Abstract
Purpose of review
In contrast to other saturated fatty acids, very long-chain saturated fatty acids (VLSFAs) have received limited attention The purpose of this review is to summarize the associations of VLSFAs, including arachidic acid, behenic acid and lignoceric acid, with cardiovascular disease outcomes and type 2 diabetes; to discuss the findings implications; and to call for future studies of the VLSFAs.
Recent findings
Increased levels of circulating levels of VLSFAs have been found associated with lower risks of incident heart failure, atrial fibrillation, coronary heart disease, mortality, sudden cardiac arrest, type 2 diabetes and with better aging. The VLSFA associations are paralleled by associations of plasma ceramide and sphingomyelin species carrying a VLSFA with lower risks of heart failure, atrial fibrillation and mortality, suggesting VLSFAs affect the biological activity of ceramides and sphingomyelins thereby impacting health. For diabetes, there is no such parallel and the associations of VLSFAs with diabetes may be confounded or mediated by triglyceride and circulating palmitic acid, possible biomarkers of de novo lipogenesis.
Summary
In many ways, the epidemiology has preceded our knowledge of VLSFAs biology. We hope this review will spur interest from the research community in further studying these potentially beneficial fatty acids.
Keywords: very long-chain saturated fatty acids, cardiovascular disease, type 2 diabetes, mortality, aging
INTRODUCTION
In this review, we define very long-chain saturated fatty acids (VLSFAs) as saturated fatty acids with 20 carbons or more. The VLSFAs are simple aliphatic (straight) chain fatty acids that differ from the most abundant saturated fatty acids, palmitic acid (16:0) and stearic acid (18:0), by their chemistry, biological properties, and dietary sources in addition to their length. This review will focus on arachidic acid (20:0), behenic acid (22:0), and lignoceric acid (24:0), 3 VLSFAs that can be measured in the circulation. Longer VLSFAs (26 to 38 carbons) are primarily present in the skin, brain and Meibomian glands (1) and not considered here.
Circulating VLSFAs originate both from the diet and endogenous metabolism. Low amounts of VLSFAs are found in some nuts and seeds and their oils (2). Peanuts, macadamia nuts and canola oil have the most total VLSFA, with peanuts highest in 22:0 and 24:0 and canola oil highest in 20:0. Among the main commercial oils, sunflower oil has noticeably more 22:0 (2, 3). Other commercial oils, including corn, olive, soy, safflower, contain low amounts of 20:0. Short-term feeding trials have shown that supplementary peanuts (4) and macadamia nuts (5) raise circulating levels of VLSFAs. In addition, peanut intake is associated with circulating VLSFAs in prospective cohorts (6, 7) suggesting usual peanut intake influences VLSFA levels. The VLSFAs can also be synthesized endogenously from 18:0 (Fig 1). 18:0 itself may originate from 16:0, the main end product of the fatty acid synthase and the main saturate in the diet. The synthesis of VLSFAs occurs in the endoplasmic reticulum by action of several elongases in the ELOVL (Elongation of very long chain fatty acids) family of enzymes (Fig 1) (8). The relative contribution of diet and metabolism to levels of circulating VLSFAs is not known and may differ for the different VLSFA given diminishing absorption with increasing chain length of saturated fatty acids.
Figure 1:
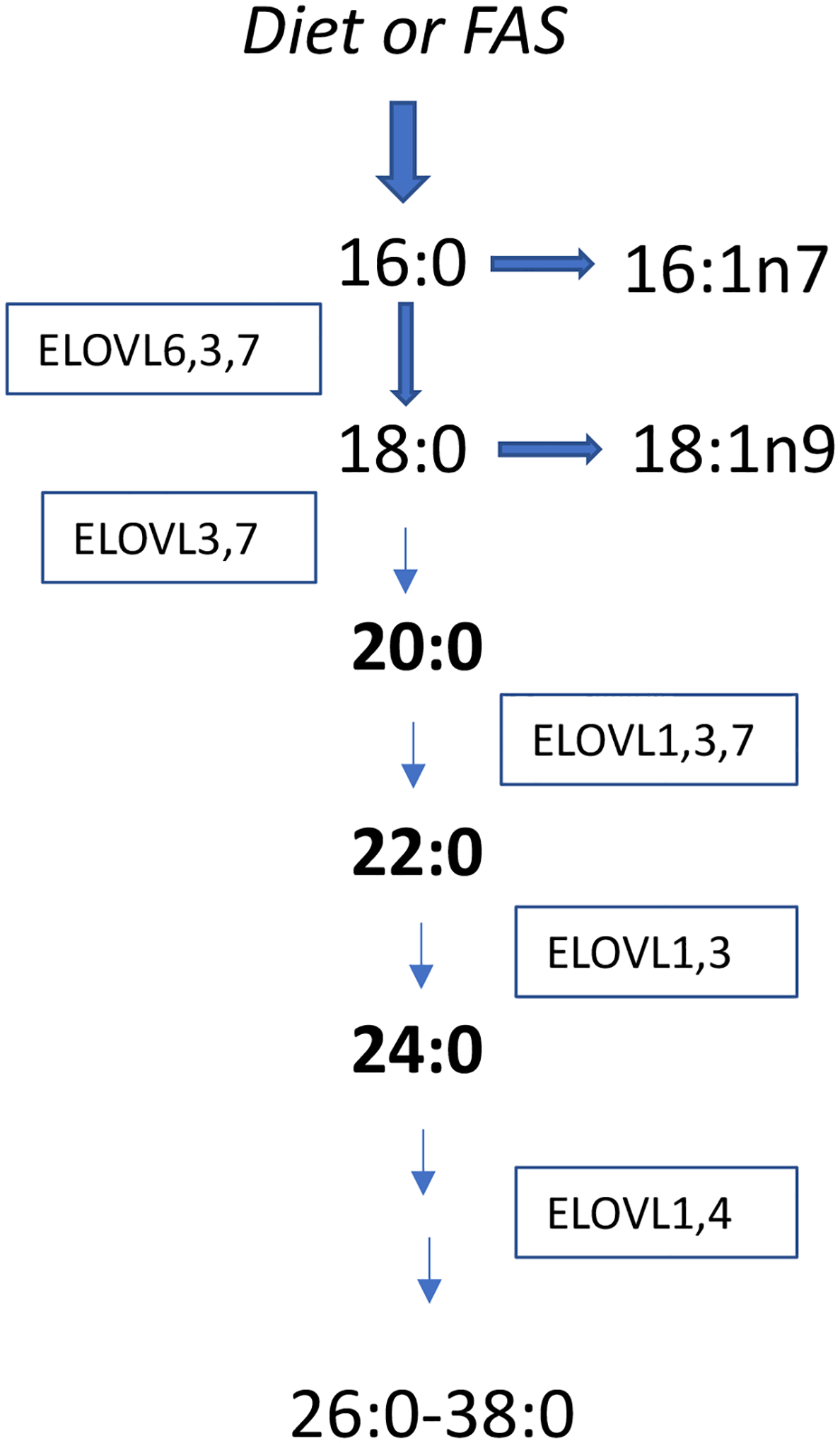
Endogenous production of VLSFAs.
Very long chain saturated fatty acids (VLSFAs) are produced from 18:0 by elongases in the ELOVL (Elongation of very long chain fatty acids) family of enzymes. Seven ELOVL enzymes exist in humans and overlap in the elongation steps they can catalyze. The 18:0 can come from 16:0 or from the diet. The VLSFAs are in low abundance unlike 18:0 and 16:0, and only a small proportion of 18:0 is elongated to 20:0 and longer saturates.
The VLSFAs have not been extensively studied and until recently were mostly known for their role in hereditary peroxisomal disorders and their use in special food formulations (such as Olestra). However, in 2014 we reported that increased erythrocyte levels of VLSFAs were associated with a reduction in risk of sudden cardiac arrest (9) and in the following years, VLSFAs have emerged as fatty acids associated with reduced risks of diabetes, heart disease mortality and aging (reviewed below). The purpose of this review is to summarize the epidemiologic evidence, highlighting findings from prospective studies, and discuss the implications of the VLSFA associations.
The assessment of overall VLSFA dietary intake would be a challenge and is rarely done, therefore we focus on studies that used circulating levels of VLSFAs, an objective measure of combined diet and metabolism.
VLSFAs & TYPE 2 DIABETES
The Fatty Acids and Outcomes Research Consortium (FORCE), a consortium of about 35 world-wide prospective studies, has made major contributions to our knowledge of the association of circulating fatty acids with incident type 2 diabetes. For each project, an analytical plan is provided to the cohorts, and analysts conduct analysis of their own cohort data. Aggregate results from these harmonized analyses are then pooled by a lead analyst. FORCE has provided valuable information on circulating fatty acids and incident diabetes, including n3 PUFAs (10), n6 PUFAs (11), dairy fatty acids (12) and fatty acids from de novo lipogenesis (13). In addition, in a report focusing on VLSFAs that included 12 cohorts with these fatty acids, Fretts et al reported associations of each VLSFA with lower risk of incident type 2 diabetes; for example, participants with levels of 24:0 in the 90th percentile were at 25% (95% CI: 17 to 31%) lower risk of incident type 2 diabetes than those with levels of 24:0 in the 10th percentile, after adjustment for demographics, lifestyle factors and adiposity measures (14). The FORCE findings, obtained with 12 prospective studies with data on VLSFAs from the US, Europe, Iceland, Australia and Taiwan are more robust and generalizable than would be achieved with a single cohort. Recently, these findings were replicated in the Nurses’ Health Study and the Health Professionals Follow-Up Study cohorts (7). As noted in an earlier report from one of the contributing studies (6), the associations of VLSFAs with diabetes in the FORCE paper were greatly attenuated by adjustment for triglycerides levels and levels of 16:0. We discuss possible implications in the discussion section.
In the Chinese Guangzhou Nutrition and Health Study cohort, with 216 incident type 2 diabetes, levels of erythrocyte 20:0 were found associated with an elevated risk of incident diabetes (15), however 16:0 was also associated with an unexpected, reduced risk; these findings suggest the possibility of fundamental differences, dietary or metabolic, from other populations including the Taiwan-based Chin-Shan Community Cardiovascular Cohort Study (CCCC) that contributed to the FORCE report. In addition, in a recent case-control study in Wuhan, China, with 900 cases of type 2 diabetes, total plasma VLSFAs were associated with reduced risk of type 2 diabetes, and as observed elsewhere, the associations were attenuated by adjustment for triglycerides (16). The cross-sectional design of this recent Chinese study does not inform the directionality of the VLSFA-diabetes associations and results need to be replicated in further studies of VLSFAs and diabetes in Asian populations.
Among the studies that contributed to FORCE, the EPIC-Interact study, a large case-control study of diabetes nested in the EPIC cohorts, stands out for its broad geographic representation of 8 European countries and contribution to the field of diabetes (17). EPIC-Interact includes about 12,400 cases of incident type 2 diabetes and a random sample of 16,150 controls without diabetes. To measure plasma phospholipid fatty acids in over 28K samples, a high-throughput method was developed and validated (18). In an early report, the investigators observed VLSFAs were associated with lower risk of incident diabetes while saturated fatty acids with 18 carbons or less were associated with higher risk (19). In another recent report from the EPIC study, Fumiaki et al reduced data on 27 fatty acids to principal components. The 1st component, characterized by high concentrations of linoleic acid, odd-chain fatty acids and VLSFAs, and low concentration of palmitic acid, was associated with lower risk of incident diabetes (20). While the principal components were derived agnostically, the association of the 1st component with reduced risk of incident diabetes effectively resumes known fatty acid-diabetes associations. Interestingly, removal of VLSFAs from the 1st component had the most effect on attenuating the association with diabetes highlighting the potential importance of these fatty acids to type 2 diabetes risk.
VLSFAs & CARDIOVASCULAR DISEASE
VLSFAs have been associated with reduced risk of several cardiovascular outcomes. In this regard, the Cardiovascular Health Study (CHS), a cohort of older adults in the U.S. with large numbers of cardiovascular events and where plasma phospholipid fatty acids have been measured with good precision at baseline and twice more during follow-up, has provided much needed epidemiological information on the VLSFAs. In the CHS cohort, 20:0 constitutes on average 0.5% of total plasma phospholipid fatty acids, while 22:0 constitutes 1.7% and 24:0 1.4% (21). This contrasts with the most abundant long-chain saturated fatty acids, 16:0 and 18:0 that account for 25.4% and 13.4% of total fatty acids respectively in CHS. Adjacent VLSFAs on the elongation pathway (Figure 1) show high intercorrelations (0.64 between 20:0 and 22:0, and 0.89 between 22:0 and 24:0) suggesting coordinated elongation. In CHS, increased levels of plasma phospholipid VLSFAs are associated with decreased risks of incident heart failure (21), incident atrial fibrillation (22), as well as decreased risk of mortality (23).
Heart failure.
The CHS study of VLSFAs and heart failure included 1304 incident heart failure events during an average 11 years of follow-up (21). Among those with ejection fraction data, 489 had heart failure with preserved ejection fraction and 310 had reduced ejection fraction. In time-dependent analyses of serial measurements, each VLSFA was associated with reduced risk of heart failure. For example, compared to the lowest quartile of the fatty acid distribution, plasma phospholipid levels of 24:0 in the highest quartile were associated with 33% (95% CI: 19 to 45%) reduction in risk of incident heart failure. The associations were independent of demographics, lifestyle factors, other fatty acids, and medical conditions, including coronary heart disease, atrial fibrillation, and diabetes. The associations appeared similar for heart failure with preserved or reduced ejection fraction although confidence intervals were large. Unlike the VLSFA-diabetes associations mentioned above, the VLSFA-heart failure associations were independent of circulating levels of 16:0 and fasting triglycerides.
In an older case-control study nested in the Physicians’ Health Study, plasma phospholipid levels of 20:0 and 22:0 were not associated with incident heart failure (24). The levels of 20:0 and 22:0 were on average 2 to 3 times lower than in CHS and measured with less precision. 24:0 was not measured. Lower levels and single measurement may have affected the power to detect VLSFA-associations. Further studies are needed to replicate the CHS findings.
Atrial fibrillation
Atrial fibrillation is a common arrhythmia that affect the synchronicity of the heart rhythm between the atria and the heart ventricles. The typical quivering of the atrial results in blood pools that can promote the formation of blood clots and increase the risk of stroke (23). In the CHS study of VLSFAs and atrial fibrillation, 707 incident atrial fibrillation events were identified among participants without coronary heart disease during the follow-up. High levels of each VLSFA were associated with reduced risk of incident atrial fibrillation. The associations were independent of other risk factors for atrial fibrillation including heart failure and diabetes and of other fatty acids including 16:0. For example, plasma phospholipid levels of 24:0 in the highest quartile compared to the lowest one were associated with 32% (95% CI: 15 to 45%) reduction in risk of incident atrial fibrillation. These striking associations need replication.
Coronary heart disease.
The associations of total plasma and erythrocyte VLFSAs with CHD risk were examined in case-control studies nested in the Nurses’ Health Study and the Health Professionals Follow-up Study cohorts (25). The study included 794 total cases, defined as non-fatal myocardial infarction or CHD death. In multivariable pooled analyses, summed levels of plasma VLSFAs were associated with reduced risk of incident CHD (52% decreased risk comparing highest to lowest quintile, 95% CI: 28 to 68%). When using erythrocyte VLSFAs, only 20:0 was associated with reduced risk possibly due to greater measurement error in erythrocyte than plasma measurements. Similarly, in a case-control study nested in the PREDIMED trial (Prevención con Dieta Mediterránea), that included 136 cases of CHD (defined as angina or MI), erythrocyte membrane 24:0 and 22:0 were associated with lower risk of incident CHD (26). These 2 studies suggest VLSFAs are associated with lower risk of atherosclerotic events.
Sudden cardiac arrest.
In a case-control study in the greater Seattle area that included 265 cases of sudden cardiac arrest and 415 controls from the same community, increased levels of erythrocyte VLSFAs in bloods collected at the time of the cardiac arrest were associated with reduced risk of sudden cardiac arrest (9) (for example 1 SD higher 22:0 was associated with 29% lower risk [12 to 43%]). These findings need to be replicated in a prospective study.
VLSFAs & HEALTHY AGING AND MORTALITY
In addition to heart disease phenotypes, circulating VLSFAs are associated with lower mortality and better aging.
Mortality
In the CHS cohort, high levels of plasma phospholipid 22:0 and 24:0 were associated with reduced risk of mortality, CVD mortality as well as non-CVD mortality independent of demographics, lifestyle risk factors, medical conditions, and self-reported health status (23). In a study of Framingham Offspring Cohort participants without prevalent CVD, 4 erythrocyte fatty acids predicted mortality during 11 years follow-up: high levels of 22:0, 14:0 (myristic acid) and the omega3 index (sum of docosahexaenoic acid and eicosapentaenoic acid) were associated with reduced risk, and 16:1n7 (palmitoleic acid) with increased mortality (27). Although predictive models are agnostic and based solely on correlations, the selection of 22:0 is in general agreement with the report from Fretts (23), and these 2 studies suggest overall health benefits of the VLSFAs.
Cognition and aging
In addition to associations with lower mortality, circulating VLSFAs may be associated with better aging. In the Atherosclerosis Risk in Communities (ARIC) cohort, higher proportions of plasma phospholipid VLSFAs in midlife were associated with less 20-y decline in a word fluency test suggesting possible protection from cognitive decline (28). Recently, we investigated the relationship of VLSFAs to healthy aging among the CHS participants who had aged successfully up to the time of the fatty acid measurement (free of chronic disease including cardiovascular disease, cancer, lung disease and severe kidney disease, and free of physical dysfunction and cognitive decline). Compared with the lowest quintile, levels of 24:0 in the highest quintile of the fatty acid distribution were associated with 16% (5%−27%) lower risk of an unhealthy event during follow-up (median 6.4 years), after adjustment for demographics, lifestyle factors, and clinical conditions (29). Findings were very similar for 22:0, while 20:0 showed no association with this end point. Adjustment for markers of lipogenesis (fasting triglyceride and circulating 16:0) did not change the findings suggesting lipogenesis was not a major driver of these associations.
DISCUSSION
Observational (epidemiological) studies cannot in and out of themselves provide proof of causality or mechanisms. Here we discuss possible implications of the associations of circulating VLSFAs.
Measurement of circulating VLSFAs.
In vivo VLSFAs are primarily incorporated into carrier molecules and are acylated to sphingolipids, phosphoglycerolipids, triacylglycerols, or sterol esters. Small amounts of VLSFAs also circulate as non-esterified fatty acid attached to albumin (30). However, in the cohorts mentioned here, fatty acids were released from the carrier backbone and quantitated by gas chromatography after transformation to fatty acid methyl ester. This classic method has the advantages of correctly identifying the fatty acids, generally with high precision, even if they are in low abundance. The disadvantage is the information about the lipid backbone is lost. We think it is necessary to identify the lipid carrier(s) of the VLSFAs to move beyond associations toward mechanistic understanding.
GWAS of circulating VLSFAs.
In a meta-analysis of genome-wide association results in the CHARGE consortium and in populations of Chinese ancestry, circulating VLSFAs were associated with genetic variation in two sphingolipid-related enzymes, SPTLC3 (Serine Palmitoyl Transferase Long Chain Base Subunit 3) and CERS4 (Ceramide Synthase 4) (31, 32). SPTLC3 is the first and rate-limiting enzyme in the de novo biosynthesis of ceramide and produces the sphingoïd backbone. CERS4 attaches a fatty acid to the backbone. Ceramides can then be used for the de novo synthesis of sphingomyelins and complex sphingolipids. The genetics findings suggest circulating plasma VLSFA levels may reflect in part their utilization in ceramides and other sphingolipids. Coordinated synthesis of 24:0 by ELOVL1 and ceramide synthesis by CERS2 has also been suggested (33).
Ceramides and sphingomyelins with VLSFAs.
Sphingolipids are a large family of lipids with multiple biological activities (34, 35). They usually include one saturated or monounsaturated fatty acid acylated to the lipid backbone and there is evidence that the length of the saturated fatty acid influences the biological activity of ceramides (36, 37). Additionally, epidemiological findings suggest VLSFAs impart different properties to ceramides and sphingomyelins than 16:0 resulting in better health outcomes (reviewed below).
In the CHS cohort, plasma ceramides and sphingomyelins with a VLSFA are associated with reduced risks of incident atrial fibrillation (38) and incident heart failure (39). In contrast, ceramides and sphingomyelins with 16:0 are associated with higher risks of atrial fibrillation and heart failure. Ceramide plays a role in apoptosis (40) and apoptosis in the context of heart remodeling and fibrosis is part of the pathophysiology of both heart failure and atrial fibrillation (41, 42). We speculate this may explain the association of ceramide with 16:0 with higher risks of both outcomes; and should ceramides with VLSFAs actually protect from apoptosis, it may explain their association with lower risks of both heart failure and atrial fibrillation. Parallel association of sphingomyelin and ceramide species may reflect the generation of ceramides from sphingomyelins by sphingomyelinases (43). And at least for the outcomes of heart failure and atrial fibrillation, the sphingomyelin associations may explain at least in part the associations of plasma phospholipid VLSFAs with these outcomes.
Differences in associations between ceramide with 16:0 and ceramide with 24:0 has led investigators to use the ratio of these ceramides to predict outcomes, assuming the relative proportion of these ceramides determine the health effects. In patients with stable coronary artery disease and those with an acute coronary event, the ratio ceramide-16:0/ceramide-24:0 was found associated with cardiovascular death (44). Associations of ceramides and sphingomyelins with mortality were also observed in the population-based CHS cohort. Fretts et al recently reported a reduced risk of total mortality with increased levels of plasma ceramides with 22:0 and 24:0 and with sphingomyelins with a VLSFA; and increased mortality with ceramide and sphingomyelin with 16:0 (manuscript revised for Clinical Chemistry).
Ceramides and diabetes
While some of the associations of plasma phospholipid VLSFAs were paralleled by associations of ceramides and sphingomyelins with VLSFAs, this does not appear to be the case for the outcome of incident type 2 diabetes. All circulating ceramides, including those with a VLSFA, are associated with increased risk of incident diabetes in the Strong Heart Family Study, a cohort study among American Indians (45), in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) cohort (46), as well as in the CHS cohort (manuscript revised for the Journal of Lipid Research). Circulating ceramides are also cross-sectionally associated with fasting insulin and fasting glucose in multivariable analyses (47, 48), in agreement with a large body of experimental studies on ceramides and insulin resistance (49). Therefore, the associations of circulating VLSFAs with lower diabetes risk do not seem to be explained by the presence of VLSFAs in ceramides and sphingomyelins.
VLSFAs and de novo lipogenesis
Several studies reported that circulating VLSFAs were inversely associated with plasma triglyceride levels and plasma phospholipid levels of 16:0 in multivariable, cross-sectional analyses (6, 14, 50). 16:0 is the primary output of fatty acid synthetase and circulating levels, together with triglycerides may be markers of de novo lipogenesis. The associations of VLSFAs with type 2 diabetes in the FORCE consortium were greatly attenuated after adjustment for triglycerides and 16:0 suggesting one of several possibilities; the associations of VLSFAs with diabetes may be confounded by triglycerides and 16:0; or increased levels of VLSFAs are biomarkers of reduced lipogenesis which leads to lower diabetes risk; or VLSFAs are more directly involved in reducing lipogenesis.
Conclusions
We and others have shown beneficial associations of circulating VLSFAs on heart disease, diabetes, mortality and aging. It is clear that VLSFAs (20 to 24 carbons) need to be distinguished from other saturated fatty acids for their potential health benefits. The epidemiologic studies raised many questions and knowledge of the VLSFAs biology is limited. Further studies are needed to understand how the endogenous production of VLSFAs is regulated, the influence of dietary intake on circulating and tissue levels, the extent to which VLSFAs are incorporated into lipids other than sphingolipids, and to understand the influence of VLSFAs on sphingolipid metabolism, membrane function and potentially on de novo lipogenesis. We hope this review will promote interest in these unique saturated fatty acids and the lipids that carry them.
Key points.
Very long-chain saturated fatty acids (VLSFAs) in the circulation are associated with lower risks of heart failure, atrial fibrillation, coronary heart disease, mortality, sudden cardiac arrest, type 2 diabetes, and with healthy aging
The VLSFA associations with heart failure, atrial fibrillation and mortality may be due to ceramides and sphingomyelins carrying a VLSFA
The VLSFA associations with type 2 diabetes suggest different explanations and could be due to confounding or mediation by de novo lipogenesis
Acknowledgments
Financial support and sponsorship
This work was supported by a grant from the NHLBI
Funding:
This work was funded by grant R01HL146499 from the National Institute of Health (NHLBI)
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
References and Recommended Reading
- 1.Yeboah GK, Lobanova ES, Brush RS, Agbaga MP. Very long chain fatty acid-containing lipids: a decade of novel insights from the study of ELOVL4. J Lipid Res. 2021;62:100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Department of Agriculture ARS, Nutrient Data Laboratory. USDA National Nutrient Database for Standard Reference, Legacy. Version Current: April 2018. Internet: http://www.ars.usda.gov/nutrientdata. US Department of Agriculture (USDA); 2018. [Google Scholar]
- 3.Chernova AI, Gubaev RF, Singh A, Sherbina K, Goryunova SV, Martynova EU, et al. Genotyping and lipid profiling of 601 cultivated sunflower lines reveals novel genetic determinants of oil fatty acid content. BMC Genomics. 2021;22(1):505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam C, Wong D, Cederbaum S, Lim B, Qu Y. Peanut consumption increases levels of plasma very long chain fatty acids in humans. Mol Genet Metab. 2012;107(3):620–2. [DOI] [PubMed] [Google Scholar]
- 5.Garg ML, Blake RJ, Wills RB. Macadamia nut consumption lowers plasma total and LDL cholesterol levels in hypercholesterolemic men. J Nutr. 2003;133(4):1060–3. [DOI] [PubMed] [Google Scholar]
- 6.Lemaitre RN, Fretts AM, Sitlani CM, Biggs ML, Mukamal K, King IB, et al. Plasma phospholipid very-long-chain saturated fatty acids and incident diabetes in older adults: the Cardiovascular Health Study. Am J Clin Nutr. 2015;101(5):1047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ardisson Korat AV, Malik VS, Furtado JD, Sacks F, Rosner B, Rexrode KM, et al. Circulating Very-Long-Chain SFA Concentrations Are Inversely Associated with Incident Type 2 Diabetes in US Men and Women. J Nutr. 2020;150(2):340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Most recent publication on VLSFAs and type 2 diabetes
- 8.Sassa T, Kihara A. Metabolism of very long-chain Fatty acids: genes and pathophysiology. Biomol Ther (Seoul). 2014;22(2):83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemaitre RN, King IB, Rice K, McKnight B, Sotoodehnia N, Rea TD, et al. Erythrocyte very long-chain saturated fatty acids associated with lower risk of incident sudden cardiac arrest. Prostaglandins Leukot Essent Fatty Acids. 2014;91(4):149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian F, Ardisson Korat AV, Imamura F, Marklund M, Tintle N, Virtanen JK, et al. n-3 Fatty Acid Biomarkers and Incident Type 2 Diabetes: An Individual Participant-Level Pooling Project of 20 Prospective Cohort Studies. Diabetes Care. 2021;44(5):1133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu JHY, Marklund M, Imamura F, Tintle N, Ardisson Korat AV, de Goede J, et al. Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 2017;5(12):965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imamura F, Fretts A, Marklund M, Ardisson Korat AV, Yang WS, Lankinen M, et al. Fatty acid biomarkers of dairy fat consumption and incidence of type 2 diabetes: A pooled analysis of prospective cohort studies. PLoS Med. 2018;15(10):e1002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imamura F, Fretts AM, Marklund M, Ardisson Korat AV, Yang WS, Lankinen M, et al. Fatty acids in the de novo lipogenesis pathway and incidence of type 2 diabetes: A pooled analysis of prospective cohort studies. PLoS Med. 2020;17(6):e1003102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fretts AM, Imamura F, Marklund M, Micha R, Wu JHY, Murphy RA, et al. Associations of circulating very-long-chain saturated fatty acids and incident type 2 diabetes: a pooled analysis of prospective cohort studies. Am J Clin Nutr. 2019;109(4):1216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Findings from the FORCE consortium on VLSFAs and diabetes.
- 15.Lin JS, Dong HL, Chen GD, Chen ZY, Dong XW, Zheng JS, et al. Erythrocyte Saturated Fatty Acids and Incident Type 2 Diabetes in Chinese Men and Women: A Prospective Cohort Study. Nutrients. 2018;10(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo C, Liu H, Wang X, Xia L, Huang H, Peng X, et al. The associations between individual plasma SFAs, serine palmitoyl-transferase long-chain base subunit 3 gene rs680379 polymorphism, and type 2 diabetes among Chinese adults. Am J Clin Nutr. 2021;114(2):704–12. [DOI] [PubMed] [Google Scholar]; *Recent information on VLSFAs and diabetes in a Chinese population. Results from a large case-control study.
- 17.InterAct C, Langenberg C, Sharp S, Forouhi NG, Franks PW, Schulze MB, et al. Design and cohort description of the InterAct Project: an examination of the interaction of genetic and lifestyle factors on the incidence of type 2 diabetes in the EPIC Study. Diabetologia. 2011;54(9):2272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang LY, Summerhill K, Rodriguez-Canas C, Mather I, Patel P, Eiden M, et al. Development and validation of a robust automated analysis of plasma phospholipid fatty acids for metabolic phenotyping of large epidemiological studies. Genome Med. 2013;5(4):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kroger J, Schulze MB, et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014;2(10):810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imamura F, Sharp SJ, Koulman A, Schulze MB, Kroger J, Griffin JL, et al. A combination of plasma phospholipid fatty acids and its association with incidence of type 2 diabetes: The EPIC-InterAct case-cohort study. PLoS Med. 2017;14(10):e1002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemaitre RN, McKnight B, Sotoodehnia N, Fretts AM, Qureshi WT, Song X, et al. Circulating Very Long-Chain Saturated Fatty Acids and Heart Failure: The Cardiovascular Health Study. J Am Heart Assoc. 2018;7(21):e010019. [DOI] [PMC free article] [PubMed] [Google Scholar]; *First and only report on VLSFAs and heart failure; to compare to ref #21
- 22.Fretts AM, Mozaffarian D, Siscovick DS, Djousse L, Heckbert SR, King IB, et al. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc. 2014;3(3):e000889. [DOI] [PMC free article] [PubMed] [Google Scholar]; *First and only report on VLSFAs and atrial fibrillation; to compare to ref #22
- 23.Fretts AM, Mozaffarian D, Siscovick DS, King IB, McKnight B, Psaty BM, et al. Associations of Plasma Phospholipid SFAs with Total and Cause-Specific Mortality in Older Adults Differ According to SFA Chain Length. J Nutr. 2016;146(2):298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto C, Hanson NQ, Tsai MY, Glynn RJ, Gaziano JM, Djousse L. Plasma phospholipid saturated fatty acids and heart failure risk in the Physicians’ Health Study. Clin Nutr. 2013;32(5):819–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malik VS, Chiuve SE, Campos H, Rimm EB, Mozaffarian D, Hu FB, et al. Circulating Very-Long-Chain Saturated Fatty Acids and Incident Coronary Heart Disease in US Men and Women. Circulation. 2015;132(4):260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papandreou C, Sala-Vila A, Galie S, Muralidharan J, Estruch R, Fito M, et al. Association Between Fatty Acids of Blood Cell Membranes and Incidence of Coronary Heart Disease. Arterioscler Thromb Vasc Biol. 2019;39(4):819–25. [DOI] [PubMed] [Google Scholar]
- 27.McBurney MI, Tintle NL, Vasan RS, Sala-Vila A, Harris WS. Using an erythrocyte fatty acid fingerprint to predict risk of all-cause mortality: the Framingham Offspring Cohort. Am J Clin Nutr. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Prediction of mortality in the Framingham study using fatty acids. An example of the importance of fatty acids in general and 22:0 in particular.
- 28.Li D, Misialek JR, Jing M, Tsai MY, Eckfeldt JH, Steffen LM, et al. Plasma phospholipid very-long-chain SFAs in midlife and 20-year cognitive change in the Atherosclerosis Risk in Communities (ARIC): a cohort study. Am J Clin Nutr. 2020;111(6):1252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bockus LB, Biggs ML, Lai HTM, de Olivera Otto MC, Fretts AM, McKnight B, et al. Assessment of Plasma Phospholipid Very-Long-Chain Saturated Fatty Acid Levels and Healthy Aging. JAMA Netw Open. 2021;4(8):e2120616. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Intriguing recent report suggesting that VLSFAs promote healthy aging
- 30.Choi JK, Ho J, Curry S, Qin D, Bittman R, Hamilton JA. Interactions of very long-chain saturated fatty acids with serum albumin. J Lipid Res. 2002;43(7):1000–10. [DOI] [PubMed] [Google Scholar]
- 31.Lemaitre RN, King IB, Kabagambe EK, Wu JH, McKnight B, Manichaikul A, et al. Genetic loci associated with circulating levels of very long-chain saturated fatty acids. J Lipid Res. 2015;56(1):176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu J, Manichaikul A, Hu Y, Chen YI, Liang S, Steffen LM, et al. Meta-analysis of genome-wide association studies identifies three novel loci for saturated fatty acids in East Asians. Eur J Nutr. 2017;56(4):1477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohno Y, Suto S, Yamanaka M, Mizutani Y, Mitsutake S, Igarashi Y, et al. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc Natl Acad Sci U S A. 2010;107(43):18439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hannun YA, Obeid LM. Many ceramides. J Biol Chem. 2011;286(32):27855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canals D, Salamone S, Hannun YA. Visualizing bioactive ceramides. Chem Phys Lipids. 2018;216:142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stiban J, Perera M. Very long chain ceramides interfere with C16-ceramide-induced channel formation: A plausible mechanism for regulating the initiation of intrinsic apoptosis. Biochim Biophys Acta. 2015;1848(2):561–7. [DOI] [PubMed] [Google Scholar]
- 37.Grosch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Prog Lipid Res. 2012;51(1):50–62. [DOI] [PubMed] [Google Scholar]
- 38.Jensen PN, Fretts AM, Hoofnagle AN, Sitlani CM, McKnight B, King IB, et al. Plasma Ceramides and Sphingomyelins in Relation to Atrial Fibrillation Risk: The Cardiovascular Health Study. J Am Heart Assoc. 2020;9(4):e012853. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Report showing ceramides and sphingomyelins with a VLSFA are associated with lower risk of atrial fibrillation; to compare to ref# 22
- 39.Lemaitre RN, Jensen PN, Hoofnagle A, McKnight B, Fretts AM, King IB, et al. Plasma Ceramides and Sphingomyelins in Relation to Heart Failure Risk. Circ Heart Fail. 2019;12(7):e005708. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Report showing ceramides and sphingomyelins with a VLSFA are associated with lower risk of heart failure; to compare to ref #21
- 40.Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res. 2009;50 Suppl:S91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nattel S. Molecular and Cellular Mechanisms of Atrial Fibrosis in Atrial Fibrillation. JACC Clin Electrophysiol. 2017;3(5):425–35. [DOI] [PubMed] [Google Scholar]
- 42.Piek A, de Boer RA, Sillje HH. The fibrosis-cell death axis in heart failure. Heart Fail Rev. 2016;21(2):199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shamseddine AA, Airola MV, Hannun YA. Roles and regulation of neutral sphingomyelinase-2 in cellular and pathological processes. Adv Biol Regul. 2015;57:24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laaksonen R, Ekroos K, Sysi-Aho M, Hilvo M, Vihervaara T, Kauhanen D, et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J. 2016;37(25):1967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fretts AM, Jensen PN, Hoofnagle A, McKnight B, Howard BV, Umans J, et al. Plasma Ceramide Species Are Associated with Diabetes Risk in Participants of the Strong Heart Study. J Nutr. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen GC, Chai JC, Yu B, Michelotti GA, Grove ML, Fretts AM, et al. Serum sphingolipids and incident diabetes in a US population with high diabetes burden: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Am J Clin Nutr. 2020;112(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lemaitre RN, Yu C, Hoofnagle A, Hari N, Jensen PN, Fretts AM, et al. Circulating Sphingolipids, Insulin, HOMA-IR, and HOMA-B: The Strong Heart Family Study. Diabetes. 2018;67(8):1663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jensen PN, Fretts AM, Yu C, Hoofnagle AN, Umans JG, Howard BV, et al. Circulating sphingolipids, fasting glucose, and impaired fasting glucose: The Strong Heart Family Study. EBioMedicine. 2019;41:44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park M, Kaddai V, Ching J, Fridianto KT, Sieli RJ, Sugii S, et al. A Role for Ceramides, but Not Sphingomyelins, as Antagonists of Insulin Signaling and Mitochondrial Metabolism in C2C12 Myotubes. J Biol Chem. 2016;291(46):23978–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng JS, Sharp SJ, Imamura F, Koulman A, Schulze MB, Ye Z, et al. Association between plasma phospholipid saturated fatty acids and metabolic markers of lipid, hepatic, inflammation and glycaemic pathways in eight European countries: a cross-sectional analysis in the EPIC-InterAct study. BMC Med. 2017;15(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]


