Abstract
Importance:
Inhaled nitric oxide (iNO) is commonly administered for selectively-inhaled pulmonary vasodilation and prevention of oxidative injury following lung transplantation (LT). Inhaled epoprostenol (iEPO) has been introduced worldwide as a cost-saving alternative to iNO without high-grade evidence for this indication.
Objective:
To investigate if iEPO will lead to similar rates of severe/grade-3 primary graft dysfunction (PGD-3) as the use of iNO after LT.
Design, Setting, and Participants:
Health-system funded, randomized, blinded (to participants, clinicians, data managers and statistician), parallel-designed, equivalence clinical trial in 201 adult patients that underwent single or bilateral LT from May 30, 2017 to March 21, 2020.
Interventions:
Participants were grouped into five strata according to key prognostic clinical features and randomized per stratum to receive either iNO or iEPO at the time of LT via 1:1 treatment allocation. Allocated treatment was initiated before lung allograft reperfusion and continuously administered until cessation criteria were met in the intensive care unit.
Main Outcomes and Measures:
The primary outcome was PGD-3 development at 24-, 48- or 72-hours after LT. The primary analysis was for equivalence using a two one-sided test (TOST) procedure (90% Confidence Interval, CI) with a margin of 19% for between-group PGD-3 risk difference. Secondary outcomes included duration of mechanical ventilation; hospital and intensive care unit lengths-of-stay; incidence and severity of acute kidney injury; postoperative tracheostomy placement; and in-hospital, 30-day and 90-day mortality rates. An intention-to-treat analysis was performed for the primary and secondary outcomes, supplemented by per-protocol analysis for the primary outcome.
Results:
201 randomized patients met eligibility criteria at the time of LT. In the intention-to-treat population, 103 received iEPO and 98 received iNO. The primary outcome occurred in 46 patients (44.7%) in the iEPO group and 39 (39.8%) in the iNO group, leading to a risk difference of 4.9% (TOST 90% CI, −6.4, 16.2; P = 0.019 for equivalence). There were no significant between-group differences for secondary outcomes.
Conclusions and Relevance:
Among patients undergoing lung transplantation, use of iEPO was associated with similar risks for PGD-3 development and other postoperative outcomes compared to those that received iNO.
Trial Registration:
ClinicalTrials.gov identifier: NCT03081052
Introduction
Inhaled nitric oxide (iNO) is administered after lung transplantation (LT) to promote lung-allograft function1,2 by improving oxygenation and lowering pulmonary vascular resistance.3–5,6 Consequently, iNO may help mitigate severe primary graft dysfunction (PGD grade-3 or PGD-3) development,7 which is diagnosed within 72-hours after LT8 and is strongly associated with short- and long-term mortality.9,10 Although iNO is not approved by the U.S. Food and Drug Administration (FDA) for this indication, international guidelines support its use after LT.8 In a recent survey of 74 LT centers worldwide, the primary inhaled pulmonary vasodilator (iPVD) was iNO in 73% (n=54 centers) followed by aerosolized or inhaled epoprostenol (iEPO) in 12% (n=9).11
Unfortunately, iNO cost exceeds millions of dollars annually for large healthcare systems nationwide and iNO is approximately seven-fold more expensive than iEPO.12 Accordingly, iEPO has emerged as a cost-saving iNO-alternative at several institutions. Although similar anti-oxidative and vasodilatory properties of iEPO have been reported in LT,13,14 evidence supporting use is not based on robust comparisons with iNO that evaluate for clinically-meaningful outcomes. Furthermore, available data interpretation is complicated by retrospective observational studies, differing epoprostenol formulations15 and aerosol-generating devices,16 and lack of standardized criteria for discontinuing either agent.
Given the serious nature of PGD-3 development and significant economic considerations of continued iNO use, clinician-investigators designed and conducted a randomized trial funded by the health system to determine if iEPO delivery would result in similar rates of PGD-3 and other outcomes after LT, compared with iNO.
Methods
Design
This was a parallel-designed, clinical trial that randomly assigned LT recipients (“participants”) to receive either iNO or iEPO. This study is registered as part of the INSPIRE-FLO trial (Clinicaltrials.gov identifier: NCT03081052; see Supplement 1 for trial protocol). Two surgical populations were evaluated under this registration with separate, independent analysis plans. Analysis for the LT population is described here.
Funding
Research-related activities were funded through Duke University Health System. A separate process was established to ensure trial medication costs were covered by insurance providers. First, a blanket approval for trial enrollment was obtained for patients insured through the Centers for Medicare & Medicaid Services (CMS) before trial commencement. For other eligible patients, an enrollment request letter was sent to private insurers. The Institutional Review Board approved the protocol without a data safety monitoring board as both medications were on formulary and either could be used as standard-care. Adverse events were reviewed each quarter by the PI and research team while blinded to treatment assignment.
Participants
End-stage lung disease patients, 18-years and older, with insurance approval for enrollment were screened for eligibility upon transplant listing, approached for consent and randomized at the time of consent. Notable exclusions included combined-organ transplantation and presence of extracorporeal membrane oxygenation (ECMO) before LT. Given the variable duration from randomization to potential treatment initiation during LT, participants were included in the primary analysis if they were not withdrawn, did not die or develop changes to eligibility post-randomization.
Randomization and Blinding
Five randomization strata were created based on primary indication and single or bilateral lung-allograft transplantation. Within each strata, participants were assigned to receive either iNO or iEPO at the time of LT via 1:1 treatment allocation with block sizes of four. Randomization sequence was generated before trial commencement using nQuery Advisor® version 7 (Statsols, Inc., Cork, Ireland). Upon notification from the transplant coordinator that a participant would be undergoing LT, the research team would contact the study respiratory therapist and pharmacist. The pharmacist would access the password-protected randomization sequence list and prepare the allocated treatment.
Using an inline system for blinding iEPO and iNO delivery adopted from Preston et al.17 (Supplement 2), blinding was preserved for all participants and clinicians involved in patient care. Additionally, allocated treatment was masked in the electronic record in a separate clinical documentation platform developed for this study (Maestro-Care®, Epic-Systems, Madison, WI). All research team members with database access (data managers) were blinded to treatment assignment. Following study completion, an independent statistician created a blinded-treatment assignment code for use during analysis and the study statistician remained blinded to the assignment until all analyses were completed.
Intervention
A blinded 50-millilter syringe solution of either 5% sodium chloride (if randomized to iNO) or 30,000 nanograms/ml epoprostenol (Veletri®, Actelion Pharmaceuticals, South San Francisco, CA) was prepared by the study pharmacist. Epoprostenol syringe-concentration was based on standard compounding by the pharmacy department. The study respiratory therapist would obtain the syringe from pharmacy, verbally confirm the solution identity, and place the syringe in a dedicated refrigerator. Fifteen minutes before reperfusion of the first transplanted lung, the study respiratory therapist would initiate the treatment in the operating room. Participants randomized to iNO (iNOMax®, Mallinkrodt Pharmaceuticals, St. Louis, MO) would continuously receive 20 parts-per-million while the iEPO group would continuously receive 50 nanograms/kg/min (ideal body weight) delivered using a syringe pump and vibrating mesh aerosolizer (Aerogen Pro-X®, Galway, Ireland). iEPO dosing derived from a dose-response study that displayed improved oxygenation between 10–50 ng/kg/min in acute respiratory distress syndrome18 and has been adopted at multiple institutions for previous studies.14,17,19–22
After LT, the study therapist accompanied the clinical-care team to the ICU and ensured appropriate treatment delivery and blinding. In the ICU, a non-study respiratory therapist then assumed direct patient care. The study therapist remained immediately available to manage treatment delivery and was notified to wean each treatment by protocol once discontinuation criteria were identified (Supplement 1).
Standardized Care for Lung Transplantation
Standardized care for LT management at our institution, including intensive care, infection prophylaxis and immunosuppression, has been reviewed.23 Relevant protocols for mechanical ventilation (Supplement 1) and ECMO management (Supplement 2) are included.
Outcomes
The primary outcome was PGD-3 development based on daily grading assigned at 24-, 48-, or 72-hour timepoints after ICU arrival following LT. Based on PGD guidelines,8 grade-3 is diagnosed by poor systemic oxygenation (defined by partial pressure of arterial oxygen-to-fraction of inspired oxygen, PaO2:FiO2 < 200 or ECMO use) and radiographic evidence for lung-allograft edema.8
Secondary outcomes included duration of mechanical ventilation measured from ICU arrival to endotracheal extubation, censored for those that underwent postoperative tracheostomy placement. Acute kidney injury (AKI) was determined by the Kidney Disease-Improving Global Outcomes (KDIGO) criteria (Supplement 2) up to seven days post-LT based on studies that have supported iEPO24 and iNO25 for renal protection. Other outcomes included hospital and ICU lengths-of-stay (LOS) and early postoperative mortality (in-hospital, 30-days, 90-days). Additionally, we compared daily average values for mean pulmonary arterial pressures between groups through postoperative day 3.
Statistical Methods
The statistical analysis plan (Supplement 1) is written in accordance with journal guidelines.26 Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
The trial was designed to demonstrate clinical equivalence between iEPO and iNO by a prespecified lower and upper bound around the PGD-3 outcome measure. The margin of equivalence was set to 19% with an anticipated PGD-3 incidence of 30% for the iNO group based on previous PGD-3 event rates that used the first 72-hours after LT as the primary outcome timeframe.7,10,27–30 PGD-3 assessment was performed while participants remained in the hospital and loss to follow-up was not factored into sample-size determination. Thus, 200 participants allocated 1:1 would be sufficient to establish equivalence for the prespecified margin at 80% power. Alpha was controlled at 0.05 for all comparisons. Two one-sided tests (TOST) were used for primary outcome analyses while utilizing two-sided hypothesis testing for secondary outcomes.
An intention-to-treat analysis was planned for the primary and secondary outcomes, supplemented by per-protocol analysis for the primary outcome. Baseline characteristics were summarized for each treatment group and reported as mean (standard deviation, SD) or median (interquartile range, IQR) for continuous variables and as count (%) for categorical ones. Summaries were used to assess randomization performance and protocol adherence. Using the TOST procedure, the primary outcome was determined by calculating the point estimate and corresponding 90% confidence intervals, CI, for the risk difference between iEPO and iNO. If the CI were contained inside the equivalence margin, then there would be sufficient evidence to conclude that PGD-3 rates in each group would be similar (P<0.05). Relative risk, RR, estimates for PGD-3 development if treated with iNO compared with iEPO (95% CI) were reported. Based on baseline characteristics, the balance of patient factors was evaluated between treatment groups. All covariates meeting P<0.15 association between treatment groups were considered for variable selection to build a stepwise, multivariable regression model for PGD-3 to adjust the treatment difference for these potential confounders. Secondary outcomes were assessed for treatment differences under typical null hypothesis-utilizing univariable effect estimates and corresponding two-sided 95% CI. Binary secondary outcomes (tracheostomy, AKI, mortality) were assessed via risk differences and RR, while continuous secondary outcomes (ICU and hospital LOS) were assessed via risk differences and mean ratios estimated from log-linear regression models. Kaplan-Meier point estimates (95% CI) were used to determine mechanical ventilation duration censored for postoperative tracheostomy placement.
Finally, a post-hoc analysis was performed to determine overall and between-group PGD-3 rates using two commonly-reported sub-intervals of the 72-hour outcome timeframe: 48- or 72-hours9,29 and 72-hours alone.31
Results
Population
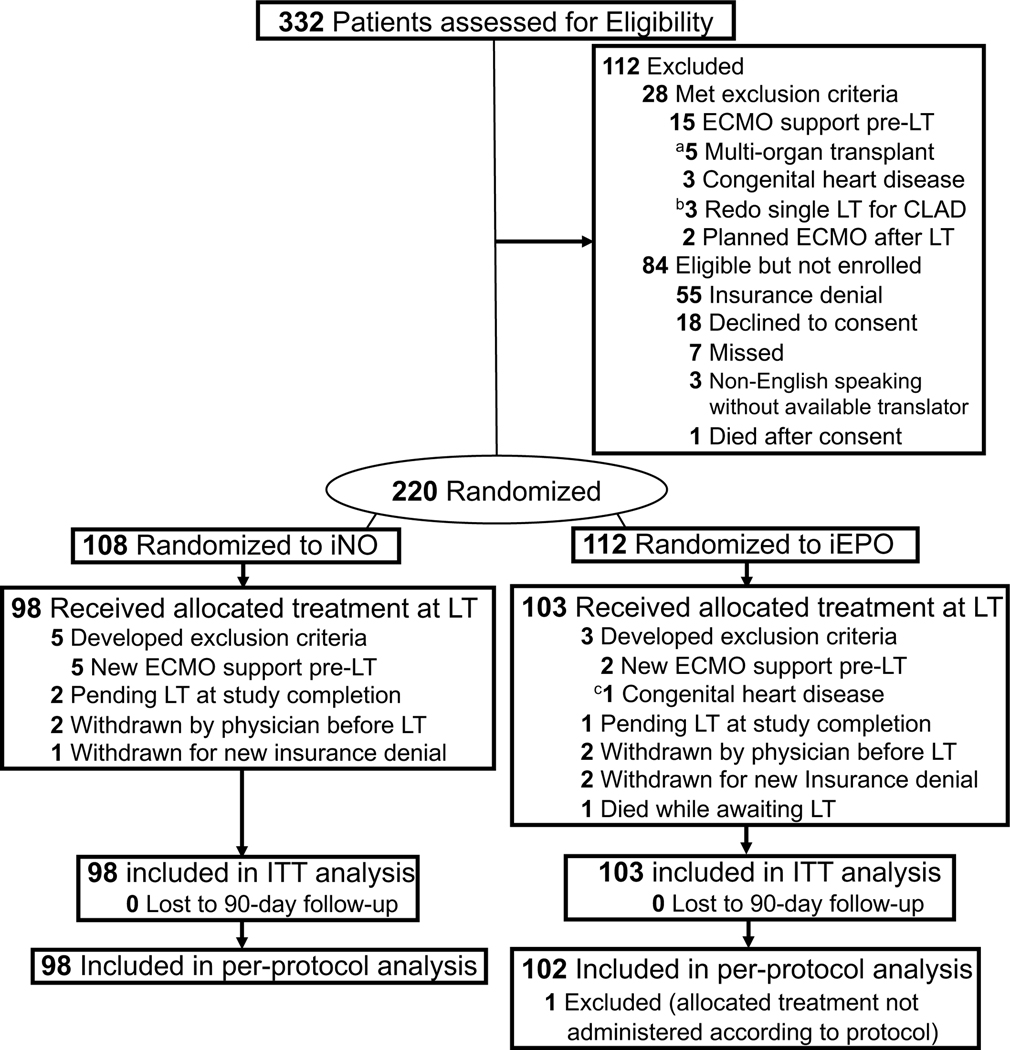
From May 29, 2017 to March 21, 2020, 332 patients were screened. Of these, 112 patients did not meet eligibility criteria during screening, where 28 patients (8.4%) met exclusion criteria and 84 (25.3%) were eligible but not enrolled due to various reasons (Figure 1). Of 220 randomized participants, 19 (8.6%) developed changes to eligibility before they could receive the allocated treatment (8 needed ECMO before LT, 4 were withdrawn-by-physician for clinical deterioration, 3 were withdrawn for insurance denial, 3 were awaiting LT at time of study completion, and 1 died), leaving 98 patients who were allocated to the iNO group and 103 to the iEPO group (n=201). Final 90-day follow-up for mortality was performed on June 19, 2020.
Figure 1. Flow of Participants in a Study of Inhaled Pulmonary Vasodilators for Adult Lung Transplantation.
In all analyses, patients were analyzed according to their randomized group. Participants were excluded from the intention-to-treat analysis if they were withdrawn, developed exclusion criteria after randomization, or remained on the LT list and were not transplanted. Those that received the allocated treatment at the time of LT were included in an intention-to-treat analysis. Study enrollment was completed once sample-size was achieved. None of the participants were lost to 90-day follow-up.
aMultiple organ transplantation included 1 patient for lung-kidney and 4 patients for lung-liver.
bPatient with diagnosis that did not fit one of the five randomization strata
cIneligible for enrollment, consented and randomized, then ineligibility was noted before LT.
CLAD, Chronic lung allograft dysfunction; ITT, Intention-to-treat; LT, Lung transplantation
Baseline and clinical characteristics for participants and organ-donors in the intention-to-treat analysis are shown (Table 1). Common indications for LT were restrictive (62.3%) and obstructive diseases (20.9%), which are proportionally consistent with the 2016 LT registry report.32 There were 173 participants (86.1%) who underwent bilateral LT and 28 (13.9%) for single LT. Indications for single or bilateral LT were similar between treatment groups, indicating success of the stratified randomization.
Table 1.
Baseline Participant Characteristics
| iNO (N=98) | iEPO (N=103) | |
|---|---|---|
| Patient Demographics and History | ||
| Age, years | 64 (54, 68) | 64 (51, 69) |
| Sex, male | 59 (60.2%) | 70 (68.0%) |
| Race | ||
| Caucasian/White | 83 (84.7%) | 91 (88.3%) |
| African American | 12 (12.2%) | 9 (8.7%) |
| Other | 3 (3.1%) | 3 (2.9%) |
| BMI | 25.0 (22.1, 26.7) | 26.0 (22.8, 27.3) |
| HTN | 48 (49.0%) | 43 (41.7%) |
| PH Diagnosis | 42 (42.9%) | 54 (52.4%) |
| Severity of PH | ||
| Mild | 6 (6.1%) | 7 (6.8%) |
| Moderate | 29 (29.5%) | 33 (32.0%) |
| Severe | 7 (7.1%) | 14 (13.6%) |
| DM (Types 1 or 2) | 17 (17.3%) | 25 (24.3%) |
| COPD | 34 (34.7%) | 45 (43.7%) |
| aPreoperative LVEF (%) | ||
| Normal (≥ 50%) | 94 (95.9%) | 100 (97.1%) |
| Mild Dysfunction (40%−49%) | 0 (0.0%) | 1 (1.0%) |
| Moderate Dysfunction (30%−39%) | 0 (0.0%) | 1 (1.0%) |
| Previous Sternotomy for Cardiac Surgery | 4 (4.1%) | 2 (1.9%) |
| Previous Lung Transplant | 6 (6.1%) | 4 (3.9%) |
| Lung Allocation Score | 42.0 (36.9, 51.9) | 42.8 (37.2, 52.4) |
| Common Indications for Lung Transplant. | ||
| Group A - Obstructive lung disease | 21 (21.4%) | 21 (20.3%) |
| Group B - Pulmonary vascular disease | 2 (1.9%) | 1 (1.0%) |
| Group C - Infectious lung disease | 8 (8.2%) | 15 (14.6%) |
| Group D - Restrictive lung disease | 63 (64.2%) | 63 (61.2%) |
| bOther | 4 (4.1%) | 3 (2.9%) |
| Preoperative Laboratory Values | ||
| Estimated GFR (ml/min) | 85 (70, 98) | 88 (75, 100) |
| Hemoglobin (g/dL) | 12.30 (1.65) | 12.57 (1.73) |
| Creatinine (mg/dL) | 0.9 (0.7, 1.0) | 0.9 (0.7, 1.0) |
| Class 1 PRA > 0 | 17 (17.3%) | 17 (16.5%) |
| Class 1 PRA % (among those >0) | 17 (7, 75) | 29 (17, 57) |
| Class 2 PRA > 0 | 13 (13.3%) | 13 (12.6%) |
| Class 2 PRA % (among those >0) | 38 (26, 49) | 30 (22, 40) |
| Right Heart Catheterization Values | ||
| Cardiac Index (L/min/m2) | 2.8 (2.5, 3.2) | 2.9 (2.7, 3.3) |
| Mean PAP (mm Hg) | 22.8 (18.3, 27.7) | 24.7 (20.0, 29.7) |
| Procedural Characteristics | ||
| cBilateral Lung Transplantation | 84 (85.7%) | 89 (86.4%) |
| Obstructive Lung Disease | 26 (26.5%) | 31 (30.1%) |
| Restrictive Lung Disease | 52 (53.1%) | 53 (51.5%) |
| Pulm. Vascular Disease | 2 (2.0%) | 1 (1.0%) |
| Other Diagnosisd | 4 (4.1%) | 4 (3.9%) |
| cSingle LT – Restrictive Lung Disease | 14 (14.3%) | 14 (13.6%) |
| Concurrent Cardiac Operation | 7 (7.1%) | 7 (6.8%) |
| Intraoperative CPB used | 19 (19.4%) | 19 (18.4%) |
| Intraoperative ECMO Used | 33 (33.7%) | 27 (26.2%) |
| Ischemia time, Single LT only, minutes | 325 (304, 353) | 325 (261, 340) |
| eIschemia time, 2nd Lung only, minutes | 395 (349, 489) | 432 (352, 495) |
| fUse of Transmedics OCS™/EVLP | 4 (4.1%) | 3 (2.9%) |
| Donor Characteristics | ||
| Age, years | 35 (26, 46) | 35 (27, 47) |
| Sex donor-to-recipient mismatch | ||
| Matched | 78 (79.6%) | 69 (67.0%) |
| Female Donor-to-Male Recipient | 6 (6.1%) | 16 (15.5%) |
| Male Donor-to-Female Recipient | 14 (14.3%) | 18 (17.5%) |
| Race | ||
| Caucasian/White | 72 (73.5%) | 74 (71.8%) |
| African American/Black | 16 (16.3%) | 17 (16.5%) |
| Other | 10 (10.2%) | 12 (11.7%) |
| gBMI of donor-recipient % mismatch | −4.5 (−20.4, 10.0) | −7.5 (−20.3, 7.2) |
| Donor PaO2: FiO2 ratio | 443 (396, 494) | 425 (378, 495) |
| Donor cigarette use > 20 pack years | 11 (11.5%) | 10 (9.7%) |
| Donation after Cardiac Death | 10 (10.2%) | 13 (12.6%) |
| Donation after Brain Death | 88 (89.8%) | 90 (87.4%) |
| Cause of Brain Death | ||
| Anoxia | 33 (33.7%) | 30 (29.1%) |
| CVA/Stroke | 26 (26.5%) | 29 (28.2%) |
| Head Trauma | 37 (37.8%) | 41 (39.8%) |
| CNS Tumor | 1 (1.1%) | 0 (0.0%) |
| Other | 1 (1.1%) | 3 (2.9%) |
Preoperative LVEF available in 94/98 patients in iNO group and 102/103 participants from iEPO group
Includes diagnoses that were not otherwise classifiable under Group A-D
Randomization strata are based on single or bilateral LT and primary diagnosis for LT
Other Bilateral LT diagnosis in iNO group (N=4): Bronchiolitis obliterans syndrome (N=2), Occupational fiberglass exposure (N=1), Adult Respiratory Distress Syndrome (N=1);
Other Bilateral LT diagnosis in iEPO group (N=4): Bronchiolitis obliterans syndrome (N=2), Coal worker’s pneumoconiosis (N=2)
By convention, for Bilateral LT, the ischemia time of the second lung only is reported
Organ care system (Transmedics OCS™) use during lung-allograft transport after donor harvest has shown promise in reducing PGD-3 rates.39
Negative percent indicates recipient BMI is less than donor BMI.
BMI, Body mass index; CPB; Cardiopulmonary bypass; CNS, Central nervous system; CVA, Cerebrovascular Accident; ECMO, Extracorporeal membrane oxygenation; EVLP, Ex-vivo lung perfusion; LVEF, Left ventricular ejection fraction; GFR, glomerular filtration rate; OCS, Organ care system; PaO2:FiO2, Partial pressure of arterial oxygen-to-Fraction of inspired oxygen; PAP, pulmonary arterial pressure; PRA, panel-reactive antibody
For donor characteristics, donor-to-recipient sex mismatch was observed in 20 participants (20.4%) in the iNO group and in 34 (33.0%) in the iEPO group with a predominance of male donor-to-female recipient mismatch for both groups.
Intervention
Median (IQR) duration from randomization to treatment was 5-days (1, 20-days). Once initiated, allocated treatment durations, blood transfusion or ECMO support were similar between groups (Table 2). Delayed chest closure was observed in 15 patients (15.3%) who received iNO and in 7 (6.8%) who received iEPO (P=0.053).
Table 2.
Participant Characteristics Relevant to Outcome Determination after Treatment Exposure
| Parameter | iNO (N=98) | iEPO (N=103) | P-value |
|---|---|---|---|
| dDuration of treatment, hours | 45.7 (33.1, 102.6) | 46.6 (30.4, 83.6) | 0.43a |
| Delayed Chest closure | 15 (15.3%) | 7 (6.8%) | 0.053b |
| eECMO Present on ICU arrival | 17 (17.4%)) | 16 (15.5%) | 0.73b |
| fECMO placed within 72 hours | 7 (7.1%) | 6 (5.8%) | 0.70c |
| gPRBC transfusion, Units | 2 (0, 4) | 2 (1, 4) | 0.62a |
p-Value Key:
Wilcoxon
Chi-Square
Equal Variance t-test.
In the per-protocol population, iEPO group had n=102, with median duration of 46.6 hours (IQR, 30.7, 83.6) after LT before discontinuation of iEPO.
ECMO placement between ICU arrival and 72-hours after LT.
ECMO placement in the operating room during LT that continued into the intensive care unit
Transfusion data missing for 1 iNO patient
ECMO, Extracorporeal membrane oxygenation; ICU, Intensive care unit; PRBC, Packed red blood cells
Outcomes
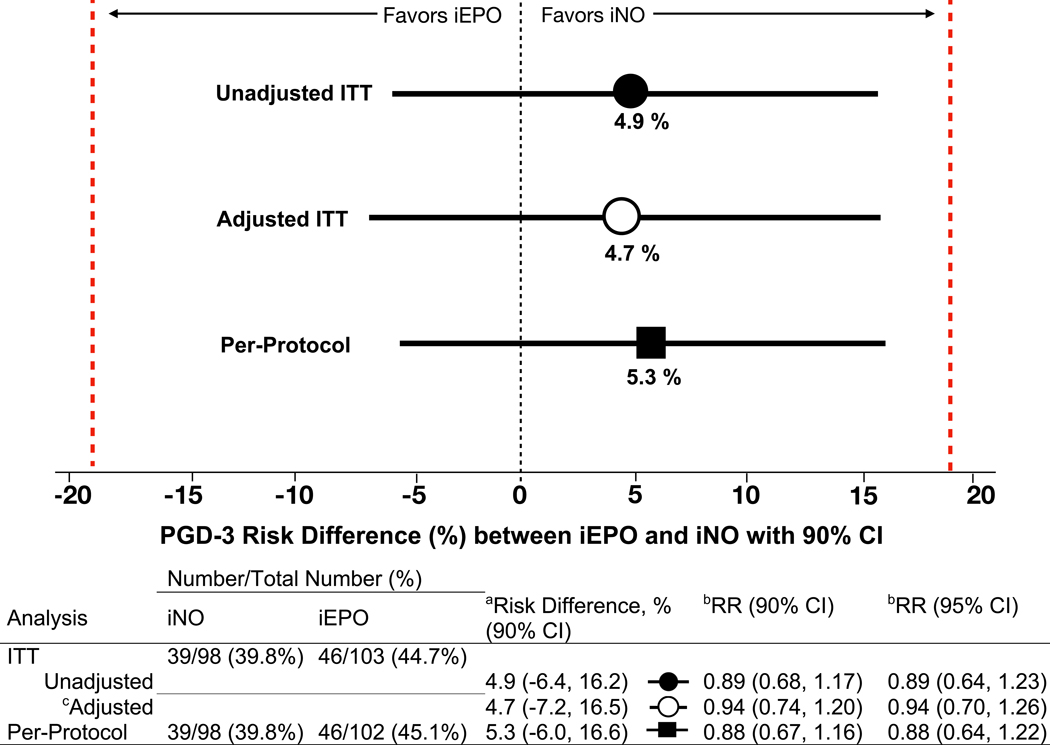
In the unadjusted intention-to-treat analysis, PGD-3 incidence was 39.8% (n=39) in the iNO group and 44.7% (n=46) in the iEPO group for a risk difference of 4.9% (90% CI, −6.4%, 16.2%; P=0.019 in support for equivalence). The results of the adjusted intention-to-treat analysis using a stepwise data-driven, model-building approach (Supplement 3, eTable 1) and per-protocol analysis confirmed those of the unadjusted intention-to-treat analysis (Figure 2).
Figure 2. Risk differences and relative risks of PGD-3 development between iNO and iEPO treatment groups.
To determine the presence of clinical equivalence between iNO and iEPO, a lower and upper bounds of −19% and +19% was prespecified (red lines).
aRisk difference is the absolute difference between PGD-3 rates between groups and is determined by the two one-sided test (TOST) procedure. Setting α at 0.05 and testing the upper and lower bounds separately, equivalence is concluded only if both tests are significant. To transform this procedure into a single confidence interval, 1–2α (90%) is used and the TOST CI becomes the intersection of the two one-sided confidence intervals. A more conservative 95% CI (1-α) was determined and also demonstrated exclusion of the lower and upper bounds of the margin in support of equivalence for the unadjusted ITT (−8.6%, +18.3%), adjusted ITT (−9.5%, +18.8%) and per-protocol (−8.2%, +18.8%) analyses.
bRelative risk, RR, is the risk of developing PGD-3 if treated with iNO compared with iEPO.
cMultivariable logistic regression adjusting for delayed chest closure and donor-recipient sex mismatch from the selected model (Supplement 3, eTable 1). Risk difference and RR are derived from the multivariable logistic regression model. Differences between adjusted and unadjusted risk difference and RR are due to the difference in comparing two patients in the adjusted analysis with the same sex-mismatch and chest closure status. However, number of events and their distribution between the unadjusted and adjusted analyses remain the same.
CI, Confidence interval; iEPO, Inhaled epoprostenol; iNO, Inhaled nitric oxide; ITT, Intention-to-treat.
For secondary outcomes (Table 3), there were no significant between-group differences for mortality, AKI, tracheostomy placement, LOS or duration of mechanical ventilation censored for tracheostomy placement (Supplement 3, eFigure 1). No important differences were found in adverse events (Supplement 3, eTable 2) or daily mean pulmonary arterial pressures (Supplement 3, eFigure 2).
Table 3.
Secondary Outcomes
| Outcome | iNO (N=98) | iEPO (N=103) | Risk Difference, % (95% CI) | aRelative Risk (95% CI) | P-value |
|---|---|---|---|---|---|
| Mortality | |||||
| 30-Day | 2 (2.0%) | 1 (1.0%) | −1.0 (−4.0, 2.0) | 2.10 (0.19, 22.81) | 0.61 |
| 90-Day | 4 (4.1%) | 4 (3.9%) | 0.2 (−6.0, 5.0) | 1.05 (0.27, 4.09) | 0.94 |
| In-Hospital | 5 (5.1%) | 7 (6.8%) | 1.7 (−5.0, 8.0) | 0.75 (0.25, 2.29) | 0.61 |
| Tracheostomy | 22 (22.4%) | 29 (28.2%) | 5.8 (−6.0 to 18.0) | 0.80 (0.49, 1.29) | 0.35 |
| bAKI of any stage | 72 (73.5%) | 67 (65.0%) | −8.5 (−20.0, 5.0) | 1.12 (0.93, 1.34) | 0.23 |
| bAKI stages 2 or 3 | 29 (29.6%) | 24 (23.3%) | −6.3 (−18.0, 6.0) | 1.26 (0.79, 2.00) | 0.33 |
| d HL Location Shift (95% CI) | e Mean Ratio (95% CI) | ||||
| ICU LOS (days) | 4 (2, 10) | 4 (2, 10) | 0 (−1, 1) | 1.19 (0.76, 1.87) | 0.45 |
| Hospital LOS (days) | 23 (16, 38) | 23 (15, 38) | 0 (−3, 3) | 1.03 (0.75, 1.41) | 0.86 |
| Duration of mechanical ventilation (hours) | |||||
| fKM Median (95% CI) Estimates | 19 (15, 24) | 22 (17, 36) | 0.75g | ||
Relative Risk (with p-values) of developing the outcome if participants receive iNO rather than iEPO.
Kidney Disease-Improving Global Outcomes AKI grading include stages 1, 2 or 3 in ascending order of severity. AKI stage 2 and 3 are more commonly associated with poor outcomes after Lung Transplantation (LT) and AKI incidence is independent of PGD-3 occurrence.40
Hodges-Lehmann non-normal difference estimator
Mean ratio with P-values from log-linear models.
Measured from 197 patients (four patients had pre-LT tracheostomy). For those that received postoperative tracheostomy, time-to-extubation interval was censored at the time of tracheostomy placement to avoid underestimating the distribution of time-to-end of mechanical ventilation.
Log-rank P-value
AKI, Acute kidney injury; ICU, Intensive care unit; KM, Kaplan-Meier analysis; LOS, Length-of-stay.
Post-hoc analysis
Using the intention-to-treat population, the PGD-3 incidence at 48- or 72-hours was 26.5% (n=26) in the iNO group and 28.2% (n=29) in the iEPO group, for a risk difference of 1.6% (90% CI, −8.8%, 12.0% for equivalence). The PGD-3 incidence at the 72-hour mark was 16.3% (n=16) in the iNO group and 21.3% (n=22) in the iEPO group for a risk difference of 5.0% (90% CI, −4.5%, 14.6% for equivalence).
Discussion
In this trial of adult patients undergoing LT who prophylactically received iPVD to promote lung-allograft function, iEPO was associated with similar PGD-3 development as seen with iNO. Furthermore, no significant between-group differences were observed in durations of mechanical ventilation, LOS, tracheostomy and AKI incidences, or mortality up to 90-days.
Financial support for this study originated from the large economic impact of iNO use in this population on the health system. While specific contract-pricing is not disclosed, based on 2017 pricing, the cost-per-hour in a 70-kg adult for Veletri® was $6.52 while the non-contracted price for iNOmax® was $220.46.12 While contract pricing may lower the hourly cost, the contract cost-per-hour of iNO remained 7-fold more than that of iEPO at our institution. In another study, McGinn reviewed 98 cardiothoracic surgical patients and found the median iEPO cost-per-patient was also seven-fold higher cost with iNO ($364 [IQR, $226-$865] vs. $2,563 [IQR, $1875-$8625], respectively; P<0.01).33 While costs vary between institutions, the annual estimated iEPO expenditures can range from $200,000-$1,000,000 while that of iNO can exceed $3,000,000-$8,000,000.12
To our knowledge, this is the largest randomized trial comparing iEPO to iNO after adult LT using the PGD-3 outcome, which is diagnosed within an established timeframe after LT. PGD-3 may be modified through lowering of the pulmonary vascular resistance and limiting oxidative injury of the lung allograft. PGD-3 development can be devastating for long-term functional status with increased risk for redo LT from chronic lung-allograft dysfunction.10 Benefits of iNO for PGD-3 prevention through prophylactic use were reported in a placebo-controlled trial where iNO was initiated before lung-allograft reperfusion, discontinued within 48-hours, and was associated with lower PGD biomarkers and two-fold lower PGD-3 incidence compared with placebo (45% versus 17%, P<0.035).7 These authors defined PGD-3 development within 72-hours after LT using radiographic evidence of allograft edema, P:F ratio < 200, and no other cause for allograft dysfunction (i.e., anastomotic venous obstruction, infection or cardiogenic allograft edema).7 Where our study implemented clinical protocols to wean the allocated iPVD when indicated, this study utilized a fixed 48-hour duration. Interestingly, we found similar between-group median durations for postoperative iPVD use that approximated 48-hours (Table 2).
Equivalence testing was chosen, rather than noninferiority alone, as both medications are used in LT centers worldwide and guidelines support use of either medication to promote allograft function.34 The choice of the margin was based on the potential loss of relative efficacy that was acceptable with iNO in return for non-efficacy advantages with iEPO. Thus, considerations encompassed risk differences of previous studies, powering for an important outcome and feasibility in accomplishing the study based on annual operations. Additionally, the 2016 FDA guidance document for noninferiority trials was reviewed, which endorsed using a margin that was less than the risk difference of best-available evidence between active control (i.e., iNO) and placebo.35 FDA guidance was also reviewed for equivalence testing using the TOST procedure and allowable margin selection up to 20%.36 Thus, the prespecified equivalence margin for this study satisfied these considerations.
Bias was minimized in this study through concealed allocation, analysis by randomized assignment, and medication blinding to participants, clinicians, data managers and statistician. Performing an appropriately powered and designed prospective investigation in this population is pragmatically challenging as most operations often occur at night, which creates logistical challenges for implementation of research-related activities. Furthermore, changes in clinical culture to adopt iEPO as a potential iNO alternative was critical for the successful implementation of a parallel-design with clinician blinding. In fact, protocol non-adherence occurred in only a single participant who was allocated to iEPO, switched to iloprost (long-acting prostacyclin) to accommodate a procedure, then transitioned back to iEPO.
Importantly, this study was funded through the health system without external support and medication costs were remunerated through insurance providers in the same manner as non-trial patients as both medications were standard-care. While blanket approval for CMS patients was obtained, private insurers were contacted individually, resulting in 58 eligible patients who were denied enrollment (Figure 1). Three of these were denied after an initial approval allowed for randomization. Furthermore, a separate documentation platform was developed to facilitate medication blinding during clinical-care and unblinding for medical billing after participant hospital discharge.
After randomization, participants who were not withdrawn for clinical deterioration, insurance denial, or death, were included in the intention-to-treat analysis. Modification of a classic intention-to-treat analysis has been previously described in trials of other critically-ill populations37 and those undergoing other cardiothoracic operations.38 Furthermore, given the standard use of iNO or iEPO during LT, analyzing all participants “as randomized” without iPVD blinding and risking potential for cross-over to the non-randomized treatment could have led to significant interpretation bias. Post-randomization changes to eligibility before LT mainly included new ECMO support, which was an established exclusion criteria since pre-LT ECMO support routinely continued after LT and would have confounded PGD-3 assessment.
A post-hoc analysis was performed to evaluate for PGD-3 development using two validated sub-intervals of the 72-hour assessment timeframe.9,29,31 Compared with overall PGD-3 rates for the primary outcome (42.2%), lower rates were demonstrated at 48- and 72-hours (27.4%, consistent with a previous report of 30% using this timeframe29) and at 72-hours (18.9%). The first 24-hours was included in the primary outcome definition as the allocated treatment could be weaned during this early postoperative period. In fact, 25% of participants were weaned by 31-hours after ICU arrival. Thus, evaluating the primary outcome at 48- or 72-hours while excluding the 24-hour mark could have potentially resulted in missed events in the early post-LT period related to interventions. Thus, we were able to capture all participants that experienced PGD-3 while receiving allocated treatment. Importantly, between-group PGD-3 risk differences at each sub-interval were similar to that of the intention-to-treat analysis, suggesting similar between-group effects on PGD-3 development at these differing timepoints along the 72-hour window.
Limitations
This study has several limitations. Although this study represents one of the largest randomized perioperative clinical trials in adult LT to date, it was of moderate size and powered for equivalence between treatment groups for the primary outcome. Second, the prespecified equivalence margin was based on a range of incidences that may have been considered too large. While confidence intervals for the RR of developing PGD-3 with iEPO versus iNO included the null hypothesis for unadjusted, adjusted and per-protocol analyses, the risk difference point estimate favored iNO in all three analyses. Thus, iEPO could conceivably be clinically-inferior while simultaneously meeting the statistical criteria for equivalence. While this is a potential interpretation, the current study represents the best available randomized evidence and suggests no between-groups differences were observed for PGD-3 development. Third, given the complexity of risk factors in this patient population, and the anticipation that the randomization would balance most potential confounders, an adjustment model was not prespecified. Instead, a stepwise data-driven, model-building approach was performed to adjust for significant outcome-effect modifiers. While this data-driven approach may not account for all potential sources of confounding, it does account for the most significant of them, and ones that would have been strong enough to bias our findings. For example, delayed chest closure, which heralds a more complex clinical course, occurred more often in the iNO group and was identified as a PGD-3 effect modifier and used to adjust the intention-to-treat analysis. Fourth, given the unique funding mechanism, a multicenter investigation could not be supported. Fifth, this study was not placebo-controlled. However, iPVD use was part of standard-care at our institution and placebo would have led to considerable cross-over to the treatment arm, thus complicating the interpretation between intention-to-treat and per-protocol analyses. Sixth, while prophylactic iPVD administration is part of our standard-care, this practice is not universal and some high-volume LT centers may selectively initiate iPVD in high-risk patients or after lung-allograft reperfusion injury. Nevertheless, high-risk patients were represented in this study and balanced between groups. Finally, this report was limited to 90-day outcomes and 1-year follow-up with a cost-effectiveness analysis is underway.
Conclusions
Among patients undergoing LT, use of iEPO was associated with similar risks for PGD-3 development and other outcomes compared to those that received iNO.
Supplementary Material
Keypoints.
Question:
In adult patients who receive inhaled pulmonary vasodilators during lung transplantation (LT), is there a difference in the rates of severe primary graft dysfunction (PGD-3) between those that receive inhaled epoprostenol (iEPO) and those that receive inhaled nitric oxide (iNO)?
Findings:
In this randomized clinical trial of 201 LT recipients, PGD-3 determined at 24-, 48- or 72-hours after LT occurred in 46 (44.7%) receiving iEPO and in 39 (39.8%) receiving iNO. This 4.9% risk difference (90% CI, −6.4, 16.0) was included within the margin to favor equivalence.
Meaning:
PGD-3 development after LT was similar between iEPO and iNO groups.
Acknowledgments
Study data were collected and managed using REDCap™ hosted at Duke University.
Role of Funder for Trial activities. Duke University Health System (“the Funder”) was responsible for funding of research related-activities described in the study. Otherwise, the Funder was not involved in the design and conduct of the study; the collection, management, analysis or interpretation of the data; the preparation, review or approval of the manuscript; or decision to submit for publication.
COI and Disclosures past 3-years.
KG: grant support from NIH T32 GM008600, Duke Health, and the International Anesthesia Research Society-Mentored Research Training Grant.
JC: None
MCW: None
JCH: None
JMR: None
BAB: None
JAK: Concluded research support from Mallinkrodt Pharmaceuticals (March - September 2019)
JHL: Steering committees for Instrumentation Labs, Merck, Octapharma
MGH: Consulting and/or research support for Lung Bioengineering, Paragonix, Intuitive Surgical, and Gift of Life. Concluded research support from Mallinkrodt Pharmaceuticals (March - September 2019).
Author access to data. KG and MCW had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. MCW conducted and was responsible for the data analysis.
Footnotes
Credits. The authors would like to thank the Duke Office of Clinical Research for developing the clinical documentation platform through our electronic health record, Epic/MaestroCare®, which facilitated clinical documentation ordering and masking of the allocated treatment; the Patient Revenue Management Organization and personnel who worked with the investigators to create a system whereby patients were not responsible for medication costs and that health system support could be operationalized to implement the trial; the Centers for Medicare and Medicaid Services who recognized the importance of our investigation and provided approval for eligible patient enrollment and provided guidance by example for select private insurance companies to follow. We would also like to thank the Clinical Research Unit of the Duke Department of Anesthesiology & Critical Care, the CTICU respiratory care providers from the Department of Respiratory Care Services, and the Investigational Drug Services Pharmacy and personnel who were responsible for implementation of research-related activities during hours of operation that were often late at night, on weekends and holidays. Please see supplemental online content for nonauthor collaborators.
References
- 1.Pinsky DJ, Naka Y, Chowdhury NC, et al. The nitric oxide/cyclic GMP pathway in organ transplantation: critical role in successful lung preservation. Proc Natl Acad Sci U S A. Dec 6 1994;91(25):12086–90. doi: 10.1073/pnas.91.25.12086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricciardolo FL. Multiple roles of nitric oxide in the airways. Thorax. Feb 2003;58(2):175–82. doi: 10.1136/thorax.58.2.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shargall Y, Guenther G, Ahya VN, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part VI: treatment. J Heart Lung Transplant. Oct 2005;24(10):1489–500. doi: 10.1016/j.healun.2005.03.011 [DOI] [PubMed] [Google Scholar]
- 4.Bacha EA, Sellak H, Murakami S, et al. Inhaled nitric oxide attenuates reperfusion injury in non-heartbeating-donor lung transplantation. Paris-Sud University Lung Transplantation Group. Transplantation. May 27 1997;63(10):1380–6. doi: 10.1097/00007890-199705270-00002 [DOI] [PubMed] [Google Scholar]
- 5.Gielis JF, Quirynen L, Briede JJ, Roelant E, Cos P, Van Schil PEY. Pathogenetic role of endothelial nitric oxide synthase uncoupling during lung ischaemia-reperfusion injury. Eur J Cardiothorac Surg. Aug 1 2017;52(2):256–263. doi: 10.1093/ejcts/ezx125 [DOI] [PubMed] [Google Scholar]
- 6.Minamoto K, Pinsky DJ, Fujita T, Naka Y. Timing of nitric oxide donor supplementation determines endothelin-1 regulation and quality of lung preservation for transplantation. Am J Respir Cell Mol Biol. Jan 2002;26(1):14–21. doi: 10.1165/ajrcmb.26.1.4649 [DOI] [PubMed] [Google Scholar]
- 7.Moreno I, Vicente R, Mir A, et al. Effects of inhaled nitric oxide on primary graft dysfunction in lung transplantation. Transplant Proc. Jul-Aug 2009;41(6):2210–2. doi: 10.1016/j.transproceed.2009.05.019 [DOI] [PubMed] [Google Scholar]
- 8.Snell GI, Yusen RD, Weill D, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. Oct 2017;36(10):1097–1103. doi: 10.1016/j.healun.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 9.Christie JD, Bellamy S, Ware LB, et al. Construct validity of the definition of primary graft dysfunction after lung transplantation. J Heart Lung Transplant. Nov 2010;29(11):1231–9. doi: 10.1016/j.healun.2010.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. Mar 1 2007;175(5):507–13. doi: 10.1164/rccm.200608-1079OC [DOI] [PubMed] [Google Scholar]
- 11.Tomasi R, Betz D, Schlager S, et al. Intraoperative Anesthetic Management of Lung Transplantation: Center-Specific Practices and Geographic and Centers Size Differences. J Cardiothorac Vasc Anesth. Feb 2018;32(1):62–69. doi: 10.1053/j.jvca.2017.05.025 [DOI] [PubMed] [Google Scholar]
- 12.Rao V, Ghadimi K, Keeyapaj W, Parsons CA, Cheung AT. Inhaled Nitric Oxide (iNO) and Inhaled Epoprostenol (iPGI2) Use in Cardiothoracic Surgical Patients: Is there Sufficient Evidence for Evidence-Based Recommendations? J Cardiothorac Vasc Anesth. Dec 13 2017;doi: 10.1053/j.jvca.2017.12.014 [DOI] [PubMed] [Google Scholar]
- 13.Khan TA, Schnickel G, Ross D, et al. A prospective, randomized, crossover pilot study of inhaled nitric oxide versus inhaled prostacyclin in heart transplant and lung transplant recipients. J Thorac Cardiovasc Surg. Dec 2009;138(6):1417–24. doi: 10.1016/j.jtcvs.2009.04.063 [DOI] [PubMed] [Google Scholar]
- 14.Fiser SM, Cope JT, Kron IL, et al. Aerosolized prostacyclin (epoprostenol) as an alternative to inhaled nitric oxide for patients with reperfusion injury after lung transplantation. J Thorac Cardiovasc Surg. May 2001;121(5):981–2. doi: 10.1067/mtc.2001.115668 [DOI] [PubMed] [Google Scholar]
- 15.Torbic H, Szumita PM, Anger KE, Nuccio P, Lagambina S, Weinhouse G. Clinical and Economic Impact of Formulary Conversion From Inhaled Flolan to Inhaled Veletri for Refractory Hypoxemia in Critically Ill Patients. Ann Pharmacother. Feb 2016;50(2):106–12. doi: 10.1177/1060028015621308 [DOI] [PubMed] [Google Scholar]
- 16.Dhanani J, Fraser JF, Chan HK, Rello J, Cohen J, Roberts JA. Fundamentals of aerosol therapy in critical care. Crit Care. Oct 7 2016;20(1):269. doi: 10.1186/s13054-016-1448-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Preston IR, Sagliani KD, Roberts KE, et al. Comparison of acute hemodynamic effects of inhaled nitric oxide and inhaled epoprostenol in patients with pulmonary hypertension. Pulm Circ. Jan 2013;3(1):68–73. doi: 10.4103/2045-8932.109916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Heerden PV, Barden A, Michalopoulos N, Bulsara MK, Roberts BL. Dose-response to inhaled aerosolized prostacyclin for hypoxemia due to ARDS. Chest. Mar 2000;117(3):819–27. doi: 10.1378/chest.117.3.819 [DOI] [PubMed] [Google Scholar]
- 19.Torbic H, Szumita PM, Anger KE, Nuccio P, LaGambina S, Weinhouse G. Inhaled epoprostenol vs inhaled nitric oxide for refractory hypoxemia in critically ill patients. J Crit Care. Oct 2013;28(5):844–8. doi: 10.1016/j.jcrc.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 20.Ammar MA, Bauer SR, Bass SN, Sasidhar M, Mullin R, Lam SW. Noninferiority of Inhaled Epoprostenol to Inhaled Nitric Oxide for the Treatment of ARDS. Ann Pharmacother. Oct 2015;49(10):1105–12. doi: 10.1177/1060028015595642 [DOI] [PubMed] [Google Scholar]
- 21.DeGrado JR, Szumita PM, Schuler BR, et al. Evaluation of the Efficacy and Safety of Inhaled Epoprostenol and Inhaled Nitric Oxide for Refractory Hypoxemia in Patients With Coronavirus Disease 2019. Crit Care Explor. Oct 2020;2(10):e0259. doi: 10.1097/CCE.0000000000000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonti R, Pike CW, Cobb N. Responsiveness of Inhaled Epoprostenol in Respiratory Failure due to COVID-19. J Intensive Care Med. Mar 2021;36(3):327–333. doi: 10.1177/0885066620976525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray AL, Mulvihill MS, Hartwig MG. Lung transplantation at Duke. J Thorac Dis. Mar 2016;8(3):E185–96. doi: 10.21037/jtd.2016.02.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johannes T, Ince C, Klingel K, Unertl KE, Mik EG. Iloprost preserves renal oxygenation and restores kidney function in endotoxemia-related acute renal failure in the rat. Crit Care Med. Apr 2009;37(4):1423–32. doi: 10.1097/CCM.0b013e31819b5f4e [DOI] [PubMed] [Google Scholar]
- 25.Baylis C. Nitric oxide deficiency in chronic kidney disease. Am J Physiol Renal Physiol. Jan 2008;294(1):F1–9. doi: 10.1152/ajprenal.00424.2007 [DOI] [PubMed] [Google Scholar]
- 26.Gamble C, Krishan A, Stocken D, et al. Guidelines for the Content of Statistical Analysis Plans in Clinical Trials. JAMA. Dec 19 2017;318(23):2337–2343. doi: 10.1001/jama.2017.18556 [DOI] [PubMed] [Google Scholar]
- 27.Cornfield DN, Milla CE, Haddad IY, Barbato JE, Park SJ. Safety of inhaled nitric oxide after lung transplantation. J Heart Lung Transplant. Aug 2003;22(8):903–7. doi: 10.1016/s1053-2498(02)00809-4 [DOI] [PubMed] [Google Scholar]
- 28.Botha P, Jeyakanthan M, Rao JN, et al. Inhaled nitric oxide for modulation of ischemia-reperfusion injury in lung transplantation. J Heart Lung Transplant. Nov 2007;26(11):1199–205. doi: 10.1016/j.healun.2007.08.008 [DOI] [PubMed] [Google Scholar]
- 29.Diamond JM, Lee JC, Kawut SM, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. Mar 1 2013;187(5):527–34. doi: 10.1164/rccm.201210-1865OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Liu Y, Su L, Jiang SJ. Recipient-related clinical risk factors for primary graft dysfunction after lung transplantation: a systematic review and meta-analysis. PLoS One. 2014;9(3):e92773. doi: 10.1371/journal.pone.0092773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christie JD, Kotloff RM, Ahya VN, et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med. Jun 1 2005;171(11):1312–6. doi: 10.1164/rccm.200409-1243OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yusen RD, Edwards LB, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Lung and Heart-Lung Transplant Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant. Oct 2016;35(10):1170–1184. doi: 10.1016/j.healun.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 33.McGinn K, Reichert M. A Comparison of Inhaled Nitric Oxide Versus Inhaled Epoprostenol for Acute Pulmonary Hypertension Following Cardiac Surgery. Ann Pharmacother. Jan 2016;50(1):22–6. doi: 10.1177/1060028015608865 [DOI] [PubMed] [Google Scholar]
- 34.Van Raemdonck D, Hartwig MG, Hertz MI, et al. Report of the ISHLT Working Group on primary lung graft dysfunction Part IV: Prevention and treatment: A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. Oct 2017;36(10):1121–1136. doi: 10.1016/j.healun.2017.07.013 [DOI] [PubMed] [Google Scholar]
- 35.U.S. Dept of Health and Human Services, Food & Drug Administration. FDA Noninferiority Clinical Trials to Establish Effectiveness: Guidance for Industry. https://www.fda.gov/media/78504/download
- 36.Equivalence Testing for SE Evaluations - US Food and Drug Administration. https://www.fda.gov/media/124669/download
- 37.Girardis M, Busani S, Damiani E, et al. Effect of Conservative vs Conventional Oxygen Therapy on Mortality Among Patients in an Intensive Care Unit: The Oxygen-ICU Randomized Clinical Trial. JAMA. Oct 18 2016;316(15):1583–1589. doi: 10.1001/jama.2016.11993 [DOI] [PubMed] [Google Scholar]
- 38.Mathew JP, Mackensen GB, Phillips-Bute B, et al. Randomized, double-blinded, placebo controlled study of neuroprotection with lidocaine in cardiac surgery. Stroke. Mar 2009;40(3):880–7. doi: 10.1161/STROKEAHA.108.531236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warnecke G, Van Raemdonck D, Smith MA, et al. Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): a randomised, open-label, non-inferiority, phase 3 study. Lancet Respir Med. May 2018;6(5):357–367. doi: 10.1016/S2213-2600(18)30136-X [DOI] [PubMed] [Google Scholar]
- 40.Shashaty MGS, Forker CM, Miano TA, et al. The association of post-lung transplant acute kidney injury with mortality is independent of primary graft dysfunction: A cohort study. Clin Transplant. Oct 2019;33(10):e13678. doi: 10.1111/ctr.13678 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.