Abstract
Morphometric evidence derived from studies of mast cells, pancreatic acinar cells, and other cell types supports a model in which the post Golgi processes that generate mature secretory granules can be resolved into three steps: 1) fusion of small, Golgi-derived progranules to produce immature secretory granules which have a highly constrained volume; 2) transformation of such immature granules into mature secretory granules, a process often associated with a reduction in the maturing granule's volume, as well as changes in the appearance of its content; and 3) fusion of secretory granules of the smallest size, termed “unit granules”, forming granules whose volumes are multiples of the unit granule's volume.
Mutations which perturb this process can cause significant pathology. For example, CHS/Lyst mutations result in giant secretory granules in a number of cell types in humans with the Chediak-Higashi syndrome and in “beige” (Lystbg/Lystbg) mice. Analysis of the secretory granules of mast cells and pancreatic acinar cells in Lyst-deficient beige mice suggests that beige mouse secretory granules retain the ability to fuse randomly with other secretory granules no matter what the size of the fusion partners. By contrast, in normal mice, the pattern of granule-granule fusion occurs exclusively by the addition of unit granules, either to each other or to larger granules. The normal pattern of fusion is termed unit addition and the fusion evident in cells with CHS/Lyst mutations is called random addition.
The proposed model of secretory granule formation has several implications. For example, in neurosecretory cells, the secretion of small amounts of cargo in granules constrained to a very narrow size increases the precision of the information conveyed by secretion. By contrast, in pancreatic acinar cells and mast cells, large granules composed of multiple unit granules permit the cells to store large amounts of material without requiring the amount of membrane necessary to package the same amount of cargo into small granules. In addition, the formation of mature secretory granules that are multimers of unit granules provides a mechanism for mixing in large granules the contents of unit granules which differ in their content of cargo.
Keywords: condensing vacuole, Golgi, Lyst, secretion, secretory granule
INTRODUCTION
Many cells, such as fibroblasts, plasma cells and hepatocytes, constitutively export specialized macromolecules directly from the Golgi within simple small vesicles [1-3]. Other cell types, whose function includes the regulated secretion of various products, utilize secretory organelles that are variously termed “secretory vesicles” or “secretory granules” (Fig. 1) and which have a complex life history. For simplicity, throughout this review we will use the term “secretory granules” or “granules” interchangeably to refer to these secretory organelles. Cells that package products destined for secretion within secretory granules include exocrine and endocrine cells, neurons, and hematopoietic cells. Many but not all such secretory granules have electron dense content, which may show little evident organization (Fig. 1A,C) or, in other instances, as in eosinophils [4] and human mast cells [5] can exhibit considerable structure. The contents of secretory granules range from proteins and proteoglycans to small molecules such as acetylcholine and histamine.
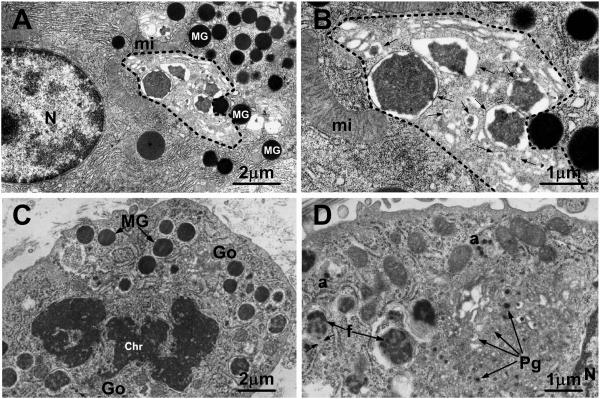
Fig. 1.
(A,B) Transmission electron micrographs of a pancreatic acinar cell from a mouse killed 8 h after pilocarpine administration to induce secretion of granules. Many mature granules (MG in A) are present in the cytoplasm outside of the progranule zone (indicated by dashed line), which is limited by the outermost of the Golgi cisternae, the mature granules, and the rough endoplasmic reticulum. Within the progranule zone are several membrane-delimited structures of various sizes (arrows in B), containing electron dense content that is more variegated in appearance than that in the mature granules; the smallest of these structures are interpreted as progranules and the larger ones may represent the products of fusion of progranules. mi mitochondrion; N nucleus. Bars: (A) 2 μm; (B) 1 μm. (A and B are reproduced in modified form from S. Lew, I. Hammel and S. J. Galli. Cell Tissue Res. 278: 327-336, 1994, with the permission of the publisher.) (C,D) Transmission electron micrographs of mast cells in the subcutaneous tissue of a 35 mm rat embryo (C) or a newborn rat (D). In (C), a mast cell in mitosis exhibits some mature secretory granules (MG) with homogeneously electron dense content. Chr chromatin. Numerous progranules (Pg in D) are present in the Golgi zone (Go in C). In (D), several progranules appear to have aggregated at “a” and larger structures (f), representing immature granules, appear to contain progranules in various stages of fusion; the arrows near f indicate the close association of the rough endoplasmic reticulum with one of the immature granules. N nucleus. Bars: (C) 2 μm; (D) 1 μm. (C & D are figures 3 & 4, reproduced in modified form from J. W. Combs. J Cell Biol. 31: 563-75, 1966, with the permission of the publisher.)
The now classical model of secretory granule formation holds that proteins are transported from the rough endoplasmic reticulum (RER) to the proximal or cis side of the Golgi cisternae, then through the Golgi to its opposite, trans-face [2,6-11]. While moving through the Golgi cisternae, the proteins undergo a number of modifications, and are ultimately packaged in transport vesicles or “progranules” with variably electron-dense content. Morphological evidence has indicated that progranules, the smallest of which in some cells have volumes 1-2 orders of magnitude smaller than that of their respective mature secretory granules (Fig. 1), can fuse to form structures which have been termed by various authors “condensing vacuoles” or “immature granules” [12-18]. Further modifications create the mature secretory granule [6,7,19-21].
The ultimate fate of the secretory granule is release of the granule contents from the cell, accomplished by fusion of the limiting membrane of the secretory granule with the plasma membrane. A specific site of such fusion, termed the porosome, has been characterized in a number of cell types [22]. Moreover, in some cell types, secretion of granule contents can occur by the transient docking and fusion of the granule membrane with the plasma membrane [22,23]. The period of time between secretory granule formation and release varies considerably depending on a variety of cell-specific factors. In the case of mast cells, granules may persist in the cytoplasm for days to months before being released by the cell [24]. By contrast, the life time of secretory zymogen granules of pancreatic acinar cells is typically a matter of hours in non-fasting animals [6,25].
Important features of the process of secretory granule formation were identified in the 1960s and early 1970s using a combination of transmission electron microscopy (EM) and EM radioautography. Perhaps because there was no question about the ultimate fate of secretory granules, little or no attention was paid to events in the life history of a secretory granule between its origin and its ultimate destiny at the plasma membrane. Furthermore, analyses of the size of secretory granules within a single cell type usually assumed a normal (Gaussian) distribution of granule size. This assumption, while useful for comparing secretory granules sizes among different cell types, was incorrect and, as we shall describe, interfered with the discovery of some interesting features of the life history of secretory granules.
The “Unit Granule” and evidence of granule-granule fusion
The notion that little happened to secretory granules between the time they were formed in the Golgi and secreted by the cell was challenged first on the basis of detailed morphometric analyses of secretory granules in mast cells [26-29] and subsequently in pancreatic acinar cells [17,30,31], as well as in several additional cell types [4,32,33]. The key assumption underlying these studies was that secretory granules are organelles which develop and then persist over a period of time, not structures whose important features can be appreciated simply by single measurements of their diameters. This implies that secretory granules should be studied in ways which permit accurate assessment of their volume rather than their diameter and, to the extent feasible, the volume of secretory granules should be studied over their lifetimes.
To characterize the true distribution of secretory granule size in rat mast cells, a computer-assisted morphometric approach was employed in which cross-sectional areas of granules were measured directly on randomly obtained transmission electron micrographs. In such a setting, most of the sections would not be truly equatorial and some would sample only a small part of the organelle (Fig. 2). The measured granule profile areas (ai) were then converted to their “equivalent volumes” (V1), where V1 = (4π/3)(ai/π)3/2 and the noise in the histogram of organelle equivalent volumes was smoothed by a moving bin of continuous averaging [26,27].
Fig. 2.
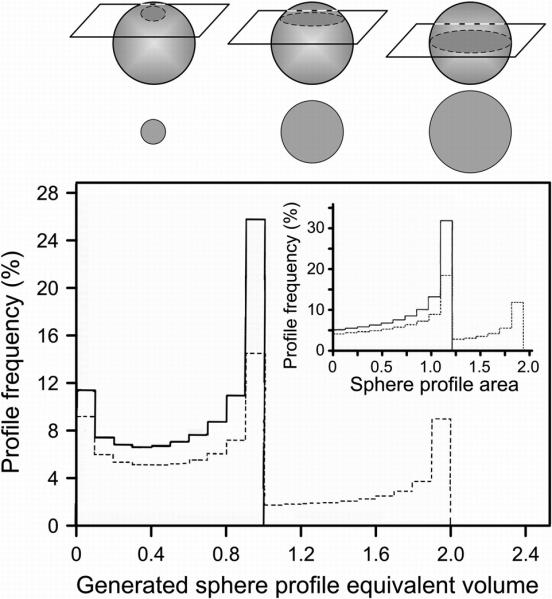
Model histograms of equivalent volumes calculated from (as shown in inset) cross section areas obtained by random sectioning of two populations of computer-generated spheres: a uniform population of spheres of 1-unit volume (solid lines) and a 1: 1 mixture of spheres of 1- and 2-unit volumes (dashed lines). The drawing above the histograms shows how the sectioning of spheres of identical volume will produce (left to right) small, intermediate or equatorial cross section areas, depending on the location of the section plan relative to the equator of the sphere. (Modified from I. Hammel, D. Lagunoff, M. Bauza, and E. Chi. Cell Tissue Res. 228: 51-59, 1983, with the permission of the publisher.)
The key assumption in this approach is that it is possible to identify the true size of the organelle of interest by an appropriate analysis of multiple cross-sectional areas of the structure, most of which are not true equatorial sections. This assumption was tested by computer simulation of the section areas which would be obtained from the random sectioning of a 1:1 mixture of perfect spheres of volumes of 1 and 2 unit volumes, converting these sphere profile areas into the equivalent sphere volumes, and plotting a histogram showing the frequencies of cross section areas and equivalent sphere volumes [27]. The peaks in this histogram identified correctly that the parent population of spheres was comprised of spheres of 1 and 2 unit volumes (Fig. 2). This method can be used not only for true spheres but also for ellipsoid structures with an axial ratio, defined as the largest diameter divided by the diameter perpendicular to the largest diameter, of < 1.3 [34].
This morphometric approach also depends on the ability of the moving bin method of continuous averaging of equivalent volumes to identify peaks in the distribution which closely approximate the true volumes of the structures sectioned [27,35]. In the initial study of rat peritoneal mast cells, the same peaks were identified in the moving bin histograms of granule equivalent volumes using three different bin sizes and based on data derived from two different total numbers of granules (Fig. 3). In each instance, the mean distance between the peaks in the distribution was roughly equivalent to the volume defined by the first peak.
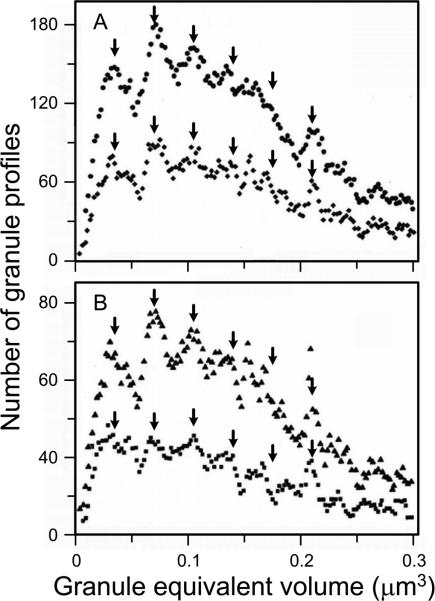
Fig. 3.
Moving-bin histogram of granule equivalent volumes calculated by using transmission electron micrographs to measure cross section areas of cytoplasmic granules in rat peritoneal mast cells. In (A), data for 2327 granules are presented using bin sizes of 0.0036 μm3 (μ3) (upper curve) and 0.0072 μ3 (lower curve). In (B), a bin size of 0.0045 μ3 is used to compare data obtained from 2327 granules (upper curve) vs. the first 1050 granules measured (lower curve). The arrows (V1, V2, V3, etc.) are placed at integral multiples of the mean intermodal distance calculated for each curve, a value that defines the magnitude of the “unit volume”. (Modified from I. Hammel, D. Lagunoff, M. Bauza, and E. Chi. Cell Tissue Res. 228: 51-59, 1983, with the permission of the publisher.)
These results showed that mature secretory granules in mast cells have a periodic, multimodal volume distribution and that the successive modes are integral multiples of the smallest volume identified. Secretory granules of this smallest volume class were termed “unit granules” and their volume was designated “V1” whereas those with volumes two, three or four times that of the unit granule were designated “V2”, “V3” and “V4”, respectively (Fig. 3). Application of this approach to mature secretory granules of rat [27,36-38], mouse [39], mole rat [40] and human [5,28] mast cells, mouse and rat pancreatic acinar cells [30,31,39], rat eosinophils [4], and rat neuroendocrine cells [32,33], demonstrated that this pattern of the distribution of sizes of mature secretory granules was common to each of these different secretory cells.
These findings support the hypothesis that the secretory granules of these cell types, after initial formation as unit granules, undergo fusion within the cell prior to secretion, resulting in the other size classes of secretory granules [27,31]. The alternative explanation compatible with the results of the morphometric analysis is that secretory granules of different size classes, which vary as multiples of the unit granule volume, are produced by the Golgi. This alternative hypothesis was less appealing conceptually, inconsistent with the sizes of vesicles within the Golgi area, and less compatible with the results of subsequent pulse labeling studies of secretory granules (see below).
Although multiple peaks had previously been seen in frequency distributions of secretory granule size [41-46], the presentation of data for diameters rather than equivalent volumes obscured the relationships among the peaks. Similarly, demonstration of the quantal release of acetylcholine at the neuromuscular junction [47] provided evidence for a non-continuous variation in the release of acetylcholine but was unable to distinguish between the simultaneous release of multiple vesicles of a fixed small size and the release of vesicles of varying size formed by fusion of unit granules prior to secretion. The use of equivalent volumes, and the improved covariances resulting from using areas rather than diameters to calculate the volumes, permitted the identification of the periodic character of the multimodal distributions.
Several lines of evidence, in addition to morphometry performed on specimens examined by conventional EM, support the basic hypothesis that secretory granule size distribution in a given cell type reflects the fusion of unit granules over time prior to their secretion. Measurements of the enzyme content of individual secretory granules in pancreatic acinar cells indicate that enzyme content varies roughly as a multiple of the smallest content observed [48]. Data derived from measurements of time-resolved patch-clamp capacitance of the plasma membrane [49] confirmed the multimodal distribution of the sizes of secreted granules in rat mast cells. In the patch-clamp studies, histograms of the sizes of the step increases in plasma membrane surface area, reflecting the addition of perigranule membrane of secretory granules to the plasma membrane during exocytosis of mast cell secretory granules, showed at least four peaks where the spacing between peaks averaged 1.3 ± 0.4 μm2. Similar results were documented in patch clamp studies of human and horse eosinophils [50]. Finally, additional evidence that mature secretory granules have volumes which are different multiples of the smallest size class was provided by direct measurements of the secretory granules in mouse pancreatic acinar cells by electron microscopy of “wet” glutaraldehyde-fixed specimens [51]. The latter study eliminated the unlikely possibility that multimodality was the result of the post-fixation preparation of the tissue for conventional transmission EM.
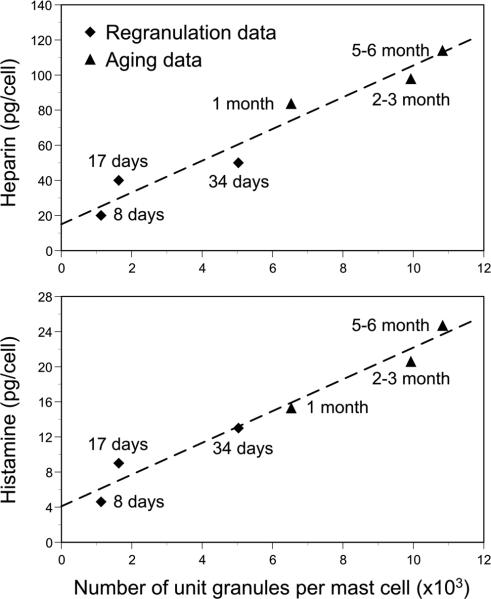
One prediction of the model of granule enlargement by granule-granule fusion in cells in which unit granules are continually being produced is that in cell types which retain their granules for long periods of time, the size of secretory granules will increase with the age of the animals. In mast cells, granules are thought largely to be retained within the cell until it receives a “degranulation signal”, such as challenge with IgE and specific antigen or by some other agonist of mast cell degranulation [52-54]. Analysis of peritoneal mast cells in rats of different ages showed that the unit granule volume remained constant over time but the mean size of the mature secretory granules and the total mass of granules per cell progressively increased [40]. Moreover, there was a close correlation between the progressive increase in the total granule volume of a cell over time and the cell's content of the granule-stored mediators, histamine and heparin (Fig. 4, “Aging data”) [36,55]. A similar close correlation between the number of unit granules per mast cell and the content of histamine and heparin was observed by in rats sacrificed for analysis of peritoneal mast cells by transmission EM 8, 17 or 34 days after the mast cells were induced to degranulate in vivo by 4 injections (i.p.) of polymyxin B (Fig. 4, “Regranulation data”) [37,56].
Fig. 4.
Heparin (upper panel) and histamine (lower panel) content of rat peritoneal mast cells as a function of number of unit granules per mast cell. “Regranulation data” (indicated by solid diamonds) were derived from rats sacrificed for analysis of peritoneal mast cells by transmission electron microscopy 8, 17 or 34 days after the mast cells were induced to degranulate in vivo by 4 injections (i.p.) of polymyxin B (mg/kg body weight of 2.8 on day 1, 5.7 and days 2 and 3 and 12.0 and day 4). “Aging data” (indicated by solid triangles) were derived from control rats (not injected with mast cell activating agents) sacrificed for analysis of peritoneal mast cells by transmission electron microscopy at 1, 2-3 or 5-6 months of age. (Data in upper panel are from P. G. Krüger and D. Lagunoff. Int Arch Allergy Appl Immunol. 65: 291-299, 1981a and P. G. Krüger and D. Lagunoff. Int Arch Allergy Appl Immunol. 65: 278-290, 1981b, with the permission of the publisher: S. Karger, Basel; figure in lower panel is reproduced from I. Hammel, D. Lagunoff, and P. G. Krüger. Exp Cell Res. 184: 518-523, 1989, with the permission of the publisher.)
The simplest and, we suggest, most likely explanation of these observations is that the Golgi continuously produces unit granules whose size and content are tightly constrained and largely constant over the lifetime of the cell whereas, over time, the progressive fusion of these unit granules results in larger granules. These processes thus are responsible for both an increased mass of granules, and an increased content of histamine, heparin and, presumably, other mediators which are stored in the granules.
Evidence for two models of secretory granule formation, unit addition and random fusion
The Monte Carlo method can be used to predict the distribution of secretory granule sizes which would be observed in two possible models of granule-granule fusion, analogous to “addition” and “condensation” models of polymerization [57,58]: unit addition, in which the only fusogenic secretory granule is the unit granule, which can fuse with existing secretory granules of any size; and 2) random addition, in which secretory granule fusion can occur between granules of any size. Unit addition results in a distribution of secretory granule sizes in which some multiple of the unit granule size is the most frequent size. In the random addition model, the most frequent volume in the size distribution will be the unit granule. Furthermore, very large granules will occur more frequently in the random addition model than in the unit addition model, since in the former case large granules can fuse with other large granules. The correspondence of the distributions of the actual measurements with the two theoretical distributions can be assessed using a standard statistical method, the Kolmogorov-Smirnov test [31,37].
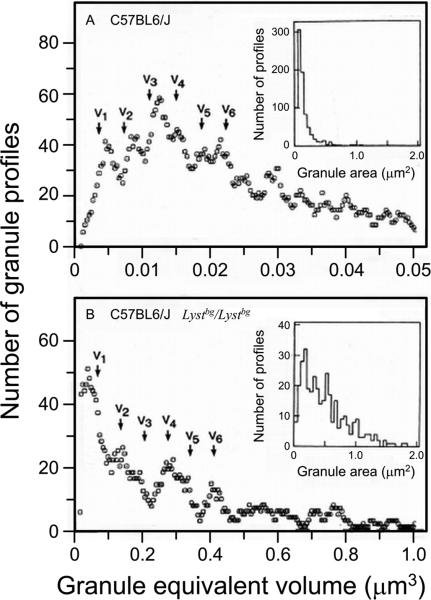
Beige mice have a genetic syndrome characterized by unusually large secretory granules within mast cells (Fig. 5) and certain other cell types [59-62]. When mast cell secretory granules were analyzed in beige versus the corresponding wild type mice, the size distribution of secretory granules in wild type mast cells conformed to that expected if the granule-granule fusion was by unit addition (Fig. 6A). By contrast, in beige mice, the secretory granule size distribution conformed to the prediction of a random patter of granule fusion (Fig. 6B). The same was true for the analysis of the size distributions of pancreatic acinar cell secretory granules in wild type versus beige mice, although the differences between the secretory granules of beige and wild type pancreatic acinar cells were not as obvious upon visual inspection of the transmission electron micrographs of these cells as they were in the case of mast cells [39]. As noted above, mast cells can retain their secretory granules for weeks to months, whereas pancreatic acinar cells typically secrete their granules in a matter of hours. Since mast cell secretory granules reside in the cell for much longer periods prior to secretion than do acinar cell granules, mast cell granules have more time to undergo fusion prior to secretion, with the consequent generation of extremely large granules in such cells in the mutant animals.
Fig. 5.
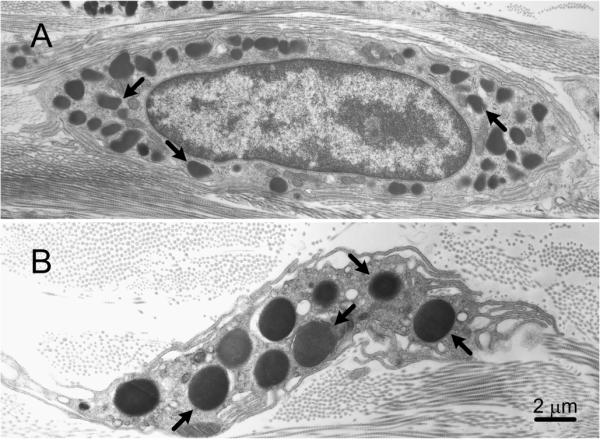
Dermal mast cells from (A) a C57BL/6 wild type mouse and (B) a C57BL/6-Lystbg/Lystbg (“beige”) mouse. The secretory granules (some indicated by arrows) in the beige mouse mast cell are much larger and fewer in number than those in the wild type mouse mast cell. Bar (for A and B): 2 μm.
Fig. 6.
Moving bin histograms demonstrating periodic, multimodal distributions of control (A) or beige (B) mouse dermal mast cells granule equivalent volumes. The arrows (V1, V2, V3, etc.) are placed at integral multiples of the mean intermodal distance, a value that defines the magnitude of the “unit volume”. Insets show the distribution of the measured granule cross-sectioned areas. In (A), 826 granules (in 24 mast cells) were analyzed with a bin size of 0.0021 μm3 moved every 0.0003 μm3. In (B), 344 granules (in 28 mast cells) were analyzed with a bin size of 0.035 μm3 moved every 0.005 μm3. Note the difference in the volume ordinates in (A) and (B). (Reproduced from I. Hammel, A. M. Dvorak and S. J. Galli. Lab Invest. 56: 321-328, 1987, with the permission of the publisher.)
The mutation in beige mice is a null allele of Lyst (lysosomal trafficking regulator [63] and the ortholog of the CHS (Chediak-Higashi Syndrome) gene in humans [64,65]. Lyst, a cytoplasmic protein of ~ 424-kD, is the founding member of the family of BEACH proteins, named because they contain a BEACH (BEige And CHediak) domain, which includes the amino acid sequence WIDL [66]. Lyst initially was thought to be cytosolic protein, since the predicted protein sequence lacks an obvious transmembrane domain or a signal sequence [66]. However, it appears very likely that Lyst requires interactions with membranes, presumably mediated via interactions with other proteins, to perform its physiological roles [67].
LvsB is the ortholog of Lyst in the amoeba Dictyostelium discoideum. In this species, GFP-labeled LvsB was localized to postlysosomes, secretory organelles that eventually fuse with the plasma membrane and release their contents [68,69]. Functional evaluation in LvsB-null amoebae revealed that the loss of LvsB resulted in inappropriate heterotypic fusion of different intracellular compartments [69]. Notably, LvsB null cells exhibited both enlarged acid lysosomes and very large postlysosomes [69]. The authors concluded that Lyst proteins act as negative regulators of fusion and suggested that Lyst may provide specificity for endosomal fusion [69]. In a contradictory study analyzing a lvsB knock-out in Dictyostelium, Charette and Cosson [70] found that a lack of LvsB resulted in a slowing of the maturation of lysosomes into fusion-competent postlysosomes and a reduction in the number and in the secretion of postlysosomes, but the small increase in the size of the postlysosomes, measured by confocal microscopy, was not significant.
The reason for the differences in the results obtained in the studies by Kypri et al., [69] and Charette and Cosson [70] is not clear. However, the morphometric data derived from the studies of the secretory granules of mast cells and pancreatic acinar cells in beige (Lystbg/Lystbg) versus wild type mice, namely, the very large size of the granules in the mutant mice and the evidence that the Lystbg/Lystbg granules of any size retain the ability to fuse with other granules, suggest that Lyst is a negative regulator of granule-granule fusion in mice. The fact that the size of the unit granule in beige mouse mast cells is ~ 18 fold that in wild type mast cells [39] further suggests that Lyst also may negatively regulate fusional events in the formation of the unit granule (see below).
How Lyst performs such functions is not yet understood. Using a yeast two-hybrid screen, followed by an in vitro binding assay, Tchernev et al., [67] provided evidence that human Lyst can interact with proteins that regulate vesicle docking and fusion during exocytosis, including 14-3-3, casein kinase II, and the soluble NSF attachment protein (i.e., SNAP) receptor (SNARE) complex protein HRS. Grimberg et al., [71], using rat basophilic leukemia (RBL) cells, showed that RBL cells lacking synaptotagmin III, which can participate in interactions involving SNARE complex proteins, exhibited unit granules of normal size but an increased number of very large secretory granules. The authors interpreted this finding as suggesting that such cells might have a defect in the removal and recycling of proteins from immature granules during the process of granule maturation. However, the definitive molecular mechanisms which explain why the Lyst protein is so critical for the physiologic unit addition model of granule-granule fusion, and how its absence results in the striking abnormalities of structure and function observed in the cells of beige mice, remain to be identified.
Formation of unit granules: progranule fusion, immature granule maturation, and membrane conservation
The evidence reviewed above indicates that unit granules exist and that, in mast cells, pancreatic acinar cells, and other cell types, the populations of mature secretory granules in individual cells are comprised of unit granules and larger granules whose volumes are multiples of unit granules. The phenotypic effects of Lyst mutations suggest that Lyst participates in a mechanism that controls granule-granule fusion by restricting the fusion mechanism to unit granules. But what is the mechanism that controls the volume of the unit granule? Lyst appears to be involved in this process, in that unit granules in beige (Lystbg/Lystbg) mouse mast cells are ~ 18 fold the volume of those in wild type mast cells [39]. However, the process that results in the formation of unit granules is likely to be quite complex. The first stage of the process is the production and fusion of many progranules (> 100 in the case of mouse pancreatic acinar cells [17]) to form a structure termed by various authors a “condensing vacuole” [7,72] or an “immature granule” [14,15,17,18,73]. Second, in many cell types, there is also a significant reduction of the volume and, in some cases, a reorganization of the contents, of the condensing vacuole/immature granule, in a process that has been termed “condensation” [6,7,19-21]. In some instances, condensation includes formation of amyloid-like aggregates [74]. Finally, there must be a balancing of the progranule fusion and condensation processes to achieve a mature unit granule whose volume is very narrowly constrained, e.g., the coefficients of variation of rat and mouse pancreatic acinar cell unit granules are < 0.2 and in the range of 0.08-0.16, respectively [31,51].
Arguably the most likely points of control in this process are the production by the Golgi of progranules of relatively uniform size and the regulation, by either a “counter” or a “timer”, of the number of such uniform progranules that fuse to form an immature unit granule. Using data drawn primarily from the analysis of these processes in rat and mouse mast cells and mouse pancreatic acinar cells, we will comment on two aspects of the production of unit granules: progranule fusion and immature granule maturation and the accompanying process of membrane conservation.
Progranule fusion and immature granule maturation
In mast cells, ultrastructural observations [26,27,36,38,39,55,56,73,75] suggest that mast cell secretory granules are derived from the coalescence of numerous progranules with small volumes (1.8 ×10−4-5.2×10−4 μm3 in rat mast cells) yielding an immature granule with heterogeneous content. In rat mast cells, such immature granules have a volume of ~ 0.065 μm3, so that each mature granule formed from a single immature granule must be derived from the fusion of at least 100 progranules. EM observations suggest that a similar process also occurs during the formation of immature granules in pancreatic acinar cells [25,29,31,39], eosinophils [76] and other cell types [7]. In this review, we use the term “immature granule” to refer to structures in pancreatic acinar cells which are found outside of the progranule zone, that is defined as outside the area delimited by the outermost of the Golgi cisternae, the mature granules, and the rough endoplasmic reticulum [17], but which lack the homogenously electron dense content characteristic of the mature granules in this cell type [17]. Morphometric analysis of mouse pancreatic acinar cells demonstrated that the smallest progranules observed in the progranule zone following secretion induced by pilocarpine had a volume of 0.0094 ± 0.0002 μm3 [17]. This measurement indicated that the immature granules identified in this cell type, that have a mean volume of 1.0-1.2 μm3, would require fusion of 106-128 of progranules, assuming that the size of the immature granule was determined solely by the fusion of progranules [17].
In many cell types, including pancreatic acinar cells [6,72] and mouse and rat mast cells [12,55,56,73,77], the immature granules contain variable mixtures of electron dense and electron lucent content (Fig. 1B) and, in some cell types, membrane vesicles, whereas the mature secretory granules appear homogeneously electron-dense (Figures 1A,C and 5). In mouse pancreatic acinar cells, morphometric data indicated that the unit mature granule had a volume of 0.13 μm3 compared to 1.0-1.2 μm3 for the unit immature granule, representing a “condensation factor” in the maturation of an immature granule into a mature unit granule (i.e., ratio of immature granule volume to mature unit granule volume) of between 7.7:1 and 9.2:1 [17]. The notion that a process of condensation can result in a reduction of granule volume without substantial change in the protein content of the granule is supported by the work of Goncz et al., [78] who estimated the protein content of individual CVs and mature zymogen granules in rat pancreatic acinar cells by X-ray microscopy.
Pulse labeling of mouse pancreatic acinar cells with 3H-glycine also supports the conclusion that immature granules undergo a progressive reduction in size during their maturation [25]. In these experiments, the 3H label was preferentially located in condensing vacuoles/immature granules at early intervals after injection of 3H-glycine and in mature zymogen granules at later intervals, and the mean volume of 3H-labelled granules diminished over the 6 hour period of study. However, by only two hours after injection of 3H-glycine, the size distributions of the 3H-labeled granules and the mature non-labeled granules were quite similar. These data support the assumption of Caro and Palade [79] that the condensing vacuoles that are formed by the fusion of progranules in pancreatic acinar cells undergo a size reduction upon maturation.
Comparing the proportion of granule profile areas that bore 3H-label at 1, 2 or 6 hours after administration of 3H-glycine to wild type versus beige (Lystbg/Lystbg) mice showed that the percentage of granules that were 3H-labelled increased progressively in either case [25]. However, at each interval tested, the proportion of 3H-labelled granules was substantially higher in the pancreatic acinar cells of beige than wild type mice; values for beige mice were ~4.8-, 4.6-, and 1.8-fold higher than those for wild type mice at 1, 2 and 6 hours after administration of 3H-glycine [25]. Because beige mice had only ~ 39% fewer granules per pancreatic acinar cell than wild type mice, we think it unlikely that this finding reflects solely the distribution of the same amount of 3H-label to a smaller number of granules. Rather, we think that the findings are better explained as another consequence of the normal “unit addition” mechanism of granule-granule fusion which is observed in wild type mice being replaced, in beige mice, by a “random fusion” model, as the latter pattern of granule enlargement would result in a faster distribution of 3H-label to a higher proportion of the total zymogen granule population than the former.
Secretory granule development has also been analyzed in mast cells following the extensive induced secretion of their granules. After degranulation in vivo, mast cells replenish their secretory granules in a prolonged process with several morphologic phases, resulting in mast cells of varying appearance and granule content [5,37,56]. In rat peritoneal mast cells, the mast cells identified immediately following induced secretion exhibited a substantial decrease in cell volume associated with granule loss. One month later, the total number of granules had returned to baseline but the total volume of granules had not returned to the original value (Fig. 4, “Regranulation data”) [37].
Moreover, after degranulation in vivo, rat peritoneal mast cell immature granules with heterogeneous content by EM were multimodally distributed with a unit granule size of 0.083 μm3. By contrast, one month following mast cell activation, the mature granules were homogeneous in content by EM and exhibited a unit granule volume of 0.048-0.052 μm3. The unit granule size of rat mast cells one month after degranulation thus was similar to that of normal (i.e., non-degranulated) adult rat mast cells (i.e., 0.044 μm3 in 3 month old rats examined at baseline before mast cell activation) but about 40% less than the unit granule volume calculated for the immature granules observed soon after degranulation [37].
The reduction in the calculated volume of the unit granule associated with granule maturation, and the fact that immature granules with heterogeneous content by EM were multimodally distributed, suggests that in rat mast cells, immature granules may be able to undergo fusion even before the process of granule condensation is complete, at least under certain circumstances. This conclusion is consistent with morphometric [4,32], morphological [80-83], fluorescence imaging [84,85], biochemical [19] and patch-clamp [86] analyses which also have suggested that, in at least some cell types, secretory granules with the appearance of immature granules can fuse with other granules and/or the plasma membrane.
It should be emphasized that not all immature secretory granules undergo a reduction in volume as they develop into mature granules. For example, studies of rat bone marrow and peritoneal eosinophils [4] and of melanotrophic cells of the intermediate lobe of rat pituitary [32] suggest that morphologically immature granules are able to fuse but that the morphological changes which occur during granule maturation in these cells types are not associated with a reduction in the volume of the maturing granule. Thus, the changes observed during the maturation of secretory granules in these cell types appear to reflect mainly intragranule processes that occur independently of a significant change in granule volume.
Membrane conservation
Several lines of evidence indicate that, as one would expect based on simple geometry, the formation of immature granules from multiple small progranules, and the subsequent maturation of the granule, can result in substantial conservation of the membranes of these structures. Such membrane conservation would be particularly impressive in those instances in which granule maturation is associated with a marked reduction in the granule's volume. A consideration of the mechanisms that can contribute to such membrane conservation during the life cycle of secretory granules in different cell types, and how these mechanisms also may regulate the final composition of mature secretory granule membranes, is beyond the scope of this review but has been discussed in detail by others [8,20,21].
However, we do wish to call attention to three aspects of this process that seem relevant to the model of secretory granule formation and enlargement reviewed herein. First, this process will result in considerable conservation of the amount of membrane needed to package the cargo of secretory cells. Assuming that the surfaces of progranules and mature granules are smooth, morphometric data from studies of mouse pancreatic acinar cells indicate that the formation of a single mature unit granule (volume = 0.13 μm3) by the fusion of 106-128 progranules of the smallest size class (volume = 0.0094 μm3) will be associated with a conservation of ~ 95% of the membrane surface area required to produce the initial population of progranules [17].
Second, as reviewed extensively by others [20,21], membrane conservation may also substantially alter the composition and therefore the functional properties of the secretory granule membrane. One consequence of such selective modification of the surface membrane of secretory granules may be to regulate their ability to fuse with other granules, as well as with the plasma membrane.
Finally, whatever the details of the processes of membrane conservation and selective alteration of the composition of secretory granule surface membranes that occur during the life cycle of the mature secretory granule, these processes are compatible with the development and stable existence in the cell of a population of mature secretory granules which have volumes that represent multiples of the volume of the unit granule.
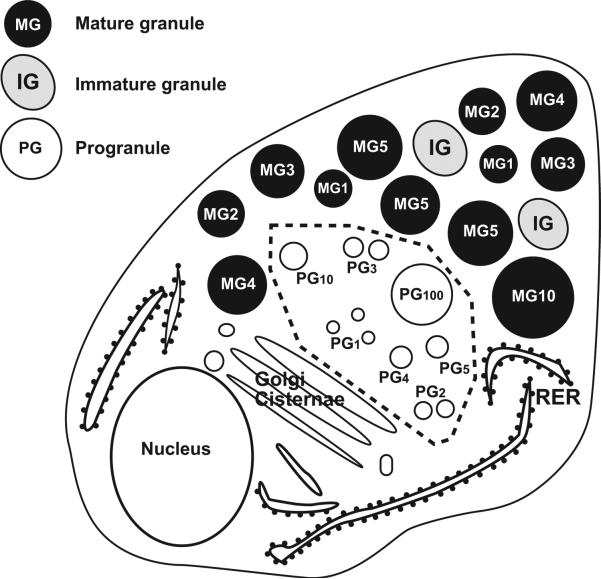
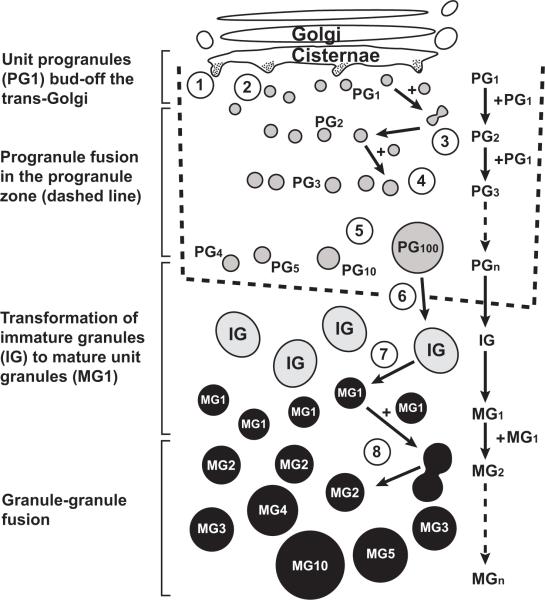
Figures 7 and 8 schematically depict our model of secretory granule formation and enlargement in mouse pancreatic acinar cells.
Fig. 7.
Diagram of a pancreatic acinar cell illustrating subcellular landmarks and ultrastructural criteria used for morphometric analysis. The progranule zone (-----) is limited by the outermost of the Golgi cisternae, the mature granules (MGs), and the rough endoplasmic reticulum (RER). Note that some profiles classified by these criteria as products of fused progranules (PGs) can appear identical in ultrastructure to some profiles classified as “immature granules” (IGs) because the distinction is based on whether the structure is in the progranule zone (the unit progranules and products of fused progranules) or outside this zone (the immature granules). The numbers associated with unit progranules (PG1), structures interpreted as products of fused progranules (PG2-100), or mature granules (MG1-10) indicate the sizes of the structures in equivalent granule unit volumes, with all of these structures illustrated as if the plane of section was through the equator of the structures. Thus, a MG2 would have a volume twice that of an MG1. (Reproduced from S. Lew, I. Hammel and S. J. Galli. Cell Tissue Res. 278: 327-336, 1994, with the permission of the publisher.)
Fig. 8.
Diagram of the formation of mature secretory granules in pancreatic acinar cells. 1-5: Formation of immature granules (IGs) in the progranule zone of the Golgi region (the progranule zone is that area limited by the outermost Golgi cisternae, the rough endoplasmic reticulum, and the mature secretory granules (MGs)). (1) Formation of a “unit progranule” (PG1) at the Golgi cisterna. (2) Separation of a “unit progranule” from Golgi cisterna. (3) Fusion of two unit progranules to form a structure comprised of the volume and contents of two unit progranules (PG1 + PG1 forms PG2). (4) Formation of a PG3 by fusion of a PG2 and a PG1. (5) Formation of progressively larger structures that represent fused progranules (PGn). (6) Passage of structures comprised of many fused progranules outside of the progranule zone, changing the operational designation of this structure to “immature secretory granule” (IG). The individual unit progranules and structures comprised of fused progranules are drawn as if the plane of section was through the equator of the structures and so that their diameters are in correct proportion for structures whose volumes vary by the integral units depicted. Note that the addition of a quantal unit of volume (particularly to a large structure comprised of multiple fused progranules) produces a change in the two-dimensional appearance of the structure which appears relatively modest by visual inspection. (7) “Condensation” of immature granule, outside of the progranule zone, to form a mature “unit” secretory granule (MG1) with homogeneously electron dense content. (8) Progressive aggregation of mature unit granules to each other (MG1 + MG1 forms MG2) or to larger mature granules (e.g., MG1 + MG4 forms MG5 [not shown]). The individual mature granules are drawn so that their diameters are in correct proportion for structures whose volumes vary by the integral units depicted. In this figure, only mature unit granules are shown as being able to fuse with other mature granules. However, in some cell types, there is evidence that immature granules (i.e., immature unit granules) may be able to fuse even before the process of granule maturation is complete (see text). (Reproduced from S. Lew, I. Hammel and S. J. Galli. Cell Tissue Res. 278: 327-336, 1994, with the permission of the publisher.)
Functional implications
Are there any advantages for cells to secrete granules of a tightly constrained minimal size (the unit granule), or, for cells in which there is a period of post-Golgi granule-granule fusion, for storing and secreting granule that vary in size as integral multiples of that minimal size? We think that there are several possibilities. In some cells, such as neurosecretory cells, most granules appear to be very small (volume < 0.004 μm3) and are comprised of a single unit granule, and each granule typically contains a single secretory product (e.g., a single neurotransmitter) [87-90]. The maintenance of a population of granules that is composed solely or primarily of very small granules may allow more precise control of neurotransmitter secretion than could be achieved if multiple size classes of granules were present.
By contrast, certain neurohormone- or hormone- secreting cells contain small granules of a wider range of sizes (volume = 0.004-0.065 μm3) and the individual granules are comprised of one to a few unit granules [32,33]. This may facilitate the simultaneous secretion of a single type of small peptide hormone together with proportional amounts of other secretory products which can have autocrine, paracrine or systemic effects; in such cells, there also would be some membrane conservation during formation of mature granules.
Finally, cells such as pancreatic acinar cells and mast cells typically contain large granules (volume > 0.065 μm3), most of which are comprised of multiple unit granules [27,31,39] and which contain hydrolytic enzymes and many other biologically active compounds. This permits short to long term storage of cargo and then the simultaneous secretion of large amounts of enzymes and other products. In such cells, there also would be extensive membrane conservation during the assembly of the cells’ complement of mature granules, due to the formation of mature granules.
We propose that, in general, the discrete secretion of small amounts of cargo packed into small granules constrained to a very narrow size increases the precision of the information conveyed by secretion. By contrast, large granules composed of multiple unit granules can store a large amount of material in the cell cytoplasm without requiring the amount of membrane that would be required to pack the same amount of cargo into smaller granules. In addition, the formation and storage of mature secretory granules that are multimers of unit granules provides a potential mechanism for mixing in large granules the contents of unit granules which differ in their content of cargo. For example, a particular cell type may produce unit granules which vary in content based on varying micro-environmental cues. When such granules undergo unit addition to the existing complement of granules, the new content then is packaged with the old, ready for secretion together upon appropriate stimulation of the cell.
One can attempt to investigate some of the proposed advantages listed above by comparing particular physiological responses which might be influenced by aspects of our model of secretory granule formation in wild type mice and in mice with mutations that alter either the volume of the unit granule, its coefficient of variation, or the pattern of granule-granule fusion (e.g., resulting in unit addition versus random patterns of fusion). However, this is easier said than done. For example, Lystbg/Lystbg mice develop giant secretory granules and appear to exhibit a random mechanism of granule-granule fusion [39], as well as many other phenotypic abnormalities [66]. Yet it may be challenging to prove to what extent the diverse phenotypic abnormalities in beige mice are due to the effects of a lack of Lyst on secretory granule size or pattern of fusion per se, as opposed to other direct or indirect consequences of the Lyst deficiency.
Conclusions and future directions
The evidence now seems compelling that the model of secretory granule enlargement reviewed herein is correct, and is common not only to mast cells and pancreatic acinar cells but to many different cell types and species of organisms. This model was initially proposed based on morphological and morphometric evidence derived from transmission electron micrographs of rat peritoneal mast cells. However, it is now supported by the results of patch clamp measurements performed in living secretory cells, biochemical assessment of the contents of secretory granules, and measurements of wet preparations of secretory cells examined by electron microscopy.
While the evidence in support of the proposed model is strong, the molecular mechanisms which account for the key features of the model largely remain to be defined. Important questions to be addressed include why it is only (or primarily) the unit immature or mature granules that are fusogenic with other granules of any size class, and why a deficiency in Lyst results in giant secretory granules and a random pattern of granule-granule fusion. In addition to elucidating the functions of Lyst in this model, it will also be of interest to examine to what extent the processes of unit granule formation and granule-granule aggregation and fusion are altered by abnormalities in other molecules which regulate vesicle trafficking and/or the formation of secretory granules, including the adaptor protein-3 (AP-3) complex [91-97] protein tyrosine phosphatase MEG2 (PTP-MEG) [98], and molecules involved in the Rab cycle [99], as well as particular SNAREs and SNAPs [62,67,100-104].
In particular, it will be of interest to search for similarities and differences in the granule-granule fusion processes that result in the formation of mature secretory granules in exocrine cells, such as pancreatic acinar cells, and hematopoietic cells, such as mast cells and eosinophils, as opposed to endocrine or neuroendocrine cells. Both morphological and biochemical evidence supports the occurrence of homotypic fusion of immature secretory granules as part of the secretory granule maturation pathway in the regulated secretory program of neuroendocrine cells, and there also is evidence that this pathway is distinct from that leading to the formation of vesicles which mediate constitutive secretion in the same cells [2,105]. The model described in this review pertains to the generation and enlargement of granules which participate in regulated secretion, and it will be important to analyze the relevance of this model, if any, to the constitutive pathway of cell secretion.
It will be challenging to elucidate how maintaining tight control over the size of the unit granule in individual cell types, and sustaining a unit addition model of granule-granule fusion, confer physiologic benefits and therefore a selective advantage. However, such knowledge not only will provide new insights into the importance of physiological mechanisms of secretory granule formation and enlargement, but also may point to additional and perhaps therapeutically accessible consequences of the abnormalities caused by mutations that substantially perturb the normal processes.
ACKNOWLEDGEMENTS
We are grateful to the many colleagues who have participated in the work described in this review, especially Ann Dvorak, who collaborated on several of the early studies reviewed here. This work was supported by NIH grants AI23990, AI070813 and CA72074 (to S.J.G.).
REFERENCES
- 1.Tartakoff A, Vassalli P, Detraz M. Comparative studies of intracellular transport of secretory proteins. J Cell Biol. 1978;79:694–707. doi: 10.1083/jcb.79.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lippincott-Schwartz J, Roberts TH, Hirschberg K. Secretory protein trafficking and organelle dynamics in living cells. Annu Rev Cell Dev Biol. 2000;16:557–89. doi: 10.1146/annurev.cellbio.16.1.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polishchuk EV, Di Pentima A, Luini A, et al. Mechanism of constitutive export from the Golgi: bulk flow via the formation, protrusion, and en bloc cleavage of large trans-Golgi network tubular domains. Mol Biol Cell. 2003;14:4470–85. doi: 10.1091/mbc.E03-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elmalek M, Hammel I. Morphometric evidence that the maturation of the eosinophil granules is independent of volume change. J Submicrosc Cytol. 1987;19:265–8. [PubMed] [Google Scholar]
- 5.Dvorak AM, Hammel I, Schulman ES, et al. Differences in the behavior of cytoplasmic granules and lipid bodies during human lung mast cell degranulation. J Cell Biol. 1984;99:1678–87. doi: 10.1083/jcb.99.5.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Case RM. Synthesis, intracellular transport and discharge of exportable proteins in the pancreatic acinar cell and other cells. Biol Rev Camb Philos Soc. 1978;53:211–354. doi: 10.1111/j.1469-185x.1978.tb01437.x. [DOI] [PubMed] [Google Scholar]
- 7.Farquhar MG, Palade GE. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981;91:77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tooze SA. Biogenesis of secretory granules. Implications arising from the immature secretory granule in the regulated pathway of secretion. FEBS Lett. 1991;285:220–4. doi: 10.1016/0014-5793(91)80805-d. [DOI] [PubMed] [Google Scholar]
- 9.Borgonovo B, Ouwendijk J, Solimena M. Biogenesis of secretory granules. Curr Opin Cell Biol. 2006;18:365–70. doi: 10.1016/j.ceb.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Raposo G, Marks MS, Cutler DF. Lysosome-related organelles: driving post-Golgi compartments into specialisation. Curr Opin Cell Biol. 2007;19:394–401. doi: 10.1016/j.ceb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JJ, Koshimizu H, Loh YP. Biogenesis and transport of secretory granules to release site in neuroendocrine cells. J Mol Neurosci. 2009;37:151–9. doi: 10.1007/s12031-008-9098-y. [DOI] [PubMed] [Google Scholar]
- 12.Combs JW, Lagunoff D, Benditt EP. Differentiation and proliferation of embryonic mast cells of the rat. J Cell Biol. 1965;25:577–92. doi: 10.1083/jcb.25.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sesso A, Assis JE, Kuwajima VY, et al. Freeze-fracture and thin-section study of condensing vacuoles in rat pancreatic acinar cells. Acta Anat (Basel) 1980;108:521–39. doi: 10.1159/000145351. [DOI] [PubMed] [Google Scholar]
- 14.Laurie GW, Leblond CP, Martin GR. Intracellular localization of basement membrane precursors in the endodermal cells of the rat parietal yolk sac. II. Immunostaining for type IV collagen and its precursors. J Histochem Cytochem. 1982;30:983–90. doi: 10.1177/30.10.6752264. [DOI] [PubMed] [Google Scholar]
- 15.Clermont Y, Rambourg A, Hermo L. Segregation of secretory material in all elements of the Golgi apparatus in principal epithelial cells of the rat seminal vesicle. Anat Rec. 1992;232:349–58. doi: 10.1002/ar.1092320304. [DOI] [PubMed] [Google Scholar]
- 16.Clermont Y, Xia L, Rambourg A, et al. Transport of casein submicelles and formation of secretion granules in the Golgi apparatus of epithelial cells of the lactating mammary gland of the rat. Anat Rec. 1993;235:363–73. doi: 10.1002/ar.1092350305. [DOI] [PubMed] [Google Scholar]
- 17.Lew S, Hammel I, Galli SJ. Cytoplasmic granule formation in mouse pancreatic acinar cells. Evidence for formation of immature granules (condensing vacuoles) by aggregation and fusion of progranules of unit size, and for reductions in membrane surface area and immature granule volume during granule maturation. Cell Tissue Res. 1994;278:327–36. doi: 10.1007/BF00414176. [DOI] [PubMed] [Google Scholar]
- 18.Karam S, Leblond CP. Origin and migratory pathways of the eleven epithelial cell types present in the body of the mouse stomach. Microsc Res Tech. 1995;31:193–214. doi: 10.1002/jemt.1070310304. [DOI] [PubMed] [Google Scholar]
- 19.Tooze SA, Flatmark T, Tooze J, et al. Characterization of the immature secretory granule, an intermediate in granule biogenesis. J Cell Biol. 1991;115:1491–503. doi: 10.1083/jcb.115.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arvan P, Castle D. Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem J. 1998;332(Pt 3):593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorr SU, Venkatesh SG, Darling DS. Parotid secretory granules: crossroads of secretory pathways and protein storage. J Dent Res. 2005;84:500–9. doi: 10.1177/154405910508400604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jena BP. Porosome: the secretory portal in cells. Biochemistry. 2009;48:4009–18. doi: 10.1021/bi9002698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho SJ, Cho J, Jena BP. The number of secretory vesicles remains unchanged following exocytosis. Cell Biol Int. 2002;26:29–33. doi: 10.1006/cbir.2001.0848. [DOI] [PubMed] [Google Scholar]
- 24.Padawer J. Mast cells: extended lifespan and lack of granule turnover under normal in vivo conditions. Exp Mol Pathol. 1974;20:269–80. doi: 10.1016/0014-4800(74)90059-8. [DOI] [PubMed] [Google Scholar]
- 25.Hammel I, Dvorak AM, Fox P, et al. Defective cytoplasmic granule formation. II. Differences in patterns of radiolabeling of secretory granules in beige versus normal mouse pancreatic acinar cells after [3H]glycine administration in vivo. Cell Tissue Res. 1998;293:445–52. doi: 10.1007/s004410051136. [DOI] [PubMed] [Google Scholar]
- 26.Hammel I, Rabinowitz H, Nir I. Morphometric studies on normal and immune mast-cells. Israel J Med Sci. 1981;17:301. (abs) [Google Scholar]
- 27.Hammel I, Lagunoff D, Bauza M, et al. Periodic, multimodal distribution of granule volumes in mast cells. Cell Tissue Res. 1983;228:51–9. doi: 10.1007/BF00206264. [DOI] [PubMed] [Google Scholar]
- 28.Hammel I, Dvorak AM, Peters SP, et al. Differences in the volume distributions of human lung mast cell granules and lipid bodies: evidence that the size of these organelles is regulated by distinct mechanisms. J Cell Biol. 1985;100:1488–92. doi: 10.1083/jcb.100.5.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammel I, Shiloh-Rabinovich H, Nir I. Two populations of mast cells on fibroblast monolayers: correlation of quantitative microscopy and functional activity. J Cell Sci. 1988;91(Pt 1):13–9. doi: 10.1242/jcs.91.1.13. [DOI] [PubMed] [Google Scholar]
- 30.Weintraub H, Abramovici A, Amichai D, et al. Morphometric studies of pancreatic acinar granule formation in NCTR-Balb/c mice. J Cell Sci. 1992;102(Pt 1):141–7. doi: 10.1242/jcs.102.1.141. [DOI] [PubMed] [Google Scholar]
- 31.Hammel I, Lagunoff D, Wysolmerski R. Theoretical considerations on the formation of secretory granules in the rat pancreas. Exp Cell Res. 1993;204:1–5. doi: 10.1006/excr.1993.1001. [DOI] [PubMed] [Google Scholar]
- 32.Kalina M, Elmalek M, Hammel I. Intragranular processing of proopiomelanocortin in the intermediate cells of the rat pituitary glands. A quantitative immunocytochemical approach. Histochemistry. 1988;89:193–8. doi: 10.1007/BF00489924. [DOI] [PubMed] [Google Scholar]
- 33.Bialik GM, Abassi ZA, Hammel I, et al. Evaluation of atrial natriuretic peptide and brain natriuretic peptide in atrial granules of rats with experimental congestive heart failure. J Histochem Cytochem. 2001;49:1293–300. doi: 10.1177/002215540104901012. [DOI] [PubMed] [Google Scholar]
- 34.Hennig A, Elias H. Theoretical and experimental investigations of sections of rotatory ellipsoids. 1963. pp. 133–45. [PubMed]
- 35.Rahamimoff R, Yaari Y. Delayed release of transmitter at the frog neuromuscular junction. J Physiol. 1973;228:241–57. doi: 10.1113/jphysiol.1973.sp010084. Z Wiss Mikrosk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammel I, Lagunoff D, Krüger PG. Recovery of rat mast cells after secretion: a morphometric study. Exp Cell Res. 1989;184:518–23. doi: 10.1016/0014-4827(89)90349-2. [DOI] [PubMed] [Google Scholar]
- 37.Hammel I, Lagunoff D, Krüger PG. Studies on the growth of mast cells in rats. Changes in granule size between 1 and 6 months. Lab Invest. 1988;59:549–54. [PubMed] [Google Scholar]
- 38.Hammel I, Arizono N, Galli SJ. Mast cells in rat dermis and jejunal lamina propria show a five-fold difference in unit granule volume. Cell Tissue Res. 1991;265:329–34. doi: 10.1007/BF00398080. [DOI] [PubMed] [Google Scholar]
- 39.Hammel I, Dvorak AM, Galli SJ. Defective cytoplasmic granule formation. I. Abnormalities affecting tissue mast cells and pancreatic acinar cells of beige mice. Lab Invest. 1987;56:321–8. [PubMed] [Google Scholar]
- 40.Amihai D, Trachtenberg S, Terkel J, et al. The structure of mast cell secretory granules in the blind mole rat (Spalax ehrenbergi). J Struct Biol. 2001;136:96–100. doi: 10.1006/jsbi.2001.4429. [DOI] [PubMed] [Google Scholar]
- 41.Henderson WR, Chi EY. Ultrastructural characterization and morphometric analysis of human eosinophil degranulation. J Cell Sci. 1985;73:33–48. doi: 10.1242/jcs.73.1.33. [DOI] [PubMed] [Google Scholar]
- 42.Meyrick B, Reid L. Ultrastructure of cells in the human bronchial submucosal glands. J Anat. 1970;107:281–99. [PMC free article] [PubMed] [Google Scholar]
- 43.Nadelhaft I. Measurement of the size distribution of zymogen granules from rat pancreas. Biophys J. 1973;13:1014–29. doi: 10.1016/S0006-3495(73)86042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Batten T, Ball JN, Benjamin M. Ultrastructure of the adenohypophysis in the teleost Poecilia latipinna. Cell Tissue Res. 1975;161:239–61. doi: 10.1007/BF00220372. [DOI] [PubMed] [Google Scholar]
- 45.Itoh T. Quantitative study of secretory granules of the parotid gland acinar cell of the rat. J Electron Microsc (Tokyo) 1977;26:19–28. [PubMed] [Google Scholar]
- 46.Seiden D. Specific granules of the rat atrial muscle cell. Anat Rec. 1979;194:587–602. doi: 10.1002/ar.1091940411. [DOI] [PubMed] [Google Scholar]
- 47.Matteson DR, Kriebel ME, Llados F. A statistical model indicates that miniature end-plate potentials and unitary evoked end-plate potentials are composed of subunits. J Theor Biol. 1981;90:337–63. doi: 10.1016/0022-5193(81)90316-7. [DOI] [PubMed] [Google Scholar]
- 48.Mroz EA, Lechene C. Pancreatic zymogen granules differ markedly in protein composition. Science. 1986;232:871–3. doi: 10.1126/science.2422756. [DOI] [PubMed] [Google Scholar]
- 49.Alvarez de Toledo G, Fernandez JM. Patch-clamp measurements reveal multimodal distribution of granule sizes in rat mast cells. J Cell Biol. 1990;110:1033–9. doi: 10.1083/jcb.110.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hartmann J, Scepek S, Lindau M. Regulation of granule size in human and horse eosinophils by number of fusion events among unit granules. J Physiol. 1995;483(Pt 1):201–9. doi: 10.1113/jphysiol.1995.sp020578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hammel I, Anaby D. Imaging of zymogen granules in fully wet cells: evidence for restricted mechanism of granule growth. Microsc Res Tech. 2007;70:790–5. doi: 10.1002/jemt.20467. [DOI] [PubMed] [Google Scholar]
- 52.Lagunoff D. Membrane fusion during mast cell secretion. J Cell Biol. 1973;57:252–9. doi: 10.1083/jcb.57.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dvorak AM. Histamine content and secretion in basophils and mast cells. Prog Histochem Cytochem. 1998;33:III–IX. 169–320. doi: 10.1016/s0079-6336(98)80006-5. [DOI] [PubMed] [Google Scholar]
- 54.Galli SJ, Kalesnikoff J, Grimbaldeston MA, et al. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–86. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 55.Krüger PG, Lagunoff D. Effect of age on mast cell granules. Int Arch Allergy Appl Immunol. 1981;65:291–9. doi: 10.1159/000232768. [DOI] [PubMed] [Google Scholar]
- 56.Krüger PG, Lagunoff D. Mast cell restoration. A study of the rat peritoneal mast cells after depletion with polymyxin B. Int Arch Allergy Appl Immunol. 1981;65:278–90. [PubMed] [Google Scholar]
- 57.Flory P. Principles of Polymer Chemistry. Cornell University Press; Ithica: 1953. [Google Scholar]
- 58.Odian G. Principles of Polymerization. 4th Edition Wiley Interscience; New Jersey: 2004. [Google Scholar]
- 59.Chi EY, Lagunoff D. Abnormal mast cell granules in the beige (Chediak-Higashi syndrome) mouse. J Histochem Cytochem. 1975;23:117–22. doi: 10.1177/23.2.46876. [DOI] [PubMed] [Google Scholar]
- 60.Chi EY, Lagunoff D, Koehler JK. Abnormally large lamellar bodies in type II pneumocytes in Chediak-Higashi syndrome in beige mice. Lab Invest. 1976;34:166–73. [PubMed] [Google Scholar]
- 61.Chi EY, Ignacio E, Lagunoff D. Mast cell granule formation in the beige mouse. J Histochem Cytochem. 1978;26:131–7. doi: 10.1177/26.2.624833. [DOI] [PubMed] [Google Scholar]
- 62.Huizing M, Boissy RE, Gahl WA. Hermansky-Pudlak syndrome: vesicle formation from yeast to man. Pigment Cell Res. 2002;15:405–19. doi: 10.1034/j.1600-0749.2002.02074.x. [DOI] [PubMed] [Google Scholar]
- 63.Barbosa MD, Nguyen QA, Tchernev VT, et al. Identification of the homologous beige and Chediak-Higashi syndrome genes. Nature. 1996;382:262–5. doi: 10.1038/382262a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nagle DL, Karim MA, Woolf EA, et al. Identification and mutation analysis of the complete gene for Chediak-Higashi syndrome. Nat Genet. 1996;14:307–11. doi: 10.1038/ng1196-307. [DOI] [PubMed] [Google Scholar]
- 65.Perou CM, Moore KJ, Nagle DL, et al. Identification of the murine beige gene by YAC complementation and positional cloning. Nat Genet. 1996;13:303–8. doi: 10.1038/ng0796-303. [DOI] [PubMed] [Google Scholar]
- 66.Shiflett SL, Kaplan J, Ward DM. Chediak-Higashi Syndrome: a rare disorder of lysosomes and lysosome related organelles. Pigment Cell Res. 2002;15:251–7. doi: 10.1034/j.1600-0749.2002.02038.x. [DOI] [PubMed] [Google Scholar]
- 67.Tchernev VT, Mansfield TA, Giot L, et al. The Chediak-Higashi protein interacts with SNARE complex and signal transduction proteins. Mol Med. 2002;8:56–64. [PMC free article] [PubMed] [Google Scholar]
- 68.Harris E, Wang N, Wu Wl WL, et al. Dictyostelium LvsB mutants model the lysosomal defects associated with Chediak-Higashi syndrome. Mol Biol Cell. 2002;13:656–69. doi: 10.1091/mbc.01-09-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kypri E, Schmauch C, Maniak M, et al. The BEACH protein LvsB is localized on lysosomes and postlysosomes and limits their fusion with early endosomes. Traffic. 2007;8:774–83. doi: 10.1111/j.1600-0854.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 70.Charette SJ, Cosson P. A LYST/beige homolog is involved in biogenesis of Dictyostelium secretory lysosomes. J Cell Sci. 2007;120:2338–43. doi: 10.1242/jcs.009001. [DOI] [PubMed] [Google Scholar]
- 71.Grimberg E, Peng Z, Hammel I, et al. Synaptotagmin III is a critical factor for the formation of the perinuclear endocytic recycling compartment and determination of secretory granules size. J Cell Sci. 2003;116:145–54. doi: 10.1242/jcs.00186. [DOI] [PubMed] [Google Scholar]
- 72.Jamieson JD, Palade GE. Condensing vacuole conversion and zymogen granule discharge in pancreatic exocrine cells: metabolic studies. J Cell Biol. 1971;48:503–22. doi: 10.1083/jcb.48.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Combs JW. Maturation of rat mast cells. An electron microscope study. J Cell Biol. 1966;31:563–75. doi: 10.1083/jcb.31.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maji SK, Perrin MH, Sawaya MR, et al. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science. 2009;325:328–32. doi: 10.1126/science.1173155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ginsburg H, Lagunoff D. The in vitro differentiation of mast cells. Cultures of cells from immunized mouse lymph nodes and thoracic duct lymph on fibroblast monolayers. J Cell Biol. 1967;35:685–97. doi: 10.1083/jcb.35.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bainton DF, Farquhar MG. Segregation and packaging of granule enzymes in eosinophilic leukocytes. J Cell Biol. 1970;45:54–73. doi: 10.1083/jcb.45.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ginsburg H, Nir I, Hammel I, et al. Differentiation and activity of mast cells following immunization in cultures of lymph-node cells. Immunology. 1978;35:485–502. [PMC free article] [PubMed] [Google Scholar]
- 78.Goncz KK, Behrsing R, Rothman SS. The protein content and morphogenesis of zymogen granules. Cell Tissue Res. 1995;280:519–30. doi: 10.1007/BF00318356. [DOI] [PubMed] [Google Scholar]
- 79.Caro LG, Palade GE. Protein synthesis, storage, and discharge in the pancreatic exocrine cell, an autoradiographic study. J Cell Biol. 1964;20:473–95. doi: 10.1083/jcb.20.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Theron JJ, Biagio R, Meyer AC, et al. Ultrastructural observations on the maturation and secretion of granules in atrial myocardium. J Mol Cell Cardiol. 1978;10:567–72. doi: 10.1016/0022-2828(78)90014-7. [DOI] [PubMed] [Google Scholar]
- 81.Kosmelj K, Cedilnik A, Veranie P, et al. Intergranule fusion in rat pars intermedia. Image Anal Stereol. 2001;20:79–85. [Google Scholar]
- 82.Wendler F, Page L, Urbe S, et al. Homotypic fusion of immature secretory granules during maturation requires syntaxin 6. Mol Biol Cell. 2001;12:1699–709. doi: 10.1091/mbc.12.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amatore C, Arbault S, Bonifas I, et al. Correlation between vesicle quantal size and fusion pore release in chromaffin cell exocytosis. Biophys J. 2005;88:4411–20. doi: 10.1529/biophysj.104.053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Angleson JK, Cochilla AJ, Kilic G, et al. Regulation of dense core release from neuroendocrine cells revealed by imaging single exocytic events. Nat Neurosci. 1999;2:440–6. doi: 10.1038/8107. [DOI] [PubMed] [Google Scholar]
- 85.Aravanis AM, Pyle JL, Tsien RW. Single synaptic vesicles fusing transiently and successively without loss of identity. Nature. 2003;423:643–7. doi: 10.1038/nature01686. [DOI] [PubMed] [Google Scholar]
- 86.Scepek S, Lindau M. Exocytotic competence and intergranular fusion in cord blood-derived eosinophils during differentiation. Blood. 1997;89:510–7. [PubMed] [Google Scholar]
- 87.Payne CM. Phylogenetic considerations of neurosecretory granule contents: role of nucleotides and basic hormone/transmitter packaging mechanisms. Arch Histol Cytol. 1989;52(Suppl):277–92. doi: 10.1679/aohc.52.suppl_277. [DOI] [PubMed] [Google Scholar]
- 88.Montana V, Malarkey EB, Verderio C, et al. Vesicular transmitter release from astrocytes. Glia. 2006;54:700–15. doi: 10.1002/glia.20367. [DOI] [PubMed] [Google Scholar]
- 89.Scalettar BA. How neurosecretory vesicles release their cargo. Neuroscientist. 2006;12:164–76. doi: 10.1177/1073858405284258. [DOI] [PubMed] [Google Scholar]
- 90.Hook V, Funkelstein L, Lu D, et al. Proteases for processing proneuropeptides into peptide neurotransmitters and hormones. Annu Rev Pharmacol Toxicol. 2008;48:393–423. doi: 10.1146/annurev.pharmtox.48.113006.094812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mangini NJ, Vanable JW, Jr., Williams MA, et al. The optokinetic nystagmus and ocular pigmentation of hypopigmented mouse mutants. J Comp Neurol. 1985;241:191–209. doi: 10.1002/cne.902410207. [DOI] [PubMed] [Google Scholar]
- 92.Faundez V, Horng JT, Kelly RB. A function for the AP3 coat complex in synaptic vesicle formation from endosomes. Cell. 1998;93:423–32. doi: 10.1016/s0092-8674(00)81170-8. [DOI] [PubMed] [Google Scholar]
- 93.Kretzschmar D, Poeck B, Roth H, et al. Defective pigment granule biogenesis and aberrant behavior caused by mutations in the Drosophila AP-3beta adaptin gene ruby. Genetics. 2000;155:213–23. doi: 10.1093/genetics/155.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lyerla TA, Rusiniak ME, Borchers M, et al. Aberrant lung structure, composition, and function in a murine model of Hermansky-Pudlak syndrome. Am J Physiol Lung Cell Mol Physiol. 2003;285:L643–53. doi: 10.1152/ajplung.00024.2003. [DOI] [PubMed] [Google Scholar]
- 95.Na C-L, Duan CX, Apsley KS, et al. Abnormal alveolar type 2 cell phenotype in AP-3 mutant mocha and pearl mice: an electron microscopy study. Microsc Microanal. 2003;9:1426–27. [Google Scholar]
- 96.Grabner CP, Price SD, Lysakowski A, et al. Regulation of large dense-core vesicle volume and neurotransmitter content mediated by adaptor protein 3. Proc Natl Acad Sci U S A. 2006;103:10035–40. doi: 10.1073/pnas.0509844103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Charette SJ, Cosson P. Altered composition and secretion of lysosome-derived compartments in Dictyostelium AP-3 mutant cells. Traffic. 2008;9:588–96. doi: 10.1111/j.1600-0854.2008.00706.x. [DOI] [PubMed] [Google Scholar]
- 98.Wang X, Huynh H, Gjorloff-Wingren A, et al. Enlargement of secretory vesicles by protein tyrosine phosphatase PTP-MEG2 in rat basophilic leukemia mast cells and Jurkat T cells. J Immunol. 2002;168:4612–9. doi: 10.4049/jimmunol.168.9.4612. [DOI] [PubMed] [Google Scholar]
- 99.Rink J, Ghigo E, Kalaidzidis Y, et al. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–49. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 100.Benado A, Nasagi-Atiya Y, Sagi-Eisenberg R. Protein trafficking in immune cells. Immunobiology. 2009;214:403–21. doi: 10.1016/j.imbio.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 101.Hou JC, Min L, Pessin JE. Insulin granule biogenesis, trafficking and exocytosis. Vitam Horm. 2009;80:473–506. doi: 10.1016/S0083-6729(08)00616-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jena BP. Membrane fusion: role of SNAREs and calcium. Protein Pept Lett. 2009;16:712–7. doi: 10.2174/092986609788681869. [DOI] [PubMed] [Google Scholar]
- 103.Malsam J, Kreye S, Sollner TH. Membrane fusion: SNAREs and regulation. Cell Mol Life Sci. 2008;65:2814–32. doi: 10.1007/s00018-008-8352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wickner W, Schekman R. Membrane fusion. Nat Struct Mol Biol. 2008;15:658–64. doi: 10.1038/nsmb.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morvan J, Tooze SA. Discovery and progress in our understanding of the regulated secretory pathway in neuroendocrine cells. Histochem Cell Biol. 2008;129:243–52. doi: 10.1007/s00418-008-0377-z. [DOI] [PMC free article] [PubMed] [Google Scholar]