Abstract
Human T-lymphotropic virus type 1 (HTLV-1) infection causes adult T-cell lymphoma/leukemia (ATL) following a prolonged clinical incubation period, despite a robust adaptive immune response against the virus. Early immune responses that allow establishment of the infection are difficult to study without effective animal models. We have developed a cytotoxic T-lymphocyte (CTL) assay to monitor the early events of HTLV-1 infection in rabbits. Rabbit skin fibroblast cell lines were established by transformation with a plasmid expressing simian virus 40 (SV40) large T antigen and used as autochthonous targets (derived from same individual animal) to measure CTL activity against HTLV-1 infection in rabbits. Recombinant vaccinia virus (rVV) constructs expressing either HTLV-1 envelope surface unit (SU) glycoprotein 46 or Tax proteins were used to infect fibroblast targets in a 51Cr-release CTL assay. Rabbits inoculated with Jurkat T cells or ACH.2 cells (expressing ACH HTLV-1 molecule clone) were monitored at 0, 2, 4, 6, 8, 13, 21, and 34wk post-infection. ACH.2-inoculated rabbits were monitored serologically and for viral infected cells following ex vivo culture. Proviral load analysis indicated that rabbits with higher proviral loads had significant CTL activity against HTLV-1 SU as early as 2wk post-infection, while both low- and high-proviral-load groups had minimal Tax-specific CTL activity throughout the study. This first development of a stringent assay to measure HTLV-1 SU and Tax-specific CTL assay in the rabbit model will enhance immunopathogenesis studies of HTLV-1 infection. Our data suggest that during the early weeks following infection, HTLV-1-specific CTL responses are primarily targeted against Env-SU.
Introduction
Cytotoxic T-lymphocyte (CTL) responses are a primary host defense against viral infections. Accurate measures of CTL activity are used to monitor the cellular immune response against viral infections, and are critical to studies seeking to test vaccines or provide information about immunopathogenic mechanisms of viral diseases. Many assays have been developed to measure virus-specific major histocompatibility complex (MHC)-restricted CTL responses, from chromium-release assays to cytokine-detection assays. CTL assays are typically performed by mixing CTL cells with their cognate targets in various ratios and measuring a cell-death event. The lack of suitable target cells is often a problem when developing a MHC-restricted CTL assay, particularly in experimental animal models using outbred animals such as rabbits. Typically, when testing human- and mouse-specific MHC-restricted CTL responses, B- and T-cell lines are used as target cells following immortalization with Epstein-Barr virus and herpes saimiri virus, respectively. These viruses only infect a limited number of domestic species and are not effective in immortalizing target cells in species such as dogs and cats (1).
Herein we developed a CTL assay to measure human T-lymphotropic virus type 1 (HTLV-1)-specific CTL responses in a rabbit model of infection. HTLV-1 is a deltaretrovirus and causative agent of adult T-cell leukemia/lymphoma (ATL), and a number of neurologic- or lymphocyte-mediated disorders [reviewed in (2)]. The HTLV-1 genome encodes for structural proteins and enzymes (Gag, Env, reverse transcriptase [RT], protease, and integrase [IN]) (3–8), as well as regulatory and nonstructural proteins. The pX region of the viral genome, through alternative splicing of mRNA, encodes for regulatory or accessory gene products. One such product is the transactivating protein (Tax), which is a known target of the cellular immune response against the virus. The pX genome region also encodes for the regulatory protein (Rex), and the nonstructural proteins p30, p12, p13, and the antisense encoded HBZ (2). The HTLV-1 envelope is expressed as a glycosylated precursor that is cleaved by cellular proteases into an extracellular glycosylated surface unit (SU) gp46 and a transmembrane unit (TM) gp21 (9,10).
A number of animal models of HTLV-1 have provided fundamental information about host responses to the infection. The virus consistently infects rabbits (11,12), some nonhuman primates (13,14), and to a lesser extent rats (15,16). The rabbit model has provided important knowledge of the immune response against HTLV-1 infection, but is limited by the lack of assays to measure specific cellular immune responses. To generate a CTL assay in this important animal model we generated immortalized rabbit skin fibroblasts using simian virus (SV-40). Rabbit fibroblasts were then used as autochthonous targets to measure CTL activity against an infectious molecular clone of HTLV-1. Recombinant vaccinia virus (rVV) constructs expressing either HTLV-1 envelope SU gp46 or Tax were used to infect fibroblast targets in a 51Cr-release CTL assay. Specific CTL activity was measured from HTLV-1-infected rabbits at early stages of infection. The development of a stringent assay to measure HTLV-1 SU gp46 and Tax-specific CTL activity in a reproducible animal model provides an important tool to monitor early immune responses to HTLV-1 infection. Serologic and proviral load analysis indicated that rabbits with higher proviral loads had significant CTL activity against HTLV-1 SU gp46 as early as 2wk post-infection, while both low- and high-proviral-load groups had minimal Tax-specific CTL activity throughout the study. Our data suggest that during the early weeks following infection, the HTLV-1 specific CTL response is first directed against Env-SU gp46.
Materials and Methods
Animals, cells, and inocula
Six female 12-wk-old New Zealand white rabbits were obtained from commercial sources (Harlan, Indianapolis, IN). The rabbits were kept in accordance with an animal care and use protocol approved by The Ohio State University Animal Care and Use Committee. Peripheral blood mononuclear cells (PBMCs) were isolated from 10–20mL of whole blood at each time point from the lateral auricular artery. Percoll (Sigma-Aldrich Corp., St. Louis, MO) at 1.065 g/mL was used to isolate rabbit lymphocytes. PBMCs were kept in RPMI 1640 (GIBCO BRL, Grand Island, NY), 15% FBS (GIBCO BRL), 1% penicillin-streptomycin (GIBCO BRL), 1% sodium pyruvate (GIBCO BRL), 2% L-glutamine (GIBCO BRL), recombinant human IL-2 (10U/mL) (NIH AIDS Reagent Catalog #136; 10,000U/mL), and 250 mL of 2-mercaptoethanol. ACH.2 cells (5 × 106) containing the ACH full molecular clone of HTLV-1 were used to inoculate each rabbits as previously described (17). Identical numbers of HTLV-1-negative Jurkat T lymphocytes were inoculated in the control rabbit.
Immortalization of primary rabbit fibroblasts
Following application of an appropriate anesthetic, skin biopsies were taken from all six rabbits under aseptic conditions. The biopsies were trimmed and minced in Hank’s balanced salt solution (GIBCO BRL) in the presence of collagenase (1mg/mL; Boehringer Mannheim, Indianapolis, IN) and trypsin-EDTA (0.25% trypsin, 1mMEDTA; GIBCO BRL). Rabbit skin fibroblasts were then incubated in a CO2 incubator at 37°C for 30 min. The solution was then transferred to sterile 15-mL conical tubes and centrifuged at 500×g for 10 min. The primary rabbit fibroblasts were resuspended in 82% Dulbecco’s MEM (GIBCO BRL), 15% fetal calf serum (FBS) (GIBCO BRL), 1% sodium pyruvate (GIBCO BRL), 2% L-glutamine (GIBCO BRL) and 0.1% enrofloxacin (Baytril; Bayer, Shawnee Mission, KN). The cells were transferred to flasks and incubated at 37°C and 5% CO2 and reseeded weekly in fresh media.
Rabbit primary skin fibroblasts were monitored for proper morphology and confirmed by staining for vimentin by immmunohistochemistry using an avidin-biotin complex method. The primary antibody used was a mouse monoclonal vimentin antibody (clone V9) (Dako, Carpintera, CA) at a 1:100 dilution. The secondary antibody used was a biotinylated horse anti-mouse antibody (Vector, Burlingame, CA) at a dilution of 1:200.
To produce immortalized rabbit target cells for the CTL assay, 5×106 skin fibroblast cells obtained from each rabbit were electroporated with 1 µg of pBR-SV plasmid as previously described (1). Immortalized rabbit cell lines unique to each rabbit were maintained for subsequent cytotoxic assays.
Immunostaining for SV40 large T antigen
Immunofluorescence assays were used to confirm large T-antigen expression in the rabbit skin fibroblasts. Rabbit primary fibroblasts were grown on microscope slides in sterile conditions. After a 12-h incubation period, rabbit primary fibroblasts were fixed with an ice-cold 1:1 mix of acetone and methanol on ice for 1 h. After the 1-h incubation on ice, the rabbit primary fibroblasts were rehydrated at room temperature for 3 min. After rehydration, the rabbit primary fibroblasts were stained with mouse monoclonal SV40 large T antigen antibody (DP02; Calbiochem; San Diego, CA) at 1:10 in phosphate-buffered saline (PBS) with 0.1% FBS (GIBCO BRL) and 0.1% sodium azide (PBN). The slides were incubated for 1 h at 37°C, then washed three times in PBN before staining with secondary antibody rabbit anti-mouse IgG fluorescein isothiocyanate (FITC) (F7506; Sigma-Aldrich). The slides were then incubated for 1 h at 37°C, washed three times, rehydrated in water, and mounted with 50% glycerol in PBS (GIBCO BRL) for viewing with a fluorescence microscope.
Recombinant vaccinia virus vectors expressing HTLV-1 SU and Tax
Recombinant vaccinia virus vectors (rVV) were used to deliver HTLV-1 genes expressing the surface unit of envelope (SU) and transactivating protein (Tax) in each unique rabbit primary skin fibroblast cell line. Vaccinia virus vectors rVV-wt (wild type), rVV-SU, and rVV-Tax were used to infect rabbit fibroblast cell lines at a multiplicity of infection (MOI) of 5. The infection was allowed to proceed for 3 h to allow sufficient HTLV-1 SU and Tax expression. Western blot analysis confirmed expression of each protein.
During the 51Cr-release assay, each unique rabbit skin fibroblast cell line was infected at an MOI of 3 with VV-wt, rVV-SU, or rVV-Tax, incubated for 3 h, and washed three times with RPMI 1640 complete media. After the third wash, the rabbit primary skin fibroblasts were incubated for an additional 30 min at 37°C in 5% CO2 to test spontaneous chromium release. Following this incubation, the wells were washed one more time before addition of rabbit PBMCs at the indicated effector-to-target ratios (E:T).
Detection of provirus load by real-time PCR
To quantify provirus load in rabbit PBMCs, genomic DNA was isolated according to the manufacturer’s protocol (Qiagen, Germantown, MD). The extracted DNA was tested for quality and quantified (NanoDrop Technologies, Wilmington, DE). HTLV-1 Tax forward and reverse primers were used at a final concentration of 300 nM. Dual-labeled probe was used at a concentration of 200 nM. The real-time techniques and conditions have been previously reported (18). The forward Tax primer 5′-CGG ATA CCC AGT CTA CGT GTT T-3′ and the reverse primer 3′-TGG ACG CGT TAT CGG CTC AG-5′ were used to amplify genomic DNA. The molecular probe used was 5′-VIC-TGC TGG CAC CAG ACT TGC CCT C-TAMRA-3′. Standard curves performed in the same plate as the unknown test samples were generated from an HTLV-1Taxgenestandard constructed bysubcloning a 161-bp fragment (nucleotides 6985–7145; NCBI accession no. AF03 3817) from the pACH HTLV-1 proviral clone into the pCR® 4-TOPO vector (Invitrogen, Carlsbad, CA).
For each test run, a standard curve was generated from triplicate samples of log10 dilutions of plasmid DNA in DNAse/RNAse-free water. The lower limit of detection was estimated to be 82 copies/µg of DNA. The normalized copy number per cell was calculated based on the estimation of 1 µg DNA = DNA from 134,600 cells.
Detection of p19 matrix antigen from ex-vivo-cultured PBMCs
HTLV-1 infection in rabbit PBMCs was tested using a p19 matrix antigen (MA) enzyme-linked immunosorbent assay (ZeptoMetrix, Buffalo, NY) performed on supernatants from 3-d PBMC cultures (1.0×106 per well) as previously described (19).
Serologic response to HTLV-1 infection
Reactivity to specific viral antigenic determinants was detected using a commercial HTLV-1 Western immunoblot assay (GeneLabs Diagnostics, Singapore) adapted for rabbit plasma by the use of alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (1:1000 dilution; bio-Mérieux, Inc., Durham, NC). Plasma (diluted 1:100) showing reactivity to HTLV-1 Gag (p24 or p19) and Env (p21 or gp46) antigens was classified as positive for HTLV-1 seroreactivity.
Results
Characterization of rabbit primary skin fibroblasts
To overcome MHC restrictions and create autochthonous targets (derived from the same rabbit) we immortalized rabbit primary skin fibroblasts from each rabbit used in the study. The rabbit primary skin fibroblast cell lines were developed from biopsies taken from the rabbits prior to HTLV-1 infection, therefore each cell line was unique to each rabbit. The pBR-SV plasmid, which expresses SV40 large T antigen, was electroporated into primary rabbit fibroblasts. The generated immortalized primary rabbit skin fibroblast cell lines were expanded through 35 passages (Fig. 1A). The fibroblasts were stained for vimentin, a cellular intermediate filament protein (Fig. 1B). Immunofluorescence assays (IFA) were conducted to confirm SV40 large-T-antigen expression in the rabbit skin fibroblasts (Fig. 1C).
FIG. 1. Immortalized rabbit primary skin fibroblasts.
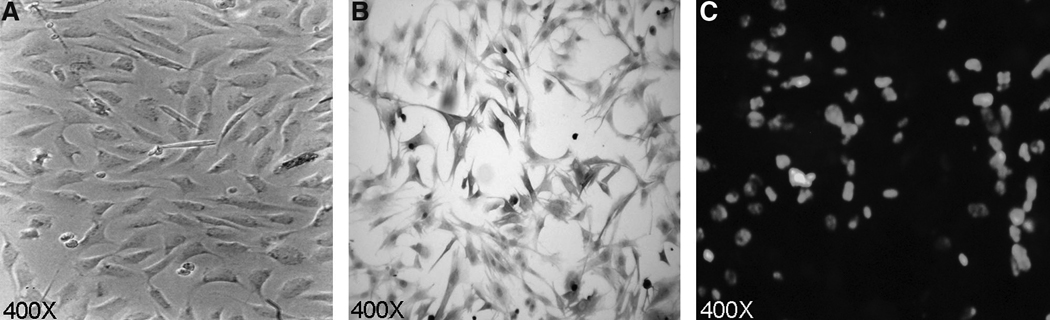
(A) Photomicrograph of immortalized rabbit primary skin fibroblasts taken at 400×magnification by an inverted microscope. (B) Immunohistochemistry: Photomicrograph of rabbit primary skin fibroblasts taken at 400× magnification by an inverted microscope. The rabbit primary skin fibroblasts were positive for vimentin. (C) Immunofluorescence: Photomicrograph of rabbit primary skin fibroblasts taken at 400× magnification by a fluorescence microscope. Rabbit primary skin fibroblasts were positive for large-T-antigen expression.
HTLV-1 SU and Tax expression in CTL target cells
We used rVV vectors, which expressed either HTLV-1 SU or Tax in the immortalized rabbit fibroblasts. Each of these HTLV-1 proteins are known targets of the cell-mediated immune response in humans (20,21), and Western blot analysis confirmed that our immortalized rabbit fibroblasts expressed HTLV-1 SU and Tax 6 h following infection (Fig. 2A and B). Immortalized fibroblasts expressing wild-type vaccinia virus served as a negative control (Fig. 2A and B).
FIG. 2. Expression of HTLV-1 Tax and SU in primary rabbit fibroblasts.
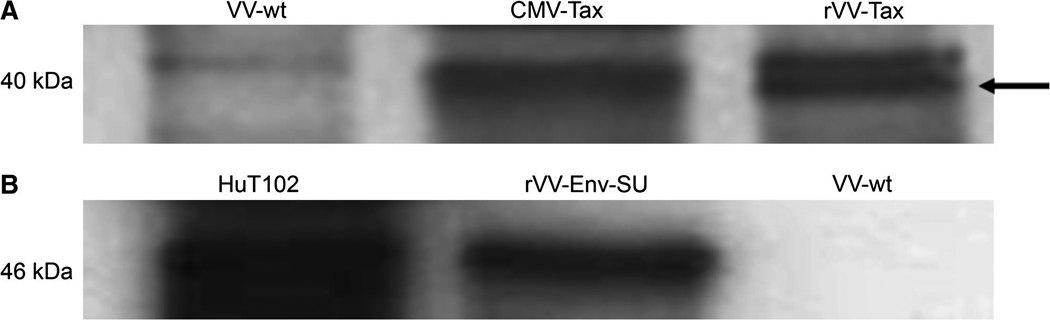
(A) Western blot analysis depicting Tax expression from CMV-Tax-transfected and rVV-Tax-infected rabbit primary skin fibroblasts. The arrow indicates the Tax-specific band at 40 kDa. rVV-wt-infected rabbit primary skin fibroblasts did not express Tax. (B) Western blot analysis depicting Env-SU expression in HTLV-1-positive HuT102 cells and rVV-Env-SU-infected rabbit primary skin fibroblasts. rVV-wt did not express Env-SU.
HTLV-1 infection in rabbits
We then used six female 12-wk-old New Zealand white rabbits to establish persistently infected rabbits and a control rabbit. ACH.2 cells (5×106) containing the ACH full molecular clone of HTLV-1 were used to inoculate each rabbit in the virus-infected group. Identical numbers of HTLV-1-negative Jurkat T lymphocytes were inoculated in the control rabbit. The animals were then monitored for virological and serological parameters of infection.
Rabbit serum samples were tested for reactivity to specific viral antigenic determinants by HTLV-1 Western immunoblot assay. All ACH.2-inoculated rabbits seroconverted by 4wk after exposure, and remained positive throughout the 34-wk study period (Fig. 3). The control rabbit (ROS1) inoculated with Jurkat T lymphocytes had no virus-specific reactivity throughout the study.
FIG. 3. Anti-HTLV-1 Western blot.
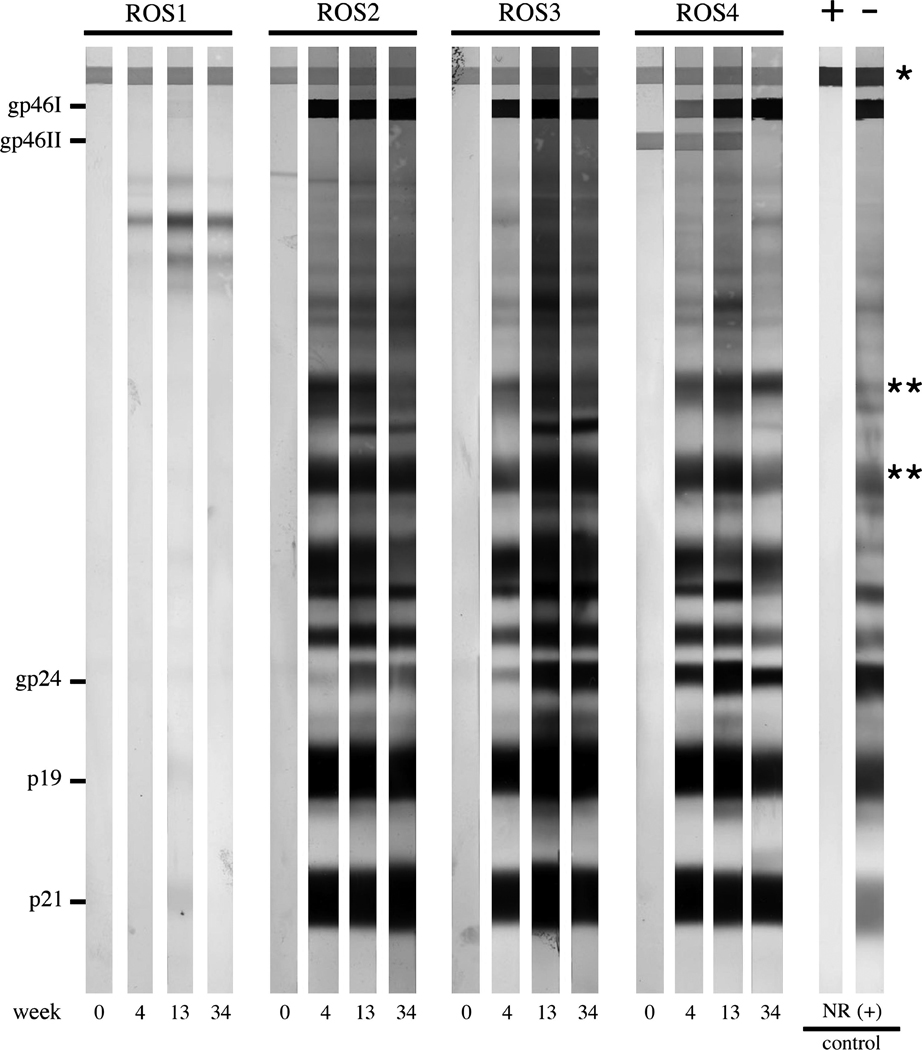
Representative results from selected animals are shown. ROS1 was inoculated with HTLV-1-negative Jurkat T lymphocytes, and never seroconverted. ROS-2, -3, and -4 were inoculated with HTLV-1-positive ACH.2 cells. All ACH.2-inoculated rabbits seroconverted by week 4 (gp46I, glycoprotein 46 HTLV-1 Env surface unit; gp46II, glycoprotein 46 HTLV-2 Env surface unit; gp24, HTLV-1 capsid; p19, HTLV-1 matrix; p21, HTLV-1 Env transmembrane unit; asterisks denote serum loading control bands, indicating comparable concentrations of serum immunoglobulin levels among the samples).
The production of HTLV-1 p19 matrix protein (MA) from ex-vivo cultured PBMCs was used as an indicator of active virus replication from exposed rabbits. Production of p19 MA served as a corollary to HTLV-1 virus expression. Rabbit lymphocytes isolated from 10–20mL of whole blood were put into culture for 3 d and stimulated with fresh recombinant human IL-2 (10U/mL) (NIH AIDS Reagent Catalog #136; 10,000U/mL). The supernatant from the ex-vivo culture was collected and used in a p19 MA ELISA. ROS1 had no detectable level of p19 expression for the entire length of the study. All ACH.2-inoculated rabbits had detectable levels of ex-vivo p19 MA at multiple points during the study (Fig. 4). The average ex-vivo p19 production from cultured lymphocytes was 292 (week 8), 743 (week 13), 83 (week 21), and 53 (week 34) pg/mL. The highest detectable level of ex-vivo p19 production in all ACH.2-inoculated rabbits was seen at 13wk post-infection. At 13wk post-infection ROS4 and ROS6 had the highest ex-vivo p19 production, at 1151 and 1050 pg/mL, respectively. As expected, as the maturation of the immune response developed against the virus infection, ex-vivo p19 production declined to baseline levels at later time points in all HTLV-1-infected rabbits. At the end of the study all ACH.2-inoculated rabbits had similar ex-vivo p19 production values.
FIG. 4. Ex-vivo p19 production from cultured rabbit lymphocytes.
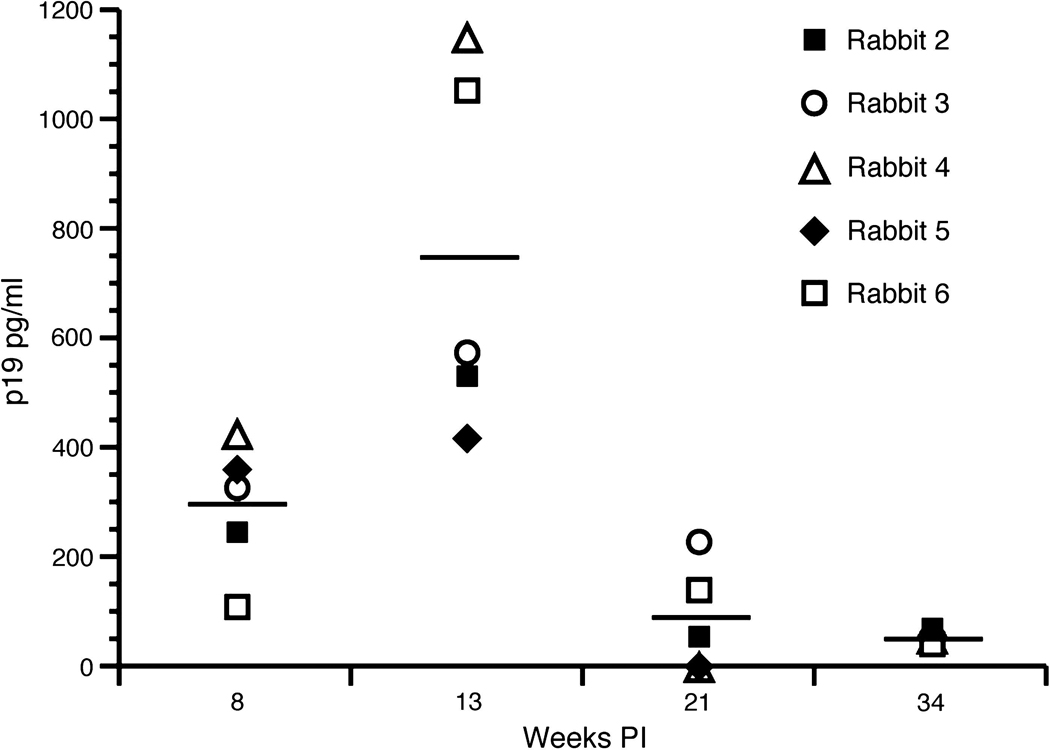
Rabbit lymphocytes were isolated from PBMCs and cultured for 24 h. p19 MA, a measure of virus expression, was measured with an HTLV-1 ELISA. p19 expression was highest at 13wk post-infection in all rabbits. At 34wk post-infection ex-vivo p19 production was at a similar level in all rabbits.
We used real-time PCR to quantitatively measure the expression of our molecular clone of HTLV-1 from rabbit PBMC samples throughout the course of infection. All ACH.2-inoculated rabbits were negative for proviral copies at week 0, and ROS1, inoculated with HTLV-1-negative Jurkat T lymphocytes, remained negative in this assay throughout the study. ACH.2-inoculated rabbits developed a range of proviral copy numbers ranging from 13 (ROS4) to 286 (ROS5) HTLV-1-infected cells per 10,000 PBMCs, typical of the natural infection in asymptomatic human subjects. The average proviral copy numbers following infection were 84 (week 8), 116 (week 13), 89 (week 21), and 166 (week 34) infected lymphocytes per 10,000 PBMCs (Fig. 5). ROS5 was the only rabbit in which the proviral copy numbers increased every week following infection. At 21wk post-infection ROS3 and ROS4 had no detectable proviral copies.
FIG. 5. Proviral load.
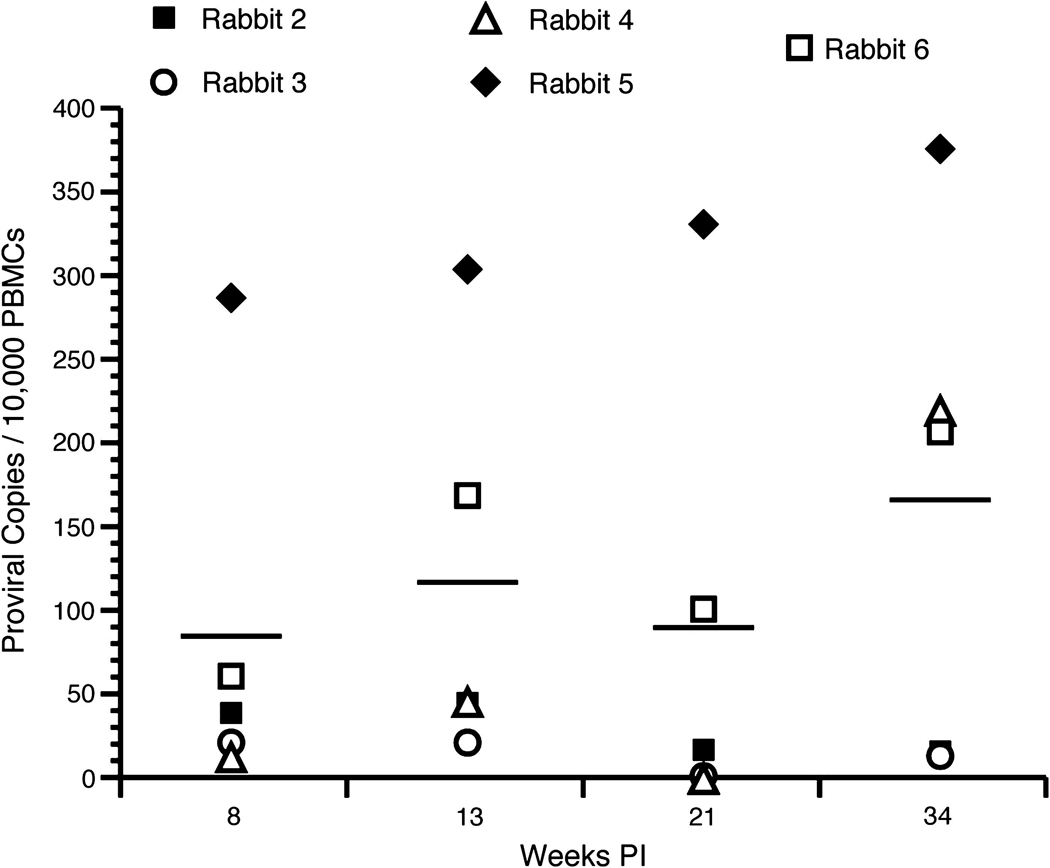
Genomic DNA was isolated from PBMCs and used in a real-time PCR assay to detect provirus. The average proviral load was 84, 116, 89, and 166 proviral copies per 10,000 PBMCs at 8, 13, 21, and 34wk post-infection, respectively. ROS1, the negative control, was below the level of detection for the entire length of the study.
CTL assay
In our CTL assay we detected HTLV-1-specific CTL lysis of immortalized rabbit fibroblasts infected with both rVV-SU and rVV-Tax. The assay conditions were purposely highly stringent and did not rely upon expansion of effector cells ex vivo with HTLV-1 antigens. The CTL responses to both viral target proteins were low and variable and peaked at early time points after infection (Fig. 6). An SU-specific CTL response was observed initially at 2wk post-infection in rabbits ROS4 and ROS6. ROS4 and ROS6 had Env-SU-specific CTL lysis percentages of 24.6 and 6.1, respectively. ROS4 and ROS6 were the only rabbits in which we detected multiple SU-specific CTL responses over the course of infection. The Tax-specific CTL response appeared at 4wk post-infection. ROS3 and ROS6 had HTLV-1-Tax-specific CTL lysis percentages of 3.2 and 6, respectively. ROS6 was the only rabbit in which we detected both SU- and Tax-specific CTL responses over the course of the study.
FIG. 6. Specific lysis of rVV-SU- and Tax-infected immortalized rabbit fibroblasts.
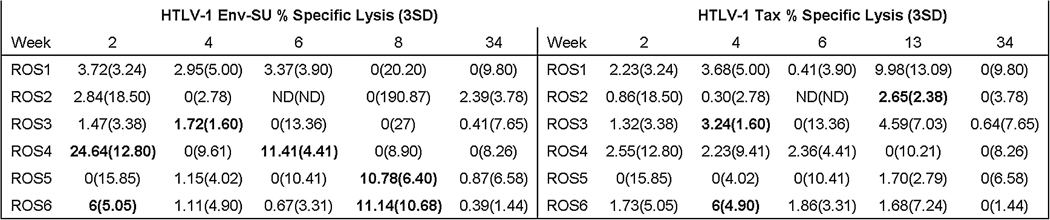
Freshly isolated PBMCs were placed into culture with rVV-infected autochthonous immortalized rabbit primary skin fibroblasts expressing either SU gp46, Tax, or VV-wt proteins at a 40:1 E:T ratio. Specific lysis of rabbit fibroblasts detected above 3 standard deviations (3 SD) of the VV-wt-infected control was counted as true lysis, and appears to the left of the 3 SD in parentheses. Note detection of anti-SU CTL responses more often and earlier than anti-Tax CTL responses. Bold type indicates true specific lysis.
Discussion
Developing an MHC-restricted CTL assay for use in an outbred species such as the rabbit model of HTLV-1 infection is an important tool for understanding early HTLV-1-infection specific cell-mediated immune responses. Studies from HTLV-1-infected subjects are limited and do not provide opportunities to examine the earliest HTLV-1-specific CTL responses following infection because the time of virus exposure is typically unknown. Our study further developed the rabbit model of HTLV-1 infection by using immortalized rabbit primary skin fibroblasts to develop an autochthonous CTL assay to test early immune responses to this important human retrovirus. We used recombinant vaccinia virus (rVV) constructs to express the known HTLV-1 CTL target proteins SU gp46 and Tax in fibroblast targets. Following establishment of HTLV-1 infection, we serially monitored rabbits for their CTL activity using a stringent format. Our data indicated that rabbits with higher proviral loads had significant CTL activity against HTLV-1 SU gp46 as early as 2wk post-infection, while both the low- and high-proviral-load groups had minimal Tax-specific CTL activity. Our assay to measure HTLV-1 SU- and Tax-specific CTL activity in the rabbit model provides a new tool to understand the early events of HTLV-1 infection, which determine subsequent pathogenic outcomes associated with this persistent retroviral infection.
In our CTL assay we were able to detect Env-SU specific CTL lysis as early as 2wk following infection, and more frequently than CTL activity against Tax. Our ability to detect Env-SU-gp46-specific CTL lysis in our assay earlier and more frequently is likely a reflection of early viral proteins required during the early period of viral spread. In chronically infected human subjects cell-mediated immune responses have been documented against both viral proteins (22–33), but have not been evaluated during early viral spread following virus exposure. Our data suggest that during the early weeks following infection, the HTLV-1-specific CTL response is first directed against Env-SU gp46. This is likely because during early infection, viral spread is dependent upon active virus replication and cell-to-cell transfer to establish a targeted population of cells that act as reservoirs for subsequent clonal expansion during more chronic phases of disease (34–36). This phase of infection is important for establishment of the viral infection prior to a more robust and maturing immune response against HTLV-1. Other reports have shown that Tax is the immunodominant protein (37), and the focus of the cell-mediated immune response. However, these reports often focus on patients who have been infected for many decades. This might explain why we did not detect more robust Tax-specific CTL lysis during early infection.
An important caveat of our study method is the lack of viral antigen pulsing of effector cells prior to testing in our CTL assay. In addition, we used stringent criteria (3 SD above background as the positive cut-off). The inclusion of antigen pulsing combined with our more stringent assay could have extended the sensitivity of our assay. In addition, we tested the response to intravenous exposure of rabbits to HTLV-1 infection as a model of blood-product-associated infection in humans. The natural route of HTLV-1 infection is from breast-feeding and sexual transmission. Our animal model has the potential in future studies to test the early CTL responses against HTLV-1 by these mucosal routes of exposure. It will be important in future studies to compare early CTL activity following oral versus intravenous transmission of HTLV-1, as breast-feeding has been epidemiologically linked to favorable subsequent development of ATL versus HAM/TSP (38–40). It will also be important to define the specific cell type mediating target cell lysis in our assay using more refined effector cell separation methods.
Conclusion
In conclusion, we have developed a stringent CTL assay in rabbits to monitor the early events of HTLV-1 infection using autochthonous immortalized rabbit primary skin fibroblast cell lines as MHC-restricted targets. This is the first study to examine the earliest HTLV-1-specific cell-mediated immune responses following infection in the rabbit model. Our data suggest that early HTLV-1-specific CTLs are primarily directed against Env-SU proteins following infection. In the future, with the development of improved reagents for rabbits, and the continued development of our CTL assay, we will be able to expand knowledge of early target cells of the infection, and immunological events of the immune responses following infection.
Acknowledgment
This work was supported by National Cancer Grant no. CA100730, awarded to Dr. Michael D. Lairmore.
Footnotes
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.Koksoy S, Phipps AJ, Hayes KA, Mathes LE. SV40 immortalization of feline fibroblasts as targets for MHC-restricted cytotoxic T-cell assays. Vet Immunol Immunopathol. 2001;7:285–295. doi: 10.1016/s0165-2427(01)00272-0. [DOI] [PubMed] [Google Scholar]
- 2.Lairmore M, Franchini G. Human T-cell leukemia virus types 1 and 2. In: Knipe DM, editor. Fields Virology. 5th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins; 2007. pp. 2071–2105. [Google Scholar]
- 3.Bertola F, Manigand C, Picard P, Goetz M, Schmitter JM, Precigoux G. N-terminal domain of HTLV-I integrase. Complexation and conformational studies of the zinc finger. J Pept Sci. :588–597. doi: 10.1002/psc.356. 20017. [DOI] [PubMed] [Google Scholar]
- 4.Heidecker G, Hill S, Lloyd PA, Derse D. A novel protease processing site in the transframe protein of human T-cell leukemia virus type 1 PR76(gag-pro) defines the N terminus of RT. J Virol. 2002;76:13101–13105. doi: 10.1128/JVI.76.24.13101-13105.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le B, I, Blot V, Bouchaert I, Salamero J, Goud B, Rosenberg AR, Dokhelar MC. Intracellular distribution of human T-cell leukemia virus type 1 Gag proteins is independent of interaction with intracellular membranes. J Virol. 2002;76:905–911. doi: 10.1128/JVI.76.2.905-911.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mariani VL, Beckham SS. Identification of the RT-RH/IN cleavage site of HTLV-I. Biochem Biophys Res Commun. 2003;300:268–270. doi: 10.1016/s0006-291x(02)02848-6. [DOI] [PubMed] [Google Scholar]
- 7.Muller B, Krausslich HG. Characterization of human T-cell leukemia virus type I integrase expressed in Escherichia coli. Eur J Biochem. 1999;259:79–87. doi: 10.1046/j.1432-1327.1999.00026.x. [DOI] [PubMed] [Google Scholar]
- 8.Trentin B, Rebeyrotte N, Mamoun RZ. Human T-cell leukemia virus type 1 reverse transcriptase (RT) originates from the pro and pol open reading frames and requires the presence of RT-RNase H (RH) and RT-RH-integrase proteins for its activity. J Virol. 1998;72:6504–6510. doi: 10.1128/jvi.72.8.6504-6510.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee TH, Coligan JE, McLane MF, et al. Serological cross-reactivity between envelope gene products of type I and type II human T-cell leukemia virus. Proc Natl Acad Sci USA. 1984;81:7579–7583. doi: 10.1073/pnas.81.23.7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider J, Yamamoto N, Hinuma Y, Hunsmann G. Sera from adult T-cell leukemia patients react with envelope and core polypeptides of adult T-cell leukemia virus. Virology. 1984;132:1–11. doi: 10.1016/0042-6822(84)90086-2. [DOI] [PubMed] [Google Scholar]
- 11.Akagi T, Takeda I, Oka T, Ohtsuki Y, Yano S, Miyoshi I. Experimental infection of rabbits with human T-cell leukemia virus type 1. Jpn J Cancer Res. 1985;76:86–94. [PubMed] [Google Scholar]
- 12.Lairmore MD, Roberts B, Frank D, Rovnak J, Weiser MG, Cockerell GL. Comparative biological responses of rabbits infected with human T-lymphotropic virus type I isolates from patients with lymphoproliferative and neuro-degenerative disease. Int J Cancer. 1992;50:124–130. doi: 10.1002/ijc.2910500125. [DOI] [PubMed] [Google Scholar]
- 13.Murata N, Hakoda E, Machida H, Ikezoe T, Sawada T, Hoshino H, Miyoshi I. Prevention of human T cell lymphotropic virus type 1 infection in Japanese macaques by passive immunization. Leukemia. 1996;10:1971–1974. [PubMed] [Google Scholar]
- 14.Nakamura H, Hayami M, Ohta Y, et al. Protection of cynomolgus monkeys against infection by human T-cell leukemia virus type-1 by immunization with viral env gene products produced in Escherichia coli. Int J Cancer. 1987;40:403–407. doi: 10.1002/ijc.2910400320. [DOI] [PubMed] [Google Scholar]
- 15.Suga T, Kameyama T, Shimotohno K, et al. Infection of rats with HTLV-1: A small-animal model for HTLV-1 carriers. Int J Cancer. 1991;49:764–769. doi: 10.1002/ijc.2910490522. [DOI] [PubMed] [Google Scholar]
- 16.Ibrahim F, Fiette L, Gessain A, Buisson N, Dethe G, Bomford R. Infection of rats with human T-cell leukemia virus type-1: Susceptibility of inbred strains, antibody response and provirus location. Int J Cancer. 1994;58:446–451. doi: 10.1002/ijc.2910580324. [DOI] [PubMed] [Google Scholar]
- 17.Collins ND, Newbound GC, Ratner L, Lairmore MD. In vitro CD4(+) lymphocyte transformation and infection in a rabbit model with a molecular clone of human T-cell lymphotropic virus type 1. J Virol. 1996;70:7241–7246. doi: 10.1128/jvi.70.10.7241-7246.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miley WJ, Suryanarayana K, Manns A, Kubota R, Jacobson S, Lifson JD, Waters D. Real-time polymerase chain reaction assay for cell-associated HTLV type I DNA viral load. AIDS Res Hum Retroviruses. 2000;16:665–675. doi: 10.1089/088922200308891. [DOI] [PubMed] [Google Scholar]
- 19.Hiraragi H, Kim SJ, Phipps AJ, et al. Human T-lymphotropic virus type 1 mitochondrion-localizing protein p13II is required for viral infectivity in vivo. J Virol. 2006;80:3469–3476. doi: 10.1128/JVI.80.7.3469-3476.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pique C, Connan F, Levilain JP, Choppin J, Dokhelar MC. Among all human T-cell leukemia virus type 1 proteins, tax, polymerase, and envelope proteins are predicted as preferential targets for the HLA-A2-restricted cytotoxic T-cell response. J Virol. 1996;70:4919–4926. doi: 10.1128/jvi.70.8.4919-4926.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bangham CR. The immune control and cell-to-cell spread of human T-lymphotropic virus type 1. J Gen Virol. 2003;84:3177–3189. doi: 10.1099/vir.0.19334-0. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson S, Shida H, McFarlin DE, Fauci AS, Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature. 1990;348:245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson S, Reuben J, Streilen R, Palker T. Induction of CD4+ human T lymphotropic virus type 1 specific cytotoxic T lymphocytes from patients with HAM/TSP. J Immunol. 1991;146:1155–1162. [PubMed] [Google Scholar]
- 24.Koenig S, Woods RM, Brewah YA, et al. Characterization of MHC class I restricted cytotoxic T-cell responses to Tax in HTLV-I infected patients with neurologic diseases. J Immunol. 1993;156:3874–3883. [PubMed] [Google Scholar]
- 25.Elovaara I, Koenig S, Brewah AY, Woods RM, Lehky T, Jacobson S. High human T cell lymphotropic virus type 1 (HTLV-1)-specific precursor cytotoxic T lymphocyte frequencies in patients with HTLV-1-associated neurological disease. J Exp Med. 1993;177:1567–1573. doi: 10.1084/jem.177.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehky TJ, Cowan EP, Lampson LA, Jacobson S. Induction of HLA class I and class II expression in human T-lymphotropic virus type I-infected neuroblastoma cells. J Virol. 1994;68:1854–1863. doi: 10.1128/jvi.68.3.1854-1863.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biddison WE, Kubota R, Kawanishi T, et al. Human T cell leukemia virus type I (HTLV-1)-specific CD8(+) CTL clones from patients with HTLV-I-associated neurologic disease secrete proinflammatory cytokines, chemokines, and matrix metalloproteinase. J Immunol. 1997;159:2018–2025. [PubMed] [Google Scholar]
- 28.Kubota R, Kawanishi T, Matsubara H, Manns A, Jacobson S. Demonstration of human T lymphotropic virus type I (HTLV- I) tax-specific CD8(+) lymphocytes directly in peripheral blood of HTLV-I-associated myelopathy tropical spastic paraparesis patients by intracellular cytokine detection. J Immunol. 1998;161:482–488. [PubMed] [Google Scholar]
- 29.Kubota R, Nagai M, Kawanishi T, Osame M, Jacobson S. Increased HTLV type 1 tax specific CD8+ cells in HTLV type 1-asociated myelopathy/tropical spastic paraparesis: correlation with HTLV type 1 proviral load. AIDS Res Hum Retroviruses. 2000;16:1705–1709. doi: 10.1089/08892220050193182. [DOI] [PubMed] [Google Scholar]
- 30.Parker CE, Nightingale S, Taylor GP, Weber J, Bangham CRM. Circulating anti-Tax cytotoxic T lymphocytes from human T-cell leukemia virus type I-infected people, with and without tropical spastic paraparesis, recognize multiple epitopes simultaneously. J Virol. 1994;68:2860–2868. doi: 10.1128/jvi.68.5.2860-2868.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daenke S, Kermode AG, Hall SE, Taylor G, Weber J, Nightingale S, Bangham CRM. Hight activated and memory cytotoxic T-cell responses to HTLV-1 in healthy carriers and patients with tropical spastic paraparesis. Virology. 1996;217:139–146. doi: 10.1006/viro.1996.0101. [DOI] [PubMed] [Google Scholar]
- 32.Schonbach C, Nokihara K, Bangham CRM, et al. Identification of HTLV-1-specific CTL directed against synthetic and naturally processed peptides in HLA-B*3501 transgenic mice. Virology. 1996;226:102–112. doi: 10.1006/viro.1996.0632. [DOI] [PubMed] [Google Scholar]
- 33.Hanon E, Hall S, Taylor GP, et al. Abundant Tax protein expression in CD4+ T cells infected with human T-cell lymphotropic virus type I (HTLV-I) is prevented by cytotoxic T lymphocytes. Blood. 2000;95:1386–1392. [PubMed] [Google Scholar]
- 34.Cavrois M, Leclercq I, Gout O, Gessain A, Wainhobson S, Wattel E. Persistent oligoclonal expansion of human T-cell leukemia virus type 1 infected circulating cells in patients with tropical spastic paraparesis/HTLV-1 associated myelopathy. Oncogene. 1998;17:77–82. doi: 10.1038/sj.onc.1201906. [DOI] [PubMed] [Google Scholar]
- 35.Mortreux F, Kazanji M, Gabet AS, de Thoisy B, Wattel E. Two-step nature of human T-cell leukemia virus type 1 replication in experimentally infected squirrel monkeys (Saimiri sciureus) J Virol. 2001;75:1083–1089. doi: 10.1128/JVI.75.2.1083-1089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zane L, Sibon D, Mortreux F, Wattel E. Clonal expansion of HTLV-1 infected cells depends on the CD4 versus CD8 phenotype. Front Biosci. 2009;14:3935–3941. doi: 10.2741/3502. [DOI] [PubMed] [Google Scholar]
- 37.Goon PK, Biancardi A, Fast N, et al. Human T cell lymphotropic virus (HTLV) type-1-specific CD8+ T cells: Frequency and immunodominance hierarchy. J Infect Dis. 2004;189:2294–2298. doi: 10.1086/420832. [DOI] [PubMed] [Google Scholar]
- 38.Ureta-Vidal A, Angelin-Duclos C, Tortevoye P, et al. Mother-to-child transmission of human T-cell-leukemia/lymphoma virus type I: implication of high antiviral antibody titer and high proviral load in carrier mothers. Int J Cancer. 1999;82:832–836. doi: 10.1002/(sici)1097-0215(19990909)82:6<832::aid-ijc11>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Perez MP, Munoz-Juarez L, Cardenas FC, Zarranz Imirizaldu JJ, Carranceja JC, Garcia-Saiz A. Human T-cell leukemia virus type I infection in various recipients of transplants from the same donor. Transplantation. 2003;75:1006–1011. doi: 10.1097/01.TP.0000058470.15921.CA. [DOI] [PubMed] [Google Scholar]
- 40.Mahieux R, Gessain A. HTLV-1 and associated adult T-cell leukemia/lymphoma. Rev Clin Exp Hematol. 2003;7:336–361. [PubMed] [Google Scholar]


