Abstract
Objective
The relative risk of SARS-CoV-2 infection and COVID-19 disease severity among people with rheumatic and musculoskeletal diseases (RMD) compared to those without RMD remains uncertain. We sought to quantify the risk for SARS-CoV-2 infection and describe clinical outcomes of COVID-19 in people with RMD.
Methods
A systematic literature review was conducted using 14 databases from January 1st, 2019 to February 13th, 2021. We included observational studies and experimental trials in RMD patients reporting comparative rates of SARS-CoV-2 infection, hospitalization, oxygen supplementation/ICU admission/mechanical ventilation, or mortality attributed to COVID-19. Methodological quality was evaluated using the Joanna Briggs Institute Critical Appraisal Tools or the Newcastle-Ottawa scale. Risk ratios (RRs) and odds ratios (ORs) were calculated, as applicable for each outcome, using the Mantel-Haenszel formula with random effect models.
Results
Of 5799 abstracts screened, 100 studies met criteria for inclusion in the systematic review and 54/100 had a low risk-of-bias. Among studies included in the meta-analyses, we found an increased prevalence of SARS-CoV-2 infection in people with RMD (RR 1.53 (95%CI 1.16, 2.01)) compared with the general population. Odds of hospitalization, ICU admission, and mechanical ventilation were similar in patients with and without RMD, whereas odds of mortality was increased (OR 1.74 (95%CI 1.08, 2.80)). A smaller number of studies reported adjusted risk for outcomes and variably demonstrated increased risk for no difference in risk.
Conclusion
Patients with RMDs had higher rates of SARS-CoV-2 infection and increased odds of mortality.
Keywords: Rheumatic disease, COVID-19, prevalence, hospitalization, mortality
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has resulted in unprecedented morbidity and mortality due to Coronavirus disease 2019 (COVID-19). Risk factors associated with poor outcomes from COVID-19 in the general population include older age, sex, and chronic illnesses (1, 2).
Patients with rheumatic diseases may be at an increased risk of infection due to their underlying disease, associated comorbidities, and use of potentially immunosuppressive treatments (3). Furthermore, concern exists regarding the potential for individuals with rheumatic disease to experience more severe COVID-19 disease and poorer outcomes. However, one year after the first cases of COVID-19 were described, the applicability of this heuristic to SARS-CoV-2 infection, and the magnitude of any such risk, remains uncertain. Data directly addressing these questions are limited and lack clarity due to the rapid publication of many small studies during the pandemic, frequently underpowered to show clinically significant effects.
The present systematic review and meta-analysis aimed to quantify the risk for contracting SARS-CoV-2 infection and to describe the outcomes of COVID-19 in patients with rheumatic diseases.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement for reporting was used for this study (4). The protocol was registered with PROSPERO a priori (CRD42020205668).
Data sources and literature search
A systematic search of the literature was conducted by a medical librarian in the BioRxiv, China National Knowledge Infrastructure (CKNI), Cochrane Library, Disaster Lit, Global Health, Google Scholar, LitCovid, MedRxiv, Ovid Embase, Ovid Medline, Pubmed, Scopus, Wanfang, and Web of Science Core Collection databases to find relevant articles published from January 1, 2019 to February 13, 2021. Databases were searched using a combination of controlled vocabulary and free text terms for COVID-19 and rheumatic disease. The search was peer-reviewed by a second medical librarian using PRESS (Peer Review of Electronic Search Strategies) (5). Details of the full search strategy are listed in Supplementary Table 1. Details of rheumatic and musculoskeletal diseases (RMDs) included are shown in Supplementary Table 2. The bibliography of included studies were reviewed to identify additional relevant literature.
Study selection eligibility criteria
Citations from all databases were imported in an Endnote x9 library (Clarivate Analytics, Philadelphia, PA), where duplicates were removed. The de-duplicated results were imported into Covidence v2627 (Covidence, Melbourne, Victoria, Australia) for screening and data extraction. Two independent screeners performed a title and abstract review with a third screener to resolve disagreements. The full texts of the resulting papers were then reviewed for inclusion by two independent screeners with a third screener to resolve disagreements.
Observational studies and experimental trials were eligible for inclusion if they reported data regarding adult and/or pediatric patients with rheumatic disease and included SARS-CoV-2 infection or subsequent clinical course as outcomes. Publications were excluded if they did not report quantifiable data regarding patients with rheumatic disease, did not include original primary data, did not focus on human subjects, did not report data on outcomes related to SARS-CoV2 infection or its associated clinical course, represented duplicate or retracted data, were case reports or series, or were not yet published as a full-text study. No restrictions were placed based upon language, and review of foreign language articles were performed by individuals fluent in the language. Outcomes of interest included SARS-CoV-2 infection and COVID-19 outcomes (hospitalization, oxygen requirement, ICU admission, mechanical ventilation, or death).
Data collection process
Two reviewers independently extracted data using Qualtrics software, version Oct-May 2020–2021 (Qualtrics, Provo, UT). A third reviewer reviewed the forms to resolve any conflicts. Data extracted included study characteristics (first author, year of publication, country of origin, study design, sample size, and study sponsor), baseline characteristics of the participants (age, sex, race/ethnicity, comorbidities (extracted as each individual comorbidity reported and additionally grouped as diabetes, respiratory, cardiovascular, smoking, and others)), SARS-CoV-2 infection, and COVID-19 outcomes (hospitalization, oxygen requirement, ICU admission, mechanical ventilation, or death).
Risk of bias in individual studies
Two reviewers independently assessed the risk of bias. Risk of bias was assessed using the Joanna Briggs Institute (JBI) checklists for prevalence and analytical cross-sectional studies, and the Newcastle-Ottawa Scale for case-control and cohort studies (6–8) The JBI checklists for prevalence and cross-sectional analytical studies were divided into 3 categories (0–3 = high risk, 4–6 = some concerns, 7–9 = low risk and 0–3 = high risk, 4–6 = some concerns, 7–8 = low risk, respectively). The comparability domain of the Newcastle‐Ottawa Scale was the primary differentiation point for a study’s risk of bias in this context and was used to determine global risk of bias (0 = high risk, 1 = some concerns, and 2 = low risk) (9, 10). Disagreements were resolved by a third reviewer.
Data analysis
Studies included in the systematic review were further evaluated for suitability for the meta-analyses if they reported comparative data for patients with and without RMDs. For COVID-19 prevalence, studies were included if they reported the number of COVID-19 cases among patients in the RMD group as well as the number of COVID-19 cases within the overall regional populations. For hospitalization, ICU admission, mechanical ventilation, and death, studies were included if they provided raw counts of each outcome among patients with RMDs diagnosed clinically with COVID-19 as well as counts among a comparator group of patients without RMD diagnosed clinically with COVID-19. Data were insufficient to meta-analyze risk for oxygen supplementation. Studies were excluded if the non-RMD comparator group was selected from a non-representative sample such as a cluster of similar diseases (e.g., inflammatory bowel disease patients) or family members.
Meta-analyses were performed using R version 4.1.0 and the “meta” package version 4.18-1. Risk estimates were calculated using Mantel-Haenszel formula with random effect models. The risk estimates with 95% confidence intervals (CIs) were reported as risk ratios (RRs) for prevalence, and odds ratios (ORs) for hospitalization, oxygen requirement, ICU admission, mechanical ventilation, or mortality. Heterogeneity among studies was assessed using I2 and Cochran Chi-square tests. We performed sensitivity analyses to evaluate the effect of study design (limiting to cohort or cross-sectional design studies), risk of bias (limited to low risk of bias studies), country of study, and study size (limiting to studies with >20 RMD patients). Funnel plots and Egger tests were used to evaluate publication bias. P <0.05 was considered as statistically significant.
Role of Funding Source
The COVID-19 Global Rheumatology Alliance receives financial support, unrelated to this project, from Amgen, Janssen, AbbVie, Gilead, Novartis, UCB Pharma, Pfizer, GlaxoSmithKline, and Bristol-Myers Squibb. The study received support from the American College of Rheumatology (ACR).
Results
The search resulted in 13,076 articles; after duplicates were removed, 5,799 remained for title/abstract screening. Full text review was undertaken on 534 articles (Figure 1). Of these, 98 articles met the inclusion criteria. An additional two studies were identified via review of the bibliographies of included articles, resulting in 100 included studies (Supplementary Table 3)(Supplementary References 1–100). Studies were excluded due to wrong study design, no original data, irrelevant outcomes, wrong study population, duplicates, irrelevant exposures, conference abstracts, and duplicate study data (Supplementary Table 4). Six percent of studies were international. The majority of single region/country studies were from European nations (63%), with the rest from: Asia (14%), North America (13%), Worldwide (6%), South America (1%). The majority (75%) focused on adult populations, however 14% of studies included pediatric and adult populations and 1% included pediatric populations only. In 10% of studies, the age range was unspecified.
Figure 1.

PRISMA flowchart
Risk of bias assessment
Overall, the majority of studies had a low risk of bias. Of the 4 studies assessed with the Newcastle-Ottawa Scale for case control studies, 2 had a low risk of bias, 1 had some concerns, and 1 had a high risk of bias, (Supplementary Table 5). Of the 46 studies assessed with the Newcastle-Ottawa Scale for cohort studies, 33 had a low risk of bias, 6 had some concerns, and 7 had a high risk of bias, (Supplementary Table 6). Of the 37 studies assessed with the JBI checklist for prevalence studies, 25 had a low risk of bias, 8 had some concerns, and 4 had a high risk of bias (Supplementary Table 7). Of the 13 studies assessed with the JBI checklist for analytical cross-sectional studies, 8 had a low risk of bias, 2 had some concerns, and 3 had a high risk of bias, (Supplementary Table 7).
SARS-CoV-2 infection
Forty-six studies reported comparative rates of SARS-CoV-2 infection in people with RMD (Supplementary Table 8). Fifteen showed increased rates, 27 no difference, and four decreased rates. Twenty-three studies met the inclusion criteria for the meta-analysis (Supplementary Table 9). The pooled relative risk based upon unadjusted data demonstrated an increased risk of COVID-19 infection among patients with RMD, RR 1.53 (95%CI 1.16 to 2.01) (Figure 2). We observed moderately high heterogeneity (I2 = 73% (95%CI 59% to 82%), p<0.01) but did not detect evidence of publication bias (Supplementary Figure 1).
Figure 2.

Risk of SARS-CoV-2 infection in patients with rheumatic diseases versus those without rheumatic disease
Eight studies reported adjusted comparative risk measures, with two of these reporting two outcomes. Five reported increased risk: Pablos et al., OR 1.3 (95%CI 1.15 to 1.52), Zhong et al., OR 2.68 (95% CI 1.14 to 6.27), Francesconi et al., OR 1.64 (95%CI1.32 to 2.05) for rheumatoid arthritis (RA), Topless et al., OR 1.34 (95%CI 1.02 to 1.77) for RA, and Chen et al., OR 10.898 (95%CI 5.43 to 21.89)(11–14). Five showed no difference in risk, Topless et al., OR 1.01 (95%CI 0.83 to 1.23) for gout, Jung et al., OR 1.131 (95%CI 0.57 to 2.24), Francesconi et al., OR 1.09 (95%CI 0.72 to 1.66) for connective tissue disease (CTD), Salvarani et al., OR 0.94 (95%CI 0.66 to 1.34), and Kipps et al., RR 0.32 (95%CI 0.05 to 2.28) (12, 15–18). Unless otherwise specified above, studies reported risk for patients with multiple RMDs as a combined group.
Hospitalization
Seventy studies reported rates of hospitalization among patients with RMD who had COVID-19, diagnosed clinically and/or by PCR testing (Supplementary Table 10). Among these, 11 studies compared patients with RMD to the general population (n=2) or other non-RMD comparator populations (n=9), (Supplementary Table 11). Three studies showed an increased risk among patients with RMD and seven showed no significant effect. No studies found a decreased risk for hospitalization among patients with RMD. Meta-analysis of 10 comparative studies reporting unadjusted hospitalization rates did not find an increased odds of hospitalization among patients with rheumatic disease, OR 1.25 (95%CI 0.68 to 2.31), (Figure 3A).
Figure 3.

(A) Risk of Hospitalization, (B) ICU admission and (C) Mechanical Ventilation for COVID-19 in patients with rheumatic diseases versus those without rheumatic disease
Among the five studies reporting adjusted risk estimates, three included patients with clinical symptom or PCR-confirmed COVID-19 diagnoses, and two included only patients with PCR-confirmed diagnoses of COVID-19. Data from two studies demonstrated an increased risk: Cordtz et al., adjusted hazard ratio (aHR) 1.46 (95%CI 1.15 to 1.86), Reilev et al., adjusted OR (aOR) 1.5 (95%CI 1.1 to 1.9). D’Silva et al., reported an increased risk in one matched model (RR 1.14 (95%CI 1.03 to 1.26)), and a neutral risk in an extended matched model (RR 1.06 (0.96 to 1.17))(19–21). Finally, two studies reported no significant difference in risk, Serling-Boyd et al., aHR 0.87 (95%CI 0.68 to 1.11) and D’Silva et al. aORs for model 1: 1.27 (95%CI 0.61 to 2.64); model 2 1.22 (95%CI 0.56 to 2.63); model 3: 1.10 (95%CI 0.51 to 2.38)(22, 23).
Oxygen supplementation, intensive care and mechanical ventilation
Sixty-two studies (Supplementary Table 12) reported the proportion of patients requiring new oxygen supplementation (n=28), ICU admission (n=52), and mechanical ventilation (n=42) during hospitalization for COVID-19 diagnosed clinically and/or by PCR testing.
In terms of oxygen supplementation, three studies reported comparative findings in patients with and without RMD (Supplementary Table 11); one of these showed an increased risk among patients with RMD and the other two found no significant difference between the groups. No studies reported adjusted analyses.
Regarding ICU admission, 11 studies compared rates between patients with and without RMD (Supplementary Table 11), with two studies showing increased risk among patients with RMD and the remainder showing no statistical difference. Two studies reported adjusted risk estimates, including Serling-Boyd et al., (aHR 1.27 (95%CI 0.86 to 1.86)), which found no effect, and D’Silva et al., which evaluated three adjusted models, all demonstrating a positive association between RMD status and ICU admission/mechanical ventilation (aOR model 1: 3.26 (95%CI 1.17 to 9.09); aOR model 2: 3.11 (95%CI 1.07 to 9.05); aOR model 3: 2.92 (95%CI 1.002 to 8.49))(22, 23).
Finally, 8 studies compared rates of mechanical ventilation between patients with RMD and those without RMD (Supplementary Table 11). Seven studies showed no significant difference based upon RMD status, including the study by Serling-Boyd et al., which reported an adjusted HR of 1.51 (95%CI 0.93 to 2.44)(23). However, D’Silva et al., found that risk for ICU admission/mechanical ventilation was significantly increased in unadjusted OR: 3.22 (95%CI 1.16 to 8.92) and adjusted models (as described above)(22).
Pooled estimates from 7 studies reporting unadjusted comparative ICU admission rates, and 7 studies reporting unadjusted mechanical ventilation rates are shown in Figure 3B and 3C. Overall, the odds of ICU admission or mechanical ventilation were not significantly different between patients with and without RMD.
Mortality
Seventy-one studies reported mortality (Supplementary Table 13). Of these, 16 studies reported comparative mortality rates to the general population (n=7) or non-RMD comparator populations (n=9) (Supplementary Table 14). Of these, 5 reported increased risk, 9 reported a neutral effect, and 2 reported a decreased risk for RMD. Meta-analysis of 13 studies reporting comparative mortality rates showed an unadjusted odds ratio of 1.74 (95%CI 1.08 to 2.80), (Figure 4A). Moderately high heterogeneity was observed (I2 83% (95%CI 71% to 89%)), but we did not detect evidence of publication bias (Supplementary Figure 2). Among six studies focused solely on hospitalized patients, the unadjusted odds of mortality was 1.26 (95%CI 1.03 to 1.53), (Figure 4B).
Figure 4.
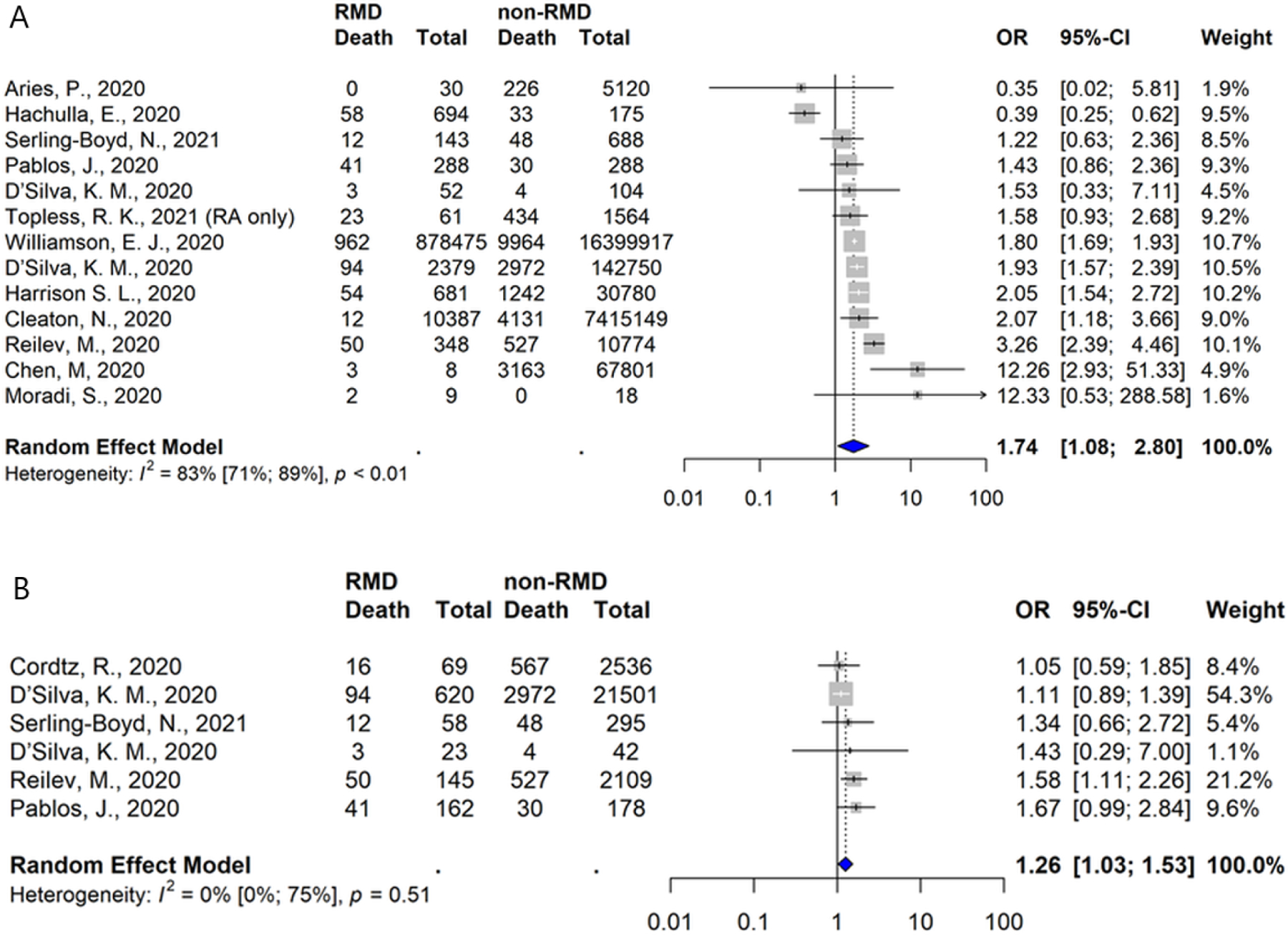
Risk of Mortality from COVID-19 in patients with rheumatic diseases versus those without rheumatic disease (A) overall and (B) among studies focused on hospitalized patients only
Seven studies reported adjusted risk estimates. Compared to the general population, Williamson et al., reported a HR of 1.19 (95%CI 1.11 to 1.27) for RA/systemic lupus erythematosus/psoriasis, Topless et al., ORs of 1.9 (95%CI 1.2 to 3.0) for RA and 1.2 (95%CI 0.9 to 1.7) for gout, and Reilev et al., an OR of 0.9 (95%CI 0.6 to 1.3) for RA/CTD (18, 21, 24). Compared to non-RMD comparators D’Silva et al., reported a RR of 1.08 (95%CI 0.81 to 1.44), Harrison et al., an OR of 1.17 (95%CI 0.85 to 1.60), Serling-Boyd et al., a HR of 1.02 (95%CI 0.53 to 1.95) and the FAI2R/SFR/SNFMI/SOFREMIP/CRI/IMIDIATE consortium an OR of 1.45 (95%CI 0.87 to 2.42)(20, 23, 25, 26).
Sensitivity Analyses
Our sensitivity analyses demonstrated overall stability in the pooled estimates for each of the outcomes when we limited the samples based upon study design (cohort or cross-sectional studies only), risk of bias (low risk of bias studies only), country of study (excluding Italy, which had a disproportionate number of studies reporting prevalence of SARS-CoV2 infection), or study size (limited to studies with >20 patients with RMD only). Not unexpectedly, 95% confidence intervals were observed to widen as sample sizes decreased (Supplementary Figures 3–10).
Discussion
In this study, we report COVID-19 outcomes in people with RMDs. In unadjusted meta-analyses, the relative risk of developing SARS-CoV-2 infection was 52% higher in patients with RMDs compared to the general population. People with RMDs were also at higher odds of a poor outcome following COVID-19 infection, with a 74% increase in odds of mortality compared to patients without RMD. Other measures of severity, including hospitalization, oxygenation requirements, ICU admission, and mechanical ventilation were not significantly higher among people with RMDs versus comparators.
Our study focused on RMDs as a combined group which limits extrapolating our findings to any individual with an RMD. This group is composed of many different diseases that have different organ manifestations, severity and treatments. Prior studies have reported differences in COVID-19 outcomes in specific rheumatic diseases (27, 28). Some RMDs (e.g., gout) may be associated with increased general COVID-19 risk factors, such as cardiovascular disease, but none of the studies included in our meta-analysis included patients with gout (29). Previous reports have also suggested a differential association of baseline use of rheumatic disease medications on outcomes (30, 31). Other factors such as age, sex, comorbidities, and disease activity have also been shown to influence COVID-19 outcomes in people with RMDs (31, 32). Due to the heterogeneity of study designs, it was not possible to statistically combine the results of included studies to generate additional pooled estimates for the overall influence of these risk factors on outcomes.
The discrepancy between the observed increased risk of COVID-19 infection and associated mortality in the absence of a corresponding increased risk of hospitalization, ICU admission, and mechanical ventilation may appear surprising. However, these findings may relate to overall power to detect differences given the smaller number of studies reporting these outcomes which may be more difficult to systematically assess. Our pooled analysis focusing only on studies of hospitalized patients (Figure 4B), allowed for comparison of RMD with non-RMD groups that maybe more similar in terms of risk factor profile (e.g., age, presence of multi-morbidity) than a general population comparator group, and still found a significantly increased odds of mortality. It is important to take into consideration, however, the smaller number of studies and smaller effect size.
This is not the first systematic literature review and meta-analysis performed assessing outcomes in patients with immune-mediated diseases. Wang, et al. performed a meta-analysis on 14 studies of COVID-19 in RMD published through October 2020, reporting a 53% increased risk of SARS-CoV-2 infection with no increased risk of death or other markers of poor outcomes (33). Xu et al. performed a meta-analysis of 31 studies of COVID-19 in rheumatic disease to August 2020 evaluating comparative risks across regions of the world but did not include a comparison to a non-RMD group (34). Akiyama, et al. performed a meta-analysis of 62 studies in patients with autoimmune diseases and COVID-19 published through July 2020; however, this study combined a more heterogeneous group of autoimmune diseases, such as inflammatory bowel disease and multiple sclerosis, which may have different outcomes as compared to RMDs (35). They showed an increased risk of SARS-CoV-2 infection in RMDs but no increase in severe outcomes in autoimmune diseases. The interpretation of the results of these previous meta-analyses is limited by the low number of included studies and patients, as evidenced by the generally wide confidence intervals for the reported risk estimates.
Applying these results to clinical care is complex, but this analysis suggests that people with RMDs are at a greater risk of developing SARS-CoV-2 infection and severe COVID-19 as compared to the general population. The reasons for this lie outside the scope of the present work, but three plausible explanations should be considered. First, bias from greater baseline exposure to the healthcare system or a lower threshold for seeking care when symptomatic could falsely inflate the rate of COVID-19 among people with RMD. Second, people with RMD may have a greater burden of comorbidities, which have previously been associated with worse outcomes. Finally, it may be that immune dysregulation related to RMD treatments or to the RMDs themselves may result in higher rates of symptomatic infections and higher rates of severe outcomes. (20, 30, 36). All three of these explanations may account for prior observations that higher RMD disease activity is associated with worse outcomes in COVID-19, since such patients are more likely to be identified, more likely to have comorbidities, and more likely to have immune dysregulation or immunosuppressive therapies (31). Regardless of the cause, people with RMDs should be encouraged to be vaccinated against SARS-CoV-2 and to employ risk mitigation strategies as able (37, 38).
There are considerable strengths to this study. Our study comprehensively identified potential studies from 14 databases through February 2021, making it the most current literature review and meta-analysis of COVID-19 in RMDs. We assembled a geographically diverse study team, enabling inclusion of studies in all available languages. This is particularly relevant in COVID-19 which exhibits wide regional variation in outcomes (39). To ensure reliability of the search and data extraction process, these tasks were performed manually; machine learning methods are being developed to streamline this process and these approaches have potential strengths but remain exploratory at this time (40). Despite these strengths, our study had several limitations. The included studies demonstrated significant heterogeneity in design and reporting, as evidenced by the formal tests of heterogeneity performed in the meta-analysis. The study protocol was created a priori; the increased volume of relevant materials rapidly published during the COVID-19 pandemic resulted in an amendment to the protocol to exclude case reports and case series. COVID-19 outcomes have changed, and generally improved, over time, which may limit the comparability of cohorts assembled at different periods during the pandemic (41, 42). Due to the small number of studies reporting adjusted risks, our meta-analysis was confined to an analysis of unadjusted numbers. However, we have presented the complementary adjusted risks from individual studies reporting these. The interpretation of unadjusted risks is complicated by the potential imbalance of other risk factors between the RMD and general populations.
In conclusion, we have performed the most comprehensive systematic literature review and meta-analysis to date assessing COVID-19 outcomes in people with RMDs. Our analyses show that people with RMD have higher rates of SARS-CoV-2 infection and mortality from COVID-19 in unadjusted analyses. This may be mediated through factors other than the RMD itself.
Supplementary Material
Acknowledgements:
The authors would like to thank all members of the COVID-19 Global Rheumatology Alliance
Funding, grant/award info:
The COVID-19 Global Rheumatology Alliance receives financial support, unrelated to this project, from Amgen, Janssen, AbbVie, Gilead, Novartis, UCB Pharma, Pfizer, GlaxoSmithKline, and Bristol-Myers Squibb. The study received support from the American College of Rheumatology (ACR).
Footnotes
ACR/EULAR Disclaimer Statement: The views expressed here are those of the authors and participating members of the COVID-19 Global Rheumatology Alliance and do not necessarily represent the views of the American College of Rheumatology (ACR), the European League Against Rheumatism (EULAR), the (UK) National Health Service (NHS), the National Institute for Health Research (NIHR), or the (UK) Department of Health, or any other organization.
Ethical approval information: Not applicable
Patient and Public Involvement: Patients and/or the public were involved in the design, conduct, reporting, and dissemination plans of this research. LP is a patient partner and provided feedback on the study protocol, contributed to data collection, and writing the manuscript.
Registration
PROSPERO: CRD42020205668
Competing interests:
Richard Conway: Speakers bureau: Janssen, Roche, Sanofi, Abbvie.
Alyssa A Grimshaw: None declared.
Maximilian F Konig: None declared.
Alí Duarte-García: Grant/research support from the Rheumatology Research Foundation and the Centers for Disease Control and Prevention.
Michael Putman: None declared. Grant/research support from the Rheumatology Research Foundation.
Eimear Duff: None declared.
Patricia Harkins: None declared.
Candice Low: None declared.
Diego M. Cabrera: None declared.
Adam Kilian: None declared.
Jean Liew: Grant/research support from: Pfizer.
Rebecca Grainger: Participated in speakers bureau or advisory board for Pfizer, Cornerstones, Janssen, Novartis, Abbvie.
Kristen Young: None declared.
Laurie Proulx: LP is on the executive of the Canadian Arthritis Patient Alliance, an organization that receives most of its funding through unrestricted and restricted educational grants from pharmaceutical companies.
Kaicheng Wang: None declared.
Bimba Hoyer: None declared.
Alfred Kim: Participated in consulting, advisory board, or speaker’s bureau for Alexion Pharmaceuticals, Aurinia Pharmaceuticals, Annexon Biosciences, Exagen Diagnostics, Inc., and GlaxoSmithKilne and received funding under a sponsored research agreement unrelated to the data in the paper from GlaxoSmithKline.
Arundathi Jayatilleke: None declared.
Yu Pei Eugenia Chock: None declared.
Sebastian Sattui: None declared. Grant/research support from the Vasculitis Clinical Research Consortium and Vasculitis Foundation.
Manuel F. Ugarte.Gil: Research support from Pfizer and Janssen. Not related to this paper.
Chung Mun Alice Lin: None declared.
Leanna Wise: Speaker’s Bureau and consulting for Aurinia Pharm, unrelated to this work
Jeffrey Sparks: Consultancy for AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Optum, and Pfizer unrelated to this work
Zachary Wallace: Consulting for MedPace and Viela Bio. Grant Support from Bristol-Myers Squibb and Sanofi/Principia. All unrelated to this work.
Namrata Singh: None declared. Research support from the Rheumatology Research Foundation and the American Heart Association
Evelyn Hsieh: None Declared.
Data sharing statement:
Request for access to data should be made to the Data Access and Sharing Committee of the COVID-19 Global Rheumatology Alliance.
References
- 1.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ (Clinical research ed). 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. The European respiratory journal. 2020;55(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Listing J, Gerhold K, Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology (Oxford). 2013;52(1):53–61. [DOI] [PubMed] [Google Scholar]
- 4.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed). 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. Journal of clinical epidemiology. 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- 6.Wells G, Shea B, O’Connell D, Robertson J, Peterson J, Welch V. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta- analysis [Internet]. [cited 2021. Mar 17]. [Google Scholar]
- 7.Moola S. Chapter 7: systematic reviews of etiology and risk in: Aromataris E, Munn Z, eds Joanna Briggs institute reviewer’s manual. The Joanna Briggs Institute, 2017. [Google Scholar]
- 8.Monteiro F, Canavarro MC, Pereira M. Prevalence and correlates of psychological distress of middle-aged and older women living with HIV. Psychology Health & Medicine. 2017;22(9):1105–17. [DOI] [PubMed] [Google Scholar]
- 9.Viswanathan M, Patnode CD, Berkman ND, Bass EB, Chang S, Hartling L, et al. Recommendations for assessing the risk of bias in systematic reviews of health-care interventions. Journal of clinical epidemiology. 2018;97:26–34. [DOI] [PubMed] [Google Scholar]
- 10.Putman M, Chock YPE, Tam H, Kim AHJ, Sattui SE, Berenbaum F, et al. Antirheumatic Disease Therapies for the Treatment of COVID-19: A Systematic Review and Meta-Analysis. Arthritis & Rheumatology. 2021;73(1):36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen M, Wei Y, Zhang Q, Wan Q, Chen X. Epidemiology and clinical characteristics of COVID-19 in rheumatic diseases at a tertiary care hospital in Wuhan, China. Clinical and experimental rheumatology. 2020;01. [PubMed] [Google Scholar]
- 12.Francesconi P, Cantini F, Profili F, Mannoni A, Bellini B, Benucci M. COVID-19 epidemiology in rheumatic diseases in Tuscany: A case-control study. Joint, bone, spine : revue du rhumatisme. 2021;88 (3) (no pagination)(105131). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong J, Shen G, Yang H, Huang A, Chen X, Dong L, et al. COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. The Lancet Rheumatology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pablos JL, Abasolo L, Alvaro-Gracia JM, Blanco FJ, Blanco R, Castrejon I, et al. Prevalence of hospital PCR-confirmed COVID-19 cases in patients with chronic inflammatory and autoimmune rheumatic diseases. Annals of the rheumatic diseases. 2020;(no pagination)(annrheumdis-2020–217763). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung SY, Kim MS, Kim MC, Choi SH, Chung JW, Choi ST. Effect of hydroxychloroquine pre-exposure on infection with SARS-CoV-2 in rheumatic disease patients: a population-based cohort study. Clinical Microbiology and Infection. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kipps S, Paul A, Vasireddy S. Incidence of COVID-19 in patients with rheumatic disease: is prior health education more important than shielding advice during the pandemic? Clinical rheumatology.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvarani C, Mancuso P, Gradellini F, Viani N, Pandolfi P, Reta M, et al. Susceptibility to COVID-19 in Patients Treated With Antimalarials: A Population Based Study in Emilia-Romagna, Northern Italy. Arthritis and Rheumatology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topless RK, Phipps-Green A, Leask M, Dalbeth N, Stamp LK, Robinson PC, et al. Gout, Rheumatoid Arthritis, and the Risk of Death Related to Coronavirus Disease 2019: An Analysis of the UK Biobank. ACR Open Rheumatol. 2021;3(5):333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cordtz R, Lindhardsen J, Soussi BG, Vela J, Uhrenholt L, Westermann R, et al. Incidence and severeness of COVID-19 hospitalisation in patients with inflammatory rheumatic disease: a nationwide cohort study from Denmark. Rheumatology. 2020;28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Silva KM, Jorge A, Cohen A, McCormick N, Zhang Y, Wallace ZS, et al. COVID-19 Outcomes in Patients with Systemic Autoimmune Rheumatic Diseases (SARDs) Compared to the General Population: A US Multi-Center Comparative Cohort Study. Arthritis & rheumatology. 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reilev M, Kristensen KB, Pottegård A, Lund LC, Hallas J, Ernst MT, et al. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. International journal of epidemiology. 2020;49(5):1468–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Silva KM, Serling-Boyd N, Wallwork R, Hsu T, Fu X, Gravallese EM, et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US ‘hot spot’. Annals of the rheumatic diseases. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serling-Boyd N, D’Silva KM, Hsu TYT, Wallwork R, Fu X, Gravallese EM, et al. Coronavirus disease 2019 outcomes among patients with rheumatic diseases 6 months into the pandemic. Annals of the rheumatic diseases. 2020;(no pagination)(annrheumdis-2020–219279). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison SL, Fazio-Eynullayeva E, Lane DA, Underhill P, Lip GYH. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: A federated electronic medical record analysis. PLoS Medicine. 2020;17 (9) (no pagination)(e1003321). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Florence A, Nassim AA, Jean-David A, Didier A, Yannick A, Blanca AB, et al. Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: Data from the French RMD COVID-19 cohort of 694 patients. Annals of the rheumatic diseases. 2020;(no pagination)(218310). [Google Scholar]
- 27.Conway R, Nikiphorou E, Demetriou C, Low C, Leamy K, Ryan J, et al. POS1162 PREDICTORS OF HOSPITALISATION IN PATIENTS WITH RHEUMATIC DISEASE AND COVID-19 IN IRELAND: DATA FROM THE COVID-19 GLOBAL RHEUMATOLOGY ALLIANCE PHYSICIAN-REPORTED REGISTRY. Annals of the rheumatic diseases. 2021;80(Suppl 1):859–60.33568387 [Google Scholar]
- 28.Pablos JL, Galindo M, Carmona L, Lledo A, Retuerto M, Blanco R, et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Annals of the rheumatic diseases. 2020. [DOI] [PubMed] [Google Scholar]
- 29.Dalbeth N, Robinson PC. Patients with gout: an under-recognised group at high risk of COVID-19. The Lancet Rheumatology. 2021;3(5):e317–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sparks JA, Wallace ZS, Seet AM, Gianfrancesco MA, Izadi Z, Hyrich KL, et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: Results from the COVID-19 Global Rheumatology Alliance physician registry. Annals of the rheumatic diseases. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strangfeld A, Schäfer M, Gianfrancesco MA, Lawson-Tovey S, Liew JW, Ljung L, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Annals of the rheumatic diseases. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasseli R, Mueller-Ladner U, Hoyer BF, Krause A, Lorenz HM, Pfeil A, et al. Older age, comorbidity, glucocorticoid use and disease activity are risk factors for COVID-19 hospitalisation in patients with inflammatory rheumatic and musculoskeletal diseases. RMD open. 2021;7 (1) (no pagination)(e001464). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Q, Liu J, Shao R, Han X, Su C, Lu W. Risk and clinical outcomes of COVID-19 in patients with rheumatic diseases compared with the general population: a systematic review and meta-analysis. Rheumatology international. 2021;41(5):851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu C, Yi Z, Cai R, Chen R, Thong BY, Mu R. Clinical outcomes of COVID-19 in patients with rheumatic diseases: A systematic review and meta-analysis of global data. Autoimmunity reviews. 2021;20(4):102778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Annals of the rheumatic diseases. 2020. [DOI] [PubMed] [Google Scholar]
- 36.Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. The New England journal of medicine. 2021;384(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Annals of the rheumatic diseases. 2020;79(6):685–99. [DOI] [PubMed] [Google Scholar]
- 38.Fraenkel L, Bathon JM, England BR, St.Clair EW, Arayssi T, Carandang K, et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis care & research. 2021;73(7):924–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazumder A, Arora M, Sra MS, Gupta A, Behera P, Gupta M, et al. Geographical variation in case fatality rate and doubling time during the COVID-19 pandemic. Epidemiology and infection. 2020;148:e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van de Schoot R, de Bruin J, Schram R, Zahedi P, de Boer J, Weijdema F, et al. An open source machine learning framework for efficient and transparent systematic reviews. Nature Machine Intelligence. 2021;3(2):125–33. [Google Scholar]
- 41.Anesi GL, Jablonski J, Harhay MO, Atkins JH, Bajaj J, Baston C, et al. Characteristics, Outcomes, and Trends of Patients With COVID-19-Related Critical Illness at a Learning Health System in the United States. Annals of internal medicine. 2021;174(5):613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roth GA, Emmons-Bell S, Alger HM, Bradley SM, Das SR, de Lemos JA, et al. Trends in Patient Characteristics and COVID-19 In-Hospital Mortality in the United States During the COVID-19 Pandemic. JAMA Network Open. 2021;4(5):e218828–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Request for access to data should be made to the Data Access and Sharing Committee of the COVID-19 Global Rheumatology Alliance.


