Graphical Abstract
Keywords: VITT, TTS, Platelet Factor 4, mRNA vaccine, mRNA-1273
To the Editor:
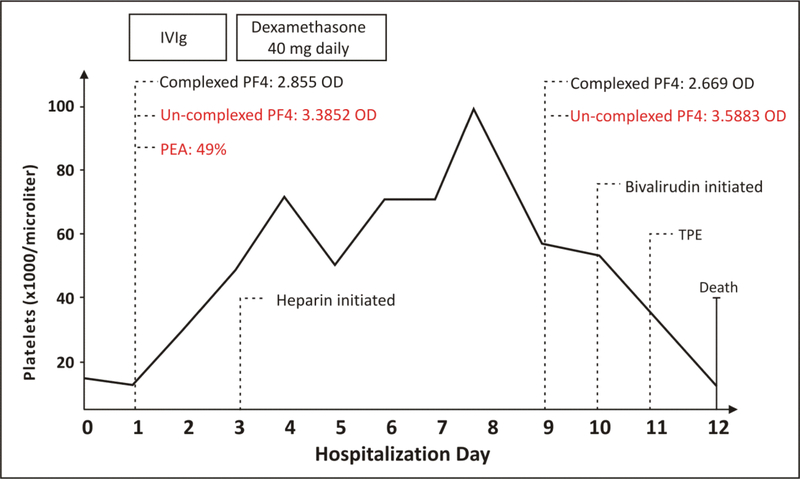
Recently, we reported on a 65-yr-old male who developed bilateral pulmonary emboli and lower extremity deep venous thromboses associated with severe thrombocytopenia (14,000/μL) ten days after receiving the second dose of the COVID-19 mRNA–1273 vaccine1. The patient was treated with intravenous immunoglobulin G and steroids for presumed immune thrombocytopenia, followed by unfractionated heparin. HIT testing obtained at two time points, immediately upon admission (first sample: prior to heparin therapy) and nine days into admission (second sample: after initiation of heparin therapy), demonstrated high optical densities (OD) in PF4-polyanion ELISA testing of 2.855 and 2.669, respectively1. A conventional serotonin release assay (that utilizes low concentrations of heparin) performed on the second sample was positive (51%; no functional testing was performed on the initial sample)1. Despite the cessation of heparin and initiation of bivalirudin treatment, the patient worsened due to the development of cerebral venous sinus thrombosis, shock, lactic acidemia, compartment syndrome, sepsis and ultimately died on day 12 of admission1. In the report, we suggested the possibility of vaccine-induced immune thrombotic thrombocytopenia (VITT), the first case of this type after mRNA vaccination. However, more recently, due to the rarity of this event (0.00855 per million mRNA-based COVID-19 vaccines2), and current dogma that VITT is an adenoviral-vector associated syndrome, the CDC recently concluded that this likely represented “…a background rate of spontaneous HIT or TTS associated with a different risk factor than cases associated with Ad26.COV2.S vaccination”2.
A challenge in distinguishing between VITT and the “background rate” of thrombotic thrombocytopenia due to anti-PF4 antibodies (i.e., spontaneous HIT) is a lack of tests capable of differentiating the two3; antibodies from both entities are detected in current ELISA and functional assays. In a just-published report in the American Journal of Hematology4, Kanack and colleagues make the novel finding that binding of antibodies to un-complexed PF4 can distinguish between these two syndromes. Thus, post-mortem, the un-complexed PF4 ELISA was used to further characterize our patient’s anti-PF4 antibodies. To avoid confounding antibodies that may have developed after heparin exposure, the pre-heparin (first) blood sample was initially tested. Figure 1 shows that this sample demonstrated a high OD of 3.3852, consistent with VITT antibodies, and was also found to be platelet-activating in the PEA (PF4-dependent P-selectin expression assay), an assay that uses PF4-treated platelets for the sensitive detection of VITT4 and HIT antibodies5. The follow-up sample continued to be strongly positive in the un-complexed PF4 ELISA (OD 3.5883), also consistent with VITT. These data are consistent with the possibility that non-adenoviral, mRNA-based vaccines can cause VITT in rare instances. To add to this case, the CDC reports two additional patients with a clinical/laboratory picture consistent with VITT after mRNA-1273 vaccination, including thrombosis (at unusual sites: cerebral venous sinus in one and mesenteric artery thrombosis in the other), thrombocytopenia, highly elevated d-dimers, and strong positive HIT ELISAs (OD>1.0) in both patients2. To the best of our knowledge samples from these patients have not been tested against un-complexed PF4 targets. In addition, a recent case of VITT has been reported after HPV vaccination (recombinant human papillomavirus quadrivalent vaccine) which uses non-adenoviral VLP (virus-like particle) vaccine technology6. Thus, we believe the emerging data on VITT after non-adenoviral vector vaccines highlighted by our case suggests that VITT should remain on the differential diagnosis for thrombotic thrombocytopenic reactions seen after multiple different vaccine types so that an accurate diagnosis can be made, and appropriate treatment interventions promptly instituted.
Figure 1. mRNA-1273 vaccine-associated antibodies recognize un-complexed PF4 targets.
Key testing, intervention, and platelet count trending are provided. The abscissa denotes days of hospitalization, and the ordinate shows the platelet count. Complexed PF4--PF4-polyanion ELISA (Lifecodes PF4 IgG); Un-complexed PF4-- Antibody binding to un-complexed PF4 targets in an ELISA format; PEA- PF4-dependent P-selectin Expression Assay; TPE- Therapeutic plasma exchange. Newly generated data since the prior report on this patient1 is indicated in red. Some data are reproduced with permission from Sangli et al. Thrombosis with Thrombocytopenia After the Messenger RNA-1273 vaccine. Annals of Internal Medicine. 2021 Oct;174(10):1480–1482. doi: 10.7326/L21-0244. Epub 2021 Jun 29 ©American College of Physicians.
Acknowledgments
We would like to thank Bandana Singh, PhD for the performance of the PEA study. This work was supported, in part, by National Institutes of Health grants HL158932 (AP).
Funding
The study was supported in part by HL158932 (National Institutes of Health).
Footnotes
Conflict of Interest disclosure
AP reports pending/issued patents (Mayo Clinic, Retham Technologies and Versiti), equity ownership in Retham Technologies, and serving on the advisory board of Veralox Therapeutics. The remaining authors declare no competing financial interests.
Data sharing statement
Data will be made available upon reasonable request to the corresponding author
References
- 1.Sangli S, Virani A, Cheronis N, et al. Thrombosis With Thrombocytopenia After the Messenger RNA-1273 Vaccine. Ann Intern Med. 2021;174(10):1480–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.See I, Lale A, Marquez P, et al. Case Series of Thrombosis With Thrombocytopenia Syndrome After COVID-19 Vaccination-United States, December 2020 to August 2021. Ann Intern Med. 2022. Jan 18;M21–4502. doi: 10.7326/M21-4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warkentin TE and Greinacher A. Laboratory testing for VITT antibodies. Seminars in Hematology. Mar 7, 2022. 10.1053/j.seminhematol.2022.03.003 [DOI] [PubMed] [Google Scholar]
- 4.Kanack AJ, Singh B, George G, et al. Persistence of Ad26.COV2.S-associated vaccine-induced immune thrombotic thrombocytopenia (VITT) and specific detection of VITT antibodies. Am J Hematol. 2022. Feb 7. doi: 10.1002/ajh.26488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samuelson Bannow B, Warad DM, Jones CG, et al. A prospective, blinded study of a PF4-dependent assay for HIT diagnosis. Blood. 2021;137(8):1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansen S, Laegreid IJ, Ernstsen SL, et al. Thrombosis and thrombocytopenia after HPV vaccination. J Thromb Haemost. 2021. Nov 24. doi: 10.1111/jth.15604. [DOI] [PMC free article] [PubMed] [Google Scholar]