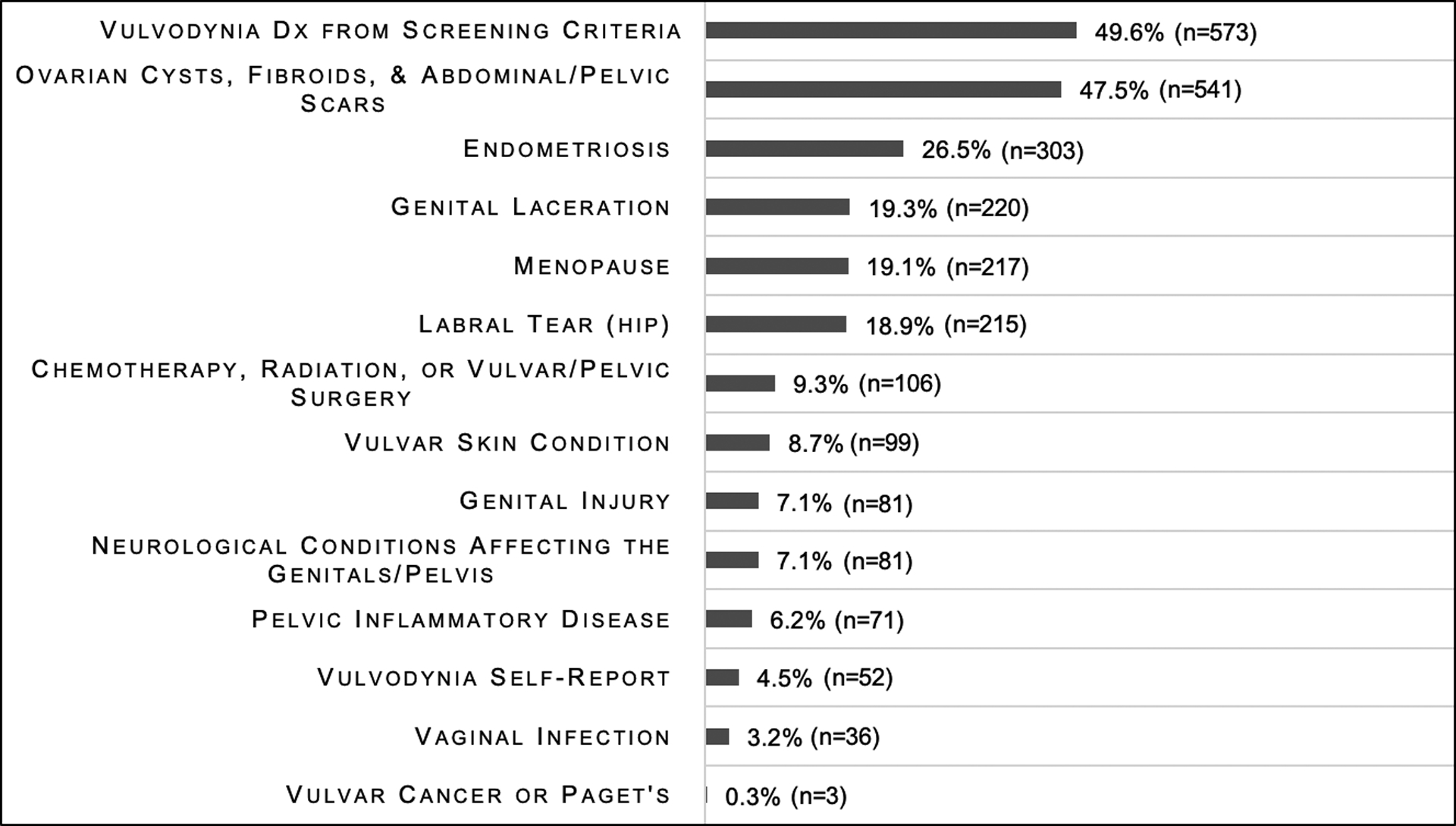
Prevalence of conditions associated with dyspareunia in women with Ehlers-Danlos syndromes and hypermobility spectrum disorders.
Each question had a slightly different sample size due to missing data. Sample sizes range from 1137 to 1146.
Irritable bowel syndrome (Maeland et al., 2011; Reed et al., 2012a; Vieira-Baptista et al., 2014), fibromyalgia (Reed et al., 2012a; Vieira-Baptista et al., 2014), temporomandibular joint dysfunction (Murray et al., 2013; Vieira-Baptista et al., 2014), interstitial cystitis (Castori et al., 2013a; Reed et al., 2012a; Seneviratne et al., 2017), mast cell activation syndrome (McDonald & Rapkin, 2012; Regauer et al., 2015; Seneviratne et al., 2017), and migraine (Hakim & Grahame, 2004; Puledda et al., 2015; Vieira-Baptista et al., 2014) are known comorbidities of EDS and vulvodynia. Figure 5 shows the number of each comorbid condition in the total sample with fibromyalgia having the highest rate at 40% (n=458).
Figure 5:
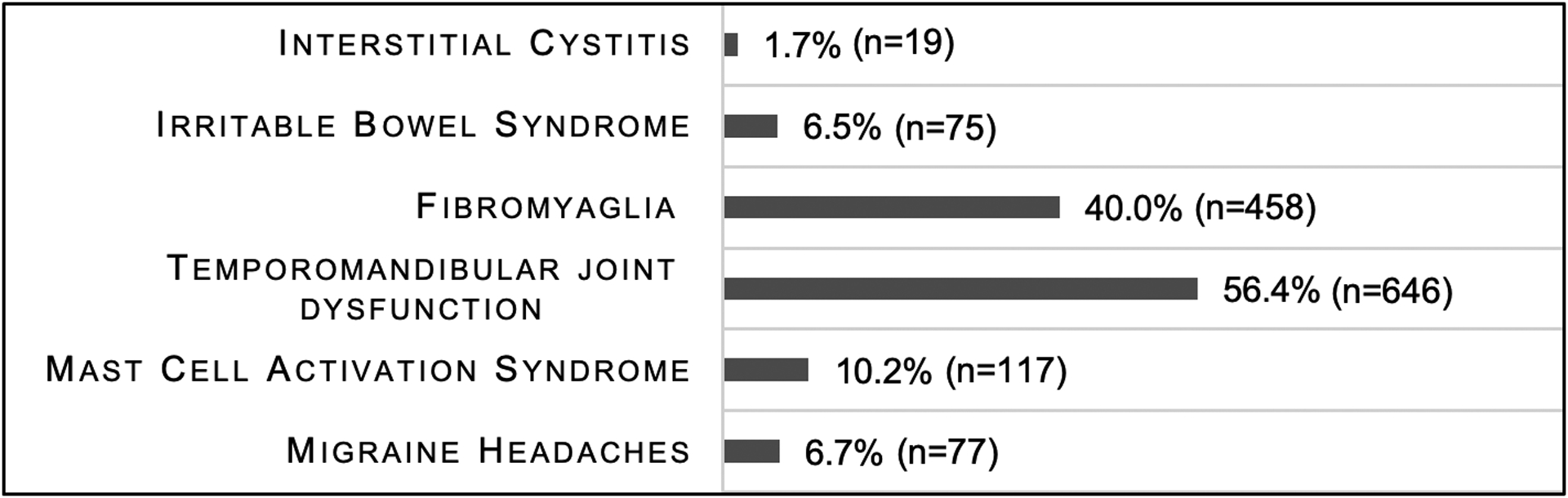
Frequency of comorbid conditions associated with vulvodynia and Ehlers-Danlos Syndromes or hypermobility spectrum disorders (N=1146).
Participants’ demographics were significantly associated with the odds of whether or not they had dyspareunia or screened positive for vulvodynia (Table 2). The odds of having dyspareunia varied significantly based on age and whether or not a participant was White (p=0.006). Age had a nonlinear relationship with the odds of women having dyspareunia. Younger women and older women had higher odds of having dyspareunia than middle age women; with women in their mid-forties having the lowest odds (Figure 6). White participants had decreased odds of having dyspareunia compared to participants who were not White. Similarly, the odds of screening positive for vulvodynia varied significantly based on demographics. Age had a linear relationship with the odds of screening positive for vulvodynia, with each 1-year increase in age the odds decreased. Participants in the U.S. had decreased odds of screening positive for vulvodynia compared to participants who resided outside the U.S. he odds of having dyspareunia or screening positive for vulvodynia did not vary based on whether or not a participant was hispanic of latino, or if the participant had hEDS/HSD compared to other types of EDS. The effect of specific races other than White, countries outside of the U.S., and EDS types not including hEDS/HSD on the odds of reporting dyspareunia or screening positive for vulvodynia were unable to be evaluated due to small group size.
Table 2:
Logistic Odds of Having Dyspareunia and Vulvodynia Based on Demographics (N=1146).
| Variable | Adjusted OR | 95% Cl | P |
|---|---|---|---|
| Dyspareunia (p=0.006) * | |||
| Age | 0.902 | 0.841 – 0.968 | 0.004* |
| US Resident | 0.836 | 0.464 – 1.506 | 0.550 |
| White | 0.708 | 0.511 – 0.980 | 0.037* |
| hEDS | 0.855 | 0.542 – 1.347 | 0.499 |
| Hispanic or Latino | 1.232 | 0.747 – 2.033 | 0.414 |
| Vulvodynia (p=0.001) * | |||
| Age | 0.981 | 0.977 – 0.998 | 0.018* |
| US Resident | 0.668 | 0.498 – 0.895 | 0.007* |
| White | 0.848 | 0.498 – 1.444 | 0.533 |
| hEDS | 0.768 | 0.506 – 1.167 | 0.216 |
| Hispanic or Latino | 1.491 | 0.947 – 2.349 | 0.085 |
Abbreviations: OR, odds ratio; CI, confidence interval; hEDS, Hypermobility type Ehlers-Danlos syndrome
significant findings at p< .05 level
The odds of participants with EDS or HSD screening positive for vulvodynia varied significantly based on which comorbid conditions shared EDS or HSD and vulvodynia (p<0.001) (Table 3) they had when adjusting for age and residing in the U.S. Participants that had fibromyalgia, interstitial cystitis, temporomandibular joint dysfunction, migraine headaches, or interstitial cystitis had increased odds of screening positive for vulvodynia (Table 3). However, the sample of participants with interstitial cystitis was small (n=19). Irritable bowel syndrome and mast cell activation syndrome did not correlate with screening positive for vulvodynia.
Table 3:
Logistic odds of having vulvodynia based on the presence of comorbid conditions common in Ehlers-Danlos syndromes (and hypermobility spectrum disorders) and vulvodynia (p=0.000).
| Variable | N | Adjusted OR | 95% Cl | P |
|---|---|---|---|---|
| Age | 1146 | 0.984 | 0.974 – 0.995 | 0.003* |
| US Resident | 1146 | 0.639 | 0.476 – 0.858 | 0.002* |
| Temporomandibular Joint Dysfunction | 651 | 1.286 | 1.008 – 1.639 | 0.043* |
| Fibromyalgia | 463 | 1.885 | 1.457 – 2.416 | 0.000* |
| Migraine Headaches | 78 | 1.548 | 0.939 – 2.554 | 0.087 |
| Mast Cell Activation Syndrome | 118 | 0.928 | 0.622 – 1.384 | 0.714 |
| Interstitial Cystitis | 19 | 5.652 | 1.607 – 19.883 | 0.007* |
| Irritable Bowel Syndrome | 75 | 0.632 | 0.381 – 1.048 | 0.075 |
significant findings at p< .05 level
Discussion
The two new findings in this study are: 1) women with EDS or HSD may have a higher rate of vulvodynia (50%) than women in the U.S. population at large (8%); and 2) EDS or HSD and vulvodynia have comorbid conditions in common. Dyspareunia is a hallmark of vulvodynia. Our sample had a high rate of dyspareunia (63.7%) that was consistent with three previous studies on EDS that reported it to be as high as 77% (Castori et al., 2012; Hugon-Rodin et al., 2016; Hurst et al., 2014). However, this study also examined the rate of other conditions associated with dyspareunia in women with EDS (Castori et al., 2012; Hugon-Rodin et al., 2016; Hurst et al., 2014; Tinkle et al., 2017). Our finding that 50% of women with EDS or HSD may also have vulvodynia, over six times the rate of the general population, is important because this group has a high chronic pain burden (Schlaeger et al., 2019a; Schlaeger et al., 2019b; Tinkle et al., 2017; Voermans et al., 2010). Women with EDS have fragile lax tissues (Ehlers-Danlos syndrome: National Library of Science (US), 2019; Malfait et al., 2017; Tinkle et al., 2017) and report spontaneous vaginal lacerations due to the poor integrity of their collagen which may promote scarring and dyspareunia. Lax ligaments in EDS and HSD can cause pelvic instability (Aydeniz et al., 2010; Hunt et al., 2007; Tinkle et al., 2017) which can result in hypertonic pelvic floor muscles in an attempt to stabilize the pelvis (Hartmann & Sarton, 2014; Morin et al., 2017; Prather et al., 2009). Hypertonic pelvic floor muscles have been found to contribute to vulvodynia (Bornstein et al., 2016), which may explain the high rate of vulvodynia in women with EDS and HSD. Our data suggests that these women suffer chronic pain that interferes with their intimate relationships.
The four questions used to screen for vulvodynia in lieu of a pelvic exam: 1) Do you have or have you had pain with sexual intercourse or vaginal penetration?; 2) Do you have or have you had vaginal pain with tampon insertion?; 3) Do you have or have you had vaginal itching and burning or pain with sitting?; and, 4) Do you have or have you had vaginal itching and burning or pain when wearing certain types of tight clothing such as pants/trousers or nylons/tights? (Vulvodynia Screening Criteria) (Harlow et al., 2009; Reed et al., 2006) were instrumental in enabling us to perform this survey in a large sample of women from around the world. Identifying that dyspareunia occurs and vulvodynia may occur at a higher rate in EDS and HSD highlights the importance of ensuring adequate gynecologic screening for dyspareunia.
Demographics may have an impact on the odds of having dyspareunia and vulvodynia. The U shaped curve (Figure 6) relationship between age and the odds of having dyspareunia may be explained by the increased odds of vulvodynia in younger women (< 48 years old) and the increased odds of atrophic vaginitis in post-menopausal women (> 48 years old). We found a linear relationship between age and the odds of screening positive for vulvodynia, where a woman’s odds of screening positive decreased as a woman aged. Younger women may be at higher risk for screening positive for vulvodynia due to the use of combined estrogen-progestin oral contraceptives, which may be associated with a higher rate of vulvodynia (Bornstein et al., 2016). Decreased odds for vulvodynia in women who reside in the U.S. may be explained by culturally specific issues that may influence the manifestation of vulvodynia which requires further examination. Whether or not women had hEDS/HSD or another type of EDS did not impact the odds of dyspareunia or vulvodynia. Our findings suggest that women with EDS and HSD should be screened for dyspareunia and vulvodynia, and that dyspareunia and vulvodynia impact this group universally.
Figure 6:
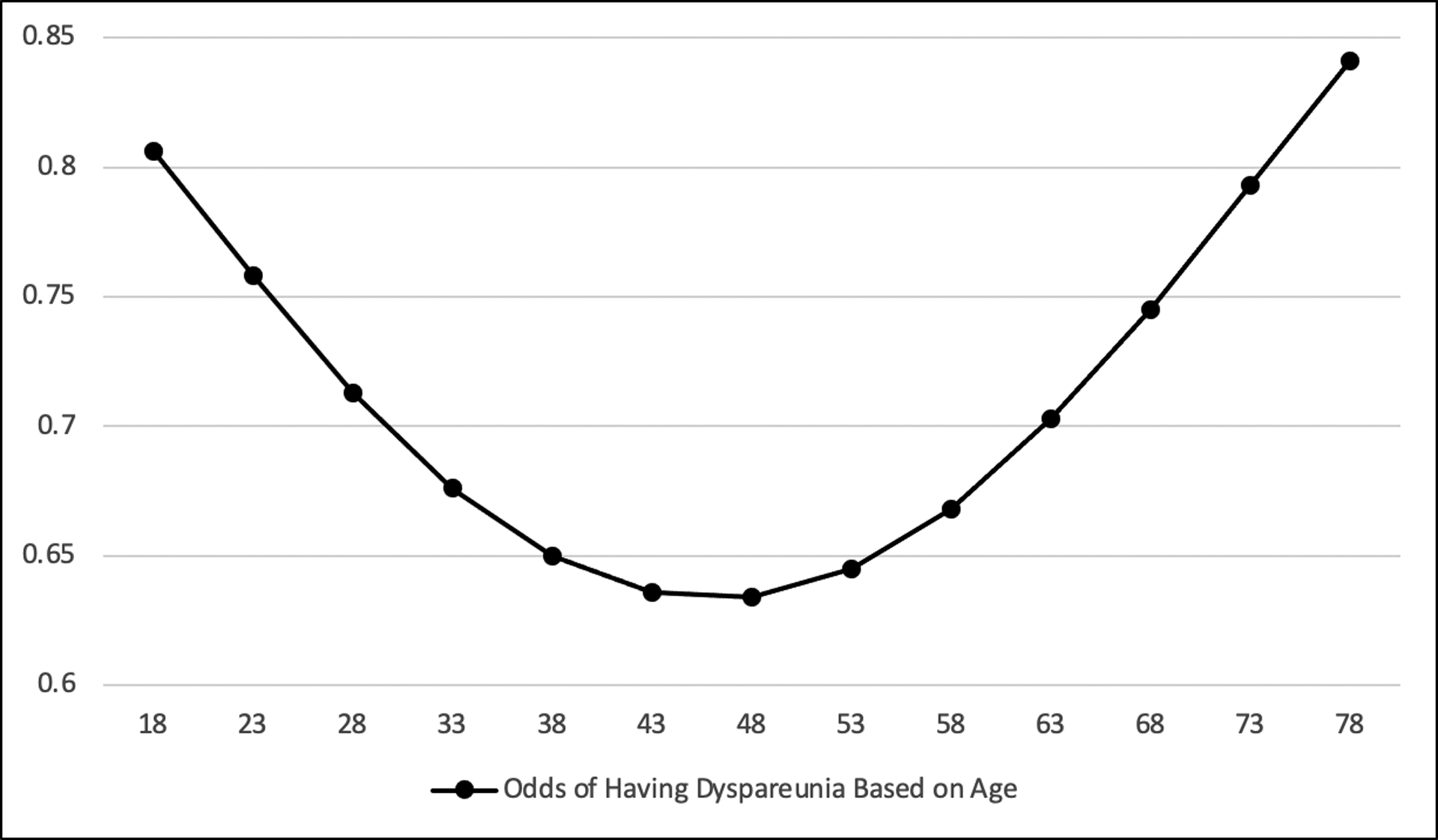
Logistic odds of having dyspareunia based on age (N=1118).
Sample size of 1118 due to listwise deletion of missing observations.
It is well known that women suffering from chronic pain conditions may have a higher prevalence of shared comorbid conditions such as fibromyalgia, temporomandibular joint disorder, irritable bowel syndrome, interstitial cystitis, migraine, and vulvodynia (Backonja & Argoff, 2005; Bornstein et al., 2016; Bouhassira et al., 2005; Biasi et al., 2014; Puledda et al., 2015; Reed et al., 2012a). It is hypothesized this is due to sensitization of the central and or peripheral nervous system (Backonja & Argoff, 2005; Camerota et al., 2011; Castori, 2016). However, our study identified that these same comorbid conditions are indeed shared by women with EDS or HSD and vulvodynia. Comorbid conditions have been reported in EDS and HSD (Hakim & Grahame, 2004; Maeland et al., 2011; Murray et al., 2013; Puledda et al., 2015; Seneviratne et al., 2017) and women with vulvodynia (McDonald & Rapkin, 2012; Regauer et al., 2015; Vieira-Baptista et al., 2014) separately, but never together. Women with fibromyalgia, temporomandibular joint dysfunction, migraine, or interstitial cystitis had higher odds of having dyspareunia and vulvodynia than those without the respective comorbid conditions. This finding suggests that both EDS or HSD and vulvodynia may have a neuropathic component which affects treatment options (Bornstein et al., 2016; Castori et al., 2013a; Castori et al., 2013b; Schlaeger et al., 2019a). Clinical presentation is typically complex, with multiple comorbidities, rendering it unlikely that an individual or cohort would respond to one single treatment option.
Our study’s large sample size is noteworthy and would have not been possible in a prospective design. A limitation of this study was that all diagnoses were self-reported or diagnosed through a reliable and validated screening tool and were not verified by a healthcare provider (Harlow et al., 2009; Reed et al., 2006). Using self-report and a screening tool to examine the rate of conditions could increase the rate of false positives. However, the burden of performing pelvic exams would have made the large sample size difficult, if not impossible to obtain. In addition, our findings are roughly in line with the “real world” experience in clinics and daily living of affected individuals, which documents a wide array of chronic pain manifestations in people with EDS and HSD.
Conducting EDS research is difficult as most types are classified as a rare disease (National Insititutes of Health, 2017; Steinmann et al., 2002) which hampers case finding. Using social media enabled us to examine gynecological conditions in women with EDS or HSD in a large population from around the globe, thus suggesting the utility and benefit of using an online survey format. Our sample was a convenience sample of only women who had access to providers that diagnosed EDS or HSD, used social media, and could read English. Participants in this study were predominantly from the U.S., White, had internet access, and used social media; and the study was conducted in English. The Vulvodynia Screening Criteria enabled us to identify women who may have vulvodynia in a large sample who also have a rare disease.
Conclusion
The findings of our study suggest important information for clinicians caring for women with EDS or HSD. EDS, HSD, and vulvodynia have shared comorbid conditions that include fibromyalgia, interstitial cystitis, and temporomandibular joint dysfunction. The complex nature of EDS, HSD and vulvodynia make their treatment a clinical challenge. The first step towards treatment is recognizing that women with EDS or HSD may have vulvodynia and dyspareunia. Recognizing the high rate of vulvodynia in this population may provide insights into risk factors for vulvodynia as well as the development of new treatments.
Disclosure of Funding:
This publication was made possible in part by Grant Number R01 HD091210, R01 HD089935, and 1F31 NR019529-01A1 from the National Institutes of Health, National Institute of Child Health and Human Development (NICHD) and the National Institute for Nursing Research (NINR). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NICHD or NINR. The final peer-reviewed manuscript is subject to the National Institutes of Health Public Access Policy. This publication is Co-sponsored by the Rockefeller University Heilbrunn Family Center for Research Nursing through the generosity of the Heilbrunn Family and the National Center for Advancing Translational Sciences, National Institutes of Health, through Rockefeller University, Grant # UL1 TR001866.
Bios of Authors:
Jennifer E. Glayzer, BSN, RN, is a PhD student at the University of Illinois Chicago, College of Nursing, Department of Human Development Nursing Science, jshenk5@uic.edu
Barbara L. McFarlin, PhD, CNM, RDMS, FACNM, FAAN, is a Professor at the University of Illinois Chicago, College of Nursing, Department of Human Development Nursing Science, bmcfar1@uic.edu,
Marco Castori, MD, PhD, Head of the Division of Medical Genetics, Fondazione IRCCS-Casa Sollievo Della Sofferenza in Italy, m.castori@operapadrepio.it
Marie L. Suarez, PhD, Research Project Director at the University of Illinois Chicago, College of Nursing, Department of Human Development Nursing Science, mlsuarez@uic.edu
Monya C. Meinel, BA, Research Coordinator at the University of Illinois Chicago, College of Nursing, Department of Human Development Nursing Science, meinemo@uic.edu,
William H. Kobak, MD, Director of Urogynecology and Associate Professor of Obstetrics and Gynecology at the University of Illinois Chicago, College of Medicine, wkobak@uic.edu
Alana D. Steffen, PhD, Research Assistant Professor and Senior Biostatistician at the University of Illinois Chicago, College of Nursing, Department of Population Health Nursing Science, steffena@uic.edu
Judith M. Schlaeger, PhD, RN, CNM, LAc, FAAN, Associate Professor at the University of Illinois Chicago, College of Nursing, Department of Human Development Nursing Science, jschlaeg@uic.edu
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
Data Availability:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Alimi Y, Iwanaga J, Oskouian RJ, Loukas M, & Tubbs RS (2018). The clinical anatomy of dyspareunia: A review. Clinical Anatomy, 31(7), 1013–1017. 10.1002/ca.23250 [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists & Committee on Gynecologic Practice (2016). Committee Opinion No 673: Persistent Vulvar Pain. Obstetrics and Gynecology, 128(3), e78. 10.1097/AOG.0000000000001645 [DOI] [PubMed] [Google Scholar]
- Andrews JC (2011). Vulvodynia interventions--systematic review and evidence grading. Obstetrical and Gynecological Survey, 66(5), 299–315. 10.1097/OGX.0b013e3182277fb7 [DOI] [PubMed] [Google Scholar]
- Aydeniz A, Dikensoy E, Cebesoy B, Altindag O, Gursoy S, & Balat O (2010). The relation between genitourinary prolapse and joint hypermobility in Turkish women. Archives of Gynecology and Obstetrics, 281(2), 301–304. 10.1007/s00404-009-1103-3 [DOI] [PubMed] [Google Scholar]
- Backonja MM, & Argoff CE (2005). Neuropathie Pain-Definition and Implications for Research and Therapy. Journal of Neuropathic Pain & Symptom Palliation, 1(2), 11–17. 10.3109/J426v01n02_03 [DOI] [Google Scholar]
- Biasi G, Di Sabatino V, Ghizzani A, & Galeazzi M (2014). Chronic pelvic pain: comorbidity between musculoskeletal pain and vulvodynia. Reumatismo, 66(1). 10.4081/reumatismo.2014.768 [DOI] [PubMed] [Google Scholar]
- Bornstein J, Goldstein AT, Stockdale CK, Bergeron S, Pukall C, Zolnoun D, Coady D, Consensus vulvar pain terminology committee of the International Society for the Study of Vulvovaginal Disease, the International Society for the Study of Women’s Sexual Health, & the International Pelvic Pain, Society (2016). 2015 ISSVD, ISSWSH and IPPS Consensus Terminology and Classification of Persistent Vulvar Pain and Vulvodynia. Obstetrics and Gynecology, 127(4), 745–751. 10.1097/AOG.0000000000001359 [DOI] [PubMed] [Google Scholar]
- Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, Cunin G, Fermanian J, Ginies P, Grun-Overdyking A, Jafari-Schluep H, Lanteri-Minet M, Laurent B, Mick G, Serrie A, Valade D, & Vicaut E (2005). Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain, 114(1–2), 29–36. 10.1016/j.pain.2004.12.010 [DOI] [PubMed] [Google Scholar]
- Camerota F, Celletti C, Castori M, Grammatico P, & Padua L (2011). Neuropathic pain is a common feature in Ehlers-Danlos syndrome. Journal of Pain and Symptom Management, 41(1), e2–4. 10.1016/j.jpainsymman.2010.09.012 [DOI] [PubMed] [Google Scholar]
- Castori M (2016). Pain in Ehlers-Danlos syndromes: manifestations, therapeutic strategies and future perspectives. Expert Opinion on Orphan Drugs, 4(11), 1145–1158. 10.1080/21678707.2016.1238302 [DOI] [Google Scholar]
- Castori M, Celletti C, & Camerota F (2013a). Ehlers–Danlos syndrome hypermobility type: a possible unifying concept for various functional somatic syndromes. Rheumatology International, 33(3), 819–821. 10.1007/s00296-011-2275-2 [DOI] [PubMed] [Google Scholar]
- Castori M, & Hakim A (2017). Contemporary approach to joint hypermobility and related disorders. Current Opinion in Pediatrics, 29(6), 640–649. 10.1097/MOP.0000000000000541 [DOI] [PubMed] [Google Scholar]
- Castori M, Morlino S, Celletti C, Ghibellini G, Bruschini M, Grammatico P, Blundo C, & Camerota F (2013b). Re-writing the natural history of pain and related symptoms in the joint hypermobility syndrome/Ehlers-Danlos syndrome, hypermobility type. American Journal of Medical Genetics. Part A, 161A(12), 2989–3004. 10.1002/ajmg.a.36315 [DOI] [PubMed] [Google Scholar]
- Castori M, Morlino S, Dordoni C, Celletti C, Camerota F, Ritelli M, Morrone A, Venturini M, Grammatico P, & Colombi M (2012). Gynecologic and obstetric implications of the joint hypermobility syndrome (a.k.a. Ehlers-Danlos syndrome hypermobility type) in 82 Italian patients. American Journal of Medical Genetics Part A, 158A(9), 2176–2182. 10.1002/ajmg.a.35506 [DOI] [PubMed] [Google Scholar]
- Castori M, Tinkle B, Levy H, Grahame R, Malfait F, & Hakim A (2017). A framework for the classification of joint hypermobility and related conditions. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 175(1), 148–157. 10.1002/ajmg.c.31539 [DOI] [PubMed] [Google Scholar]
- Chopra P, Tinkle B, Hamonet C, Brock I, Gompel A, Bulbena A, & Francomano C (2017). Pain management in the Ehlers-Danlos syndromes. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 175(1), 212–219. 10.1002/ajmg.c.31554 [DOI] [PubMed] [Google Scholar]
- Copetti M, Morlino S, Colombi M, Grammatico P, Fontana A, & Castori M (2019). Severity classes in adults with hypermobile Ehlers–Danlos syndrome/hypermobility spectrum disorders: a pilot study of 105 Italian patients. Rheumatology, 58(10), 1722–1730. 10.1093/rheumatology/kez029 [DOI] [PubMed] [Google Scholar]
- Demes JS, McNair B, & Taylor MR (2020). Use of complementary therapies for chronic pain management in patients with reported Ehlers‐Danlos syndrome or hypermobility spectrum disorders. American Journal of Medical Genetics Part A, 182(11), 2611–2623. 10.1002/ajmg.a.61837 [DOI] [PubMed] [Google Scholar]
- Ehlers-Danlos syndrome: National Library of Science (US). (2019). Retrieved 10/20/2019 from https://ghr.nlm.nih.gov/condition/ehlers-danlos-syndrome#sourcesforpage
- Gilliam E, Hoffman JD, & Yeh G (2020). Urogenital and pelvic complications in the Ehlers-Danlos syndromes and associated hypermobility spectrum disorders: A scoping review. Clinical Genetics, 97(1), 168–178. 10.1111/cge.13624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AT, Pukall CF, Brown C, Bergeron S, Stein A, & Kellogg-Spadt S (2016). Vulvodynia: Assessment and Treatment. Journal of Sexual Medicine, 13(4), 572–590. 10.1016/j.jsxm.2016.01.020 [DOI] [PubMed] [Google Scholar]
- Grahame R, Bird HA, & Child A (2000). The revised (Brighton 1998) criteria for the diagnosis of benign joint hypermobility syndrome (BJHS). Journal of Rheumatology, 27(7), 1777–1779. [PubMed] [Google Scholar]
- Hakim AJ, & Grahame R (2004). Non-musculoskeletal symptoms in joint hypermobility syndrome. Indirect evidence for autonomic dysfunction? Rheumatology, 43(9), 1194–1195. 10.1093/rheumatology/keh279 [DOI] [PubMed] [Google Scholar]
- Harlow BL, Vazquez G, MacLehose RF, Erickson DJ, Oakes JM, & Duval SJ (2009). Self-reported vulvar pain characteristics and their association with clinically confirmed vestibulodynia. Journal Women’s Health, 18(9), 1333–1340. 10.1089/jwh.2008.1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann D, & Sarton J (2014). Chronic pelvic floor dysfunction. Best Practice and Research. Clinical Obstetrics and Gynaecology, 28(7), 977–990. 10.1016/j.bpobgyn.2014.07.008 [DOI] [PubMed] [Google Scholar]
- Hugon-Rodin J, Lebegue G, Becourt S, Hamonet C, & Gompel A (2016). Gynecologic symptoms and the influence on reproductive life in 386 women with hypermobility type ehlers-danlos syndrome: a cohort study. Orphanet Journal of Rare Diseases, 11(1), 124. 10.1186/s13023-016-0511-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D, Clohisy J, & Prather H (2007). Acetabular labral tears of the hip in women. Physical Medicine and Rehabilitation Clinics of North America, 18(3), 497–520, ix-x. 10.1016/j.pmr.2007.05.007 [DOI] [PubMed] [Google Scholar]
- Hurst BS, Lange SS, Kullstam SM, Usadi RS, Matthews ML, Marshburn PB, Templin MA, & Merriam KS (2014). Obstetric and gynecologic challenges in women with Ehlers-Danlos syndrome. Obstetrics and Gynecology, 123(3), 506–513. 10.1097/AOG.0000000000000123 [DOI] [PubMed] [Google Scholar]
- Latthe P, Latthe M, Say L, Gülmezoglu M, & Khan KS (2006). WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health, 6(1), 1–7. 10.1186/1471-2458-6-177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeland S, Assmus J, & Berglund B (2011). Subjective health complaints in individuals with Ehlers-Danlos syndrome: a questionnaire study. Disability and Rehabilitation, 48(6), 720–724. 10.1016/j.ijnurstu.2010.10.007 [DOI] [PubMed] [Google Scholar]
- Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, Bloom L, Bowen JM, Brady AF, Burrows NP, Castori M, Cohen H, Colombi M, Demirdas S, De Backer J, De Paepe A, Fournel-Gigleux S, Frank M, Ghali N, Giunta C, Grahame R, Hakim A, Jeunemaitre X, Johnson D, Juul-Kristensen B, Kapferer-Seebacher I, Kazkaz H, Kosho T, Lavallee ME, Levy H, Mendoza-Londono R, Pepin M, Pope FM, Reinstein E, Robert L, Rohrbach M, Sanders L, Sobey GJ, Van Damme T, Vandersteen A, van Mourik C, Voermans N, Wheeldon N, Zschocke J, & Tinkle B (2017). The 2017 international classification of the Ehlers-Danlos syndromes. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 175(1), 8–26. 10.1002/ajmg.c.31552 [DOI] [PubMed] [Google Scholar]
- McDonald JS, & Rapkin AJ (2012). Multilevel local anesthetic nerve blockade for the treatment of generalized vulvodynia: a pilot study. Journal of Sexual Medicine, 9(11), 2919–2926. 10.1111/j.1743-6109.2012.02909.x [DOI] [PubMed] [Google Scholar]
- McIntosh LJ, Mallett VT, Frahm JD, Richardson DA, & Evans MI (1995). Gynecologic disorders in women with Ehlers-Danlos syndrome. Journal of the Society for Gynecologic Investigation, 2(3), 559–564. 10.1177/107155769500200309 [DOI] [PubMed] [Google Scholar]
- Morin M, Binik YM, Bourbonnais D, Khalife S, Ouellet S, & Bergeron S (2017). Heightened Pelvic Floor Muscle Tone and Altered Contractility in Women With Provoked Vestibulodynia. Journal of Sexual Medicine, 14(4), 592–600. 10.1016/j.jsxm.2017.02.012 [DOI] [PubMed] [Google Scholar]
- Murray B, Yashar BM, Uhlmann WR, Clauw DJ, & Petty EM (2013). Ehlers-Danlos syndrome, hypermobility type: A characterization of the patients’ lived experience. American Journal of Medical Genetics Part A, 161A(12), 2981–2988. 10.1002/ajmg.a.36293 [DOI] [PubMed] [Google Scholar]
- National Institutes of Health (2017). FAQs About Rare Disease. https://rarediseases.info.nih.gov/diseases/pages/31/faqs-about-rare-diseases
- Prather H, Dugan S, Fitzgerald C, & Hunt D (2009). Review of anatomy, evaluation, and treatment of musculoskeletal pelvic floor pain in women. PM&R: The Journal of Injury, Function and Rehabilitation, 1(4), 346–358. 10.1016/j.pmrj.2009.01.003 [DOI] [PubMed] [Google Scholar]
- Puledda F, Vigano A, Celletti C, Petolicchio B, Toscano M, Vicenzini E, Castori M, Laudani G, Valente D, Camerota F, & Di Piero V (2015). A study of migraine characteristics in joint hypermobility syndrome a.k.a. Ehlers-Danlos syndrome, hypermobility type. Neurological Sciences, 36(8), 1417–1424. 10.1007/s10072-015-2173-6 [DOI] [PubMed] [Google Scholar]
- Reed BD, Haefner HK, Harlow SD, Gorenflo DW, & Sen A (2006). Reliability and validity of self-reported symptoms for predicting vulvodynia. Obstetrics and Gynecology, 108(4), 906–913. 10.1097/01.AOG.0000237102.70485.5d [DOI] [PubMed] [Google Scholar]
- Reed BD, Harlow SD, Sen A, Edwards RM, Chen D, & Haefner HK (2012a). Relationship between vulvodynia and chronic comorbid pain conditions. Obstetrics and Gynecology, 120(1), 145. 10.1097/AOG.0b013e31825957cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed BD, Harlow SD, Sen A, Legocki LJ, Edwards RM, Arato N, & Haefner HK (2012b). Prevalence and demographic characteristics of vulvodynia in a population-based sample. American Journal of Obstetrics and Gynecology, 206(2), 170 e171–179. 10.1016/j.ajog.2011.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regauer S, Eberz B, & Beham-Schmid C (2015). Mast cell infiltrates in vulvodynia represent secondary and idiopathic mast cell hyperplasias. Journal of Pathology, Microbiology and Immunology, 123(5), 452–456. 10.1111/apm.12372 [DOI] [PubMed] [Google Scholar]
- Sacheti A, Szemere J, Bernstein B, Tafas T, Schechter N, & Tsipouras P (1997). Chronic pain is a manifestation of the Ehlers-Danlos syndrome. Journal of Pain and Symptom Management, 14(2), 88–93. 10.1016/s0885-3924(97)00007-9 [DOI] [PubMed] [Google Scholar]
- Schlaeger JM, Patil CL, Steffen AD, Pauls HA, Roach KL, Thornton PD, Hartmann D, Kobak WH, Yao Y, Suarez ML, Hughes TL, & Wilkie DJ (2019a). Sensory pain characteristics of vulvodynia and their association with nociceptive and neuropathic pain: an online survey pilot study. Pain Reports, 4(2), e713. 10.1097/PR9.0000000000000713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaeger JM, Pauls HA, Powell-Roach KL, Thornton PD, Hartmann D, Suarez ML, Kobak WH, Hughes TL, Steffen AD, & Patil CL (2019b). Vulvodynia, “A Really Great Torturer”: A Mixed Methods Pilot Study Examining Pain Experiences and Drug/Non-drug Pain Relief Strategies. Journal of Sexual Medicine, 16(8), 1255–1263. 10.1016/j.jsxm.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubart JR, Schaefer E, Hakim AJ, Francomano CA, & Bascom R (2019). Use of cluster analysis to delineate symptom profiles in an Ehlers-Danlos syndrome patient population. Journal of Pain and Symptom Management, 58(3), 427–436. 10.1016/j.jpainsymman.2019.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehusen DA, Baird DC, & Bode DV (2014). Dyspareunia in women. American Family Physician, 90(7), 465–470. https://www.ncbi.nlm.nih.gov/pubmed/25369624 [PubMed] [Google Scholar]
- Seneviratne SL, Maitland A, & Afrin L (2017). Mast cell disorders in Ehlers-Danlos syndrome. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 175(1), 226–236. 10.1002/ajmg.c.31555 [DOI] [PubMed] [Google Scholar]
- Sorensen J, Bautista KE, Lamvu G, & Feranec J (2018). Evaluation and Treatment of Female Sexual Pain: A Clinical Review. Cureus, 10(3), e2379. 10.7759/cureus.2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann B, Royce PM, & Superti-Furga A (2002). The Ehlers-Danlos syndrome. Connective tissue and its heritable disorders, 2, 431–523. [Google Scholar]
- Tinkle B, Castori M, Berglund B, Cohen H, Grahame R, Kazkaz H, & Levy H (2017). Hypermobile Ehlers-Danlos syndrome (a.k.a. Ehlers-Danlos syndrome Type III and Ehlers-Danlos syndrome hypermobility type): Clinical description and natural history. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 175(1), 48–69. 10.1002/ajmg.c.31538 [DOI] [PubMed] [Google Scholar]
- Tommola P, Unkila-Kallio L, & Paavonen J (2010). Surgical treatment of vulvar vestibulitis: a review. Acta Obstetricia et Gynecologica Scandinavica, 89(11), 1385–1395. 10.3109/00016349.2010.512071 [DOI] [PubMed] [Google Scholar]
- Vieira-Baptista P, Lima-Silva J, Cavaco-Gomes J, & Beires J (2014). Prevalence of vulvodynia and risk factors for the condition in Portugal. International Journal of Gynecology & Obstetrics, 127(3), 283–287. 10.1016/j.ijgo.2014.05.020 [DOI] [PubMed] [Google Scholar]
- Voermans NC, Knoop H, Bleijenberg G, & van Engelen BG (2010). Pain in ehlers-danlos syndrome is common, severe, and associated with functional impairment. Journal of Pain and Symptom Management, 40(3), 370–378. 10.1016/j.jpainsymman.2009.12.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


