Abstract
The impact of liver metastases on immune checkpoint-inhibitor effectiveness in patients with solid-tumor malignancies has been the focus of several recent clinical and translational studies. We review the literature describing the immune functions of the liver and particularly the mechanistic observations in these studies. The initial clinical observation was that pembrolizumab appeared to be much less effective in melanoma and non–small cell lung cancer (NSCLC) patients with liver metastasis. Subsequently other clinical studies have extended and reported similar findings with programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1) inhibitors in many cancers. Two recent translational studies in animal models have dissected the mechanism of this systemic immune suppression. In both studies CD11b+ suppressive macrophages generated by liver metastasis in a two-site MC38 model appear to delete CD8+ T cells in a FasL-dependent manner. In addition, regulatory T-cell (Treg) activation was observed and contributed to the distal immunosuppression. Finally, we discuss some of the interventions reported to address liver immune suppression, such as radiation therapy, combination checkpoint blockade, and Treg depletion.
Introduction
Not all sites of metastasis in solid tumors are equal in their impact on treatment outcome; some, such as liver metastasis, have a disproportionate impact on survival and treatment response in the context of immunotherapy. Herein we review the recent experimental and translational advances demonstrating that systemic anticancer immune responses can be shaped by the liver.
The liver is anatomically, histologically, and functionally unique (1). It processes blood flowing from the gut through its complex lobular architecture, detoxifying and extracting nutrients from the portal venous blood. It is immunologically unique in the number and types of conventional and nonconventional antigen-processing cells which enable the liver to simultaneously tolerize T cells to the innocuous nutrient and commensal bacteria antigen load from the gut while also, in general, enabling potent immune responses to pathogenic microbes. Anatomically, the liver has portal inflow and a central vein outflow arranged in a hexagonal lobular structure with fenestrated capillaries that allow direct contact between circulating T cells and hepatocytes. Also uniquely amongst organs, the liver is able to regenerate up to 90% of its volume and is able to scale to body size (2). This compensatory hyperplasia is not due to a specialized stem-cell population as in the gut, skin, or bone marrow but is present in midzone hepatocytes and can be activated following infection, injury, or hepatectomy (3, 4).
The uniquely tolerogenic immune orientation of the liver was initially described by Calne and colleagues in a set of experiments in pigs where it was shown that major histocompatibility complex (MHC)–mismatched liver allografts could be successfully grafted (5). This ability to accept mismatched allografts is without parallel in other solid-organ transplants (reviewed in ref. 6). In humans, liver transplant recipients require less immunosuppression than do renal or cardiac transplant recipients. In addition, patients with liver allografts can be weaned off immunosuppression (which is not possible in most instances in renal or cardiac grafts) and liver allografts can tolerize the recipient to other types of grafts such as renal, cardiac, or skin allografts (5). Interestingly, injection of antigen into the portal vein (as compared with the inferior vena cava) results in tolerance (7). These data highlight that the liver can regulate systemic immune responses.
Several proposed mechanisms exist for liver graft tolerance. One model is that antigen-presenting cells (APC) in the liver, Kupffer cells, stellate cells, and liver sinusoidal endothelial cells (LSEC) present antigens in a tolerogenic fashion. Allospecific T cells which are educated within the liver are consequently either tolerized or deleted. Supportive of this model, delivery of ovalbumin, a strongly immunogenic protein for mammals, into the stomach leads to tolerization to future exposure; this oral tolerization can be abolished if blood from the gut is diverted from the liver using a mesenteric-portal shunt (8). In ex-vivo models, LSECs can present ovalbumin to T cells and activate them but bias them towards a tolerogenic cytokine program (9). Indeed LSECs from orally administered ovalbumin-tolerized mice can be transferred into mice and can be tolerized to ovalbumin which is typically associated with a marked immune reaction (9). Liver APCs appear to be able to tolerize when injected into the portal vein (10). An additional mechanism for tolerance is the induction of regulatory T cells (Treg) by LSECs as seen in nanoparticle delivery of antigen to LSEC in an experimental allergic encephalomyelitis model (11). Natural Killer (NK) cell- hepatocyte interaction via NKG2A has also been shown to induce Tregs (12) in the liver. In addition, the liver acts as a graveyard for activated T cells that become trapped and eventually destroyed in the liver by clonal deletion (13). These data as well as others reviewed here (14) collectively highlight the immune functionality of the liver and its suppressive milieu.
It has been postulated that some viruses [e.g., hepatitis C virus, hepatitis B virus (HBV)] “hijack” the liver’s tolerogenic mechanisms resulting in ineffective or transient priming of CTLs and subsequent incomplete clearance of infection. Interestingly, virus-specific CD4 and CD8 T-cell responses are able to successfully clear the virus in a minority of patients, with viral control being associated with a greater breadth and depth to the T-cell response. Failure of these T-cell responses is associated with viral persistence (15). There are multiple inhibitory pathways that contribute to T-cell dysfunction in chronic hepatitis, including: (i) extrinsic regulation through Tregs and cytokines; (ii) intrinsic regulation through coinhibitory molecules like programmed death-1 (PD-1) or CTL-associated antigen-4 (CTLA-4); and (iii) deletion of T cells that are able to recognize the virus (16-18). These mechanisms enable virus persistence, and highlight the Achilles heel of hepatic immune tolerance.
T-cell priming in lymphoid tissue and the tumor microenvironment
Recent research has highlighted the importance of antigen cross-presentation with costimulation by dendritic cells (DC) to naïve T cells (19, 20) for effective immunotherapy. In lymphoid tissue or in the tumor microenvironment (TME), type-1 conventional DC (cDC1) are crucial for cross-presentation to CD8+ T cells and deletion of these cells abrogates antitumor immunity (21). While these cells are rare, the frequency of these cells in the TME is a crucial determinant of T-cell infiltration, immune checkpoint inhibitor (ICI) response and of survival. Another potent DC subtype in the TME is the cDC2 which present antigen to CD4 cells and can in some cases prime the immune system for tumor rejection in the context of ICI (22). Phenotypically, cDC1 express the BATF3 transcription factor and are CD11c+, MHC-II+, CD8α+, (resident) CD103+, (migratory) CD24+, XCR1+, CLEC9A+, DEC205+, and essentially prime CD8+ T cells while cDC2 express CD11c+, MHC-II+, CD11b+ (high), CD172a+, and prime CD4+ cells (19).
T-cell priming in the liver
In contrast to the efficient priming described above, in the liver, priming often results in tolerized or anergic T cells. Portal vein blood is replete with microbial products from the gut, including endotoxic lipopolysaccharide (LPS). The presence of persistent low-level LPS in portal blood coming into the liver results in tonic stimulation of toll-like receptor 4 (TLR4) on multiple liver-cell types. The liver contains a large and diverse population of APCs including a large population of plasmacytoid and myeloid DC, resident hepatic macrophages called Kupffer cells, LSECs, and hepatic stellate cells (HSC). All of these cells are capable of presenting antigens to T cells. Kupffer cells, representing the vast majority of all tissue-resident macrophage populations in the entire body (23), are predominantly tolerogenic. They present antigen to T cells along with PGE2 (24), nitric oxide (25), and IL10 and especially in the presence of low levels of LPS, do so without costimulation. Kupffer cells have been implicated in portal vein and oral antigen tolerance. Although Kupffer cells are typically tolerogenic, when TLR 3 or TLR9 ligands are present they can prime T cells (26). LSECs, unlike Kupffer cells are fully capable of cross-presenting antigens to T cells but express PD-L1 and as a result also promote T-cell anergy (26). While hepatocytes are also able to interact with T cells directly due to the unique fenestrated capillary network in the liver, their cross-presentation of antigen in the absence of costimulation can result in T-cell anergy (27, 28). In this regard, there is interesting data (29) that HBV core or envelope antigen presentation by Kupffer cells results in efficient priming and Teff differentiation, while HBV core or envelope presentation by hepatocytes results in dysfunctional CD8+ cells that could be rescued by IL2 but not PD-1 blockade. These data cumulatively show that hepatic tolerance is carried out by an immunosuppressive network of diverse cell types and the complexity of hepatic priming.
Clinical data with liver metastasis treated with ICI
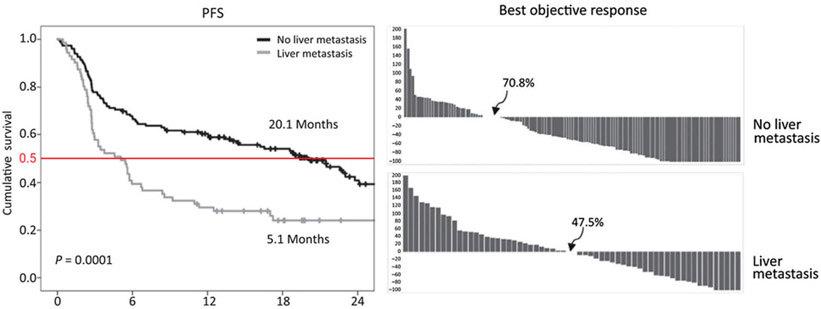
The initial observation of reduced response to PD-1 blockade in patients with liver metastasis was made by Tumeh and colleagues in 2015 (30); it was noted that in 112 patients treated with pembrolizumab, the overall response rate (ORR) was of 16% and median progression-free survival (PFS) of 2.79 months in patients with liver metastasis versus an ORR of 40% and a median PFS of 5.58 months in all patients. An update by Goldinger and colleagues (31) in a larger sample (n = 336), multivariate analysis confirmed the predictive value of liver metastasis. In a subsequent publication, it was demonstrated that the liver metastasis subgroup had a reduced PFS (median PFS, 5.1 months) compared with patients without liver metastasis (median PFS, 20.1 months; P <0.0001); this finding was confirmed in a separate validation cohort (32). These findings were replicated in patients with non–small cell lung cancer (NSCLC); here, the PFS was also significantly reduced in patients with liver metastasis [median PFS 1.82 months; 95% confidence interval (CI), 1.36–2.02] compared with those without liver metastasis (median PFS 4.03 months; 95% CI, 2.12–5.09) and objective response rates were significantly lower as well (Fig. 1; ref. 32).
Figure 1.
Kaplan–Meier OS curves for melanoma patients without (black line) and with liver metastasis (gray line) and waterfall response curves for melanoma without and with liver metastasis. All patients were treated with pembrolizumab immunotherapy as described in Tumeh and colleagues (32). Figure originally published in Cancer Immunology Research (32).
Given the differences in clinical response, Tumeh and colleagues (32) investigated if biopsy samples from patients with melanoma liver metastasis had reduced CD8+ T-cell infiltration at the invasive margin (33) which had been reported to correlate with PD-1 response. Indeed, in 61 patients, fewer CD8+ T cells were found in nonresponders as compared with responders to PD-1 (as previously reported). In addition, The CD8+ T-cell count at the invasive margin was also significantly lower in the liver metastasis group compared with the nonliver metastasis group (liver metastases group mean count 547, nonliver metastases group, mean count 1,441; P < 0.016). Additionally in 35 nonliver (mostly skin) biopsies from patients who also had liver metastasis, reduced CD8+ T-cell infiltration was seen at the invasive margin in these distant nonliver metastases.
In a separate clinical trial of patients with melanoma, renal cell carcinoma (RCC) or NSCLC treated with nivolumab (CA 209–003), Topalian and colleagues examined the impact of liver metastasis on survival at 5 years and found in a multivariate model that the overall survival (OS) OR was 0.31 (95% CI, 0.12–0.83; P = 0.02; ref. 34). In yet another study, done in Australia, 140 patients with melanoma treated with ipilimumab and a PD-1 antibody were evaluated (35). In this study, patients with liver metastases had an inferior ORR (ORR, 0.33; P = 0.02), PFS [PFS, hazard ratio (HR), 4.03; P < 0.01], and OS (OS, HR, 3.17; P = 0.01) compared with those without liver metastasis. A larger recently published study (36), examined the effect of liver metastases on 1,009 patients with advanced melanoma. Here, liver metastasis was associated with a HR of 2.22 for death (95% CI, 1.48–3.33; P < 0.001) and 2.0 for progression (95% CI, 2.00; 1.36–2.79; P < 0.001) in a Cox proportional hazard model. The authors concluded that the presence of liver metastasis, elevated lactate dehydrogenase (LDH) and Eastern Cooperative Oncology Group (ECOG) performance status had a more significant impact in multivariate analysis than American Joint Committee on Cancer (AJCC) version 8 stage. Interestingly, the same group reported that MAP Kinase–targeted therapy was not associated with the same detrimental impact in patients with melanoma with liver metastasis (37).
The presence of liver metastases is also associated with worse prognosis in other tumor types. In NSCLC, Sridhar and colleagues (38) examined 569 patients enrolled in the ATLANTIC and 1,108 trials who were treated with the PD-L1 inhibitor durvalumab at 10 mg/kg every 2 weeks. A multivariate Cox proportional hazards analysis found that the presence of liver metastasis was associated with worse ORR, PFS, and OS. In the ATLANTIC study, the HR for OS in the multivariate Cox model was 2.20 for patients with liver metastasis as compared with those without (P < 0.0001), while in the 1,108-trial study, the HR for OS was 1.91 (P = 0.0001) for a similar comparison in a multivariate Cox analysis. Recently a meta-analysis of 10 trials with PD-1/PD-L1 in NSCLC was conducted and showed a HR of 1.73 for liver metastasis (HR = 1.73; 95% CI, 1.35–2.20; I2 = 69.4%; P < 0.001; ref. 39).
In urothelial cancer, in patients treated with the PDL-1 inhibitors atezolizumab (n = 405), durvalumab (n = 242), and avelumab (n = 198) in the second line following platinum-based chemotherapy, liver metastasis had a HR of 1.55 for OS in a multivariate analysis and have been incorporated into a prognostic model (40). In the first-line setting, patients treated with ICI also had worse outcomes if liver metastases were present (n = 370, liver metastasis ORR 17% versus no liver metastasis 30%; ref. 41). Collectively, these publications highlight that patients with liver metastasis derive reduced clinical benefit from ICI across multiple cancer types.
Translational Modeling of Liver Metastases
Murine tumor models are critical to our understanding of novel clinical observations as they enable rigorous reverse-translational hypothesis testing and development. While murine cell line-derived allograft (CDX) models have been useful for targeted therapy, these models are not as useful for modeling immune therapies. Genetically Engineered Mouse models (GEMM) are also useful in expressing a specific oncogene or knockout in an immune-intact mouse. However, they have limitations including long latency periods and unpredictable metastatic biology making them less tractable for studying metastasis site (42). Given these issues, the workhorse of cancer immunotherapy research for the last 2 decades has been the single-tumor inoculation of syngeneic cell lines. Indeed, these models have led to the successful development of modern immunotherapeutic such as ICIs and chimeric antigen receptor (CAR) T-cell therapies (43, 44). To date, the generation and use of syngeneic cell lines matched with many different mouse genetic backgrounds has enabled the study of ICI, with response rates similar to those seen in the clinical setting (45).
Although the single-tumor syngeneic immunocompetent mouse model has been useful, alternative models are needed to recapitulate the complexity of human cancer. For instance, immunotherapy is deployed predominantly in metastatic or unresectable disease, which accounts for approximately 90% of cancer-related deaths (46), raising the possibility that the majority of single-site mouse models today do not capture the complexities of metastatic-cancer immunobiology. The observation of reduced systemic immunotherapy efficacy in patients with liver metastases suggests tumor involvement in specific organs can impact systemic antitumor immunity or influence the tumor-immune microenvironment at distant tumor sites. Since the immune system is interconnected, it is certainly possible that immunity, or suppression, from one anatomic site can transfer to another. The earliest efforts that studied the observation known as concomitant tumor immunity involved inoculums of tumor cells at two separate subcutaneous (SC) sites. The inoculation of tumors at a primary site can result in the immunogenic tumor-bearing host rejecting inoculums of a similar tumor at a distant site, in a process mediated by T cells that were capable of memory and trafficking (47-49). If antitumor immunity can be induced distantly, it raises the question of whether the reverse can occur- can tumors within select organ tissues actively suppress concomitant tumor immunity at distant sites, adding to the immunologic challenge of treating metastatic cancer?
The liver has been reported to be locally immunosuppressive and systemically tolerogenic in the context of infectious disease and transplantation, as reviewed above. In cancer research, most preclinical work focuses on the locally suppressive intrahepatic TME. Greten and colleagues have developed several murine models that examined the immunosuppressive liver TME in the context of hepatocellular carcinoma (HCC) and showed inhibitory modulation of intrahepatic NK and NK T cells results in the loss of antitumor immunity within the liver (50, 51). A list of other preclinical models that studied the local liver TME is summarized in a recent review (52). These models, however, do not accurately capture the impact of liver metastases on systemic antitumor immunity, which is needed to address the tolerogenic organ’s impact on cancer immunotherapy in patients with liver metastases.
To address these issues, a murine model developed by Kershaw and colleagues. was the first to incorporate 2 syngeneic tumors orthotopically implanted at different tissue sites to investigate their influence on each other’s response to immunotherapy (53). Using the Renca RCC model, mice were inoculated with a SC tumor and a second tumor in the kidney, liver, or SC tissue. Interestingly, the mice with two SC tumors had a significantly better response to a triple immune-agonists antibody therapy (anti-CD40, anti-CD137, and anti-DR5) than those with other tumor configurations. Although the authors did not investigate the mechanism of the synchronous liver-tumor mediated suppression, their data demonstrated that the kidney tumors increased the infiltrating M2 macrophages and reduced the infiltrating NK, CD4+, and CD8+ T cells at the concurrent SC tumor site. This process was reversible by blocking the M2-associated chemokine CCL2, supporting the hypothesis that the kidney tumors may coordinate the migration of M2 macrophages toward the SC tumors (53).
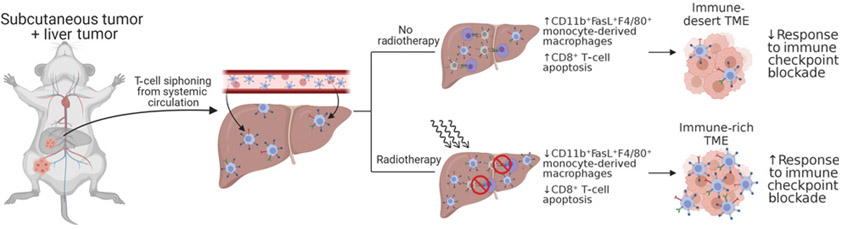
Recently, 2 reports investigated in greater detail the mechanism of liver metastases suppression of systemic antitumor immunity and cancer immunotherapy response using the PD-1 blockade-responsive syngeneic MC38 carcinoma model. In the largest translational study to date on this topic, Yu and Green and colleagues (37) examined T-cell receptor sequencing in patients with breast cancer, colorectal cancer, prostate cancer, melanoma, and NSCLC. They found that patients with liver metastasis but not lung metastases had significantly diminished numbers of intratumor T-cell clones as well as diminished diversity of T-cell clones, suggesting that liver metastases regulated adaptive immune responses. Data from their preclinical model further support that liver metastases created a systemic “immune desert” devoid of functional antitumor CD8+ T cells via Fas ligand–mediated clonal deletion of tumor-antigen–specific T cells by CD11b+ suppressive macrophages (Figs. 2-4). In addition to the suppressive monocytes, Lee and colleagues (54) demonstrated that liver metastases induced CTLA-4, PD-1, and ICOS- high Tregs which were critical in inducing coordinated immune suppression (Figs. 5 and 6). Depletion of the Treg compartment through conditional Foxp3 knockout or depleting anti–CTLA-4 antibodies reversed liver metastases–associated suppression and restored immunotherapy efficacy. Although both Treg and monocyte compartments underwent significant phenotypic and transcriptomic changes as a result of liver metastasis, perturbations of Tregs directly resulted in a shift in the monocytes towards antitumor immunity (increased costimulatory CD80/86). In contrast, intratumoral depletion of monocytes using liposomal chlodronate did not change the Tregs, suggesting the Tregs may be the ultimate driver of the liver-metastases suppressive nexus (37). Notably, all above preclinical studies converge on suppressive CD11b+ myeloid cells as playing a key role in the systemic liver metastases TME. Strategies that address this population of cells will likely yield improved treatment outcomes.
Figure 2.
Schematic diagram showing experimental protocol for mice with subcutaneous + liver-tumor treated with no radiotherapy or with radiotherapy showing changes in the subcutaneous tumor as depicted in Yu and colleagues (37). Figure created with BioRender.com.
Figure 4.
Liver metastasis induces systemic loss of antigen-specific T cells. Immunofluorescent staining of CD8+ cells in MC38 subcutaneous tumors from mice with subcutaneous tumors only or with subcutaneous and liver tumors. Analysis was done at 10 days after therapy initiation. Figure previously published in Nature Medicine (37); reprinted with permission.
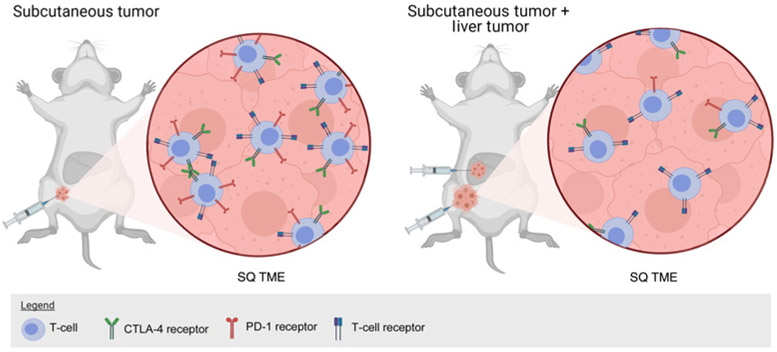
Figure 5.
Mouse model with MC38 colon cancer syngeneic tumor implanted into the subcutaneous tissue or subcutaneous and liver as depicted in Lee JC and colleagues (54). In liver and subcutaneous tumor-bearing mice, there are reduced PD-1hi/CTLA-4hi CD8+ T cells, and these tumors have reduced response to PD-1 blockade. Figure created with BioRender.com.
Figure 6.
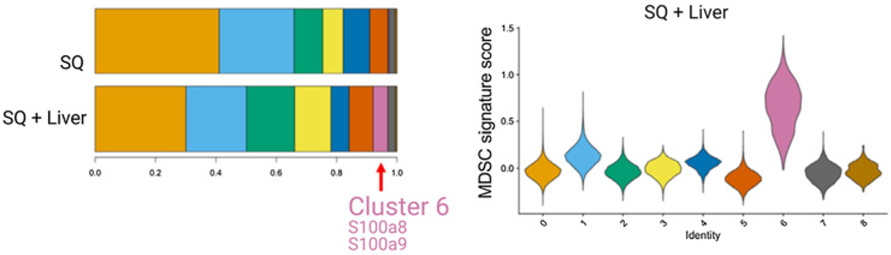
Single-cell RNA sequencing in Lee and colleagues (54) showed a myeloid population consistent with an MDSC phenotype in liver MC38 tumor mice, cluster 6 shown in the histogram and in violin plots showing relative MDSC score ordered by monocyte/myeloid cell subclusters. Figure previously published in Science Immunology (54); reprinted with permission.
Therapeutic Implications for Liver Immunity Nexus
These studies collectively suggest that liver metastases drive therapeutic resistance. Given this finding, it was hypothesized that the elimination of liver metastases by surgical resection or the use of radiotherapy may restore cancer immunotherapy efficacy. Both primary and metastatic liver tumors can be treated using invasive or noninvasive local, regional, or systemic approaches. These approaches have diverse impacts on spontaneous and therapeutic immunity, and, it is not established which approaches are best in combination with cancer immunotherapy clinically.
Surgical resection of primary and metastatic liver tumors is frequently utilized in fit patients with oligometastatic disease and sufficient hepatic reserve. There is limited preclinical evidence examining the impact of liver-tumor resection on therapeutic antitumor immunity, but preclinical modeling suggests that resection can remodel systemic anticancer immunity and eliminate immunosuppression (55). Correlative studies in patients undergoing resection of liver tumors have mainly focused on acute postoperative immunosuppression that underlies perioperative increased infection risk and have not evaluated the impact on adaptive immune system (56). However, the clinical benefits of resection are apparent. Resection of earlystage HCC can be curative, and the resection of oligometastatic colorectal cancer liver metastases in select patients has been associated with prolonged OS (57). Ongoing clinical trials are examining the resection of liver metastases in combination with adoptive cell transfer in HCC (NCT02709070) and immunotherapy in colorectal cancer (NCT03844750).
Stereotactic body radiotherapy (SBRT) is an effective noninvasive treatment modality for both primary and metastatic liver tumors appropriate for patients who are not surgical candidates (58-60). Preclinical studies have suggested that radiotherapy and immunotherapy can synergize to promote improve ICI efficacy in subcutaneous tumor models (61, 62) although the sequence of immunotherapy and radiation is important (63) and PD-1 blockade prior to radiation appears to be deleterious due to destruction of immune cells in the PD-1–stimulated mouse. In preclinical models, radiotherapy has been shown to eliminate immunosuppressive populations including Tregs and myeloid-derived suppressor cells (MDSC; ref. 64). Further, tumoral irradiation can increase antigen expression and exposure, and has been found to increase the T-cell repertoire in both preclinical models and patients (65, 66). Finally, radiotherapy can act as an immunologic adjuvant by inducing the formation of tumor micronuclei, generating cytoplasmic DNA, and causing lipid oxidation which stimulate immune responses (67). Moreover, liver SBRT can promote systemic antitumor immunity in a preclinical models of liver metastases (37); although the dose of radiation used, 8Gy is considerably lower than that used with SBRT. Liver SBRT appears to be safe in combination with immune checkpoint blockade (68). There are case reports suggesting that liver SBRT may induce abscopal responses (69, 70). In larger clinical trials however, out of field responses are rare; in a recent trial of SBRT + pembrolizumab, out-of-field responses occurred in 13.5% of patients (71). Despite these data, it should be noted that abscopal responses are rare in patients with cancer and the complexity of the immune response to radiation is incompletely understood.
Radiofrequency ablation and cryotherapy are minimally invasive approaches that have demonstrated clinical utility in the management of small primary and secondary liver metastases (59). Preclinical evaluations have shown that in subcutaneous models, radiofrequency and cryotherapy can induce an in situ vaccination which potentiates spontaneous and therapeutic antitumor immunity (72, 73). Moreover, both global and tumor antigen-specific T-cell responses have been seen in the peripheral blood of patients with HCC undergoing radiofrequency ablation (74). Further, other minimally invasive immunogenic approaches for the management of liver metastases, such as histotripsy are being developed (75). A large number of clinical trials are examining whether radiofrequency ablation is safe in combination with immunotherapy (76).
Transarterial chemoembolization (TACE) and transarterial radio-embolization (TARE) are effective treatment approaches for patients with localized or regional liver tumors. The smaller caliber of blood vessels in rodents introduces technical challenges that have limited the investigation of these modalities in preclinical models. Correlative studies have found that TACE may diminish the frequency of immunosuppressive Tregs in the blood of patients with HCC (77). It is not yet established if TACE or TARE is safe or effective in combination with immune checkpoint blockade.
Finally, combinatorial approaches of systemic therapy are being developed, and these may modulate antitumor immunity and overcome resistance to immune checkpoint blockades. Lee and colleagues showed that combination anti–CTLA-4 and anti–PD-1 overcame hepatic immunosuppression induced by liver metastases (54). The combination of BRAF inhibitors and MEK inhibitors demonstrate activity in BRAFV600 mutant melanoma, and cytotoxic chemotherapy is active in patients with liver metastases (37). Ongoing and completed clinical trials examining the combination of these agents with immunotherapy are ongoing (78). Other promising approaches include intratumoral IL12 (79).
In summary, there are multiple clinically efficacious approaches to treat liver metastases and correlative studies suggest that these liver-directed approaches may modulate systemic antitumor immunity. Ongoing efforts are underway to understand how best to combine these approaches with cancer immunotherapy. Additional unanswered questions revolve around the sequencing of therapies. Additional clinical trials in both primary liver cancer as well as metastatic cancer patients with liver metastases are needed to understand which strategy may best help these patients, where liver involvement dysregulates the cancer immunity cycle.
Conclusions
The development of liver metastasis is associated with a multitude of immune alterations, many of which induce antigen-specific systemic immune suppression. These alterations reduce the effectiveness of ICI in preclinical models and populations of patients with otherwise ICI-sensitive malignancies. Current preclinical models point to MDSCs and Tregs as potential culprits and suggest that combined immunotherapy or surgery/radiation approaches may be useful to combat these immune-suppressive changes.
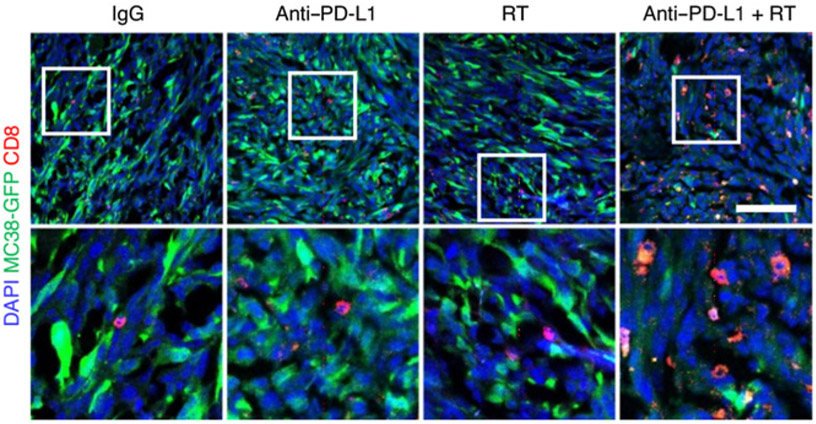
Figure 3.
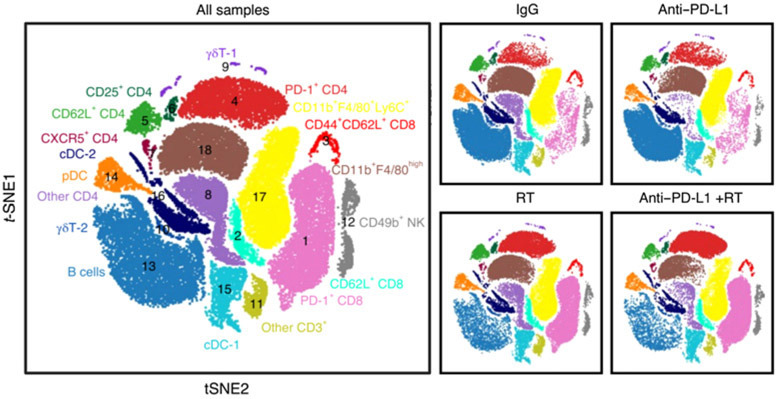
Effect of radiotherapy on immune-cell populations in the liver. Large plot; viSNE analysis of CyTOF immunophenotyping of livers from mice with both subcutaneous and liver tumors, treated with IgG, anti–PD-L1, liver-directed radiotherapy, or combination therapy, as depicted in Yu and colleagues (37). Figure previously published in Nature Medicine (37); reprinted with permission.
Footnotes
Authors’ Disclosures
J.C. Lee reports grants from NIH, A.P. Giannini Foundation, Parker Institute for Cancer Immunotherapy, and UCSF Liver Center (NIH P30DK026743) during the conduct of the study. R.H. Pierce is the Chief Scientific Officer for Sensei Biotherapeutics, a biotechnology company focused on developing therapeutics in immuno-oncology, and has either been a paid consultant for, scientific advisory board member for, or received sponsored research funding (during employment as faculty at the Fred Hutchinson Cancer Research Center, November 2017 to March 2020) from the following entities: OncoSec Medical (former employee, then consultant), Immunomic Therapeutics, Inc., Juno Therapeutics, Inc., Pulse Biosciences, AbbVie, Calithera Biosciences, Minerva Biosciences, AstraZeneca, Curis, Inc., Exicure Therapeutics, X4-Pharma, Incyte Pharmaceuticals, and Neoleukin Therapeutics, all outside the submitted work. A.I. Daud reports grants from Merck, BMS, Pfizer, Roche, and Incyte outside the submitted work, as well as patents with OncoSec and equity in Neuvogen and Trex. No disclosures were reported by the other authors.
References
- 1.Juza RM, Pauli EM. Clinical and surgical anatomy of the liver: a review for clinicians. Clin Anat 2014;27:764–9. [DOI] [PubMed] [Google Scholar]
- 2.Andersson ER. In the zone for liver proliferation. Science 2021;371:887–8. [DOI] [PubMed] [Google Scholar]
- 3.He L, Pu W, Liu X, Zhang Z, Han M, Li Y, et al. Proliferation tracing reveals regional hepatocyte generation in liver homeostasis and repair. Science 2021;371: eabc4346. [DOI] [PubMed] [Google Scholar]
- 4.Wei Y, Wang YG, Jia Y, Li L, Yoon J, Zhang S, et al. Liver homeostasis is maintained by midlobular zone 2 hepatocytes. Science 2021;371:eabb1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, et al. Induction of immunological tolerance by porcine liver allografts. Nature 1969;223:472–6. [DOI] [PubMed] [Google Scholar]
- 6.Benseler V, McCaughan GW, Schlitt HJ, Bishop GA, Bowen DG, Bertolino P. The liver: a special case in transplantation tolerance. Semin Liver Dis 2007;27:194–213. [DOI] [PubMed] [Google Scholar]
- 7.Triger DR, Cynamon MH, Wright R. Studies on hepatic uptake of antigen. I. Comparison of inferior vena cava and portal vein routes of immunization. Immunology 1973;25:941–50. [PMC free article] [PubMed] [Google Scholar]
- 8.Yang R, Liu Q, Grosfeld JL, Pescovitz MD. Intestinal venous drainage through the liver is a prerequisite for oral tolerance induction. J Pediatr Surg 1994;29:1145–8. [DOI] [PubMed] [Google Scholar]
- 9.Limmer A, Ohl J, Wingender G, Berg M, Jüngerkes F, Schumak B, et al. Cross-presentation of oral antigens by liver sinusoidal endothelial cells leads to CD8 T cell tolerance. Eur J Immunol 2005;35:2970–81. [DOI] [PubMed] [Google Scholar]
- 10.Gorczynski RM. Immunosuppression induced by hepatic portal venous immunization spares reactivity in IL-4 producing T lymphocytes. Immunol Lett 1992;33:67–77. [DOI] [PubMed] [Google Scholar]
- 11.Carambia A, Freund B, Schwinge D, Bruns OT, Salmen SC, Ittrich H, et al. Nanoparticle-based autoantigen delivery to Treg-inducing liver sinusoidal endothelial cells enables control of autoimmunity in mice. J Hepatol 2015;62:1349–56. [DOI] [PubMed] [Google Scholar]
- 12.Jinushi M, Takehara T, Tatsumi T, Yamaguchi S, Sakamori R, Hiramatsu N, et al. Natural killer cell and hepatic cell interaction via NKG2A leads to dendritic cell-mediated induction of CD4+ CD25+ T cells with PD-1-dependent regulatory activities. Immunology 2007;120:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev 2000;174:47–62. [DOI] [PubMed] [Google Scholar]
- 14.Horst AK, Neumann K, Diehl L, Tiegs G. Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell Mol Immunol 2016;13:277–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt J, Blum HE, Thimme R. T-cell responses in hepatitis B and C virus infection: similarities and differences. Emerg Microbes Infect 2013;2: e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga ME, Kaminski M, et al. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog 2009;5:e1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopes AR, Kellam P, Das A, Dunn C, Kwan A, Turner J, et al. Bim-mediated deletion of antigen-specific CD8 T cells in patients unable to control HBV infection. J Clin Invest 2008;118:1835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park J-J, Wong DK, Wahed AS, Lee WM, Feld JJ, Terrault N, et al. HBV-specific and global T-cell dysfunction in chronic hepatitis B. Gastroenterology 2016;150:684–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol 2020;20:7–24. [DOI] [PubMed] [Google Scholar]
- 20.Spranger S, Dai D, Horton B, Gajewski TF. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell 2017. 8;31:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 2014;26: 638–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binnewies M, Mujal AM, Pollack JL, Combes AJ, Hardison EA, Barry KC, et al. Unleashing type-2 dendritic cells to drive protective antitumor CD4+ T cell immunity. Cell 2019;177:556–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int 2006;26:1175–86. [DOI] [PubMed] [Google Scholar]
- 24.Callery MP, Mangino MJ, Flye MW. Arginine-specific suppression of mixed lymphocyte culture reactivity by Kupffer cells–a basis of portal venous tolerance. Transplantation 1991;51:1076–80. [DOI] [PubMed] [Google Scholar]
- 25.Roland CR, Walp L, Stack RM, Flye MW. Outcome of Kupffer cell antigen presentation to a cloned murine Th1 lymphocyte depends on the inducibility of nitric oxide synthase by IFN-gamma. J Immunol 1994;153:5453–64. [PubMed] [Google Scholar]
- 26.Huang L-R, Wohlleber D, Reisinger F, Jenne CN, Cheng R-L, Abdullah Z, et al. Intrahepatic myeloid-cell aggregates enable local proliferation of CD8 (+) T cells and successful immunotherapy against chronic viral liver infection. Nat Immunol 2013;14:574–83. [DOI] [PubMed] [Google Scholar]
- 27.Arnold B Parenchymal cells in immune and tolerance induction. Immunol Lett 2003;89:225–8. [DOI] [PubMed] [Google Scholar]
- 28.Herkel J, Jagemann B, Wiegard C, Lazaro JFG, Lueth S, Kanzler S, et al. MHC class II-expressing hepatocytes function as antigen-presenting cells and activate specific CD4 T lymphocyutes. Hepatology 2003;37:1079–85. [DOI] [PubMed] [Google Scholar]
- 29.Bénéchet AP, De Simone G, Di Lucia P, Cilenti F, Barbiera G, Le Bert N, et al. Dynamics and genomic landscape of CD8+ T cells undergoing hepatic priming. Nature 2019;574:200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tumeh PC, Rosenblum M, Handley N, Tsai K, Rodriguez RRS, Khurana N, et al. Abstract 2857: Metastatic site and response to pembrolizumab (anti-PD1 antibody) in melanoma. Cancer Res 2015;75:2857. [Google Scholar]
- 31.Goldinger SM, Tsai KK, Tumeh P, Hamid O, Nosrati A, Loo K, et al. Correlation between metastatic site and response to anti-Programmed Death-1 (PD-1) agents in melanoma. J Clin Oncol 201634. [Google Scholar]
- 32.Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res 2017;5:417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol 2019;5:1411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pires da Silva I, Lo S, Quek C, Gonzalez M, Carlino MS, Long GV, et al. Site-specific response patterns, pseudoprogression, and acquired resistance in patients with melanoma treated with ipilimumab combined with anti-PD-1 therapy. Cancer 2020;126:86–97. [DOI] [PubMed] [Google Scholar]
- 36.Waninger JJ, Ma VT, Journey S, Skvarce J, Chopra Z, Tezel A,et al. Validation of the American Joint Committee on cancer eighth edition staging of patients with metastatic cutaneous melanoma treated with immune checkpoint inhibitors. JAMA Netw Open 2021;4:e210980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med 2021;27:152–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sridhar S, Paz-Ares L, Liu H, Shen K, Morehouse C, Rizvi N, et al. Prognostic significance of liver metastasis in durvalumab-treated lung cancer patients. Clin Lung Cancer 2019;20:e601–8. [DOI] [PubMed] [Google Scholar]
- 39.Huang Y, Zhu L, Guo T, Chen W, Zhang Z, Li W, et al. Metastatic sites as predictors in advanced NSCLC treated with PD-1 inhibitors: a systematic review and meta-analysis. Hum Vaccin Immunother 2020;17:1278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonpavde G, Manitz J, Gao C, Tayama D, Kaiser C, Hennessy D, et al. Five-factor prognostic model for survival of post-platinum patients with metastatic urothelial carcinoma receiving PD-L1 inhibitors. J Urol 2020;204:1173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balar AV, Castellano D, O’Donnell PH, Grivas P, Vuky J, Powles T, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18:1483–92. [DOI] [PubMed] [Google Scholar]
- 42.Day CP, Merlino G, Van Dyke T. Preclinical Mouse Cancer Models: A Maze of Opportunities and Challenges. Cell 2015;163:39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996;271:1734 LP–1736. [DOI] [PubMed] [Google Scholar]
- 44.Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 2012;119:4133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mosely SIS, Prime JE, Sainson RCA, Koopmann J-O, Wang DYQ, Greenawalt DM, et al. Rational selection of syngeneic preclinical tumor models for immunotherapeutic drug discovery. Cancer Immunol Res 2017;5:29–41. [DOI] [PubMed] [Google Scholar]
- 46.Garner H, de Visser KE. Immune crosstalk in cancer progression and metastatic spread: a complex conversation. Nat Rev Immunol 2020;20:483–97. [DOI] [PubMed] [Google Scholar]
- 47.Bursuker I, North RJ. Generation and decay of the immune response to a progressive fibrosarcoma. II. Failure to demonstrate postexcision immunity after the onset of T cell-mediated suppression of immunity. J Exp Med 1984;159:1312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin Y-C, Chang L-Y, Huang C-T, Peng H-M, Dutta A, Chen T-C, et al. Effector/memory but not naive regulatory T cells are responsible for the loss of concomitant tumor immunity. J Immunol 2009;182:6095–104. [DOI] [PubMed] [Google Scholar]
- 49.Gershon RK, Carter RL, Kondo K. On concomitant immunity in tumour-bearing hamsters. Nature 1967;213:674–6. [DOI] [PubMed] [Google Scholar]
- 50.Eggert T, Wolter K, Ji J, Ma C, Yevsa T, Klotz S, et al. Distinct functions of senescence-associated immune responses in liver tumor surveillance and tumor progression. Cancer Cell 2016;30:533–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, et al. Gut microbiome–mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018;360:eaan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown ZJ, Heinrich B, Greten TF. Mouse models of hepatocellular carcinoma: an overview and highlights for immunotherapy research. Nat Rev Gastroenterol Hepatol 2018;15:536–54. [DOI] [PubMed] [Google Scholar]
- 53.Devaud C, John LB, Westwood JA, Yong CSM, Beavis PA, Schwendener RA, et al. Cross-talk between tumors can affect responses to therapy. OncoImmunology 2015;4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee JC, Mehdizadeh S, Smith J, Young A, Mufazalov IA, Mowery CT, et al. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci Immunol 2020;5:eaba0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allen BM, Hiam KJ, Burnett CE, Venida A, DeBarge R, Tenvooren I, et al. Systemic dysfunction and plasticity of the immune macroenvironment in cancer models. Nat Med 2020;26:1125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Efron PA, Matsumoto T, McAuliffe PF, Scumpia P, Ungaro R, Fujita S, et al. Major hepatectomy induces phenotypic changes in circulating dendritic cells and monocytes. J Clin Immunol 2009;29:568–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stewart CL, Warner S, Ito K, Raoof M, Wu GX, Lu W-P, et al. Cytoreduction for colorectal metastases: liver, lung, peritoneum, lymph nodes, bone, brain. when does it palliate, prolong survival, and potentially cure? Curr Probl Surg 2018;55:330–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soliman H, Ringash J, Jiang H, Singh K, Kim J, Dinniwell R, et al. Phase II trial of palliative radiotherapy for hepatocellular carcinoma and liver metastases. J Clin Oncol 2013;31:3980–6. [DOI] [PubMed] [Google Scholar]
- 59.Wahl DR, Stenmark MH, Tao Y, Pollom EL, Caoili EM, Lawrence TS, et al. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol 2016;34:452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jackson WC, Tao Y, Mendiratta-Lala M, Bazzi L, Wahl DR, Schipper MJ, et al. Comparison of stereotactic body radiation therapy and radiofrequency ablation in the treatment of intrahepatic metastases. Int J Radiat Oncol Biol Phys 2018;100:950–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immunemediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009;15:5379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wei J, Montalvo-Ortiz W, Yu L, Krasco A, Ebstein S, Cortez C,et al. Sequence of αPD-1 relative to local tumor irradiation determines the induction of abscopal antitumor immune responses. Sci Immunol 2021;6:eabg0117. [DOI] [PubMed] [Google Scholar]
- 64.Filatenkov A, Baker J, Mueller AMS, Kenkel J, Ahn G-O, Dutt S, et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res 2015;21:3727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Formenti SC, Rudqvist N-P, Golden E, Cooper B, Wennerberg E, Lhuillier C, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med 2018;24:1845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rudqvist N-P, Pilones KA, Lhuillier C, Wennerberg E, Sidhom J-W, Emerson RO, et al. Radiotherapy and CTLA-4 blockade shape the TCR repertoire of tumor-infiltrating T cells. Cancer Immunol Res 2018;6:139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun 2017;8:15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xie C, Duffy AG, Brar G, Fioravanti S, Mabry-Hrones D, Walker M, et al. Immune checkpoint blockade in combination with stereotactic body radiotherapy in patients with metastatic pancreatic ductal adenocarcinoma. Clin Cancer Res 2020;26:2318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hiniker SM, Chen DS, Reddy S, Chang DT, Jones JC, Mollick JA, et al. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Translational Oncology 2012;5:404–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res 2013;1:365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luke JJ, Lemons JM, Karrison TG, Pitroda SP, Melotek JM, Zha Y,et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol 2018;36:1611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.den Brok MHMGM, Sutmuller RPM, van der Voort R, Bennink EJ, Figdor CG, Ruers TJM, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res 2004;64:4024–9. [DOI] [PubMed] [Google Scholar]
- 73.Waitz R, Solomon SB, Petre EN, Trumble AE, Fassò M, Norton L, et al. Potent induction of tumor immunity by combining tumor cryoablation with anti–CTLA-4 therapy. Cancer Res 2012;72:430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mizukoshi E, Yamashita T, Arai K, Sunagozaka H, Ueda T, Arihara F, et al. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology 2013;57:1448–57. [DOI] [PubMed] [Google Scholar]
- 75.Qu S, Worlikar T, Felsted AE, Ganguly A, Beems MV, Hubbard R, et al. Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy. J Immunother Cancer 2020;8:e000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greten TF, Mauda-Havakuk M, Heinrich B, Korangy F, Wood BJ. Combined locoregional-immunotherapy for liver cancer. J Hepatol 2019;70:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ren Z, Yue Y, Zhang Y, Dong J, Liu Y, Yang X, et al. Changes in the peripheral blood treg cell proportion in hepatocellular carcinoma patients after transarterial chemoembolization with microparticles. Front Immunol 2021;12:624789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dummer R, Lebbé C, Atkinson V, Mandalà M, Nathan PD, Arance A, et al. Combined PD-1, BRAF and MEK inhibition in advanced BRAF -mutant melanoma: safety run-in and biomarker cohorts of COMBI-i. Nat Med 2020;26:1557–63. [DOI] [PubMed] [Google Scholar]
- 79.Fernandez-Penas P, Carlino M, Tsai K, Atkinson V, Shaheen M, Thomas S, et al. 799 Durable responses and immune activation with intratumoral electroporation of pIL-12 plus pembrolizumab in actively progressing anti-PD-1 refractory advanced melanoma: KEYNOTE 695 interim data. BMJ Specialist Journals 2020. [Google Scholar]