Abstract
During development and tissue homeostasis, cells must communicate with their neighbors to ensure coordinated responses to instructional cues. Cues such as morphogens and growth factors signal at both short and long ranges in temporal- and tissue-specific manners to guide cell fate determination, provide positional information, and to activate growth and survival responses. The precise mechanisms by which such signals traverse the extracellular environment to ensure reliable delivery to their intended cellular targets are not yet clear. One model for how this occurs suggests that specialized filopodia called cytonemes extend between signal-producing and -receiving cells to function as membrane-bound highways along which information flows. A growing body of evidence supports a crucial role for cytonemes in cell-to-cell communication. Despite this, the molecular mechanisms by which cytonemes are initiated, how they grow, and how they deliver specific signals are only starting to be revealed. Herein, we discuss recent advances toward improved understanding of cytoneme biology. We discuss similarities and differences between cytonemes and other types of cellular extensions, summarize what is known about how they originate, and discuss molecular mechanisms by which their activity may be controlled in development and tissue homeostasis. We conclude by highlighting important open questions regarding cytoneme biology, and comment on how a clear understanding of their function may provide opportunities for treating or preventing disease.
Keywords: HH, WNT, BMP, FGF, Signaling filopodia, Signal transduction
Overview
Organ and tissue development rely on coordinated dispersal of morphogen signals from cellular organizing centers that provide instructional cues to govern cell fate. Signaling proteins contributing to tissue morphogenesis include Hedgehog (Hh/HH) family members, Transforming Growth Factor-β (TGF- β) and Bone Morphogenic Protein (BMP) family members, WNTs, NOTCH, and members of the Epidermal Growth Factor (EGF) and Fibroblast Growth Factor (FGF) families. These molecules direct distinct transcriptional programs in target cells, oftentimes through concentration- and signal duration-dependent manners [1–5].
Establishment of terminal cell fate across different tissues results from coordinated input from different combinations of morphogen signals. For example, limb development and digit specification are orchestrated through WNT, FGF, BMP, and HH signaling, craniofacial development is instructed primarily by HH and WNT activity [6–8], and pancreatic and endocrine system development are dictated by FGF, BMP, HH, NOTCH, and WNT signals [9]. One of the best examples of the multi-faceted roles of signaling molecules is found in central nervous system (CNS) development. Here, HH, WNT, FGF, and BMP collectively signal to dictate cell fate at early developmental stages and to induce proliferation, promote cell survival, and guide axon pathfinding at later developmental stages [10–22].
A tractable genetic model system in which coordinated developmental signaling can be studied is provided by the Drosophila wing imaginal disc, a sac-like epithelial tissue that is composed of several types of progenitor cells that communicate with one another to drive wing morphogenesis. Epithelial cells of the wing disc signal to a segment of the tracheal system called the Air Sac Primordium (ASP), which develops into the adult dorsal air sacs. Myoblasts found in this tissue form the adult flight muscles, which are provided oxygen by the air sacs. For each of these components of the flight system to develop properly, numerous pathways must be induced in precise temporal- and tissue-specific manners. Pathways active during wing cell specification include Hh, the BMP homolog Decapentaplegic (Dpp), FGF homolog Branchless (Bnl), WNT homolog Wingless (Wg), and the NOTCH ligand Delta [23–27]. Dysregulation of any of these pathways during wing morphogenesis results in overt patterning defects that compromise wing development, underscoring the importance of organized multi-pathway input for proper fate determination.
In addition to their crucial contributions during development, morphogens also play key roles promoting tissue homeostasis. Morphogens act in the stem cell niche to maintain stemness and promote proliferation or differentiation in response to distinct cues. This is exemplified by maintenance of the intestinal epithelium. Repopulation of cells in the crypts occurs through continuous regeneration from stem cells located at the crypt base. WNT and NOTCH signals maintain the undifferentiated state of cells in the stem cell niche and BMPs promote differentiation of intestinal epithelial cells to replace dying cells in the crypts [28]. Indian Hedgehog (IHH) is expressed by differentiating epithelial cells in the midcrypt region where it signals to the mesenchymal intestinal stem cells (ISCs) [29]. IHH pathway activation indirectly reduces WNT-mediated proliferation, thus stimulating ISC differentiation [29]. Similar mechanisms are found in homeostasis and repair of other adult tissues including the brain, skin, prostate, and bladder [30]. Given the myriad of important functions that depend upon proper activity of these signaling proteins, it is not surprising that their dysregulation can lead to developmental disorders and cancers [31–36].
Despite the importance of controlled signal deployment and delivery for tissue development and homeostasis, the precise mechanisms by which morphogens and growth factors are transported between sending and receiving cells remain a topic of debate. Interest in the mechanisms by which morphogens travel from a signaling source was spurred by discovery of the Spemann–Mangold organizer. This pioneering work demonstrated that specific regions of a developing embryo could influence development of other embryonic regions upon their transplantation [37]. Subsequently, the Dalcq–Pasteels hypothesis suggested that signals emanating from organizing tissues would form a double gradient, with one set of molecules spreading from the vegetal pole of the embryo and a second set simultaneously forming from the dorsal cortex [38]. In 1952, the term “morphogen” was coined to describe the molecules making up these developmental maps [39]. The French Flag representation of morphogenetic gradient formation was proposed to explain how cell fate is established by dynamic equilibrium of exposure to signals across a developing tissue. In this model, a tri-colored flag design depicts how positional information is conferred upon fields of cells across a concentration gradient [40]. Source cells produce morphogens which organize into these gradients to dictate positional information [1]. These gradients can stretch to be quite broad, which allows for tissue patterning to be instructed at significant distances from a morphogen source. For example, in the developing limb bud, signals from the zone of polarizing activity (ZPA) can reach cells situated as far as 200 μm away from the signaling source [41]. How such long-range signals reach their intended targets is not yet clear, but has been proposed to occur through processes including free and assisted diffusion, cell-to-cell transfer through repeated cycles of endocytosis and exocytosis (transcytosis), or direct engagement of membrane-associated signaling molecules [1, 42–44].
While diffusion of signals from organizing tissues is the simplest model for gradient formation, many mature signals are insoluble due to the presence of transmembrane domains or specific lipid modifications that promote their association with signal-producing-cell membranes. For example, NOTCH ligands are transmembrane proteins that remain tethered to their site of production, and thus require direct cell–cell interaction for receptor–ligand engagement and signal activation [45]. HH family members are modified by both cholesterol and long-chain fatty acid modifications [46–48] and WNT family members harbor a palmitoylation modification [49, 50]. A recent study on FGF transport in the Drosophila wing imaginal disc revealed that the Bnl/FGF is GPI-anchored to the producing-cell membrane, requiring direct contact with receiving cells to activate signaling through its receptor Breathless (Btl) [51]. These observations, along with the inability of passive diffusion models to fully account for the stringency and robustness observed across physiological morphogen gradients, suggest a requirement for a mode of direct, contact-mediated morphogen delivery to target cells [52–54].
Two models have been proposed to address this need. The first is planar transcytosis, in which morphogens are directly transferred between neighboring cells through repeated cycles of receptor-mediated endocytosis, membrane recycling, and exocytosis [42, 55]. In this model, degradation dynamics of endocytosed signaling molecules in the signal-receiving cells directly dictate the concentration gradient across target tissues. Although this process allows membrane-tethered and insoluble morphogens to forgo travel through the extracellular space, it does not fully account for longer range signaling that is seen during physiological development in tissues such as the limb [52].
A second model, which is gaining increasing experimental support, postulates that morphogens travel between signal-producing and -receiving cells along specialized filopodia called cytonemes [43]. These actin-based, cell surface protrusions emanate from morphogen-producing and/or -receiving cells and act as molecular highways for direct exchange of developmental signaling molecules. Since their characterization in 1999, there has been an explosion in interest that has led to numerous advances in our understanding of cytoneme biology. Interrogation of cytoneme-mediated signaling in the developing Drosophila wing disc identified pathways using cytonemes to include Hh, Dpp/BMP, Bnl/FGF, Spitz/EGF, Wg/WNT, and NOTCH [43, 56–61]. Drosophila tissues in which cytonemes have been observed include the wing disc, abdominal epidermis, female germline stem cell niche, and the developing eye [43, 59–61].
Evidence supporting involvement of cytonemes in morphogen transport in vertebrate systems is also mounting. Sonic Hedgehog (SHH)-containing cytonemes have been documented in the developing chick limb bud and WNT-transporting cytonemes have been identified in zebrafish embryos [62–65]. Excitingly, a recent study demonstrated the presence of SHH-transporting cytonemes with lengths up to 80 μm in the Axolotl blastema during limb regeneration, suggesting involvement of the structures in cellular response to injury [66]. In vitro analysis of cytoneme function has also increased, providing opportunities for interrogating the molecular mechanisms that control cytoneme behavior. Recent mechanistic investigations have focused on SHH-mediated cytoneme formation using cultured murine cells [67] and WNT-transporting cytonemes have been studied in murine intestinal organoids [63, 65]. The expansion of models available for cytoneme research provides opportunities to identify protein partners contributing to cytoneme formation and signal transport along them.
Cytonemes as specialized signaling filopodia
Decades ago, long, thin cellular protrusions were observed in developing sea urchin larvae and hypothesized to be involved in cellular motility [68, 69]. Years later, examination of the structures during sea urchin gastrulation led to the hypothesis that they did not influence cell motility, but instead facilitated cell–cell communication [70]. This hypothesis was strengthened by studies in Drosophila wing imaginal discs, which revealed thread-like projections extending from signaling centers toward fields of responding cells. Like the long filopodia observed in sea urchin, these structures, called cytonemes, were determined to be actin-based and highly dynamic. Furthermore, cytonemes could be induced ex vivo by exposing explants of Drosophila wing imaginal discs and mouse limb buds to an FGF source, supporting that they were responsive to extracellular cues. Because Drosophila cytonemes were observed to extend from organizing centers expressing Hh and Dpp/BMP, they were proposed to facilitate direct delivery of morphogens from signal-producing to signal-responding cells [43].
A persistent question about the cytoneme model centers around how they differ from the well-studied conventional filopodia that aid in cellular communication, motility, and wound healing. Conventional filopodia are actin-based membrane extensions that are generally ~ 0.1–0.3 μm in diameter and rarely reach lengths greater than 10 μm [71]. A variety of conventional filopodia with specialized functions have been described. These include myopodia (neuromuscular synapse formation), podosomes (matrix degradation), and invadopodia (cancer cell invasion) [72–74]. Despite differences in their functions, sizes, and behaviors, these protrusions all share a core structure of bundled actin filaments and extend from the cell surface following regulation by actin- and cytoskeletal-associated proteins [72–74]. Although cytonemes have similar diameters to these conventional filopodia, they have the potential to extend to significantly longer lengths. For example, anterior/posterior (A/P)-oriented cytonemes in Drosophila wing discs demonstrate average lengths of greater than 20 μm [75]. Incredibly, cytonemes extending from wing discs grown in culture have been documented to extend up to 700 μm to contact co-cultured, signal-responding cells [43]. This ability to grow to significant lengths, coupled with their morphogen cargo and distinct polarization relative to cellular organizing centers, has resulted in cytonemes being classified as a specialized type of cellular extension [43, 76]. Whether cytonemes are appropriately classified as specialized filopodia, or if they represent a new class of cellular extensions that are specifically suited to grow to incredible lengths to shape morphogen gradients, are open and controversial questions in developmental biology. Moreover, other cellular extensions including tunneling nanotubes (TNTs), intercellular bridges (IBs), and airinemes share both physical and functional characteristics with cytonemes.
TNTs are actin-based membrane protrusions with a diameter of 0.05–0.7 μm that can span lengths of several cell diameters [77, 78]. TNTs are reported to form through two different mechanisms, the first being cytoneme-like where a filopodium directed toward a neighboring cell makes a stable contact. The second mode of TNT formation occurs when two cells in direct membrane contact migrate away from each other while maintaining a point of contact through a TNT bridge [77, 79, 80]. Plasma membrane and cytoplasmic components are transferred freely between the connected cells through the bridge, thereby establishing a direct cytoplasmic connection. TNTs can also expand to facilitate vesicular transfer of individual vesicles and multi-vesicular bodies (MVBs). TNTs also allow for exchange of organelles including mitochondria, which provide a potential mechanism for energy replenishment during generation of these long structures [77, 78, 81]. Once formed, TNT bridges facilitate Ca2+ signaling and electrical synchronization between cells to promote downstream signaling in the contexts of development and immune cell communication. Studies of chick and quail cranial explants have shown that during development, migrating neural crest cells form intercellular TNTs for electrical coupling [82–84]. In the immune system, macrophages and T cells use TNTs for Ca2+ signaling, natural killer cells lyse their targets via perforin-containing TNTs, and dendritic cells use them to promote inflammation [80, 85–87]. In addition, TNTs formed by immune cells can be hijacked by Human Immunodeficiency Virus (HIV) and prion proteins for more efficient transmission [79, 88, 89].
The second type of cellular connection that shares similarity with the cytoneme is the IB. IBs, which are also referred to as ring canals, are similar in appearance to cytonemes and TNTs due to their ability to reach lengths of up to 350 μm [90]. They were first documented in spermatids and continue to be observed primarily in germ cells [91–95]. IBs are important for spermatogenesis and fertility and, like TNTs, are implicated in molecular and organelle transport and Ca2+ signaling [95–97]. The process of IB formation is what sets them apart from other actin-based structures. They are remnants of incomplete cytokinesis or syncytia, and as such, can be much thicker than filopodia with diameters ranging between 0.2 and 10 μm [98].
Airinemes are cytoneme-like signaling extensions reported to be crucial for NOTCH signaling during patterning of zebrafish stripes. Stripes are generated by two types of pigment-producing cells: yellow pigment-producing xanthophores and black pigment-producing melanophores. Melanophores express the receptor NOTCH, while xanthophores produce the NOTCH ligand DELTA [99]. Both are transmembrane proteins that require direct interaction between communicating cells. NOTCH receptor–ligand engagement promotes lateral inhibition, such that signal-sending cells instruct their signaling partners to adopt a different fate [45]. In this case, NOTCH signaling both communicates a survival signal and promotes clearance of melanophores from the inter-stripe region, resulting in accurate spatial patterning of the stripes [99, 100]. For airinemes to communicate over a distance, a xanthophore first forms a large DELTA-containing membrane bleb that is positive for the phospholipid phosphatidylserine (pS) [100, 101]. A nearby macrophage recognizes the pS-containing membrane and engulfs the bleb, forming an airineme vesicle [101]. The macrophage then migrates up to 189 μm away to deposit the large, signal-containing vesicle on the surface of an NOTCH-expressing melanophore [101].
Notch-mediated lateral inhibition occurring between distantly localized signaling partners is also observed in Drosophila where Delta-expressing cells in the wing disc activate Notch receptors on nonadjacent cells through transient cytoneme-like filopodia to instruct spatial patterning of sensory bristles [57, 58]. Filopodia-mediated, long distance Notch signaling also occurs in ovarian germ cells in flies, which use the structures to deliver Delta to distantly localized somatic cells to induce stem cell niche formation [102]. Whether the Drosophila Notch filopodia are true cytonemes or fly airineme-like structures has not yet been firmly established.
Despite sharing some physical and functional characteristics with TNTs, IBs, and airinemes, key differences set cytonemes apart from these other signaling extensions (Table 1). First, cytonemes do not appear to facilitate direct cytoplasmic transfer as is observed for TNTs and IBs. As will be discussed below, they are thought to provide signaling information by forming synapse-like connections with signal-receiving cells. A second difference is that, due to their narrow diameter, cytonemes are generally too thin to facilitate transfer of large organelles, so do not appear to transfer mitochondria like TNTs [103]. Airinemes differ from cytonemes, because they require both microfilaments and microtubules, while cytonemes do not [100]. Nevertheless, all these structures play important roles in cell-to-cell communication, underscoring the need for direct, contact-based signaling in development and tissue homeostasis.
Table 1.
Cellular extensions contributing to cell–cell communication
| Cytonemes | Tunneling nanotubes | Intercellular bridges | Airinemes | |
|---|---|---|---|---|
|
| ||||
| Diameter | ∼ 200 nm | 50–700 nm | 200 nm- 10 μm | < 1 μm |
| Length | 4–700 μm Average 10–25 μm |
10–200 μm | Up to 350 μm | ∼ 10–250 μm |
| Cell/tissue type | Developing and adult tissues Stem cell niche |
Migrating neural crest cells Immune cells: macrophages, T cells, natural killer (NK) cells, dendritic cells |
Germ cells Developing tissues |
Extend from Xanthophores to melanophores |
| Formation | Extension toward cell signaling partners | Extension toward neighboring cell Contact maintained between cells migrating apart |
Incomplete cytokinesis/syncytia | Macrophage engulfs membrane bleb, migrates to deposit on signaling cell |
| Function | Morphogen and growth factor transport Maintenance of stem cell niche Tissue homeostasis |
Exchange of organelles Ca2+ signaling Electrical synchronization NK-mediated cell lysis, inflammation |
Spermatogenesis and fertility Molecular/organelle transport Ca2+ signaling |
Zebrafish stripe patterning |
| References | 43, 56, 59–67, 75, 76, 104, 105, 106 | 77–87 | 90–98 | 99–101, 107 |
Mechanisms of cytoneme initiation
Despite improved imaging technologies and the growing number of model organisms in which cytonemes have been observed, the precise molecular mechanisms by which cytonemes are initiated remain unclear. Intriguingly, both in vitro and in vivo studies indicate that morphogen expression can promote cytoneme initiation. Transient overexpression of SHH, the NOTCH ligand JAGGED, FGF2, or WNT3A in mouse NIH3T3 cells increases cytoneme occurrence rates [67, 108]. Similar observations have been reported in Drosophila where Dpp overexpression leads to increased numbers of cytonemes extending from the A/P organizer of the larval wing disc [75]. Dpp also influences cytoneme directionality. Whereas cytonemes of control discs projected specifically toward A/P and dorsal/ventral (D/V) axes, cytonemes induced by ectopic Dpp grew outwards in all directions [75]. The conserved ability of morphogen signaling proteins to induce, stabilize, and/or guide cytonemes suggests an ability of morphogens to communicate with cytoskeletal regulators in signal-sending cells. The specific signals facilitating this communication are not yet known. However, the molecular mechanisms driving traditional filopodia initiation may provide clues about how cytonemes initiate in morphogen-expressing cells.
Contributions of GTPases to filopodial extensions
Filopodia can be initiated by activation of cell surface receptors that recruit and activate cytoskeleton polymerization machinery. Recruitment is typically orchestrated by rapid and specific activities of kinases and GTPases downstream of the receptors [109] (Fig. 1a). Rapid responses are achieved, because effector kinases, GTPases, and downstream formins that control actin polymerization are poised for signal-induced activation [109, 110]. The Rho GTPase family member Cdc42 is the most common GTPase effector regulating filopodia initiation [110, 111]. GTPases are commonly activated by guanine nucleotide exchange factors (GEFs) that promote the exchange of GDP for GTP on the small G protein [112] (Fig. 1a). GEFs can be activated by cytokines, growth factors, GPCR ligands, proteoglycans, and integrins to stimulate Cdc42 at the site of receptor engagement [112].
Fig. 1.
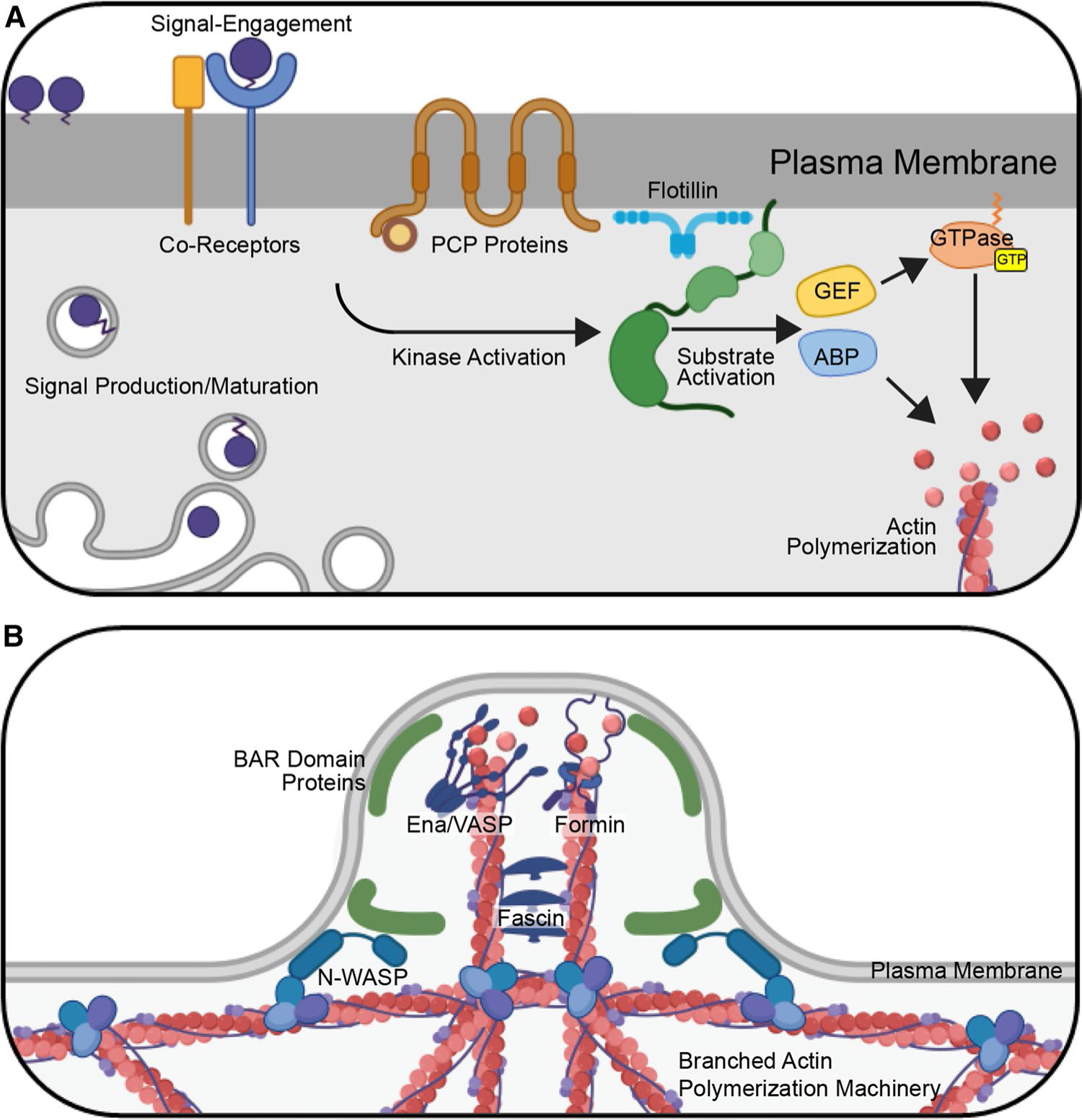
Initiation of Cytoneme Formation. A Morphogen initiation of cytoneme budding. Following maturation and secretory transport, morphogens engage cognate deployment proteins and co-receptors at the cell surface to initiate intracellular signaling through effector kinases and GTPases. Kinases activate substrates including Actin-Binding Proteins (ABPs) and Guanine nucleotide Exchange Factors (GEFs), which in turn promote GTP binding to small GTPases. GTPase effectors polymerize actin at these sites. For WNT signaling, Planar Cell Polarity (PCP) proteins contribute to deployment. Flotillin is frequently detected at sites of morphogen clustering, suggesting that it may contribute to aggregation of transmembrane and lipid-modified molecules involved in this process. B Cytoneme extension. Following activation of actin-polymerization machinery, actin is assembled into linear bundles that expand the cell surface to form a cytoneme bud. BAR domain-containing proteins are activated by Cdc42 to induce membrane curvature and concentrate actin machinery at these sites. In these cases, BAR–domain protein interactor Wiskott-Aldrich Syndrome Protein (N-WASP) promotes branched actin assembly to provide a scaffold for linear actin filament assembly. The unbranched actin polymerization, accomplished by Ena/VASP family members and/or formin proteins, then acts to extend the nascent cytoneme. Linear actin filaments are cross-linked upon binding by Fascin
Once activated, GTPases associate with the plasma membrane where they interact with downstream effectors including kinases, scaffolding proteins, and actin nucleation and polymerization machinery to orchestrate cytoskeletal reorganization for filopodial extension [110, 113] (Fig. 1a). A prominent Cdc42 GTPase effector in the nucleation of actin in filopodia is the membrane-binding, F-BAR domain protein Transducer of Cdc42-dependent Actin assembly (TOCA-1), which binds to Cdc42-GTP at sites where outgrowth will occur [114] (Fig. 1b). The subsequent formation of filopodia occurs in a stepwise process, starting with recruitment and activation of molecules that exist in autoinhibited conformations within the cytoplasm [114]. First, TOCA-1 recruits and activates Wiskott-Aldrich syndrome protein (N-WASP), an actin nucleation promoting factor [115]. Upon activation, N-WASP recruits the actin-nucleating ARP2/3 complex, which accepts and incorporates actin monomers into growing polymers [116]. This results in nucleation of a branched actin structure at the site of filopodial outgrowth [114] (Fig. 1b). Formins and/or Enabled/vasodilator-stimulated phosphoprotein (Ena/VASP) family members can attach to the growing ends of actin filaments at this site and polymerize linear filaments to form plasma membrane protrusions that establish nascent filopodial structures [109, 114, 117] (Fig. 1b). In the final step, the actin cross-linker protein Fascin bundles the parallel actin filaments during extension of the growing filopodium [114] (Fig. 1b).
Pathway-specific cytoneme initiation
It is reasonable to hypothesize that morphogens with the ability to impact cytoneme occurrence might tap into actin-regulatory pipelines to promote cytoneme initiation and growth. The specific cytoskeletal regulators engaged are likely dictated by the morphogen being transported, which may act through receptors, co-receptors, and/or adaptor proteins at the cell surface. In the case of HH, studies in both murine and Drosophila models indicate that HH, its deployment protein Dispatched (Disp/DISP), and co-receptors Interference Hedgehog/Cell adhesion-associated, Down-regulated by Oncogenes (Ihog/CDON) and Brother of Ihog/Brother of CDON (Boi/BOC) can all promote cytoneme occurrence [67, 108, 118, 119]. Disp overexpression in cultured Drosophila cells promotes cytonemes by slowing retraction rates of Hh-containing extensions [108]. In cultured murine cells, expression of BOC or CDON increases cytoneme occurrence rates to a level comparable to what is observed upon SHH overexpression. DISP forms a complex with SHH and BOC or CDON in cytonemes that is essential for ligand delivery through the structures, such that loss of either DISP or BOC/CDON decreases cytoneme-based SHH signal delivery to target cells [67]. As such, HH may signal through DISP/co-receptor complexes in ligand-producing cells to boost cytoneme occurrence and/or stability for efficient delivery to cellular targets. Consistent with this hypothesis, both BOC and CDON have been reported to interact with ABL tyrosine kinase, which is linked with increased filopodia persistence in fibroblasts [120–122]. During neurite outgrowth, the ABL–BOC interaction results in SHH-mediated c-Jun N-terminal Kinase (JNK) activation [121]. During myogenesis, the ABL–CDON interaction activates p38MAPK via scaffold protein JNK-interacting protein 4 (JIP4/JLP) [122].
JNKs are established regulators of actin that are activated during various developmental programs to remodel the cytoskeleton for stress fiber formation, microtubule stabilization, axon regeneration, regulation of smooth muscle contractility, and cellular migration [123]. Treatment of murine embryonic dorsal root ganglia neurons with an actin depolymerizing agent promotes JNK activation to restore cytoskeletal integrity and facilitate axon regeneration [124]. Moreover, in rat cortical neurons, JNK activation results in formation of mature filopodia by phosphorylating the actin cross-linker MARCKS-like protein 1 (MARCKSL1), which in turn facilitates actin bundling [125].
Despite these capabilities, the role of JNK activity in regulation of cytonemes remains unclear. In Drosophila, JNK activation appears to be inhibitory toward formation of Hh-containing cytoneme-like projections in the hematopoietic niche [126]. In this context, loss of the transcription factor Relish, which represses JNK, leads to filopodial loss and ‘trapping’ of Hh in the niche. This occurs due to increased activity of JNK effectors Ena and Fascin/Singed, which play key regulatory roles in actin polymerization and bundling [126]. However, Fascin activity is required for formation of cytonemes by mammalian embryonic stem cells [127] and the family of Ena/VASP proteins effectively promote filopodia formation in multiple cellular contexts [128]. As such, cytoneme formation likely relies on a balance of signaling activity by these effectors.
In addition to being able to impact actin dynamics through kinase activation, BOC and CDON have also been reported to influence cytoskeletal dynamics by promoting GTPase activation. During commissural axon guidance, the GEF regulator Engulfment and Cell Motility (ELMO) binds DOCK3/4 GEFs to stabilize their interaction with Rac1-GTPase for Rac1 activation. In the absence of SHH, BOC sequesters ELMO and prevents ELMO-mediated Rac1 activity. However, upon axon exposure to SHH, BOC binding to ELMO is blocked to allow for Rac1-GTP binding for cytoskeletal remodeling to induce growth cone turning toward the SHH source [129]. Studies in C2C12 myoblasts suggest that CDON may also be able to influence actin remodeling by activating the GTPase Cdc42 [130]. This is thought to occur through CDON binding the Cdc42 regulator BNIP-2, which binds both Cdc42 and ARHGAP1 to promote Cdc42-GTP binding during myogenesis [131]. However, in vivo studies suggest that SHH does not signal through Cdc42 to influence cytonemes in the chick limb bud, because conditional inactivation of the GTPase did not disrupt cytoneme formation in the limb bud mesenchyme [62]. A lack of Cdc42 involvement is also reported for FGF-induced cytonemes in cultured mesenchymal cells. Instead, the atypical GTPase RhoD is activated downstream of FGF2/4/8 to induce its effector formin mDia3C to promote N-WASP-independent actin polymerization [132]. Formins are also involved in cytonemes in the Drosophila wing disc where Diaphanous (Dia) promotes cytoneme-mediated Dpp signaling in the ASP [104].
Recent reports suggest that WNT uses similar strategies to influence cytoneme dynamics for its transport. WNT binding to the receptor tyrosine kinase ROR2 is important for cytoneme initiation in zebrafish embryos, murine intestinal organoids, and human cancer cell lines [63]. Like what is observed for DISP, BOC, and CDON, ROR2 overexpression increases cytoneme incidence in fibroblasts. In these cells, WNT8A clusters co-localized with ROR2 at the plasma membrane prior to cytoneme initiation from the cluster sites [63]. WNT–ROR2 clusters are hypothesized to work by concentrating actin regulators for filopodial extension at sites of ligand enrichment and may also improve efficiency of WNT8A presentation at receiving-cell membranes to ensure robust signal initiation [63, 105]. Mechanistic studies suggest ROR2 promotes cytoneme extension by recruiting planar cell polarity (PCP) proteins and Casein Kinase 1 (CK1) to the plasma membrane. This facilitates CK1-mediated phosphorylation of the PCP protein VANGL2 (Van-Gogh-like). WNT8A, ROR2, and VANGL2 are then loaded into cytoneme buds where they stimulate JNK activation to promote cytoneme outgrowth in a manner that is dependent upon the I-BAR domain protein IRSp53 [63, 65]. In rat hippocampal neuronal cultures, JNK3 is recruited to the actin cytoskeleton upon WNT7A treatment to promote axon branching and increased motility of axonal filopodia [133]. Because these JNK effects on WNT-mediated cytoneme formation differ from what is seen with Hh cytonemes in the Drosophila hematopoietic niche [126], it is likely that the impact of JNKs on cytoneme formation varies based on morphogen and tissue contexts.
In addition to working through JNK, WNT has also been proposed to initiate cytoneme formation through the Rho GTPase Cdc42 in a PCP-dependent manner [134]. WNT-ROR2-induced cytonemes of zebrafish fibroblasts require Cdc42 activity, because introduction of dominant-negative Cdc42 led to a significant loss in cytoneme occurrence and WNT8A signal initiation in receiving cells [63]. In this context, Cdc42 activation led to actin nucleation and polymerization by N-WASP and IRSp53 [134]. A potential avenue for activation of these intracellular signaling molecules is suggested by the discovery that WNT co-receptors, LGR4 and LGR5, can induce cytoneme formation in Human Embryonic Kidney (HEK) cells [135]. The long protrusions induced by LGR5 are positive for Fascin and are enriched for VASP at their tips [135]. As such, the ability of LGR4/5 to influence cytoneme outgrowth may indicate that cytoneme formation occurs downstream of ligand engagement with these co-receptors.
Another molecule that has been linked to morphogen-specific cytoneme initiation in the Drosophila wing disc is Flotillin-2/Reggie-1 (Flo2), which marks cholesterol-rich plasma membrane microdomains [59, 106]. Flo2 is present in Hh-containing wing disc cytonemes where it contributes to proper cytoneme length and Hh gradient establishment [59]. The size of the gradient of Wg in the Drosophila wing disc is also reported to depend upon Flo2, because its knockdown resulted in a shortened range of Wg dispersion and compaction of target gene expression [136]. Flotillins function as protein scaffolds for clustering tyrosine kinases, Rho GTPases, and adhesion molecules to regulate cellular polarity, extracellular matrix interaction, and cytoskeletal regulation [137]. Consistent with Flo2 involvement in cytoneme formation in Drosophila, overexpression of its homolog, FLOT2, in mammalian epithelial cell lines can induce filopodia-like cell surface protrusions [138]. It is tempting to speculate that Flotillins promote the clustering of molecules involved in cytoneme formation at specific membrane microdomains that are enriched for molecules with post-translational modifications including GPI-linkage, cholesterolylation, palmitoylation, and myristoylation (Fig. 1a). These modifications are common on GTPases, kinases, and some morphogens, and can potently influence their interaction with the plasma membrane [137]. Recruitment of cytoskeletal regulation machinery where signals are accumulating on the cell surface could then promote focused cytoneme outgrowth at these sites.
Cytoneme extension and stabilization
One of the most striking characteristics of dynamic cytonemes is their length variability. In the Drosophila wing disc, ASP cytonemes range from 12 to 50 μm, with an average length of ~ 23 μm [61]. Cytonemes extending from myoblasts are approximately 25 μm long and cytonemes from apical epithelia are on average ~ 20 μm, but can reach lengths of over 80 μm [56, 75]. Similar dynamic ranges are found in vertebrates. Cytonemes in zebrafish embryos average between 10 and ~ 17 μm and cytonemes extending from cells of the developing chick limb bud can range from 34 to 150 μm [62, 63, 134]. To date, murine cytonemes have only been analyzed in cultured cells, and range in length from 4 μm to upwards of 50 μm [65, 67, 132]. As such, cytoneme lengths are likely tissue- and context-dependent.
The precise molecular mechanisms governing cytoneme extension in differing cell and tissue contexts are not known. However, mutagenesis studies suggest that formins are likely responsible for the long, flexible nature of cytonemes due to their function in nucleating unbranched actin filaments and preventing capping of filament ends [109]. Perturbations of actin-regulatory proteins including Capping Protein (CP), SCAR/WAVE, or Pico/Lamellipodin shortens cytonemes formed by Ihog-expressing wing disc cells from an average length of over 60 μm to under 30 μm [59]. Moreover, the activated form of the Drosophila formin Dia enriches in tips of cytonemes extending from the ASP and is required for effective communication between the ASP and wing disc epithelium [104]. Loss of actin-regulatory proteins can lead to collapse of the Hh morphogen gradient across the developing wing disc, supporting that regulation of cytoneme length through actin modulation directly impacts morphogen spread [59].
In addition to being influenced by cell autonomous actin modulation, cytoneme length is also impacted by interactions with components of the extracellular matrix (ECM). In Drosophila, loss of PCP proteins Van Gogh (Vang) and Prickle (Pk) significantly decreases lengths of FGF-containing cytonemes that extend between the ASP and underlying wing disc [139]. This was attributed to alteration of ECM composition, which showed reduced levels of the matrix protein laminin and the ECM heparan sulfate proteoglycans (HSPGs) Division abnormally delayed (Dally) and Dally-like protein (Dlp). HSPG disruption prevented cytoneme extension and pathfinding, suggesting crucial roles for the ECM in promoting cytoneme action [139]. Moreover, cytonemes from cells of the Drosophila wing disc fail to extend over tissue that is deficient for glypican biosynthesis, further supporting potent modulation of cytoneme extension by PCP and ECM proteins [59]. Communication between the ECM and PCP proteins appears to be an evolutionarily conserved mode of controlling cytoneme length. Recent work in vertebrate model systems supports a role for VANGL2 in driving actin polymerization in WNT-containing cytonemes, because its overexpression in cultured zebrafish fibroblasts led to an approximate 187% increase in cytoneme lengths [65]. ECM components are similarly important during zebrafish development, as evidenced by the HSPG Glypican-4 (GPC4) being required to maintain cytoneme lengths in the WNT5B- and WNT11F2-producing endoderm [140].
Two hypotheses have been proposed to explain how the ECM promotes cytoneme extension. The first suggests that the ECM stores attractants for cytoneme pathfinding akin to how the ECM influences axon guidance [139]. This is best described by the model of restricted diffusion, in which secreted molecules interact with ECM proteins like HSPGs to establish an extracellular gradient for navigation through the tissue [141]. A second hypothesis is that GPI-anchored glypicans of the HSPG family facilitate direct interactions between the ECM and cytoneme membranes and serve as substrates upon which cytonemes can travel [139]. The HSPGs Dally and Dlp cluster on the cell surface of cultured Drosophila cells and Dlp clusters on Hh-transporting cytonemes [142]. In the Drosophila wing disc, Dally and Dlp interact with and stabilize ectopically expressed Ihog on cytoneme membranes by slowing cytoneme elongation and retraction velocities [143]. Similarly, BOC and CDON enrich in microdomains along portions of chick limb bud cytonemes that remain static, suggesting that they bind to extracellular components to stabilize the structures as they extend [62].
Morphogen loading and transport
Models explaining how morphogens load into cytonemes include free diffusion from the plasma membrane onto cytoneme membrane, extension from the plasma membrane at sites of ligand accumulation, endocytic recycling for vesicular and multi-vesicular body (MVB)-mediated entry, and active transport (Fig. 2) [63, 67, 106, 144].
Fig. 2.

Morphogen Loading and Transport. A Morphogen enrichment in budding cytonemes. In polarized cells, morphogens accumulating on apical membrane are recycled in a dynamin-dependent manner for sorting to basal cytonemes. The vesicles are sorted by Rab GTPases directly to basolateral membrane for cytoneme loading through accumulating at sites of budding cytonemes or for incorporation into multi-vesicular bodies for active transport along cytoneme extensions. B Morphogen entry into existing cytonemes. Vesicles containing signaling proteins are loaded into mature cytonemes for transport to cytoneme tips by Myosin-10 (MYO10)
Contributions of morphogen diffusion and membrane clustering to cytoneme loading
In the Drosophila ASP, the FGF family member Bnl has been observed to scatter along the surface of cytonemes where it is tethered by a GPI anchor, suggesting that it enters through diffusion along the membrane [51, 144]. Similarly, SHH-N, which receives an amino-terminal lipid modification but lacks a carboxyl-terminal lipid attachment, can localize to the extracellular face of cytoneme membranes in chick limb buds, suggesting that it may also enter cytonemes passively through membrane association [62]. However, studies of dually lipid-modified HH proteins in the Drosophila wing disc and cultured mouse cells revealed localization of the morphogen inside vesicular structures, raising the possibility that specific lipid modifications may impact cytoneme loading behavior [67, 106].
Studies carried out in zebrafish embryos demonstrate that WNT8A-ROR2 form clusters at the plasma membrane that initiate cytoneme formation, allowing for their incorporation into the nascent cytoneme tip during outgrowth [63]. These WNT8A-positive accumulations co-localize with TOCA-1 at the plasma membrane prior to protrusion of a cytoneme bud [134]. WNT ligand remains at the tips of cytonemes during outgrowth, supporting a model in which actin-regulatory signaling downstream of ligand accumulation promotes localized actin assembly [63, 134]. This allows for protrusion of the ligand-containing portions of the plasma membrane in the form of cytoneme outgrowth, resulting in efficient, direct delivery of the morphogen to distant cells (Fig. 2a).
Contribution of endocytosis to cytoneme loading
Mechanistic interrogation of morphogen membrane recycling suggests that the endocytic pathway may play a role in cytoneme loading of some signaling proteins. Studies in Drosophila polarized epithelia revealed that Dpp-, Hh-, and Wg-containing vesicles all have features indicative of having undergone receptor-mediated endocytosis from the apical cell surface [145, 118, 146] (Fig. 2). Dpp and its receptor Thickveins (Tkv) co-localize with the endocytic marker human transferrin receptor (hTfR) in basolateral puncta in wing epithelia [145], and Wg signals are reported to transfer through the wing disc epithelium via internalized vesicles called argosomes [146, 147]. In the signal-producing cells of the Drosophila wing disc, Hh localizes to basolateral MVBs that provide exovesicles that carry ligand to signal-receiving target cells [106] (Fig. 2). MVB formation and vesicular trafficking rely on activity of the endosomal sorting complex required for transport (ESCRT) protein complexes I, II, and III [148]. ESCRT protein Vps32 labels Hh-containing exovesicles found in the extracellular space of Drosophila wing discs, and depletion of various ESCRT family members attenuates long-range Hh signaling [149]. Similarly, activity of ESCRT-III protein CHMP1A promotes SHH release in extracellular vesicles during mouse brain development to ensure proper neural patterning [150]. Disp, Ihog, and Dlp are reported to enrich with Hh in exovesicles within the Drosophila wing disc, and DISP and CDON/BOC enrich with SHH in vesicles inside cultured mouse cell cytonemes [67, 106]. Additional support for involvement of endocytic recycling in priming cells to send a long-range signal is provided by the Drosophila ASP and wing disc where Dynamin, which pinches off the endocytic bud, is required for endocytic recycling of both Hh and Dpp. Accordingly, its mutation alters Hh gradient formation in vivo [118, 104, 151] (Fig. 2).
Consistent with the important role of membrane recycling for Hh deployment, the transporter-like protein Disp, which neutralizes the membrane-tethering activity of Hh lipid modifications, stimulates recycling endocytosis of Hh proteins from the apical surface of ligand-producing cells in the Drosophila wing disc [152]. Disp-Hh complexes that internalize from apical membrane then target back to the membrane in an Rab4/5-dependent manner to release Hh for long-range signaling [118, 152] (Fig. 2). Importantly, cytonemes that contribute to long-range signaling across polarized epithelial tissue typically originate from basolateral membranes, and Hh signal-receiving cells of the developing wing disc endocytose Hh in complex with its receptor Ptc from basolateral membrane [56, 58, 118, 119, 153]. As such, Disp likely contributes to long-range signaling through redirecting apical Hh to basolateral membranes for cytoneme loading. Consistent with this hypothesis, Disp loss ablates long-range Hh signaling activity, but does not disrupt short-range juxtacrine signaling in the wing imaginal disc [118, 154]. Intriguingly, the apical-basal distribution of cellular extensions is controlled by a gradient of active Rac1-GTPase, with higher activity occurring in basolateral compartments and correlating with filopodia and lamellipodia formation [155]. It remains to be seen if Rac1 activity is required for basolateral cytoneme extension, and whether Disp may signal to Rac1 to promote Hh cytoneme activity. Notably, in mice, Rac1 is activated to induce cytoskeletal remodeling by noncanonical SHH signaling in ligand-receiving cells [156], suggesting that Hh may be able to link with this small G protein in signal-producing cells to influence cytoneme growth.
Recycling of Hh prior to cytoneme entry suggests a model whereby lipid-modified morphogens first accumulate on the plasma membrane where they interact with packaging and release machinery that promotes their basolateral retargeting for long-range signaling [118] (Fig. 2). Consistent with this hypothesis, in the Drosophila wing disc lipid-modified Wg proteins that signal at short range occur apically, while long-range signals are sent basolaterally [157]. However, unlike Hh, Wg does not rely on Dynamin for its recycling, which may indicate that individualized modes of membrane recycling and ligand transport exist for different morphogen signals [151]. Interestingly, a recent study suggests that intracellular trafficking of specific morphogens can provide control over the amount of morphogen permitted to exit a signal-producing cell. In this study, graded increases in expression of Dpp, Hh, or Wg in the Drosophila wing disc failed to affect wing disc morphology, because consistent levels of ligand were targeted to basolateral membrane regardless of production level [158]. As such, intracellular trafficking machinery may ensure that appropriate concentrations of morphogen are packaged and redistributed for cytoneme-mediated transport.
Contributions of active transport to cytoneme signaling
The actin-based motor Myosin 10 (MYO10) enriches at cytoneme tips with SHH-N in the developing chick limb bud and with cholesterol-modified SHH in cultured mouse cells, suggesting that it promotes SHH movement to cytoneme tips [62, 67] (Fig. 2b). Both in vitro and in vivo studies support a requirement for MYO10 in SHH signal propagation, because cultured mouse cells lacking MYO10 or expressing a cargo-binding-deficient mutant fail to effectively enrich SHH in cytonemes or induce a response in signal-receiving cells. Consistent with these observations, neural tubes of MYO10-deficient mice show reduced Gli1 induction and patterning defects indicative of attenuated SHH morphogen gradient activity [67]. However, while there is support for MYO10 involvement in SHH transport in vertebrate systems, Drosophila lack an MYO10 homolog, suggesting that a different molecular mechanism may drive Hh transport along fly cytonemes [159]. Nevertheless, evidence for a conserved role for MYO10 in cytoneme-based morphogen transport in vertebrates is mounting. In the developing zebrafish neural plate, MYO10 colocalizes with WNT8A and cargo-receptor EVI-WLS at cytoneme tips where they accumulate to promote pathway activation in receiving cells [134]. MYO10 has also been observed to traffic along LGR5-induced cytonemes in HEK cells, suggesting a conserved mechanism of active transport [135].
Morphogen reception by target cell cytonemes
Cytonemes extend from both signal-sending and signal-receiving cells and are thought to transfer morphogens through direct contact at morphogenetic synapses [54, 160, 161]. Cytoneme contact has been observed in both fixed and live cellular imaging in fly and vertebrate models [67, 104]. Live imaging studies reveal that cytoneme associations can occur as transient connections suggestive of scan and release activity, or stable connections that may be reinforced by adhesion protein or co-receptor functions [59, 62, 67, 162] (Fig. 3). Connections can occur between cytoneme tips or through cytoneme–cell body association, but the regulatory events determining how contact is made are not yet clear (Fig. 4). Whereas live imaging of murine NIH3T3 cells overexpressing SHH and MYO10 revealed stable contact between cytoneme tips [67], analysis of Ihog-stabilized cytonemes in the Drosophila wing disc using GFP reconstitution across synaptic partners (GRASP) revealed multiple cytoneme-cytoneme contact points along the lengths of the extensions [162] (Fig. 3a). In the latter study, homophilic Ihog interactions worked in trans to stabilize multiple discrete connections, indicating that enrichment of specific proteins in cytonemes can influence connection behavior [162].
Fig. 3.

Stabilization of Cytoneme Contacts. A Cytoneme stabilization through additive contact. Stability can be conferred by protein–protein interactions in trans. Cytoneme-localized transmembrane proteins can bind heparan sulfate proteoglycans (HSPGs) (green). For Hh (dark purple), HSPGs contribute to Ihog (blue) interactions in trans to stabilize cytoneme-cytoneme contacts (left). HSPGs in the ECM or on cytoneme membrane can also cooperate in the higher affinity Ihog–Hh interaction (center). Highest affinity interactions are achieved between the Ihog, ligand, and Hh receptor Ptc (orange) to allow for morphogen transfer and pathway activation. B Stability of cytoneme contact through CAMs. HH co-receptors BOC/Boi and CDON/Ihog also function as CAMs to stabilize these points of interaction. Dpp transport is supported by wing disc expression of CAM Capricious (Caps)/Tartan (Trn) and by ASP expression of neuronal CAMs Neuroligin 2 (Ngl2) and Neuroglian (Nrg) at cytoneme tips
Fig. 4.
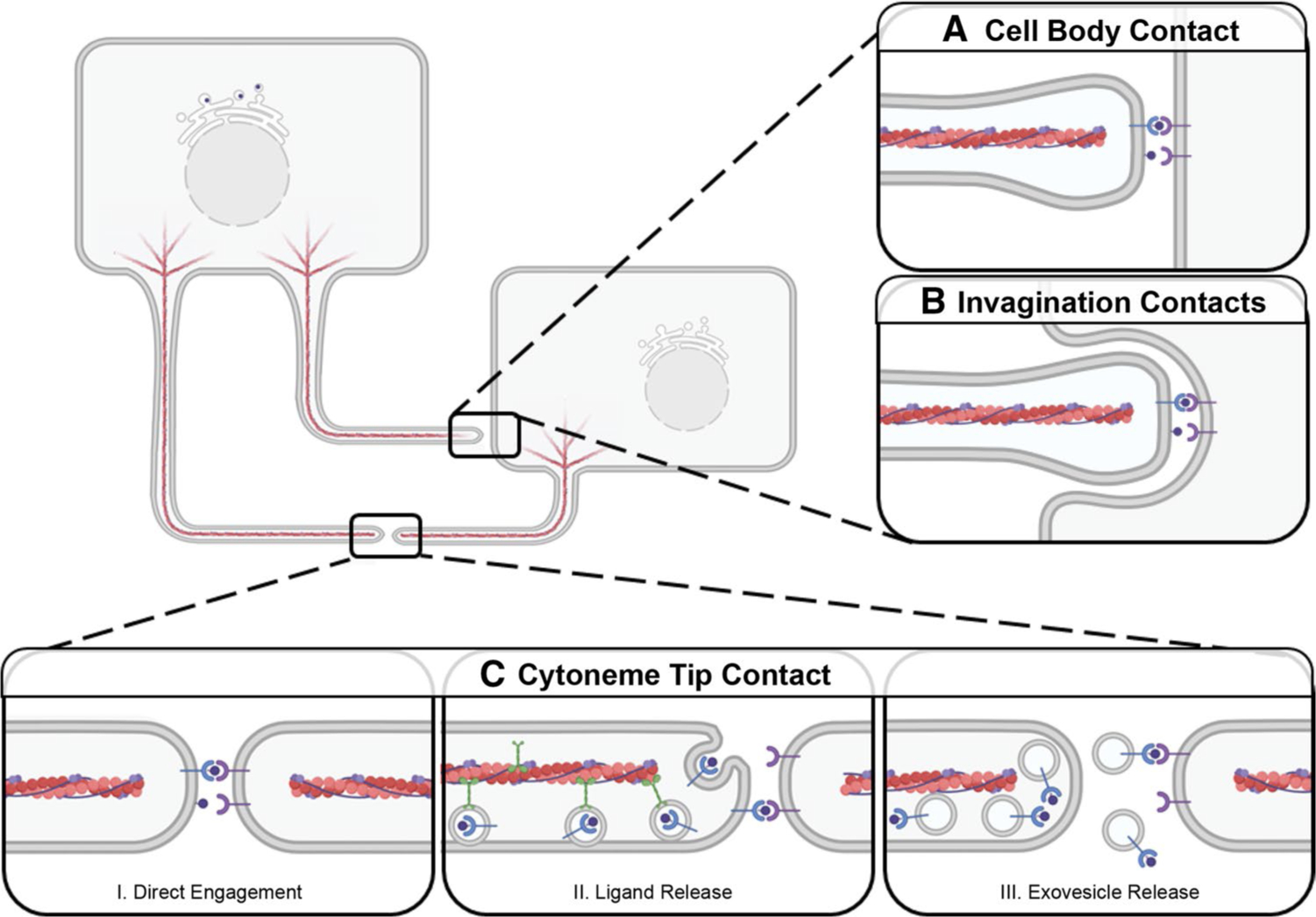
Morphogen Reception by Target Cells. Cytonemes can deliver signal through contact with receiving-cell bodies (A,B) or other cytonemes (C). A Signal-containing cytonemes directly contact the cell body. B Cytonemes extend into plasma membrane invaginations on the cell body. C Cytoneme tips contact to form a morphogenetic synapse where signals are presented through: (I) Incorporation into the plasma membrane at the cytoneme tip. (II) Transport along cytonemes for release from the tip. (III) Vesicular transport along the cytoneme for exovesicle-based release at a morphogenetic synapse for signaling
Signal-sending cytonemes can also directly contact signal-receiving-cell bodies to extend into plasma membrane invaginations. This has been observed in cultured mouse cells and in vivo in Drosophila [67, 103] (Fig. 4b). It is not yet clear how a mode of cytoneme contact is selected. It is possible that cytoneme-to-cell body contact is favored when producing-cell cytonemes outnumber cytonemes extending from receiving cells. Cytoneme insertion into receiving-cell plasma membrane invaginations may also be favored in situations requiring delivery of higher concentration of morphogen to achieve a desired signaling threshold.
To receive a cytoneme-based signal, a target cell must express the proper receptor to confer responsiveness to morphogens enriched in the delivering cytoneme. In the Drosophila ASP, the receptors for the ligands Dpp, Spitz/EGF, and Bnl/FGF segregate to distinct signal-receiving cytonemes that only interact with the signal-sending cytonemes that contain their cognate ligand [61, 104]. However, ASP cytonemes that contain Btl are also positive for Ptc, suggesting that some signal/receptor clustering can occur [163].
During somite formation in chick, the WNT receptor Frizzled 7 (FZD) enriches in puncta on cytoneme-like structures originating from the WNT-responsive dermomyotome [164], and in flies, Fzd-positive myoblast cytonemes orient directly toward Wg-producing cells in the wing disc [56]. Furthermore, the Dpp receptor Tkv and the Hh receptor Ptc enrich in puncta that move along cytonemes [75, 119]. In some tissues, the density of cytonemes from signal-receiving cells appears to shift relative to signal strength and receptor enrichment. For example, in the Drosophila ASP, Btl/FGFR-containing cytonemes form a gradient across signal-receiving tissue, such that cytoneme numbers decrease as distance from the Bnl/FGF source increases [144]. As such, signal-receiving-cell cytonemes constitute an important part of concentration gradient establishment.
Cytonemes of both signal-sending and signal-receiving cells are proposed to identify targets through sensing chemoattractant signals. This was first demonstrated in Drosophila where cytonemes of the wing imaginal disc extended toward the signaling center located at the A/P border [43]. More recent studies of dynamic cytonemes suggest a “random walk” model in which cytonemes orient in a deliberate manner through stabilization of connections that form between compatible molecules [76]. A mathematically generated model of WNT transport supports that random walk behavior has the capacity to rapidly form a robust concentration gradient that recapitulates what occurs in developing tissues [165]. The dynamic nature of cytonemes increases the likelihood that morphogen-enriched filopodial tips will encounter receptor-enriched cytonemes or cell membrane through which they establish stable contact. Cytoneme dynamics are influenced by rapid bursts of extension and retraction from the originating cell body [165]. In the conventional filopodia, extension and retraction dynamics are controlled through a balance between the functions of actin assembly and disassembly machinery. Similar mechanisms are likely in place to control cytoneme dynamics, because in the chick limb bud, Cofilin enriches at tips of Fascin-containing cytonemes. Its retrograde movement toward the cell body correlates with rapid retraction of dynamic cytonemes in this tissue [62].
Once successful contact is made between a dynamic cytoneme and a target cell cytoneme or membrane, the interaction may be stabilized to allow for signaling to occur (Fig. 3). For the Hh pathway, the adhesion protein Ihog and the HSPGs Dally and Dlp are thought to be critical for stable contact between Hh-sending and -receiving cytonemes in the Drosophila wing disc [119] (Fig. 3a). Ihog was first demonstrated to function in Hh signal reception by acting as a co-receptor with Ptc in signal-receiving cells [166–169]. More recently, its function as a cell adhesion molecule (CAM) was demonstrated to promote longevity of cytoneme contacts to ensure Hh gradient establishment in the wing discs [59, 162, 169]. Ihog contributes to these activities through both hetero- and homo-typic interactions that are governed by binding affinity [162]. Lower affinity Ihog–Ihog interactions promote cytoneme-to-cytoneme association and high-affinity Ihog–Hh interactions promote morphogen transfer [162] (Fig. 3a). The highest affinity interactions occur through the heterotypic Ptc–Ihog–Hh complex, which drives associations that lead to pathway activation in signal-receiving cells [162] (Fig. 3a). This model suggests that cytoneme pathfinding may occur through establishment of ligand–co-receptor and ligand–receptor interactions that improve efficiency of signal transport.
Although this type of competitive binding coordination has not yet been reported for other signaling pathways, cytonemes containing other morphogens are reported to use CAMs to stabilize cytoneme attachments in a manner similar to what occurs with neuronal synapses. For example, Dpp-containing cytonemes from the Drosophila wing disc are stabilized by the presence of the CAM Capricious (Caps) or its paralog Tartan (Trn) at their tips (Fig. 3b). Disruption of Caps or Trn function leads to defective Dpp uptake and signaling in the ASP, leading to altered morphogenesis [104]. Additional proteins common to neuronal synapses including the adhesion protein Neuroligin 2 (Nlg2) and the neuronal CAM Neuroglian (Nrg) are also reported to play roles in Dpp cytoneme synapse function (Fig. 3b). Knockdown of either Nlg2 or Nrg in the signal-receiving ASP decreases cytoneme number, which results in alteration of ASP morphology [104, 170].
In murine embryonic stem cells (ESCs), WNT-receiving cytonemes are stabilized through engagement of co-receptors LRP5/6 [127]. Similar mechanisms are in place for FGF signal transmission, because a GPI-anchored form of the Drosophila FGF Bnl at ASP cytoneme tips binds its receptor Btl in a CAM-like manner to stabilize synapses. Removal of the domain of Bnl facilitating CAM activity blocked formation of long, polarized cytonemes from both source and recipient cells, suggesting Bnl–Btl binding facilitates forward and reverse signaling to reinforce cytoneme function [51].
Further similarity between cytonemes and neuronal signaling is highlighted by a recent report demonstrating that trafficking of Tkv-containing puncta along ASP cytonemes is regulated by Ca2+ [170]. The Dpp-producing wing disc relies on Ca2+ signaling generated by the Voltage-Gated Calcium Channel (VGCC) and inward-rectifying K+ channel (Irk2). These ion gradients promote activity of Synaptotagmin Ca2+-binding protein 1 (Syt1) to target Dpp-containing vesicles toward the plasma membrane, where Ca2+-dependent Synaptobrevin (Syb), an R-SNARE family member, mediates vesicle docking for Dpp release [170, 171]. The Dpp-containing, pre-synaptic vesicles from wing discs contain glutamate, which is taken up by the vesicular glutamate transporter (VGlut). Glutamate release promotes postsynaptic Ca2+ uptake by ASP cytonemes via the non-NMDA ionotropic glutamate receptor (GluRII). Uptake of Ca2+ by the signal-receiving cytonemes then stimulates activity of Synaptotagmin 4 (Syt4) to promote receptor internalization for pathway activation [170] (Fig. 5). Evidence of glutamatergic signaling in mammalian cytonemes has been recently established in murine ESCs [127]. This cell population extends cytonemes toward WNT-producing trophoblast stem cells (TSCs) for self-renewal [127]. Upon formation of a stable cytoneme contact, ionotropic glutamate receptor (iGluR) generates a Ca2+ flux within the ESC cytoneme [127]. As such, intracellular signaling activity once thought to be specific to neurons may be more broadly employed to allow for morphogen signaling in developing tissues and stem cell compartments [172].
Fig. 5.

Glutamatergic Signaling at Morphogenetic Synapses. Cytonemes from a signal-producing cell are primed for signaling through uptake of glutamate molecules (green circles) through the Vesicular Glutamate Transporter (VGlut). At the cytoneme membrane, Ca2+ ions (blue circles) are imported by the Voltage-Gated Calcium Channel (VGCC) and K+ ions (orange circles) are exported by Inward-Rectifying K+ Channel (Irk2) to establish an ion gradient. The Ca2+-binding protein Synaptotagmin 1 (Syt1) targets signal-containing vesicles to the plasma membrane for vesicle docking by Ca2+-dependent R-SNARE family members. Release of vesicular contents (signals and/or exosomes) at the synapse results in release of glutamate into this site. Glutamate binds the non-NMDA ionotropic glutamate receptor (GluRII) to promote its activity for Ca2+ uptake by the signal-receiving cell. Ca2+-binding Synaptotagmin 4 (Syt4) functions on the extracellular surface of the signal-receiving cytoneme to facilitate signal reception. The neuronal synaptic adhesion protein Neuroligin 2 (Nlg2) and neuronal CAM Neuroglian (Nrg) function on the ASP to stabilize this interaction
The future of cytoneme research
As summarized above, the cytoneme-based model for morphogen transport continues to gain experimental support. There is much research effort focused on identifying the mechanisms controlling cytoneme initiation, morphogen loading, pathfinding, and signal delivery, because several questions remain unanswered. Key among them are defining when and where cytonemes initiate and determining how cytoneme behavior is tuned between signal-sending and -receiving cells in organizing tissues to ensure pattern formation. An additional aspect of cytoneme biology that is likely to be an intense area of research focus going forward will be to define the series of events that occur following signal reception by a receiving-cell cytoneme. Is signal initiated immediately upon receptor binding at a cytoneme tip, or do ligand/receptor complexes return to the cell body to signal from discrete membrane locations or vesicles, as has been proposed for the WNT signalosome [173]? Notably, for HH and WNT pathways, the co-receptors BOC/Boi, CDON/Ihog, and ROR2, function in both producing and receiving cells, suggesting that shared protein partners between connecting cytonemes may enhance signal transfer [63, 65, 67]. Determination of how other morphogen classes ensure successful signal transfer will require further research.
As it becomes increasingly clear that cytonemes are crucial for dispersion of many types of morphogens and growth factors, an understanding of the underlying molecular mechanisms governing cytoneme behavior will be paramount. The aspects of cytoneme regulation that are universally conserved during initiation, elongation, and stabilization, and activities that are morphogen-specific must be determined.
Due to the central role morphogens play during embryogenesis, it is likely that cytoneme dysfunction contributes to developmental disorders. Thus, a clear understanding of the regulatory mechanisms controlling their activity may provide novel opportunities for prevention or treatment of devastating developmental syndromes. Cytoneme biology is also likely relevant to cancer progression. EGFR and RET tumor models in Drosophila reveal decreased tumor growth and increased survival when cytoneme formation is disrupted in neoplastic cells and surrounding tissue [174]. In humans, many tumor types express and secrete morphogens that are thought to contribute to tumor–stroma communication during tumor growth and metastasis [175]. Because morphogen expression in tumors can drive cytoneme formation [67, 75], it is possible their high-level expression may facilitate direct lines of communication between cancer cells and surrounding tissue to facilitate tumor growth. In astrocytoma, microtubule-containing extensions are observed between tumor cells and documented to function as routes for tumor invasion and proliferation [176]. These results lend credence to the idea that cytonemes not only act as highways for morphogen transport in development and tissue homeostasis, but can be coopted in pathological progression.
Acknowledgements
Figures were created using BioRender.com.
Funding
This work was supported by a National Institutes of Health grant from the National Institute of General Medical Sciences R35GM122546 (SKO) and by ALSAC of St. Jude Children’s Research Hospital. The content is solely the responsibility of the authors and does not necessarily reflect the views of the funding agencies.
Footnotes
Conflict of interests The authors declare no conflicts of interest.
Declarations
Ethics approval and consent to participate Not applicable. No experiments were performed for this manuscript. It is a review of published literature.
Consent for publication Not applicable. We have not included any figures that have previously been published elsewhere. The authors of this manuscript provide consent to Springer to publish this review.
Data availability
Not applicable. This is a review of published literature.
References
- 1.Crick F (1970) Diffusion in embryogenesis. Nature 225(5231):420–422. 10.1038/225420a0 [DOI] [PubMed] [Google Scholar]
- 2.Zeng X, Goetz JA, Suber LM, Scott WJ Jr, Schreiner CM, Robbins DJ (2001) A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature 411(6838):716–720. 10.1038/35079648 [DOI] [PubMed] [Google Scholar]
- 3.Yu SR, Burkhardt M, Nowak M, Ries J, Petrásek Z, Scholpp S, Schwille P, Brand M (2009) Fgf8 morphogen gradient forms by a source-sink mechanism with freely diffusing molecules. Nature 461(7263):533–536. 10.1038/nature08391 [DOI] [PubMed] [Google Scholar]
- 4.Zhou S, Lo WC, Suhalim JL, Digman MA, Gratton E, Nie Q, Lander AD (2012) Free extracellular diffusion creates the Dpp morphogen gradient of the Drosophila wing disc. Curr Biol 22(8):668–675. 10.1016/j.cub.2012.02.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribes V, Briscoe J (2009) Establishing and interpreting graded Sonic Hedgehog signaling during vertebrate neural tube patterning: the role of negative feedback. Cold Spring Harb Perspect Biol 1(2):a002014. 10.1101/cshperspect.a002014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuniga A (2015) Next generation limb development and evolution: old questions, new perspectives. Development (Cambridge, England) 142(22):3810–3820. 10.1242/dev.125757 [DOI] [PubMed] [Google Scholar]
- 7.Kurosaka H, Iulianella A, Williams T, Trainor PA (2014) Disrupting hedgehog and WNT signaling interactions promotes cleft lip pathogenesis. J Clin Investig 124(4):1660–1671. 10.1172/jci72688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu D, Helms JA (1999) The role of sonic hedgehog in normal and abnormal craniofacial morphogenesis. Development (Cambridge, England) 126(21):4873–4884 [DOI] [PubMed] [Google Scholar]
- 9.Gittes GK (2009) Developmental biology of the pancreas: a comprehensive review. Dev Biol 326(1):4–35. 10.1016/j.ydbio.2008.10.024 [DOI] [PubMed] [Google Scholar]
- 10.Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP (1993) Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75(7):1417–1430. 10.1016/0092-8674(93)90627-3 [DOI] [PubMed] [Google Scholar]
- 11.Liem KF Jr, Tremml G, Roelink H, Jessell TM (1995) Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell 82(6):969–979. 10.1016/0092-8674(95)90276-7 [DOI] [PubMed] [Google Scholar]
- 12.Marti E, Takada R, Bumcrot DA, Sasaki H, McMahon AP (1995) Distribution of Sonic hedgehog peptides in the developing chick and mouse embryo. Development (Cambridge, England) 121(8):2537–2547 [DOI] [PubMed] [Google Scholar]
- 13.Vaccarino FM, Schwartz ML, Raballo R, Nilsen J, Rhee J, Zhou M, Doetschman T, Coffin JD, Wyland JJ, Hung YT (1999) Changes in cerebral cortex size are governed by fibroblast growth factor during embryogenesis. Nat Neurosci 2(3):246–253. 10.1038/6350 [DOI] [PubMed] [Google Scholar]
- 14.Charrier JB, Lapointe F, Le Douarin NM, Teillet MA (2001) Anti-apoptotic role of Sonic hedgehog protein at the early stages of nervous system organogenesis. Development (Cambridge, England) 128(20):4011–4020 [DOI] [PubMed] [Google Scholar]
- 15.Chenn A, Walsh CA (2002) Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science (New York, NY) 297(5580):365–369. 10.1126/science.1074192 [DOI] [PubMed] [Google Scholar]
- 16.Megason SG, McMahon AP (2002) A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development (Cambridge, England) 129(9):2087–2098 [DOI] [PubMed] [Google Scholar]
- 17.Britto J, Tannahill D, Keynes R (2002) A critical role for sonic hedgehog signaling in the early expansion of the developing brain. Nat Neurosci 5(2):103–110. 10.1038/nn797 [DOI] [PubMed] [Google Scholar]
- 18.Kunz M, Herrmann M, Wedlich D, Gradl D (2004) Autoregulation of canonical Wnt signaling controls midbrain development. Dev Biol 273(2):390–401. 10.1016/j.ydbio.2004.06.015 [DOI] [PubMed] [Google Scholar]
- 19.Lewis PM, Gritli-Linde A, Smeyne R, Kottmann A, McMahon AP (2004) Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev Biol 270(2):393–410. 10.1016/j.ydbio.2004.03.007 [DOI] [PubMed] [Google Scholar]
- 20.Rios I, Alvarez-Rodríguez R, Martí E, Pons S (2004) Bmp2 antagonizes sonic hedgehog-mediated proliferation of cerebellar granule neurons through Smad5 signalling. Development (Cambridge, England) 131(13):3159–3168. 10.1242/dev.01188 [DOI] [PubMed] [Google Scholar]
- 21.Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M (2003) The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell 113(1):11–23. 10.1016/s0092-8674(03)00199-5 [DOI] [PubMed] [Google Scholar]
- 22.Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y (2003) Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science (New York, NY) 302(5652):1984–1988. 10.1126/science.1089610 [DOI] [PubMed] [Google Scholar]
- 23.Lee JJ, von Kessler DP, Parks S, Beachy PA (1992) Secretion and localized transcription suggest a role in positional signaling for products of the segmentation gene hedgehog. Cell 71(1):33–50. 10.1016/0092-8674(92)90264-d [DOI] [PubMed] [Google Scholar]
- 24.Spencer FA, Hoffmann FM, Gelbart WM (1982) Decapentaplegic: a gene complex affecting morphogenesis in Drosophila melanogaster. Cell 28(3):451–461. 10.1016/0092-8674(82)90199-4 [DOI] [PubMed] [Google Scholar]
- 25.Kelvin DJ, Simard G, Connolly JA (1989) FGF and EGF act synergistically to induce proliferation in BC3H1 myoblasts. J Cell Physiol 138(2):267–272. 10.1002/jcp.1041380207 [DOI] [PubMed] [Google Scholar]
- 26.Sharma RP, Chopra VL (1976) Effect of the Wingless (wg1) mutation on wing and haltere development in Drosophila melanogaster. Dev Biol 48(2):461–465. 10.1016/0012-1606(76)90108-1 [DOI] [PubMed] [Google Scholar]
- 27.Muskavitch MA (1994) Delta-notch signaling and Drosophila cell fate choice. Dev Biol 166(2):415–430. 10.1006/dbio.1994.1326 [DOI] [PubMed] [Google Scholar]
- 28.Santos AJM, Lo YH, Mah AT, Kuo CJ (2018) The intestinal stem cell niche: homeostasis and adaptations. Trends Cell Biol 28(12):1062–1078. 10.1016/j.tcb.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Dop WA, Uhmann A, Wijgerde M, Sleddens-Linkels E, Heijmans J, Offerhaus GJ, van den Bergh Weerman MA, Boeckxstaens GE, Hommes DW, Hardwick JC, Hahn H, van den Brink GR (2009) Depletion of the colonic epithelial precursor cell compartment upon conditional activation of the hedgehog pathway. Gastroenterology 136 (7):2195–2203.e2191–2197. doi: 10.1053/j.gastro.2009.02.068 [DOI] [PubMed] [Google Scholar]
- 30.Petrova R, Joyner AL (2014) Roles for Hedgehog signaling in adult organ homeostasis and repair. Development (Cambridge, England) 141(18):3445–3457. 10.1242/dev.083691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopan R, Ilagan MX (2009) The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137(2):216–233. 10.1016/j.cell.2009.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aster JC, Pear WS, Blacklow SC (2017) The varied roles of notch in cancer. Annu Rev Pathol 12:245–275. 10.1146/annurev-pathol-052016-100127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Que J (2020) BMP signaling in development, stem cells, and diseases of the gastrointestinal tract. Annu Rev Physiol 82:251–273. 10.1146/annurev-physiol-021119-034500 [DOI] [PubMed] [Google Scholar]
- 34.Salazar VS, Gamer LW, Rosen V (2016) BMP signalling in skeletal development, disease and repair. Nat Rev Endocrinol 12(4):203–221. 10.1038/nrendo.2016.12 [DOI] [PubMed] [Google Scholar]
- 35.Clevers H, Nusse R (2012) Wnt/β-catenin signaling and disease. Cell 149(6):1192–1205. 10.1016/j.cell.2012.05.012 [DOI] [PubMed] [Google Scholar]
- 36.Skoda AM, Simovic D, Karin V, Kardum V, Vranic S, Serman L (2018) The role of the Hedgehog signaling pathway in cancer: a comprehensive review. Bosn J Basic Med Sci 18(1):8–20. 10.17305/bjbms.2018.2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spemann H, Mangold H (1923) Induction of embryonic primordia by implantation of organizers from a different species. Int J Dev Biol 45(1):13–38 [PubMed] [Google Scholar]
- 38.Dalcq AaP JL (1937) Une conception nouvelle des bases physiologiques de la morphogénèse. Arch Biol 48:669–711 [Google Scholar]
- 39.Turing A (1952) The Chemical Basis of Morphogenesis (1952). B Jack Copeland:519 [Google Scholar]
- 40.Wolpert L (1969) Positional information and the spatial pattern of cellular differentiation. J Theor Biol 25(1):1–47. 10.1016/s0022-5193(69)80016-0 [DOI] [PubMed] [Google Scholar]
- 41.Honig LS (1981) Positional signal transmission in the developing chick limb. Nature 291(5810):72–73. 10.1038/291072a0 [DOI] [PubMed] [Google Scholar]
- 42.Dierick HA, Bejsovec A (1998) Functional analysis of Wingless reveals a link between intercellular ligand transport and dorsal-cell-specific signaling. Development (Cambridge, England) 125(23):4729–4738 [DOI] [PubMed] [Google Scholar]
- 43.Ramirez-Weber FA, Kornberg TB (1999) Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell 97(5):599–607. 10.1016/s0092-8674(00)80771-0 [DOI] [PubMed] [Google Scholar]
- 44.Stapornwongkul KS, Vincent JP (2021) Generation of extracellular morphogen gradients: the case for diffusion. Nat Rev Genet 22(6):393–411. 10.1038/s41576-021-00342-y [DOI] [PubMed] [Google Scholar]
- 45.Bray SJ (2016) Notch signalling in context. Nat Rev Mol Cell Biol 17(11):722–735. 10.1038/nrm.2016.94 [DOI] [PubMed] [Google Scholar]
- 46.Lee JJ, Ekker SC, von Kessler DP, Porter JA, Sun BI, Beachy PA (1994) Autoproteolysis in hedgehog protein biogenesis. Science (New York, NY) 266(5190):1528–1537. 10.1126/science.7985023 [DOI] [PubMed] [Google Scholar]
- 47.Pepinsky RB, Zeng C, Wen D, Rayhorn P, Baker DP, Williams KP, Bixler SA, Ambrose CM, Garber EA, Miatkowski K, Taylor FR, Wang EA, Galdes A (1998) Identification of a palmitic acid-modified form of human Sonic hedgehog. J Biol Chem 273(22):14037–14045. 10.1074/jbc.273.22.14037 [DOI] [PubMed] [Google Scholar]
- 48.Long J, Tokhunts R, Old WM, Houel S, Rodgriguez-Blanco J, Singh S, Schilling N, A JC, Ahn NG, Robbins DJ, (2015) Identification of a family of fatty-acid-speciated sonic hedgehog proteins, whose members display differential biological properties. Cell Rep 10(8):1280–1287. 10.1016/j.celrep.2015.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR 3rd, Nusse R (2003) Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423(6938):448–452. 10.1038/nature01611 [DOI] [PubMed] [Google Scholar]
- 50.Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC (2012) Structural basis of Wnt recognition by Frizzled. Science (New York, NY) 337(6090):59–64. 10.1126/science.1222879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Du L, Sohr A, Roy S (2021) Glypiated FGF directs contact-mediated bidirectional signaling to self-regulate tissue-specific dispersion. bioRxiv [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kornberg TB, Guha A (2007) Understanding morphogen gradients: a problem of dispersion and containment. Curr Opin Genet Dev 17(4):264–271. 10.1016/j.gde.2007.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kornberg TB (2012) The imperatives of context and contour for morphogen dispersion. Biophys J 103(11):2252–2256. 10.1016/j.bpj.2012.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kornberg TB (2017) Distributing signaling proteins in space and time: the province of cytonemes. Curr Opin Genet Dev 45:22–27. 10.1016/j.gde.2017.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.González-Gaitán M (2003) Signal dispersal and transduction through the endocytic pathway. Nat Rev Mol Cell Biol 4(3):213–224. 10.1038/nrm1053 [DOI] [PubMed] [Google Scholar]
- 56.Huang H, Kornberg TB (2015) Myoblast cytonemes mediate Wg signaling from the wing imaginal disc and Delta-Notch signaling to the air sac primordium. eLife 4:e06114. doi: 10.7554/eLife.06114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Joussineau C, Soulé J, Martin M, Anguille C, Montcourrier P, Alexandre D (2003) Delta-promoted filopodia mediate long-range lateral inhibition in Drosophila. Nature 426(6966):555–559. 10.1038/nature02157 [DOI] [PubMed] [Google Scholar]
- 58.Cohen M, Georgiou M, Stevenson NL, Miodownik M, Baum B (2010) Dynamic filopodia transmit intermittent Delta-Notch signaling to drive pattern refinement during lateral inhibition. Dev Cell 19(1):78–89. 10.1016/j.devcel.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 59.Bischoff M, Gradilla AC, Seijo I, Andrés G, Rodríguez-Navas C, González-Méndez L, Guerrero I (2013) Cytonemes are required for the establishment of a normal Hedgehog morphogen gradient in Drosophila epithelia. Nat Cell Biol 15(11):1269–1281. 10.1038/ncb2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rojas-Ríos P, Guerrero I, González-Reyes A (2012) Cytoneme-mediated delivery of hedgehog regulates the expression of bone morphogenetic proteins to maintain germline stem cells in Drosophila. PLoS Biol 10(4):e1001298. 10.1371/journal.pbio.1001298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roy S, Hsiung F, Kornberg TB (2011) Specificity of Drosophila cytonemes for distinct signaling pathways. Science (New York, NY) 332(6027):354–358. 10.1126/science.1198949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanders TA, Llagostera E, Barna M (2013) Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning. Nature 497(7451):628–632. 10.1038/nature12157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mattes B, Dang Y, Greicius G, Kaufmann LT, Prunsche B, Rosenbauer J, Stegmaier J, Mikut R, Özbek S, Nienhaus GU, Schug A, Virshup DM, Scholpp S (2018) Wnt/PCP controls spreading of Wnt/β-catenin signals by cytonemes in vertebrates. eLife 7. doi: 10.7554/eLife.36953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosenbauer J, Zhang C, Mattes B, Reinartz I, Wedgwood K, Schindler S, Sinner C, Scholpp S, Schug A (2020) Modeling of Wnt-mediated tissue patterning in vertebrate embryogenesis. PLoS Comput Biol 16(6):e1007417. 10.1371/journal.pcbi.1007417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brunt L, Greicius G, Rogers S, Evans BD, Virshup DM, Wedgwood KCA, Scholpp S (2021) Vangl2 promotes the formation of long cytonemes to enable distant Wnt/β-catenin signaling. Nat Commun 12(1):2058. 10.1038/s41467-021-22393-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Z, Denans N, Liu Y, Zhulyn O, Rosenblatt HD, Wernig M, Barna M (2021) Optogenetic manipulation of cellular communication using engineered myosin motors. Nat Cell Biol 23(2):198–208. 10.1038/s41556-020-00625-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hall ET, Dillard ME, Stewart DP, Zhang Y, Wagner B, Levine RM, Pruett-Miller SM, Sykes A, Temirov J, Cheney RE, Mori M, Robinson CG, Ogden SK (2021) Cytoneme delivery of sonic hedgehog from ligand-producing cells requires Myosin 10 and a dispatched-BOC/CDON co-receptor complex. eLife 10. 10.7554/eLife.61432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gustafson T, Wolpert L (1961) Cellular mechanisms in the morphogenesis of the sea urchin larva. The formation of arms. Exp Cell Res 22:509–520. 10.1016/0014-4827(61)90127-6 [DOI] [PubMed] [Google Scholar]
- 69.Karp GC, Solursh M (1985) Dynamic activity of the filopodia of sea urchin embryonic cells and their role in directed migration of the primary mesenchyme in vitro. Dev Biol 112(2):276–283. 10.1016/0012-1606(85)90398-7 [DOI] [PubMed] [Google Scholar]
- 70.Miller J, Fraser SE, McClay D (1995) Dynamics of thin filopodia during sea urchin gastrulation. Development (Cambridge, England) 121(8):2501–2511 [DOI] [PubMed] [Google Scholar]
- 71.Mattila PK, Lappalainen P (2008) Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol 9(6):446–454. 10.1038/nrm2406 [DOI] [PubMed] [Google Scholar]
- 72.Ritzenthaler S, Suzuki E, Chiba A (2000) Postsynaptic filopodia in muscle cells interact with innervating motoneuron axons. Nat Neurosci 3(10):1012–1017. 10.1038/79833 [DOI] [PubMed] [Google Scholar]
- 73.Tarone G, Cirillo D, Giancotti FG, Comoglio PM, Marchisio PC (1985) Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res 159(1):141–157. 10.1016/s0014-4827(85)80044-6 [DOI] [PubMed] [Google Scholar]
- 74.Chen WT (1989) Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J Exp Zool 251(2):167–185. 10.1002/jez.1402510206 [DOI] [PubMed] [Google Scholar]
- 75.Hsiung F, Ramirez-Weber FA, Iwaki DD, Kornberg TB (2005) Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature 437(7058):560–563. 10.1038/nature03951 [DOI] [PubMed] [Google Scholar]
- 76.Kornberg TB, Roy S (2014) Cytonemes as specialized signaling filopodia. Development (Cambridge, England) 141(4):729–736. 10.1242/dev.086223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH (2004) Nanotubular highways for intercellular organelle transport. Science (New York, NY) 303(5660):1007–1010. 10.1126/science.1093133 [DOI] [PubMed] [Google Scholar]
- 78.Korenkova O, Pepe A, Zurzolo C (2020) Fine intercellular connections in development: TNTs, cytonemes, or intercellular bridges? Cell stress 4(2):30–43. 10.15698/cst2020.02.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Köhler K, Oddos S, Eissmann P, Brodsky FM, Hopkins C, Onfelt B, Sattentau Q, Davis DM (2008) Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol 10(2):211–219. 10.1038/ncb1682 [DOI] [PubMed] [Google Scholar]
- 80.Chauveau A, Aucher A, Eissmann P, Vivier E, Davis DM (2010) Membrane nanotubes facilitate long-distance interactions between natural killer cells and target cells. Proc Natl Acad Sci USA 107(12):5545–5550. 10.1073/pnas.0910074107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jackson MV, Morrison TJ, Doherty DF, McAuley DF, Matthay MA, Kissenpfennig A, O’Kane CM, Krasnodembskaya AD (2016) Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem cells (Dayton, Ohio) 34(8):2210–2223. 10.1002/stem.2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Teddy JM, Kulesa PM (2004) In vivo evidence for short- and long-range cell communication in cranial neural crest cells. Development (Cambridge, England) 131(24):6141–6151. 10.1242/dev.01534 [DOI] [PubMed] [Google Scholar]
- 83.McKinney MC, Stark DA, Teddy J, Kulesa PM (2011) Neural crest cell communication involves an exchange of cytoplasmic material through cellular bridges revealed by photoconversion of KikGR. Dev Dyn 240(6):1391–1401. 10.1002/dvdy.22612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang X, Veruki ML, Bukoreshtliev NV, Hartveit E, Gerdes HH (2010) Animal cells connected by nanotubes can be electrically coupled through interposed gap-junction channels. Proc Natl Acad Sci USA 107(40):17194–17199. 10.1073/pnas.1006785107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hase K, Kimura S, Takatsu H, Ohmae M, Kawano S, Kitamura H, Ito M, Watarai H, Hazelett CC, Yeaman C, Ohno H (2009) M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat Cell Biol 11(12):1427–1432. 10.1038/ncb1990 [DOI] [PubMed] [Google Scholar]
- 86.Watkins SC, Salter RD (2005) Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity 23(3):309–318. 10.1016/j.immuni.2005.08.009 [DOI] [PubMed] [Google Scholar]
- 87.Chinnery HR, Pearlman E, McMenamin PG (2008) Cutting edge: Membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. Journal of immunology (Baltimore, Md : 1950) 180 (9):5779–5783. doi: 10.4049/jimmunol.180.9.5779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eugenin EA, Gaskill PJ, Berman JW (2009) Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: a potential mechanism for intercellular HIV trafficking. Cell Immunol 254(2):142–148. 10.1016/j.cellimm.2008.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT, Chenouard N, de Chaumont F, Martino A, Enninga J, Olivo-Marin JC, Männel D, Zurzolo C (2009) Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol 11(3):328–336. 10.1038/ncb1841 [DOI] [PubMed] [Google Scholar]
- 90.Caneparo L, Pantazis P, Dempsey W, Fraser SE (2011) Intercellular bridges in vertebrate gastrulation. PLoS ONE 6(5):e20230. 10.1371/journal.pone.0020230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burgos MH, Fawcett DW (1955) Studies on the fine structure of the mammalian testis. I. Differentiation of the spermatids in the cat (Felis domestica). The Journal of biophysical and biochemical cytology 1 (4):287–300. doi: 10.1083/jcb.1.4.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fawcett DW, Ito S, Slautterback D (1959) The occurrence of intercellular bridges in groups of cells exhibiting synchronous differentiation. J Biophys Biochem Cytol 5(3):453–460. 10.1083/jcb.5.3.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hime GR, Brill JA, Fuller MT (1996) Assembly of ring canals in the male germ line from structural components of the contractile ring. J Cell Sci 109(Pt 12):2779–2788 [DOI] [PubMed] [Google Scholar]
- 94.Rehain-Bell K, Love A, Werner ME, MacLeod I, Yates JR 3rd, Maddox AS (2017) A Sterile 20 family kinase and its co-factor CCM-3 regulate contractile ring proteins on germline intercellular bridges. Curr Biol 27(6):860–867. 10.1016/j.cub.2017.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Greenbaum MP, Yan W, Wu MH, Lin YN, Agno JE, Sharma M, Braun RE, Rajkovic A, Matzuk MM (2006) TEX14 is essential for intercellular bridges and fertility in male mice. Proc Natl Acad Sci USA 103(13):4982–4987. 10.1073/pnas.0505123103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ventelä S, Toppari J, Parvinen M (2003) Intercellular organelle traffic through cytoplasmic bridges in early spermatids of the rat: mechanisms of haploid gene product sharing. Mol Biol Cell 14(7):2768–2780. 10.1091/mbc.e02-10-0647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xing F, Qu S, Liu J, Yang J, Hu F, Drevenšek-Olenik I, Pan L, Xu J (2020) Intercellular Bridge Mediates Ca(2+) Signals between Micropatterned Cells via IP(3) and Ca(2+) Diffusion. Biophys J 118(5):1196–1204. 10.1016/j.bpj.2020.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haglund K, Nezis IP, Stenmark H (2011) Structure and functions of stable intercellular bridges formed by incomplete cytokinesis during development. Communicative & integrative biology 4(1):1–9. 10.4161/cib.4.1.13550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hamada H, Watanabe M, Lau HE, Nishida T, Hasegawa T, Parichy DM, Kondo S (2014) Involvement of Delta/Notch signaling in zebrafish adult pigment stripe patterning. Development (Cambridge, England) 141(2):318–324. 10.1242/dev.099804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Eom DS, Bain EJ, Patterson LB, Grout ME, Parichy DM (2015) Long-distance communication by specialized cellular projections during pigment pattern development and evolution. eLife 4. 10.7554/eLife.12401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eom DS, Parichy DM (2017) A macrophage relay for long-distance signaling during postembryonic tissue remodeling. Science (New York, NY) 355(6331):1317–1320. 10.1126/science.aal2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yatsenko AS, Shcherbata HR (2021) Distant activation of Notch signaling induces stem cell niche assembly. PLoS Genet 17(3):e1009489. 10.1371/journal.pgen.1009489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wood BM, Baena V, Huang H, Jorgens DM, Terasaki M, Kornberg TB (2021) Cytonemes with complex geometries and composition extend into invaginations of target cells. The Journal of cell biology 220 (5). 10.1083/jcb.202101116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roy S, Huang H, Liu S, Kornberg TB (2014) Cytoneme-mediated contact-dependent transport of the Drosophila decapentaplegic signaling protein. Science (New York, NY) 343(6173):1244624. 10.1126/science.1244624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mattes B, Scholpp S (2018) Emerging role of contact-mediated cell communication in tissue development and diseases. Histochem Cell Biol 150(5):431–442. 10.1007/s00418-018-1732-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gradilla AC, González E, Seijo I, Andrés G, Bischoff M, González-Mendez L, Sánchez V, Callejo A, Ibáñez C, Guerra M, Ortigão-Farias JR, Sutherland JD, González M, Barrio R, Falcón-Pérez JM, Guerrero I (2014) Exosomes as Hedgehog carriers in cytoneme-mediated transport and secretion. Nat Commun 5:5649. 10.1038/ncomms6649 [DOI] [PubMed] [Google Scholar]
- 107.Eom DS (2020) Airinemes: thin cellular protrusions mediate long-distance signalling guided by macrophages. Open Biol 10(8):200039. 10.1098/rsob.200039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bodeen WJ, Marada S, Truong A, Ogden SK (2017) A fixation method to preserve cultured cell cytonemes facilitates mechanistic interrogation of morphogen transport. Development (Cambridge, England) 144(19):3612–3624. 10.1242/dev.152736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Merino F, Pospich S, Raunser S (2020) Towards a structural understanding of the remodeling of the actin cytoskeleton. Semin Cell Dev Biol 102:51–64. 10.1016/j.semcdb.2019.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Clayton NS, Ridley AJ (2020) Targeting Rho GTPase signaling networks in cancer. Front Cell Dev Biol 8:222. 10.3389/fcell.2020.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sinha S, Yang W (2008) Cellular signaling for activation of Rho GTPase Cdc42. Cell Signal 20(11):1927–1934. 10.1016/j.cellsig.2008.05.002 [DOI] [PubMed] [Google Scholar]
- 112.Buchsbaum RJ (2007) Rho activation at a glance. J Cell Sci 120(Pt 7):1149–1152. 10.1242/jcs.03428 [DOI] [PubMed] [Google Scholar]
- 113.Ridley AJ (2006) Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol 16(10):522–529. 10.1016/j.tcb.2006.08.006 [DOI] [PubMed] [Google Scholar]
- 114.Lee K, Gallop JL, Rambani K, Kirschner MW (2010) Self-assembly of filopodia-like structures on supported lipid bilayers. Science (New York, NY) 329(5997):1341–1345. 10.1126/science.1191710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ho HY, Rohatgi R, Lebensohn AM, Le M, Li J, Gygi SP, Kirschner MW (2004) Toca-1 mediates Cdc42-dependent actin nucleation by activating the N-WASP-WIP complex. Cell 118(2):203–216. 10.1016/j.cell.2004.06.027 [DOI] [PubMed] [Google Scholar]
- 116.Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW (1999) The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97(2):221–231. 10.1016/s0092-8674(00)80732-1 [DOI] [PubMed] [Google Scholar]
- 117.Brühmann S, Ushakov DS, Winterhoff M, Dickinson RB, Curth U, Faix J (2017) Distinct VASP tetramers synergize in the processive elongation of individual actin filaments from clustered arrays. Proc Natl Acad Sci USA 114(29):E5815–e5824. 10.1073/pnas.1703145114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Callejo A, Bilioni A, Mollica E, Gorfinkiel N, Andres G, Ibanez C, Torroja C, Doglio L, Sierra J, Guerrero I (2011) Dispatched mediates Hedgehog basolateral release to form the long-range morphogenetic gradient in the Drosophila wing disk epithelium. Proc Natl Acad Sci USA 108(31):12591–12598. 10.1073/pnas.1106881108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.González-Méndez L, Seijo-Barandiarán I, Guerrero I (2017) Cytoneme-mediated cell-cell contacts for Hedgehog reception. eLife 6. 10.7554/eLife.24045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Woodring PJ, Litwack ED, O’Leary DD, Lucero GR, Wang JY, Hunter T (2002) Modulation of the F-actin cytoskeleton by c-Abl tyrosine kinase in cell spreading and neurite extension. J Cell Biol 156(5):879–892. 10.1083/jcb.200110014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vuong TA, Leem YE, Kim BG, Cho H, Lee SJ, Bae GU, Kang JS (2017) A Sonic hedgehog coreceptor, BOC regulates neuronal differentiation and neurite outgrowth via interaction with ABL and JNK activation. Cell Signal 30:30–40. 10.1016/j.cellsig.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 122.Bae GU, Kim BG, Lee HJ, Oh JE, Lee SJ, Zhang W, Krauss RS, Kang JS (2009) Cdo binds Abl to promote p38alpha/beta mitogen-activated protein kinase activity and myogenic differentiation. Mol Cell Biol 29(15):4130–4143. 10.1128/mcb.00199-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Benoit B, Baillet A, Poüs C (2021) Cytoskeleton and Associated Proteins: Pleiotropic JNK Substrates and Regulators. International journal of molecular sciences 22 (16). doi: 10.3390/ijms22168375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Valakh V, Frey E, Babetto E, Walker LJ, DiAntonio A (2015) Cytoskeletal disruption activates the DLK/JNK pathway, which promotes axonal regeneration and mimics a preconditioning injury. Neurobiol Dis 77:13–25. 10.1016/j.nbd.2015.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Björkblom B, Padzik A, Mohammad H, Westerlund N, Komulainen E, Hollos P, Parviainen L, Papageorgiou AC, Iljin K, Kallioniemi O, Kallajoki M, Courtney MJ, Mågård M, James P, Coffey ET (2012) c-Jun N-terminal kinase phosphorylation of MARCKSL1 determines actin stability and migration in neurons and in cancer cells. Mol Cell Biol 32(17):3513–3526. 10.1128/mcb.00713-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ramesh P, Dey NS, Kanwal A, Mandal S, Mandal L (2021) Relish plays a dynamic role in the niche to modulate Drosophila blood progenitor homeostasis in development and infection. eLife 10. doi: 10.7554/eLife.67158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Junyent S, Garcin CL, Szczerkowski JLA, Trieu TJ, Reeves J, Habib SJ (2020) Specialized cytonemes induce self-organization of stem cells. Proc Natl Acad Sci USA 117(13):7236–7244. 10.1073/pnas.1920837117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kwiatkowski AV, Rubinson DA, Dent EW, Edward van Veen J, Leslie JD, Zhang J, Mebane LM, Philippar U, Pinheiro EM, Burds AA, Bronson RT, Mori S, Fässler R, Gertler FB (2007) Ena/VASP Is Required for neuritogenesis in the developing cortex. Neuron 56(3):441–455. 10.1016/j.neuron.2007.09.008 [DOI] [PubMed] [Google Scholar]
- 129.Makihara S, Morin S, Ferent J, Côté JF, Yam PT, Charron F (2018) Polarized Dock Activity Drives Shh-Mediated Axon Guidance. Dev Cell 46(4):410–425.e417. 10.1016/j.devcel.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 130.Han JW, Lee HJ, Bae GU, Kang JS (2011) Promyogenic function of Integrin/FAK signaling is mediated by Cdo, Cdc42 and MyoD. Cell Signal 23(7):1162–1169. 10.1016/j.cellsig.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 131.Kang JS, Bae GU, Yi MJ, Yang YJ, Oh JE, Takaesu G, Zhou YT, Low BC, Krauss RS (2008) A Cdo-Bnip-2-Cdc42 signaling pathway regulates p38alpha/beta MAPK activity and myogenic differentiation. J Cell Biol 182(3):497–507. 10.1083/jcb.200801119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Koizumi K, Takano K, Kaneyasu A, Watanabe-Takano H, Tokuda E, Abe T, Watanabe N, Takenawa T, Endo T (2012) RhoD activated by fibroblast growth factor induces cytoneme-like cellular protrusions through mDia3C. Mol Biol Cell 23(23):4647–4661. 10.1091/mbc.E12-04-0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang G, Liu Y, Yang K, Liu R, Zhu S, Coquinco A, Wen W, Kojic L, Jia W, Cynader M (2012) Isoform-specific palmitoylation of JNK regulates axonal development. Cell Death Differ 19(4):553–561. 10.1038/cdd.2011.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stanganello E, Hagemann AI, Mattes B, Sinner C, Meyen D, Weber S, Schug A, Raz E, Scholpp S (2015) Filopodia-based Wnt transport during vertebrate tissue patterning. Nat Commun 6:5846. 10.1038/ncomms6846 [DOI] [PubMed] [Google Scholar]
- 135.Snyder JC, Rochelle LK, Marion S, Lyerly HK, Barak LS, Caron MG (2015) Lgr4 and Lgr5 drive the formation of long actin-rich cytoneme-like membrane protrusions. J Cell Sci 128(6):1230–1240. 10.1242/jcs.166322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Katanaev VL, Solis GP, Hausmann G, Buestorf S, Katanayeva N, Schrock Y, Stuermer CA, Basler K (2008) Reggie-1/flotillin-2 promotes secretion of the long-range signalling forms of Wingless and Hedgehog in Drosophila. EMBO J 27(3):509–521. 10.1038/sj.emboj.7601981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Head BP, Patel HH (1838) Insel PA (2014) Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochem Biophys Acta 2:532–545. 10.1016/j.bbamem.2013.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Neumann-Giesen C, Falkenbach B, Beicht P, Claasen S, Lüers G, Stuermer CA, Herzog V, Tikkanen R (2004) Membrane and raft association of reggie-1/flotillin-2: role of myristoylation, palmitoylation and oligomerization and induction of filopodia by overexpression. Biochem J 378(Pt 2):509–518. 10.1042/bj20031100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Huang H, Kornberg TB (2016) Cells must express components of the planar cell polarity system and extracellular matrix to support cytonemes. eLife 5. 10.7554/eLife.18979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hu B, Rodriguez JJ, Kakkerla Balaraju A, Gao Y, Nguyen NT, Steen H, Suhaib S, Chen S, Lin F (2021) Glypican 4 mediates Wnt transport between germ layers via signaling filopodia. J Cell Biol 220 (12). 10.1083/jcb.202009082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yan D, Lin X (2009) Shaping morphogen gradients by Proteoglycans. Cold Spring Harb Perspect Biol. 10.1101/cshperspect.a002493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vyas N, Goswami D, Manonmani A, Sharma P, Ranganath HA, VijayRaghavan K, Shashidhara LS, Sowdhamini R, Mayor S (2008) Nanoscale organization of hedgehog is essential for long-range signaling. Cell 133(7):1214–1227. 10.1016/j.cell.2008.05.026 [DOI] [PubMed] [Google Scholar]
- 143.Simon E, Jiménez-Jiménez C, Seijo-Barandiarán I, Aguilar G, Sánchez-Hernández D, Aguirre-Tamaral A, González-Méndez L, Ripoll P, Guerrero I (2021) Glypicans define unique roles for the Hedgehog co-receptors boi and ihog in cytoneme-mediated gradient formation. eLife 10. 10.7554/eLife.64581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Du L, Sohr A, Yan G, Roy S (2018) Feedback regulation of cytoneme-mediated transport shapes a tissue-specific FGF morphogen gradient. eLife 7. doi: 10.7554/eLife.38137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Teleman AA, Cohen SM (2000) Dpp gradient formation in the Drosophila wing imaginal disc. Cell 103(6):971–980. 10.1016/s0092-8674(00)00199-9 [DOI] [PubMed] [Google Scholar]
- 146.Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, Budnik V (2009) Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell 139(2):393–404. 10.1016/j.cell.2009.07.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Greco V, Hannus M, Eaton S (2001) Argosomes: a potential vehicle for the spread of morphogens through epithelia. Cell 106(5):633–645. 10.1016/s0092-8674(01)00484-6 [DOI] [PubMed] [Google Scholar]
- 148.Rusten TE, Vaccari T, Stenmark H (2011) Shaping development with ESCRTs. Nat Cell Biol 14(1):38–45. 10.1038/ncb2381 [DOI] [PubMed] [Google Scholar]
- 149.Matusek T, Wendler F, Polès S, Pizette S, D’Angelo G, Fürthauer M, Thérond PP (2014) The ESCRT machinery regulates the secretion and long-range activity of Hedgehog. Nature 516(7529):99–103. 10.1038/nature13847 [DOI] [PubMed] [Google Scholar]
- 150.Coulter ME, Dorobantu CM, Lodewijk GA, Delalande F, Cianferani S, Ganesh VS, Smith RS, Lim ET, Xu CS, Pang S, Wong ET, Lidov HGW, Calicchio ML, Yang E, Gonzalez DM, Schlaeger TM, Mochida GH, Hess H, Lee WA, Lehtinen MK, Kirchhausen T, Haussler D, Jacobs FMJ, Gaudin R, Walsh CA (2018) The ESCRT-III Protein CHMP1A Mediates Secretion of Sonic Hedgehog on a Distinctive Subtype of Extracellular Vesicles. Cell Rep 24(4):973–986.e978. 10.1016/j.celrep.2018.06.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kicheva A, Pantazis P, Bollenbach T, Kalaidzidis Y, Bittig T, Jülicher F, González-Gaitán M (2007) Kinetics of morphogen gradient formation. Science (New York, NY) 315(5811):521–525. 10.1126/science.1135774 [DOI] [PubMed] [Google Scholar]
- 152.D’Angelo G, Matusek T, Pizette S, Therond PP (2015) Endocytosis of Hedgehog through dispatched regulates long-range signaling. Dev Cell 32(3):290–303. 10.1016/j.devcel.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 153.Chen W, Huang H, Hatori R, Kornberg TB (2017) Essential basal cytonemes take up Hedgehog in the Drosophila wing imaginal disc. Development (Cambridge, England) 144(17):3134–3144. 10.1242/dev.149856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Burke R, Nellen D, Bellotto M, Hafen E, Senti KA, Dickson BJ, Basler K (1999) Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell 99(7):803–815. 10.1016/s0092-8674(00)81677-3 [DOI] [PubMed] [Google Scholar]
- 155.Couto A, Mack NA, Favia L, Georgiou M (2017) An apicobasal gradient of Rac activity determines protrusion form and position. Nat Commun 8:15385. 10.1038/ncomms15385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Polizio AH, Chinchilla P, Chen X, Kim S, Manning DR, Riobo NA (2011) Heterotrimeric Gi proteins link Hedgehog signaling to activation of Rho small GTPases to promote fibroblast migration. J Biol Chem 286(22):19589–19596. 10.1074/jbc.M110.197111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gallet A, Staccini-Lavenant L, Thérond PP (2008) Cellular trafficking of the glypican Dally-like is required for full-strength Hedgehog signaling and wingless transcytosis. Dev Cell 14(5):712–725. 10.1016/j.devcel.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 158.Hatori R, Wood BM, Oliveira Barbosa G, Kornberg TB (2021) Regulated delivery controls Drosophila Hedgehog, Wingless, and Decapentaplegic signaling. eLife 10. 10.7554/eLife.71744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Odronitz F, Kollmar M (2007) Drawing the tree of eukaryotic life based on the analysis of 2269 manually annotated myosins from 328 species. Genome Biol 8(9):R196. 10.1186/gb-2007-8-9-r196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kornberg TB, Roy S (2014) Communicating by touch–neurons are not alone. Trends Cell Biol 24(6):370–376. 10.1016/j.tcb.2014.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.González-Méndez L, Gradilla AC, Guerrero I (2019) The cytoneme connection: direct long-distance signal transfer during development. Development (Cambridge, England) 146 (9). 10.1242/dev.174607 [DOI] [PubMed] [Google Scholar]
- 162.Yang S, Zhang Y, Yang C, Wu X, El Oud SM, Chen R, Cai X, Wu XS, Lan G, Zheng X (2021) Competitive coordination of the dual roles of the Hedgehog co-receptor in homophilic adhesion and signal reception. eLife 10. 10.7554/eLife.65770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hatori R, Kornberg TB (2020) Hedgehog produced by the Drosophila wing imaginal disc induces distinct responses in three target tissues. Development (Cambridge, England) 147 (22). 10.1242/dev.195974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sagar PF, Wiegreffe C, Scaal M (2015) Communication between distant epithelial cells by filopodia-like protrusions during embryonic development. Development (Cambridge, England) 142(4):665–671. 10.1242/dev.115964 [DOI] [PubMed] [Google Scholar]
- 165.Bressloff PC, Kim H (2019) Search-and-capture model of cytoneme-mediated morphogen gradient formation. Phys Rev E 99(5–1):052401. 10.1103/PhysRevE.99.052401 [DOI] [PubMed] [Google Scholar]
- 166.Lum L, Yao S, Mozer B, Rovescalli A, Von Kessler D, Nirenberg M, Beachy PA (2003) Identification of Hedgehog pathway components by RNAi in Drosophila cultured cells. Science (New York, NY) 299(5615):2039–2045. 10.1126/science.1081403 [DOI] [PubMed] [Google Scholar]
- 167.Izzi L, Lévesque M, Morin S, Laniel D, Wilkes BC, Mille F, Krauss RS, McMahon AP, Allen BL, Charron F (2011) Boc and Gas1 each form distinct Shh receptor complexes with Ptch1 and are required for Shh-mediated cell proliferation. Dev Cell 20(6):788–801. 10.1016/j.devcel.2011.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bae GU, Domené S, Roessler E, Schachter K, Kang JS, Muenke M, Krauss RS (2011) Mutations in CDON, encoding a hedgehog receptor, result in holoprosencephaly and defective interactions with other hedgehog receptors. Am J Hum Genet 89(2):231–240. 10.1016/j.ajhg.2011.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zheng X, Mann RK, Sever N, Beachy PA (2010) Genetic and biochemical definition of the Hedgehog receptor. Genes Dev 24(1):57–71. 10.1101/gad.1870310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Huang H, Liu S, Kornberg TB (2019) Glutamate signaling at cytoneme synapses. Science (New York, NY) 363(6430):948–955. 10.1126/science.aat5053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.González-Méndez L, Gradilla AC, Sánchez-Hernández D, González E, Aguirre-Tamaral A, Jiménez-Jiménez C, Guerra M, Aguilar G, Andrés G, Falcón-Pérez JM, Guerrero I (2020) Polarized sorting of Patched enables cytoneme-mediated Hedgehog reception in the Drosophila wing disc. EMBO J 39(11):e103629. 10.15252/embj.2019103629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hinoi E, Takarada T, Ueshima T, Tsuchihashi Y, Yoneda Y (2004) Glutamate signaling in peripheral tissues. Eur J Biochem 271(1):1–13. 10.1046/j.1432-1033.2003.03907.x [DOI] [PubMed] [Google Scholar]
- 173.Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C (2007) Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science (New York, NY) 316(5831):1619–1622. 10.1126/science.1137065 [DOI] [PubMed] [Google Scholar]
- 174.Fereres S, Hatori R, Hatori M, Kornberg TB (2019) Cytoneme-mediated signaling essential for tumorigenesis. PLoS Genet 15(9):e1008415. 10.1371/journal.pgen.1008415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wendler F, Stamp GW, Giamas G (2016) Tumor-Stromal Cell Communication: Small Vesicles Signal Big Changes. Trends in cancer 2(7):326–329. 10.1016/j.trecan.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 176.Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, Blaes J, Weil S, Horstmann H, Wiestler B, Syed M, Huang L, Ratliff M, Karimian Jazi K, Kurz FT, Schmenger T, Lemke D, Gömmel M, Pauli M, Liao Y, Häring P, Pusch S, Herl V, Steinhäuser C, Krunic D, Jarahian M, Miletic H, Berghoff AS, Griesbeck O, Kalamakis G, Garaschuk O, Preusser M, Weiss S, Liu H, Heiland S, Platten M, Huber PE, Kuner T, von Deimling A, Wick W, Winkler F (2015) Brain tumour cells interconnect to a functional and resistant network. Nature 528(7580):93–98. 10.1038/nature16071 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable. This is a review of published literature.


