Abstract
Lung transplant recipients (LTR) with coronavirus disease 2019 (COVID-19) may have higher mortality than non-lung solid organ transplant recipients (SOTR), but direct comparisons are limited. Risk factors for mortality specifically in LTR have not been explored. We performed a multicenter cohort study of adult SOTR with COVID-19 to compare mortality by 28 days between hospitalized LTR and non-lung SOTR. Multivariable logistic regression models were used to assess comorbidity-adjusted mortality among LTR vs. non-lung SOTR and to determine risk factors for death in LTR. Of 1,616 SOTR with COVID-19, 1,081 (66%) were hospitalized including 120/159 (75%) LTR and 961/1457 (66%) non-lung SOTR (p = .02). Mortality was higher among LTR compared to non-lung SOTR (24% vs. 16%, respectively, p = .032), and lung transplant was independently associated with death after adjusting for age and comorbidities (aOR 1.7, 95% CI 1.0–2.6, p = .04). Among LTR, chronic lung allograft dysfunction (aOR 3.3, 95% CI 1.0–11.3, p = .05) was the only independent risk factor for mortality and age >65 years, heart failure and obesity were not independently associated with death. Among SOTR hospitalized for COVID-19, LTR had higher mortality than non-lung SOTR. In LTR, chronic allograft dysfunction was independently associated with mortality.
Keywords: clinical research/practice, infection and infectious agents - viral, infectious disease, lung (allograft) function/dysfunction, lung disease: infectious, lung transplantation/pulmonology, organ transplantation in general
1 ∣. INTRODUCTION
Solid organ transplant recipients (SOTR) are at risk for poor outcomes from coronavirus disease 2019 (COVID-19) due to the frequent medical comorbidities and the potential impact of immunosuppression.1,2 Lung transplant recipients (LTR) with COVID-19 are unique among SOTR because severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly infects the allograft. Small observational cohort studies suggest that LTR with COVID-19 may have higher mortality than non-lung SOTR. Mortality as high as 46% has been reported in LTR, compared to ~20% for SOTR as a collective group.1,3-6 However, heterogeneity in follow-up duration both within and between these studies precludes meaningful assessment of differences between LTR and non-lung SOTR with COVID-19. Although advanced age, obesity, chronic lung disease, and heart failure appear to be the key drivers of COVID-19-related mortality among SOTR and the general population,1,7-9 risk factors for mortality specifically among LTR have not been assessed in large cohorts.
We previously reported a multicenter registry of SOTR with COVID-19, all of whom were followed for 28 days (or until death if <28 days) using standardized data collection tools.1 This registry remained open for an additional 7 months to accrue further cases. Here, we report the 28-day morbidity and mortality among SOTR who were hospitalized for COVID-19, directly compare outcomes between LTR and non-lung SOTR, and evaluate risk factors for death specifically in LTR.
2 ∣. MATERIALS AND METHODS
As previously described,1 the study was approved by the University of Washington Institutional Review Board with waiver of informed consent (STUDY00009698). The Institutional Review Board issued a “not human subjects research” designation for contributors reporting de-identified data accessed during routine clinical care. Study sites underwent review by local institutional review boards if needed to access data or to maintain a key to identify patient records at their discretion.
2.1 ∣. Study design
This multicenter cohort study included recipients of any solid organ transplant who were diagnosed with COVID-19 between February 28, 2020 and December 31, 2020 by SARS-CoV-2 antigen or polymerase chain reaction testing of an upper or lower respiratory sample. Providers were notified of the study through postings on message boards hosted by the American Society of Transplantation, the American Society of Transplant Surgeons, and the International Society for Heart and Lung Transplantation. Contributors at individual study sites entered de-identified patient data into REDCap (Research Electronic Data Capture), a secure, web-based data capture software program, which was hosted at the University of Washington.10,11 Clinical information regarding baseline demographics and details of disease presentation were collected in an initial survey, and a second follow-up survey collected clinical data at 28 days after COVID-19 diagnosis (Table S1). The registry remained open to collect follow-up surveys through January 31, 2021. Patients for whom both the initial and follow-up surveys were completed were included in the present study.
2.2 ∣. Statistical analyses
Primary analysis assessed patients who were hospitalized within the 28 days following the first positive SARS-CoV-2 test and who were not already hospitalized for another indication prior to or concurrent with diagnosis (consort diagram, Figure S1). Demographic and baseline characteristics were assessed as counts and percentages for categorical values and as a mean (standard deviation) for continuous variables. Chi-square and Student's t tests were used to compare baseline categorical and continuous variables, respectively. Chi-squared and Fisher's exact tests were used to compare the incidence of death (primary outcome) and seven secondary outcomes between LTR and non-lung SOTR by 28 days after COVID-19 diagnosis. Secondary outcomes included intensive care unit (ICU) admission, mechanical ventilation, acute kidney injury, bacterial pneumonia, fungal pneumonia, bloodstream infection, and acute cellular rejection.
Univariable logistic regression was used to assess risk factors for mortality among three hospitalized cohorts: (1) LTR only, (2) non-lung SOTR only, and (3) LTR and non-lung SOTR combined, and multivariable logistic regression was used to determine risk factors for mortality among the entire SOTR cohort. Variables that were selected a priori for consideration of inclusion in our multivariable model (based on our hypothesis and/or prior studies showing significance) included receipt of lung transplant (vs. non-lung transplant), age, and comorbidities (hypertension, diabetes mellitus, heart failure, obesity, chronic kidney disease, coronary artery disease, and chronic lung disease). We evaluated other covariates with at least a prevalence of 5% and a p < .2 in the univariable analysis of the entire SOTR cohort for inclusion; based on this, use of mammalian target of rapamycin (mTOR) inhibitor was included as well. Age was assessed as a dichotomous variable (age ≤65 years vs. >65 years) based on clinical relevance and in accordance with prior studies.12,13 Interactions between lung transplant history and other covariates were considered in the final model. A separate multivariable logistic regression model was used to identify risk factors for mortality specifically in the LTR cohort. For consistency, this model contained the same covariates as the multivariable model used to assess risk factors for mortality in the full SOTR cohort, with the exception that type of lung transplant (single lung vs. bilateral lung) was assessed in place of lung transplant vs. no lung transplant and chronic lung allograft dysfunction (CLAD) was assessed in place of chronic lung disease. Complete data were available for the majority of variables; multiple imputation by chained equations was used to handle the missing data for multivariable models.14 All results were considered statistically significant at a two-sided p value ≤ .05. All analyses were performed using Stata version 16.1 (Stata Corporation, College Station, TX) and R version 4.0.2.
3. ∣. RESULTS
3.1 ∣. Study population
There were 1,701 cases of COVID-19 in SOTR entered into the registry during the study period. Cases were excluded from analysis if there was no laboratory-confirmed COVID-19 diagnosis (n = 8), >10% of requested data were missing on initial or follow-up surveys (n = 5), data were duplicated in another entry (n = 9), the contributor reported that the case was diagnosed after December 31, 2020 (n = 1), or follow-up reports for a living patient were submitted earlier than 28 days after COVID-19 diagnosis (n = 62). Of the 1616 SOTR who met inclusion criteria, 1081 (66%) were hospitalized within 28 days of COVID-19 diagnosis, including 120/159 (75%) LTR and 961/1457 (66%) non-lung SOTR (p = .02, Table S2). These 1081 patients were included in the primary analysis (consort diagram, Figure S1).
3.2 ∣. Baseline characteristics of hospitalized LTR and non-lung SOTR
Baseline demographics, clinical characteristics, and therapeutic interventions in hospitalized lung and non-lung SOTR are summarized in Table 1. Thirty-four LTR (29%) received a single lung transplant and 16 (14%) LTR had CLAD at baseline. Most LTR were receiving a calcineurin inhibitor (114, 97%) and corticosteroids (117, 98%) at the time of presentation, and 84 (70%) were receiving a regimen that included a calcineurin inhibitor, corticosteroid, and anti-metabolite. Reduction in immunosuppression after COVID-19 diagnosis was less common in LTR vs. non-lung SOTR (64/120 [57%] vs. 686/961 [74%]), and LTR were more likely to receive treatment with corticosteroids, remdesivir, and/or convalescent plasma (Table 1).
TABLE 1.
Baseline characteristics of hospitalized SOTR with COVID-19
| Features of hospitalized SOTR | Lung (n = 120) | Non-lung (n = 961) | p value |
|---|---|---|---|
| Mean age in years (SD) | 60.6 (13.0) | 961 (57.0) | .005 |
| Age >65 years, n (%) | 48 (40.0) | 289 (30.0) | .03 |
| Mean day of hospitalization relative to first positive SARS-CoV–2 test (SD) | 2.3 (4.8) | 2.3 (4.8) | |
| Male, n (%) | 62 (51.7) | 610 (63.5) | .01 |
| Race, n (%) | .05 | ||
| Indigenous peoples | 0 (0.0) | 18 (1.9) | |
| Asian | 3 (2.5) | 44 (4.6) | |
| Black or African Descent | 29 (24.2) | 328 (34.1) | |
| White or Caucasian | 75 (62.5) | 498 (51.8) | |
| Pacific Islander | 0 (0.0) | 4 (0.4) | |
| Other or unknown | 13 (10.8) | 69 (7.2) | |
| Geographic location, n (%) | <.001 | ||
| Northeastern United States | 28 (23.3) | 298 (23.3) | |
| Midwestern United States | 12 (10.0) | 91 (9.5) | |
| Southern United States | 33 (27.5) | 319 (33.2) | |
| Western United States | 42 (35.0) | 181 (18.8) | |
| International | 5 (4.2) | 28 (2.9) | |
| Organ n (%)a | |||
| Heart | — | 131 (13.6) | |
| Liver | — | 154 (16.0) | |
| Kidney | — | 72 (70.0) | |
| Other | — | 3 (0.3) | |
| Single lung transplant | 34 (28.6) | — | |
| Comorbidities, n (%) | |||
| Hypertension | 60 (50.0) | 776 (80.8) | <.001 |
| Diabetes mellitus | 56 (46.7) | 489 (50.9) | .38 |
| Heart failure | 8 (6.7) | 58 (6.0) | .79 |
| Obesity (BMI ≥30 kg/m2)b | 29 (24.8) | 347 (37.2) | .01 |
| Chronic kidney disease | 62 (51.7) | 336 (35.0) | <.001 |
| Coronary artery disease | 23 (19.2) | 171 (17.8) | .71 |
| Chronic lung disease | — | 55 (5) | |
| Chronic lung allograft dysfunction | 16 (13.3) | — | |
| Immunosuppression, n (%)c,d,e,f | |||
| Recent induction therapy, n (%) | 1 (0.8) | 62 (6.5) | .01 |
| CNI, anti-metabolite, steroids | 84 (70.0) | 485 (50.5) | <.001 |
| Any CNI containing regimen | 117 (97.5) | 878 (91.4) | .02 |
| Any anti-metabolite containing regimen | 86 (71.7) | 727 (75.7) | .34 |
| Any steroid containing regimen | 117 (97.5) | 674 (70.2) | <.001 |
| Any mTOR inhibitor containing regimen | 9 (7.5) | 52 (5.4) | <.001 |
| Covid–19 treatments and interventions | |||
| Reduction in immunosuppressiong | 64 (56.6) | 686 (74.1) | <.001 |
| Corticosteroids (≥6-mg dexamethasone equivalents/day) | 67 (55.8) | 304 (31.6) | <.001 |
| Remdesivir | 65 (54.2) | 253 (26.3) | <.001 |
| Convalescent plasma | 34 (28.3) | 158 (16.4) | .001 |
Abbreviations: BMI, body mass index; CNI, calcineurin inhibitor; mTOR, mammalian target of rapamycin; SOTR, solid organ transplant recipients; SD, standard deviation.
Lung includes 1 lung/liver, 2 heart/lung recipients and 1 lung/kidney/islet cell. Heart includes 14 heart/kidney and 1 heart/kidney/small bowel recipients. Liver includes 34 liver/kidney and 2 liver/pancreas/small bowel. Kidney includes 18 kidney/pancreas recipients. Other includes 1 vascular composite allograft and 2 small bowel recipients.
Type of lung transplant (single vs. double) was available for 119 lung recipients.
BMI was available for 117 lung and 932 non-lung recipients.
Induction of immunosuppression includes receipt of any of the following agents in the three months prior to Covid-19 diagnosis: antithymocyte globulin (35 non-lung, 0 lung), alemtuzumab ( 2 non-lung, 0 lung), basiliximab (15 non-lung, 1 lung), ≥ 500mg methylprednisolone/day × 3 or more days (21 non-lung, 0 lung), rituximab (4 lung, 0 non-lung), plasmapheresis (1 lung, 0 non-lung), or bortezomib (1 lung, 0-non-lung). Some patients received more than one induction agent.
CNI in lung recipients: tacrolimus (n = 113), cyclosporine (n = 4). CNI in non-lung recipients: tacrolimus (n = 821), cyclosporine (n = 58).
Antimetabolite in lung recipients: mycophenolate (n = 73), azathioprine (n = 13). Antimetabolite in non-lung recipients: mycophenolate (n = 690), azathioprine (n = 34), leflunomide (n = 4).
Maintenance corticosteroid dose equivalents in lung recipients: ≤20 mg prednisone/day (n = 111), >20 mg prednisone/day (n = 6). Maintenance steroid dose equivalents in non-lung recipients: ≤20 mg prednisone/day (n = 664), >20 mg prednisone/day (n = 30).
Reduction in immunosuppression in lung recipients: hold anti-metabolite (n = 54), decrease anti-metabolite (n = 4), change calcineurin inhibitor goal (n = 13), hold and decrease mTOR inhibitor (n = 2) hold all immunosuppression (n = 2). Reduction in immunosuppression in nonlung recipients: hold anti-metabolite (n = 521), decrease anti-metabolite (n = 86), change calcineurin inhibitor goal (n = 222), hold or decrease mTOR inhibitor (n = 11), delay or withhold belatacept (n = 4), decrease prednisone n = 1), hold all immunosuppression (n = 18). Some patients experienced more than one change.
3.3 ∣. Crude incidence of outcomes by 28 days in hospitalized lung and non-lung SOTR
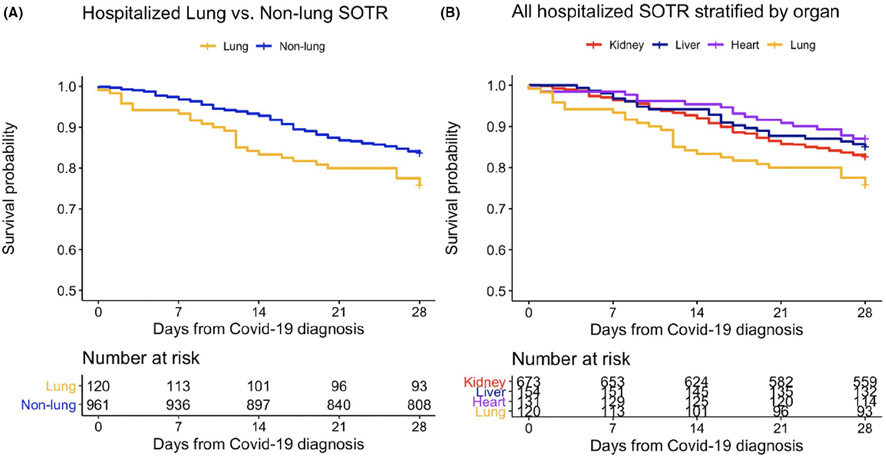
We compared the crude incidence of death (primary outcome) and seven secondary outcomes, assessed by 28 days, in hospitalized lung and non-lung SOTR (Table 2). LTR and non-lung SOTR had similar rates of admission to the ICU (52/1120 [44%] vs. 346/925 [37%], p = .18), need for mechanical ventilation (33/120 [28%] vs. 250/961 [27%], p = .74) and bacterial pneumonia (12/120 [10%] vs. 72/961 [8%], p = .33). Fungal pneumonia occurred more commonly among LTR vs. non-lung SOTR (3/120 [3%] vs. 1/961[0.4%], p = .01). Crude 28-day mortality was significantly higher among LTR compared to non-lung SOTR (29/120 [24%] vs. 157/961 [16%], p = .032). Unadjusted survival curves demonstrate time to death during the first 28 days after COVID-19 diagnosis in hospitalized lung and non-lung SOTR (Figure 1A) and in all hospitalized SOTR, stratified by organ (Figure 1B).
TABLE 2.
Outcomes among hospitalized solid organ transplant recipients with COVID-19 (lung vs. non-lung)
| Lung (n = 120) |
Non-lung (n = 961) | p value | |
|---|---|---|---|
| Death by 28 days | 29 (24.2) | .032* | |
| ICU admission | 52 (43.7) | ||
| Mechanical ventilation | 33 (27.5) | ||
| Extracorporeal membrane oxygenation | |||
| Acute kidney injuryb | 40 (33.3) | 394 (41.0) | |
| Bacterial pneumoniac | 12 (10.0) | 72 (7.5) | |
| Fungal pneumoniad | 3 (2.5) | 4 (0.4) | .01* |
| Bloodstream infectione | 2 (1.7) | 52 (5.4) | .08 |
| Acute cellular rejection | 2 (1.7) | 9 (1.0) | .45 |
ICU admission status was available for 119 lung and 925 non-lung recipients.
Acute kidney injury defined as >50% increase in creatinine from baseline.
Includes cases in which a pathogen was isolated on respiratory culture; excludes empirically treated cases. Respiratory bacterial pathogens in lung recipients included Pseudomonas spp. (n = 10), Klebsiella spp. (n = 1), Escherichia coli (n = 1), Stenotrophomonas maltophilia (n = 1). Pathogens in non-lung recipients included Pseudomonas (n = 24), Klebsiella spp. (n = 11), E. coli (n = 7), Stenotrophomonas maltophila (n = 4), Burkholderia spp. (n = 5), Citrobacter spp. (n = 3), Enterobacter spp. (n = 3), Acinetobacter spp. (n = 2), Proteus spp. (n = 1), Achromobacter spp. (n = 1), Hafnia spp. (n = 1), Capnocytophyga spp.(n = 1), Staphylococcus aureus (n = 25), and Streptococcus spp. (n = 5). Some patients had more than one pathogen isolated.
Includes cases in which a pathogen was identified; excludes diagnoses based on galactomannan or imaging findings alone. Aspergillus was isolated in 2 lung recipients and 3 non-lung recipients. One non-lung recipient had Rhizopus and one non-lung recipient had Cryptococcus.
Bloodstream pathogens in lung recipients included Stenotrophomonas maltophila (n = 1) and Enterococcus spp. (n = 1). Bloodstream pathogens in non-lung recipients included Pseudomonas spp. (n = 8), E. coli (n = 10), Enterobacter spp. (n = 2), Stenotrophomonas maltophila (n = 1), Proteus spp. (n = 1), Staphylococcus aureus (n = 22), Streptococcus spp. (n = 1), Enterococcus spp. (n = 8), and Candida spp. (n = 6). Excludes possible contaminants: coagulase-negative staphylococcus (0 lung, 18 non-lung recipients) Bacillus spp (1 non-lung recipient), and Corynebacterium spp (1 non-lung recipient).
Statistically significant at p ≤ .05.
FIGURE 1.
Unadjusted Kaplan–Meier curves for all-cause mortality within 28-days after COVID-19 diagnosis for hospitalized solid organ transplant recipients. Abbreviation: solid organ transplant recipients (SOTR). Note: Lung includes 1 lung/liver and 2 heart/lung recipients. Heart includes 14 heart/kidney and 1 heart/kidney/small bowel recipients. Liver includes 33 liver/kidney, 2 liver/pancreas/small bowel. Kidney includes 17 kidney/pancreas recipients. Recipients of other organs (1 vascular composite allograft and 2 small bowel recipients) not included
3.4 ∣. Risk factors for 28-day mortality: Lung vs. non-lung SOTR
In LTR, there was no association between death by 28 days and hypertension, diabetes, coronary artery disease, heart failure, or chronic kidney disease in either univariable (Table 3) or multivariable (Table 4) analysis. After adjusting for comorbidities, CLAD (aor 3.3, 95% CI 1.0–11.3, p = .05) was independently associated with increased mortality. Adjusted mortality trended higher in LTR over age 65 (aOR 1.5, 95% CI 0.6–4.2, p = .4) and those with obesity (aOR 2.7, 95% CI 0.9–7.6 p = .07). Surrogates of immunosuppression intensity (receipt of induction therapy in the 3 months prior to diagnosis or receipt of triple therapy with a calcineurin inhibitor, anti-metabolite, and steroids) were not associated with mortality.
TABLE 3.
Risk factors for 28-day mortality in hospitalized lung and non-lung solid-organ transplant recipients with COVID-19, univariable logistic regression models
| Lung (n = 120) |
Non-lung (n = 961) |
All SOTR (n = 1081) |
||||
|---|---|---|---|---|---|---|
| Covariate | Unadjusted OR (95% CI) |
p-value | Unadjusted OR ratio (95% CI) |
p-value | Unadjusted OR ratio (95% CI) |
p-value |
| Age >65 years | 1.3 (0.6–3.0) | .6 | 2.3 (1.6–3.3) | <.001 | 2.1 (1.6–3.0) | <.001 |
| Male sex | 0.6 (0.3–1.3) | .2 | 1.2 (0.8–1.7) | .44 | ||
| Black racea | 0.5 (0.2–1.6) | .3 | 1.1 (0.7–1.5) | .74 | 1.0 (0.7–1.4) | .84 |
| Latinx or Hispanic ethnicity | 1.0 (0.3–2.7) | >.9 | 0.9 (0.6–1.4) | .7 | 0.9 (0.6–1.3) | .6 |
| Region (vs. Midwest) | ||||||
| Northeast | 1.7 (0.3–9.5) | .6 | 0.9 (0.5–1.6) | .7 | 0.9 (0.5–1.6) | .79 |
| South | 1.6 (0.3–8.9) | .5 | 0.8 (0.5–1.4) | .4 | 0.8 (0.5–1.5) | .54 |
| West | 1.8 (0.3–9.4) | .5 | 0.8 (0.4–1.6) | .6 | 1.0 (0.5–1.8) | .91 |
| Organa | ||||||
| Lung | 2.6 (1.0–2.6) | .04 | ||||
| Single lung transplant | 2.2 (0.9–5.3) | .08 | ||||
| Heart | – | 0.7 (0.5–1.3) | .3 | 0.7 (0.4–1.2) | .15 | |
| Liver | – | 0.9 (0.5–1.4) | .6 | 0.8 (0.5–1.3) | .40 | |
| Kidney | – | 1.1 (0.8–1.7) | .5 | 0.9 (0.7–1.3) | ||
| Comorbidities | ||||||
| Hypertension | 0.6 (0.3–1.5) | .3 | 2.2 (1.3–3.7) | .004 | 1.4 (0.9–2.1) | .12 |
| Diabetes mellitus | 0.8 (0.3–1.8) | .5 | 1.9 (1.3–2.7) | .001 | 1.6 (1.2–2.2) | .003 |
| Heart failure | 1.05 (0.2–5.5) | >.9 | 3.8 (2.2–6.6) | <.001 | 3.2 (1.9–5.5) | <.001 |
| Obesity (BMI ≥30 kg/m2) | 2.0 (0.8–5.2) | .2 | 1.6 (1.1–2.2) | .02 | 1.5 (1.1–2.1) | .008 |
| Chronic kidney disease | 1.2 (0.5–2.8) | .7 | 1.3 (0.9–1.8) | .2 | 1.29 (0.9–1.8) | .11 |
| Coronary artery disease | 0.6 (0.2–2.0) | .4 | 1.6 (1.1–2.4) | .03 | 1.4 (1.0–2.1) | .07 |
| Chronic lung disease | 2.9 (1.0–8.7) | .06 | 4.1 (2.4–7.3 | <.001 | 4.0 (2.4–6.6) | <.001 |
| CLAD | 2.9 (1.0–8.7) | .06 | ||||
| Immunosuppression | ||||||
| Recent induction therapy | – | >.99 | 0.5 (0.2–1.3) | .2 | 0.5 (0.2–1.2) | .1 |
| CNI, anti-metabolite, and steroids use | 1.9 (0.7–5.1) | .2 | 0.9 (0.6–1.2) | .5 | 1.0 (0.7–1.4) | >.9 |
| Any CNI containing regimen | – | >.99 | 1.2 (0.7–2.2) | .5 | 1.3 (0.7–2.5) | .4 |
| Any anti-metabolite containing regimen | 1.7 (0.6–4.6) | .3 | 0.9 (0.6–1.3) | .5 | 0.9 (0.7–1.3) | .8 |
| Any steroid containing regimen | 0.6 (0.1–7.2) | .7 | 1.1 (0.7–1.6) | .7 | 1.1 (0.8–1.6) | .5 |
| Any mTOR containing regimen | 0.4 (0.0–3.1) | .4 | 0.2 (0.1–0.8) | .03 | 0.3 (0.1–0.8) | .02 |
Abbreviations: BMI, body mass index; CI, confidence interval; CLAD, chronic lung allograft dysfunction; CNI, calcineurin inhibitor; mTOR, mammalian target of rapamycin; OR, odds ratio.
Refers to patients who received a transplant of the specified organ, with or without transplant other organs: lung (n = 120), heart (n = 133), liver (n = 155), kidney (n = 723). Reference is no transplant of the listed organ.
TABLE 4.
Risk factors for 28-day mortality: multivariable logistic regression
| Lung (n = 120) |
All SOTR (n = 1081) |
|||
|---|---|---|---|---|
| Covariate | aOR ratio (95% CI) | p value | aOR ratio (95% CI) | p value |
| Age >65 years | 1.5 (0.6–4.2) | .4 | 2.0 (1.4–2.8) | <.001* |
| Lung transplant | — | — | 1.7 (1.0–2.8) | .04 |
| Single lung transplant | 2.6 (1.0–7.2) | .06 | — | — |
| Comorbidities | ||||
| Hypertension | 0.6 (0.3–1.7) | .3 | 1.3 (0.8–2.0) | .3 |
| Diabetes mellitus | 0.6 (0.2–1.7) | .3 | 1.3 (0.9–1.7) | .3 |
| Heart failure | 0.9 (0.2–7.0) | >.9 | 2.3 (1.2–3.9) | .007 |
| Obesity (BMI ≥30 kg/m2) | 2.7 (0.9–7.6) | .07 | 1.7 (1.2–2.4) | .005 |
| Chronic kidney disease | 1.0 (0.4–2.6) | >.9 | 1.1 (0.8–1.5) | .6 |
| Coronary artery disease | 0.5 (0.2–1.9) | .3 | 1.1 (0.7–1.7) | .7 |
| Chronic lung disease | — | — | 2.7 (1.5–4.6) | <.001 |
| CLAD | 3.3 (1.0–11.3) | .05* | — | — |
| Immunosuppression | ||||
| mTOR containing regimen | 0.3 (0.03–3.5) | .4 | 0.3 (1.0–0.8) | .05 |
Abbreviations: aOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; CLAD, chronic lung allograft dysfunction; mTOR, mammalian target of rapamycin; SOTR, solid organ transplant recipients.
Denotes statistically significant at α ≤ 0.05.
Among non-lung SOTR, the strongest predictors of death in univariable analysis included age >65 years (OR 2.3, 95% CI 1.6–3.8 p < .001) and comorbidities: heart failure (OR 3.8, 95% CI 2.2–6.6, p < .001), obesity (OR 1.6, 95% CI 1.1–2.2, p = .02), and chronic lung disease (OR 4.1, 95% CI 2.4–7.3, p < .001). Hypertension, diabetes mellitus, coronary artery disease, and chronic kidney disease were also associated with increased mortality; mTOR inhibitor use was associated with lower 28-day mortality (Table 3). There was no association between death and the receipt of any specific organ type (heart, liver, or kidney), induction immunosuppressive therapy in the three months preceding COVID-19 diagnosis, or three-drug maintenance immunosuppression with a CNI, corticosteroid, and anti-metabolite.
3.5 ∣. Lung transplantation as an independent risk factor for mortality in hospitalized patients: multivariable analysis of all hospitalized SOTR
Lung, but not other organ transplant type, was associated with increased 28-day mortality in univariable analysis of all hospitalized SOTR (Table 3). Lung transplantation remained independently associated with death after adjusting for age and comorbidities (aOR 1.7, 95% CI 1.0–2.8, p = .04, Table 4), but several other factors were more strongly associated with mortality: age >65 years (aOR 2.1, 95% CI 1.5–3.0), heart failure (aOR 2.3, 95% CI 1.3–3.9, p = .007), obesity (aOR 1.7, 95% CI 1.2–2.4, p = .005), and chronic lung disease (aOR 2.7, 95% CI 1.5–4.6, p < .001). Diabetes mellitus, coronary artery disease, and chronic kidney disease were not associated with mortality in the multivariable model (Table 4). Baseline mTOR inhibitor use at the time of COVID-19 diagnosis was associated with decreased mortality (aOR 0.3, 95% CI 0.1–0.8, p = .03) but the number of SOTR receiving these agents was small (61 SOTR: nine lung and 52 non-lung).
4 ∣. DISCUSSION
In this large multicenter cohort study of SOTR hospitalized for COVID-19, receipt of a lung (compared to non-lung organ) transplant was independently associated with increased mortality after controlling for comorbidities. Among LTR, single lung transplant and CLAD were independently associated with death. Age >65 years, obesity, and extra-pulmonary comorbidities which were important risk factors for mortality in non-lung SOTR were not independently associated with mortality among LTR.
In the present study, 24% of hospitalized LTR died within 28 days of COVID-19 diagnosis. Smaller observational studies in hospitalized LTR conducted early in the COVID-19 pandemic reported mortality rates ranging from 13% to 46%, though these studies were heterogeneous, and duration of follow-up varied within and between studies.3,6,15-17 Most investigations of LTR with COVID-19 were conducted either outside the United States or were limited to a single center. This study focused on LTR from a broad range of locations primarily in the United States and provides a more precise estimate of mortality in a larger and more geographically diverse population than previously reported cohorts. Combining data from the present study with those from previous studies with ≥10 LTR, we calculated a pooled weighted average mortality of 31% among LTR hospitalized with COVID-19 (Table S3). Thus, we have identified hospitalized lung transplant recipients as a specific subpopulation of SOTR in whom new therapies or novel interventions are urgently needed to improve outcomes.
We observed a 50% increase in the odds of death in LTR compared to non-lung SOTR hospitalized for COVID-19. Lung transplant was more strongly associated with death than several comorbidities, including coronary artery disease, chronic kidney disease, and diabetes mellitus, conditions which have been associated with both COVID-19 and all-cause mortality in the general population.18 However, the magnitude of risk conferred by lung transplant was lower than that of other high-risk conditions, including age >65 years, obesity, chronic lung disease, and heart failure, all of which independently conferred at least a two-fold increase in the odds of mortality. The strong association between these high-risk conditions and mortality is consistent with multiple investigations in both SOTR and the general population.13,19 Notably, hospitalization occurred in a higher proportion of lung recipients (75%) than non-lung SOTR (66%). While this could indicate predisposition to severe COVID-19 after SARS-CoV-2 infection in LTR, this study was not designed to determine differences in the incidence of hospitalization between organ groups.
There are multiple potential mechanisms that may contribute to increased mortality in hospitalized LTR with COVID-19. Compared to non-lung SOTR, LTR typically receive more intensive maintenance immunosuppression,20 leading to a theoretic increased risk for severe complications of COVID-19 and other infections. For example, 70% of LTR in this study were receiving a three-drug regimen containing a calcineurin inhibitor, anti-metabolite, and corticosteroids, compared to 50% of non-lung SOTR. However, neither this combination regimen nor any of its components were associated with death in either lung or non-lung SOTR. Likewise, induction immunosuppressive therapy in the 3 months preceding COVID-19 diagnosis was not associated with mortality, although the numbers of those who received induction immunosuppression in the preceding three months were small. While there is no standardized measure of immunosuppression intensity, these data imply that features of baseline immunosuppression are not prominent predictors of mortality in SOTR with COVID-19. It is worth nothing that maintenance immunosuppression with mTOR inhibitors was associated with reduced mortality, particularly in non-lung SOTR. In vitro investigations have shown that mTOR inhibitors may enhance autophagy of cells infected with SARS-CoV-2 and inhibit translation of SARS-CoV-2 structural proteins.22 Thus, it is biologically plausible that mTOR inhibitors may offer protection against severe COVID-19, but the efficacy of mTOR inhibitors has not been assessed in prospective randomized trials and the clinical benefits of mTOR inhibitors requires further exploration.
Anti-metabolites and/or calcineurin inhibitors were held or reduced after COVID-19 diagnosis in 57% of LTR and 74% of non-lung SOTR. However, because underlying disease severity likely influenced management decisions, we were unable to accurately evaluate the relationship between immunosuppression reduction and mortality due to substantial confounding by indication. LTR were more likely to receive treatment with corticosteroids, remdesivir, or convalescent plasma and more frequent use of these agents may reflect more severe disease in LTR, but assessment of the relationships between various treatments and mortality is again limited by confounding.
Direct infection of the transplanted organ in LTR may also account for differences in mortality between LTR and non-lung SOTR. Chronic lung disease and other medical conditions that decrease pulmonary reserve such as heart failure and obesity were the strongest predictors of death in all SOTR with COVID-19, indicating the importance of underlying pulmonary status on COVID-19 prognosis. Like heart failure and obesity, lung transplantation, even if uncomplicated, may have an unfavorable effect on lung disease in COVID-19. Disruption of thoracic lymphatic drainage during lung transplant surgery predisposes LTR to pulmonary edema, and denervation of the transplanted lung during procurement results in impairment of the cough reflex and mucociliary clearance.21 Thus, altered physiology following lung transplantation, like other underlying conditions that impact native lung function, may contribute to the excess mortality in LTR.
The risk factors for mortality among hospitalized LTR with COVID-19 differed from those of non-lung SOTR. Among LTR, lung-associated parameters, including single lung transplant and the presence of CLAD had the greatest association with mortality. Obesity, heart failure and age >65 years, which were strong predictors of death in the non-lung cohort, were not significantly associated with mortality in LTR. However, there was still a trend toward increased mortality among LTR with obesity and advanced age, and the study may not have been powered to detect the effect of these conditions. At the same time, these observations suggest that conditions traditionally considered to be high risk may be less important when lung function is already compromised, as in LTR. The effect of age and other comorbidities on outcomes of COVID-19 in LTR requires additional investigation.
Strengths of our study include the relatively large sample size, geographic and institutional diversity, rigorous and comprehensive statistical analyses, and standardized data collection with 28-day follow-up. We acknowledge potential limitations. Mild or asymptomatic SARS-CoV-2 infections may not have come to the attention of transplant providers, and therefore, the registry may be biased toward more severe cases. Thus, the incidence of hospitalization in this cohort (75% of lung recipients and 66% of non-lung recipients) may overestimate the true incidence of hospitalization. To account for this potential bias, we limited our analysis to only patients who were hospitalized after the diagnosis of COVID-19 to include a more homogenous study population and focus on the most vulnerable patients. In order to perform a study of such magnitude in a timely fashion during a global pandemic, an accessible and concise data collection tool was necessary. This pragmatic study design precluded collection of potentially relevant but identifiable health information, such as date of organ transplant, and the need for efficiency prevented acquisition of detailed clinical information, including measurements required to calculate standardized metrics of disease severity (i.e., the sequential organ failure assessment [SOFA]).
In summary, LTR hospitalized with COVID-19 had higher 28-day mortality compared to non-lung SOTR. While lung transplantation remained an independent risk factor for death after adjusting for age and comorbidities, several traditional comorbidities were stronger predictors of mortality in the entire SOTR cohort. Mortality in LTR with COVID-19 was closely linked to features of the allograft—single lung transplant and CLAD—rather than age or extra-pulmonary comorbidities. These findings should be corroborated in other cohorts, and future investigations should assess the long term outcomes of COVID-19 in LTR who survive the acute illness.
Supplementary Material
ACKNOWLEDGMENTS
The following are the members of the UW COVID-19 SOT Study Team, without whom this work would not have been possible: Cameron Lawrence BS, William Bennett MD, Jennifer Leandro, Afrah Sait MD, Amy Rumore PharmD, Patricia West PhD, Amy Jeng MD, Valida Bajrovic MD, Erin P Bilgili BS, Tracy Anderson-Haag, PharmD, BCPS, Abigail Nastase, Abbas Badami MD, Jesus Alvarez-Garcia MD, Lyndsey Bowman-Anger PharmD, Lovelyn Julien MPH, Carlos Ortiz-Bautista MD, Rachel Friedman-Morocco MD, Kiran Gajurel MD, Lizbeth Cahuayme-Zuniga MD, Mark Wakefield MD, Monica Fung MD, Nicole Theodoropoulos MD MS, Sally T Chuang MD, Srividya Bhandaram MD, Massimiliano Veroux MD PhD, Bhavna Chopra MD, Diana Florescu MD, Danielle Witteck, Daniela Diaz, Kathryn Ripley NP-C, Kapil Saharia MD MPH, Sanjeev Akkina MD, Todd P. McCarty MD, Ally Webb PharmD, Akanksha Arya MD, Giridhar Vedula MD, Jose-Marie El-Amm MD, M. Katherine Dokus, Arun Narayanan MD, Priscila Cilene Leon Bueno de Camargo MD, Rosemary Ouseph MD, Andrew Breuckner PharmD, Alfred Luk MD, Avinash Aujayeb MBBS MRCP, Daniel Ganger MD, Douglas S. Keith MD, Federica Meloni MD, Ghady Haidar MD, Lori Zapernick, Megan Moraels MD, Nitender Goyal MD, Tanvi Sharma MD MPH, Uma Malhotra MD, Alexander Kuo MD, Ana P Rossi MD MPH, Angelina Edwards MD, Brian Keller MD PhD, Christy Beneri DO, Darby Derringer PharmD, Edward Dominguez MD, Elise Carlson PharmD, Faris Hashim MD, Haris Murad MD, Heinrike Wilkens MD, Henry Neumann MD, Imran Gani MD, Joseph Kahwaji MD, Joyce Popoola FRCP, Marian Michaels MD MPH, Niyati Jakharia MD, Oveimar De la Cruz MD, Alfredo Puing MD, Reza Motallebzadeh, Ravi Velagapudi MD, Rajan Kapoor MD, Sridhar Allam MD, Fernanda Silveira MD MD, Surabhi Vora MD MPH, Ursala M Kelly MD, Uttam Reddy MD, Vikas Dharnidharka MD MPH, Hani Wadei MD, and Lominadze Zurabi MD. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (T32AI118690 to M.R.H. and O.S.K.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding information
National Institute of Allergy and Infectious Diseases, Grant/Award Number: T32AI118690
Abbreviations:
- BMI
body mass index
- COVID-19
coronavirus disease 2019
- CLAD
chronic lung allograft dysfunction
- ICU
intensive care unit
- LTR
lung transplant recipients
- mTOR
mammalian target of rapamycin
- SOTR
solid organ transplant recipients
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
Footnotes
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. M.R.H. reports receiving speaking honoraria from Cigna LifeSource and Thermo Fisher Scientific. M.G.I. received research support, paid to Northwestern University, from AiCuris, Janssen, and Shire; he is a paid consultant for Adagio, AlloVir, Celltrion, Cidara, Genentech, Roche, Janssen, Shionogi, Viracor Eurofins; he is also a paid member of DSMBs from Janssen, Merck, SAB Biotherapeutics, Sequiris, Takeda, and Vitaeris. J.D.G. has research support from Gilead, Lilly and Regeneron and served as an advisory board member for Gilead. The other authors have no conflicts of interest to disclose.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Kates OS, Haydel BM, Florman SS, et al. COVID-19 in solid organ transplant: a multi-center cohort study. Clin Infect Dis. 2020. 10.1093/cid/ciaa1097 [DOI] [Google Scholar]
- 2.Fisher AM, Schlauch D, Mulloy M, et al. Outcomes of COVID-19 in hospitalized solid organ transplant recipients compared to a matched cohort of non-transplant patients at a national healthcare system in the United States. Clin Transplant. 2021;35(4):e14216. 10.1111/ctr.14216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coll E, Fernández-Ruiz M, Sánchez-Álvarez JE, et al. COVID-19 in transplant recipients: the Spanish experience. Am J Transplant. 2020;21(5):1825–1837. 10.1111/ajt.16369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rinaldi M, Bartoletti M, Bussini L, et al. COVID-19 in solid organ transplant recipients: no difference in survival compared to general population. Transpl Infect Dis. 2020;23(1):e13421. 10.1111/tid.13421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caillard S, Anglicheau D, Matignon M, et al. An initial report from the French SOT COVID Registry suggests high mortality due to Covid-19 in recipients of kidney transplants. Kidney Int. 2020;98(6):1549–1558. 10.1016/j.kint.2020.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aversa M, Benvenuto L, Anderson M, et al. COVID-19 in lung transplant recipients: a single center case series from New York City. Am J Transplant. 2020;20(11):3072–3080. 10.1111/ajt.16241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webb GJ, Marjot T, Cook JA, et al. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5(11):1008–1016. 10.1016/S2468-1253(20)30271-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808. 10.1111/ajt.15941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nair V, Jandovitz N, Hirsch JS, et al. An early experience on the effect of solid organ transplant status on hospitalized COVID-19 patients. Am J Transplant. 2020. 10.1111/ajt.16460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208- 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen NT, Chinn J, Nahmias J, et al. Outcomes and mortality among adults hospitalized With COVID-19 at US medical centers. JAMA Netw Open. 2021;4(3):e210417. 10.1001/jamanetworkopen.2021.0417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Royston P Multiple imputation of missing values. Stata J. 2004;4(3):227–241. [Google Scholar]
- 15.Messika J, Eloy P, Roux A, et al. COVID-19 in lung transplant recipients. Transplantation. 2021;105(1):177–186. 10.1097/TP.0000000000003508 [DOI] [PubMed] [Google Scholar]
- 16.Verleden GM, Godinas L, Lorent N, et al. COVID-19 in lung transplant patients: a case series. Am J Transplant. 2020;20(11):3234–3238. 10.1111/ajt.16212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miarons M, Larrosa-Garcnía M, García-García S, et al. COVID-19 in solid organ transplantation: a matched retrospective cohort study and evaluation of immunosuppression management. Transplantation. 2021;105(1):138–150. 10.1097/TP.0000000000003460 [DOI] [PubMed] [Google Scholar]
- 18.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hachem RR. The role of the immune system in lung transplantation: towards improved long-term results. J Thorac Dis. 2019;11(Suppl 14):S1721–S1731. 10.21037/jtd.2019.04.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feltracco P, Falasco G, Barbieri S, Milevoj M, Serra E, Ori C. Anesthetic considerations for nontransplant procedures in lung transplant patients. J Clin Anesth. 2011;23(6):508–516. 10.1016/j.jclinane.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 22.Karam BS, Morris RS, Bramante CT, et al. mTOR inhibition in COVID-19: A commentary and review of efficacy in RNA viruses. J Med Virol. 2021;93(4):1843–1846. 10.1002/jmv.26728 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.