Abstract
Background:
Sepsis-induced gut microbiome alterations contribute to sepsis-related morbidity and mortality. Given evidence for improved post-sepsis outcomes in females compared to males, we hypothesized that female mice maintain microbiota resilience versus males.
Methods:
Mixed-sex C57BL/6 mice underwent cecal ligation and puncture (CLP) with antibiotics, saline resuscitation, and daily chronic stress (DCS), and were compared to naïve (non-sepsis/no antibiotics) controls. For this work, the results of young (3-5 month old) and old (18-22 month old) adult mice were analyzed by sex, independent and dependent of age. Mice were sacrificed at day 7 and 14, and 16S rRNA gene sequencing was performed on fecal bacterial DNA. Alpha and beta-diversity were determined by Shannon index and Bray-Curtis with principal coordinate analysis, respectively. False discovery rate (FDR) correction was implemented to account for potential housing effect.
Results:
In control mice, there was no difference in alpha- or beta-diversity between male and female mice (FDR=0.76 and 0.99, respectively). However, CLP+DCS male mice had a decrease in microbiota alpha-diversity at 7 days post-CLP (Shannon FDR=0.005) that was sustained at 14 days post-CLP (Shannon FDR=0.001), compared to baseline. In addition, male mice maintained differences in beta-diversity even at day 14 compared to controls (FDR<0.0001). In contrast, female mice had a decreased microbiota alpha-diversity (Shannon FDR=0.03) and beta-diversity (FDR=0.02) 7 days post-CLP, but recovered their alpha- and beta-diversity by post-CLP day 14 (Shannon FDR=0.5 and FDR=0.02, respectively). Further analysis of females revealed that only young female mice were not different (beta-diversity) post-CLP day 14 to controls.
Conclusions:
Although sepsis-induced perturbations of the intestinal microbiota occur initially in both male and female C57BL/6 mice, females demonstrate different microbiota by day 14. This may be seen primarily in younger females. This difference in recovery may play a role in outcome differences between sexes after sepsis.
Level of Evidence:
Basic Science.
Study Type:
Experimental murine model.
Keywords: fecal bacteria, resilience, 16S rRNA, persistent inflammation immunosuppression and catabolism syndrome, PICS
Background
Globally, sepsis is a leading cause of death (1) and the World Health Organization (WHO) has made sepsis a health priority (2). Additionally, sepsis accounts for >$20 billion of total hospital costs in the United States (3). Although early sepsis survival has improved over the past decade, this decrease in mortality has yielded a rapidly expanding population of sepsis survivors who develop chronic critical illness (CCI) (4). CCI is characterized by persistent organ dysfunction requiring prolonged intensive care unit (ICU) treatment. Subsequently, CCI frequently manifests thereafter in the host as low-grade systemic inflammation, global immunosuppression and cachexia/muscle wasting (5), known as the Persistent Inflammation, Immunosuppression and Catabolism Syndrome (PICS) endotype (4, 5). Sepsis survivors with CCI/PICS are often discharged to long-term acute care facilities, where they experience repeat infections and ~40% 1-year post-discharge mortality (6, 7). Specific treatments for these sepsis survivors are lacking, due in part to: 1) inadequate knowledge of its pathobiology, and, 2) a paucity of studies that are able to address sepsis in a manner that befits precision medicine, including but not limited to sex (8, 9). In fact, it is now recognized that “[a] better understanding of sex-and gender-dependent differences may serve to increase translational research success” (8).
Although there is still significant debate as to whether females have improved outcomes after sepsis (8, 9), studies indicate that females are less likely to have post-sepsis morbidity and mortality, even after menopause (10, 11). In fact, one study demonstrated that in patients less than 90 years of age, women had significantly decreased one-year mortality compared to males (10). Regardless, it is clear that females and males respond differently to infection (8-10). Future successful interventions for sepsis will need to utilize personalized/precision medical therapies, which will require a better understanding of the differences in host response due to severe infection.
One key aspect to septic outcomes is the host microbiome (12, 13). The microbiota is the collection of trillions of microorganisms that form a symbiont and pathobiont relationship with its host. Its role in various human diseases, including post-trauma recovery, has gained much traction recently given its interaction with the immune system and host metabolic processes (14-16). The ability to study this ecosystem is predicated on taking advantage of the unique 16S ribosomal subunit gene in prokaryotic organisms. Due to the slow evolutionary change imparted on this gene, sequencing allows reconstruction of bacterial phylogeny present in a host microbiota. Subsequently, the abundance (total number), richness (the assortment of bacterial species in an ecosystem, ex. your gut microbiome), and diversity (the amount of individual bacteria from each of the bacterial species present) can be determined. Microbial community diversity can be measured within a cohort (alpha-diversity) or between cohorts from different environments (beta-diversity) (17). Through bioinformatic analysis, any noted changes based on host, treatment, etc. can be determined to give greater insight into how the microbiome may play a role in human disease.
Critical illness has been shown to disturb the host microbiome (12), and evidence exists demonstrating that a host dysbiome worsens outcomes after sepsis (13). Importantly, it is not simply the changes in host microbiome that contributes to its pathology, but the ability (or inability) of the microbiome to recover from perturbations, known as microbiota resilience, that can determine host morbidity and mortality (18). Furthermore, sex is considered one the most important factors affecting the gut microbiota (11). For example, investigators have conducted sex-specific fecal microbiota transplantation (FMT) animal studies from C57BL/6J conventional mice in germ-free mice of the opposite sex. Their results illustrated that female recipients lost significantly more weight after receiving male microbiota compared with receiving female microbiota, suggesting a sexually dimorphic impact of the microbiome on metabolic regulation (19).
Using a murine model of surgical/abdominal sepsis that combines cecal ligation and puncture with daily chronic stress (CLP+DCS), and is associated with PICS and CCI, our goal was to determine differences in the diversity of the murine microbiome at post-CLP days 7 and 14 days between female and male mice. Furthermore, we specifically sought to determine if differences existed in the gut microbiota in female versus male adult mice in post-sepsis recovery, as differences in microbiota resilience are potentially targets of therapy (16), and may help to explain sex-based differences in post-sepsis recovery.
Methods
Animals.
All animal experiments were approved by the University of Florida Institutional Animal Care and Use Committee and followed Animal Research Reporting of In Vivo Experiments (ARRIVE) guidelines (https://www.nc3rs.org.uk/arrive-guidelines). The animals were cared for and used according to the Guide for the Care and Use of Laboratory Animals (20). C57BL/6J (B6) mice of both sexes were purchased from Jackson Laboratory (JAX; Bar Harbor, ME) and the National Institute on Aging (NIA; Baltimore, MD). Mice were cared for by the University of Florida Animal Care Services and housed in transparent cages (three to four animals of same sex/age per cage) under specific pathogen-free conditions in a single room. Animals were provided standard irradiated pelleted diet and water ad libitum for the duration of the study. Prior to initiation of the experiment, mice were acclimated to a 12-hour light-dark cycle for a minimum of 14 days, and NIA and JAX old adult mice were housed together. Due to the coprophagic nature of mice, this assured that mice in the same cage would have similar microbiota composition and structure (21). Only animals of the same sex, age, and treatment group were housed together. For this work, young (3-5 months) and old (18-22 months) adult murine microbiome results were combined and analyzed by the variable of sex.
Intra-Abdominal Sepsis and Daily Chronic Stress Model.
In order to recapitulate the human condition of CCI and PICS, a murine model of sepsis and persistent inflammation, previously described by our laboratory, was utilized (22). General anesthesia was induced with inhaled isoflurane and cecal ligation and puncture (CLP) was performed via a midline laparotomy with exteriorization of the cecum to induce a model of sepsis. The cecum was ligated with 2-0 silk suture 1 cm from its tip, a 25-gauge needle was used to puncture the cecum, and the laparotomy was closed in one layer with surgical clips. Buprenorphine analgesia was provided for 48 hours post-surgery. Imipenem monohydrate (25 mg/kg in 1 mL 0.9% normal saline) was administered subcutaneously 2 hours post-CLP and then continued twice daily for 72 hours. Subsequently, we added a component of daily chronic stress (DCS) to mimic the ICU stay of human patients who develop CCI (19). DCS was conducted by placing mice in weighted plexiglass animal restraint holders (Kent Scientific; Torrington, CT) for 2 hours daily commencing the day after CLP. The CLP+DCS mice, along with mixed-sex naïve mice (no CLP, no DCS, no antibiotics, and no fluid resuscitation) (old n=13, young n=13), were euthanized on day 7 or 14 post-CLP+DCS. Mice were euthanized via cervical dislocation following isoflurane inhalation, after which stool from the descending colon (during exploratory laparotomy after euthanasia the distal portion of the colon was incised and feces was pushed directly into a cryogenic vial) was collected under sterile conditions in a laminar flow hood into sterile Eppendorf tubes (Fisher Scientific) and snap frozen in liquid nitrogen immediately. Subsequently, these samples were stored in a −80°C freezer.
Bacterial DNA Isolation and 16S rRNA Gene Sequencing.
Whole genome bacterial DNA was isolated from individual animal stool using the QIAamp Fast DNA Stool Mini Kit (Qiagen, Germantown, MD) per manufacturer guidelines. Library preparation was performed utilizing the Quick-16S NGS (Next Generation Sequencing) Library Prep Kit (Zymo Research; Irvine, CA) according to manufacturer protocol. The V3-V4 hypervariable region of the 16S rRNA gene was amplified and paired-end adapter sequences with unique 8 nucleotide barcodes were used during library preparation to allow multiplexing. The final PCR products were quantified with quantitative PCR and pooled based on equal molarity. The final pooled library was purified utilizing the Mag-Bind TotalPure NGS kit (Omega Bio-tek, Inc; Norcross, GA); quality control and quantification was performed with TapeStation (Agilent Technologies; Santa Clara, CA) and Qubit (Thermo Fisher Scientific; Waltham, WA). Sequencing was subsequently completed using the Illumina MiSeq™ sequencer (Ilumina, Inc; San Diego, CA), producing paired end reads, 300 bases long, which produced 11,834,108 total reads (each end) for the 48 samples.
Plasma Testosterone and Estrogen Assay.
Blood was collected in K2-EDTA tubes. Plasma samples were collected after centrifugation of blood samples at 1800 x g for 10 minutes and stored at −80° C until used for quantitative determination of testosterone and estradiol using the Testosterone and Estradiol Assay kits (Bio-Techne, Minneapolis, MN). Standard curves were created using a four-parameter logistic (4-PL) curve fit (Softmax Pro 3.1 software; Molecular Devices Corporation, Sunnyvale, CA).
Statistical analyses.
Paired end reads were joined using fastq-join from ea-utils (https://expressionanalysis.github.io/ea-utils/) with a minimum overlap of 6bp and up to 8% maximum difference. The joined reads were quality filtered using Quantitative Insights into Microbial Ecology (QIIME) version 1.9.1 (23) with a score of Q20 or higher in at least 75% of the input read length. The first and last 10 bases of each read were then trimmed and any read <100 bases long were removed via Trimmomatic (http://www.usadellab.org/cms/?page=trimmomatic). An additional filtering step was applied to remove PhiX and mouse/host sequences using KneadData (http://huttenhower.sph.harvard.edu/kneaddata). Pre-indexed bowtie2 files for PhiX and Mus musculus Ensembl GRCm38 from Illumina’s iGenomes collection were used as references. The surviving reads were fed to QIIME to pick OTUs at 97% similarity level using an open-reference picking strategy with the Greengenes reference dataset (release 13.8). Chimeric sequences were detected and removed using ChimeraSlayer (http://microbiomeutil.sourceforge.net/#A_CS). OTUs that had ≤ 0.005% of the total number of sequences were excluded according to Bokulich and colleagues (23) and the final set contained 13,455,787 reads. Taxonomic assignment was carried out using the RDP (Ribosomal Database Project) classifier version 2.2 (24) through QIIME with confidence set to 50%. Counts were then normalized and log10 transformed according to the following formula (25):
where RC is the read count for a particular Operational Taxonomic Unit (OUT) in a particular sample, n is the total number of reads in that sample, the sum of x is the total number of reads in all samples, and N is the total number of samples.
Principal Coordinate Analysis (PCoA) was performed on Bray-Curtis distance of the normalized and log10 transformed counts using the phyloseq R package (26). Alpha-diversity was assessed using Chao1 and Shannon indexes using rarefied counts (set to 89,087 reads representing the minimum number of reads among all samples). All statistical analyses were performed in R version 4.0.3 (27). A linear Mixed-Effects Model (lme function) in the R lme4 package (version 1.1-25), with the REML method was used to fit a generalized mixed linear model of the following form: var ~ state + 1∣cage, where var is PCoA axis, Chao1 index, Shannon index, taxa normalized, and log10 transformed count (considering only taxa present in at least 25% of the samples) or log10 read count in each sample. The latter was performed to ensure the sparsity of 16S rRNA count data did not contribute to sample clustering. State is defined as male/female and post-CLP+DCS (7 days or 14 days)/naïve and one ∣cage indicates that we used the cage as a random effect to account for cohousing effects (25). P-values were obtained from Analysis of Variance (ANOVA) on the above model and were false discovery rate (FDR) corrected to control for potential cage effect using the Benjamini & Hochberg approach (28). A one-way ANOVA with Tukey’s multiple comparisons test was performed to compare plasma testosterone and estradiol levels between indicated cohorts using Prism 8.0 (GraphPad Software, San Diego, CA.) A p value ≤0.05 was considered statistically significant.
Results
Female B6 mice recover from sepsis and stress-induced microbiota diversity changes over time compared to non-recovering male mice.
We have previously demonstrated the impact of age on the stability of the murine microbiota after CLP+DCS (29). However, it is unknown what impact sex has in relation to microbiome changes after sepsis over the adult age range. Given clinical data demonstrating survival differences after sepsis between male and female patients, we sought to determine whether the changes in the host microbiota in a murine model of sepsis/PICS are based on the sex of the host. For this work, we analyzed the results of our young and old adult mice, combined looking at the results by sex only, and subsequently, looking at the differences in sex in old and young mice separately. Because of the small sample sizes, this latter analysis must be considered preliminary.
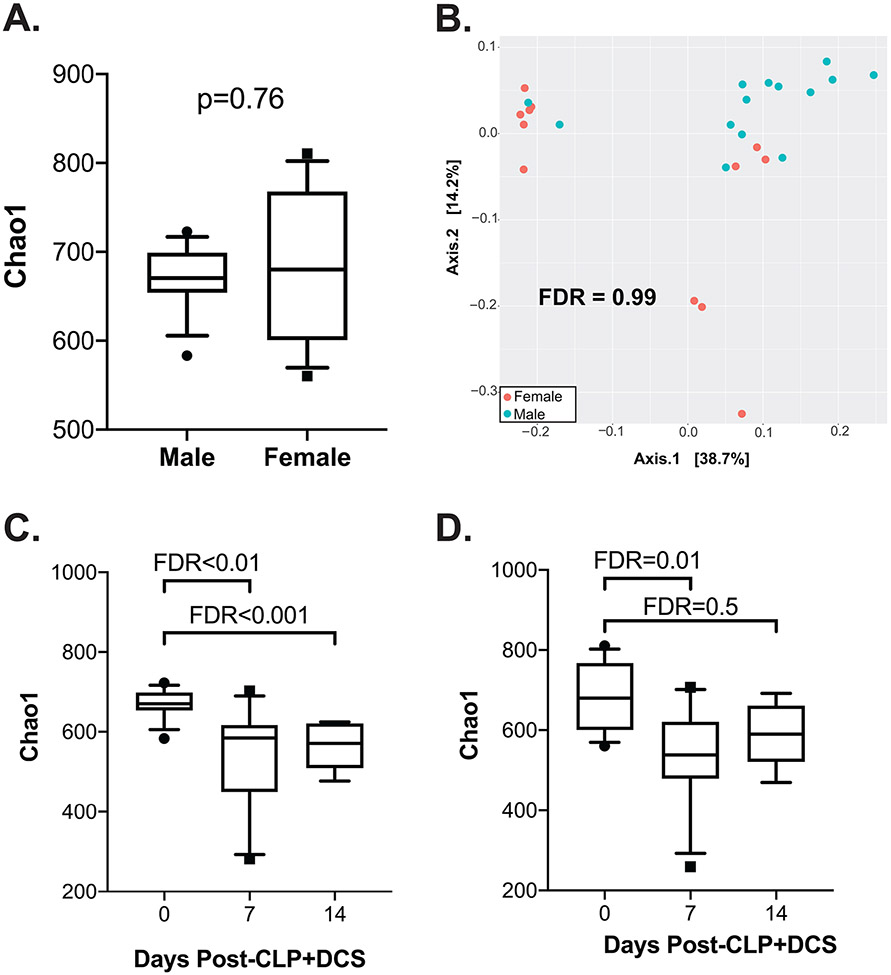
Mice of both sexes underwent the CPL+DCS model as indicated. In order to determine similarities of baseline murine microbiota based on sex, 16S rRNA gene sequencing was performed followed by a measure of alpha and beta diversity between the cohorts. This demonstrated no difference in alpha-diversity in naïve (healthy, control) male versus female mice by the Chao1 index (FDR=0.76; Figure 1A), nor beta-diversity as measured by the Bray-Curtis dissimilarity index (FDR=0.99; Figure 1B). Given this, we next sought to determine temporal changes within each sex over time (0, 7, and 14 days post CLP+DCS). This demonstrated that compared to their naïve (time 0) counterparts, male B6 mice had decreased alpha-diversity at 7 days (Chao1 FDR=0.003; Shannon FDR=0.005; Figure 1C) that persisted at 14 days post CLP+DCS (Chao1 FDR<0.001; Shannon FDR=0.001; Figure 1C). Interestingly, although the alpha-diversity in female mice 7 days post-CLP+DCS was decreased (Chao1 FDR=0.01; Shannon FDR=0.03), this difference disappeared by day 14 (Chao1 FDR=0.5; Shannon FDR=0.5; Figure 1D). Finally, PCoA on Bray-Curtis dissimilarity did not identify a significant difference in beta-diversity in male B6 mice at 7 days (FDR=0.08; Figure 2A), but was present at 14 days post-CLP+DCS (FDR<0.001; Figure 2B), demonstrating a delayed shift in the microbiota. However, as was the case with alpha-diversity in the female cohort, B6 female mice demonstrated a significant difference in beta-diversity by PCoA at 7 days (FDR=0.02; Figure 2C), which generally recovered by 14 days (FDR=0.07; Figure 2D). These data demonstrate the association of sex with changes in the murine microbiota after sepsis and daily chronic stress.
Figure 1.
16S rRNA gene sequencing of the intestinal microbiota at baseline (“naïve”) prior to CLP+DCS demonstrates no significant difference in alpha diversity by Chao1 (A) or beta diversity by Bray-Curtis dissimilarity index (B). However, 7- and 14-days post-CLP+DCS demonstrated a significant difference in intestinal microbiota alpha diversity in male mice compared to baseline (C). While female mice also demonstrated a CLP-DCS induced microbiota shift in diversity at 7 days, this change in diversity appears restored after 14 days (D). False discovery rate (FDR), cecal ligation and puncture (CLP), daily chronic stress (DCS).
Figure 2.
Principal coordinate analysis (PCoA) of Bray-Curtis dissimilarity index is presented, which demonstrates no difference in beta-diversity in male B6 mice post-CLP+DCS at 7 days (A) but at 14 days is significantly different (B). In contrast, female B6 mice had a shift in the beta diversity at 7 days (C), which appeared restored by 14 days (D). False discovery rate (FDR), cecal ligation and puncture (CLP), daily chronic stress (DCS), not significant (NS).
Alterations in alpha diversity associate with age and sex-based differences in mice recovering from sepsis
In order to extend observations from our prior work that demonstrated stability of the murine microbiota in young mice compared to old after sepsis(29), but in the context of sex and temporal differences, changes in alpha-diversity of the cohort were determined in subsets of the mice based on both age and sex (young male, old male, young female, and old female) pre-CLP+DCS as well as 7- and 14-days post-CLP+DCS. This demonstrated that after sepsis, the alpha-diversity of both young male (Figure 3A) and young female mice remains stable compared to old, which maintains a persistent level of alpha-diversity even at 14 days after CLP+DCS (Figure 3). Specifically, old male mice had a significant decrease in Chao1 alpha-diversity 14 days after CLP+DCS (FDR<0.01; Figure 3B) and old female mice had a significant decrease in alpha-diversity 7- and 14-days post-CLP+DCS (FDR<0.001; Figure 3D).
Figure 3.
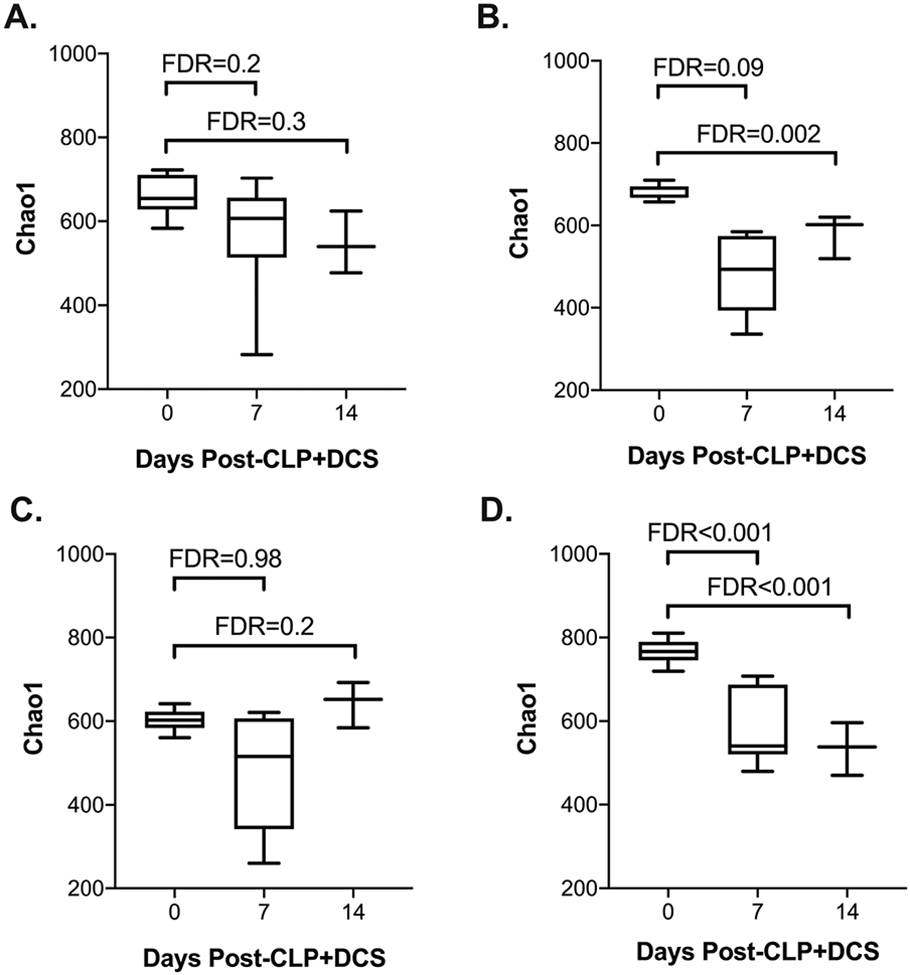
Alpha diversity by Chao1 of young and old mice who underwent CLP+DCS were analyzed for each sex and demonstrate that (A) young male mice do not have alterations in the alpha diversity of their intestinal microbiota after CLP+DCS but their old male counterparts do (B; FDR=0.002). Similarly, young female mice who undergo CLP+DCS have stable alpha diversity after CLP+DCS (C) but old female mice were noted to have decreased alpha diversity over time after CLP+DCS (D; FDR <0.001). False discovery rate (FDR), cecal ligation and puncture (CLP), daily chronic stress (DCS).
In order to determine if age drives the difference in beta-diversity seen in male and female mice (Figure 2), Bray-Curtis dissimilarity index was next performed as previously described for male and female mice based on age with the PCoA plots illustrated in Figure 4. Given the difference in beta-diversity in male mice at 14 days post-CLP+DCS, subset analysis of this group based on age was performed to determine if this was a driver of the observation. Indeed, young male mice at 14-days post-CLP+DCS did not have a difference in their intestinal microbiota beta-diversity (FDR=0.9; Figure 4A); however, only old male mice had a significant difference in beta-diversity (FDR<0.0001; Figure 4B). Finally, given the differences in beta-diversity in female mice 7 days post-CLP+DCS, PCoA analysis was likewise performed on the cohort comparing young to old mice. This demonstrated that there was a difference in beta-diversity in young female mice at 7 days post-CLP+DCS (FDR=0.04; Figure 4C), but not old female mice (FDR=0.99; Figure 4D). However, at 14-days post CLP+DCS, the beta diversity of young female mice remained different compared to their baseline (FDR=0.02; data not shown), while old female mice now had a significant difference in beta-diversity at 14 days post-CLP+DCS compared to pre-CLP+DCS (FDR<0.0001; data not shown).
Figure 4.
Principal coordinate analysis (PCoA) of Bray-Curtis dissimilarity index is presented for young and old male mice 14 days after CLP+DCS (A and B, respectively) as well as young and old female mice at 7 days post-CLP+DCS (C and D, respectively). This demonstrated a difference in the beta diversity of the old male mice at 14 days post-CLP+DCS (FDR<0.0001) and young female mice at 7 days post-CLP+DCS (FDR=0.04). False discovery rate (FDR), cecal ligation and puncture (CLP), daily chronic stress (DCS).
Late taxonomic changes in male mice after sepsis, as well as stress-induced microbiota changes, are dominated by a decrease in Clostridialis and increased Lactobacillus.
Subsequent analysis of the 16S sequencing from each sex cohort was performed to determine taxa associated with the diversity changes. The 7-day post-CLP+DCS changes in diversity in females compared to naive was dominated by a 65-log fold decrease in the order Clostridiales (FDR<0.001), which comprised the top 10 altered taxa. Conversely, the 7-day post-CLP+DCS changes in diversity in males was dominated by a 278-log fold decrease in the order Bacteroidales (FDR<0.001; supplemental Table 1). Given recovery of the female intestinal microbiota as evidenced by no difference in diversity compared to naïve, we sought to determine the taxa responsible for the persistent shift in diversity among male mice at 14 days post-CLP+DCS. The change was notably driven by a 449-log fold decrease in the order Clostridialis (FDR<0.001) and 474-log fold increase in genus Lactobacillus (supplemental Table 2).
Plasma estradiol and testosterone levels are unaffected by sepsis and stress-induced changes in the intestinal microbiota of B6 mice.
Given prior reports of the impact of sepsis on sex hormones and the possibility that the identified intestinal microbiota changes may be secondary to changes in these hormones induced by stress (8-11, 30), plasma was isolated from male and female mice after CLP+DCS. This demonstrated no significant difference in estradiol levels between naïve vs 7d post-CLP+DCS and 14d post-CLP+DCS (p=0.2 and 0.99, respectively; Figure 5A). Similarly, estradiol levels were similar in male B6 mice at 7 and 14 days post-CLP+DCS compared to their naïve control (p=0.99 and 0.91, respectively; Figure 5A). Plasma testosterone levels were measured in female and male B6 mice after CLP+DCS. This demonstrated stable testosterone levels in female mice at 7 and 14 days post-CLP+DCS (p=0.99 and 0.99, respectively; Figure 5B) compared to their naïve control as well as stability of testosterone levels in male mice 7 and 14 days post-CLP+DCS (p=0.23 and 0.1, respectively; Figure 5B). It should be noted that we were not able to determine the stage of the estrous cycle in the naïve and CLP+DCS female mice (31), although this may not be necessary for all sex-based murine research (32). In addition, it has been demonstrated that while there are clear increases in plasma estradiol in female mice during estrous (approximately 4 days), differences in plasma E2 levels between males and females during the non-estrous female days are not as clear cut (31, 32). These data indicate that sepsis and daily chronic stress induced changes in the murine intestinal microbiota may not be directly related to changes plasma sex hormone concentrations, at least in this wide age-range of animals.
Figure 5.
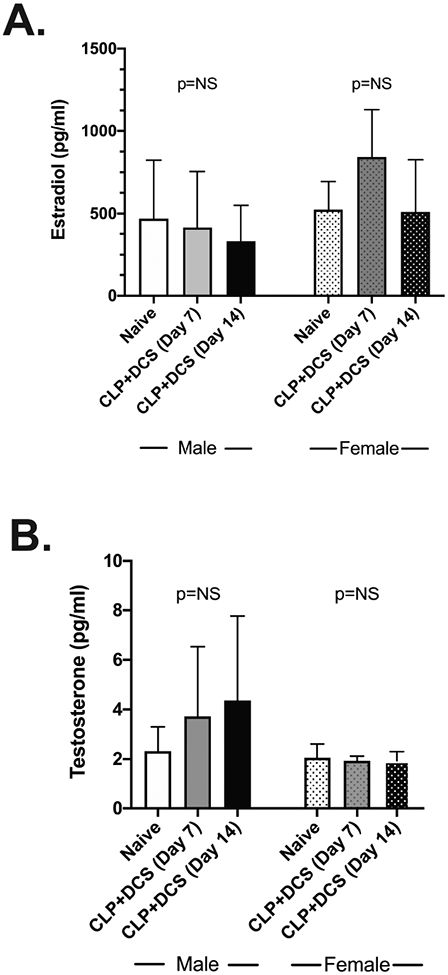
Plasma measurement of estradiol (A) and testosterone (B) in male and female B6 mice at baseline, 7 days, and 14 days post-CLP+DCS demonstrated no significant difference in plasma levels within sexes at the indicated time points. Cecal ligation and puncture (CLP), daily chronic stress (DCS), not significant (NS).
Discussion
Using our mouse surgical-sepsis model (CLP+DCS) emulating the human condition of CCI and the PICS endotype (19), we determined that although perturbations of the intestinal microbiota occur initially in both male and female C57BL/6 mice, the microbiota of males and females by day 14 post-sepsis are not equivalent. This could potentially be due to improved microbial resilience by two weeks after severe infection in females. This resilience may be also driven by age, given the stability of the young female and male microbiota in these age groups. Regardless, our results may help to better explain sex differences in post-sepsis ICU survival given that females may have better survival compared to males (8-11). These unique sex-related changes may be imparted on the host microbiota by the impact of sepsis and chronic stress. Such changes may lead to a chronic inflammatory state seen in PICS (4, 5). The impact of CPL+DCS on microbiota diversity may not be directly reliant on plasma testosterone or estradiol concentration given the lack of differences of these hormones during our murine model of surgical sepsis.
Despite the lack of differences in circulating estradiol in our cohort of animals, the protective link between estrogen and the microbiome is well established (33, 34). On one end, estrogen is positively correlated with gut microbiota species richness and α-diversity in both males and females (35). This relationship is supported by a reduction of diversity under estrogen deprivation states such as menopause (36). Importantly, this relationship has been reported to be responsible for the sexually dimorphic protection against metabolic stress in cycling females and to mediate the preventive effect against chronic low-grade inflammation (37). Of note, the gut microbiome has also been reported to regulate production of estradiol, particularly in postmenopausal women, an aspect that is termed the “estrabolome” and defined as the aggregate of enteric bacterial genes whose products are capable of metabolizing estrogens, which has been linked to breast cancer (38). Thus, this all suggests a complex relationship as well as potential source of variability when trying to link estradiol levels to microbiome changes across different disease states and ages.
Our study is limited in that we were not able to monitor the estrogen cycle of the mice, ensuring that all cycling female were at the same stage of their estrus cycle when measurements were taken (39). Of note, mice in general are considered acyclic or have lower estrogen starting at 18 months of age (39). However, our primary goal was to determine if there were differences in the concentrations of testosterone or estradiol at days 7 and 14 after surgical sepsis that may explain changes in the microbiota. Additionally, it has been stated that having mice all at the same stage of estrus may not be necessary for all sex-based murine research (32). Despite the inability to “normalize” females in their estrus cycle, the determination of plasma estradiol and testosterone levels supports that microbiota changes were primarily due to CLP+DCS. Additionally, future studies addressing only DCS may be useful to differentiate changes secondary to sepsis from those induced by chronic stress. However, our model has been validated to replicate the clinical patient scenario and is thus advantageous in the current study (22, 40, 41). Finally, we were limited in the total number of elderly mice available for these studies, which may have an impact on the observed changes in alpha and beta diversity. Future studies should focus on these older age groups with microbial reconstitution studies in an effort to “rescue” older mice from a state of PICS.
In conclusion, given prior clinical studies whereby females, for unknown reasons, recover more expeditiously after sepsis and ICU stress than males. It is imperative that the factors explaining these differences between the sexes are determined. Herein we identify that changes in the intestinal microbiota occur after sepsis with chronic daily stress and, while changes in diversity are initially present in both male and female mice, female mice demonstrate a unique microbiota at 2 weeks after sepsis (versus male mice), and this may be due to microbial resilience that is not present in the male mice. While further studies are required, our work may explain the persistent inflammatory state in males versus females that may make them more susceptible to CCI/PICS.
Supplementary Material
Funding
This work was supported, in part, by the following National Institutes of Health grants: R01 GM-113945, R01 GM-104481 (LLM), R01 AG-037984 (TCF), and P50 GM-111152 (LLM, PAE), awarded by the National Institute of General Medical Sciences (NIGMS). In addition, this work was supported, in part, by a postgraduate training grant T32 GM-008721 (DBD, JM, LK, BF) in burns, trauma, and perioperative injury by NIGMS and the McKnight Brain Research Foundation. Finally, this work was supported by NIH R21 AG072011 (OL).
Footnotes
Conflict of Interest
The authors declare that they have no relevant conflicts of interests.
This work was accepted for an oral presentation at the 80th Annual Meeting of the American Association for the Surgery of Trauma and Clinical Congress of Acute Care Surgery, 2021.
Supplemental Data. Top taxonomic changes in male mice 7 and 14 days post-CLP+DCS highlighting which order/genus dominated certain fold increases/decreases.
References
- 1.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41(5):1167–74. [DOI] [PubMed] [Google Scholar]
- 2.Organization WH, editor Improving the prevention, diagnosis and clinical management of sepsis (WHA 70.7). Seventieth World Health Assembly; 2017; Geneva, Switzerland. [Google Scholar]
- 3.Torio CM, Moore BJ. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013: Statistical Brief #204. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. 2016. [Google Scholar]
- 4.Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72(6):1491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stortz JA, Murphy TJ, Raymond SL, Mira JC, Ungaro R, Dirain ML, et al. Evidence for Persistent Immune Suppression in Patients Who Develop Chronic Critical Illness After Sepsis. Shock (Augusta, Ga. 2018;49(3):249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stortz JA, Mira JC, Raymond SL, Loftus TJ, Ozrazgat-Baslanti T, Wang Z, et al. Benchmarking clinical outcomes and the immunocatabolic phenotype of chronic critical illness after sepsis in surgical intensive care unit patients. J Trauma Acute Care Surg. 2018;84(2):342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brakenridge SC, Efron PA, Cox MC, Stortz JA, Hawkins RB, Ghita G, et al. Current Epidemiology of Surgical Sepsis: Discordance Between Inpatient Mortality and 1-year Outcomes. Ann Surg. 2019;270(3):502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang MQ, Macala KF, Fox-Robichaud A, Mendelson AA, Lalu MM, Sepsis Canada National Preclinical Sepsis P. Sex- and Gender-Dependent Differences in Clinical and Preclinical Sepsis. Shock (Augusta, Ga. 2021;56(2):178–87. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Montroy J, Sharma R, Fergusson DA, Mendelson AA, Macala KF, et al. The Effects of Biological Sex on Sepsis Treatments in Animal Models: A Systematic Review and a Narrative Elaboration on Sex- and Gender-Dependent Differences in Sepsis. Crit Care Explor. 2021;3(6):e0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J, Tong L, Yao J, Guo Z, Lui KY, Hu X, et al. Association of Sex With Clinical Outcome in Critically Ill Sepsis Patients: A Retrospective Analysis of the Large Clinical Database MIMIC-III. Shock (Augusta, Ga. 2019;52(2):146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim YS, Unno T, Kim BY, Park MS. Sex Differences in Gut Microbiota. World J Mens Health. 2020;38(1):48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haak BW, Wiersinga WJ. The role of the gut microbiota in sepsis. Lancet Gastroenterol Hepatol. 2017;2(2):135–43. [DOI] [PubMed] [Google Scholar]
- 13.Kang H, Thomas RM. Bacteria and Sepsis: Microbiome to the Rescue? J Clin Med. 2021;10(16):3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas RM, Jobin C. The Microbiome and Cancer: Is the 'Oncobiome' Mirage Real? Trends Cancer. 2015;1(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16(6):341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SM, DeFazio JR, Hyoju SK, Sangani K, Keskey R, Krezalek MA, et al. Fecal microbiota transplant rescues mice from human pathogen mediated sepsis by restoring systemic immunity. Nat Commun. 2020;11(1):2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knight R, Vrbanac A, Taylor BC, Aksenov A, Callewaert C, Debelius J, et al. Best practices for analysing microbiomes. Nat Rev Microbiol. 2018;16(7):410–22. [DOI] [PubMed] [Google Scholar]
- 18.Dogra SK, Dore J, Damak S. Gut Microbiota Resilience: Definition, Link to Health and Strategies for Intervention. Front Microbiol. 2020;11:572921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fransen F, van Beek AA, Borghuis T, Meijer B, Hugenholtz F, van der Gaast-de Jongh C, et al. The Impact of Gut Microbiota on Gender-Specific Differences in Immunity. Front Immunol. 2017;8:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Committee NRC. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press (US); 2011. Available from: https://www.ncbi.nlm.nih.gov/books/NBK54050/. [PubMed] [Google Scholar]
- 21.Franklin CL, Ericsson AC. Microbiota and reproducibility of rodent models. Lab Anim (NY). 2017;46(4):114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stortz JA, Hollen MK, Nacionales DC, Horiguchi H, Ungaro R, Dirain ML, et al. Old Mice Demonstrate Organ Dysfunction as well as Prolonged Inflammation, Immunosuppression, and Weight Loss in a Modified Surgical Sepsis Model. Crit Care Med. 2019;47(11):e919–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, et al. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods. 2013;10(1):57–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(16):5261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCafferty J, Muhlbauer M, Gharaibeh RZ, Arthur JC, Perez-Chanona E, Sha W, et al. Stochastic changes over time and not founder effects drive cage effects in microbial community assembly in a mouse model. ISME J. 2013;7(11):2116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PloS One. 2013;8(4):e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 28.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- 29.Mankowski RT, Thomas RM, Darden DB, Gharaibeh RZ, Hawkins RB, Cox MC, et al. Septic Stability? Gut Microbiota in Young Adult Mice Maintains Overall Stability After Sepsis Compared to Old Adult Mice. Shock (Augusta, Ga. 2021;55(4):519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valeri F, Endres K. How biological sex of the host shapes its gut microbiota. Front Neuroendocrinol. 2021;61:100912. [DOI] [PubMed] [Google Scholar]
- 31.Saito T, Ciobotaru A, Bopassa JC, Toro L, Stefani E, Eghbali M. Estrogen contributes to gender differences in mouse ventricular repolarization. Circ Res. 2009;105(4):343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beery AK. Inclusion of females does not increase variability in rodent research studies. Curr Opin Behav Sci. 2018;23:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aguilera M, Galvez-Ontiveros Y, Rivas A. Endobolome, a New Concept for Determining the Influence of Microbiota Disrupting Chemicals (MDC) in Relation to Specific Endocrine Pathogenesis. Front Microbiol. 2020;11:578007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoon K, Kim N. Roles of Sex Hormones and Gender in the Gut Microbiota. J Neurogastroenterol Motil. 2021;27(3):314–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flores R, Shi J, Fuhrman B, Xu X, Veenstra TD, Gail MH, et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med. 2012;10:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vieira AT, Castelo PM, Ribeiro DA, Ferreira CM. Influence of Oral and Gut Microbiota in the Health of Menopausal Women. Front Microbiol. 2017;8:1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaliannan K, Robertson RC, Murphy K, Stanton C, Kang C, Wang B, et al. Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome. 2018;6(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parida S, Sharma D. The Microbiome-Estrogen Connection and Breast Cancer Risk. Cells. 2019;8(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brooks HL, Pollow DP, Hoyer PB. The VCD Mouse Model of Menopause and Perimenopause for the Study of Sex Differences in Cardiovascular Disease and the Metabolic Syndrome. Physiology (Bethesda). 2016;31(4):250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Efron PA, Darden DB, Wang Z, Nacionales DC, Lopez MC, Hawkins RB, et al. Transcriptomic responses from improved murine sepsis models can better mimic human surgical sepsis. FASEB J. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mankowski RT, Thomas RM, Darden DB, Gharaibeh RZ, Hawkins RB, Cox MC, et al. Septic Stability? Gut Microbiota in Young Adult MICE Maintains Overall Stability After Sepsis Compared to Old Adult MICE. Shock (Augusta, Ga. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.