Abstract
Glucagon-like peptide 1 (GLP-1) is well known as a gut hormone, but also acts as a neuropeptide, produced in a discrete population of caudal brainstem neurons that project widely throughout the brain. GLP-1R receptors (GLP-1Rs) are expressed in many brain areas of relevance to energy balance, and stimulation of GLP-1Rs at many of these sites potently suppresses food intake. This review surveys the current evidence for GLP-1R effects on feeding behavior at a wide array of brain sites, and discusses behavioral and neurophysiological mechanisms for these effects identified thus far. Taken together, it is clear that GLP-1R activity in the brain can impact feeding by diverse means, including mediation of gastrointestinal satiation and/or satiety signaling, suppression of motivation for food reward, induction of nausea, and mediation of restraint stress-induced hypophagia, but many questions about the organization of this system remain.
1. Introduction
Glucagon-like peptide 1 (GLP-1) is well known as an incretin hormone, and the anorexigenic effects of GLP-1 itself as well as long-acting GLP-1 receptor (GLP-1R) agonists have been a prominent focus for the last several decades. Many of GLP-1’s physiological effects, and at least some of its behavioral effects are likely mediated by GLP-1Rs expressed in the periphery, but a large body of research has shown that GLP-1Rs in a number of different brain regions potently affect feeding and related behaviors. Two influential papers published in 1996 demonstrated that i.c.v. injection of GLP-1 strongly suppressed food intake in rats (Tang-Christensen et al., 1996; Turton et al., 1996), at doses far below those required for effects when GLP-1 is administered systemically, supporting the idea that the hypophagia was mediated by brain GLP-1Rs. These receptors are widely expressed, and found in many brain regions well known to influence food intake (Merchenthaler et al., 1999). Since those initial reports, a large body of literature has focused on identifying brain regions in which GLP-1Rs contribute to feeding control, determining whether endogenous GLP-1R activation plays a role, and unraveling the neurophysiological and behavioral mechanisms for the effects of GLP-1R stimulation on feeding (see Figure 1). For each location at which GLP-1Rs are now known to affect feeding, the answers to the subsequent questions have varied, and possible species and sex differences further complicate the picture. Some regions have been examined in considerably more depth than others, however, the available data support the broad conclusion that brain GLP-1Rs influence ingestive behavior in a number of ways, including effects on satiation and satiety, food reward, and mediation of stress and illness responses. This review will discuss work on GLP-1R action in brain regions from caudal to rostral, and consider what these findings tell us about the role of the brain GLP-1 system.
Figure 1.
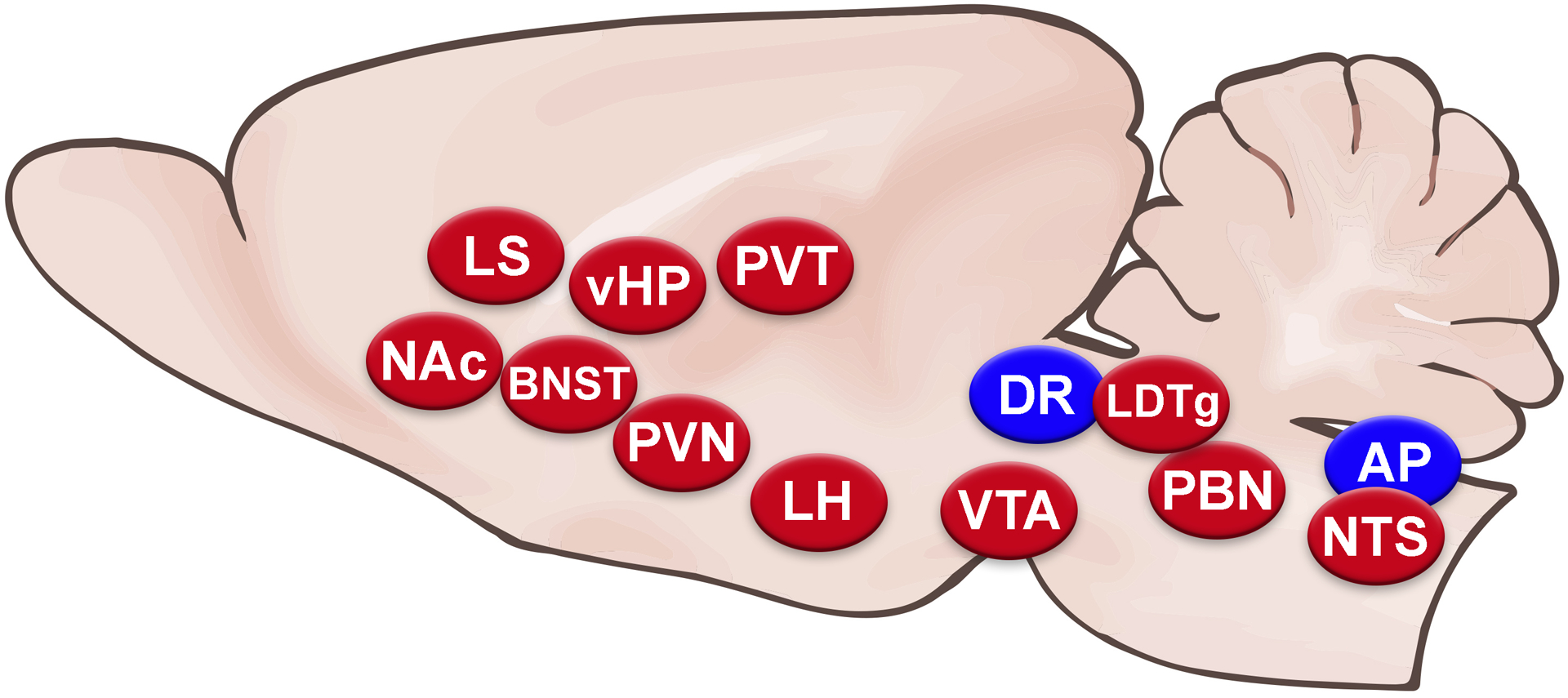
Sagittal rodent brain cartoon depicting brain nuclei where GLP-1Rs have been shown to affect food intake. Blue ovals represent those at which GLP-1R agonist application suppresses feeding, while red ovals represent those at which both 1) GLP-1R agonist application suppresses feeding and 2) antagonist injection or receptor knockdown increases feeding, providing evidence that endogenous GLP-1R stimulation at these sites plays a role in feeding control.
In discussing the effects of brain GLP-1R stimulation, this review assumes the endogenous source of ligand for these receptors to originate from caudal brainstem preproglucagon (PPG) neurons in most if not all cases. The reasons for this are discussed in depth in another review in this issue (Trapp & Brierley) but some of the evidence will be explained briefly here. First, active GLP-1 in the circulation is so rapidly metabolized to the inactive form that little to no active GLP-1 released by the intestine is likely to arrive in the brain, given the relatively low maximum concentrations this hormone reaches after meals (Holst & Deacon, 2005). Second, measurement of active GLP-1 content in the brain of mice confirms concentrations to be many-fold higher in brain tissue than in circulation, and only detectable in brain regions that receive PPG neuron projections (Holt et al., 2019). In that study, selective lesion of PPG neurons in the nucleus of the solitary tract (NTS) substantially reduced active GLP-1 measured in those brain regions that do receive PPG projections. These findings support the idea that the active GLP-1 found in the brain comes from PPG neurons and not from the periphery. It is also consistent with earlier pharmacologic data showing that in rats, blockade of brain GLP-1Rs by i.c.v delivery of the antagonist, Exendin (9-39) (Ex9), can block the ability of i.c.v.-injected GLP-1 to suppress feeding but not i.p. GLP-1 (Williams et al., 2009). This distinction suggests that even if some amount of intestine-derived GLP-1 does access the brain, it does not stimulate GLP-1Rs sufficiently to alter feeding. Therefore, the behaviorally relevant endogenous ligand of most brain GLP-1Rs must come from PPG neurons.
It has been proposed that peripheral GLP-1 stimulates neuronal GLP-1 release, but recent data refutes this and suggests that instead, these are two distinct systems. First, PPG neurons do not express GLP-1Rs and do not show an electrophysiologic response to GLP-1R agonists (Card, et al., 2018; Hisadome et al., 2010), so peripheral GLP-1 could not stimulate PPG neuron directly. Instead, some have suggested that peripheral GLP-1 activates vagal afferents which then excite PPG neurons (e.g., Müller et al., 2019). However, Brierley and colleagues (2021) have presented compelling evidence that this is not the case. In that study, they showed that there is little innervation of NTS PPG neurons by GLP-1R-expressing vagal afferents. They also reported that NTS PPG neurons received very little innervation from GLP-1R-expressing neurons in the area postrema (AP), a circumventricular organ where circulating GLP-1 or systemic long-acting GLP-1R agonists could act, eliminating a second possible mechanism for peripheral-central interaction. Together, the evidence suggests that the peripheral and central GLP-1 systems suppress feeding behavior independently.
Degradation-resistant GLP-1R agonists are now in use in humans for treatment of type 2 diabetes and obesity (Grill, 2020; Aroda, 2018), and whether these long-acting GLP-1R agonist therapies suppress food intake through CNS GLP-1Rs is a separate question (also discussed in the review by Trapp & Brierley). When labeled versions of these compounds are delivered peripherally, they can be observed to access circumventricular organs and some hypothalamic nuclei (Gabery et al. 2020; Secher et al. 2014). Blockade or knockout of CNS GLP-1Rs can attenuate the hypophagic effect of peripheral degradation-resistant agonist treatment (Kanoski et al., 2011; Sisley et al. 2014), suggesting that brain GLP-1R stimulation plays some role in the feeding response. Similar to speculation about mechanism of action of intestine-derived GLP-1, it has been suggested that these degradation-resistant GLP-1R agonists could engage the central GLP-1 system by activating PPG neurons, but this does not appear to be the case. As noted above, NTS PPG neurons do not express GLP-1Rs, so a direct effect of these drugs on PPG neurons is not possible, and neither Exendin-4 (Ex-4) nor semaglutide induce c-fos, a measure of neuronal activation, in PPG neurons (Brierley et al., 2021; Holt et al., 2020). Therefore, it seems that if long-acting GLP-1R agonists work even in part through brain GLP-1Rs, they are likely doing so by accessing some of these receptors directly. Further research is needed to determine the most relevant brain locations, identify specific cell types involved, and determine whether central pathways recruited by long-acting GLP-1R agonists interact with those engaged by PPG neurons and neuronal GLP-1 action.
2. Hindbrain & Midbrain Regions
2.1. Nucleus of the Solitary Tract
Fourth-i.c.v application of GLP-1 agonists effectively suppress feeding (Hayes et al., 2008), and because of the rostro-caudal flow of CSF in the ventricular system, we can assume that these effects are mediated by receptors in the caudal brainstem. GLP-1Rs are expressed at high density in the nucleus of the solitary tract (NTS) (Cork et al., 2015; Merchenthaler et al., 1999), and this nucleus has an established role in the control of food intake (Grill & Hayes, 2012), so it was a natural candidate for investigation. Multiple studies have shown that injection of the degradation-resistant GLP-1R agonist Ex4 directly into the NTS suppresses food intake (Alhadeff & Grill, 2014; Hayes et al., 2011; Richard et al., 2015), however, this could be a pharmacologic effect and does not necessarily reflect a physiological role for these receptors. Loss-of function studies resolve this. Injection of the antagonist, Ex9, directly into the caudal NTS, at a dose subthreshold for effect when delivered to the ventricle, increases food intake in rats, supporting the hypothesis that endogenous stimulation of NTS GLP-1Rs limits feeding (Hayes et al., 2009). Taking a different loss-of-function approach in a subsequent study, Alhadeff and colleagues (2017) knocked down GLP-1R expression in the NTS with adeno-associated virus (AAV) delivery of shRNA. This manipulation caused a small but significant increase in food intake in rats 2 weeks after AAV delivery relative to subjects that received a control AAV. Together, these findings offer strong support for a physiological role of NTS GLP-1Rs in suppressing food intake.
What are the behavioral mechanisms through which NTS GLP-1Rs exert this effect? As the first stop in the brain for vagal afferents carrying information about meal-related gastrointestinal (GI) signals, the NTS is positioned to play a prominent role in satiation (the process of meal termination) and satiety (the continued inhibition of feeding after a meal has ended). NTS PPG neurons, themselves, are activated by vagal afferents and signals including the prototypical satiation signal cholecystokinin (CCK) (Hisadome et al., 2010), so it is reasonable to hypothesize that NTS GLP-1Rs play a mediatory role in satiation and/or satiety, and the evidence bears this out. Knock-down of NTS GLP-1R expression was shown to selectively increase meal size, suggesting that these receptors normally promote satiation (Alhadeff et al., 2017b). Hayes and colleagues (2009) asked whether caudal brainstem GLP-1Rs mediate the intake-suppressive effects of either intra-duodenal nutrient infusion or gastric balloon distention, which both strongly promote satiation and satiety. In that study, 4th-i.c.v. pretreatment with Ex9 attenuated the effect of gastric distention but not the duodenal nutrient load. While this pharmacologic manipulation was not restricted to the NTS, we can assume that 4th-i.c.v. delivery effectively blocked NTS GLP-1Rs, and therefore these data suggest that NTS GLP-1Rs mediate the effects of some, though not all, GI negative feedback signals.
Nausea is a well-known side effect of GLP-1 agonists in humans (Bettge et al., 2017; also discussed in another review in this issue, Borner et al.), and evidence of visceral illness as a consequence of GLP-1R stimulation can be observed in rodent models, as well, using approaches including conditioned taste aversion (CTA) and measurement of pica. For any treatment that seems to promote satiation or satiety, but especially for one such as this, it is important to ask whether that intake suppression may be a result of nausea or aversion rather than a normal physiological process. This has been investigated for NTS GLP-1Rs, and the results are inconsistent. Medial NTS injection of Ex4 has been shown to induce pica and support the formation of a CTA (Kanoski et al., 2012), however, others have shown that at least some doses of Ex4 delivered to the NTS can suppress food intake without inducing pica (Alhadeff & Grill, 2014; Richard et al., 2015). Therefore, while NTS GLP-1Rs may be involved in mediation of visceral illness responses, this can be uncoupled from food intake effects.
While the NTS has traditionally been considered to have a role in homeostatic control of feeding, responding to meal-related GI signals and adiposity signals such as leptin (Grill & Hayes, 2012), this nucleus has direct and poly-synaptic links to brain regions commonly considered to mediate reward and motivation. More recent data have shown that indeed, the NTS can influence food reward, and GLP-1Rs in the NTS impact ingestive behavior through this mechanism. One approach to assessing food reward focuses on motivation by examining operant responding for a palatable food reinforcer on a progressive ratio (PR) schedule. The ratio requirement increases with each subsequent reinforcer, and the breakpoint – the last completed ratio before the subject “gives up” – is taken as a measure of motivation. A second common approach is to assess conditioned place preference (CPP), in which subjects are trained to associate a specific location with palatable food. After training, preference for that vs. a location that has never been paired with food is evaluated. CPP involves associative learning, memory, and motivated approach behavior, so interpretation is not always straightforward, but it can be instructive to compare results obtained in this task with others. Two groups have shown that NTS activation of GLP-1Rs can alter behavior in both of these tests; NTS injection of Ex4 suppresses PR responding for sucrose pellets as well as expression of a high-fat palatable food-induced CPP (Alhadeff & Grill, 2014; Richard et al., 2015). Importantly, the endogenous activation of NTS GLP-1Rs seems to impact food reward, because shRNA-mediated knockdown of GLP-1Rs in the NTS increased PR responding for sucrose (Alhadeff et al., 2017).
Several mechanisms for these caudal hindbrain GLP-1R effects on feeding have been identified. The GLP-1R is a GPCR, and a great deal of investigation of GLP-1R signaling done in pancreatic cells shows that it couples primarily to Gαs and increases cAMP (Pabreja et al., 2014). It is perhaps unsurprising, then, that in the hindbrain, GLP-1R agonist application increases PKA activity. In a series of experiments using a variety of kinase inhibitors and promoters, Hayes and colleagues (2011) showed that the intake-suppressive effects of hindbrain GLP-1R activation are mediated through PKA-induced suppression of AMPK and activation of MAPK, and a subsequent study from this group showed that GLP-1R-induced PI3K/PIP3-induced translocation of Akt to the membrane plays a role as well (Rupprecht et al., 2013). The neurochemical phenotypes of some of the NTS neurons involved in mediating GLP-1R effects have also been identified. Richard and colleagues (2015) showed that PPG neuron fibers have close appositions with noradrenergic A2 neurons in the NTS, many of which project to the ventral tegmental area (VTA), the major source of dopamine in the mesolimbic reward pathway, and that NTS application of Ex4 potently increases tyrosine hydroxylase and D2 receptor expression in the VTA, possibly through this pathway. Recently, NTS GABA neurons were shown to express GLP-1Rs, and acute silencing of these neurons via a selectively expressed inhibitory Designer Receptor Exclusively Activated by Designer Drugs (DREADD) approach attenuated the effects of GLP-1R agonist treatment (Fortin et al., 2020). GLP-1Rs are not exclusively expressed on neurons, and in fact, there is evidence that GLP-1R signaling in NTS astrocytes impacts feeding, as well (Reiner et al., 2016; covered in depth in another review in this issue, Hayes & Stein, 2021).
2.2. Area Postrema
Neighboring the NTS, neurons of the area postrema (AP) also express GLP-1Rs (Cork et al., 2015; Merchenthaler et al., 1999), and this nucleus has a role in mediating the effects of satiation and satiety signals as well. It is therefore a reasonable candidate for mediation of feeding effects of GLP-1R stimulation, and as a circumventricular organ, has been suggested as a potential mediatory site for peripheral GLP-1 agonist treatment. This is a difficult nucleus to isolate in pharmacology studies, because its location and small size render attempts at injection directly into the AP likely deliver drug to the NTS, as well. Indirect evidence of AP mediation of effects comes from studies in the rat showing that peripheral degradation-resistant agonist administration induces c-Fos in AP neurons, many of which are catecholaminergic and project to brain areas associated with autonomic control (Yamamoto et al., 2003). This could be a secondary effect, not necessarily mediated by direct stimulation of GLP-1Rs on AP neurons, but electrophysiologic data in mice demonstrate that GLP-1 directly activates AP neurons through AC/cAMP pathways (Kawatani et al., 2018). Few studies have attempted to examine the role of AP GLP-1Rs in food intake control, and data from experiments focusing on the AP are mixed, rendering it difficult to draw a firm conclusion. In one study, lesion of the AP attenuated the feeding-suppressive effects of hepatic portal vein GLP-1 infusion (Punjabi et al., 2014), however, in another report, AP lesion did not attenuate the effect of systemic liraglutide, the long-acting GLP-1 agonist (Fortin et al., 2020). It may be that the AP has a role in effects of GLP-1 itself, but that the effects of degradation-resistant agonists are mediated elsewhere. Recent work from Zhang and colleagues (2020) suggests that GLP-1R-expressing AP neurons are one of several AP cell types that mediate nausea.
2.3. Parabrachial nucleus
GLP-1R expression in the parabrachial nucleus (PBN) (Cork et al., 2015; Merchenthaler et al., 1999) as well as PPG neuron projections to this area (Alhadeff et al., 2014; Richard et al., 2014) in both rats and mice have made this nucleus another target for investigation. The PBN is a complex nucleus with many subdivisions that play a role in a wide variety of physiological and behavioral functions. Of most relevance here, it is a key node for integrating information about GI signals and taste, receiving projections from both rostral and caudal NTS (Baird et al., 2001). The lateral PBN (lPBN) in particular has been examined as a site for GLP-1R effects on feeding, and it has been shown that indeed, delivery of ventricle-subthreshold doses of Ex4 at this location can suppress intake of chow, high-fat diet, chocolate pellets, and saccharine, without inducing pica (Alhadeff et al., 2014; Richard et al., 2014). Conversely, in these reports, blockade of lPBN GLP1-Rs with site-specific Ex9 injection increased chow and high-fat diet intake, showing that endogenous activation of these receptors plays a role in limiting ingestion. The behavioral mechanisms for these effects on feeding have not been as thoroughly investigated as for the NTS, so it is unclear whether lPBN GLP-1Rs may be involved in satiation or satiety. However, Alhadeff and colleagues (2014) showed that lPBN Ex4 administration reduced PR responding for chocolate pellets, suggesting that modulation of motivation for food may mediate the effects of these GLP-1Rs on feeding behavior. Richard and colleagues (2014) investigated potential neurophysiological mechanisms for these effects, and reported that bath application of Ex4 to brain slices increased the firing rate of lPBN neurons. In that study, lateral i.c.v. injection of Ex4 increased CGRP and IL-6 mRNA expression in the lPBN, effects that could be indirect given that this manipulation would have accessed GLP-1Rs throughout the brain. However, they also reported that PPG neuron fibers were observed in close apposition to CGRP neurons in the lPBN, so it is plausible that these neurons mediate at least some of the behavioral effects observed.
2.4. Dorsal Raphe
Brain 5-HT neurons and 5-HT receptors have long been thought to play a role in energy balance based in part on the appetite suppressive effects of 5-HT agonists and reuptake inhibitors in both humans and rodent models (Berger et al., 2009; Burke & Heisler, 2015). The DR is home to the largest 5-HT neuron population, which projects widely throughout the brain into many other nuclei known to influence ingestive behavior. GLP-1Rs are expressed on 5-HT neurons in the DR (Cork et al., 2015; Merchenthaler et al., 1999), though PPG neuron projections to this location are not dense and vary by sub-region (Anderberg et al., 2017; Llewellyn-Smith et al., 2013). Limited data are available to support a physiological role of DR GLP-1Rs in ingestive behavior, but one report showed that injection of GLP-1 or Ex4 into the DR of rats strongly suppressed feeding (Anderberg et al., 2017). Ex4 application to brain slices increased excitability of DR 5-HT neurons, suggesting a potential neurophysiological mechanism for these effects. Without more detailed analysis of the feeding effects, it is difficult to speculate on the underlying behavioral mechanisms for this intake suppression, and malaise has not been ruled out for this site. It is also unclear whether the reported effects reflect a physiological role for these receptors or if this is entirely pharmacologic in nature.
2.5. Lateral Dorsal Tegmental Area
The lateral dorsal tegmental area (LDTg) is not a brain area traditionally associated with control of food intake, but rather has been implicated in effects of stimulant drugs of abuse (e.g., methamphetamine, nicotine) through modulation of the dopamine neurons of the ventral tegmental area (VTA) (Dobbs & Cunningham, 2014; Lodge & Grace, 2006). GLP-1Rs are expressed in this nucleus (Merchenthaler et al., 1999) and Reiner and colleagues (2018) showed that in the rat, the LDTg does receive projections from a small number of PPG neurons. They also reported that intra-LDTg Ex4 injection suppresses feeding by selective reduction in meal size, with no induction of pica (Reiner et al., 2018). Importantly, blockade of LDTg GLP-1Rs attenuated the food intake suppression normally induced by a nutrient preload. Together, these results suggest that endogenous stimulation of these LDTg receptors has a role in mediating the satiation response to GI signals. Based on the connectivity of this nucleus with the mesolimbic reward pathway, it would be reasonable to hypothesis an effect on motivation for palatable food. However, one study has shown that intra-LDTg injection of Ex4 can suppress cocaine-seeking operant responding while the same doses have no effect on sucrose-seeking behavior (Hernandez et al., 2020). There may be a role for LDTg GLP-1Rs in a different aspect of food reward, or this dissociation may reflect different roles for GLP-1 action in this nucleus in food and drug reward.
2.6. Ventral Tegmental Area
As the location of the dopamine neurons whose projections form the mesolimbic reward pathway, the VTA is well known for its role in reward and motivation. This nucleus influences responses to drugs of abuse but also natural rewards, including palatable food (Meye & Adan, 2014). In rats, PPG neurons project to the VTA (Alhadeff et al., 2012; Rinaman, 2010) and a moderate level of GLP-1R expression is observed there (Merchenthaler et al., 1999). Intra-VTA injection of Ex4, at low doses, has been shown to suppress intake of chow, high-fat diet, and sucrose, without inducing pica (Alhadeff et al., 2012; Dickson et al., 2012). The finding that blockade of GLP-1Rs in the VTA increases high-fat diet intake (Alhadeff et al., 2012) supports the idea that this is a physiological, and not just a pharmacologic effect, of these receptors. Consistent with the role of the VTA in motivation, activation of GLP-1Rs here suppressed PR responding for sucrose (Dickson et al., 2012). Meal pattern analysis in rats consuming high-fat food showed that VTA Ex4 injection suppresses feeding primarily through reduction in meal size, supporting a possible role for these receptors in satiation (Mietlicki-Baase et al., 2013). The same paper described electrophysiological evidence that GLP-1Rs in the VTA act pre-synaptically via AMPA receptors to increase glutamatergic transmission to VTA dopamine neurons. A recent paper examining the effect of i.c.v. Ex4 administration supports the idea that GLP-1R stimulation modulates activity of VTA dopamine neurons, specifically by suppressing their activity in response to palatable food-associated cues (Konanur et al., 2020) Given that i.c.v. delivery would access GLP-1Rs throughout the brain, this is not necessarily a direct effect of VTA GLP-1Rs, and this result is not consistent with the previous finding that GLP-1R stimulation targeted exclusively to this site increases excitatory drive to VTA dopamine neurons, so further research is needed to elucidate the mechanisms at play here.
3. Hypothalamic & Thalamic Regions
3.1. Hypothalamic Nuclei
The hypothalamus has naturally been a target of considerable investigation, because of its longstanding importance in energy balance and because GLP-1Rs are expressed at high density in many hypothalamic nuclei (Cork et al., 2015; Merchenthaler et al., 1999). Because another review in this issue covers hypothalamic GLP-1 action (Kabahizi et al.), this review will discuss only some of this work in brief.
The paraventricular nucleus (PVN) was one of the first hypothalamic nuclei to be targeted, likely because GLP-1Rs are expressed at high density here, and PPG neurons strongly innervate this nucleus (Cork et al., 2015; Gu et al., 2013; Merchenthaler et al., 1999; Rinaman, 2010). Indeed, intra-PVN injection of GLP-1 suppresses food intake in rats, at doses that do not support the formation of a conditioned taste aversion (McMahon & Wellman, 1998), and PVN injection of Ex9 produces a small but significant elevation of food intake (Katsurada et al., 2014), supporting a role for endogenous activation of GLP-1Rs at this site. The evidence in mouse models is mixed. Selective knockout of GLP-1R in Sim1 neurons, mainly found in the PVN, has no impact on food intake (Burmeister et al., 2017a). That lack of effect could be due to developmental compensation, because Liu and colleagues (2017) showed that virally-mediated knockdown of PVN GLP-1Rs in the adult mouse increases daily food intake. PPG neurons are strongly activated by stress (Rinaman, 1999; Terrill et al., 2019), so a number of studies have examined brain GLP-1R mediation of stress responses. The PVN is an obvious candidate for such investigations, and indeed, knockout of GLP-1Rs in Sim1 neurons attenuates a number of physiological responses to stress (Ghosal et al., 2017). In that study, mice lacking GLP-1Rs in Sim1 neurons did not show the typical body weight loss induced by chronic stress, suggesting that they may be protected from stress-induced anorexia (Ghosal et al., 2017). Corticotrophin releasing hormone (CRH) neurons of the PVN were identified as targets of GLP-1 action decades ago (Larsen et al., 1997). More recent work shows that GLP-1R stimulation in the PVN increases excitatory synaptic strength in CRH neurons, through a PKA-mediated increase in AMPA receptor trafficking to the membrane (Liu et al., 2017), suggesting this as a possible mechanism for the behavioral effects of GLP-1Rs in the PVN. This would be consistent with an earlier finding that in rats that were deprived of food for 24 hours before the food intake test, 3rd-i.c.v. pretreatment with the non-selective CRH receptor antagonist, α-helical CRH, completely prevented the usual intake suppression seen after 3rd-i.c.v. GLP-1 administration (Gotoh et al., 2005).
The lateral hypothalamus (LH) is another candidate mediator for GLP-1R action on ingestive behavior, especially compelling because of its well established involvement in food intake as well as reward and motivated behavior in general (Stuber & Wise, 2016). Research on the effects of GLP-1Rs in the lateral hypothalamus (LH) suggests complex sex differences in brain GLP-1 function, which have largely gone unstudied. Very few reports on brain GLP-1R effects have used females or compared sexes. Evidence of possible sex differences for brain GLP-1R effects were originally shown with i.c.v. agonist administration: estrogens increased sensitivity to lateral-i.c.v. GLP-1 effects on chow intake in female rats (Maske et al., 2017) and female rats were more sensitive to lateral-i.c.v. Ex4-induced suppression in motivation for food (Richard et al., 2016). In the LH, GLP-1R agonist injection suppressed motivation for food as measured by PR responding for sucrose, but surprisingly, female rats required a higher dose to show this effect than males (López-Ferreras et al., 2018). Moreover, loss of LH GLP-1R function through antagonist injection or shRNA-mediated knockdown increased PR responding for food in males but had no effect in females (López-Ferreras et al., 2018).
Paraventricular Nucleus of the Thalamus (PVT):
The PVT has been implicated in arousal, stress responses, and reward processes including motivation for food. In rats and mice, PPG neurons project to the PVT, and neurons here express GLP-1Rs (Cork et al., 2015; Gu et al., 2013; Llewellyn-Smith et al., 2013; Merchenthaler et al., 1999; Rinaman, 2010). At this location, GLP-1R agonist injection suppresses chow and high-fat diet intake in rats, without inducing pica (Ong et al., 2017). Blockade of PVT GLP-1Rs increased food intake primarily through an increase in meal size (Ong et al., 2017). Furthermore, intra-PVT Ex4 injection suppressed PR responding for sucrose and also blocked the expression of a conditioned place preference induced by high-fat diet (Ong et al., 2017), suggesting a role for these GLP-1Rs in in food reward. Interestingly, in evaluating potential downstream mechanisms for PVT GLP-1R effects, Ong and colleagues (2017) determined that GLP-1R agonist application to brain slices had an inhibitory effect on neurons projecting from PVT to the nucleus accumbens (NAc), and this was mediated both pre- and post-synaptically. The GLP-1R is often considered an excitatory GPCR because of its established signaling through Gαs, but these and other data (Cork et al., 2015; Hällbrink et al., 2001; Williams et al., 2018) suggest that GLP-1R signaling should be considered more heterogeneous.
4. Limbic & other Forebrain Regions
4.1. Bed Nucleus of the Stria Terminalis
The BNST, part of the extended amygdala, is a complex nucleus that has been implicated in a wide array of behaviors including stress responses, drug and alcohol seeking, appetite, and mating (Ch’ng et al., 2018). In both rats and mice, PPG neurons project to the BNST and GLP-1Rs are expressed here (Cork et al., 2015; Merchenthaler et al., 1999; Rinaman, 2010; Williams et al., 2018). In mice, intra-BNST delivery of GLP-1 suppressed, and Ex9 increased chow intake (Williams et al., 2018). In that study, when mice were maintained on high-fat diet, the effect of intra-BNST GLP-1 was attenuated and Ex9 no longer had any effect. This contrasts with the ability of these treatments to strongly impact high-fat diet intake when targeted to many other brain areas described here, and these findings suggest that modulation of BNST GLP-1R function could contribute to the overconsumption typically seen on high-fat diets.
Consistent with its known involvement in stress responses, BNST blockade of GLP-1Rs can attenuate acute restraint stress-induced hypophagia in the mouse (Williams et al., 2018), and data suggest a similar function of BNST GLP-1Rs in the rat. Zheng and colleagues (2019) reduced GLP-1R expression in the anterolateral BNST of adult rats using virally-delivered shRNA, and found that this attenuated the hypophagia induced by the mild stressors of novelty and elevated open platform exposure, and also attenuated some but not all other behavioral stress responses tested. In that same study, rats with BNST GLP-1R knockdown showed no alteration in daily home cage food intake, suggesting that at least in rats, BNST GLP-1Rs may not have a physiological role in feeding under non-stressed conditions. It is plausible that the main behavioral role of GLP-1Rs in this nucleus is in responding to stress, including stress-related modulation of feeding.
Cellular mechanisms for these GLP-R effects in BNST neurons have not been fully elucidated, but we have some clues. In rat anterolateral BNST, GLP-1Rs were found to be expressed by a subset of GABA neurons, and in the dorsal part of the anterolateral BNST, GLP-1R mRNA was co-expressed with CRH mRNA, suggesting these cell types as candidate mediators of GLP-1’s effects in this nucleus (Zheng et al., 2019). Electrophysiological evidence suggests heterogeneous mechanisms; in slice preparations, 40% of identified GLP-1R-expressing neurons in mouse BNST had an excitatory response to bath application of GLP-1, while 60% of these cells were inhibited (Williams et al., 2018). Direction of response did not correlate with location within the BNST or other electrophysiological properties of the neurons, but interestingly, was correlated with response to dopamine. In that study, the projections of BNST GLP-1R-expressing neurons were assessed with virally-mediated cell type-specific anterograde tracing, and fibers from these neurons were observed in many established BNST target sites, including the lateral septum (LS), PVN, and central nucleus of the amygdala (CeA). Which of these projections, if any, mediate the feeding behavioral effects of BNST GLP-1R activation is as yet unknown.
4.2. Nucleus Accumbens
The NAc is the recipient of the VTA dopamine neuron projections that form the mesolimbic reward pathway, and this nucleus has a known role in controlling motivation for food and intake of palatable foods (Kelley, 2004). There is documented GLP-1R expression in the caudal part of the NAc in both rats and mice, though lower density than many other locations (Cork et al., 2015; Merchenthaler et al., 1999). Around the same time VTA GLP1-R effects were first investigated, several labs examined NAc effects, as well, finding that intra-NAc administration of GLP-1 or Ex4 could suppress intake of chow, high-fat diet and sucrose (Alhadeff et al., 2012; Dickson et al., 2012; Dossat et al., 2011), without inducing pica (Alhadeff et al., 2012). Increased intake after Ex9 treatment suggests that this is a function of endogenous GLP-1R stimulation in the NAc core subregion in particular (Alhadeff et al., 2012; Dossat et al., 2011). Notably, the contribution of NAc GLP-1Rs may differ in mice, because intra-NAc injection of doses of Ex9 that increased feeding when delivered to other brain nuclei, had no effect in mice (Williams et al., 2018).
Further analysis of the behavioral mechanisms for GLP-1R action in the NAc suggest involvement in mediation of GI satiation/satiety signaling as well as multiple aspects of food reward. Blockade of GLP-1Rs in the NAc core subregion increased meal size when rats consumed palatable liquid sweetened condensed milk or sucrose solutions, without increasing meal frequency, and intra-NAc Ex9 attenuated the satiating effect of intragastric nutrient infusion (Dossat et al., 2013). Site-specific injection of Ex4 into the NAc suppressed PR responding for sucrose reinforcers (Dickson et al., 2012), supporting a role in motivation, though it is unclear whether this is a function of endogenous GLP-1R stimulation at this site. Food palatability is another aspect of reward, which interacts with but is distinct from motivation for food. In rodents, analysis of licking behavior can provide clues about hedonic evaluation, and in particular, variables such as the number of licks in the first minute of a meal and the number of licks per licking burst (defined as a series of licks separated by no more than 1 s) are associated with sucrose concentration (Johnson, 2018). In experiments examining how licking is affected by intra-NAc core Ex9 injection, increased licks in the first minute and increased licks per burst during the early part of a sucrose solution meal suggested that blocking those GLP-1Rs led rats to treat this sucrose solution as though it were a higher concentration and tasted sweeter (Dossat et al., 2013). There has been relatively little investigation of the neurophysiological underpinnings of NAc GLP-1R effects on feeding, but Mietlicki-Baase and colleagues (2014) determined that effects of GLP-1R stimulation in the core subregion of the NAc are at least partially mediated by the same pre-synaptic, AMPA/kainate receptor mechanisms that were described for the VTA.
4.3. Hippocampus
The hippocampus is most closely associated with learning and memory, not food intake control, but there is a substantial body of work implicating hippocampal involvement in cognitive controls of eating (Davidson et al., 2019). The ventral hippocampus (vHP), in particular, has been identified as a site for GLP-1R effects on feeding, and this location is distinguished from all the others discussed here because while GLP-1Rs are expressed in the vHP, PPG neurons do not project here (Cork et al., 2015; Hsu et al., 2015; Merchenthaler et al., 1999). Intra-vHP injection of ventricle-subthreshold doses of Ex4 suppressed intake of chow and “Western” diet relatively high in fat and sugar, and conversely, blockade of vHP GLP-1Rs increased intake (Hsu et al., 2015). This group also showed that the food intake effects of vHP GLP-1Rs are accounted for by suppression of meal size, and that intra-vHP Ex4 administration does not support the formation of a CTA (Hsu et al., 2018; Hsu et al., 2015) suggesting that vHP GLP-1Rs are engaging normal satiation mechanisms as opposed to illness responses.
Hippocampal GLP-1Rs were also implicated in motivation for food in experiments in which intra-vHP Ex4 suppressed PR responding for moderately high fat & sucrose pellets, and conversely, virally-mediated GLP-1R knockdown in vHP increased operant responding for these reinforcers (Hsu et al., 2018; Hsu et al., 2015). Interestingly, vHP Ex4 had no effect in a different assessment of food reward, palatable food-induced CPP (Hsu et al., 2015). At least some of the feeding effects of vHP GLP-1R stimulation appear to be mediated through glutamatergic vHP neurons that project to the medial prefrontal cortex (mPFC), because inhibitory DREADD-mediated silencing of this vHP glutamate projection or mPFC NMDA receptor antagonist treatment prevented vHP Ex4 from suppressing food intake (Hsu et al., 2018).
The results of the loss of function experiments clearly suggest that endogenous activation of vHP GLP-1Rs plays a physiological role in meal size control and motivation for food, but how does the endogenous agonist reach these receptors? Hsu and colleagues (2015) proposed a volume transmission route, with PPG neurons releasing GLP-1 into the nearby lateral ventricle, through which that GLP-1 ultimately reaches the hippocampus. This mechanism could play a role for other sites, as well, and may be especially relevant for those areas where GLP-1Rs are expressed but there appears to be only weak or no PPG neuron fiber presence.
4.4. Lateral Septum
The LS, which has been implicated in drug reward and stress responses, has dense GLP-1R expression, especially in the dorsal subregion, and receives some PPG projections (Cork et al., 2015; Merchenthaler et al., 1999; Williams et al., 2018). In the rat, intra-LS Ex4 treatment reduces high-fat diet intake primarily through an effect on meal size, with no induction of pica (Terrill et al., 2016). In mice, intra-LS GLP-1 injection decreases and Ex9 injection increases the size of liquid Ensure meals (Terrill et al., 2019). In both rats and mice, intra-LS blockade of GLP-1Rs attenuates the satiating effect of an Ensure preload on subsequent chow intake, providing strong support for the idea that GLP-1Rs in this nucleus mediate at least some of the satiating effects of GI nutrients (Terrill et al., 2016, 2019). LS GLP-1R blockade increased PR responding for sucrose reinforcers in both species, as well, though the effect was more substantial in mice, and intra-LS Ex9 injection blunted the hypophagic response to restraint stress in rats and mice (Terrill et al., 2018, 2019), suggesting multiple mechanisms of action for GLP-1Rs at this site.
The identity of the LS neurons through which GLP-1R activation affects these feeding behaviors has not been well studied, but one report suggests that many of these are neurotensin-expressing neurons, and they may modulate feeding through a projection to the LH (Azevedo et al., 2020). In that study, selective chemogenetic activation of LS GLP-1R-expressing neurons suppressed feeding in mice, and the authors showed that many of these LS GLP-1R and LS neurotensin neurons project to the LH. The activity of these neurons was linked with stressful situations in which the animal could attempt active escape (Azevedo et al., 2020), so this may be a pathway involved in brain GLP-1’s mediation of stress responses. Another study, focused on the role of these LS GLP-1Rs in responses to cocaine, showed that in some parts of the LS, GLP-1R-expressing neurons overlap with fields of dopamine fibers, which originate from the VTA, raising the possibility of interaction between these two systems at this site (Reddy et al., 2016). In a slice preparation, Reddy and colleagues (2016) found that GLP-1 application increased dopamine transporter surface expression and dopamine uptake in the LS, supporting this hypothesis. However, it remains to be investigated whether these interactions with dopamine in the LS mediate any of the food intake or motivation effects of GLP-1R activation at this site.
5. Conclusions
The evidence discussed in the preceding survey of brain regions where GLP-1Rs affect feeding allows us to draw several conclusions about the brain GLP-1 system, and about brain control of ingestive behavior in general. These data also raise important questions for future research.
Taken together, it is clear that GLP-1R stimulation throughout the brain suppresses food intake through diverse behavioral mechanisms, which are in some but not all cases shared across brain regions (see Figure 2). CNS GLP-1Rs can mediate nausea, aversion and responses to stressors such as restraint, but these can be dissociated from feeding effects in some brain regions. Discussed above were a number of brain structures in which GLP-1R stimulation reduces eating without evidence of viscerosensory malaise, and in at least one location, the CeA, GLP-1R stimulation supports the formation of a CTA but has no effect on food intake (Kinzig et al., 2002). In the majority of sites where meal pattern effects have been examined, GLP-1R stimulation suppresses food intake through a reduction in meal size, and at some locations, endogenous GLP-1R activation plays a mediatory role in the intake-suppressive effects of meal-related GI signals. Suppression of food reward is a common finding across many regions, though effects on specific behavioral tasks are not always consistent across brain sites (e.g., food-CPP affected by Ex4 injection into some but not other nuclei). Therefore, the specific behavioral mechanisms underlying brain GLP-1R effects on feeding behavior should not be considered homogenous across brain sites, and each location deserves its own thorough investigation.
Figure 2.
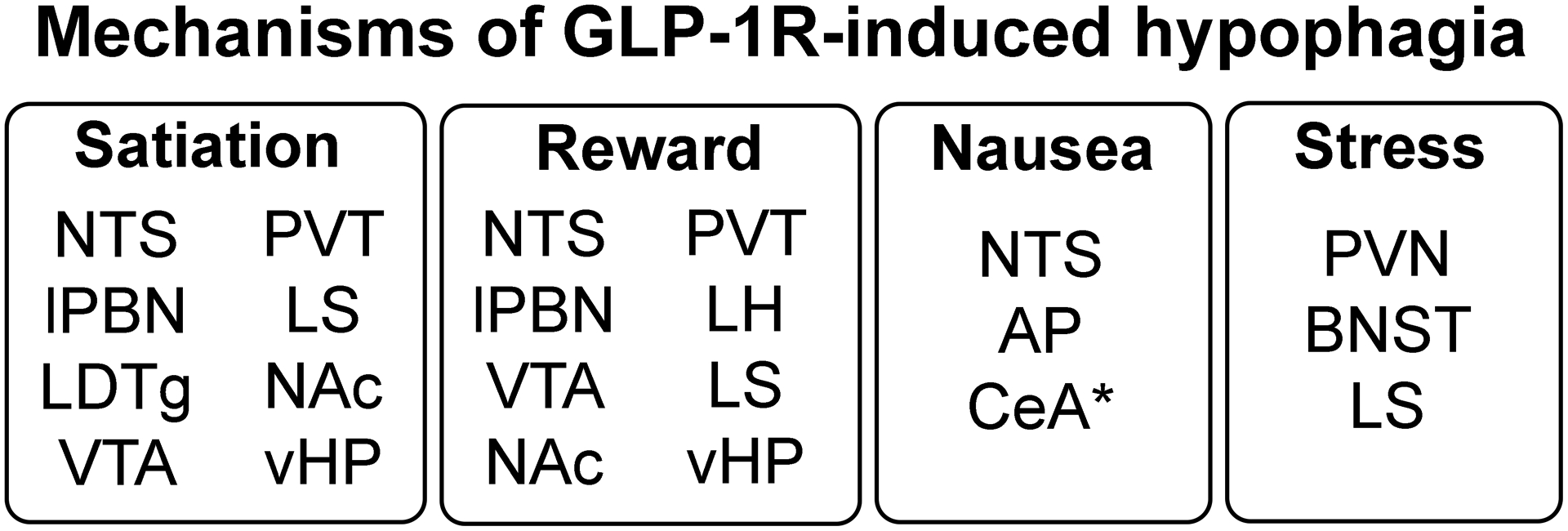
Summary of behavioral mechanisms through which brain GLP-1Rs appear to suppress food intake (amplifying satiation; suppressing food reward; inducing nausea, promoting stress responses), along with the brain areas implicated in each of these types of responses thus far. *GLP-1R stimulation in the CeA appears to induce nausea without hypophagia.
More broadly, studies examining the behavioral effects of GLP-1R stimulation have contributed to a shift in view of how the brain controls feeding. In the past, sharp distinctions were often made between “hedonic” and “homeostatic” controls of eating and the brain regions thought to underlie these mechanisms, with limited appreciation of crosstalk between these systems. This perspective was challenged by findings that GLP-1R action in brain areas like the NTS and lPBN, more often considered to mediate homeostatic feeding, could impact food reward, and that GLP-1R action in traditionally reward-associated areas like the VTA and NAc could mediate GI satiation/satiety signaling. This evidence, in addition to other research in the field, blurs the lines between hedonic and homeostatic feeding, and has advanced our understanding of how the brain responds to external and internal signals to control ingestive behavior.
This review focused on the ingestive behavioral effects of brain GLP-1Rs, but a significant body of research shows that these receptors have other behavioral effects, as well, and these data should be taken into consideration. CNS GLP-1Rs seem to be involved in drug and alcohol seeking (Jerlhag, 2019; also reviewed in this issue, Fink-Jensen), drinking behavior (McKay & Daniels, 2013; McKay et al., 2014), mating behavior (Vestlund & Jerlhag, 2020a, 2020b), and a variety of stress responses (Ghosal et al., 2017; Maniscalco & Rinaman, 2017; also reviewed in this issue, Holt & Rinaman, 2021). We do not yet know if separate populations of PPG neurons, the endogenous source of the ligand, are activated by stressors, GI signals, drugs of abuse or other stimuli, but given the numbers of activated PPG neurons identified in such studies, substantial overlap is most likely. Some target GLP-1R populations may mediate multiple types of behavioral and physiological responses, while others may be involved in only one.
There is also likely crosstalk between GLP-1R-expressing neurons located in different brain nuclei, given that reciprocal projections exist between many of these brain sites. A clear example of these anatomical relationships can be seen in a study that used transgenic mice expressing cre recombinase and a fluorescent reporter in GLP-1R neurons, in which anterograde tracing from BNST GLP-1R neurons project to the LS, where their axons surround LS GLP-1R-expressing neurons (Williams et al., 2018). It is not yet known if there are direct synaptic connections from one GLP-1R neuron to another, but even if indirect, interactions between GLP-1-receptive brain regions could impact the behavioral outcome of GLP-1R activation.
Brain GLP-1 effects may depend on the environmental context and/or internal state of the animal, as well. A few findings point to interoceptive state as an important modulator. Overnight food deprivation blocks the effects of CCK, restraint stress, and elevated platform exposure to induce c-fos in PPG neurons (Maniscalco et al., 2015; Maniscalco & Rinaman, 2013), suggesting that in a fasting state, PPG neurons are less likely to be activated and release GLP-1 at projection targets. Fasting also impairs the ability of 3rd-i.c.v. GLP-1 administration to suppress food intake (Sandoval et al., 2012), and some evidence suggests that this change in sensitivity may be mediated through brain glucose-sensing (Burmeister et al., 2017b; Sandoval et al., 2012). This suggests that GLP-1R signaling or downstream mediation of its effects is impacted as well as the availability of the endogenous ligand. It is easy to imagine this modulation by fasting to be adaptive; if food is scarce, the motivation to find and consume food should remain high even in the face of stimuli that normally suppress food seeking and eating.
In almost all laboratory research on GLP-1R effects, experiments were designed to isolate one or another behavioral response, so it is unclear how the brain GLP-1 system functions in more naturalistic situations in which animals face a more varied environment with many behavioral options. The majority of research has put the food intake suppression effects of CNS GLP-1R stimulation at the forefront, but this bears rethinking as we continue to learn more about the role of these receptors in other behavioral responses. Ultimately it may be more accurate to consider brain GLP-1Rs as part of a network that enables certain interoceptive (e.g., GI signals, homeostatic challenges) and environmental cues (e.g., signals of possible danger) to broadly suppress motivated behavior, including but not limited to food intake, in the interest of survival.
Acknowledgements
Work in the Williams laboratory is supported by NIH grant R01DK095757 to DLW.
Abbreviations
- AP
area postrema
- AAV
adeno-associated virus
- BNST
bed nucleus of the stria terminalis
- CCK
cholecystokinin
- CeA
central amygdala
- CPP
conditioned place preference
- CRH
corticotropin releasing hormone
- CTA
conditioned taste aversion
- DR
dorsal raphe
- DREADD
designer receptors exclusively activated by designer drugs
- Ex4
Exendin 4
- Ex9
Exendin (9–39)
- GI
gastrointestinal
- GLP-1
glucagon-like peptide 1
- GLP-1R
glucagon-like peptide 1 receptor
- LDTg
lateral dorsal tegmental area
- LH
lateral hypothalamus
- mPFC
medial prefrontal cortex
- NAc
nucleus accumbens
- NTS
nucleus of the solitary tract
- PPG
preproglucagon
- PR
progressive ratio
- PVN
paraventricular nucleus of the hypothalamus
- PVT
paraventricular nucleus of the thalamus
- Sim1
single-minded homolog 1
- vHP
ventral hippocampus
- VTA
ventral tegmental area
Footnotes
Data sharing is not applicable to this article because no new data were created or analysed in this study.
Nomenclature of Targets and Ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY (http://www.guidetopharmacology.org) and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos, et al., 2019; Alexander, Fabbro, et al., 2019; Alexander, Mathie, et al., 2019).
References
- 1.Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Mathie A, Peters JA, Veale EL, Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Sharman JL, Southan C, Davies JA; CGTP Collaborators. (2019) THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein-coupled receptors. Br J Pharmacol. 176 Suppl 1(Suppl 1):S21–S141. doi: 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander SPH, Fabbro D, Kelly E, Mathie A, Peters JA, Veale EL, Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Sharman JL, Southan C, Davies JA, & CGTP Collaborators. (2019). The Concise Guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander SPH, Mathie A, Peters JA, Veale EL, Striessnig J, Kelly E, Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Sharman JL, & CGTP Collaborators. (2019). The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 176, S142–S228. 10.1111/bph.14749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alhadeff AL, Baird J-P, Swick JC, Hayes MR, & Grill HJ (2014). Glucagon-like Peptide-1 receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake and motivation to feed. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 39(9), 2233–2243. 10.1038/npp.2014.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alhadeff AL, & Grill HJ (2014). Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 307(4), R465–470. 10.1152/ajpregu.00179.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alhadeff AL, Mergler BD, Zimmer DJ, Turner CA, Reiner DJ, Schmidt HD, Grill HJ, & Hayes MR (2017). Endogenous Glucagon-like Peptide-1 Receptor Signaling in the Nucleus Tractus Solitarius is Required for Food Intake Control. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 42(7), 1471–1479. 10.1038/npp.2016.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alhadeff AL, Rupprecht LE, & Hayes MR (2012). GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology, 153(2), 647–658. 10.1210/en.20111443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderberg RH, Richard JE, Eerola K, López-Ferreras L, Banke E, Hansson C, Nissbrandt H, Berqquist F, Gribble FM, Reimann F, Wernstedt Asterholm I, Lamy CM, & Skibicka KP (2017). Glucagon-Like Peptide 1 and Its Analogs Act in the Dorsal Raphe and Modulate Central Serotonin to Reduce Appetite and Body Weight. Diabetes, 66(4), 1062–1073. 10.2337/db16-0755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aroda VR (2018). A review of GLP-1 receptor agonists: Evolution and advancement, through the lens of randomised controlled trials. Diabetes, Obesity & Metabolism, 20 Suppl 1, 22–33. doi: 10.1111/dom.13162 [DOI] [PubMed] [Google Scholar]
- 10.Azevedo EP, Tan B, Pomeranz LE, Ivan V, Fetcho R, Schneeberger M, Doerig KR, Liston C, Friedman JM, & Stern SA (2020). A limbic circuit selectively links active escape to food suppression. ELife, 9, e58894. 10.7554/eLife.58894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baird JP, Travers SP, Travers JB (2001). Integration of gastric distension and gustatory responses in the parabrachial nucleus. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 281(5), R1581–93. https://doi: 10.1152/ajpregu.2001.281.5.R1581 [DOI] [PubMed] [Google Scholar]
- 12.Berger M, Gray JA, & Roth BL (2009). The Expanded Biology of Serotonin. Annual Review of Medicine, 60, 355–366. 10.1146/annurev.med.60.042307.110802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bettge K, Kahle M, Abd El Aziz MS, Meier JJ, & Nauck MA (2017). Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: A systematic analysis of published clinical trials. Diabetes, Obesity & Metabolism, 19(3), 336–347. 10.1111/dom.12824 [DOI] [PubMed] [Google Scholar]
- 14.Brierley DI, Holt MK, Singh A, de Araujo A, McDougle M, Vergara M, Afaghani MH, Lee SJ, Scott K, Maske C, Langhans W, Krause E, de Kloet A, Gribble FM, Reimann F, Rinaman L, de Lartigue G, Trapp S (2021) Central and peripheral GLP-1 systems independently suppress eating. Nat Metab.3(2), 258–273. doi: 10.1038/s42255021-00344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burke LK, & Heisler LK (2015). 5-hydroxytryptamine medications for the treatment of obesity. Journal of Neuroendocrinology, 27(6), 389–398. 10.1111/jne.12287 [DOI] [PubMed] [Google Scholar]
- 16.Burmeister MA, Ayala JE, Smouse H, Landivar-Rocha A, Brown JD, Drucker DJ, Stoffers DA, Sandoval DA, Seeley RJ, & Ayala JE (2017a). The Hypothalamic Glucagon-Like Peptide 1 Receptor Is Sufficient but Not Necessary for the Regulation of Energy Balance and Glucose Homeostasis in Mice. Diabetes, 66(2), 372–384. 10.2337/db16-1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burmeister MA, Brown JD, Ayala JE, Stoffers DA, Sandoval DA, Seeley RJ, & Ayala JE (2017b). The glucagon-like peptide-1 receptor in the ventromedial hypothalamus reduces short-term food intake in male mice by regulating nutrient sensor activity. American Journal of Physiology. Endocrinology and Metabolism, 313(6), E651–E662. doi: 10.1152/ajpendo.00113.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Card JP, Johnson AL, Llewellyn-Smith IJ, Zheng H, Anand R, Brierley DI, Trapp S, Rinaman L (2018) GLP-1 neurons form a local synaptic circuit within the rodent nucleus of the solitary tract. J Comp Neurol, 526(14), 2149–2164. doi: 10.1002/cne.24482. Epub 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ch’ng S, Fu J, Brown RM, McDougall SJ, & Lawrence AJ (2018). The intersection of stress and reward: BNST modulation of aversive and appetitive states. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 87(Pt A), 108–125. 10.1016/j.pnpbp.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 20.Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, & Trapp S (2015). Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Molecular Metabolism, 4(10), 718–731. 10.1016/j.molmet.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davidson TL, Jones S, Roy M, & Stevenson RJ (2019). The Cognitive Control of Eating and Body Weight: It’s More Than What You “Think.” Frontiers in Psychology, 10, 62. 10.3389/fpsyg.2019.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, & Skibicka KP (2012). The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: A new role for mesolimbic GLP-1 receptors. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 32(14), 4812–4820. 10.1523/JNEUROSCI.6326-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobbs LK, & Cunningham CL (2014). The role of the laterodorsal tegmental nucleus in methamphetamine conditioned place preference and locomotor activity. Behavioural Brain Research, 265, 198–202. 10.1016/j.bbr.2014.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dossat AM, Diaz R, Gallo L, Panagos A, Kay K, & Williams DL (2013). Nucleus accumbens GLP-1 receptors influence meal size and palatability. American Journal of Physiology. Endocrinology and Metabolism, 304(12), E1314–1320. 10.1152/ajpendo.00137.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dossat AM, Lilly N, Kay K, & Williams DL (2011). Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(41), 14453–14457. 10.1523/JNEUROSCI.3262-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fortin SM, Lipsky RK, Lhamo R, Chen J, Kim E, Borner T, Schmidt HD, & Hayes MR (2020). GABA neurons in the nucleus tractus solitarius express GLP-1 receptors and mediate anorectic effects of liraglutide in rats. Science Translational Medicine, 12(533). 10.1126/scitranslmed.aay8071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabery S, Salinas CG, Paulsen SJ, Ahnfelt-Rønne J, Alanentalo T, Baquero AF, Buckley ST, Farkas E, Fekete C, Frederiksen KS, Helms HCC, Jeppesen JF, John LM, Pyke C, Nøhr J, Lu TT, Polex-Wolf J, Prevot V, Raun K, … Knudsen LB (2020). Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight, 5(6). 10.1172/jci.insight.133429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosal S, Packard AEB, Mahbod P, McKlveen JM, Seeley RJ, Myers B, Ulrich-Lai Y, Smith EP, D’Alessio DA, & Herman JP (2017). Disruption of Glucagon-Like Peptide 1 Signaling in Sim1 Neurons Reduces Physiological and Behavioral Reactivity to Acute and Chronic Stress. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 37(1), 184–193. 10.1523/JNEUROSCI.1104-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grill HJ (2020). A Role for GLP-1 in Treating Hyperphagia and Obesity. Endocrinology, 161(8), bqaa093. doi: 10.1210/endocr/bqaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grill HJ, Hayes MR (2012). Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab, 16(3), 296–309. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gotoh K, Fukagawa K, Fukagawa T, Noguchi H, Kakuma T, Sakata T, Yoshimatsu H (2005). Glucagon-like peptide-1, corticotropin-releasing hormone, and hypothalamic neuronal histamine interact in the leptin-signaling pathway to regulate feeding behavior. FASEB J. 19(9), 1131–3. doi: 10.1096/fj.04-2384fje [DOI] [PubMed] [Google Scholar]
- 32.Gu G, Roland B, Tomaselli K, Dolman CS, Lowe C, & Heilig JS (2013). Glucagon-like peptide-1 in the rat brain: Distribution of expression and functional implication. The Journal of Comparative Neurology, 521(10), 2235–2261. 10.1002/cne.23282 [DOI] [PubMed] [Google Scholar]
- 33.Hällbrink M, Holmqvist T, Olsson M, Ostenson CG, Efendic S, & Langel U (2001). Different domains in the third intracellular loop of the GLP-1 receptor are responsible for Galpha(s) and Galpha(i)/Galpha(o) activation. Biochimica Et Biophysica Acta, 1546(1), 79–86. 10.1016/s0167-4838(00)00270-3 [DOI] [PubMed] [Google Scholar]
- 34.Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, Gray AJG, Bruce L, Alexander SPH, Anderton S, Bryant C, Davenport AP, Doerig C, Fabbro D, Levi-Schaffer F, Spedding M, Davies JA, NC-IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 46, D1091–1106. doi: 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayes MR, Bradley L, & Grill HJ (2009). Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology, 150(6), 2654–2659. 10.1210/en.2008-1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, De Jonghe BC, Kanoski SE, Grill HJ, & Bence KK (2011). Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metabolism, 13(3), 320–330. 10.1016/j.cmet.2011.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayes MR, Skibicka KP, & Grill HJ (2008). Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology, 149(8), 4059–4068. 10.1210/en.2007-1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez NS, Weir VR, Ragnini K, Merkel R, Zhang Y, Mace K, Rich MT, Christopher Pierce R, & Schmidt HD (2020). GLP-1 receptor signaling in the laterodorsal tegmental nucleus attenuates cocaine seeking by activating GABAergic circuits that project to the VTA. Molecular Psychiatry. 10.1038/s41380-020-00957-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hisadome K, Reimann F, Gribble FM, & Trapp S (2010). Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: Electrical properties of glucagon-like Peptide 1 neurons. Diabetes, 59(8), 1890–1898. 10.2337/db10-0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holst JJ, & Deacon CF (2005). Glucagon-like peptide-1 mediates the therapeutic actions of DPP-IV inhibitors. Diabetologia, 48(4), 612–615. 10.1007/s00125005-1705-7 [DOI] [PubMed] [Google Scholar]
- 41.Holt MK, Richards JE, Cook DR, Brierley DI, Williams DL, Reimann F, Gribble FM, & Trapp S (2019). Preproglucagon Neurons in the Nucleus of the Solitary Tract Are the Main Source of Brain GLP-1, Mediate Stress-Induced Hypophagia, and Limit Unusually Large Intakes of Food. Diabetes, 68(1), 21–33. 10.2337/db18-0729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holt MK, Cook DR, Brierley DI, Richards JE, Reimann F, Gourine AV, Marina N, Trapp S (2020). PPG neurons in the nucleus of the solitary tract modulate heart rate but do not mediate GLP-1 receptor agonist-induced tachycardia in mice. Mol Metab. 39, 101024. doi: 10.1016/j.molmet.2020.101024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsu TM, Noble EE, Liu CM, Cortella AM, Konanur VR, Suarez AN, Reiner DJ, Hahn JD, Hayes MR, & Kanoski SE (2018). A hippocampus to prefrontal cortex neural pathway inhibits food motivation through glucagon-like peptide-1 signaling. Molecular Psychiatry, 23(7), 1555–1565. 10.1038/mp.2017.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsu Ted M., Hahn JD, Konanur VR, Lam A, & Kanoski SE (2015). Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 40(2), 327–337. 10.1038/npp.2014.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jerlhag E (2019). Gut-brain axis and addictive disorders: A review with focus on alcohol and drugs of abuse. Pharmacology & Therapeutics, 196, 1–14. 10.1016/j.pharmthera.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 46.Johnson AW (2018). Characterizing ingestive behavior through licking microstructure: Underlying neurobiology and its use in the study of obesity in animal models. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience, 64, 38–47. 10.1016/j.ijdevneu.2017.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanoski SE, Fortin SM, Arnold M, Grill HJ, & Hayes MR (2011). Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology, 152(8), 3103–3112. https://doi: 10.1210/en.2011-0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, & Hayes MR (2012). The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology, 62(5–6), 1916–1927. 10.1016/j.neuropharm.2011.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katsurada K, Maejima Y, Nakata M, Kodaira M, Suyama S, Iwasaki Y, Kario K, & Yada T (2014). Endogenous GLP-1 acts on paraventricular nucleus to suppress feeding: Projection from nucleus tractus solitarius and activation of corticotropin-releasing hormone, nesfatin-1 and oxytocin neurons. Biochemical and Biophysical Research Communications, 451(2), 276–281. 10.1016/j.bbrc.2014.07.116 [DOI] [PubMed] [Google Scholar]
- 50.Kawatani M, Yamada Y, & Kawatani M (2018). Glucagon-like peptide-1 (GLP-1) action in the mouse area postrema neurons. Peptides, 107, 68–74. 10.1016/j.peptides.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 51.Kelley AE (2004). Ventral striatal control of appetitive motivation: Role in ingestive behavior and reward-related learning. Neuroscience and Biobehavioral Reviews, 27(8), 765–776. 10.1016/j.neubiorev.2003.11.015 [DOI] [PubMed] [Google Scholar]
- 52.Kinzig KP, D’Alessio DA, & Seeley RJ (2002). The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 22(23), 10470–10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Konanur VR, Hsu TM, Kanoski SE, Hayes MR, & Roitman MF (2020). Phasic dopamine responses to a food-predictive cue are suppressed by the glucagon-like peptide-1 receptor agonist Exendin-4. Physiology & Behavior, 215, 112771. 10.1016/j.physbeh.2019.112771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Larsen PJ, Tang-Christensen M, & Jessop DS (1997). Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology, 138(10), 4445–4455. 10.1210/endo.138.10.5270 [DOI] [PubMed] [Google Scholar]
- 55.Liu J, Conde K, Zhang P, Lilascharoen V, Xu Z, Lim BK, Seeley RJ, Zhu JJ, Scott MM, & Pang ZP (2017). Enhanced AMPA Receptor Trafficking Mediates the Anorexigenic Effect of Endogenous Glucagon-like Peptide-1 in the Paraventricular Hypothalamus. Neuron, 96(4), 897–909.e5. 10.1016/j.neuron.2017.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Llewellyn-Smith IJ, Gnanamanickam GJE, Reimann F, Gribble FM, & Trapp S (2013). Preproglucagon (PPG) neurons innervate neurochemically identified autonomic neurons in the mouse brainstem. Neuroscience, 229, 130–143. 10.1016/j.neuroscience.2012.09.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lodge DJ, & Grace AA (2006). The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proceedings of the National Academy of Sciences of the United States of America, 103(13), 5167–5172. 10.1073/pnas.0510715103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.López-Ferreras L, Richard JE, Noble EE, Eerola K, Anderberg RH, Olandersson K, Taing L, Kanoski SE, Hayes MR, & Skibicka KP (2018). Lateral hypothalamic GLP-1 receptors are critical for the control of food reinforcement, ingestive behavior and body weight. Molecular Psychiatry, 23(5), 1157–1168. 10.1038/mp.2017.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maniscalco JW, & Rinaman L (2017). Interoceptive modulation of neuroendocrine, emotional, and hypophagic responses to stress. Physiology & Behavior, 176, 195–206. 10.1016/j.physbeh.2017.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maniscalco JW, & Rinaman L (2013). Overnight food deprivation markedly attenuates hindbrain noradrenergic, glucagon-like peptide-1, and hypothalamic neural responses to exogenous cholecystokinin in male rats. Physiology & Behavior, 121, 35–42. doi: 10.1016/j.physbeh.2013.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maniscalco JW, Zheng H, Gordon PJ, & Rinaman L (2015). Negative Energy Balance Blocks Neural and Behavioral Responses to Acute Stress by “Silencing” Central Glucagon-Like Peptide 1 Signaling in Rats. The Journal of Neuroscience, 35(30), 10701–10714. doi: 10.1523/JNEUROSCI.3464-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maske CB, Jackson CM, Terrill SJ, Eckel LA, & Williams DL (2017). Estradiol modulates the anorexic response to central glucagon-like peptide 1. Hormones and Behavior, 93, 109–117. 10.1016/j.yhbeh.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McKay NJ, & Daniels D (2013). Glucagon-like peptide-1 receptor agonist administration suppresses both water and saline intake in rats. Journal of Neuroendocrinology, 25(10), 929–938. 10.1111/jne.12086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McKay Naomi J., Galante DL, & Daniels D (2014). Endogenous glucagon-like peptide-1 reduces drinking behavior and is differentially engaged by water and food intakes in rats. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 34(49), 16417–16423. 10.1523/JNEUROSCI.3267-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McMahon LR, & Wellman PJ (1998). PVN infusion of GLP-1-(7–36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. The American Journal of Physiology, 274(1), R23–29. 10.1152/ajpregu.1998.274.1.R23 [DOI] [PubMed] [Google Scholar]
- 66.Merchenthaler I, Lane M, & Shughrue P (1999). Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. The Journal of Comparative Neurology, 403(2), 261–280. [DOI] [PubMed] [Google Scholar]
- 67.Meye FJ, & Adan RAH (2014). Feelings about food: The ventral tegmental area in food reward and emotional eating. Trends in Pharmacological Sciences, 35(1), 31–40. 10.1016/j.tips.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 68.Mietlicki-Baase EG, Ortinski PI, Reiner DJ, Sinon CG, McCutcheon JE, Pierce RC, Roitman MF, & Hayes MR (2014). Glucagon-like peptide-1 receptor activation in the nucleus accumbens core suppresses feeding by increasing glutamatergic AMPA/kainate signaling. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 34(20), 6985–6992. 10.1523/JNEUROSCI.0115-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC, & Hayes MR (2013). The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. American Journal of Physiology. Endocrinology and Metabolism, 305(11), E1367–74. 10.1152/ajpendo.00413.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Müller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JF, Holst JJ, Langhans W, Meier JJ, Nauck M, Perez-Tilve D, Pocai A, Reimann F, Sandoval DA, Schwartz TW, & Tschöp MH (2019). Glucagon-like peptide 1 (GLP-1). Molecular Metabolism, 30(72–130). 10.1016/j.molmet.2019.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ong ZY, Liu J-J, Pang ZP, & Grill HJ (2017). Paraventricular Thalamic Control of Food Intake and Reward: Role of Glucagon-Like Peptide-1 Receptor Signaling. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 42(12), 2387–2397. 10.1038/npp.2017.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pabreja K, Mohd MA, Koole C, Wootten D, & Furness SGB (2014). Molecular mechanisms underlying physiological and receptor pleiotropic effects mediated by GLP-1R activation. British Journal of Pharmacology, 171(5), 1114–1128. 10.1111/bph.12313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Punjabi M, Arnold M, Rüttimann E, Graber M, Geary N, Pacheco-López G, & Langhans W (2014). Circulating glucagon-like peptide-1 (GLP-1) inhibits eating in male rats by acting in the hindbrain and without inducing avoidance. Endocrinology, 155(5), 1690–1699. 10.1210/en.2013-1447 [DOI] [PubMed] [Google Scholar]
- 74.Reddy IA, Pino JA, Weikop P, Osses N, Sørensen G, Bering T, Valle C, Bluett RJ, Erreger K, Wortwein G, Reyes JG, Graham D, Stanwood GD, Hackett TA, Patel S, Fink-Jensen A, Torres GE, & Galli A (2016). Glucagon-like peptide 1 receptor activation regulates cocaine actions and dopamine homeostasis in the lateral septum by decreasing arachidonic acid levels. Translational Psychiatry, 6, e809. 10.1038/tp.2016.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reiner DJ, Leon RM, McGrath LE, Koch-Laskowski K, Hahn JD, Kanoski SE, Mietlicki-Baase EG, & Hayes MR (2018). Glucagon-Like Peptide-1 Receptor Signaling in the Lateral Dorsal Tegmental Nucleus Regulates Energy Balance. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 43(3), 627–637. 10.1038/npp.2017.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reiner DJ, Mietlicki-Baase EG, McGrath LE, Zimmer DJ, Bence KK, Sousa GL, Konanur VR, Krawczyk J, Burk DH, Kanoski SE, Hermann GE, Rogers RC, & Hayes MR (2016). Astrocytes Regulate GLP-1 Receptor-Mediated Effects on Energy Balance. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 36(12), 3531–3540. 10.1523/JNEUROSCI.3579-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Richard JE, Anderberg RH, Göteson A, Gribble FM, Reimann F, & Skibicka KP (2015). Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PloS One, 10(3), e0119034. 10.1371/journal.pone.0119034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Richard JE, Anderberg RH, López-Ferreras L, Olandersson K, & Skibicka KP (2016). Sex and estrogens alter the action of glucagon-like peptide-1 on reward. Biology of Sex Differences, 7, 6. 10.1186/s13293-016-0059-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Richard JE, Farkas I, Anesten F, Anderberg RH, Dickson SL, Gribble FM, Reimann F, Jansson J-O, Liposits Z, & Skibicka KP (2014). GLP-1 receptor stimulation of the lateral parabrachial nucleus reduces food intake: Neuroanatomical, electrophysiological, and behavioral evidence. Endocrinology, 155(11), 4356–4367. 10.1210/en.2014-1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rinaman L (1999). Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. The American Journal of Physiology, 277(2), R582–590. 10.1152/ajpregu.1999.277.2.R582 [DOI] [PubMed] [Google Scholar]
- 81.Rinaman L (2010). Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Research, 1350, 18–34. 10.1016/j.brainres.2010.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rupprecht LE, Mietlicki-Baase EG, Zimmer DJ, McGrath LE, Olivos DR, & Hayes MR (2013). Hindbrain GLP-1 receptor-mediated suppression of food intake requires a PI3K-dependent decrease in phosphorylation of membrane-bound Akt. American Journal of Physiology. Endocrinology and Metabolism, 305(6), E751–759. 10.1152/ajpendo.00367.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sandoval D, Barrera JG, Stefater MA, Sisley S, Woods SC, D’Alessio DD, & Seeley RJ (2012). The anorectic effect of GLP-1 in rats is nutrient dependent. PloS One, 7(12), e51870. doi: 10.1371/journal.pone.0051870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Secher A, Jelsing J, Baquero AF, Hecksher-Sørensen J, Cowley MA, Dalbøge LS, Hansen G, Grove KL, Pyke C, Raun K, Schäffer L, Tang-Christensen M, Verma S, Witgen BM, Vrang N, & Bjerre Knudsen L (2014). The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. The Journal of Clinical Investigation, 124(10), 4473–4488. 10.1172/JCI75276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sisley S, Gutierrez-Aguilar R, Scott M, D’Alessio DA, Sandoval DA, & Seeley RJ (2014). Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. The Journal of Clinical Investigation, 124(6), 2456–2463. 10.1172/JCI72434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stuber GD, & Wise RA (2016). Lateral hypothalamic circuits for feeding and reward. Nature Neuroscience, 19(2), 198–205. 10.1038/nn.4220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang-Christensen M, Larsen PJ, Göke R, Fink-Jensen A, Jessop DS, Møller M, & Sheikh SP (1996). Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. The American Journal of Physiology, 271(4 Pt 2), R848–856. 10.1152/ajpregu.1996.271.4.R848 [DOI] [PubMed] [Google Scholar]
- 88.Terrill SJ, Holt MK, Maske CB, Abrams N, Reimann F, Trapp S, & Williams DL (2019). Endogenous GLP-1 in lateral septum promotes satiety and suppresses motivation for food in mice. Physiology & Behavior, 206, 191–199. 10.1016/j.physbeh.2019.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Terrill SJ, Jackson CM, Greene HE, Lilly N, Maske CB, Vallejo S, & Williams DL (2016). Role of lateral septum glucagon-like peptide 1 receptors in food intake. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 311(1), R124132. 10.1152/ajpregu.00460.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Terrill SJ, Maske CB, & Williams DL (2018). Endogenous GLP-1 in lateral septum contributes to stress-induced hypophagia. Physiology & Behavior, 192, 17–22. 10.1016/j.physbeh.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, & Bloom SR (1996). A role for glucagon-like peptide-1 in the central regulation of feeding. Nature, 379(6560), 69–72. 10.1038/379069a0 [DOI] [PubMed] [Google Scholar]
- 92.Vestlund J, & Jerlhag E (2020a). The glucagon-like peptide-1 receptor agonist, exendin-4, reduces sexual interaction behaviors in a brain site-specific manner in sexually naïve male mice. Hormones and Behavior, 124, 104778. 10.1016/j.yhbeh.2020.104778 [DOI] [PubMed] [Google Scholar]
- 93.Vestlund J, & Jerlhag E (2020b). Glucagon-like peptide-1 receptors and sexual behaviors in male mice. Psychoneuroendocrinology, 117, 104687. 10.1016/j.psyneuen.2020.104687 [DOI] [PubMed] [Google Scholar]
- 94.Williams DL, Baskin DG, & Schwartz MW (2009). Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology, 150(4), 1680–1687. 10.1210/en.2008-1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Williams DL, Lilly NA, Edwards IJ, Yao P, Richards JE, & Trapp S (2018). GLP1 action in the mouse bed nucleus of the stria terminalis. Neuropharmacology, 131, 83–95. 10.1016/j.neuropharm.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, Drucker DJ, & Elmquist JK (2003). Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23(7), 2939–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang C, Kaye JA, Cai Z, Wang Y, Prescott SL, & Liberles SD (2020). Area Postrema Cell Types that Mediate Nausea-Associated Behaviors. Neuron. 10.1016/j.neuron.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zheng H, Reiner DJ, Hayes MR, & Rinaman L (2019). Chronic Suppression of Glucagon-Like Peptide-1 Receptor (GLP1R) mRNA Translation in the Rat Bed Nucleus of the Stria Terminalis Reduces Anxiety-Like Behavior and Stress-Induced Hypophagia, But Prolongs Stress-Induced Elevation of Plasma Corticosterone. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 39(14), 2649–2663. 10.1523/JNEUROSCI.2180-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]


