Abstract
Purpose of Review
The prognosis of pediatric patients with metastatic solid tumors remains poor, necessitating development of novel therapeutic strategies. The biology of the pediatric tumor microenvironment (TME) presents obstacles for the efficacy of current therapeutic approaches including immunotherapies. Targeting various aspects of the TME in pediatric patients with solid tumors represents a therapeutic opportunity that may improve outcomes. Here we will discuss recent advances in characterization of the TME, and clinical advances in targeting the immune, vascular and stromal aspects of the TME.
Recent Findings
While immunotherapies have shown limited success in the treatment of pediatric solid tumor patients thus far, optimization of these approaches to overcome the TME shows promise. In addition, there is increasing focus on the myeloid compartment as a therapeutic target. Vascular endothelial growth factor (VEGF) targeting resulted in responses in some refractory pediatric solid tumors. There has been relatively little focus on stromal targeting, however emerging preclinical data are improving our understanding of underlying biology, paving the way for future therapies.
Summary
While translation of TME-targeting therapies for pediatric solid tumors is in the early stages, we are optimistic that continued exploration of approaches aimed at rebalancing the TME will lead to improved outcomes for this population.
Keywords: Pediatric, solid tumors, tumor microenvironment
Introduction
Survival rates for pediatric cancer have improved over the past four decades; however, for pediatric patients with recurrent or metastatic solid tumors outcomes remain poor [1,2]. Multimodal therapy including chemotherapy, radiation and surgery has resulted in improved survival for some pediatric solid tumors, however these gains are mostly in patients with localized disease and current treatments are associated with significant long-term toxicity [1,3]. Given the dismal prognosis of pediatric patients with metastatic solid tumors, new therapies which target the metastatic process are urgently needed.
Targeting the tumor microenvironment (TME) in pediatric solid tumors holds promise as a strategy for improving survival outcomes while limiting toxicity, and therapies are currently in the early stages of translation. In addition to tumor cells, the TME consists of immune cells, vasculature, stromal cells and extracellular matrix (ECM). Characterization of these microenvironmental components is key to identifying new therapeutic targets and optimizing the efficacy of current therapeutic approaches. Here we will highlight recent work characterizing the microenvironment of pediatric solid tumors, as well as clinical advances in TME-informed therapies.
Reversing the immune-suppressive TME in pediatric solid tumors
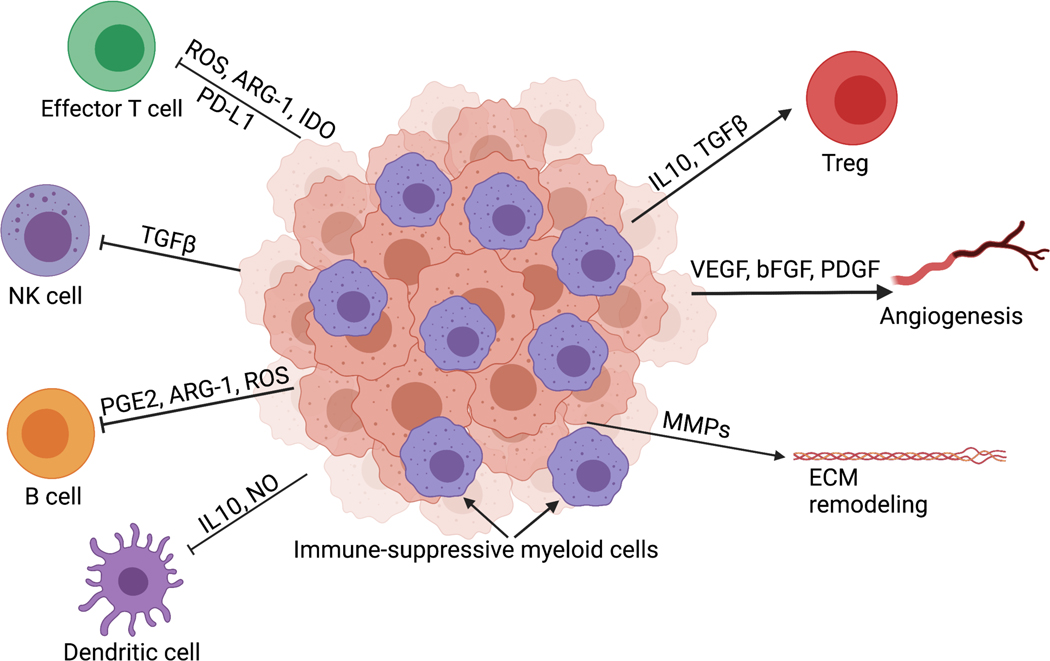
Immunotherapy has resulted in exciting advances in the treatment of chemotherapy-refractory cancers, including pediatric acute lymphoblastic leukemia and some adult solid tumors [4–6]. To date, efficacy of immunotherapy in pediatric solid tumors has been more limited, with the exception of monoclonal antibodies targeting the diganglioside GD2 in the treatment of neuroblastoma [7]. Understanding features of the immune TME in pediatric tumors is important for identifying barriers to effective immunotherapy. Relative to adult tumors, pediatric solid tumors are typically characterized by low mutational burden, low neoantigen expression, major histocompatibility complex (MHC) loss, and poor infiltration by T cells [8–11]. By contrast, myeloid cells such as tumor-associated macrophages (TAM) are abundant in many pediatric solid tumor types and are associated with inferior prognosis [11–13]. Myeloid cells are highly plastic and can acquire immune-stimulatory or immune-suppressive phenotypes in response to factors present in the TME. Immune-suppressive myeloid cells in the TME dampen effector T cell responses, inhibit antigen presentation, and promote vasculogenesis and ECM remodeling [13,14]. [Figure 1].
Figure 1: Development of the immune-suppressive TME in solid tumors.
The tumor microenvironment of pediatric solid tumors favors myeloid-mediated immune suppression. These myeloid-derived immune suppressive cells secrete factors such as transforming growth factor beta (TGFβ), prostaglandins (PGE2), arginase (ARG-1), and reactive oxygen species (ROS) that limit T cell effector function, natural killer (NK) cell activity and B cell and dendritic cell (DC) interactions limiting antigen presentation. Matrix remodeling enzymes, vascular endothelial growth factor (VEGF) and other growth factors impact vasculature and can alter the extracellular matrix. Created with BioRender.com.
Recent studies of patient samples have shown that biomarkers of immune suppression in the TME are associated with inferior outcomes. Ligon et al. characterized the immune TME in primary tumor and pulmonary metastatic lesions in osteosarcoma. Relative to primary tumors, tumor-infiltrating lymphocytes in pulmonary metastases had increased expression of immune checkpoint molecules. Tumor-infiltrating lymphocytes in the metastatic samples were concentrated at the tumor-lung interface while myeloid cells infiltrated the interior. In addition, myeloid-derived suppressor cell (MDSC)-related genes at the tumor-lung interface were upregulated, suggesting myeloid-mediated immune suppression. Expression of immune checkpoint molecules lymphocyte-activation gene 3 and programmed death-ligand 1 (PD-L1) at the tumor-lung interface, as well as colony-stimulating factor 1 receptor (CSF-1R) ubiquitously expressed on TAMs and monocytic cells, were associated with inferior progression-free survival (PFS). Conversely, expression of gene sets related to effective immune response (CD8+ T cells, T cell survival and MHC class I) were associated with longer PFS [15*]. In a recent study utilizing RNA sequencing data of primary tumor samples from patients with high-risk neuroblastoma, Bao et al. showed that both a T-cell inflamed gene expression signature and higher neoantigen load were associated with superior event-free survival and overall survival (OS) [16]. These studies underscore the importance of immune TME characteristics as prognostic biomarkers, as well as the heterogeneity of immune features within tumor subtypes.
Immune checkpoint inhibitors are monoclonal antibodies which block checkpoint proteins expressed by immune cells and cancer cells which act to inhibit cytotoxic T cell function, thereby activating anti-tumor immune responses. The success of immune checkpoint inhibition in treating adult solid tumors such as melanoma and non-small cell lung cancer has led to approval by the US Food and Drug Administration (FDA) and paved the way for clinical development in pediatrics [5,6,17]. Clinical trials investigating inhibitors of programmed cell death protein 1 (PD-1), programed cell death ligand 1 (PD-L1) and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) as single agents in pediatrics have been completed and have shown similar pharmacokinetics and toxicity profiles as in adult trials, however efficacy has been restricted to a few tumor types including Hodgkin lymphoma and tumors with high mutational burden [18**–23]. Importantly, results of recent clinical trials indicate that expression of immune checkpoint molecules alone is not sufficient to predict a clinical response in pediatric solid tumor patients, highlighting the need to identify other predictive biomarkers [20–21]. Data suggest that chemotherapy, even at low doses, and combinatorial radiation can reverse immune suppression in the TME, potentially rendering tumors more amenable to immunotherapy. [24–27]. Pediatric studies investigating immune checkpoint inhibitors in combination with other agents are underway [28*] (NCT03690869, NCT0344858, NCT04544995, NCT04216953, NCT03277924, NCT04239040, NCT02960230).
Chimeric antigen receptor (CAR) T cells are a type of adoptive cell therapy in which autologous T cells are engineered and expanded ex vivo and given back to the patient to induce an anti-tumor immune response independent of MHC. CAR T cells consist of an extracellular single chain variable fragment (scFv) which recognizes tumor-specific antigen, linked to a transmembrane domain, an intracellular signaling domain (CD3ζ) and one or more additional co-stimulatory domains. The remarkable success of CAR T cell therapy in treating relapsed pediatric B cell ALL has led to FDA approval of the CD-19 CAR product tisagenlecleucel [4]. Thus far, clinical trials investigating CAR T cell therapy for pediatric solid tumor patients have shown limited success. Challenges for the development of adoptive cellular therapies for solid tumor patients include the identification of appropriate targets to limit on-target, off-tumor toxicity, and a TME which promotes T cell exhaustion and inhibits expansion and persistence [29]. Various strategies to enhance efficacy of CAR products in the setting of an immune-suppressive TME are under investigation.
Lymphodepleting preparatory regimens increase the availability of homeostatic cytokines allowing for greater expansion of CAR T cells. Experience from clinical trials utilizing HER2 CAR T cells in pediatric patients with advanced sarcomas suggests that lymphodepleting preparatory regimens improve CAR T cell expansion and efficacy [30–32**]. Similarly, a phase 1 clinical trial utilizing a second-generation GD2 CAR T product with CD28/CD3ζ signaling domains in patients with neuroblastoma showed that chemotherapy preconditioning and higher CAR T cell doses were associated with improved clinical efficacy [33*].
Exhaustion of CAR T cells through tonic signaling may limit their persistence and efficacy in the clinic. The 4–1BB co-stimulatory domain is associated with reduced tonic signaling and exhaustion [34]. Additionally, inhibition of tonic signaling of CAR T cells in culture using dasatinib has been shown to reverse exhaustion, drive CAR T cells toward a memory-like state, and enhance efficacy in preclinical models [35**]. The upcoming GD2-CAR-PERSIST trial for pediatric patients with relapsed and refractory osteosarcoma and neuroblastoma incorporates the 4–1BB co-stimulatory domain into a GD2-CAR and utilizes a dasatinib-containing culture platform to enhance persistence (NCT04539366). Preclinical data showed that incorporation of interleukin-15 (IL-15) into CAR constructs results in reduced exhaustion and enhanced efficacy in murine models of neuroblastoma [36]. A phase 1 study of iC9.GD2.CAR.IL15 T-cells is ongoing (NCT03721068).
CAR-natural killer T (NKT) cells may have advantages over CAR-T cells in withstanding the immune-suppressive TME as NKT cells do not require priming to acquire cytotoxic function and can limit the immune-suppressive activity of TAMs [37]. In an interim analysis of a phase 1 clinical trial utilizing an anti-GD2 NKT CAR with IL-15 and cyclophosphamide/fludarabine preconditioning in children with relapsed or refractory neuroblastoma, Heczey et al reported no dose-limiting toxicities, good expansion, localization to tumors and an objective response in a patient with bone metastases [38**].
Through continued optimization of T cell therapies to enhance their fitness in the TME, the potential of these approaches in pediatric solid tumors can be fully realized.
The abundance of myeloid cells in the TME of pediatric solid tumors and their capacity to orchestrate immune responses make them an attractive therapeutic target. Repolarization of macrophages from an immune-suppressive to an immune-stimulatory phenotype holds therapeutic promise. The use of liposomal muramyl tripeptide phosphatidyl ethanolamine (L-MTP-PE) in the treatment of metastatic osteosarcoma is an illustration of this concept. L-MTP-PE is a synthetic analogue of the Bacille Calmette Guerin bacterial cell wall, which when encapsulated into liposomes reprograms resident lung monocytes and macrophages to become tumoricidal. The addition of adjuvant L-MTP-PE to standard chemotherapy was associated with superior OS in a randomized phase III Children’s Oncology Group trial [39]. Although L-MTP-PE has not gained FDA approval, the European Medicines Agency approved L-MTP-PE for patients aged 2–30 with osteosarcoma in conjunction with chemotherapy [40].
CSF-1R targeting is another potential strategy to reduce immune-suppressive myeloid cells in the TME. CSF-1R is important for myeloid cell trafficking to tumors, and their proliferation and survival [13]. Pexidartinib is a small molecule multi-tyrosine kinase inhibitor (TKI) which inhibits CSF-1R, KIT and FLT3 and is FDA-approved for adult patients with tenosynovial giant cell tumor, a mesenchymal neoplasm characterized by an abundance of infiltrating inflammatory cells which traffic to the tumor in response to CSF-1-overexpressing neoplastic cells [41]. In a Phase 1 study of Pexidartinib in pediatric and young adult patients with relapsed and refractory solid tumors, target inhibition was observed as evidenced by decreased absolute monocyte count and increased M-CSF (CSF-1) levels correlating with dose. Preliminary clinical activity was also seen, with 3 patients having stable disease for 4 or more cycles and one patient with peritoneal mesothelioma having a deep partial response [42].
Tumor cells can evade destruction by TAMs through upregulation of CD47, a “don’t eat me” signal on the cell surface. CD47 interacts with signal-regulatory protein α (SIRPα) to inhibit macrophage-mediated phagocytosis [43]. Anti-CD47 antibodies are well-tolerated and have shown promising clinical activity in the adult setting [44–45]. Preclinical work demonstrated a synergistic effect of anti-CD47 antibodies with anti-GD2 antibodies in murine models of neuroblastoma and osteosarcoma [46*]. Based on these findings, a first-in-human trial has been initiated for patients with osteosarcoma and neuroblastoma (NCT04751383).
Harnessing the ability of myeloid cells to infiltrate primary tumor and metastatic sites for adoptive cell therapy is an intriguing possibility. Klichinsky et al utilized a chimeric adenoviral vector to engineer anti-HER2 CAR-macrophages. These “CAR-Ms” showed efficacy in preclinical models and reprogrammed the TME to a pro-inflammatory state [47**]. A first-in-human study of anti-HER2 CAR-Macrophages is underway in adults with HER2-overexpressing solid tumors (NCT04660929). Preclinical work has shown that myeloid cells can be used as a platform to deliver cargo to the TME. Kaczanowska et al utilized a lentiviral vector to genetically engineer myeloid cells to express interleukin-12 (IL-12), a potent anti-tumor cytokine. Administration of these genetically engineered myeloid cells (GEMys) resulted in durable cures in a mouse model of embryonal rhabdomyosarcoma and reversed the immune-suppressive transcriptional signature in pre-metastatic lung [48**]. A first-in-human trial using IL12-GEMys to treat patients with relapsed and refractory solid tumors is in development.
Targeting the vascular compartment
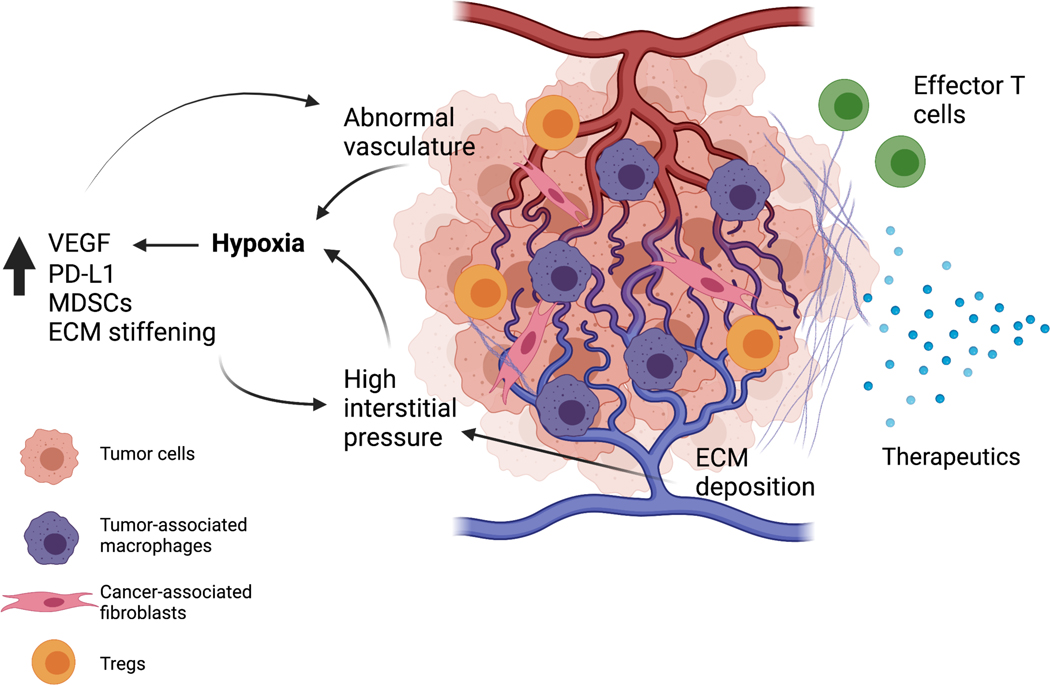
Tumor-associated blood vessels are very different in quality than normal vasculature. They are more porous, tortuous, and constricted by surrounding tumor cells and stroma. This aberrant vasculature restricts the delivery of systemic drugs to the tumor, inhibits trafficking of effector immune cells into the TME, and creates hypoxic conditions which can further drive the immune-suppressive myeloid phenotype and drive drug resistance of tumor cells [49]. [Figure 2].
Figure 2: Crosstalk between tumor-associated vasculature, extracellular matrix and immune cells in the tumor microenvironment.
Abnormal tumor-associated vasculature contributes to hypoxic conditions within the tumor microenvironment and impairs drug delivery and effector T-cell infiltration. Hypoxia contributes to an increase in vascular endothelial growth factor (VEGF), which leads to additional pathologic blood vessel formation. Hypoxia also leads to immune suppression through recruitment of myeloid-derived suppressor cells, tumor-associated macrophages, and Tregs. Both cancer-associated fibroblasts and tumor cells deposit extracellular matrix, which contributes to high interstitial pressure and exacerbates hypoxic conditions. Extracellular matrix can modulate immune cell infiltration and impact efficacy of therapeutics. Created with BioRender.com.
Clinical trials investigating antiangiogenic agents in pediatric solid tumors have largely targeted the vascular endothelial growth factor (VEGF) signaling pathway. Tumor-derived VEGF mediates neoangiogenesis in several pediatric solid tumors including neuroblastoma, Wilms’ tumor, Ewing sarcoma, osteosarcoma and rhabdomyosarcoma [50]. VEGF expression is induced by hypoxia within the TME via the transcriptional activator hypoxia-inducible factor 1-alpha (HIF)-1α [51]. VEGF signaling through VEGF receptor 1 has been implicated in the formation of the pre-metastatic niche [52]. VEGF also binds to VEGF receptor 2, which results in downstream activation of Src family kinases and mediates endothelial barrier disruption, leading to increased vascular permeability [53].
Bevacizumab, a humanized monoclonal neutralizing antibody which binds all five human VEGF isoforms, is FDA-approved for use in several types of adult cancers. As a single agent, Bevacizumab has shown encouraging results in the treatment of pediatric central nervous system tumors, including recurrent and refractory low-grade gliomas, and has shown clinical benefit in patients with vestibular schwannomas and optic pathway gliomas [54–57]. Bevacizumab has shown limited benefit in combination with radiation in pediatric patients with high grade gliomas [58] and in combination with chemotherapy in extracranial solid tumors [59–61].
However, clinical trials utilizing multi-TKIs targeting VEGF have shown that these agents are well-tolerated and show promise particularly in the treatment of pediatric patients with advanced or metastatic osteosarcoma [62–64]. The multi-TKI cabozantinib, which inhibits VEGF receptor 2 and hepatocyte growth factor receptor (MET), showed activity in a phase 2 trial in patients 12 and older with advanced Ewing sarcoma and osteosarcoma [65**]. An ongoing phase 2 Children’s Oncology Group trial evaluating cabozantinib for the treatment of refractory sarcomas, Wilms tumor and rare tumors has reported clinical activity in patients with refractory osteosarcoma [66]. Given the promising single-agent activity of cabozantinib in advanced osteosarcoma, this drug is being prioritized by the Children’s Oncology Group for incorporation with upfront chemotherapy in a phase III clinical trial [67]. Revisiting how best to modulate tumor associated vasculature and hypoxia mediated signaling remains an area of great potential.
Targeting the stromal compartment
Mounting evidence suggests that ECM remodeling is important for invasion and metastasis of solid tumors. The ECM is a three-dimensional network of macromolecules including collagens, glycoproteins and proteoglycans. This network has direct effects on cell behavior including migration, differentiation and proliferation. It also contains growth factors which, when released by matrix-remodeling enzymes, can contribute to tumor cell growth. ECM deposition by stromal and tumor cells contributes to high interstitial pressure which constricts the tumor vasculature, exacerbating hypoxic conditions. In addition, ECM can act as a physical barrier to anti-tumor drugs and effector immune cell infiltration [68] [Figure 2]. Changes in stromal populations are also among the earliest events in pre-metastatic niche development [69].
Development of stromal-targeting therapies has lagged behind other areas of TME modulation in pediatrics, however data are accumulating to support the impact of the stromal compartment on tumor progression and metastasis. Hawkins et al. described an association between Wnt/β-catenin activation in Ewing sarcoma cells, with enhanced ECM production and angiogenesis as a result of Wnt signaling and tumor/ECM crosstalk [70*]. In osteosarcoma, which exhibits abundant ECM deposition, studies of patient samples have identified ECM-related markers as prognostic [71–73]. Further, inhibition of the collagen-crosslinking enzyme lysyl oxidase-like 2 reduced tumor growth and metastasis in preclinical models [74**]. Simtuzumab, an antibody targeting lysyl oxidase-like 2, has been studied in adult cancers in combination with chemotherapy, however randomized phase II trials failed to show clinical benefit [75–76]. The anti-hypertensive drug losartan has been shown in preclinical models to normalize tumor stroma and improve drug delivery to tumors [77–80]. Losartan is currently being studied in combination with the multi-TKI sunitinib in a phase 1/1b clinical trial for patients with recurrent or refractory osteosarcoma (NCT03900793).
Conclusion
Modulating the TME represents a new frontier for the treatment of metastatic pediatric solid tumors. Increasing our understanding of the crosstalk between various aspects of the TME is necessary for the design of rational combinatorial approaches. In particular, characterization of the stromal compartment and its contribution to pediatric tumor progression and metastasis is an under-studied area that warrants further research.
Key points.
The immune-suppressive tumor microenvironment (TME) in pediatric solid tumors presents challenges for the development of effective immunotherapies.
Targeting myeloid cell populations in pediatric solid tumors to reverse immune suppression within the TME is an active area of research and we are seeing the beginning of translational efforts in this area.
Normalizing tumor vasculature via the HIF1/VEGF pathway holds promise as a TME-modulating strategy, with single agents targeting this pathway showing early signs of efficacy in pediatric patients with select subtypes of refractory tumors.
Extracellular matrix remodeling contributes to tumor progression and metastasis; however, this remains an under-studied topic in pediatric solid tumors which warrants further study.
Acknowledgements:
We thank James C. Cronk for reviewing the manuscript.
Financial support and sponsorship:
R. Kaplan and K. Wessel are supported by the Intramural Research Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health Grants: NIH NCI ZIABC011332-06 “Tumor Microenvironment in Cancer Progression” and NIH NCI ZIABC011334-10 “Biomarkers & Therapeutic Targets in Tumor Microenvironment, Angiogenesis and Metastasis”. K. Wessel is supported by the Children’s Cancer Foundation.
Footnotes
Conflicts of interest: none.
Contributor Information
Kristin M. Wessel, Tumor Microenvironment and Metastasis Section, Pediatric Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, 10 Center Drive, Bethesda, MD 20892, USA..
Rosandra N. Kaplan, Tumor Microenvironment and Metastasis Section, Pediatric Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, 10 Center Drive, Bethesda, MD 20892, USA..
References
- 1.Perkins SM, Shinohara ET, DeWees T, Frangoul H. Outcome for children with metastatic solid tumors over the last four decades. PLoS One 2014; 9(7):e100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel DA, Richardson LC, Henley SJ, et al. Pediatric cancer mortality and survival in the United States, 2001–2016. Cancer 2020;26(19): 4379–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith MA, Altekruse SF, Adamson PC, et al. Declining childhood and adolescent cancer mortality. Cancer 2014;120(16):2497–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378(5):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363(8):711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Z, Su W, Lu T, et al. First-line immune-checkpoint inhibitors in non-small cell lung cancer: current landscape and future progress. Front Pharmacol 2020;11:578091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu AL, Gilman AL, Ozkaynak MF, et al. , Children’s Oncology Group. Anti-GD2 antibody with GM-CSF, interleukin-2 and isotretinoin for neuroblastoma. N Engl J Med 2010;363:1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sweet-Cordero EA, Biegel JA. The genomic landscape of pediatric cancers: implications for diagnosis and treatment. Science 2019;363(6432):1170–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel RR, Ramkissoon SH, Ross J, Weintraub L. Tumor mutational burden and driver mutations: characterizing the genomic landscape of pediatric brain tumors. Pediatr Blood Cancer 2020;67(7):e28338. [DOI] [PubMed] [Google Scholar]
- 10.Shern JF, Chen L, Chmielecki J, et al. Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer Discov 2014;4(2):216–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terry RL, Meyran D, Ziegler DS et al. Immune profiling of pediatric solid tumors. J Clin Invest 2020;130(7):3391–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vakkila J, Jaffe R, Michelow M, Lotze MT. Pediatric cancers are infiltrated predominantly by macrophages and contain a paucity of dendritic cells: a major nosologic difference with adult tumors. Clin Cancer Res 2006;12(7):2049–2054. [DOI] [PubMed] [Google Scholar]
- 13.Koo J, Hayashi M, Verneris MR, Lee-Sherick AB. Targeting tumor-associated macrophages in the pediatric sarcoma tumor microenvironment. Front Oncol. 2020;10:581107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y, Li C, Liu T, et al. Myeloid-derived suppressor cells in tumors: From mechanisms to antigen specificity and microenvironmental regulation. Front Immunol 2020;11:1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ligon JA, Choi W, Cojocaru G, et al. Pathways of immune exclusion in metastatic osteosarcoma are associated with inferior patient outcomes. J Immunother Cancer. 2021;9(5):e001772. * Describes the immune TME in both primary and metastatic osteosarcoma and association of immune TME characteristics with patient outcomes.
- 16.Bao R, Spranger S, Hernandez K, et al. Immunogenomic determinants of tumor microenvironment correlate with superior survival in high-risk neuroblastoma. J Immunother Cancer. 2021;9(7):e002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaddepally RK, Kharel P, Pandey R, et al. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN Guidelines with the level of evidence. Cancers 2020;12(3):738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Davis KL, Fox E, Merchant MS, et al. Nivolumab in children and young adults with relapsed or refractory solid tumors or lymphoma (ADVL1412): a multicentre, open-label, single-arm phase 1–2 trial. Lancet Oncol 2020;21(4):541–550. ** Reports the safety and pharmacokinetics of the PD-1 inhibitor nivolumab in the pediatric population and defines the recommended phase 2 dose. While efficacy was limited to patients with lymphoma, the findings allow for further study of nivolumab in combination with other agents in the pediatric population.
- 19.Merchant MS, Wright M, Baird K, et al. Phase I clinical trial of ipilimumab in pediatric patients with advanced solid tumors. Clin Cancer Res 2016;22(6):1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geoerger B, Zwaan CM, Marshall LV, et al. Atezolizumab for children and young adults with previously treated solid tumours, non-Hodgkin lymphoma, and Hodgkin lymphoma (iMATRIX): a multicentre phase 1–2 study. Lancet Oncol 2020;21(1):134–144. [DOI] [PubMed] [Google Scholar]
- 21.Geoerger B, Kang HJ, Yalon-Oren M, et al. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed or refractory solid tumor or lymphoma (KEYNOTE-051): interim analysis of an open-label, single-arm phase 1–2 trial. Lancet Oncol 2020;21(1):121–133. [DOI] [PubMed] [Google Scholar]
- 22.AlHarbi M, Ali Mobark N, AlMubarak L, et al. Durable response to nivolumab in a pediatric patient with refractory glioblastoma and constitutional biallelic mismatch repair deficiency. Oncologist 2018;23(12):1401–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouffet E, Larouche V, Campbell BB, et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol 2016;34(19):2206–11. [DOI] [PubMed] [Google Scholar]
- 24.Opzoomer JW, Sosnowska D, Anstee JE, et al. Cytotoxic chemotherapy as an immune stimulus: a molecular perspective on turning up the immunological heat on cancer. Front Immunol. 2019;10:1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galluzzi L, Humeau J, Buqué A, et al. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17(12):725–741. [DOI] [PubMed] [Google Scholar]
- 26.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrera FG, Ronet C, Ochoa de Olza M, et al. Low dose radiotherapy reverses tumor immune desertification and resistance to immunotherapy. Cancer Discov 2021. Sep 3:candisc.0003.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pasqualini C, Rubino J, Brard C, et al. Phase II and biomarker study of programmed cell death protein 1 inhibitor nivolumab and metronomic cyclophosphamide in paediatric relapsed/refractory solid tumours: Arm G of AcSé-ESMART, a trial of the European Innovative Therapies for Children With Cancer Consortium. Eur J Cancer 2021;150:53–62. **This study reported that the addition of metronomic cyclophosphamide +/− irradiation to nivolumab did not improve efficacy in pediatric patients with relapsed/refractory solid tumors. Biomarker evaluation was also performed in this study and revealed low mutational load and a strong immune-suppressive TME in most tumors prior to treatment on study.
- 29.Hou AJ, Chen LC, Chen YY. Navigating CAR-T cells through the solid-tumour microenvironment. Nat Rev Drug Discov 2021;20(7):531–550. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed N, Brawley VS, Hegde M, et al. Human epidermal growth factor receptor 2 (HER2) -specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol 2015;33(15):1688–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navai A, Derenzo C, Joseph S et al. Abstract LB-147: Administration of HER2-CAR T cells after lymphodepletion safely improves T cell expansion and induces clinical responses in patients with advanced sarcomas. Cancer Res 2019. (79) (13 Supplement) LB–147. [Google Scholar]
- 32. Hegde M, Joseph SK, Pashankar F, et al. Tumor response and endogenous immune reactivity after administration of HER2 CAR T cells in a child with metastatic rhabdomyosarcoma. Nat Commun 2020;11(1):3549. **Case report describing remarkable response to HER2 CAR T cells in a patient with refractory metastatic RMS. Also includes detailed immune-monitoring studies and describes feasibility of multiple HER2 CAR T cell infusions.
- 33. Straathof K, Flutter B, Wallace R, et al. Antitumor activity without on-target off-tumor toxicity of GD2–chimeric antigen receptor T cells in patients with neuroblastoma. Sci Transl Med 2020;12(571):eabd6169. *Reports results from a phase I study of a second-generation GD2 CAR for patients with relapsed/refractory neuroblastoma. The three responders in this trial received Flu/Cy lymphodepletion and cell doses of at least 108/m2, suggesting importance of lymphodepletion and cell dose for efficacy.
- 34.Long AH, Haso WM, Shern JF, et al. 4–1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med 2015;21(6):581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weber EW, Parker KR, Sotillo E, et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science 2021;372(6537):eaba1786. **Demonstrates that CAR T cell exhaustion can be reversed through temporary cessation of signaling and identifies incorporation of transient rest as a translatable strategy for enhancing CAR T cell efficacy.
- 36.Chen Y, Sun C, Landoni E, et al. Eradication of neuroblastoma by T Cells redirected with an optimized GD2-specific chimeric antigen receptor and interleukin-15. Clin Cancer Res 2019;25(9):2915–2924. [DOI] [PubMed] [Google Scholar]
- 37.Song L, Asgharzadeh S, Salo J, et al. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest 2009;119(6):1524–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heczey A, Courtney AN, Montalbano A, et al. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat Med 2020;26(11):1686–1690. **Reports safety and feasibility of treating pediatric patients with CAR-NKT cells and describes clinical activity in a heavily pretreated patient with bone metastases.
- 39.Meyers PA, Schwartz CL, Krailo MD, et al. ; Children’s Oncology Group. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival--a report from the Children’s Oncology Group. J Clin Oncol. 2008;26(4):633–8. [DOI] [PubMed] [Google Scholar]
- 40.Meyers PA. Muramyl tripeptide-phosphatidyl ethanolamine encapsulated in liposomes (L-MTP-PE) in the Treatment of Osteosarcoma. Adv Exp Med Biol 2020;1257:133–139. [DOI] [PubMed] [Google Scholar]
- 41.Tap WD, Gelderblom H, Palmerini E, et al. ; ENLIVEN investigators. Pexidartinib versus placebo for advanced tenosynovial giant cell tumour (ENLIVEN): a randomised phase 3 trial. Lancet 2019;394(10197):478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boal LH, Glod J, Spencer M et al. Pediatric PK/PD phase I trial of pexidartinib in relapsed and refractory leukemias and solid tumors including neurofibromatosis type I–related plexiform neurofibromas. Clin Cancer Res 2020;26:6112–6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Majeti R, Chao MP, Alizadeh AA, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009;138(2):286–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Advani R, Flinn I, Popplewell L, et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin’s lymphoma. N Engl J Med 2018;379(18):1711–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sikic BI, Lakhani N, Patnaik A, et al. First-in-human, first-in-class phase I trial of the anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. J Clin Oncol. 2019;37(12):946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Theruvath J, Menard M, Smith BAH et al. Anti-GD2 antibody disrupts GD2:Siglec7 interactions and synergizes with CD47 blockade to mediate tumor eradication. bioRxiv 2021.03.19.436221. * Reports on synergy of GD2 and CD47 blockade in preclinical models of osteosarcoma and neuroblastoma, establishing rationale for a first-in-human trial.
- 47. Klichinsky M, Ruella M, Shestova O, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol 2020;38, 947–953. **Preclinical work describing generation of CAR-Macrophages and their ability to stimulate adaptive immune responses in murine solid tumor models.
- 48. Kaczanowska S, Beury DW, Gopalan V, et al. Genetically engineered myeloid cells rebalance the core immune suppression program in metastasis. Cell 2021;184(8):2033–2052.e21. **Demonstrates proof-of-concept that myeloid cells can be engineered to deliver cargo to the TME and reverse immune suppression in the pre-metastatic niche.
- 49.Schaaf MB, Garg AD, Agostinis P. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis 2018;9(2):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glade Bender J, Yamashiro DJ, Fox E. Clinical development of VEGF signaling pathway inhibitors in childhood solid tumors. Oncologist 2011;16(11):1614–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 2010;29(5):625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005;438(7069):820–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eliceiri BP, Paul R, Schwartzberg PL, et al. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4(6):915–24. [DOI] [PubMed] [Google Scholar]
- 54.Gorsi HS, Khanna PC, Tumblin M, et al. Single-agent bevacizumab in the treatment of recurrent or refractory pediatric low-grade glioma: A single institutional experience. Pediatr Blood Cancer 2018;65(9):e27234. [DOI] [PubMed] [Google Scholar]
- 55.Kalra M, Heath JA, Kellie SJ, et al. Confirmation of bevacizumab activity, and maintenance of efficacy in retreatment after subsequent relapse, in pediatric low-grade glioma. J Pediatr Hematol Oncol 2015;37(6):e341–6. [DOI] [PubMed] [Google Scholar]
- 56.Avery RA, Hwang EI, Jakacki RI, Packer RJ. Marked recovery of vision in children with optic pathway gliomas treated with bevacizumab. JAMA Ophthalmol. 2014;132(1):111–114. [DOI] [PubMed] [Google Scholar]
- 57.Hochart A, Gaillard V, Baroncini M et al. Bevacizumab decreases vestibular schwannomas growth rate in children and teenagers with neurofibromatosis type 2. J Neurooncol 2015;124:229–236. [DOI] [PubMed] [Google Scholar]
- 58.Grill J, Massimino M, Bouffet E, et al. Phase II, open-label, randomized, multicenter trial (HERBY) of bevacizumab in pediatric patients with newly diagnosed high-grade glioma. J Clin Oncol. 2018;36(10):951–958. [DOI] [PubMed] [Google Scholar]
- 59.Navid F, Santana VM, Neel M et al. A Phase II trial evaluating the feasibility of adding bevacizumab to standard osteosarcoma therapy. Int J Cancer 2017;141(7)1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Modak S, Kushner BH, Basu E, et al. Combination of bevacizumab, irinotecan, and temozolomide for refractory or relapsed neuroblastoma: Results of a phase II study. Pediatr Blood Cancer 2017;64(8): 10.1002/pbc.26448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chisholm JC, Merks JHM, Casanova M, et al. European paediatric Soft tissue sarcoma Study Group (EpSSG) and the European Innovative Therapies for Children with Cancer (ITCC) Consortium. Open-label, multicentre, randomised, phase II study of the EpSSG and the ITCC evaluating the addition of bevacizumab to chemotherapy in childhood and adolescent patients with metastatic soft tissue sarcoma (the BERNIE study). Eur J Cancer 2017;83:177–184. [DOI] [PubMed] [Google Scholar]
- 62.Grignani G, Palmerini E, Dileo P, et al. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group study. Ann Oncol 2012;23(2):508–16. [DOI] [PubMed] [Google Scholar]
- 63.Kim A, Widemann BC, Krailo M et al. Phase 2 trial of sorafenib in children and young adults with refractory solid tumors: A report from the Children’s Oncology Group. Pediatr Blood Cancer 2015;62(9):1562–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davis LE, Bolejack V, Ryan CW et al. Randomized double-blind phase II study of regorafenib in patients with metastatic osteosarcoma. J Clin Oncol 2019; 37:1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Italiano A, Mir O, Mathoulin-Pelissier S. et al. Cabozantinib in patients with advanced Ewing sarcoma or osteosarcoma (CABONE): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21: 446–55. **This trial described promising clinical activity of cabozantinib in heavily pretreated patients with relapsed/refractory Ewing sarcoma and osteosarcoma.
- 66.Ashkintala S, Widemann BC, Barkauskas DA, et al. Phase 2 trial of cabozantinib in children and young adults with refractory sarcomas, Wilms tumor, and rare tumors: Children’s Oncology Group Study (ADVL1622). J. Clin. Oncol 2021;39:15_suppl, 10010–10010. [Google Scholar]
- 67.Whittle SB, Offer K, Roberts RD, et al. Charting a path for prioritization of novel agents for clinical trials in osteosarcoma: A report from the Children’s Oncology Group New Agents for Osteosarcoma Task Force. Pediatr Blood Cancer 2021;68(9):e29188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun 2020;11(1):5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murgai M, Ju W, Eason M, et al. KLF4-dependent perivascular cell plasticity mediates pre-metastatic niche formation and metastasis. Nat Med 2017;23(10):1176–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hawkins AG, Pedersen EA, Treichel S, et al. Wnt/β-catenin-activated Ewing sarcoma cells promote the angiogenic switch. JCI Insight. 2020;5(13):e135188. *Identifies a subset of EWS cells with low EWS-FLI1 activity and increased Wnt/ β-catenin activity which participate in ECM remodeling and angiogenesis in the TME. This study provides new insight into the role of tumor-stroma crosstalk in EWS.
- 71.Mintz MB, Sowers R, Brown KM et al. An expression signature classifies chemotherapy-resistant pediatric osteosarcoma. Cancer Res 2005;65(5):1748–1754. [DOI] [PubMed] [Google Scholar]
- 72.Ito K, Nishida Y, Ikuta K, et al. Overexpression of KIAA1199, a novel strong hyaluronidase, is a poor prognostic factor in patients with osteosarcoma. J Orthop Surg Res. 2021;16(1):439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shi K, Wang SL, Shen B, et al. Clinicopathological and prognostic values of fibronectin and integrin αvβ3 expression in primary osteosarcoma. World J Surg Oncol. 2019;17(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Matsuoka K, Bakiri L, Wolff LI, et al. Wnt signaling and Loxl2 promote aggressive osteosarcoma. Cell Res. 2020. Oct;30(10):885–901. **Provides evidence for the role of ECM remodeling in osteosarcoma progression and metastasis in GEMMs and establishes an association between Wnt signaling, Loxl2 expression and outcomes in patient samples.
- 75.Hecht JR, Benson AB 3rd, Vyushkov D, et al. A phase II, randomized, double-blind, placebo-controlled study of simtuzumab in combination with FOLFIRI for the second-line treatment of metastatic KRAS mutant colorectal adenocarcinoma. Oncologist 2017;22(3):243–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Benson AB 3rd, Wainberg ZA, Hecht JR, et al. A phase II randomized, double-blind, placebo-controlled study of simtuzumab or placebo in combination with gemcitabine for the first-line treatment of pancreatic adenocarcinoma. Oncologist 2017;22(3):241–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diop-Frimpong B, Chauhan VP, Krane S et al. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. PNAS 2011; 108(7):2909–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chauhan VP, Martin JD, Liu H, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun 2013;4:2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao Y, Cao J, Melamed A. et al. Losartan treatment enhances chemotherapy efficacy and reduces ascites in ovarian cancer models by normalizing the tumor stroma. PNAS 2019; 116(6):2210–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kumar V, Boucher Y, Liu H, et al. Noninvasive assessment of losartan-induced increase in functional microvasculature and drug delivery in pancreatic ductal adenocarcinoma. Transl Oncol 2016;9(5):431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]