Abstract
Objective:
Juvenile fibromyalgia (JFM) is a prevalent chronic pain condition affecting children and adolescents worldwide during a critical period of brain development. To date, no published studies have addressed the pathophysiology of JFM. Here we characterize gray matter volume (GMV) alterations in JFM patients for the first time and investigate their functional and clinical relevance.
Methods:
34 female adolescents with JFM and 38 healthy adolescents underwent a structural magnetic resonance imaging examination and completed questionnaires assessing core JFM symptoms. Using voxel-based morphometry, we assessed between-group GMV differences and associations between GMV and functional disability, fatigue, and pain interference in JFM. We also studied whether validated brain patterns predicting pain, cognitive control, or negative emotion were amplified/attenuated in JFM patients and whether structural alterations reported in adult fibromyalgia replicated in adolescents with JFM.
Results:
Compared to controls, JFM patients showed GMV reductions in the anterior-midcingulate cortex (MCC) region associated with pain. Within the JFM group, patients reporting higher functional disability had larger GMV in inferior frontal regions linked to affective, self-referential, and language-related processing. Last, GMV reductions in JFM showed partial overlap with findings in adult fibromyalgia, specifically for the anterior/posterior cingulate cortices.
Conclusion:
Pain-related MCC reductions may be a structural hallmark of JFM, whereas alterations in regions involved in emotional, self-referential, and language-related processes may predict disease impact on patients’ well-being. The partial overlap between juvenile and adult fibromyalgia findings strengthens the importance of early symptom identification and intervention to prevent the transition to adult forms of the disease.
1. Introduction
Juvenile fibromyalgia (JFM) affects 2–6% of children and adolescents, mostly females, and is characterized by widespread musculoskeletal pain and other debilitating symptoms such as functional disability and fatigue. JFM symptoms often persist into adulthood highlighting the importance of early recognition and intervention (1,2). Research has provided insight into the clinical characteristics of JFM (2,3), but there is a research gap regarding disease pathophysiology. So far, a recent review provided the first robust evidence of central hyperexcitability in children with chronic pain and preliminarily suggested altered cortical nociceptive processing (4). Additionally, a functional magnetic resonance imaging (fMRI) study showed that adolescents with idiopathic pain had decreased activation in the thalamus and precentral and middle frontal gyri during pain processing (5).
Conversely, neuroimaging research in adult fibromyalgia has flourished over the past two decades, suggesting that chronic pain symptoms are associated with brain alterations involving multiple circuits and functional domains (6–11). At the structural level, meta-analyses in adult fibromyalgia have consistently found gray matter volume (GMV) reductions in regions involved in affective and cognitive dimensions of pain such as the anterior-mid cingulate cortex (MCC), the medial prefrontal cortex (mPFC), and the default-mode network (6,7). Such reductions have been in part correlated with age, leading researchers to suggest that atrophy in older fibromyalgia patients may result from long-term exposure to nociceptive input (7,12,13). The opposite was hypothesized for younger patients for whom recurrent over-engagement of pain modulatory circuits could result in hypertrophy earlier in the disease course followed by atrophy later in life (12,13). Importantly, GMV loss in adult fibromyalgia has been identified beyond pain-processing regions in brain circuits involved in self-referential processing, executive function, and emotion regulation (14), highlighting the importance of studying whole-brain structural alterations. Moreover, studies have shown a strong correspondence between structural and functional brain abnormalities, specifically in cingulate and dorsal mPFC regions (15,16). Therefore, studies combining structural findings with functional meta-analytic decoding, or validated brain patterns predicting relevant functional domains may help identify a link between structural abnormalities and their functional relevance in chronic pain patients.
Pathophysiological findings at an early disease stage could inform interventions that may more effectively target the multidimensional symptom constellation in JFM. In this line, fMRI research in adult fibromyalgia has linked pain catastrophizing with increased S1-anterior insula functional connectivity, which normalized after cognitive-behavioral therapy (17). Likewise, it has shown that alterations in the descending pain inhibition pathway improved with regular exercise (18). Therefore, we hypothesize that, if replicated in youth with JFM, such alterations could be modulated with similar treatment strategies as in adults. New insights regarding whether distinct pathophysiological findings predict different treatment response trajectories may help tailor first treatment actions and most appropriate treatment combinations in a personalized manner. Such knowledge might also contribute to the development of new psychobiologically-informed treatments, which could potentially alter the trajectory of JFM symptoms and foster a healthier transition into adulthood (19).
In this study, we investigated GMV abnormalities in adolescent females with JFM and associations with disease-related functional interference. We combined voxel-based morphometry (VBM) and functional meta-analytic decoding of structural data to provide insight into potential functional roles of structural alterations in JFM. Based on findings in adult fibromyalgia (6,7), we hypothesized that 1. compared to healthy adolescents, JFM patients would show alterations in the cingulate and medial prefrontal cortices and the parahippocampal gyrus, and 2. within the JFM group, individual differences in functional disability, fatigue, and pain interference would be associated with structural abnormalities in circuits involved in emotional processing and cognitive modulation of pain and emotion (anterior insula, prefrontal cortices, limbic and striatal regions) (20,21). We also investigated whether three validated brain patterns involving medial prefrontal regions and predicting pain, cognitive control, or negative emotion (22), were amplified or attenuated in JFM. Given that amplified pain is a core JFM symptom, we anticipated between-group differences specifically for the pain-predictive pattern. Finally, we tested whether structural alterations reported in adult fibromyalgia replicated in adolescents with JFM to potentially identify a link between structural abnormalities in juvenile and adult forms of the disease.
2. Methods
2.1. Participants
This study included 34 adolescent girls diagnosed with JFM (13–18 years; mean age: 16.37±1.07 years) and 38 healthy adolescent girls (13–18 years; mean age: 15.89±1.32 years). The sample size was decided assuming similar power as previous studies assessing structural differences between adults with fibromyalgia and healthy controls using VBM (studies included in meta-analyses (6,7)). We enrolled exclusively female participants because most chronic pain conditions occurring in adolescence predominantly affect girls (23) and because males with JFM are sufficiently rare as to raise questions about the potential for a distinct mechanism (24). Inclusion and exclusion criteria are detailed in Supplement 1. Participants provided informed assent and their parents or legal guardians provided written informed consent. The study protocol and consent forms were approved by the Institutional Review Board of the Cincinnati Children’s Hospital Medical Center (Study ID: 2017–7771) in compliance with the Helsinki Declaration.
2.2. Clinical measures
Developmentally appropriate and validated self-report measures were used to assess disability, fatigue, and pain interference. Participants self-reported their functional disability (physical difficulty experienced when doing their daily activities) using the 15-item Functional Disability Inventory (FDI) (25), and their fatigue symptoms over the past week completing the 10-item PROMIS Pediatric Fatigue-Short Form (26). Additionally, JFM patients completed the Brief Pain Inventory (BPI) (27), from which we extracted average pain intensity and pain interference scores. Healthy adolescents did not complete the BPI because they were selected based on having no pain.
2.3. Imaging data acquisition
MRI data acquisition is detailed in Supplement 2.
2.4. Data analysis
2.4.1. Statistical analyses of demographic and clinical variables
Differences between JFM patients and healthy adolescents in sociodemographic and clinical variables were analyzed with χ2 and two-sample t-tests in SPSSv26 software (IBM Corp, Armonk, NY, USA).
2.4.2. Voxel-based morphometry protocol
VBM quantifies gray matter at a voxel-wise, whole-brain level (28), allowing for a comprehensive measurement of gray matter throughout the brain. Here, structural data were preprocessed using MATLAB-R2021a (Math Works Inc, Natick, MA) and SPM12 (UCL, London, UK). First, a trained researcher reviewed all images to ensure they were free from acquisition artifacts and magnetic field inhomogeneities. Then, images were preprocessed using a standard pipeline combining VBM with Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra algorithm (DARTEL) (29) and including four main steps: tissue segmentation, normalization, modulation, and Gaussian smoothing. After segmentation, the resulting rigidly transformed versions of gray matter were normalized using DARTEL. Specifically, images were iteratively matched to a template generated from their own average to generate a series of templates with increasing resolution. Native space gray matter images were then registered to the highest resolution template within a high-dimensional diffeomorphic framework. Since the DARTEL method creates a study-specific template and then spatially normalizes individual images to this template, it provides better registration, specifically in the boundaries of gray and white matter, than previous VBM methods (29). Spatially normalized tissue maps were modulated by the Jacobian determinants from the corresponding flow fields to restore the volumetric information lost during the high-dimensional spatial registration. This modulation step multiplies each voxel by the relative change in volume, which allows for a comparison of absolute GMV corrected for individual brain size (30). Only normalized and modulated images were transformed to a standard MNI template and re-sliced to 1.5mm. Therefore, volume computations were not affected by this final transformation to MNI space. Similarly, multiple neuroimaging studies have used this template in samples of adolescents (e.g., (31)). Moreover, using a standard MNI templates enables us to compare our findings with the results of previous studies using this template (e.g., (12)). Finally, images were smoothed with an 8mm full-width at half-maximum isotropic Gaussian kernel. A trained researcher reviewed all processed images to ensure image quality.
2.4.3. Statistical analyses of brain structural data
To assess regional GMV differences between JFM patients and controls, we estimated a t-test model with SPM12. As a standard procedure in VBM analyses (32), we added age and total GMV as nuisance variables, and excluded all voxels with a gray matter value<.2 (maximum value: 1).
To test associations between regional GMV and clinical variables in JFM patients, we performed multiple regression models in SPM12 with the following independent variables of interest in each separate model: 1. functional disability score from the FDI, 2. fatigue symptoms, measured with the PROMIS form, and 3. pain interference score from the BPI. Each model included age and total GMV as nuisance covariates and GMV as the dependent variable. We excluded voxels with a gray matter value<.2.
To correct for multiple comparisons, we performed, for each analysis, a voxel-wise non-parametric permutation testing with 5,000 random permutations using the Threshold-Free Cluster Enhancement (TFCE) approach (33) implemented in the SPM-TFCEr214 toolbox (dbm.neuro.uni-jena.de/tfce), and the whole-brain significance threshold was set at p<.05, Family-Wise Error (FWE) corrected. The TFCE approach was introduced to increase sensitivity of voxel-based analysis (33) and is currently recommended and widely used in VBM studies (34). Compared to voxel-wise or cluster-wise inferences, TFCE inference improves power and validity and relies on minimal assumptions about data distribution (33). For completion, imaging results were also explored at an uncorrected threshold of p<.001 and a cluster extent (Ke)>50 voxels.
2.4.4. Neurosynth meta-analytic decoding
To interpret the functional role of structural regions differing in GMV between JFM patients and controls or associated with clinical symptoms in the JFM group, we performed meta-analytic decoding using the Neurosynth database (35) (neurosynth.org). This meta-analytic strategy leverages power of large datasets to compute whole-brain distributions for psychological terms. Thus, it allows performing unbiased reverse-inferences to identify terms consistently associated with a particular brain coordinate across neuroimaging studies (35). Here, we uploaded the unthresholded t-maps produced in our study onto the Neurosynth database and decoded them, which returned psychological and anatomical terms associated with the spatial pattern of each analysis (Supplementary Table 1 shows a list of the 50 neurosynth terms most correlated with each t-map and their Pearson coefficients). For interpretation purposes, we selected the 10 psychological terms (i.e., referring to psychological functions) most correlated with each map and displayed them in Figures 1 and 2 (36).
Figure 1. Regions of significantly reduced gray matter volume in adolescents with JFM.
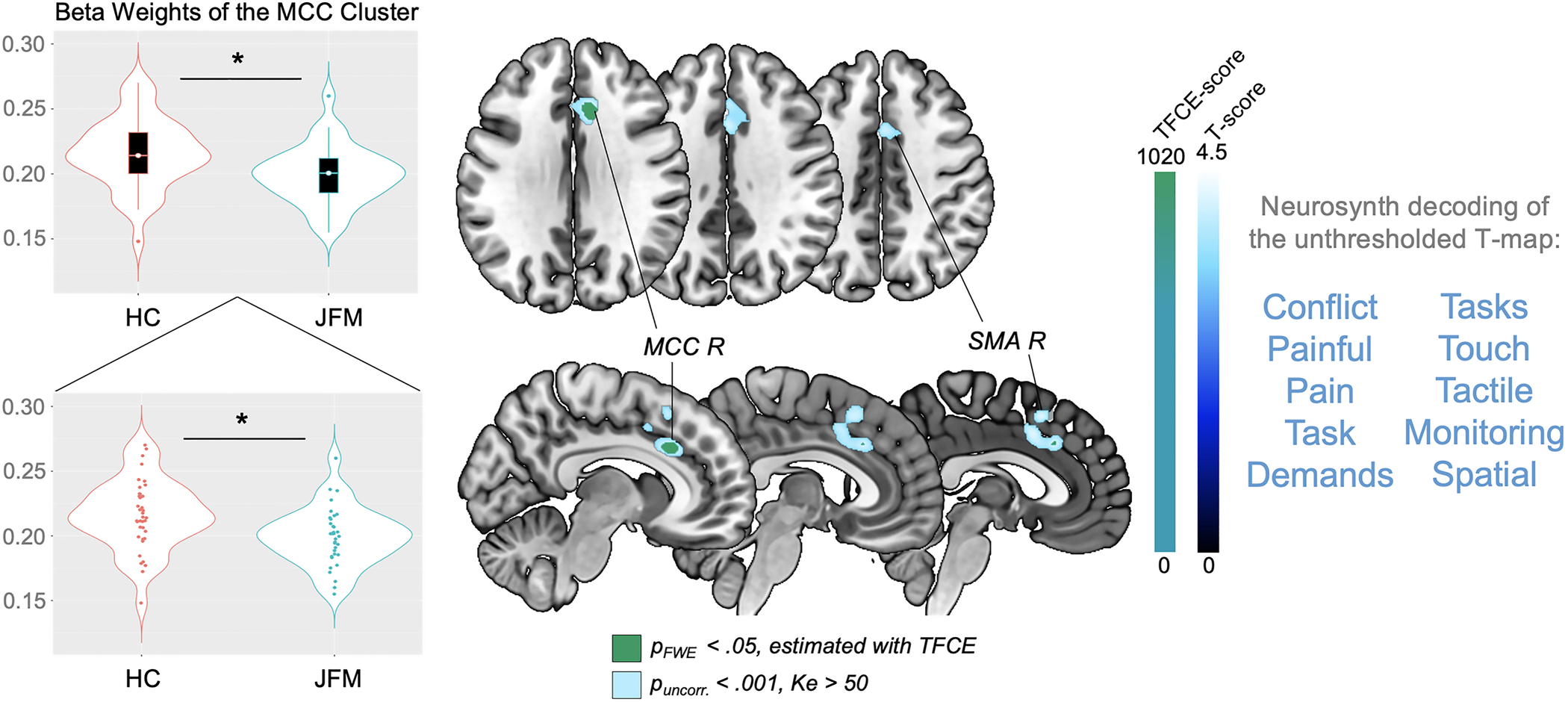
Significant between-group gray matter volume differences (contrast: JFM < Controls). Results are presented at a significance level of pFWE-corr<.05, estimated with the TFCE approach (in green) and puncorr.<.001, Ke>50 voxels (in blue). At the right of the image, we display the 10 functional annotations most associated with the unthresholded t-map, as obtained with meta-analytic decoding using Neurosynth. FWE-corr: Family-Wise Error-corrected; HC: Healthy Controls; JFM: Juvenile Fibromyalgia; Ke: Cluster extent in voxels; L: Left; MCC: Anterior-Midcingulate Cortex; R: Right; SMA: Supplementary Motor Area; TFCE: Threshold-Free Cluster Enhancement; uncorr: uncorrected.
Figure 2. Gray matter volume changes associated with clinical symptoms in adolescents with JFM.
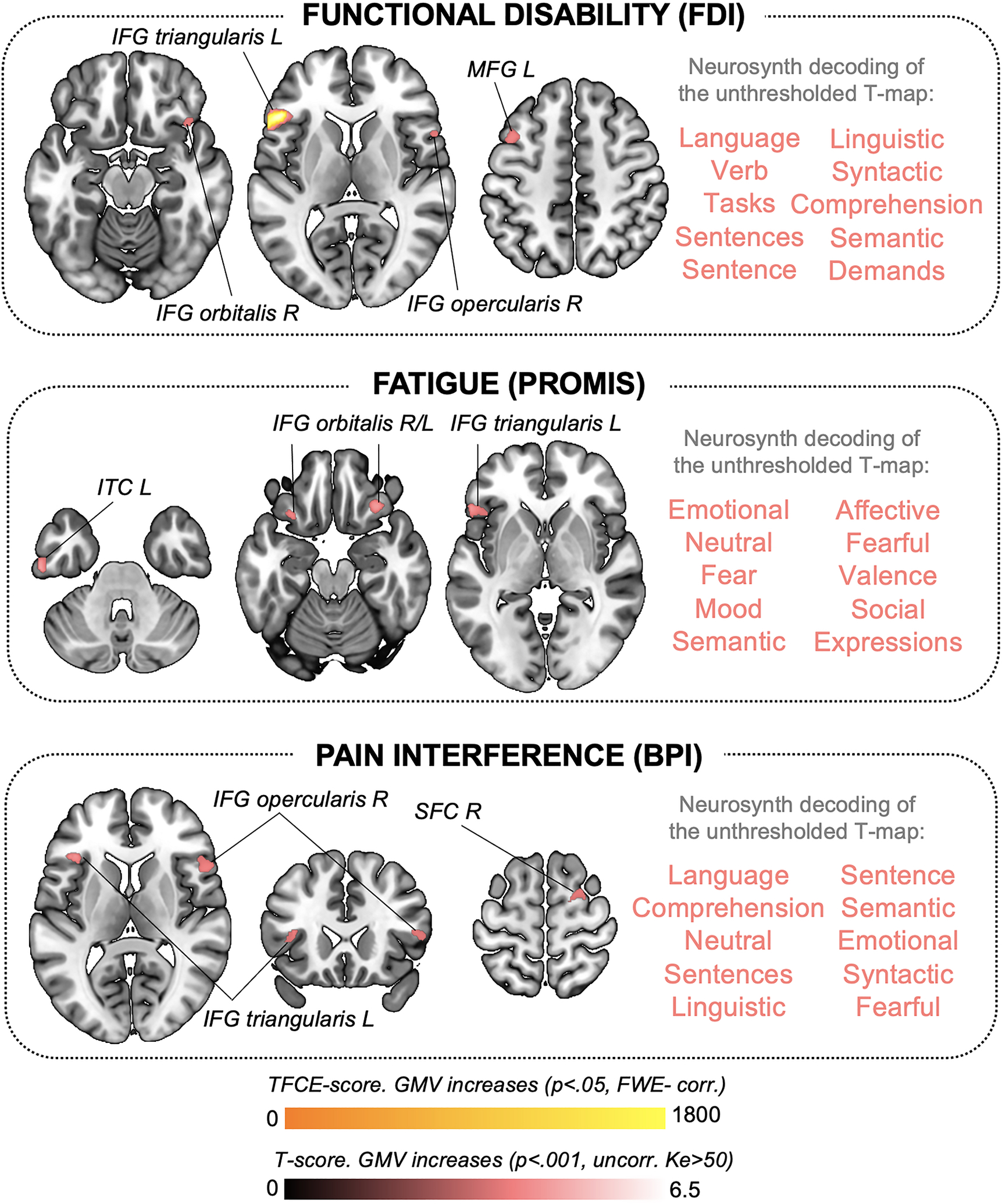
Significant correlations between gray matter volume (GMV) and clinical symptoms reflecting disease-related functional interference in adolescents with JFM, including functional disability (top), fatigue (middle), and pain interference (bottom). Results are presented at a significance level of and pFWE-corr<.05 (in yellow for GMV increases) and puncorr.<.001, Ke>50 voxels (in red for GMV increases). We did not observe negative correlations at the pFWE-corr or puncorr. level. At the right of each brain map representing our significant results, we display the 10 functional annotations most associated with the corresponding unthresholded t-map, as obtained with meta-analytic decoding using Neurosynth. BPI: Brief Pain Inventory; FDI: Functional Disability Inventory; FWE-corr: Family-Wise Error-corrected; IFG: Inferior Frontal Gyrus; ITC: Inferior Temporal Cortex; JFM: Juvenile Fibromyalgia; Ke: Cluster extent in voxels; L: Left; MFG: Middle Frontal Gyrus; PROMIS: Patient-Reported Outcomes Measurement Information System; R: Right; SFC: Superior Frontal Cortex; TFCE: Threshold-Free Cluster Enhancement; uncorr: uncorrected.
2.5. Patterns predicting pain, cognitive control, and negative emotion in JFM
Our VBM analysis showed that JFM patients had reduced GMV in the right MCC. To study the functional organization of the medial frontal cortex (MFC) surrounding this region, we tested whether the similarity between our structural maps and previously validated brain patterns predicting either pain, cognitive control, or negative emotion across 18 studies and specifically involving the MCC-dorsal MFC (22) differed between JFM patients and healthy adolescents.
The maps developed by Kragel et al. (22) corresponding to the Partial Least Squares regression coefficients for the different functional domains being predicted are available at the Canlab repository (github.com/canlab/Neuroimaging_Pattern_Masks/tree/master/Multivariate_signature_patterns/2018_Kragel_MFC_Generalizability). Here, we extracted the weighted maps corresponding to pain, cognitive control, and negative emotion in the dorsomedial aspect of the frontal cortex including anterior and posterior MCC and dorsal MFC. For each subject, we computed the dot product between our preprocessed structural images, regressed by age and total GMV, and pain, cognitive control, and negative emotion weighted maps. Dot product was calculated using publicly available code at the Canlab repository (canlab.github.io) running on MATLAB. We then ran two-sample t-tests with SPSS to assess between-group differences in pattern similarity for each weighted map.
2.6. Gray matter volume differences in regions found altered in adults with fibromyalgia
To identify potentially overlapping alterations between adult and juvenile forms of fibromyalgia, we assessed GMV alterations in regions of interest (ROIs) identified based on previous meta-analytic evidence in adult fibromyalgia patients. The selection and construction of ROIs is detailed in Supplement 3. For each subject, we computed the average parameter estimates (beta values representing GMV for each subject after removing age and total GMV effects) within each of the 6 final ROIs. Finally, we assessed between-group differences in average parameter estimates for each ROI using two-sample t-tests in SPSS.
3. Results
3.1. Demographic and clinical variables
Adolescents with JFM and healthy controls did not differ in terms of sex (all female), age, race, household income, and caregiver education level (p’s>.1, Table 1). As anticipated, JFM patients reported significantly higher functional disability (t=13.89, p<.001) and fatigue symptoms (t=12.30, p<.001) (Table 1). Since controls were selected based on having no pain (numerical rating scales for pain=0), average pain intensity and pain interference were only assessed in JFM participants (mean intensity: 5.45±1.42; mean interference: 4.29±1.55), indicating moderate intensity and mild to moderate interference. Medication details are presented in Table 1.
Table 1.
Differences between adolescents with JFM and healthy controls in demographic, brain volume and clinical variables and medication
| Controls (n = 38) | JFM (n = 34) | |||
|---|---|---|---|---|
| DEMOGRAPHIC VARIABLES | Mean ± SD | Mean ± SD | t/LRV | p-value |
| Age | 15.89 ± 1.32 | 16.37 ± 1.07 | −1.67 | 0.10 |
| Race (# Caucasian/non-Caucasian) | 35 / 3 | 32 / 2 | 0.11 | 0.74 |
| Average Household Income (1–6) | 5.13 ± 2.07 | 5.09 ± 2.02 | 0.09 | 0.93 |
| Education Caregiver 1 (1–5) | 3.97 ± 0.88 | 3.88 ± 0.91 | 0.43 | 0.67 |
| Education Caregiver 2 (1–5) | 3.74 ± 0.78 | 3.63 ± 0.98 | 0.55 | 0.58 |
| BRAIN VOLUME | Mean ± SD | Mean ± SD | t | p-value |
| Total GM volume | 787.70 ± 59.87 | 785.50 ± 53.17 | 0.16 | 0.87 |
| Total WM volume | 384.27 ± 40.42 | 399.49 ± 36.89 | −1.66 | 0.10 |
| CLINICAL VARIABLES | Mean ± SD | Mean ± SD | t | p-value |
| Functional Disability | 0.5 ± 1.25 | 22.74 ± 9.26 | −13.89 | 0.00* |
| Fatigue (PROMIS) | 14.28 ± 6.64 | 35.23 ± 6.87 | −12.30 | 0.00* |
| Pain Interference | - | 4.29 ± 1.55 | - | - |
| Average Pain Intensity | - | 5.43 ± 1.42 | - | - |
| MEDICATION | # (%) | # (%) | ||
| Pain-related drugsa | - | 12 (35.29%) | ||
| Psychiatric drugsb | - | 15 (44.12%) | ||
| Gastrointestinal drugsc | - | 7 (20.59%) | ||
| Melatonin | - | 3 (8.82%) | ||
| Antihistamines | - | 3 (8.82%) | ||
| Vitamins / Iron Supplements | - | 3 (8.82%) | ||
| Birth Control | 2 (5.26%) | 2 (5.88%) | ||
| Hypertension | 1 (2.63%) | - | ||
| Statins | 1 (2.63%) | - | ||
Note: Average Household Income: 1 = < $24,999; 2 = $25,000 – $49,999; 3 = $50,000 – $74,999; 4 = $75,000 – $99,000; 5 = $100,000 - $124,999; 6 = > $125,000. Caregiver Education Level: 1 = Less than High School; 2 = High School/GED; 3 = Partial College or Trade School; 4 = College Graduate; 5 = Post Graduate Degree.
Pain-related drugs included: antiepileptic drugs, non-steroidal anti-inflammatory drugs, muscle relaxants, acetaminophen, and/or acetylsalicylic acid;
Psychiatric drugs included: antidepressants, anxiolytics, and ADHD drugs;
Gastrointestinal drugs included: antiacids, antireflux and constipation drugs.
GM: Gray Matter; JFM: Juvenile Fibromyalgia; LRV: Likelihood Ratio Value; NRS: Numerical Rating Scale; SD: Standard Deviation; WM: White Matter.
Significant results at p<.001.
3.2. Whole-brain voxel-based morphometry analyses
3.2.1. Reductions in gray matter volume in JFM
The groups did not differ in total GMV (p=.87). Compared to controls, JFM patients had significantly less regional GMV in a cluster of the right MCC (pFWE-corr=.04, TFCE-estimated). At the uncorrected threshold (p<.001; Ke>50), this cluster extended to the left MCC and bilateral supplementary motor area (SMA). Neurosynth meta-analytic decoding revealed that the unthresholded t-map for this contrast was related, among others, to the functional terms “pain”, “painful” and “conflict” (Figure 1 and Table 2). However, correlations between the beta weights of the FWE-corrected MCC cluster and clinical variables (functional disability, fatigue, pain interference, and pain intensity) did not reveal any significant association (p’s>.3) (Supplementary Table 2), which suggests that, in our sample, GMV reductions in this area do not track clinical severity but instead identify the category of JFM patients as compared with healthy adolescents. Similarly, beta weights of the MCC cluster did not correlate with symptom duration (r=.078; p=.677), which indicates that this alteration does not depend on how long patients have been experiencing JFM symptoms.
Table 2.
Results of the whole-brain voxel-based morphometry analyses
| Structural results at p<.05 FWE-corrected, estimated with TFCE | |||||
|---|---|---|---|---|---|
| GMV differences between adolescents with JFM and controls | |||||
| Contrast | Brain Region | x, y, z | TFCE | CS | |
| JFM < Controls | MCC R | 9, 22, 30 | 1018.43 | 113 | |
| Correlations between GMV and Clinical Symptoms in adolescents with JFM | |||||
| Clinical Symptoms | Association | Brain Region | x, y, z | TFCE | CS |
| Functional Disability (FDI) | ↑GMV | IFG pars triangularis L | −54, 22, 4 | 1784.78 | 340 |
| Structural results at p<.001 uncorrected, Ke>50 voxels | |||||
| GMV differences between adolescents with JFM and controls | |||||
| Contrast | Brain Region | x, y, z | T | CS | |
| JFM < Controls | MCC R | 9, 22, 30 | 4.14 | 983 | |
| Correlations between GMV and Clinical Symptoms in adolescents with JFM | |||||
| Clinical Symptoms | Association | Brain Region | x, y, z | T | CS |
| Functional Disability (FDI) | ↑GMV | IFG pars triangularis L | −54, 22, 4 | 6.47 | 805 |
| IFG pars opercularis R | 57, 14, 15 | 4.35 | 242 | ||
| MFG L | −42, 12, 48 | 4.13 | 191 | ||
| IFG pars orbitalis R | 42, 21, −14 | 3.81 | 117 | ||
| Fatigue (PROMIS) | ↑GMV | Inferior Temporal Ctx L | −60, −16, −33 | 5.03 | 115 |
| IFG pars triangularis L | −54, 26, −3 | 4.83 | 317 | ||
| IFG pars orbitalis R | 32, 27, −21 | 4.63 | 366 | ||
| IFG pars triangularis L | −50, 28, 9 | 4.54 | 82 | ||
| IFG pars orbitalis L | −30, 21, −20 | 4.18 | 76 | ||
| Pain Interference (BPI) | ↑GMV | IFG pars triangularis L | −38, 26, 10 | 4.97 | 157 |
| IFG pars opercularis R | 52, 16, 12 | 4.21 | 264 | ||
| Superior Frontal Ctx R | 28, −3, 66 | 4.11 | 154 | ||
Note: Anatomic coordinates (x, y, z) are given in Montreal Neurological Institute space. CS: cluster size in voxels (voxel size: 1.5×1.5×1.5mm); Ctx: cortex; dlPFC: dorsolateral Prefrontal Cortex; GMV: Gray Matter Volume; IFG: Inferior Frontal Gyrus; JFM: Juvenile Fibromyalgia; Ke: Cluster extent in voxels; L: left; MCC: Anterior-Midcingulate Cortex; MFG: Middle Frontal Gyrus; NRS: Numerical Rating Scale; R: right; TFCE: Threshold-Free Cluster Enhancement.
3.2.2. Correlations between gray matter volume and clinical JFM symptoms
Functional disability correlated with increased GMV in the left inferior frontal gyrus (IFG) (pFWE-corr=.006, TFCE-estimated). At the uncorrected level (p<.001; Ke>50), functional disability was linked to larger volumes in the bilateral IFG and the left middle frontal gyrus, and we found additional associations (p<.001, Ke>50 voxels): fatigue correlated with increased GMV in the bilateral IFG and the left inferior temporal cortex. Pain interference was associated with larger GMV in the left and right inferior frontal gyri (IFG) and the right superior frontal cortex. These findings are detailed in Table 2. Figure 2 shows the anatomy of the findings and the associated meta-analytic terms based on Neurosynth decoding of each unthresholded, symptom-predicting t-map. Notably, t-maps reflecting GMV increases associated with functional disability, fatigue, and pain interference in JFM patients were strongly linked to emotional and/or language production processes.
Importantly, the symptom-GMV associations described above remained significant after including symptom duration as an additional nuisance variable, along with age and total GMV, in the multiple regression models. Thus, these associations are independent of symptom duration. As a posthoc analysis, we built an additional model with symptom duration as a predictor variable, GMV as the dependent variable, and age and total GMV as nuisance covariates. We found no significant associations at the corrected level (pFWE-corr<.05, TFCE-estimated). At the uncorrected threshold (p<.001, Ke>50 voxels), symptom duration correlated with GMV decreases in right-lateralized clusters in temporoparietal areas (Supplementary Table 3). According to Neurosynth decoding, the corresponding t-map was most associated with theory of mind and language-related processes (Supplementary Figure 1).
3.3. Reduced similarity between brain structure and a validated pain-predictive pattern involving the midcingulate cortex-dorsal medial frontal cortex in JFM
We tested whether validated brain patterns predicting either pain, cognitive control, or negative emotion specifically in the MCC-dorsal MFC region (22), differed between JFM patients and controls. We found between-group differences only in the pain pattern, which is consistent with the location of GMV between-group differences and the fact that pain is a core complaint in JFM. Specifically, the dot product between structural data and the pain weighted map was significantly lower in JFM patients compared to controls within the MCC-dorsal MFC mask (t=2.47, p=.016) (Figure 3). Thus, the anatomical MCC pattern resembled the functional pain pattern significantly less in JFM patients than in controls. We found no differences for the patterns predicting cognitive control or negative emotion (p’s>.4).
Figure 3. Differences in pattern similarity between brain structure and a validated pain-predictive pattern in MCC-dMFC circuits between JFM and healthy adolescents.
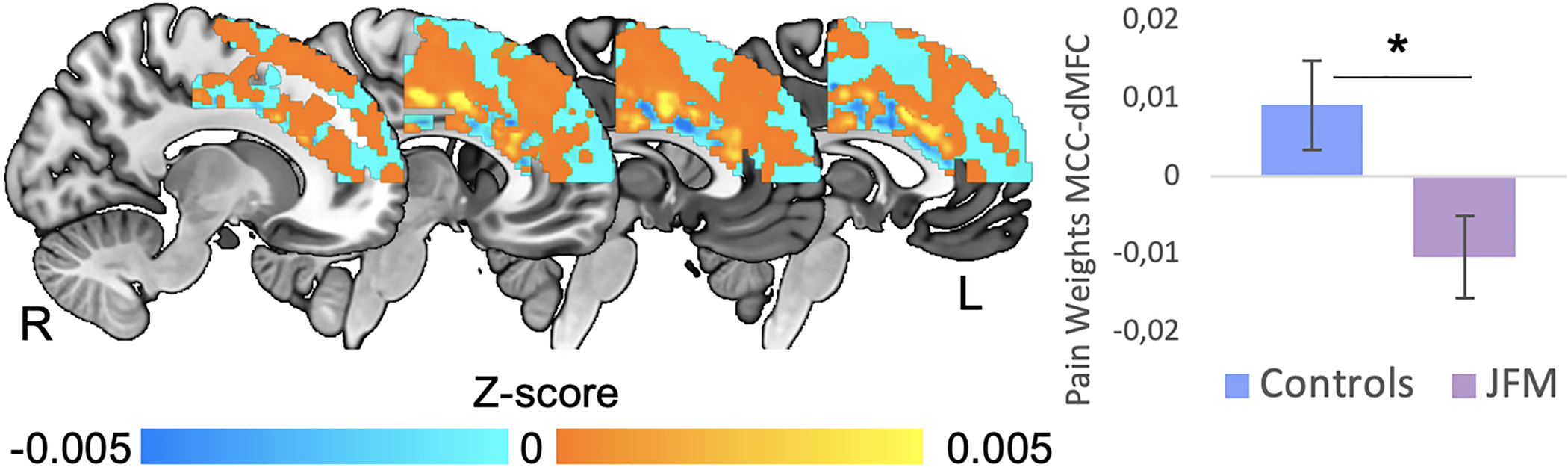
The anatomical MCC-dMFC pattern resembled the functional pain pattern significantly less in JFM patients than in healthy adolescents (t=2.47, p=.016). We found no between-group differences in the patterns predicting cognitive control and negative emotion within the MCC-dMFC (p’s>.05). *: Significant at p<.02; dMFC: dorsal Medial Frontal Cortex; L: Left; MCC: Anterior-Midcingulate Cortex; R: Right.
3.4. Gray matter volume differences between adolescents with JFM and healthy adolescents within meta-analytic regions altered in adults with fibromyalgia
In agreement with meta-analytic findings in adults (6,7), GMV reductions in two ROIs, located in the left ACC and the right PPC, replicated in adolescents with JFM (p=.02; p=.03, respectively) (Figure 4A). Neurosynth meta-analytic decoding showed that the left ACC ROI was associated with functional terms such as “pain”, “autobiographical” and “mentalizing” (the ability to understand the mental state of oneself or others), whereas the right PCC ROI was associated with “suppressed”, “declines” and “resting-state”. The remaining ROIs extracted from the meta-analyses (6,7), located in the cerebellum, the dorsal ACC and the fusiform and parahippocampal gyri, did not show evidence of alteration in JFM patients (p’s>.4), as opposed to what had been reported in adults with fibromyalgia (see Figure 4B). Notably, among our sample, two JFM participants had Chiari malformations, which is no higher than might be expected based on population prevalence (37).
Figure 4. Replication of meta-analytic gray matter volume alterations of adults with fibromyalgia in JFM.
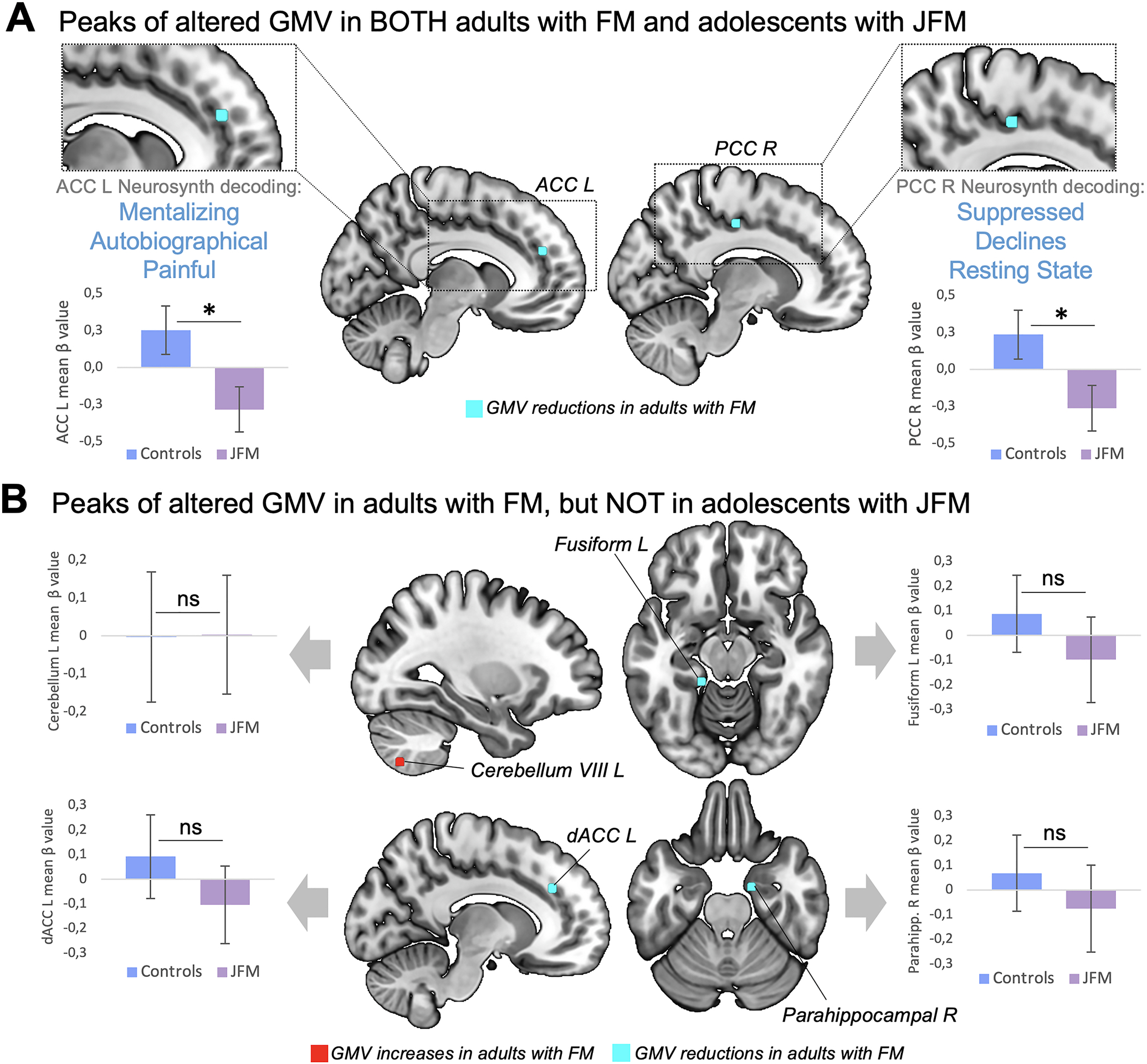
A. Peaks of gray matter volume (GMV) altered in both adults with FM and adolescents with JFM (p’s<.03). Notably, the GMV of these peaks were decreased in both adult and juvenile forms of fibromyalgia. Under to these regions, we display 3 associated functional annotations, as obtained with meta-analytic decoding using Neurosynth. B. Peaks of GMV altered in adults with FM that did not replicate in adolescents with JFM (p’s>.4). *: Significant at p<.03; ns: non-significant at p<.05. dACC: dorsal Anterior Cingulate Cortex; FM: Fibromyalgia; JFM: Juvenile Fibromyalgia; L: Left; PCC: Posterior Cingulate Cortex; R: Right.
4. Discussion
To our knowledge, this is the first study assessing brain structural alterations in JFM. Results indicate that decreased GMV in the MCC may be a key feature of JFM. This region is a core element of central acute pain processing (9,10,38–40), involved in affective encoding, cognitive interpretation, anticipation, and response selection (41). In agreement, meta-analytic decoding revealed that among all t-maps generated in our study, the one of the MCC finding was the only one associated with the terms “pain” and “painful”. Moreover, results showed that the anatomical MCC pattern resembled the validated MCC-dorsal MFC pain predictive pattern -and not the negative emotion or cognitive control ones- significantly less in JFM patients than in healthy adolescents.
We also found that adolescents with JFM exhibited GMV reductions in two of the three cingulate regions (i.e., ACC, PCC) that meta-analyses reported to be decreased in adult fibromyalgia patients. Previous studies have shown that repetitive painful stimulation leads to MCC gray matter increases in healthy subjects (42), whereas chronic pain patients display GMV reductions in this region (6,7). Our findings do not support the hypothesis that younger patients may show increased GMV as a consequence of over-engaging pain modulatory systems (12,13). Instead, results suggest that reductions in the cingulate cortex, mainly in the MCC, may be a structural hallmark of both adult and juvenile forms of fibromyalgia, independent of symptom duration. Longitudinal studies are warranted to determine whether such alterations pre-date chronic pain onset or reflect an early pain-driven alteration. Notably, other GMV alterations reported in adults did not replicate in JFM patients, which may be due to the limited power of the present investigation or may suggest that they appear later in life as a result of progression of chronic pain or medication exposure.
The MCC is not selective for pain (43,44). Research has linked activity in this area with multiple functions, including attention, cognitive control, reward-based learning, decision making, and emotional and social processing (e.g., reviews: 41,43). To deepen our understanding of the functional contributions of the MFC surrounding the region where we found GMV reductions in JFM patients, we assessed how this structural pattern resembled functional patterns predicting pain, cognitive control, and negative emotion within the MCC-dorsal MFC (22), and how this similarity differed between groups. In adolescents with JFM, the anatomical MCC pattern resembled the pain pattern significantly less than in controls, which may suggest that the anatomical hallmark of nociceptive pain processing has been attenuated to some degree in JFM patients. Whether such finding reflects the effects of excessive engagement of the pain-specific functional pattern or a reorganization of acute nociceptive processing brain circuits in patients remains to be elucidated. This finding indicates that pattern similarity reductions in patients occur beyond the cluster showing GMV reductions and reflects an overall reduction of the normal pain predictive pattern in patients. Moreover, it supports the idea of altered organization of MCC-dorsal MFC circuits. Future functional studies using experimental pain tasks are warranted to test the extent to which this alteration replicates during pain processing to further interpret this novel finding. Taken together, alterations of the pain pattern in JFM patients along with meta-analytic decoding findings specifically mentioning the terms “pain” and “painful”, suggest that this abnormality may be related to pain processing and decision making/evaluative aspects in the context of pain and less with other emotional-affective-cognitive processes.
Regarding regions associated with individual differences in patients’ clinical symptoms reflecting pain-related suffering and diminished functional ability, we found a significant association between functional disability and the left ventrolateral prefrontal cortex (vlPFC). Meta-analyses have linked the left vlPFC with language processes during emotion regulation suggesting that it might support active reinterpretation of the meaning of emotional stimuli and facilitate the selection of appropriate reappraisals (45). Likewise, the vlPFC has been associated with retrieval of semantic autobiographical memories and self-related judgments (46). In agreement, at the uncorrected level, we found that other symptoms reflecting the impact that JFM has on patients’ functional ability (i.e., functional disability, fatigue, and pain interference) all correlated with GMV increases in vlPFC. Taken together, these findings suggest that JFM patients with higher levels of pain-related suffering and impairment show augmented GMV in brain circuits involved in instantiating representations of the self and the world through language. Adding further support to this interpretation, at the uncorrected level, fatigue correlated with increased volumes in the left inferior temporal cortex, linked to visual and mnemonic processing (47), and functional disability and pain interference correlated with larger volumes in the right superior frontal cortex, involved in self-focused reappraisal (48), and in the left middle frontal gyrus, associated with attention reorienting (49). Notably, these associations were independent of symptom duration. Neurosynth decoding confirmed that these variables were associated with a brain pattern related to emotional, self-related judgment, and language-related processes. Future studies should test whether alterations in the nature, recurrence, and valence of patients’ narratives about themselves and the world may predict greater levels of suffering and disability in JFM and to which extent vlPFC circuits mediate such associations. From a neurodevelopmental perspective, frontal GMV decreases after age 11 in healthy girls, because of synaptic pruning (50). This maturation occurs earlier in ventral than in dorsal regions (50). Thus, vlPFC volume increases associated with JFM impairment may reflect a link between these symptoms and developmental inmaturity in frontal circuits specializing in emotional appraisal and regulation, which reinforces the need to consider therapeutic strategies that target these circuits, which may have the potential to reverse alterations before they become hard-wired and to mitigate the functional and psychosocial impact of pain-related symptoms on the life of adolescents with JFM.
Finally, symptom duration was associated with reduced GMV in temporoparietal areas -at an uncorrected level- including theory of mind and language-related regions. The findings suggest that not only disease-related disability but also JFM duration may be linked to alterations in regions mediating mentalizing and emotional awareness through language. Last, our between-group and symptom duration-related findings taken together do not support the hypothesis of hypertrophy in young fibromyalgia patients as a group (12,13). However, JFM patients with greater symptom severity showed GMV increases, thus, future studies with larger samples are warranted to assess whether different structural correlates underlie distinct patient clusters identified based on symptom severity.
This study has note-worthy limitations. First, we enrolled only females, thus, our findings cannot be generalized to male patients - although they are quite rare. Future studies are warranted to examine between-sex differences in JFM-related structural alterations and the ages at which these changes occur. Second, since this is the first study assessing the structural abnormalities of JFM, replication of our findings in independent samples is crucial to determine their robustness and translational utility. Likewise, the brain-symptom severity correlations presented here, although providing a cohesive picture of the alterations and opening venues for future research, are still preliminary, based on exploratory thresholds, and need replication. Nevertheless, such correlational findings highlight the importance of studying individual differences to characterize different patient profiles. Although medication regime was stable for at least 3 weeks before the MRI assessment (no modifications in type, dose, or intake regime), medication could have acted as a confounding factor. Lastly, despite the efforts to recruit diverse patient profiles, our sample had a low representation of subjects of different races and ethnicities and those with low socioeconomic status. Future community-oriented clinical research is needed to overcome the systematically high proportion of white-Caucasian participants with medium-high socioeconomic status in research samples.
In conclusion, this study provides first evidence of structural alterations in adolescents with JFM. Our findings suggest that pain-related GMV reductions in the MCC are common to JFM patients as a group, whereas alterations in regions involved in affective, self-relevant memories, and language processes predict disease impact on clinical variables related to patients’ well-being. Together, the findings reinforce the need to combine pain-specific, sensory therapies, with therapies aimed at promoting cognitive regulation of pain, negative affect, and potentially pervasive self-related narratives patients may hold of themselves. Also, the findings indicate partial overlap in the structural circuitry compromised in both juvenile and adult fibromyalgia, potentially establishing a link between juvenile and adult forms of the disease and strengthening the need for early, neurobiologically-oriented interventions to prevent the transition from juvenile to adult fibromyalgia.
Supplementary Material
Acknowledgements
This work was funded by NIH/NIAMS Grants R01 AR074795 and P30 AR076316. Marina Lopez-Sola, PhD, is hired as part of the Serra Hunter Programme of the Generalitat de Catalunya.
The authors gratefully thank Matt Lanier, Kaley Bridgewater, Kelsey Murphy, Brynne Williams, and Lacey Haas (Imaging Research Center, Department of Radiology, Cincinnati Children’s Hospital Medical Center) for their contributions to MRI data collection.
Footnotes
Conflict of interest statement
The authors have no conflict of interest to declare.
References
- 1.Ting TV, Barnett K, Lynch-Jordan A, Whitacre C, Henrickson M, Kashikar-Zuck S. 2010 American College of Rheumatology Adult Fibromyalgia Criteria for Use in an Adolescent Female Population with Juvenile Fibromyalgia. J Pediatr 2016;169:181–7.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kashikar-Zuck S, Ting TV, Arnold LM, Bean J, Powers SW, Graham TB, et al. Cognitive behavioral therapy for the treatment of juvenile fibromyalgia: a multisite, single-blind, randomized, controlled clinical trial. Arthritis Rheum 2012;64:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kashikar-Zuck S, Lynch AM, Slater S, Graham TB, Swain NF, Noll RB. Family factors, emotional functioning, and functional impairment in juvenile fibromyalgia syndrome. Arthritis Rheum 2008;59:1392–98. [DOI] [PubMed] [Google Scholar]
- 4.Pas R, Ickmans K, Van Oosterwijck S, Van der Cruyssen K, Foubert A, Leysen L, et al. Hyperexcitability of the Central Nervous System in Children with Chronic Pain: A Systematic Review. Pain Med 2018;19(12):2504–2514. [DOI] [PubMed] [Google Scholar]
- 5.Molina J, Amaro E Jr, da Rocha LGS, Jorge L, Santos FH, Len CA. Functional resonance magnetic imaging (fMRI) in adolescents with idiopathic musculoskeletal pain: a paradigm of experimental pain. Pediatr Rheumatol Online J 2017;15(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin C, Lee SH, Weng HH. Gray Matter Atrophy within the Default Mode Network of Fibromyalgia: A Meta-Analysis of Voxel-Based Morphometry Studies. Biomed Res Int 2016;7296125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi H, Yuan C, Dai Z, Ma H, Sheng L. Gray matter abnormalities associated with fibromyalgia: A meta-analysis of voxel-based morphometric studies. Semin Arthritis Rheum 2016;46(3):330–7. [DOI] [PubMed] [Google Scholar]
- 8.Napadow V, Kim J, Clauw DJ, Harris RE. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum 2012;64:2398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López-Solà M, Pujol J, Wager TD, Garcia-Fontanals A, Blanco-Hinojo L, Garcia-Blanco S, et al. Altered functional magnetic resonance imaging responses to nonpainful sensory stimulation in fibromyalgia patients. Arthritis Rheumatol 2014;66:3200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.López-Solà M, Woo CW, Pujol J, Deus J, Harrison BJ, Monfort J, et al. Towards a neurophysiological signature for fibromyalgia. Pain 2017;158:34–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pujol J, Macià D, Garcia-Fontanals A, Blanco-Hinojo L, López-Solà M, Garcia-Blanco S, et al. The contribution of sensory system functional connectivity reduction to clinical pain in fibromyalgia. Pain 2014;155:1492–503. [DOI] [PubMed] [Google Scholar]
- 12.Ceko M, Bushnell MC, Fitzcharles MA, Schweinhardt P. Fibromyalgia interacts with age to change the brain. Neuroimage Clin 2013;3:249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moayedi M, Weissman-Fogel I, Salomons TV, Crawley AP, Goldberg MB, Freeman BV, et al. Abnormal gray matter aging in chronic pain patients. Brain Res 2012;1456:82–93. [DOI] [PubMed] [Google Scholar]
- 14.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013;14:502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin F, Zheng P, Liu H, Guo H, Sun Z. Functional and anatomical connectivity-based parcellation of human cingulate cortex. Brain Behav 2018;8:e01070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tovar DT, Chavez RS. Large-scale functional coactivation patterns reflect the structural connectivity of the medial prefrontal cortex. Soc Cogn Affect Neurosci 2020;nsaa132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazaridou A, Kim J, Cahalan CM, Loggia ML, Franceschelli O, Berna C, et al. Effects of Cognitive-Behavioral Therapy (CBT) on Brain Connectivity Supporting Catastrophizing in Fibromyalgia. Clin J Pain 2017;33(3):215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellingson LD, Stegner AJ, Schwabacher IJ, Koltyn KF, Cook DB. Exercise Strengthens Central Nervous System Modulation of Pain in Fibromyalgia. Brain Sci 2016;6(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kashikar-Zuck S, King C, Ting TV, Arnold LM. Juvenile Fibromyalgia: Different from the Adult Chronic Pain Syndrome? Curr Rheumatol Rep 2016;18:19. [DOI] [PubMed] [Google Scholar]
- 20.Lutz J, Jäger L, de Quervain D, Krauseneck T, Padberg F, Wichnalek M, et al. White and gray matter abnormalities in the brain of patients with fibromyalgia: a diffusion-tensor and volumetric imaging study. Arthritis Rheum 2008;58:3960–69. [DOI] [PubMed] [Google Scholar]
- 21.Martucci KT, Mackey SC. Neuroimaging of Pain: Human Evidence and Clinical Relevance of Central Nervous System Processes and Modulation. Anesthesiology 2018;128:1241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kragel PA, Kano M, Van Oudenhove L, Ly HG, Dupont P, Rubio A, et al. Generalizable representations of pain, cognitive control, and negative emotion in medial frontal cortex. Nat Neurosci 2018;21:283–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL 3rd. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 2009;10:447–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mogil JS. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci 2020;21:353–65. [DOI] [PubMed] [Google Scholar]
- 25.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol 1991;16:39–58. [DOI] [PubMed] [Google Scholar]
- 26.PROMIS. PROMIS Instrument Development and Psychometric Evaluation Scientific Standards Version 2.0 2013; PROMIS instrument development standards. [Google Scholar]
- 27.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain 1983;17:197–210. [DOI] [PubMed] [Google Scholar]
- 28.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage 2000;11:805–21. [DOI] [PubMed] [Google Scholar]
- 29.Ashburner J A fast diffeomorphic image registration algorithm. Neuroimage 2007;38:95–113. [DOI] [PubMed] [Google Scholar]
- 30.Yaxu Y, Ren Z, Ward J, Jiang Q. Atypical Brain Structures as a Function of Gray Matter Volume (GMV) and Gray Matter Density (GMD) in Young Adults Relating to Autism Spectrum Traits. Front Psychol 2020;11:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kennedy JT, Astafiev SV, Golosheykin S, Korucuoglu O, Anokhin AP. Shared genetic influences on adolescent body mass index and brain structure: A voxel-based morphometry study in twins. Neuroimage 2019;199:261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnes J, Ridgway GR, Bartlett J, Henley SM, Lehmann M, Hobbs N, et al. Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage 2010;53:1244–55. [DOI] [PubMed] [Google Scholar]
- 33.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009;44:83–98. [DOI] [PubMed] [Google Scholar]
- 34.Li H, Nickerson LD, Nichols TE, Gao JH. Comparison of a non-stationary voxelation-corrected cluster-size test with TFCE for group-Level MRI inference. Hum Brain Mapp 2017;38(3):1269–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 2011;8:665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koban L, Jepma M, López-Solà M, Wager TD. Different brain networks mediate the effects of social and conditioned expectations on pain. Nat Commun 2019;10:4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahn EN, Muraszko KM, Maher CO. Prevalence of Chiari I Malformation and Syringomyelia. Neurosurg Clin N Am 2015;26:501–7. [DOI] [PubMed] [Google Scholar]
- 38.Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, et al. Distributed processing of pain and vibration by the human brain. J Neurosci 1994;14:4095–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol 1999;82:1934–43. [DOI] [PubMed] [Google Scholar]
- 40.Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med 2013;368:1388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vogt BA. Midcingulate cortex: Structure, connections, homologies, functions and diseases. J Chem Neuroanat 2016;74:28–46. [DOI] [PubMed] [Google Scholar]
- 42.Teutsch S, Herken W, Bingel U, Schoell E, May A. Changes in brain gray matter due to repetitive painful stimulation. Neuroimage 2008;15:42:845–9. [DOI] [PubMed] [Google Scholar]
- 43.Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 2011;12:154–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silvestrini N, Chen JI, Piché M, Roy M, Vachon-Presseau E, Woo CW, et al. Distinct fMRI patterns colocalized in the cingulate cortex underlie the after-effects of cognitive control on pain. Neuroimage 2020;217:116898. [DOI] [PubMed] [Google Scholar]
- 45.Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex 2014;24:2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci 2012;24:1742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ranganath C Working memory for visual objects: complementary roles of inferior temporal, medial temporal, and prefrontal cortex. Neuroscience 2006;139:277–89. [DOI] [PubMed] [Google Scholar]
- 48.Falquez R, Couto B, Ibanez A, Freitag MT, Berger M, Arens EA, et al. Detaching from the negative by reappraisal: the role of right superior frontal gyrus (BA9/32). Front Behav Neurosci. 2014;8:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Japee S, Holiday K, Satyshur MD, Mukai I, Ungerleider LG. A role of right middle frontal gyrus in reorienting of attention: a case study. Front Syst Neurosci 2015;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev 2006;30:718–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


