Abstract
Introduction:
We examined baseline differences in depression and antidepressant use among cognitively normal older adults in five ethnoracial groups and assessed whether depression predicted a faster progression to incident cognitive impairment across groups.
Methods:
Data from the National Alzheimer’s Coordinating Center (n = 8168) were used to examine differences between non-Hispanic Whites (nHW), African Americans (AA), Hispanics, Asians, and American Indian and Alaskan Natives in cross-sectional and longitudinal models.
Results:
AA had a lower risk of depression compared to nHW at baseline. No statistical interactions were noted between ethnoracial groups and depression. However, depression independently predicted a faster progression to incident cognitive impairment. Hispanics and Asian participants had a higher hazard for progression compared to nHW.
Discussion:
Previously established risk factors between depression and dementia were not found among AA and nHW participants. The relationship between depression and ethnoracial groups is complex and suggests differential effects on progression from cognitive normality to impairment.
Keywords: Race, Ethnicity, Depression, Alzheimer’s disease, Cognitive Impairment, Disparities
1. INTRODUCTION
The demographics of the United States (US) are dramatically and rapidly shifting, with the older adult population (≥ 65 years) doubling to 88 million by mid-century.1 Among older adults, there will be greater racial and ethnic diversity, with significant growth among African Americans (AA)/Blacks (3 to 12%), Hispanics/Latinx (8 to 22%) and Asians (4 to 9%).2 Alzheimer’s disease and related dementia (ADRD) prevalence is forecasted to reach 152 million cases globally and the prevalence of Alzheimer’s disease (AD) will increase to nearly 13 million in the US.3 Findings from studies with longitudinal cohorts indicate that AA are at twice the risk of incident AD compared to non-Hispanic Whites (nHW), despite no significant group differences in the rate of cognitive decline over time.4 Evidence from epidemiological studies suggests that there is a higher risk of dementia among AA and American Indian and Alaskan Natives (AIAN) and moderate risk for Hispanics compared to nHW.5 Emerging AD biomarker studies on ethnoracial differences suggest that if differences do exist in biofluid and imaging biomarkers, nHW cohorts tend to have more abnormal levels.6–9
Depression is a known risk factor of ADRD as researchers have documented significant associations between depression and cognitive functioning and decline, as well as cardiovascular diseases, metabolic conditions, and dementia.10–12 The 2020 Lancet Commissions’ report on Dementia Prevention, Intervention, and Care, identified the complex causal relationship between dementia and depression, while also classifying depression as a modifiable risk factor.13 Considering the rapid, global growth in the aging population, it is important to note that depression is more prevalent among older adults (age ≥ 60), is more common among women compared to men, and is a leading cause of disability worldwide.14 Numerous studies have investigated the associations between depression (symptoms, diagnosis, chronicity) and ADRD. Using data from the National Alzheimer’s Coordinating Center (NACC), investigators found that adults with active depression and mild cognitive impairment (MCI) were faster to progress to AD (median of 27 months) compared to those with MCI and a remote history of depression.15 A similar study using NACC data found cognitively normal adults with active depression were twice more likely to develop AD compared to those without active depression.16 Findings are consistent for depressive symptoms being a risk factor for cognitive impairment and decline over time.17 However, race and/or ethnicity were either not considered in analyses or included as covariates without any discussion. The increase in diversity and growth of the aging population requires inclusion and consideration of ethnoracial groups in ADRD research.18
There is a complex interaction of environmental, social, and biological factors that may lead to depressive episodes and depression as a disorder. The patterning of depression, including its prevalence and burden of disease, varies across race/ethnicity in the US. For example, analyses of data drawn from the Collaborative Psychiatric Epidemiology Surveys indicate that Mexican Americans and AA had a higher prevalence of major depression, greater chronicity of episodes, and lower treatment use compared to nHW.19 Depression was also found to be more prevalent among US-born persons who belonged to historically marginalized ethnoracial groups compared to those who were foreign-born. Survey data of older adults (≥65) from Centers for Medicare and Medicaid Services indicated that all persons of color, including Hispanic-Mexican, Hispanic-Puerto Rican, Hispanic-Cuban, multiple Hispanic ethnicities, Black or African Americans, Asian Indians, Filipinos, Native Hawaiians/ Pacific Islanders, two or more races, had a higher odds of depression compared to nHW.20 Nonetheless, these studies did not assess cognitive functioning or incident dementia.
Neither ADRD nor depression are a normal part of aging. In this study’s first aim, we examine whether baseline depression (diagnosis and symptoms) and antidepressant use differ between cognitively normal nHW, AA, AIAN, Hispanic and Asian older adults (age ≥ 65). In the second aim, we investigate whether depression and antidepressants predict progression to incident cognitive impairment. We hypothesize that AA and Hispanic older adults would present with more depression at baseline and have a higher hazard risk of progression compared to nHW.
2. METHODS
2.1. Data repository
Data were collected from 37 US-based Alzheimer’s Disease Centers (ADCs) and curated by NACC. All participants across the ADCs (33 centers currently funded) provided informed consent. We requested data from NACC in September 2020 and used the June 2020 data freeze from the Uniform Data Set (UDS).
2.2. Participants
The inclusion criteria for this study included participants aged 65 and older with a clinical dementia rating (CDR)21 score of 0 at first/baseline visit, a clinical diagnosis of normal cognition, and at least two visits. Participants were followed until dropout or first instance of progression to cognitive impairment based on a CDR ≥ 0.5. Data from 8168 cognitively normal participants met the inclusion criteria and were used. Race and ethnicity were self-reported.
2.3. Primary outcome measures
During annual clinical assessment, self-report questions assessed depression and psychiatric disorders (e.g. bipolar, schizophrenia, anxiety), and whether these disorders were active, recent, or absent. Information from the participant’s self-report along with data from the clinical interview, and observations were synthesized by the clinician to determine a depression diagnosis (yes/no). Depressive symptoms were assessed by the 15-item Geriatric Depression Scale (GDS) in which the participant answered “yes” or “no” for each item, with higher scores (range 0–15) indicating greater symptoms. Current use of an antidepressant (yes/no) was obtained from a NACC derived variable that assessed the medication form using Multum/Lexi-Comp© therapeutic drug categories and identified prescription antidepressants.
2.4. Secondary outcome measures
The participant’s collateral source completed the Neuropsychiatric Inventory Questionnaire (NPI-Q), that assessed the presence and severity of neuropsychiatric symptoms over the past month. Higher scores (range 12–36) indicated greater severity. To control for the vascular contributions, we computed a vascular composite score by summing diagnoses (yes/no) for hypertension, hyperlipidemia, type 2 diabetes, cardiovascular condition (yes=any heart attack/atrial fibrillation/cardiac bypass/congestive heart failure/angioplasty; no=none), and smoking (yes= former/current; no=never). To control for the impact of the neighborhood, we assessed the social deprivation index (SDI)22 via Zone Improvement Plan (ZIP) and Codes Tabulation Areas (ZCTAs). ZCTAs provide a more focal area level deprivation for a participant. However, NACC only provided the first three digit of a ZIP code (national and sectional center) from ADCs for participants. A mean SDI was obtained using the available three-digit ZIP code.
2.5. Statistical analyses
Descriptive statistics (mean/standard deviations; frequency/percentages) summarized numeric and categorical variables while t-test, ANOVA, and chi-square tests compared values across different ethnoracial categories. There were five ethnoracial categories in this study: nHW, AA, Hispanics, AIAN, and Asians.
Cross-sectional analyses used logistic regression to first examine the association between depression and the different ethnoracial groups with nHW serving as the reference category. A second stepwise model adjusted for age, sex, education in years, and a third model included aforementioned covariates and apolipoprotein (APOE) ε4 status. A similar set of logistic regression models were conducted for the ethnoracial groups and antidepressant use. Separate general linear models (GLMs) were conducted for assessing ethnoracial group differences on the GDS and NPIQ controlling for demographic variables and APOE ε4 status. To control for heterogeneity across ADCs, each site was accounted for in the models. Additional models controlled for vascular composite and the SDI.
In the longitudinal analyses, the outcome variable was survival time from baseline visit to the first occurrence of CDR ≥ 0.5 in annual visits. The survival time for the participants who never progressed to CDR ≥ 0.5 on subsequent follow up visits were treated as “right-censored”. In the survival analyses, we conducted both Cox Proportional Hazards Model (CPHM) and Fine and Gray Model (FGM)23; the latter included death as a competing risk. Race and depression were main effects of interest while age, education, sex, and APOE ε4 were treated as covariates. Due to the potential multicollinearity between active depression and antidepressant use, a composite variable with four categories was formed based on depression status and antidepressant use. This was used in the survival analysis models to examine the effects of both active depression and antidepressant use on the risks of developing AD. We conducted all potential two-way interactions between main effects using CPHM and FGM. Finally, separate models were run to account for ADCs, vascular composite, and SDI.
Given the small sample of AIAN participants, CPHM and FGM analyses were not performed with that group. All analyses were conducted in R statistical package (version 4.05). The Kaplan Meier curve was drawn by ggplot2 and survminer24 package. Additionally, coxph and finegray in the cmprsk package25 were used to fit the survival models.
3. RESULTS
3.1. Sample demographics
Among the 8168 participants, 7.52% nHW were diagnosed with active depression at baseline visit, which was statistically significant compared to rates of AA (4.40%) (p<0.001) (Table 1). The rate of antidepressant use was also significantly higher among nHW (17.4%), while AA (6.56%) and Asians (2.20%) had significantly lower rates of antidepressant use (p<0.001). AA, Hispanics, and Asians were younger compared to nHW at first visit (p<0.001). AA and Hispanics had less years of education than nHW, while Asians had more years of education (p<0.001). AA had the highest percentage of female participants while nHW had the lowest percentage (p<0.001). AA had a greater number of participants with APOE ε4 positive status (34.9%) while Asians had the lowest (11.5%) (p<0.001). There was an average length of 4.6 follow-up years. Hispanic and Asian older adults had significantly shorter years of follow up (p<0.001). AA and AIAN participants had a higher vascular composite score compared to nHW, while all four minoritized groups had a lower mean on the Mini Mental State Examination at baseline compared to nHW.
Table 1.
Baseline characteristics of cognitively normal participants
| All (N=8168) | nHW (n=6313) | AA (n=1250) | Hispanic (n=390) | Asian (n=186) | AIAN (n=29) | p | |
|---|---|---|---|---|---|---|---|
| Age (y) (n=8168) | 74.9±7.0 | 75.34±7.19 | 73.54±6.22* | 72.86±6.06* | 73.61±5.71* | 72.28±6.82 | <0.001 |
| Age range | (65–102) | (65–102) | (65–101) | (65–90) | (65–91) | (65–94) | |
| Sex (n=8168) | <0.001 | ||||||
| Female | 5398 (66.09%) | 3972 (62.92%)* | 1006 (80.48%)* | 277 (71.03%) | 119 (63.98%) | 24 (82.76%) | |
| Male | 2770 (33.91%) | 2341 (37.08%)* | 244 (19.52%)* | 113 (28.91%) | 67 (36.02%) | 5(17.25%) | |
| Education (y) (n=8137) | 15.8±2.9 | 16.20±2.68 | 14.66±3.09* | 13.28±4.26* | 16.81±2.68* | 15.21±2.35 | <0.001 |
| Education (n=8137) | <0.001 | ||||||
| ≤12 years | 1397 (17.17%) | 854 (13.58%)* | 364 (29.12%)* | 153 (39.53%)* | 20 (10.99%) | 6 (20.69%) | |
| > 12 years | 6740 (82.83%) | 5435 (86.42%)* | 886 (70.88%)* | 234 (60.47%)* | 162 (89.01%) | 23 (79.31%) | |
| MMSE (n=6286) | 28.9±1.4 | 29.04±1.22 | 28.12±1.87* | 28.44±1.82* | 28.81±1.25* | 27.83±1.75* | <0.001 |
| Depression (n=8168) | <0.001 | ||||||
| No | 7602 (93.7%) | 5838 (92.48%)* | 1195 (95.60%)* | 363 (93.08%) | 178 (95.70%) | 28 (96.55%) | |
| Yes | 566 (6.93%) | 475 (7.52%)* | 55 (4.40%)* | 27 (6.92%) | 8 (4.30%) | 1 (3.45%) | |
| Antidepressant (n=7994) | <0.001 | ||||||
| No | 6769 (84.68%) | 5089 (82.57%)* | 1152 (93.35%)* | 326 (84.46%) | 178 (97.80%)* | 24 (82.76%) | |
| Yes | 1225 (15.32%) | 1074 (17.43%)* | 82 (6.65%)* | 60 (15.54%) | 4 (2.20%)* | 5 (17.24%) | |
| VCS (n=8168) | 1.6±1.3 | 1.58±1.29 | 1.89±1.27* | 1.70±1.32 | 1.52±1.32 | 2.28±1.44* | <0.001 |
| APOE ε status (n=7210) | <0.001 | ||||||
| APOE ε4 − | 5133 (71.19%) | 4065 (71.68%) | 674 (65.06%)* | 249 (74.33%) | 130 (88.44%)* | 15 (71.43%) | |
| APOE ε4 + | 2077 (28.81%) | 1606 (28.32%) | 362 (34.94%)* | 86 (25.67%) | 17 (11.56%)* | 6 (28.57%) | |
| Follow-up (y) (n=8168) | 4.6±3.4 | 4.68±3.41 | 4.52±3.27 | 3.83±2.95* | 3.94±3.08* | 2.62±1.80* | <0.001 |
| Vital status (n=8168) | <0.001 | ||||||
| Alive | 6779 (82.99%) | 5122 (81.13%)* | 1089 (87.12%)* | 360 (92.31%)* | 180 (96.77%)* | 28 (96.55%) | |
| Dead | 1389 (17.01%) | 1191 (18.87%)* | 161 (12.88%)* | 30 (7.69%)* | 6 (3.23%)* | 1 (3.45%) |
Abbreviations: Mean ± Standard Deviation; nHW=non-Hispanic White; AA=African American; AIAN= American Indian and Alaskan Natives; MMSE= Mini-Mental State Examination; VCS=Vascular Composite Score; APOE ε+= Apolipoprotein ε4 allele;
group difference
3.2. Cross-sectional analyses
AA had a 43% lower odds of having active depression compared to nHW at baseline. This difference was consistent with the addition of age, sex, education and APOE ε4 to the model (Table 2). Females had a 70% higher odds of having active depression compared to men. There were no other significant ethnoracial differences. When examining antidepressant use across ethnoracial groups, AA and Asians were significantly less likely to use antidepressants compared to nHW (Table 3). This finding persisted with the adjustment of covariates. Similarly, females had a 90% higher odds of using antidepressants. In the GLMs examining depressive symptoms and neuropsychiatric symptoms, there were no differences across ethnoracial groups independently, nor while adjusting for covariates (results not presented). All cross-sectional results remained unchanged after adjusting for ADCs. Neither the vascular composite score nor SDI were statistically significant in the models.
Table 2.
Associations between ethnoracial group and active depression at baseline
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||||
| (Intercept) | 0.08 | 0.07 – 0.09 | <0.001 | 0.77 | 0.24 – 2.49 | 0.663 | 0.76 | 0.22 – 2.63 | 0.666 |
| Race/Ethnicity (Ref: nHW) | |||||||||
| AA | 0.57 | 0.42 – 0.75 | <0.001 | 0.48 | 0.35 – 0.63 | <0.001 | 0.50 | 0.36 – 0.67 | <0.001 |
| Hispanic | 0.91 | 0.60 – 1.34 | 0.662 | 0.77 | 0.50 – 1.14 | 0.215 | 0.79 | 0.50 – 1.21 | 0.305 |
| AIAN | 0.44 | 0.02 – 2.06 | 0.419 | 0.36 | 0.02 – 1.70 | 0.318 | 0.00 | NA – 0.28 | 0.949 |
| Asian | 0.55 | 0.25 – 1.06 | 0.103 | 0.55 | 0.25 – 1.05 | 0.099 | 0.60 | 0.25 – 1.20 | 0.193 |
| Age | 0.97 | 0.96 – 0.98 | <0.001 | 0.97 | 0.96 – 0.99 | <0.001 | |||
| Sex (Ref: Male) | |||||||||
| Female | 1.70 | 1.39 – 2.09 | <0.001 | 1.65 | 1.34 – 2.05 | <0.001 | |||
| Education (years) | 0.97 | 0.94 – 1.01 | 0.103 | 0.97 | 0.94 – 1.01 | 0.112 | |||
| APOE ε+ | 0.98 | 0.80 – 1.19 | 0.814 | ||||||
| n | 8168 | 8137 | 7180 | ||||||
Abbreviations: OR=Odds Ratio; CI=Confidence Interval; Ref=Reference category; nHW=non-Hispanic White; AA=African American; AIAN= American Indian and Alaskan Natives; APOE ε+= Apolipoprotein ε4 allele
Table 3.
Associations between ethnoracial group and antidepressant use at baseline
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||||
| (Intercept) | 0.21 | 0.20 – 0.23 | <0.001 | 4.43 | 1.87 – 10.54 | 0.001 | 4.47 | 1.79 – 11.23 | 0.001 |
| Race/Ethnicity (Ref: nHW) | |||||||||
| AA | 0.34 | 0.27 – 0.42 | <0.001 | 0.28 | 0.22 – 0.36 | <0.001 | 0.29 | 0.22 – 0.37 | <0.001 |
| Hispanic | 0.87 | 0.65 – 1.15 | 0.343 | 0.76 | 0.56 – 1.02 | 0.075 | 0.78 | 0.57 – 1.07 | 0.130 |
| AIAN | 0.99 | 0.33 – 2.39 | 0.979 | 0.79 | 0.26 – 1.92 | 0.629 | 0.42 | 0.07 – 1.45 | 0.241 |
| Asian | 0.11 | 0.03 – 0.25 | <0.001 | 0.10 | 0.03 – 0.23 | <0.001 | 0.12 | 0.04 – 0.29 | <0.001 |
| Age | 0.95 | 0.94 – 0.96 | <0.001 | 0.95 | 0.94 – 0.96 | <0.001 | |||
| Sex (Ref: Male) | |||||||||
| Female | 1.90 | 1.64 – 2.20 | <0.001 | 1.82 | 1.57 – 2.12 | <0.001 | |||
| Education (years) | 1.01 | 0.99 – 1.04 | 0.239 | 1.01 | 0.99 – 1.04 | 0.283 | |||
| APOE ε+ | 1.05 | 0.90 – 1.21 | 0.549 | ||||||
| n | 7994 | 7965 | 7103 | ||||||
Abbreviations: OR=Odds Ratio; CI=Confidence Interval; Ref=Reference category; nHW=non-Hispanic White; AA=African American; AIAN= American Indian and Alaskan Natives; APOE ε+= Apolipoprotein ε4 allele
3.3. Longitudinal analyses
There were 2255 (27.60%) participants who developed cognitive impairment in subsequent visits: 28.59% nHW (n=1805), 22.16% AA (n=277), 28.97% Hispanics (n=113), 26.34% Asians (n=49), and 37.95% AIAN (n=11). Unadjusted analyses using Kaplan-Meier curves revealed significant differences (X2=19.9; p<.001) in survival probability (CDR > 0) between Hispanic, AIAN and Asian groups compared to nHW (Figure 1).
Figure 1.
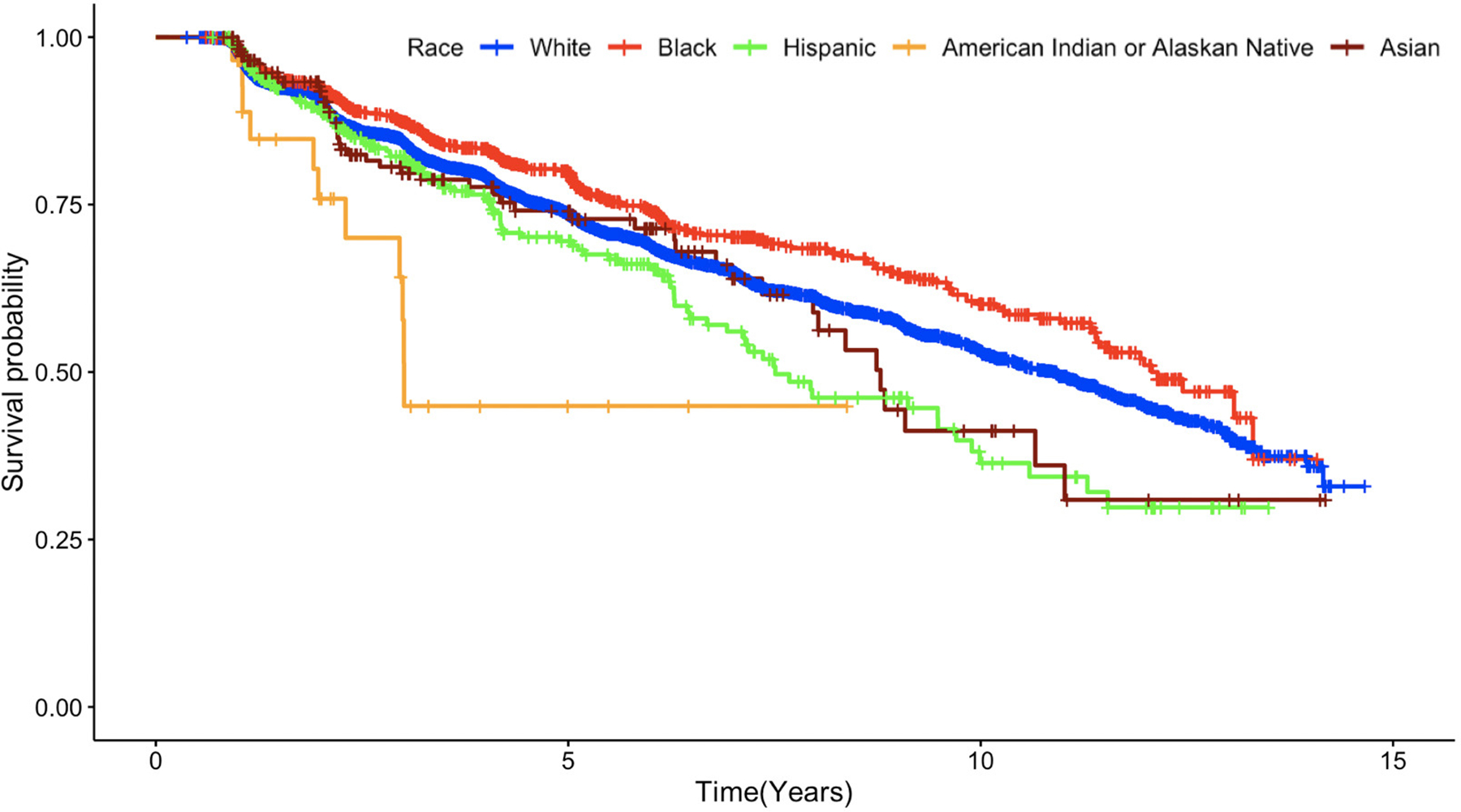
Time to incident cognitive impairment using Kaplan-Meier curves across ethnoracial groups among older adults who were cognitively normal at baseline.
In the adjusted analyses (n=244) using CPHM, compared to nHW, Hispanics had a 46% increased hazard to develop incident cognitive impairment (p<0.001), and Asians had a 34% increased hazard (p=0.080) trending towards significance (Table 4). AA had a 14% (p=0.038) lower hazard for progression compared to nHW. Depression was significantly associated with the risk of progressing to incident cognitive impairment. A participant with active depression without taking antidepressants, had a 77% greater hazard than those who did not have depression and did not take antidepressants. For participants with depression and taking an antidepressant, the hazard was decreased (45%) but still statistically significant compared to those without depression or antidepressant use. Additionally, those taking antidepressants without active depression still presented with a higher hazard (31%) of developing incident cognitive impairment. Females had a 28% lower hazard compared to men (p<0.001), while age was associated with an increased hazard of progressing (p<0.001). Positive APOE ε4 status contributed to a 52% greater hazard for progressing compared to participants without a copy of the APOE ε4 allele (p<0.001).
Table 4.
Survival analyses predicting time to incident cognitive impairment (CDR ≥0) among participants cognitively normal at baseline.
| Cox Proportional Hazards Model | Fine and Gray Model | |||||
|---|---|---|---|---|---|---|
| Race/Ethnicity | ||||||
| Ref: nHW | — | — | — | — | ||
| AA | 0.86 | 0.74, 0.99 | 0.038 | 0.85 | 0.73, 0.97 | 0.020 |
| Hispanic | 1.46 | 1.19, 1.80 | <0.001 | 1.47 | 1.21, 1.78 | <0.001 |
| Asian | 1.34 | 0.97, 1.85 | 0.080 | 1.40 | 1.03, 1.90 | 0.032 |
| Depression and Antidepressant Use | ||||||
| Ref: Depression − Antidepressant − | — | — | — | — | ||
| Depression − Antidepressant + | 1.31 | 1.13, 1.53 | <0.001 | 1.26 | 1.09, 1.45 | 0.002 |
| Depression + Antidepressant − | 1.77 | 1.41, 2.23 | <0.001 | 1.73 | 1.37, 2.17 | <0.001 |
| Depression + Antidepressant + | 1.45 | 1.18, 1.80 | <0.001 | 1.40 | 1.15, 1.71 | <0.001 |
| Gender | ||||||
| Ref: Male | — | — | — | — | ||
| Female | 0.82 | 0.75, 0.90 | <0.001 | 0.86 | 0.78, 0.94 | <0.001 |
| Age at Baseline | 1.07 | 1.06, 1.08 | <0.001 | 1.05 | 1.05, 1.06 | <0.001 |
| Education | 0.97 | 0.96, 0.99 | 0.001 | 0.98 | 0.96, 0.99 | 0.007 |
| APOE ε4+ | 1.52 | 1.38, 1.67 | <0.001 | 1.49 | 1.36, 1.63 | <0.001 |
Abbreviations: CDR=Clinical Dementia Rating; HR=Hazard Ratio; CI=Confidence Interval; Ref=Reference category; nHW=non-Hispanic White; AA=African American; AIAN= American Indian and Alaskan Natives; APOE ε+= Apolipoprotein ε4 allele
The Fine and Gray Model accounting for death as a competing event produced nearly identical results. The one notable exception showed Asians had a 40% great hazard of progression to incident cognitive impairment compared to nHW (p=0.032). Similar to the cross-sectional analyses, both models adjusted for ADCs with results unchanged. The vascular composite and SDI were not significant.
3.4. Interaction effect between depression, ethnoracial groups, and key demographics
No significant interaction effects were found between race and depression or sex and depression. However, APOE ε4 moderated the risks of progressing to incident cognitive impairment between ethnoracial groups and sex (Figure 2). APOE ε4 was associated with a decreased hazard of progressing among Hispanic (HR=0.60; p=0.035) and AA (HR=0.71; p=0.027) compared to nHW older adults. Females with APOE ε4 had a 32% greater hazard progression (p=0.008) compared to males.
Figure 2.
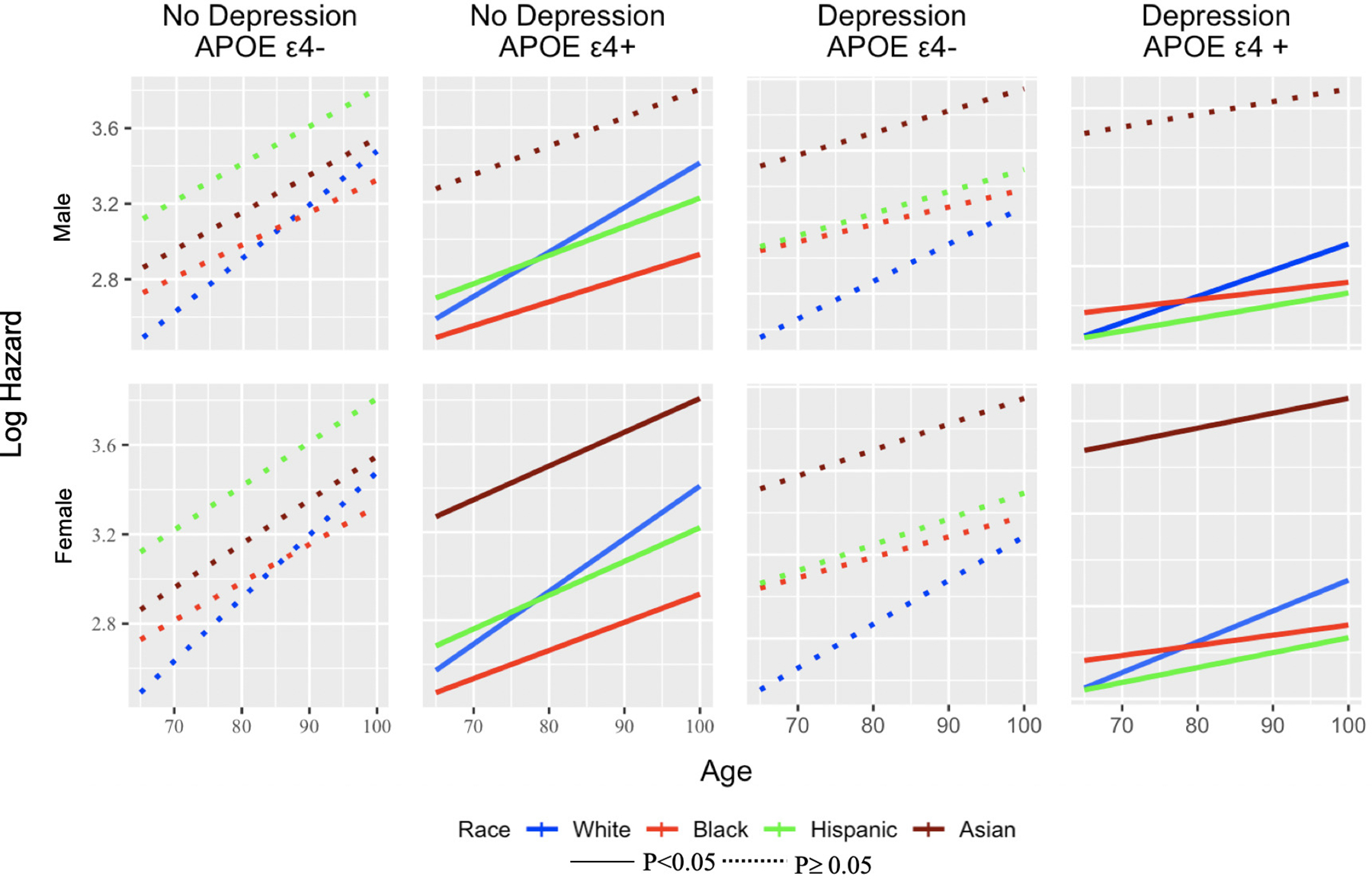
Examining differences across ethnoracial groups based on Log Hazard (y-axis) across eight groups and based on depression, APOE ε4, sex, and age. Hispanic and Black males with APOE ε4+ are less likely to progress compared with non-Hispanic White men. Females with the APOE ε4+ are more likely to progress compared to males among all the races.
4. DISCUSSION
This cohort study of cognitively normal older adults (≥ 65 years) leveraged longitudinal data from NACC UDS across 37 ADCs to examine ethnoracial differences in depression and antidepressant use and to determine if depression and antidepressant use were associated with a greater risk of progressing to incident cognitive impairment. We found that AA were less likely to have active depression, and both AA and Asians were less likely to report using antidepressants compared to nHW at baseline. There were no statistically significant ethnoracial group differences in depressive or neuropsychiatric symptoms. Longitudinal analyses accounting for death as a competing risk found that Hispanics had a 45% increased hazard and Asians had 39% increased hazard of progressing to incident cognitive impairment compared to nHW. Conversely, AA had a lower hazard for progressing to incident cognitive impairment in both longitudinal models. Statistical interactions between ethnoracial groups and depression were not significant.
Longitudinal analyses revealed that older adults with active depression and not on antidepressants had a significantly greater risk (73%) of progressing to incident cognitive impairment, compared to those without depression and antidepressant use. Participants with active depression and on antidepressants had a higher hazard for progressing compared to those without depression and antidepressant use. Conversely, those without active depression but on antidepressants still presented with an increased hazard (26%) for progression. This finding is not surprising since these participants may have had depression in the past and are actively being treated or on a maintenance regiment. A prior systematic review and meta-analysis found that those with cognitive impairment or ADRD were more likely to be prescribed antidepressants, particularly before the age of 65.26 A recent biomarker imaging study found that antidepressants modified the relationship between depression and tau pathology in cognitively normal older adults.27 However, the biological pathway and etiological interaction remains unclear.
Findings from previous research that have examined racial/ethnic differences in depression have varied as some studies indicate that there are higher rates of major depression and depressive symptoms among historically marginalized ethnoracial groups.19, 20 However, researchers have also described the “Black-White depression paradox” in which results indicate lower rates of depression among AA compared to nHW adults despite greater exposure to adverse social and economic stressors across the life course.28–31 The cross-sectional findings from this study indicate that there is no significant difference in risk of depression between Hispanic, Asian, and nHW participants and lower risk among AA compared to nHW older adults. The pattern of association in active depression between AA and nHW observed in this sample corroborates prior research. However, it is important to note that researchers have found that among AA who are depressed, they experience greater duration of depressive episodes, greater frequency of depressive episodes, as well as more severe disabling depressive symptoms.32, 33 Additionally, it is imperative we examine other factors such as exposure to stress and coping mechanism to better understand differences in ethnoracial patterning of depression and risk of ADRD.34, 35 For example, stress is known to covary with depression and inflammation, both of which are both associated with an increased risk of ADRD.36 However, this study did not examine any measures of stress (biological, psychosocial). A recent critical review37 of the paradox, interrogated extant artefactual and etiologic mechanisms (e.g. selection bias, diagnostic instrument misclassification, clinician bias, social support) and concluded there was limited evidence from the literature. It was recommended to consider additional mechanisms like John Henrysim (high-effort coping in response to prolonged stressors resulting in physiological deficits)38 and racial discrimination.
Despite the lack of statistically significant interactions between depression and ethnoracial group in the development of incident cognitive impairment, there were notable differences among ethnoracial groups. Compared to nHW, Hispanics and Asians who were cognitively normal at baseline had a greater risk for progressing while adjusting for sex, age, education, APOE ε4, along with depression and antidepressant use. The previous literature has demonstrated that Hispanics are at greater risk of developing ADRD.3 There is a paucity of research that has examined whether Asians are at a greater risk of ADRD compared to nHW. Alarmingly, a recent study of Medicare data found that AA, Hispanics, and Asians were all less likely to receive a timely dementia diagnosis compared to nHW, and Asians were less likely to receive a comprehensive evaluation compared to nHW.39 Our results also showed a lower hazard rate for AA and Hispanics with at least one copy of APOE ε4 compared to nHW older adults. Additionally, females with APOE ε4 had a greater hazard for progression. A recent study using NACC data comparing AA and nHW older adults found that age, APOE ε4, sex, and body mass index influenced the risk of AD differently by race.40 The study found the same reduced risk for AA progressing to cognitive impairment compared to nHW and stressed the use of two-and-three way interactions to better understand how biological and genetic factors may confer an elevated risk for ADRD across ethnoracial groups.
Despite AA being at twice the risk for ADRD,3, 41 the divergent results found in this study has been replicated in recent publications. For example, using NACC data of adults age 60 or older, Gleason et al., found that there was no difference between AA and nHW cognitively normal at baseline in the progression to incident cognitive impairment or dementia. They also found that AA older adults with MCI at baseline had a lower risk of progression compared to nHW.42 The survival analysis models accounted for referral source and revealed nHW were predominately recruited from clinics while AA more likely be recruited from community-based settings. An older study using NACC data from 1984–2005 found longer survival times for AA and Hispanic older adults compared to nHW older adults after a probable/possible AD diagnosis.43 It is very likely that the sampling/recruitment source influenced the survival outcomes across race/ethnicity in this sample. Vascular disease is often hypothesized to be a factor that contributes to the differential burden of dementia between AA and nHW older adults.44, 45 Prior work suggests that AA adults were twice as likely to present with comorbid cardiovascular disease and depression compared to nHW.46 We examined vascular contributions as a composite score in the models but did not find any ethnoracial group differences nor were vascular contributions significantly associated with greater risk of progression for incident cognitive impairment.
5. LIMITATIONS
There are several limitations with the observational NACC data used. The diagnosis of active depression did not allow for a uniform differentiation between depression subtypes (e.g. major depressive disorder, uni/bipolar depression, dysthymia etc.), new onset vs. life-long depression or provide detail about age of onset, number of depressive episodes, and severity. The GDS is a valid and reliable measure to complete, but has floor effects; however, measures like the Beck Depression Inventory may offer more nuance and dimensionality in depressive symptoms among community-dwelling older adults.47, 48 Relatedly, reliance on self-reported of mental health status, especially from ethnoracial groups may be influenced by stigma, a mistrust of the healthcare system, and social desirability bias. Use of culturally appropriate screens and multiple sources (e.g., collateral source) may help with to improve reliability of self-report. As Gleason and colleagues reported42, there are differences in the sampling/recruitment source for AA and nHW participants which likely extended into Hispanics and Asians. While we accounted for ADCs, we did not control for the enrollment strategies in these models. Additionally, analyses adjusted for years of education, but the NACC dataset has limited information about other social determinants of health. The SDI was not significant which was more likely a result of it being impacted by the partial three-digit ZIP available in the NACC dataset. Future studies should access and assess the full nine-digit ZIP using a neighborhood metric such as the Area Deprivation Index.49 The homogenous binning of participants into the US Census’ restrictive race and ethnicity designations (AA, nHW, Hispanic, Asian) eschews the diversity, life course, and cultural variability that contribute to identity; all of which may impact the development of depression and representation of symptoms in a specific population. Hooker and colleagues20 suggested that disaggregating racial and ethnic groups can provide a more refined understanding of prevalence rates of depression and help to better tailor interventions and policy. Despite AIAN participants presenting with a faster progression compared to nHW older adults, we were forced to exclude them from the adjusted survival models given the small sample size (n=29). However, we recognize the long standing health disparities that the AIAN population face including greater discrimination in health care access and dementia care, along with higher rates of chronic conditions like heart disease.3 We acknowledge the poor representation of AIAN across ADRD studies, however, there is a scientific mandate to do better. The use of nHW as a gold standard or comparative reference group assumes the same process occurs in both groups to impact an outcome.50 Conversely, within-group designs may serve to better understand how social forces across the life course impacts incidence and prevalence of depression and its risk on ADRD. Since data on APOE genotyping was not available on all participants, the sample size was reduced in models it was present.
ACKNOWLEDGMENTS
This work was funded by the National Institute of Health (NIH) and National Institute on Aging (NIH/NIA) grants R01AG068183 (GMB/CMR), R01AG056466 (CR), R01AG067428 (GMB), R01AG074302 (GMB/DLH), BrightFocus Foundation A2021142S (GMB). NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey,MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Footnotes
CONFLICTS OF INTEREST
All authors declare no conflicts related to this work.
Declarations of interest
GMB: None
YZ: None
CMR: None
DLH: None
MMW: None
SM: None
JD: None
AMJ: None
AW: None
JFT: None
REFERENCES
- 1.Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060: Population estimates and projections. 2017.
- 2.The Federal Interagency Forum on Aging-Related Statistics. Older Americans 2016: Key indicators of well-being. Federal Interagency Forum on Aging-Related Statistics; 2016. [Google Scholar]
- 3.Alzheimer’s Association. 2021 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2021;17. [DOI] [PubMed] [Google Scholar]
- 4.Weuve J, Barnes LL, de Leon CFM, Rajan KB, Beck T, Aggarwal NT, et al. Cognitive aging in black and white Americans: cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology (Cambridge, Mass). 2018;29:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer’s & Dementia. 2016;12:216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhry A, Rizig M. Comparing fluid biomarkers of Alzheimer’s disease between African American or Black African and white groups: A systematic review and meta-analysis. J Neurol Sci. 2020:117270. [DOI] [PubMed] [Google Scholar]
- 7.Deters KD, Napolioni V, Sperling RA, Greicius MD, Mayeux R, Hohman T, et al. Amyloid PET imaging in self-identified non-Hispanic Black participants of the Anti-Amyloid in Asymptomatic Alzheimer’s Disease (A4) study. Neurology. 2021;96:e1491–e500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris JC, Schindler SE, McCue LM, Moulder KL, Benzinger TLS, Cruchaga C, et al. Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA neurology. 2019;76:264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzales MM, Short MI, Satizabal CL, O’Bryant S, Tracy RP, Zare H, et al. Blood biomarkers for dementia in Hispanic and non-Hispanic White adults. Alzheimer’s & Dementia: Translational Research & Clinical Interventions. 2021;7:e12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byers AL, Yaffe K. Depression and risk of developing dementia. Nature Reviews Neurology. 2011;7:323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eaton WW, Armenian H, Gallo J, Pratt L, Ford DE. Depression and risk for onset of type II diabetes: a prospective population-based study. Diabetes Care. 1996;19:1097–102. [DOI] [PubMed] [Google Scholar]
- 12.Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. International Journal of Geriatric Psychiatry: A journal of the psychiatry of late life and allied sciences. 2007;22:613–26. [DOI] [PubMed] [Google Scholar]
- 13.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396:413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Depression and other common mental disorders: global health estimates. CC BY-NC-SA 3.0 IGO ed: World Health Organization; 2017. [Google Scholar]
- 15.Gallagher D, Kiss A, Lanctot K, Herrmann N. Depression and risk of Alzheimer dementia: a longitudinal analysis to determine predictors of increased risk among older adults with depression. The American Journal of Geriatric Psychiatry. 2018;26:819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim D, Wang R, Kiss A, Bronskill SE, Lanctot KL, Herrmann N, et al. Depression and Increased Risk of Alzheimer’s Dementia: Longitudinal Analyses of Modifiable Risk and Sex-Related Factors. The American Journal of Geriatric Psychiatry. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brenowitz WD, Zeki Al Hazzouri A, Vittinghoff E, Golden SH, Fitzpatrick AL, Yaffe K. Depressive Symptoms Imputed Across the Life Course Are Associated with Cognitive Impairment and Cognitive Decline. Journal of Alzheimer’s Disease. 2021:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babulal GM, Quiroz YT, Albensi BC, Arenaza-Urquijo E, Astell AJ, Babiloni C, et al. Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: update and areas of immediate need. Alzheimer’s & Dementia. 2019;15:292–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.González HM, Tarraf W, Whitfield KE, Vega WA. The epidemiology of major depression and ethnicity in the United States. J Psychiatr Res. 2010;44:1043–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hooker K, Phibbs S, Irvin VL, Mendez-Luck CA, Doan LN, Li T, et al. Depression among older adults in the United States by disaggregated race and ethnicity. The Gerontologist. 2019;59:886–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993. [DOI] [PubMed] [Google Scholar]
- 22.Butler DC, Petterson S, Phillips RL, Bazemore AW. Measures of Social Deprivation That Predict Health Care Access and Need within a Rational Area of Primary Care Service Delivery. Health Serv Res. 2013;2:539–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 24.Kassambara A, Kosinski M, Biecek P, Fabian S. Package ‘survminer’. Drawing Survival Curves using ‘ggplot2’(R package version 03 1). 2017.
- 25.Gray B, Gray MB. Package ‘cmprsk’. 2020.
- 26.Moraros J, Nwankwo C, Patten SB, Mousseau DD. The association of antidepressant drug usage with cognitive impairment or dementia, including Alzheimer disease: A systematic review and meta‐analysis. Depress Anxiety. 2017;34:217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babulal GM, Roe CM, Stout SH, Rajasekar G, Wisch JK, Benzinger TLS, et al. Depression is associated with tau and not amyloid positron emission tomography in cognitively normal adults. Journal of Alzheimer’s Disease. 2020;74:1045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mouzon DM. Can family relationships explain the race paradox in mental health? Journal of Marriage and Family. 2013;75:470–85. [Google Scholar]
- 29.Jackson JS, Torres M, Caldwell CH, Neighbors HW, Nesse RM, Taylor RJ, et al. The National Survey of American Life: A study of racial, ethnic and cultural influences on mental disorders and mental health. Int J Methods Psychiatr Res. 2004;13:196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnes DM, Bates LM. Do racial patterns in psychological distress shed light on the Black–White depression paradox? A systematic review. Soc Psychiatry Psychiatr Epidemiol. 2017;52:913–28. [DOI] [PubMed] [Google Scholar]
- 31.Jackson JS, Knight KM. Race and self-regulatory health behaviors: the role of the stress response and the HPA axis in physical and mental health disparities. Social Structures, Aging, and Self-regulation in the Elderly. 2006:189–239. [Google Scholar]
- 32.Williams DR, Gonzalez HM, Neighbors H, Nesse R, Abelson JM, Sweetman J, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean blacks, and non-Hispanic whites: results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64:305–15. [DOI] [PubMed] [Google Scholar]
- 33.Breslau J, Kendler KS, Su M, Gaxiola-Aguilar S, Kessler RC. Lifetime risk and persistence of psychiatric disorders across ethnic groups in the United States. Psychol Med. 2005;35:317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Assari S, Lankarani MM. stressful life events and risk of Depression 25 Years later: race and gender Differences. Frontiers in Public Health. 2016;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mezuk B, Rafferty JA, Kershaw KN, Hudson D, Abdou CM, Lee H, et al. Reconsidering the role of social disadvantage in physical and mental health: stressful life events, health behaviors, race, and depression. American Journal of Epidemiology. 2010;172:1238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cipriani G, Lucetti C, Carlesi C, Danti S, Nuti A. Depression and dementia. A review. Eur Geriatr Med. 2015;6:479–86. [Google Scholar]
- 37.Pamplin JR 2nd, Bates LM. Evaluating hypothesized explanations for the Black-white depression paradox: A critical review of the extant evidence. Social Science & Medicine (1982). 2021;281:114085–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hudson DL, Neighbors HW, Geronimus AT, Jackson JS. Racial Discrimination, John Henryism, and Depression Among African Americans. The Journal of Black Psychology. 2016;42:221–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsoy E, Kiekhofer RE, Guterman EL, Tee BL, Windon CC, Dorsman KA, et al. Assessment of Racial/Ethnic Disparities in Timeliness and Comprehensiveness of Dementia Diagnosis in California. JAMA Neurology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiong C, Luo J, Coble D, Agboola F, Kukull W, Morris JC. Complex interactions underlie racial disparity in the risk of developing Alzheimer’s disease dementia. Alzheimer’s & Dementia. 2020;16:589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graff-Radford NR, Besser LM, Crook JE, Kukull WA, Dickson DW. Neuropathologic differences by race from the National Alzheimer’s Coordinating Center. Alzheimer’s & Dementia. 2016;12:669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gleason CE, Norton D, Zuelsdorff M, Benton SF, Wyman MF, Nystrom N, et al. Association between enrollment factors and incident cognitive impairment in Blacks and Whites: Data from the Alzheimer’s Disease Center. Alzheimer’s & Dementia. 2019;15:1533–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehta KM, Yaffe K, Pérez-Stable EJa, Stewart A, Barnes D, Kurland BF, et al. Race/ethnic differences in AD survival in US Alzheimer’s Disease Centers. Neurology. 2008;70:1163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miles TP, Froehlich TE, Bogardus ST Jr, Inouye SK. Dementia and race: are there differences between African Americans and Caucasians? J Am Geriatr Soc. 2001;49:477–84. [DOI] [PubMed] [Google Scholar]
- 45.Barnes LL, Leurgans S, Aggarwal NT, Shah RC, Arvanitakis Z, James BD, et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology. 2015;85:528–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.González HM, Tarraf W. Comorbid cardiovascular disease and major depression among ethnic and racial groups in the United States. Int Psychogeriatr. 2013;25:833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olin JT, Schneider LS, Eaton EM, Zemansky MF, Pollock VE. The Geriatric Depression Scale and the Beck Depression Inventory as screening instruments in an older adult outpatient population. Psychol Assess. 1992;4:190. [Google Scholar]
- 48.von Glischinski M, von Brachel R, Hirschfeld G. How depressed is “depressed”? A systematic review and diagnostic meta-analysis of optimal cut points for the Beck Depression Inventory revised (BDI-II). Qual Life Res. 2019;28:1111–8. [DOI] [PubMed] [Google Scholar]
- 49.Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible—the neighborhood atlas. The New England Journal of Medicine. 2018;378:2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whitfield KE, Allaire JC, Belue R, Edwards CL. Are comparisons the answer to understanding behavioral aspects of aging in racial and ethnic groups? The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2008;63:P301–P8. [DOI] [PMC free article] [PubMed] [Google Scholar]


