Abstract
Rod cyclic nucleotide-gated (CNG) channels are formed by two protein subunits (CNGA1 and CNGB1). Calmodulin (CaM) binds to the cytosolic regulatory domain of CNGB1 and decreases the open probability of CNGA1/CNGB1 channels. The CaM binding site within bovine CNGB1 (residues 679–702) binds tightly to Ca2+-bound CaM, which promotes Ca2+-induced inactivation of CNGA1/CNGB1 channels in retinal rods. We report complete NMR chemical shift assignments of Ca2+-saturated CaM bound to the CaM-binding domain of CNGB1 (BMRB no. 51222).
Keywords: CaM, Calcium, CNGB1, Retina, Photoreceptor, NMR
Biological context
Cyclic nucleotide-gated (CNG) channels expressed in retinal rods conduct a cation current in response to changes in intracellular levels of cGMP that occur during visual phototransduction (Baylor 1996, Fesenko, Kolesnikov et al. 1985). Ca2+-dependent regulation of photoreceptor CNG channels by CaM is important for promoting light adaptation in photoreceptor cells (Bradley et al. 2005, Fain et al. 2001, Hsu and Molday 1993). Retinal CNG channels consist of two protein subunits, CNGA1 and CNGB1 (Bradley et al. 2001). The CNGA1 subunit can form a functional homotetrameric channel when expressed alone, whereas CNGB1 does not form a functional homomeric channel (Finn et al. 1998). Native CNG channels in retinal rods form a heteromeric tetramer comprised of a 3:1 stoichiometry of CNGA1:CNGB1 (Shuart, Haitin et al., 2011). Three CNGA1 subunits form a trimer that binds tightly with a single CNGB1 subunit in a Ca2+-dependent fashion. The Ca2+ sensor protein, calmodulin (CaM) binds to a cytosolic site in CNGB1 (residues 679–702) (Trudeau and Zagotta 2002) that may regulate CNGB1 binding to CNGA1 (Shuart et al. 2011) and perhaps mediate Ca2+-induced CNG channel inactivation in rod cells (Hsu and Molday 1993; Trudeau and Zagotta 2003). Defects in the Ca2+-dependent regulation of CNG channels are genetically linked to autosomal recessive retinitis pigmentosa and other inherited forms of blindness (Bareil, Hamel et al. 2001). Elucidating the CNG channel structural interaction with CaM may provide insights for the treatment of retinal diseases.
Although structures are known for CaM bound to the CNGA2 subunit from olfactory CNG channels (Contessa et al. 2005), atomic level structural information is currently not known for CaM bound to the retinal CNGB1. We report here NMR resonance assignments of Ca2+-saturated CaM bound to the CaM-binding domain of CNGB1 (hereafter called CaM/CNGB1). These assignments are a first step toward elucidating the structure of CaM bound to CNGB1.
Methods and experiments
Expression and purification of CaM
Recombinant human CaM was subcloned into pET11b expression vector (Novagen) and overexpressed in E. coli strain BL21(DE3) as described previously (Turner, Anderson et al. 2020). Uniformly 13C/15N-labeled CaM samples were overexpressed in M9 minimal media, containing 1 g/L 15NH4Cl and 3 g/L 13C-labeled glucose (Cambridge Isotopes Laboratories) as the sole nitrogen and carbon sources, respectively. The soluble fraction of the cell lysate was loaded onto a HiPrep Phenyl Sepharose 6 column that was pre-equilibrated with equilibration buffer, containing 20 mM Tris (pH 7.5), 200 mM KCl, 2 mM CaCl2. The CaM protein was eluted from the column using a buffer that contained 20 mM Tris (pH 7.5), 50 mM KCl, 2 mM EGTA. The eluted protein fraction was further loaded onto a HiPrep Q Sepharose anion exchange column that was pre-equilibrated with 50 mM Tris (pH 7.5), 25 mM KCl, 1 mM EGTA and eluted by a KCl gradient up to 625 mM. The purity and identity of the eluted protein fractions were confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. A peptide fragment of the CaM binding domain from CNGB1 (residues 679–702) was purchased from GenScript, dissolved in DMSO-d6, and quantified using UV–Vis absorption spectroscopy. A 1.7-fold excess of the peptide was added to Ca2+-bound CaM, incubated at room temperature for 30 min, and concentrated to 0.5 mM.
NMR spectroscopy
Protein samples of 15N- or 13C/15N-labeled CaM bound to unlabeled CNGB1 peptide were exchanged into NMR buffer containing 20 mM Tris-d11 (pH 7.0) with 1 mM CaCl2, and 92% H2O/8% D2O. The CaM/CNGB1 complex was concentrated to give a final concentration of 0.5 mM in a final volume of 0.3 mL. All NMR experiments were performed at 308 K on a Bruker Avance III 600 MHz spectrometer equipped with a four-channel interface and triple resonance cryogenic (TCI) probe. The 15N–1H HSQC spectrum (Fig. 1A, B) was recorded with 256 × 2048 complex points for 15N(F1) and 1H(F2). Assignment of backbone resonances was obtained by analyzing the following spectra: HNCACB, CBCA(CO)NH, HNCO and HBHA(CO)NH. The assignment of side chain (aliphatic (Fig. 1C) and aromatic) resonances was obtained by analyzing the following spectra: HCCCONH-TOCSY, HCCH-TOCSY, HBCBCGCDHD and HBCBCGCDCEHE as described previously (Ikura et al. 1991). The NMR data were processed using NMRPipe and analyzed using Sparky.
Fig. 1.
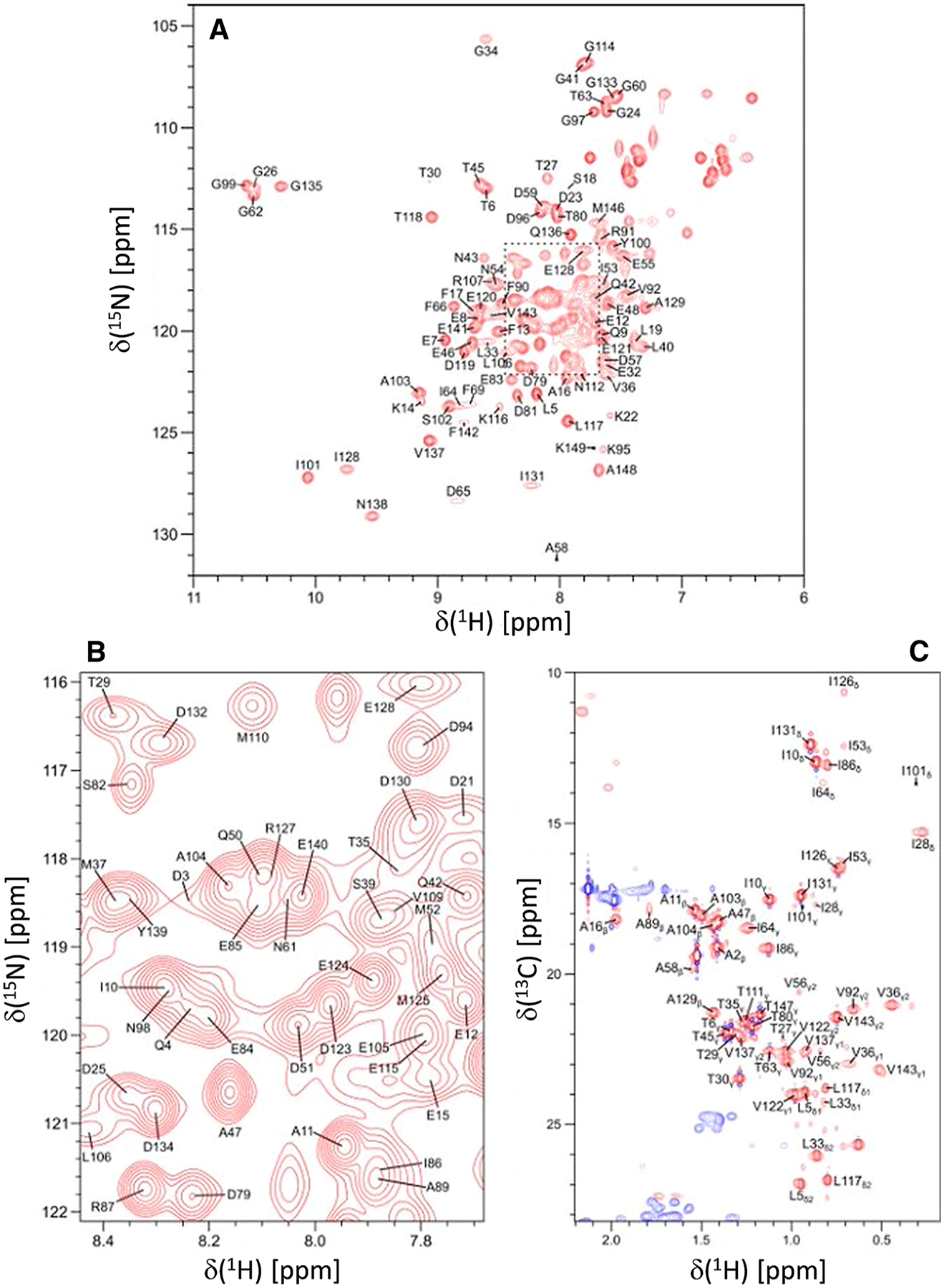
Two-dimensional NMR spectra of CaM bound to unlabeled CNGB1-CaMBD peptide. A 15N–1H HSQC spectrum recorded at 600 MHz 1H frequency was analyzed to determine backbone resonance assignments. B Expanded view of resonance assignments from the spectrally crowded region highlighted by the dashed box. C Constant-time 13C–1H HSQC spectrum was analyzed to determine side chain resonance assignments. Representative resonance assignments are indicated by residue labels; complete assignments are available as BMRB accession no. 51222
Extent of assignments and data deposition
Figure 1A, B present the 15N–1H HSQC spectrum of CaM/CNGB1 to illustrate representative backbone resonance assignments. Figure 1C presents a constant-time 13C–1H HSQC spectrum to illustrate side chain methyl resonance assignments. The NMR assignments were based on 3D heteronuclear NMR experiments performed on 13C/15N-labeled CaM bound to unlabeled CNGB1 peptide. The NMR spectra of CaM/CNGB1 exhibited well-dispersed peaks indicative of a stably folded structure. Four amide resonances (assigned to G26, G62, G99 and G135) exhibited noteworthy down-field shifts that are caused by Ca2+ binding to each of the four EF-hands (Fig. 1A). Ring current shifted methyl resonances assigned to residues I28, V36, and I101 (Fig. 1C) suggest these methyl groups are near aromatic residues in the hydrophobic core. More than 85% of the backbone resonances (1HN, 15N, 13Cα, 13Cβ, and 13CO) and 83% of aliphatic and aromatic side-chain resonances were assigned. A stretch of ten residues (residues 68–78) in the second EF-hand of CaM could not be assigned (central gap near H4 in Fig. 2), because their HSQC peaks were either broadened beyond detection or otherwise could not be detected. The observed peak broadening here suggests that these residues might undergo conformational exchange processes perhaps caused by their interaction with the bound peptide. Indeed, these same resonances are exchange broadened in CaM bound to the α-subunit of the retinal cyclic nucleotide-gated channel (CNGA2) (Contessa et al. 2005), but are not exchange broadened in free CaM (Kainosho et al. 2006). The chemical shift assignments (1H, 15N, 13C) for CaM/CNGB1 have been deposited in the BioMagResBank (http://www.bmrb.wisc.edu) under accession number 51222.
Fig. 2.
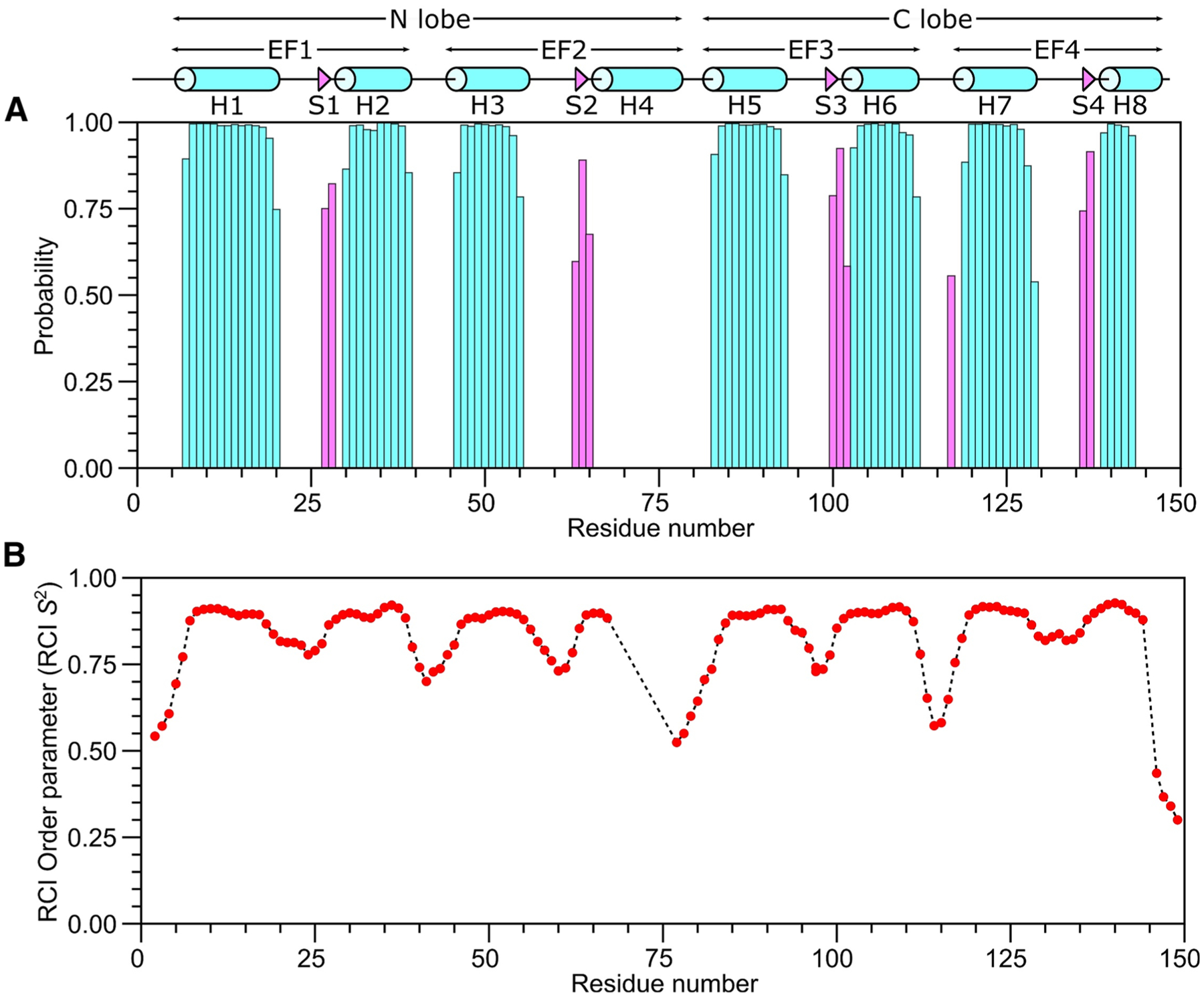
Secondary structure and order parameters of Ca2+-saturated CaM bound to unlabeled CNGB1 peptide predicted from the assigned backbone chemical shifts. A Probability of secondary structural elements (cyan for helix and magenta for strand) and B RCI order parameter (RCI-S2) of Ca2+-saturated CaM bound to unlabeled CNGB1 peptide were predicted using TALOS+ server (Shen et al. 2009). The wire diagram depicting the secondary structural elements (cylinder for helix and triangle for strand) was obtained from the CaM structure [PDB ID—2VAY (Halling et al. 2009)]
The secondary structure of CaM/CNGB1 was calculated based on the chemical shift index (Wishart et al. 1992) of each assigned amino acid residue and ANN-Secondary structure prediction using TALOS+ (Shen et al. 2009) (Fig. 2). CaM/CNGB1 contains the following α-helices: H1 (residues 7–20), H2 (residues 30–39), H3 (residues 46–55), H5 (residues 83–93), H6 (residues 103–112), H7 (residues 119–129) and H8 (residues 139–143) depicted by cylinders in Fig. 2A. Four short β-strands named S1 (residues 27–28), S2 (residues 63–65), S3 (residues 100–102) and S4 (residues 136–137) are depicted by the triangles in Fig. 2A. Preliminary NMR-derived distance restraints inferred from NOESY spectra suggest that the observed α-helices and β-strands combine to form 4 EF-hand Ca2+ binding motifs (EF1: residues 7–39, EF2: residues 45–76, EF3: residues 83–112 and EF4: residues 119–144) as seen in the crystal structure of CaM in the absence of peptide (Babu et al. 1988). In the CaM crystal structure, the N-terminal EF-hands (EF1 and EF2) interact to form what is called the N-lobe, while EF3 and EF4 interact to form the C-lobe. The binding of the CNGB1 peptide to CaM causes detectable chemical shift perturbations that are distributed uniformly throughout both the N-lobe and C-lobe of CaM (Fig. 3). Thus, the CNGB1 peptide is likely making contact with both lobes of CaM, consistent with the familiar collapsed structure of CaM bound to other peptide targets (Hoeflich and Ikura 2002). The CaM residues (A16, L19, L33, M52, A89, L106, M110 and F142) that have relatively high CSP values in Fig. 3 correspond to the residues that directly contact the CNGA2 peptide in the NMR structure of CaM/CNGA2 (Contessa et al. 2005). The NMR assignments of CaM/CNGB1 presented here are an important first step toward determining its full three-dimensional structure.
Fig. 3.
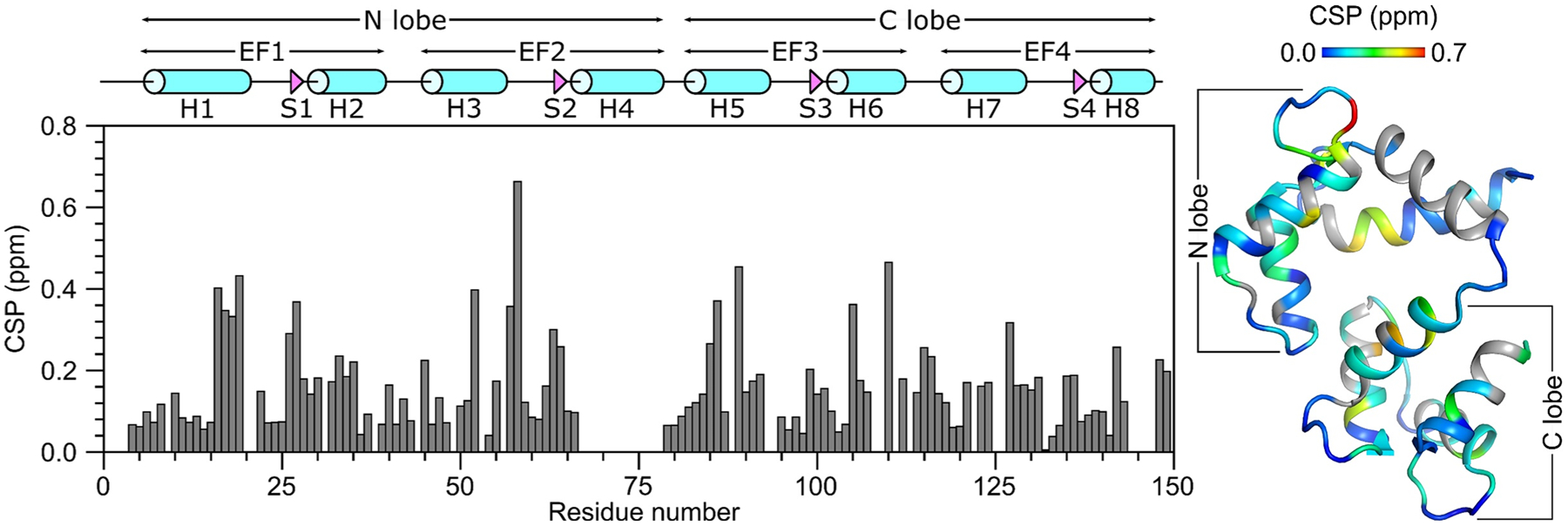
Residue-specific amide chemical shift perturbation (CSP) for Ca2+-bound CaM in the presence and absence of CNGB1 peptide. CSP was calculated as: . ΔHN and ΔN are the observed difference in the 1HN and 15N chemical shifts, respectively for CaM/CNGB1 compared to CaM alone. CSP values are mapped on to the CaM structure (PDB ID: 2VAY (Halling et al. 2009))
Acknowledgements
We thank Derrick Kaseman and Ping Yu for technical support and help with NMR experiments. Work supported by NIH Grants (EY012347) to J.B.A and (RR11973) to the UC Davis NMR facility.
Data availability
The assignments have been deposited to the BMRB under the accession code: 51222.
References
- Babu YS, Bugg CE, Cook WJ (1988) Structure of calmodulin refined at 2.2 A resolution. J Mol Biol 204:191–204 [DOI] [PubMed] [Google Scholar]
- Bareil C, Hamel CP, Delague V, Arnaud B, Demaille J, Claustres M (2001) Segregation of a mutation in CNGB1 encoding the beta-subunit of the rod cGMP-gated channel in a family with autosomal recessive retinitis pigmentosa. Hum Genet 108:328–334 [DOI] [PubMed] [Google Scholar]
- Baylor D (1996) How photons start vision. Proc Natl Acad Sci USA 93:560–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley J, Frings S, Yau K, Reed R (2001) Nomenclature for ion channel subunits. Science 294:2095–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley J, Reisert J, Frings S (2005) Regulation of cyclic nucleotide-gated channels. Curr Opin Neurobiol 15:343–349 [DOI] [PubMed] [Google Scholar]
- Contessa GM, Orsale M, Melino S, Torre V, Paci M, Desideri A, Cicero DO (2005) Structure of calmodulin complexed with an olfactory CNG channel fragment and role of the central linker: residual dipolar couplings to evaluate calmodulin binding modes outside the kinase family. J Biomol NMR 31:185–199 [DOI] [PubMed] [Google Scholar]
- Fain GL, Matthews HR, Cornwall MC, Koutalos Y (2001) Adaptation in vertebrate photoreceptors. Physiol Rev 81:117–151 [DOI] [PubMed] [Google Scholar]
- Fesenko EE, Kolesnikov SS, Lyubarsky AL (1985) Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature 313:310–313 [DOI] [PubMed] [Google Scholar]
- Finn JT, Krautwurst D, Schroeder TY, Chen TY, Reed RR, Yau KW (1998) Functional co-assembly among subunits of cyclic-nucleotide-activated, nonselective cation channels, and across species from nematode to human. Biophys J 74:1333–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halling DB, Georgiou DK, Black DJ, Yang G, Fallon JL, Quiocho FA, Pedersen SE, Hamilton SL (2009) Determinants in CaV1 channels that regulate the Ca2+ sensitivity of bound calmodulin. J Biol Chem 284:20041–20051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeflich KP, Ikura M (2002) Calmodulin in action: diversity in target recognition and activation mechanisms. Cell 108:739–742 [DOI] [PubMed] [Google Scholar]
- Hsu YT, Molday RS (1993) Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature 361:76–79 [DOI] [PubMed] [Google Scholar]
- Ikura M, Spera S, Barbato G, Kay LE, Krinks M, Bax A (1991) Secondary structure and side-chain 1H and 13C resonance assignments of calmodulin in solution by heteronuclear multidimensional NMR spectroscopy. Biochemistry 30:9216–9228 [DOI] [PubMed] [Google Scholar]
- Kainosho M, Torizawa T, Iwashita Y, Terauchi T, Mei Ono A, Guntert P (2006) Optimal isotope labelling for NMR protein structure determinations. Nature 440:52–57 [DOI] [PubMed] [Google Scholar]
- Shen Y, Delaglio F, Cornilescu G, Bax A (2009) TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR 44:213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuart NG, Haitin Y, Camp SS, Black KD, Zagotta WN (2011) Molecular mechanism for 3:1 subunit stoichiometry of rod cyclic nucleotide-gated ion channels. Nat Commun 2:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau MC, Zagotta WN (2002) Mechanism of calcium/calmodulin inhibition of rod cyclic nucleotide-gated channels. Proc Natl Acad Sci U S A 99:8424–8429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau MC, Zagotta WN (2003) Calcium/calmodulin modulation of olfactory and rod cyclic nucleotide-gated ion channels. J Biol Chem 278:18705–18708 [DOI] [PubMed] [Google Scholar]
- Turner M, Anderson DE, Nieves-Cintron M, Bartels P, Coleman AM, Yarov V, Bers DM, Navedo MF, Horne MC, Ames JB, Hell JW (2020) a-Actinin-1 promotes gating of the L-type Ca2+ Channel CaV1.2. EMBO J 39:e102622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart DS, Sykes BD, Richards FM (1992) The chemical shift index: a fast and simple method for the assignment of protein secondary structure through NMR spectroscopy. Biochemistry 31:1647–1651 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The assignments have been deposited to the BMRB under the accession code: 51222.


