Abstract
Sensitization of bladder afferents is an underlying contributor to the development and maintenance of painful bladder syndrome/interstitial cystitis. Extracellular purines and pyrimidines (e.g., ATP and UTP), released during bladder distension or from damaged cells after tissue insult, are thought to play an important role in bladder physiological and pathological states by actions at ionotropic P2X and metabotropic P2Y receptors. In the present study, we examined the ability of P2Y receptors to sensitize and modulate P2X mediated-responses in mouse bladder sensory neurons. UTP (a P2Y2 and P2Y4 agonist) increased excitability of bladder neurons by depolarizing resting membrane potential, increasing action potential firing, and facilitating responses to suprathreshold current injection as well as to P2X agonist application. These effects of UTP on bladder neuron excitability were blocked by the P2Y2 receptor antagonist suramin. UTP also facilitated bladder neuron homomeric P2X2 sustained currents and homomeric P2X3 fast currents. The facilitatory effect of UTP on P2X2 sustained currents was mediated by a G protein-coupled P2Y2 receptor/PKC pathway whereas the effect of UTP on P2X3 fast currents was G protein-independent. We also examined P2X and P2Y receptor expression in bladder neurons. P2Y2 and P2Y4 transcripts were detected in ~50% and ~20% of bladder neurons, respectively. Approximately 50% of P2X2- and P2X3-positive bladder neurons expressed P2Y2 transcripts, whereas ≤25% of the same bladder neurons expressed P2Y4 transcripts. These results \ort involvement of P2Y2 receptors in bladder sensation, suggesting an important contribution to bladder neuron excitability and hypersensitivity.
Keywords: Bladder, Sensitization, GPCR, P2X, P2Y, PKC
Introduction
Painful bladder syndrome/interstitial cystitis (PBS/IC) is characterized by urge and increased urination frequency accompanied by chronic pelvic pain in the absence of a pathobiological condition to explain symptoms (Burkman, 2004;Nickel, 2004). One endogenous mediator of bladder discomfort and pain is adenosine triphosphate (ATP), which is released from bladder urothelium during distension or chemical stimulation (Ferguson et al., 1997;Birder et al., 2003) and is released in greater amount in PBS/IC patients (Sun et al., 2001a;Sun et al., 2001b).
Both ionotropic P2X and metabotropic P2Y receptors can be activated by extracellular ATP (Ralevic and Burnstock, 1998), suggesting that P2Y receptors may also contribute to altered sensations in PBS/IC. To date, seven P2X subunits (P2X1-P2X7) have been reported (North, 2002); among these, P2X2 and P2X3 subunits are widely expressed in peripheral neurons and are thought to play an important role in the transduction of nociceptive visceral signals in general and bladder sensory information in particular (Dunn et al., 2001;Burnstock, 2006). The P2Y receptor family has eight members (P2Y1, 2, 4, 6, 11–14) to date. They respond to endogenous purine and pyrimidine nucleotides (ATP, ADP, UTP, UDP) released into the extracellular environment from various tissues (von Kugelgen, 2006;Lazarowski and Harden, 1999;Lazarowski and Boucher, 2001). P2Y receptors in sensory neurons have been reported to activate cutaneous afferents (Stucky et al., 2004), mediate internal Ca2+ release, CREB phosphorylation, and release of CGRP (Song et al., 2007;Molliver et al., 2002;Sanada et al., 2002) and contribute to mechanosensory transmission (Nakamura and Strittmatter, 1996). P2Y receptors also enhance TRPV1 channel signaling gated by capsaicin or acid and reduce its thermal threshold in peripheral sensory neurons (Moriyama et al., 2003;Tominaga et al., 2001). Thus, P2Y receptor signaling is likely to play a role in mechanosensation and/or nociception under normal and pathological conditions.
Unlike other tissues, visceral organs are innervated by two nerves. The urinary bladder is innervated by the lumbar splanchnic and pelvic nerves (Vera and Nadelhaft, 1992;Uemura et al., 1975;Uemura et al., 1973), the cell bodies of which are located, respectively, in thoracolumbar (TL) and lumbosacral (LS) dorsal root ganglia (Andersson, 2002;Applebaum et al., 1980). Because pelvic and splanchnic bladder afferent pathways have some overlapping, but also distinctly different functions (Dang et al., 2005;Xu and Gebhart, 2008), we studied both TL and LS bladder neurons in the present experiments. The goals of the study were to examine the effect of the P2Y agonist UTP on bladder neuron excitability and the interaction between bladder neuron P2X and P2Y receptors.
Materials and Methods
Animals
Male C57BL/6 mice (6–8 weeks; Taconic Labs, Germantown, NY) were used for most experiments; P2Y2 knockout mice were also used [see refs in Malin et al., 2008 for initial characterization of these mice]. Mice were housed in polypropylene cages with ad libitum access to food and water. All protocols were reviewed and approved by the Institutional Animal Care & Use Committee, the University of Pittsburgh.
Bladder neuron retrograde labeling
Mice were anesthetized with 2% inhaled isoflurane (Hospira Inc., Lake Forest, IL), the bladder exposed via a lower abdominal incision ~5 mm in length and 10 ul of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiIC18(3); Molecular Probes, Eugene, OR in 0.2mg/ml in DMSO) was injected into 3–4 sites within the bladder wall and the base around the trigone using a 30 gauge needle. Sterile cotton-tipped applicators were applied to injection sites to absorb any DiI that leaked from injection sites. The incision was closed (6.0 silk, Ethicon Inc., Somerville, NJ) and post-operative analgesia provided after surgery (buprenorphine, 0.05mg/kg, i.p.; Bedford labs, Bedford, OH).
Cell dissociation and electrophysiological recordings
Lumbosacral (LS; L6-S2) and thoracolumbar (TL; T13-L2) dorsal root ganglia (DRG) were harvested for electrophysiological whole cell recordings. After dissection of DRG, the pelvic area around the bladder was examined for evidence of DiI leakage; leakage was rarely noted and DRG from mice with evidence of leakage were not used. LS and TL DRG were incubated at 37°C, 5% CO2 for 45 min in DMEM media containing 1% penicillin/streptomycin (Gibco, Invitrogen Corp. Grand Island, NY), 1 mg/ml type 4 collagenase and 1 mg/ml neutral protease (Worthington Biochemical Corp., Lakewood, NJ). Tissue was gently triturated and collected after 5 min centrifugation at 120 X g, washed 3 times and re-suspended in DMEM media containing 0.5 mM L-glutamine, 1% penicillin/streptomycin and 10% fetal bovine serum. The cells were plated on poly-D-lysine-coated coverslips (Becton Dickinson Labware, Bedford, MA) and incubated at 37°C, 5% CO2 for 14–16 hrs. Only DiI positive bladder sensory neurons were studied. All recordings were performed within 24 hours after plating.
Coverslips were transferred to a recording chamber perfused with an external solution (in mM) consisting of NaCl 140, KCl 5, MgCl2 1, CaCl2 2, glucose 10, and HEPES 10 at pH 7.4, 310 mOsm. Glass micropipette tips were heat-polished to resistances ~ 2–3 MΩ and filled with an internal solution (in mM) consisting of KCl 130, NaCl 4, CaCl2 0.2, HEPES 10, EGTA 10, MgATP 2, and NaGTP0.5 at pH 7.25, 300 mOsm. After establishing the whole-cell configuration, membrane voltage was clamped at −70 mV using an Axopatch 200B amplifier, digitized at 1 kHz (Digidata 1350), and controlled by Clampex software (pClamp 9; all from Axon Instruments, Union City, CA). Cell capacitance was obtained by reading the value from the Axopatch 200B amplifier. In current clamp experiments, only neurons that had a resting membrane potential more negative than −50 mV and a distinct action potential overshoot >0 mV were studied.
Drugs were applied through a 3-barrel glass pipette placed close (~100 μm) to the cell using a fast-step SF-77B superfusion system (Warner Instruments, Hamden, CT). ATP, α, β-methylene (met) ATP and GTP were obtained from Sigma-Aldrich (St. Louis, MO). UTP, suramin, Guanosine- 5′-O-(2- thiodiphosphate) (GDP-β-S), and myristoylated Protein Kinase C Inhibitor 20–28 were purchased from Calbiochem (La Jolla, CA). Purinergic agonists ATP and α, β-met ATP were applied for 4 sec at a washout interval of 2 min; the P2Y agonist UTP was superfused for 40 sec before the application of agonists. Suramin and the PKC inhibitor were perfused 2 min before and during UTP application. The reagents were prepared fresh from stock solutions on the day of the experiment. All experiments were performed at room temperature (21–23°C).
Single cell RT-PCR
Bladder DRG neurons were retrogradely labeled and mice sacrificed as described above. LS and TL DRG were removed and plated as described above for electrophysiological study. After 14–16 h incubation, coverslips were placed in the patch clamp recording chamber and perfused with sterilized external solution. DiI positive neurons were collected with glass pipettes (tip diameter ~ 60–80 μm) by gentle suction and expelled into 0.2 ml microcentrifuge tubes containing 2.5 μl lysis buffer consisting of 1X first-strand buffer, 2U RNaseOut, 10 μM dNTP, 0.5% IGEPAL, and 0.05 μg/μl Oligo(dT) 12–18 primer. Tubes were incubated at 65°C for 1.5 min and held at room temperature for 2 min. Another 2.5 μl of RT-PCR buffer consisting of 50 U SuperScript II reverse transcriptase, 1X first-strand buffer, 2U RNaseOut, and 10 μM dNTP was rapidly added to each tube. Tubes were incubated at 37°C for 20 min, then at 65°C for 10 min to generate first strands of cDNA sequence. Negative controls were tubes without labeled neurons or processed with RT-PCR buffer not containing reverse transcriptase. Only RT-PCR products of the batches that passed tests of negative controls underwent further PCR steps. All single cell RT-PCR reagents were purchased from Invitrogen Inc. (Carlsbad, CA).
Multiplex PCR and gene-specific nested PCR
P2X and P2Y genes were amplified through two rounds of PCR (multiplex and nested PCR) from the cDNA library of individual mouse bladder neurons. In the first round, two external primers of targeted genes were added together into a 25 μl volume PCR solution containing 1X PCR buffer, 0.2mM dNTP, 1.6 μM primers of each gene and 1U Taq Polymerase. In the second round, 1μl of first round PCR amplicons served as a template and two internal primers of individual genes were added to the 25 μl PCR system. Both multiplex and nested PCR used the following PCR conditions: 1 cycle of 10 min at 95 °C; 32 cycles of 94 °C/30 sec, 52°C/30 sec, and 72 °C/30 sec before a final extension step at 72 °C for 10 min, after which 10 μl of the nested PCR products was electrophoresed on a 2% (w/v) agarose gel at 100V for 25 min. After electrophoresis, the gel was stained with 0.005% ethidium bromide and bands of PCR products were visualized under UV light. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as an internal positive control. Single cell RT-PCR products that failed to pass internal positive tests were eliminated from the study. All primers were purchased from Integrated DNA Technologies, Inc. (Coralville, IA) and are listed in Table S1.
Data analysis
We used Graphpad Prism 5 (Graphpad Software, San Diego, CA) to perform statistical analyses. Data are presented as mean ± SEM. Dose–response curves were generated using the following equation: Y=A+ (B−A)/{1+10^[(LogEC50−X)]}, where X is the logarithm of concentration and Y is the response and starts at A and goes to B with a standard Hill slope of 1.0. Desensitization kinetics were fitted with a standard exponential equation: Y = K0 + K1 x exp(−t/τ), where Y is the current amplitude at time t, K0 is the amplitude of the sustained component, and τ is the time constant. K0 andK1 represent the contribution to current amplitude from the fast and slow components of the current, respectively. For dichotomous variables, Fisher’s exact test was used. Unpaired two-tailed t-tests were used for parametric measures if data were collected from different animals or cells; paired t-tests were applied when comparing current amplitudes from the same cell. Comparison of normalized data from independent groups was carried by nonparametric Mann-Whitney U-test. Results were considered statistically significant when P<0.05.
Results
We first characterized the purinoceptive properties of LS (n=194) and TL (n=118) bladder neurons. Consistent with a previous study (Chen and Gebhart, 2010), the majority of LS (93%) and TL (77%) mouse bladder neurons responded to P2X receptor agonists. Three types of purinergic currents were identified: (1) sustained currents (mediated through homomeric P2X2 receptors) were detected only in ~10% of LS purinoceptive neurons; (2) rapidly activating, slow deactivating currents (mediated through heteromeric P2X2/3 receptors) predominated in both LS (~90%) and TL (~75%) purinoceptive neurons; and (3) fast activating/de-activating currents (mediated through homomeric P2X3 receptors) were detected only in ~25% of TL purinoceptive neurons. Sustained currents were evoked by the purinergic agonist ATP, but not α,β-met ATP, whereas slow and fast deactivating currents were produced by both ATP and α,β-met ATP. We found that the effects of UTP were essentially restricted to LS bladder neurons; TL bladder neurons were not greatly affected. Accordingly, data presented here were mainly collected from LS bladder neurons unless indicated otherwise.
UTP increases bladder sensory neuron excitability
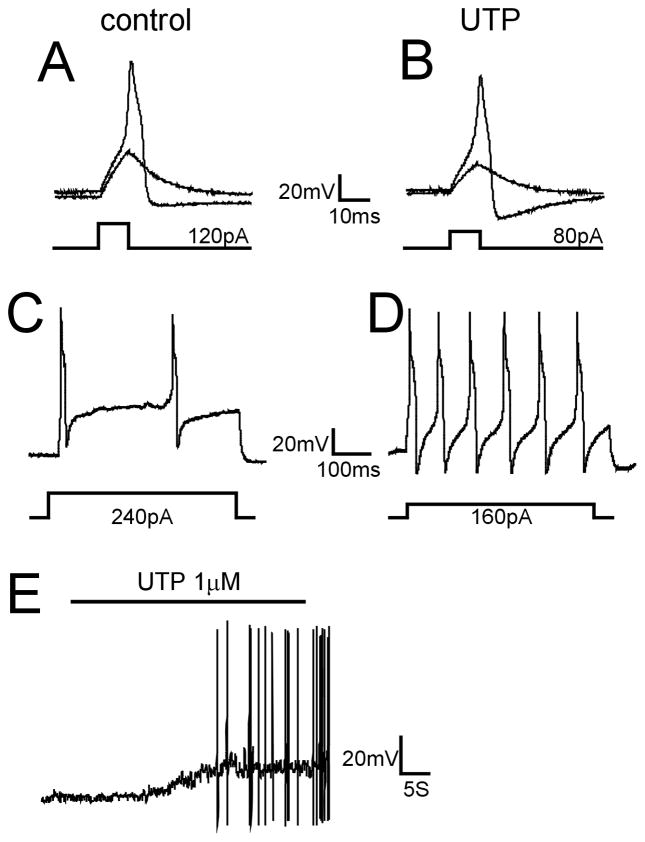
We anticipated that UTP would sensitize bladder sensory neurons, which we examined in 24 LS bladder neurons from naïve mice by injecting currents before and after UTP application (1μM, 40s) in whole-cell current clamp mode (Table 1). UTP application (1μM, 40s) depolarized LS bladder neurons from a resting membrane potential of −63.9±0.9 mV before UTP to −55.6±1.5 mV (P<0.005) after UTP. Rheobase was also significantly reduced after UTP application relative to control (from 142.4±11.6 pA to 82.2±5.2 pA; P<0.01) (examples in Fig 1A, B) and current injection @2X rheobase generated a significantly greater number of action potentials after UTP application (P<0.01; examples in Fig 1C, D). In addition, about one-half of the bladder neurons tested (10/21) exhibited sustained spontaneous activity after UTP application (example in Fig 1E).
Table 1.
Passive and active electrical properties of LS bladder neurons in the absence and presence of UTP or UTP and suramin application.
| no UTP (n=39) | UTP (n=21) | UTP+SUR (n=15) | ||
|---|---|---|---|---|
| Current Injection | RMP (mV) | −63.9±0.9 | −55.6±1.5*** | −64.9±2.8†† |
| Input Resistance (MΩ) | 242.0±16.5 | 224.9±23.0 | 259.4±17.1 | |
| Rheobase (pA) | 142.4±11.6 | 82.2±5.2** | 146.2±16.5†† | |
| AP threshold (mV) | −32.6±0.6 | −33.84±1.0 | −32.45±1.1 | |
| AP amplitude (mV) | 107.3±2.1 | 101.2±2.6 | 109.3±4.4 | |
| AP duration (ms) | 4.6±0.5 | 4.7±0.9 | 4.3±0.9 | |
| AP overshoot (ms) | 44.5±1.4 | 42.0±1.2 | 46.4±1.8 | |
| AP falling rate (mV/ms) | 19.3±1.5 | 18.6±1.6 | 20.5±1.9 | |
| AP frequency, 2X rheobase | 3.3±0.5 | 8.7±1.0** | 3.1±0.5††† | |
| Number of cells firing AP induced by UTP | 0/39 | 10/21** | 0/15†† | |
| ATP (30 μM) | Depolarization (mV) | 20.3±2.6 | 17.6±3.4 | na |
| Number of APs | 1.1±0.4 | 2.3±0.8 | na | |
| αβ-metATP (30 μM) | Depolarization (mV) | 13.3±1.7 | 12.94±1.1 | na |
| Number of APs | 0.8±0.5 | 1.1±0.6 | na |
P < 0.05;
P<0.01;
P<0.005 vs. no UTP.
P < 0.05;
P<0.01;
P<0.005 vs. UTP application.
na, not applicable
Notes: Input resistance was calculated according to the I/V relationship by injecting a series of hyperpolarizing pulses ranging from −300 to 0 pA (30 ms) in 50pA increments. To determine rheobase, a series of 10 ms current pulses in 20pA increments (1s apart) were injected. The maximum current (pA) that did not evoke an action potential was taken as rheobase. Action potential (AP) threshold was determined from the inflection point where membrane potential started to dramatically rise and the phase plot slope (the first derivative of membrane potential, dV/dt) reached 10 mV/ms (Naundorf et al., 2006). AP amplitude was measured from resting membrane potential (RMP) to the peak of the AP, AP overshoot was the amplitude from 0 mV to the peak of the AP, AP duration was determined at 50% of the AP amplitude between the rising and falling phases and the AP falling rate was the velocity of change in potential from the AP peak to RMP.
Figure 1.
UTP increases the excitability of bladder sensory neurons. Examples of responses of lumbosacral bladder neurons to current injection before and after UTP application are shown here. (A, B) The injected current required to evoke an action potential in bladder neurons was significantly reduced after UTP application from a mean142.4±11.6 pA to 82.2±5.2 pA (n=24; P<0.01). (C, D) Current injection at 2X rheobase (500ms) evoked a significantly greater number of APs after UTP application (8.7±1.0) relative to before UTP application (3.3±0.5; n=24, P<0.01). (E) Example of sustained AP firing during and after UTP application.
To further characterize the effect of UTP on bladder neuron excitability, we applied suramin (50μM), a broad P2 receptor antagonist that inhibits P2Y2 but not P2Y4, to bladder neurons (starting 2 min before and continuing during UTP application). Suramin blocked all of the UTP-produced increases in neuron excitability: reduction in membrane depolarization, decrease in rheobase and increase in number of action potentials in response to 2X rheobase current injection (all P<0.01; see Table 1). In addition, action potential (AP) discharges induced by UTP were completely inhibited by suramin (0/15). As UTP does not act as an ionotropic P2X receptor agonist (Burnstock, 2007;von Kugelgen, 2006), these findings suggest a key role for P2Y receptors in bladder sensory neuron excitability and hypersensitivity such as present in PBS/IC.
Effect of UTP on purinergic agonist-evoked responses
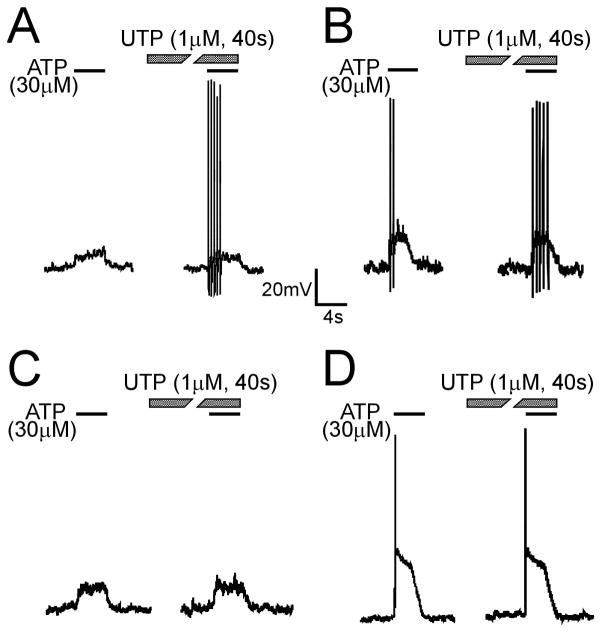
Because endogenous ATP could activate both P2X and P2Y receptors (principally P2Y2 and P2Y4 receptors), we hypothesized that there is an interaction between P2X and P2Y receptors in response to purinergic agonists. In current clamp mode, UTP application did not alter either the magnitude of membrane depolarization produced by ATP or α, β-met ATP or frequency of bladder neuron AP firing in response to these purinergic agonists (Table 1). However, purinergic responses of some bladder neurons (8/21) were sensitized by UTP. They either exhibited AP firing during application of UTP (Fig 2A) or an increased frequency of APs (Fig 2B) during purinergic agonist application (compared with responses before UTP application). Other neurons (13/21) did not exhibit significant changes (Fig 2C, D). Because the P2 antagonist suramin inhibits P2X responses (data not shown), membrane properties of bladder neurons in response to ATP or α,β-met ATP after suramin treatment could not be examined.
Figure 2.
Examples of the effect of UTP on lumbosacral bladder neuron responses to ATP. A fraction (8/21) of bladder neurons was sensitized by UTP, showing evoked AP firing (A) or increased frequency of APs (B) relative to the response to ATP before UTP application to the same cell. Other neurons (13/21) did not exhibit changes in response to ATP after UTP application (C, D).
In voltage clamp mode, the interaction between P2X and P2Y receptors in bladder neurons was examined by recording baseline inward currents in response to ATP or α, β-met ATP (30μM, 4 s). After a 2 min washout, ATP or α, β-met ATP application (4 s) was repeated during application of UTP (1μM) or nothing (control) for 40 s. After another 2 min washout, inward currents induced by a third exposure to ATP or α, β-met ATP were recorded. Approximately 10% of LS bladder neurons exhibiting ATP evoked-sustained currents do not respond to α, β-met ATP; these currents are presumably mediated by homomeric P2X2 receptors. Because no P2X2-selective agonist is available, we used ATP to examine P2X2 currents in the following experiments. ATP application was limited to a relative short duration (4 s) and immediately followed by a bath solution washout to minimize possible activation of P2Y receptors.
Because the effect of UTP was evaluated by comparing responses to the first and second application of purinergic agonist, we first calculated the kinetics of P2X current recovery from desensitization. The interval between agonist applications was increased stepwise and the current amplitude in response to the second application of agonist was normalized to that of the first application and plotted against time using a single-exponential fit. The sustained current evoked by ATP recovered very rapidly and did not show obvious decay (Fig S1A, B), whereas the slow current required around 40 s for complete recovery (Fig S1C, D). Based on the results of curve fitting (Fig S1E), the recovery time constant of the ATP-evoked slow current was 16.8 s (the time constant of the sustained current could not be determined). Similar recovery kinetics were seen for the slow current evoked by either α,β-met ATP or ATP (data not shown).
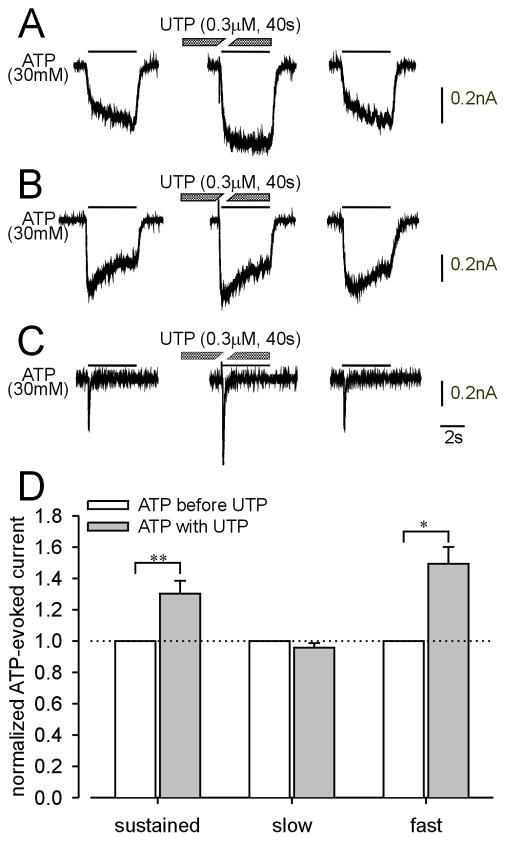
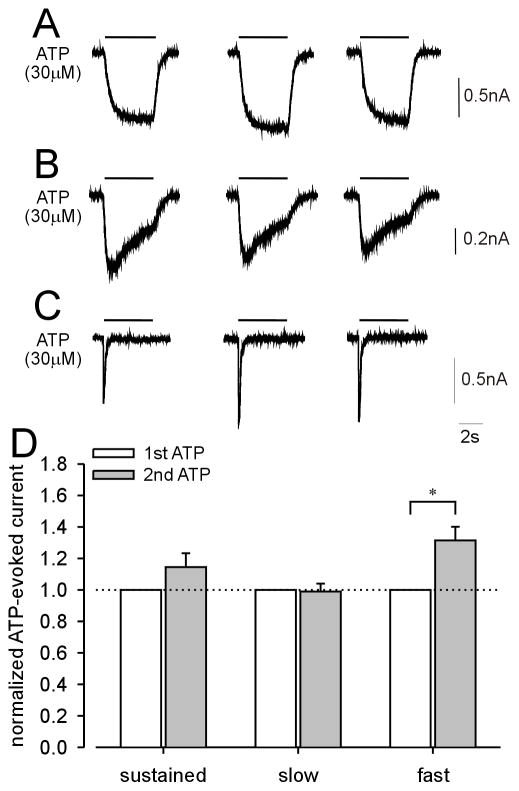
Next, we examined the effects of UTP on purinergic current kinetics. UTP application (0.3μM) for 40 s significantly facilitated ATP-evoked sustained (Fig 3A) and fast (Fig 3C) currents, but not slow currents (Fig 3B), suggesting that activation of metabotropic P2Y receptors enhances responses of homomeric P2X2 and homomeric P2X3 but not heteromeric P2X2/3 receptors in LS bladder neurons. Alternatively, this effect could instead be due to repetitive application of ATP because ATP is also an agonist at P2Y receptors, even though the application period of ATP (4 s) was relatively short. Control experiments (three repeated ATP applications as above but in the absence of UTP) revealed that ATP-evoked sustained (Fig 4A) and slow (Fig 4B) currents were not enhanced in the absence of UTP, whereas the ATP-evoked fast current was enhanced as it was in the presence of UTP (compare Figs 4C and 3C). Figs 3D and 4D summarize the effect of UTP and repetitive ATP application on ATP-evoked sustained and slow currents in LS bladder neurons and fast currents in TL bladder neurons. There was no significant effect of UTP application on α,β-met ATP-evoked slow currents in LS bladder neurons (data not shown).
Figure 3.
Examples of the effect of UTP on ATP-evoked currents in lumbosacral (A and B) and thoracolumbar (C) bladder neurons. UTP significantly facilitated sustained (A) and fast (C) currents but not slow currents (B). (D) Summary of the effect of UTP on ATP-evoked sustained (n=16), slow (n=24) and fast (n=5) currents.
Figure 4.
Examples of the effect of repeated ATP application without UTP on ATP-evoked currents in lumbosacral (LS) and thoracolumbar (TL) bladder neurons. No changes were observed in either sustained (A) or slow (B) currents after repeated ATP application in LS bladder neurons. ATP-evoked fast (C) current in TL bladder neurons show significant differences in response to repetitive ATP application. (D) Summary of repetitive ATP application on ATP-evoked sustained (n=11), slow (n=20) and fast (n=5) currents.
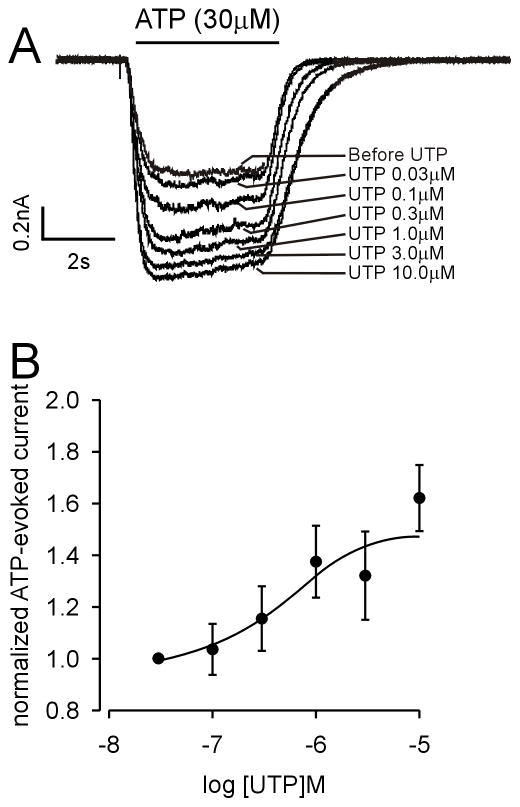
To more fully evaluate the facilitatory effect of UTP on ATP-evoked sustained currents, we determined the dose-response relationship for UTP, increasing UTP concentrations from 30nM to 10 μM. To eliminate the variation in current amplitude, we normalized P2X currents under different UTP concentrations to those associated with application of 30nM UTP. An example is given in Fig 5A; the effect of UTP on ATP-evoked sustained currents was concentration-dependent as summarized in Fig 5B (the UTP EC50 and 95% confidence interval was 0.52 μM [0.13–1.97μM]; n=7). Because there was no significant effect of UTP on slow currents, we did not undertake a dose-response study of UTP on slow currents evoked by ATP or α,β-met ATP. These outcomes confirm that UTP application facilitates homomeric P2X2-mediated sustained currents, supporting an important interaction between P2X and P2Y receptors in bladder neurons.
Figure 5.
Dose-response relationship of ATP evoked-sustained currents in response to UTP in lumbosacral bladder neurons. (A) Example of the effect of different concentrations of UTP on the ATP evoked-sustained current. (B) Normalized dose-response curve of ATP evoked-sustained currents in response to UTP. The EC50 and 95% confidence interval for UTP was 0.52 μM (0.13–1.97μM; n=7).
The metabotropic P2Y2 receptor mediates the effect of UTP
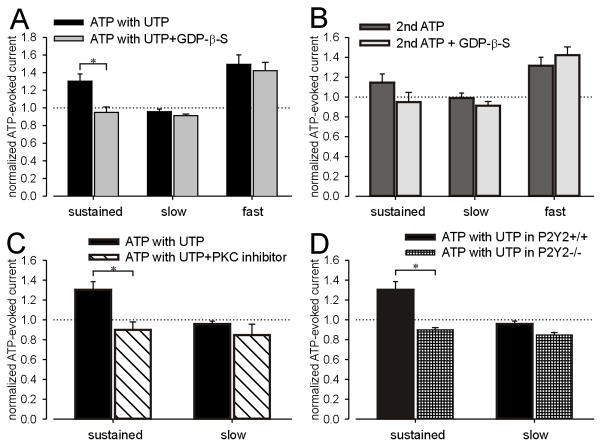
UTP is an agonist at P2Y2 and P2Y4 receptors, which are coupled to Gq and activate the phospholipase C (PLC)/protein kinase C (PKC) signaling pathway (von Kugelgen, 2006). To evaluate whether the facilitating effect of UTP described above is mediated by G-protein coupled receptors, we filled recording electrodes with an internal solution containing GDP-β-S (100μM), an inhibitor of all G-protein-mediated events in the cytoplasm, and repeated the ATP/UTP application protocol described above. As shown in Figs 6A and S2A, GDP-β-S blocked the facilitatory effect of UTP on the ATP-produced sustained, but not fast current. Repetitive ATP application in the absence of UTP also enhanced sustained and fast currents (see Fig 4D). The increase in fast current during a second application of ATP in the absence of UTP was not inhibited by GDP-β-S, whereas enhancement of the sustained current was absent in the presence of GDP-β-S (Figs 6B and S2B). These outcomes suggest that the enhancement of homomeric P2X2 sustained currents by UTP or ATP in bladder neurons is dependent on a G protein-coupled pathway, but the effect of UTP/ATP on homomeric P2X3 fast currents is not related to G protein-coupled receptors. This interpretation is consistent with previous studies reporting that P2X3 receptors can be sensitized by triphosphate nucleotides (ATP, UTP and GTP) via activation of the ecto-PKC phosphorylation site in the extracellular loop of P2X3 receptor subunit (Stanchev et al., 2006;Wirkner et al., 2005).
Figure 6.
The facilitatory effect of UTP on ATP-evoked sustained currents but not fast currents is mediated by the G protein-coupled P2Y2 receptor through a PKC-dependent pathway. Although the facilitatory effect of UTP on sustained currents was blocked by intracellular GDP-β-s (A), the increased fast currents induced by UTP/repeated ATP application were not inhibited by GDP-β-s (A and B). No significant change in either sustained or slow currents in response to repeated ATP application was found in bladder neurons containing GDP-β-s compared with controls (B). The myristoylated PKC inhibitor 20–28 (10μM) prevented the effect of UTP on the P2X2 sustained current in lumbosacral bladder neurons (C). No significant effect of UTP was observed on the slow current in the presence or absence of the PKC blocker in lumbosacral bladder neurons (C). Neurons from P2Y2 knockout mice did not show a facilitatory effect of UTP on sustained currents in lumbosacral bladder neurons compared with wild type bladder neurons (D).
Because P2Y2 and P2Y4 receptors are coupled to Gq and activate PKC, we used the myristoylated PKC inhibitor 20–28 (10μM) to determine whether the effect of UTP on P2X2 sustained currents was PKC-dependent. As shown in Figs 6C and S2C, facilitation of the sustained current by UTP was inhibited by the PKC blocker whereas the slow current amplitude was not affected. Most available P2Y receptor antagonists (e.g., PPADS, suramin, reactive blue, etc.) also block P2X receptors. Using the ATP/UTP application protocol described above and bladder neurons taken from P2Y2 knockout mice, we confirmed that P2Y2 mediates the facilitatory effect of UTP on P2X currents. Fig S2D shows significant decreases of both ATP-evoked sustained and slow currents after UTP application in LS bladder neurons from P2Y2 knockout mice. Comparison of sustained currents after UTP between wild type and knockout mice (Fig 6D) also revealed a significant facilitatory effect of UTP on purinergic currents in neurons from wild type but not P2Y2 knockout mice, indicating that P2Y2 is required for P2X2 receptor modulation by UTP.
P2X and P2Y receptor expression in bladder sensory neurons
Because the number of bladder neurons in LS and TL DRG is relatively small, we employed single cell RT-PCR and single cell nested PCR to examine P2X and P2Y receptor expression in bladder neurons. When cDNA was harvested after single cell RT-PCR, the mouse GAPDH gene was amplified by conventional PCR as an internal control. Only cells positive for the GAPDH amplicon were processed by nested PCR (cells negative for the GAPDH amplicon were thought to have an unsuccessful reverse transcription or a failed collection and were discarded).
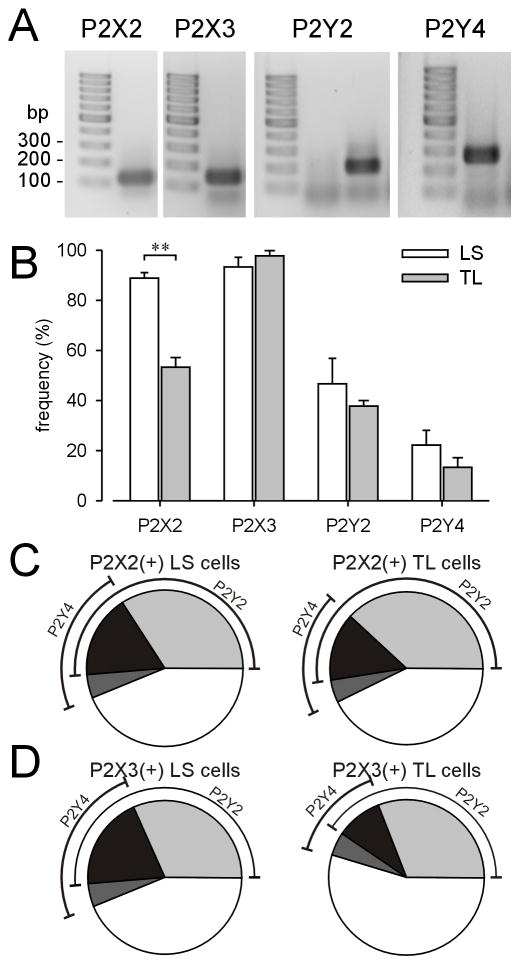
Fig 7A shows an example of positive single cell RT-PCR amplicons of P2X2, P2X3, P2Y2 and P2Y4 mRNA. Product length corresponded to the expected size of the targeted region. Fifteen LS and 15 TL bladder neurons per mouse (total 3 mice) were collected for single cell PCR. The expression pattern of P2X2 and P2X3 subunit transcripts has been discussed previously (Chen and Gebhart, 2010). The P2Y2 transcript was more abundant in LS (46.7±10.1%) and TL (37.8±2.3%) bladder neurons than the P2Y4 transcript (LS: 22.2±5.8%; TL: 13.3±3.8%). The frequencies of P2Y2 and P2Y4 transcript expression did not differ between LS and TL bladder neurons (Fig 7B). Consistent with a previous study (Chen and Gebhart, 2010), the P2X2 transcript was more frequently detected in naive LS bladder neurons (88.9%±2.2%) than in TL counterparts (53.3%±3.8%; P<0.01).
Figure 7.
Single cell nested RT-PCR of P2X2, P2X3, P2Y2 and P2Y4 receptor subunits in lumbosacral (LS) and thoracolumbar (TL) bladder neurons. (A) Examples of positive nested single cell PCR amplification of P2X and P2Y mRNAs. Product length corresponded with expected size. The frequency of P2X and P2Y transcription in bladder neurons is summarized in (B). The P2X2 subunit transcript was significantly more abundant in LS than TL bladder neurons (P<0.01). The percentage of cells expressing P2Y2 and P2Y4 transcripts in P2X2- (C) or P2X3- (D) positive neurons is illustrated in pie charts.
Fig 7C–D illustrates that the frequency of expression of P2Y2 and P2Y4 transcripts in P2X2 and P2X3 transcript-positive bladder neurons did not differ between the pelvic and splanchnic pathways. Specifically, in P2X2 transcript-positive bladder neurons (Fig 7C), 51% of LS and 52% of TL bladder neurons also expressed P2Y2 mRNA; fewer bladder neurons (22% of LS and 19% of TL) expressed P2Y4 mRNA. Seventeen percent of LS and 14% of TL bladder neurons expressing P2X2 mRNA were found to express both P2Y2 and P2Y4 transcripts. Similarly, in P2X3 transcript-positive bladder neurons (Fig 7D), 51% of LS and 40% of TL bladder neurons also expressed P2Y2 mRNA; fewer (24% of LS and 14% of TL) bladder neurons expressed P2Y4 mRNA. Twenty percent of LS and 10% of TL P2X3 mRNA-positive neurons also expressed P2Y2 and P2Y4 transcripts.
Discussion
The present study documents the presence of and interaction between P2X2 and P2Y2 receptors in mouse bladder neurons and their excitability, suggesting a previously unappreciated role for P2Y2 receptors in bladder hypersensitivity. UTP was shown to sensitize bladder sensory neurons and increase cell excitability by depolarizing resting membrane potential, decreasing rheobase and inducing action potentials. Using several experimental approaches, we established that UTP enhanced ATP-evoked sustained currents (mediated by homomeric P2X2 receptors) through the metabotropic P2Y2 receptor and the PKC pathway. This finding is significant inasmuch as mouse bladder inflammation increases four-fold the proportion of homomeric P2X2-mediated sustained currents in LS bladder neurons (Chen and Gebhart, 2010). In contrast, UTP enhancement of ATP-evoked fast currents (mediated by homomeric P2X3 receptors) was not mediated by a G protein-coupled receptor. Bladder neurons in both sensory pathways, the lumbar splanchnic and pelvic, were studied here; the interaction between P2X and P2Y receptors was restricted to the pelvic nerve pathway (i.e., LS bladder neurons).
Both ATP and UTP are released into the extracellular environment under normal conditions. Although the extracellular concentration of ATP/UTP is low in normal conditions, their release is greatly enhanced when cells are injured or respond to environmental changes (Cook and McCleskey, 2002;Lazarowski and Harden, 1999), suggesting that endogenous purines and pyrimidines act as signaling molecules in response to environmental stimuli and play a role in nociceptive transduction (Burnstock, 2006;North, 2002). ATP is released from urothelial cells in response to bladder distension (Ferguson et al., 1997;Wang et al., 2005). When administered intravesically, ATP induces bladder overactivity in conscious rats (Pandita and Andersson, 2002), suggesting a potential role of ATP in afferent control of bladder function acting on primary afferent terminals. In contrast, the effect of UTP on bladder function is not well documented. The present study shows that application of UTP sensitizes bladder sensory neurons via the P2Y2 receptor, which is consistent with other evidence that UTP and ATP excite sensory neurons and activate cutaneous afferent fibers by evoking sustained action potential firing or reducing mechanical response thresholds (Lechner and Lewin, 2009;Molliver et al., 2002;Stucky et al., 2004). The present results also indicate that activation of metabotropic P2Y receptors by relatively low concentrations and prolonged application of agonist (e.g., UTP), which does not induce an obvious inward current, can facilitate ionotropic P2X receptor-mediated responses to subsequent purinergic agonist administration. These results suggest that exposure to UTP/ATP in a low concentration may prime subsequent purinergic signaling via metabotropic P2Y receptors and elevate the sensitivity of ionotropic P2X receptors.
Studies using P2X2 knockout and P2X2/P2X3 double knockout mice indicate that both P2X2 and P2X3 play important roles in the transduction of nociceptive information from the bladder and in the regulation of normal bladder function (Cockayne et al., 2005). We previously characterized purinergic currents mediated by different P2X receptor subtypes in bladder afferents (Chen and Gebhart, 2010). In the present study, we found that only homomeric P2X2-mediated sustained currents in LS bladder sensory neurons were facilitated by UTP. The heteromeric P2X2/3-mediated slow currents in both LS and TL bladder sensory neurons were not significantly affected by UTP application. This suggests a selective effect of P2Y2 on the prolonged, sustained P2X2 current. Because the frequency of sustained homomeric P2X2 currents increases four fold in LS bladder neurons after cyclophosphamide-induced bladder inflammation in mice (Chen and Gebhart, 2010), the effect of P2Y activation on P2X2 signaling is likely to be dramatically amplified in pathological bladder disorders such as PBS/IC.
We also found that homomeric P2X3 fast currents in TL bladder neurons were enhanced by both UTP and ATP application, but this facilitatory effect could not be attenuated by a G-protein blocker. A previous report provides a comparable result in a study using human recombinant P2X3 receptors (Wirkner et al., 2005). These authors also documented that UTP facilitated homomeric P2X3 mediated-currents by a G protein-independent mechanism. The facilitation was, however, repressed by either mutation of an amino acid residue located in the extracellular PKC site or the blockade of ecto-PKC activation (Wirkner et al., 2005). Therefore, the present results support the conclusion that the effect of UTP on P2X signaling can arise by different mechanisms, suggesting a complex interaction between signaling molecules and their receptors during sensory transduction.
We examined the effect of UTP on both LS and TL bladder neurons because there is increasing evidence that pelvic and lumbar splanchnic sensory pathways innervating pelvic organs contribute differentially to visceral function and disorders (Brierley et al., 2005;Dang et al., 2005;Sugiura et al., 2005;Xu and Gebhart, 2008). We found an effect of UTP on both LS (P2X2 sustained current) and TL (P2X3 fast current) bladder neurons, suggesting a role in sensitization of both pelvic and lumbar splanchnic pathways innervating the bladder, consistent with increased activity of pelvic afferents by purinergic agonists in bladder-inflamed rat (Yu and de Groat, 2008) and sensitivity of bladder lumbar splanchnic afferents in response to chemical stimuli (Mitsui et al., 2001;Moss et al., 1997)
Among the P2Y receptor family, P2Y1, P2Y2, P2Y4, P2Y6 and P2Y11 are coupled to the Gq signaling pathway, which activates PLC and induces the release of intracellular Ca2+ stores and PKC activation; P2Y11 can also activate adenylate cyclase but is not expressed in rodents. On the other hand, P2Y12, P2Y13 and P2Y14 are coupled to Gi and inhibit adenylate cyclase (von Kugelgen, 2006). Additionally, P2Y1, P2Y2, P2Y4 and P2Y6 receptors are detected in peripheral sensory neurons located in dorsal root and nodose ganglia (Ruan and Burnstock, 2003;Gerevich and Illes, 2004). Because only P2Y2 and P2Y4 receptors are activated by UTP, we only examined expression of P2Y2 and P2Y4 in bladder neurons. A previous study on distribution of P2Y2 receptors using in situ hybridization demonstrated that P2Y2 mRNA is expressed by ~90% of small diameter rat DRG neurons and ~33% of large DRG neurons (Molliver et al., 2002); a similar study reported that 24% of rat DRG neurons expressed P2Y2 mRNA (Kobayashi et al., 2006). We found in the present study that ~50% of bladder sensory neurons, most of which are medium in size (Chen and Gebhart, 2010) but represent only about 5% of the neurons in their respective DRG, expressed P2Y2 mRNA. The P2Y4 receptor is less widely expressed than P2Y1 or P2Y2 receptors (Kobayashi et al., 2006;Ruan and Burnstock, 2003), which is consistent with the present finding that 10%-20% of bladder neurons express P2Y4 mRNA.
We confirmed that the effect of UTP on ATP-evoked P2X2 sustained currents is mediated by P2Y2 receptors because the facilitatory effect of UTP was absent in bladder neurons from P2Y2 knockout mice. Additional support for a role of P2Y2 over P2Y4 is that the effect of UTP was blocked by suramin, which is an antagonist at P2Y2 but not P2Y4 receptors (Ralevic and Burnstock, 1998). Other studies in P2Y2 knockout mice exhibit decreased calcium flux induced by UTP, reduced TRPV1 function and impaired thermal nociception (Malin et al., 2008). P2Y2 knockout mice also exhibit altered osmotic reabsorption of water, which influences volume and concentration of the urine stored in urinary bladder (Zhang et al., 2008). Interestingly, we found that UTP application had an inhibitory effect on both slow and sustained purinergic currents in P2Y2 knockout mice, suggesting that UTP may be involved in a P2Y2-independent mechanism to inhibit P2X signaling in the absence of P2Y2.
Under normal conditions, ATP released from urothelial cells in response to bladder distension activates P2X2 and P2X3 receptors in bladder afferents. Activation of P2X3-expressing sensory afferents contributes to bladder reflexes as well as sensation. In the present report, we describe a role for the ATP/UTP-gated receptor P2Y2 in the regulation of bladder neuron excitability and identify an interaction between P2X and P2Y receptors that leads to enhanced P2X signaling. Hyperexcitability and increased firing of bladder afferents are associated with urinary urgency, frequency and pain, all of which are features of PBS/IC (Nazif et al., 2007). These findings provide a new dimension to the role of nucleotide signaling in bladder function that may contribute to the pathophysiology of bladder inflammation and injury.
Supplementary Material
Figure S1. Recovery kinetics of ATP-evoked currents in lumbosacral (LS) bladder neurons. Examples of sustained (A, B) and slow (C, D) currents at different application intervals of ATP (30μM) are shown. Recovered current fractions were fitted with a single exponential (E). The time constant of the sustained current is not available because it completely recovered very rapidly. The slow current recovered with a time constant of 16.8 s.
Figure S2. The effect of intracellular GDP-β-S and a PKC inhibitor on responses of bladder neurons to UTP/ATP. The UTP/ATP effect was examined by comparing current amplitude evoked by ATP before and with UTP/repeated ATP application in the same bladder neuron. In the presence of GDP-β-S (100μM), enhancement by UTP (A) and repeated ATP (B) application of sustained (n=7 for both UTP and ATP) and slow (UTP: n=7; ATP n=9) currents in LS neurons was blocked, whereas enhancement of the fast current in TL neurons (n=5 for both UTP and ATP) was not affected. Similarly, in the presence of PKC inhibitor (10μM), no significant UTP effect on sustained (n=4) or slow (n=5) current was observed in LS neurons (C). Both ATP-evoked sustained (n=6) and slow (n=10) currents were inhibited by UTP in LS neurons from P2Y2 knockout mice (D).
Acknowledgments
This work was supported by NIH awards NS 35790 (GFG) and NS 56122 (DCM). We thank Michael Gold and Liming Fan for assistance with single cell RT-PCR, Joanne Hirt for secretarial assistance and Michael Burcham for preparation of the figures.
References
- Andersson KE. Bladder activation: afferent mechanisms. Urology. 2002;59:43–50. doi: 10.1016/s0090-4295(01)01637-5. [DOI] [PubMed] [Google Scholar]
- Applebaum AE, Vance WH, Coggeshall RE. Segmental localization of sensory cells that innervate the bladder. J Comp Neurol. 1980;192:203–209. doi: 10.1002/cne.901920202. [DOI] [PubMed] [Google Scholar]
- Birder LA, Barrick SR, Roppolo JR, Kanai AJ, de Groat WC, Kiss S, Buffington CA. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol Renal Physiol. 2003;285:F423–F429. doi: 10.1152/ajprenal.00056.2003. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Carter R, Jones W, III, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol. 2005;567:267–281. doi: 10.1113/jphysiol.2005.089714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkman RT. Chronic pelvic pain of bladder origin: epidemiology, pathogenesis and quality of life. J Reprod Med. 2004;49:225–229. [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling. Br J Pharmacol. 2006;147(Suppl 1):S172–S181. doi: 10.1038/sj.bjp.0706429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Chen X, Gebhart GF. Differential purinergic signaling in bladder sensory neurons of naïve and bladder inflamed mice. Pain. 2010 doi: 10.1016/j.pain.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne DA, Dunn PM, Zhong Y, Rong W, Hamilton SG, Knight GE, Ruan HZ, Ma B, Yip P, Nunn P, Mcmahon SB, Burnstock G, Ford AP. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol. 2005;567:621–639. doi: 10.1113/jphysiol.2005.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SP, McCleskey EW. Cell damage excites nociceptors through release of cytosolic ATP. Pain. 2002;95:41–47. doi: 10.1016/s0304-3959(01)00372-4. [DOI] [PubMed] [Google Scholar]
- Dang K, Bielefeldt K, Gebhart GF. Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons, and capsaicin. J Neurosci. 2005;25:3973–3984. doi: 10.1523/JNEUROSCI.5239-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn PM, Zhong Y, Burnstock G. P2X receptors in peripheral neurons. Prog Neurobiol. 2001;65:107–134. doi: 10.1016/s0301-0082(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes--a possible sensory mechanism? J Physiol. 1997;505 (Pt 2):503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerevich Z, Illes P. P2Y receptors and pain transmission. Purinergic Signal. 2004;1:3–10. doi: 10.1007/s11302-004-4740-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Yamanaka H, Dai Y, Obata K, Tokunaga A, Noguchi K. Neurons and glial cells differentially express P2Y receptor mRNAs in the rat dorsal root ganglion and spinal cord. J Comp Neurol. 2006;498:443–454. doi: 10.1002/cne.21066. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC. UTP as an extracellular signaling molecule. News Physiol Sci. 2001;16:1–5. doi: 10.1152/physiologyonline.2001.16.1.1. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Harden TK. Quantitation of extracellular UTP using a sensitive enzymatic assay. Br J Pharmacol. 1999;127:1272–1278. doi: 10.1038/sj.bjp.0702654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner SG, Lewin GR. Peripheral sensitisation of nociceptors via G-protein-dependent potentiation of mechanotransduction currents. J Physiol. 2009;587:3493–3503. doi: 10.1113/jphysiol.2009.175059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Davis BM, Koerber HR, Reynolds IJ, Albers KM, Molliver DC. Thermal nociception and TRPV1 function are attenuated in mice lacking the nucleotide receptor P2Y2. Pain. 2008;138:484–496. doi: 10.1016/j.pain.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui T, Kakizaki H, Matsuura S, Ameda K, Yoshioka M, Koyanagi T. Afferent Fibers of the Hypogastric Nerves Are Involved in the Facilitating Effects of Chemical Bladder Irritation in Rats. J Neurophysiol. 2001;86:2276–2284. doi: 10.1152/jn.2001.86.5.2276. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Cook SP, Carlsten JA, Wright DE, McCleskey EW. ATP and UTP excite sensory neurons and induce CREB phosphorylation through the metabotropic receptor, P2Y2. Eur J Neurosci. 2002;16:1850–1860. doi: 10.1046/j.1460-9568.2002.02253.x. [DOI] [PubMed] [Google Scholar]
- Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, Leon C, Suzuki N, Inoue K, Gachet C, Noguchi K, Tominaga M. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci. 2003;23:6058–6062. doi: 10.1523/JNEUROSCI.23-14-06058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss NG, Harrington WW, Tucker MS. Pressure, volume, and chemosensitivity in afferent innervation of urinary bladder in rats. Am J Physiol Regul Integr Comp Physiol. 1997;272:R695–R703. doi: 10.1152/ajpregu.1997.272.2.R695. [DOI] [PubMed] [Google Scholar]
- Nakamura F, Strittmatter SM. P2Y1 purinergic receptors in sensory neurons: contribution to touch-induced impulse generation. Proc Natl Acad Sci U S A. 1996;93:10465–10470. doi: 10.1073/pnas.93.19.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naundorf B, Wolf F, Volgushev M. Unique features of action potential initiation in cortical neurons. Nature. 2006;440:1060–1063. doi: 10.1038/nature04610. [DOI] [PubMed] [Google Scholar]
- Nazif O, Teichman JM, Gebhart GF. Neural upregulation in interstitial cystitis. Urology. 2007;69:24–33. doi: 10.1016/j.urology.2006.08.1108. [DOI] [PubMed] [Google Scholar]
- Nickel JC. Interstitial cystitis: a chronic pelvic pain syndrome. Med Clin North Am. 2004;88:467–81. xii. doi: 10.1016/S0025-7125(03)00151-2. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Ruan HZ, Burnstock G. Localisation of P2Y1 and P2Y4 receptors in dorsal root, nodose and trigeminal ganglia of the rat. Histochem Cell Biol. 2003;120:415–426. doi: 10.1007/s00418-003-0579-3. [DOI] [PubMed] [Google Scholar]
- Sanada M, Yasuda H, Omatsu-Kanbe M, Sango K, Isono T, Matsuura H, Kikkawa R. Increase in intracellular Ca(2+) and calcitonin gene-related peptide release through metabotropic P2Y receptors in rat dorsal root ganglion neurons. Neuroscience. 2002;111:413–422. doi: 10.1016/s0306-4522(02)00005-2. [DOI] [PubMed] [Google Scholar]
- Song Z, Vijayaraghavan S, Sladek CD. ATP increases intracellular calcium in supraoptic neurons by activation of both P2X and P2Y purinergic receptors. Am J Physiol Regul Integr Comp Physiol. 2007;292:R423–R431. doi: 10.1152/ajpregu.00495.2006. [DOI] [PubMed] [Google Scholar]
- Stanchev D, Flehmig G, Gerevich Z, Norenberg W, Dihazi H, Furst S, Eschrich K, Illes P, Wirkner K. Decrease of current responses at human recombinant P2X3 receptors after substitution by Asp of Ser/Thr residues in protein kinase C phosphorylation sites of their ecto-domains. Neurosci Lett. 2006;393:78–83. doi: 10.1016/j.neulet.2005.09.045. [DOI] [PubMed] [Google Scholar]
- Stucky CL, Medler KA, Molliver DC. The P2Y agonist UTP activates cutaneous afferent fibers. Pain. 2004;109:36–44. doi: 10.1016/j.pain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Dang K, Lamb K, Bielefeldt K, Gebhart GF. Acid-sensing properties in rat gastric sensory neurons from normal and ulcerated stomach. J Neurosci. 2005;25:2617–2627. doi: 10.1523/JNEUROSCI.2894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Keay S, De Deyne PG, Chai TC. Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. J Urol. 2001a;166:1951–1956. [PubMed] [Google Scholar]
- Sun Y, Keay S, Deyne P, Chai T. Stretch-activated release of adenosine triphosphate by bladder uroepithelia is augmented in interstitial cystitis. Urology. 2001b;57:131. doi: 10.1016/s0090-4295(01)01106-2. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci U S A. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura E, Fletcher TF, Bradley WE. Distribution of lumbar and sacral afferent axons in submucosa of cat urinary bladder. Anat Rec. 1975;183:579–587. doi: 10.1002/ar.1091830408. [DOI] [PubMed] [Google Scholar]
- Uemura E, Fletcher TF, Dirks VA, Bradley WE. Distribution of sacral afferent axons in cat urinary bladder. Am J Anat. 1973;136:305–313. doi: 10.1002/aja.1001360305. [DOI] [PubMed] [Google Scholar]
- Vera PL, Nadelhaft I. Afferent and sympathetic innervation of the dome and the base of the urinary bladder of the female rat. Brain Research Bulletin. 1992;29:651–658. doi: 10.1016/0361-9230(92)90134-j. [DOI] [PubMed] [Google Scholar]
- von Kugelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Wirkner K, Stanchev D, Koles L, Klebingat M, Dihazi H, Flehmig G, Vial C, Evans RJ, Furst S, Mager PP, Eschrich K, Illes P. Regulation of human recombinant P2X3 receptors by ecto-protein kinase C. J Neurosci. 2005;25:7734–7742. doi: 10.1523/JNEUROSCI.2028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Gebhart GF. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol. 2008;99:244–253. doi: 10.1152/jn.01049.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, de Groat WC. Sensitization of pelvic afferent nerves in the in vitro rat urinary bladder-pelvic nerve preparation by purinergic agonists and cyclophosphamide pretreatment. Am J Physiol Renal Physiol. 2008;294:F1146–F1156. doi: 10.1152/ajprenal.00592.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sands JM, Kohan DE, Nelson RD, Martin CF, Carlson NG, Kamerath CD, Ge Y, Klein JD, Kishore BK. Potential role of purinergic signaling in urinary concentration in inner medulla: insights from P2Y2 receptor gene knockout mice. Am J Physiol Renal Physiol. 2008;295:F1715–F1724. doi: 10.1152/ajprenal.90311.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Recovery kinetics of ATP-evoked currents in lumbosacral (LS) bladder neurons. Examples of sustained (A, B) and slow (C, D) currents at different application intervals of ATP (30μM) are shown. Recovered current fractions were fitted with a single exponential (E). The time constant of the sustained current is not available because it completely recovered very rapidly. The slow current recovered with a time constant of 16.8 s.
Figure S2. The effect of intracellular GDP-β-S and a PKC inhibitor on responses of bladder neurons to UTP/ATP. The UTP/ATP effect was examined by comparing current amplitude evoked by ATP before and with UTP/repeated ATP application in the same bladder neuron. In the presence of GDP-β-S (100μM), enhancement by UTP (A) and repeated ATP (B) application of sustained (n=7 for both UTP and ATP) and slow (UTP: n=7; ATP n=9) currents in LS neurons was blocked, whereas enhancement of the fast current in TL neurons (n=5 for both UTP and ATP) was not affected. Similarly, in the presence of PKC inhibitor (10μM), no significant UTP effect on sustained (n=4) or slow (n=5) current was observed in LS neurons (C). Both ATP-evoked sustained (n=6) and slow (n=10) currents were inhibited by UTP in LS neurons from P2Y2 knockout mice (D).