Abstract
PURPOSE:
Fibroblast growth factor receptor (FGFR) genomic alterations (amplification, mutations, and/or fusions) occur in ~8% of gliomas, particularly FGFR1 and FGFR3. We conducted a multicenter open-label single-arm phase II study of a selective FGFR1–3 inhibitor, infigratinib (BGJ398), in patients with FGFR-altered recurrent gliomas.
PATIENTS AND METHODS:
Adults with recurrent/progressive gliomas harboring FGFR alterations received oral infigratinib 125 mg on days 1–21/28 days. The primary endpoint was investigator-assessed 6-month progression-free survival (PFS) rate by Response Assessment in Neuro-Oncology (RANO) criteria. Comprehensive genomic profiling was performed on available pre-treatment archival tissue to explore additional molecular correlations with efficacy.
RESULTS:
Among 26 patients, the 6-month PFS rate was 16.0% (95% CI 5.0–32.5), median PFS was 1.7 months (95% CI 1.1–2.8), and objective response rate was 3.8%. However, 4 patients had durable disease control lasting longer than 1 year: among these, 3 had tumors harboring activating point mutations at analogous positions of FGFR1 (K656E) [n=2] or FGFR3 (K650E) [n=1] in pre-treatment tissue; an FGFR3-TACC3 fusion was detected in the other. Hyperphosphatemia was the most frequently reported treatment-related adverse event (all-grade, 76.9%; grade 3, 3.8%) and is a known on-target toxicity of FGFR inhibitors.
CONCLUSIONS:
FGFR inhibitor monotherapy with infigratinib had limited efficacy in a population of patients with recurrent gliomas and different FGFR genetic alterations, but durable disease control lasting more than 1 year was observed in patients with tumors harboring FGFR1 or FGFR3 point mutations or FGFR3-TACC3 fusions. A follow-up study with refined biomarker inclusion criteria and centralized FGFR testing is warranted.
Keywords: Biomarkers, Infigratinib, Efficacy, FGFR, Glioma
Introduction
Gliomas are a clinically diverse group of primary brain tumors, diagnosed in ~100,000 people/year worldwide (1,2). Glioblastomas, the most common type of primary brain tumor, are particularly aggressive with a median overall survival (OS) of ~15–18 months after standard care (3). Historically, the classification of gliomas was based on histological findings and pathological grading. However, comprehensive molecular characterization over the past decade has identified complex genetic, epigenetic, and chromosomal changes that segregate gliomas into distinct molecular subtypes, with some genetic differences impacting response to therapy (4–6). For example, methylation of the MGMT promoter is both prognostic and predictive of benefit from temozolomide (7).
Fibroblast growth factor receptor (FGFR) genomic alterations (amplification, mutations and fusions) occur in ~8% of gliomas, with most aberrations occurring in FGFR1 and FGFR3 (8). Chromosomal translocations that fuse the tyrosine kinase domains of FGFR1 or FGFR3 and TACC1 or TACC3 have been identified in 2–4% of gliomas (9–11). These FGFR fusion genes, such as FGFR3-TACC3, are capable of ligand-independent dimerization by virtue of the newly fused coiled-coil domain and have demonstrated oncogenic potential in vitro and in vivo (9). Further, FGFR3-TACC3 fusion has been reported as predictive of response to FGFR tyrosine kinase inhibitors both preclinically (9,10) and clinically in various solid tumors including gliomas (10). Less is known about the underlying role of FGFR point mutations or amplification in gliomas (12). The oncogenic effects of FGFR mutations in human cancers, including gliomas, are variable (13,14), with FGFR amplification typically failing to drive tumor addiction to FGFR signaling (9).
Infigratinib (BGJ398) is a potent ATP-competitive FGFR1–3-selective oral tyrosine kinase inhibitor (15) in development for the treatment of patients with FGFR-driven conditions, including cholangiocarcinoma, urothelial carcinoma, and achondroplasia. In clinical studies, infigratinib 125 mg on days 1–21/28 achieved disease control in 84% of patients with advanced cholangiocarcinoma harboring FGFR2 fusions/translocations (16), and in 64% of patients with advanced urothelial carcinoma harboring FGFR3 alterations (17).
We conducted a multicenter phase II study to investigate the anti-tumor activity and safety of single-agent infigratinib in patients with FGFR-altered recurrent gliomas, particularly in tumors with FGFR-TACC fusions.
Methods
Study design
This open-label, single-arm, multicenter, phase II study of infigratinib in patients with recurrent high-grade gliomas after failure of initial therapy that harbored FGFR alterations was conducted at 14 centers in the US, Spain, Switzerland, the Netherlands, and Belgium (Clinicaltrials.gov ID NCT01975701). The study was not randomized. The primary goals were to investigate efficacy, safety, and tolerability, and to explore abnormalities of FGFR and other genes in pre-treatment archival tissue as molecular predictors of efficacy.
The study was implemented and reported in accordance with the Good Clinical Practice Guidelines, with applicable local regulations, and the Declaration of Helsinki. The study protocol was approved by the ethics committee at each participating center. Patients were required to provide written informed consent.
Patients
Originally, male or female patients age 18 years or older and Eastern Cooperative Oncology Group (ECOG) performance status ≤2 with recurrent gliomas harboring any FGFR abnormality (i.e. amplification, fusions, or mutations in FGFR1, FGFR2, FGFR3 or FGFR4) determined by local or central Clinical Laboratory Improvement Amendments (CLIA)-accredited laboratories before study entry were eligible. Pre-clinical research underpinning the rationale for the study suggested that FGFR3-TACC3 fusions were predictive of response to FGFR tyrosine kinase inhibitors. When the study launched, it was assumed that FGFR amplification correlated with presence of fusions (9). The eligibility criteria were amended 17 months after enrollment started (April 2015) to require fusions or FGFR1–3 activating mutations when further pre-clinical data showed that amplification was in fact a poor surrogate for the presence of fusion (10). Prior external beam radiotherapy and/or temozolomide was required. Otherwise, unlimited prior surgeries and anti-cancer treatments including bevacizumab were permitted, except for prior treatment with an FGFR inhibitor which was not allowed. Patients receiving anticonvulsant drugs that were strong inducers of CYP3A4 (e.g. carbamazepine, phenobarbital, phenytoin) were required to discontinue therapy ≥2 weeks before enrollment. Other key entry criteria reflecting the known safety profile of infigratinib entailed normal calcium/phosphate homeostasis and no history of corneal/keratopathy or retinal disorders. There were no specified criteria regarding body weight.
Supplementary Fig. S1 shows the relationship between the different study patient populations.
Treatment
Patients were intended to receive oral infigratinib 125 mg once daily on days 1–21 of each 28-day cycle until disease progression or unacceptable toxicity. No blinding was used in the study. Two protocol-specified dose reductions (to 100 and 75 mg/day) and dose interruptions (14 days maximum) were permitted to manage treatment-emergent toxicities. Adherence to a low-phosphate diet was recommended during therapy to manage hyperphosphatemia, a known class effect of FGFR inhibition. Prophylactic use of a phosphate-binding agent, such as sevelamer, was also recommended following a protocol amendment (August 2014).
Assessments
Molecular screening of tumor samples prior to study entry for FGFR alterations was performed by either a local laboratory or sponsor-designated central laboratory as part of eligibility assessments. Separately, archival tissue (≥10 unstained formalin-fixed paraffin-embedded slides) was also collected when available and underwent comprehensive genetic profiling using a clinically-validated next-generation sequencing (NGS) platform that sequences 324 genes for mutations, indels, copy number alterations, and select gene fusions (by Foundation Medicine; Cambridge, MA, USA) (18) for post-hoc molecular correlative analysis. Central pathology review of diagnoses was not performed.
Gadolinium-enhanced magnetic resonance imaging (MRI) was performed at baseline and every 8 weeks while on study. Response (or progression) was reported by the local investigator’s interpretation of the Response Assessment in Neuro-Oncology (RANO) criteria (19). Central review was not performed. Adverse events were evaluated according to the Common Terminology Criteria for Adverse Events, version 4.03. Ophthalmologic examinations (visual acuity testing, slit-lamp examination of anterior eye segment, intraocular pressure, and fundoscopy) were performed at baseline and then on days 1 and 15 of cycle 1, and day 1 of all subsequent cycles.
Endpoints
The primary study endpoint was investigator-assessed progression-free survival (PFS) rate at 6 months according to Response Assessment in Neuro-Oncology (RANO) criteria (19). Secondary endpoints were investigator-assessed overall response rate (ORR, complete or partial responses as best outcome), PFS, overall survival (OS), safety, and tolerability.
Statistical analysis
Sample size calculations were based on simulations of the operating characteristics of the study, rather than a power consideration. Assuming a true 6-month PFS rate of 50% and a uniform accrual of one patient per month, with a sample size of 24 evaluable patients in the per-protocol set (~17 PFS events), there was about 86.1% chance to achieve success at the end of the study, where a 6-month PFS rate of <16% was defined as unacceptable efficacy, 16–25% as demonstrating limited efficacy, 25–40% as demonstrating moderate efficacy, and >40% was consistent with clinically relevant efficacy.
Efficacy analyses were performed in the full analysis set (FAS), which included all patients who received ≥1 dose of infigratinib (n = 26). ORR was evaluated in patients from the FAS who had measurable disease per RANO criteria at their baseline scan and who were also re-assessed during treatment (n = 21). Safety analyses were based on the safety set, which included all patients who received ≥1 dose of infigratinib and had ≥1 valid post-baseline safety assessment (n = 26).
The Kaplan-Meier method was used to analyze time-to-event endpoints and provide estimates of survival rates and median values with 95% confidence intervals (CI). For PFS, patients who discontinued the study and were lost to follow-up on or before the cut-off date were censored at the date of their last available tumor assessment. For OS, patients who were alive at the time of completion of the study were censored at the last contact date or end of treatment visit date if the patient elected not to be followed post-study treatment. If the patient was still being followed, the patient was censored at the data cut-off date. Statistical analyses were generated using Statistical Analysis System (SAS) software, version 9.4 or later (RRID:SCR_008567; SAS Institute Inc., Cary, NC, USA). No formal statistical comparative tests were performed.
Data Availability
The data generated in this study are available within the article and its supplementary data files.
Results
Between December 2013 and May 2016, 731 patients were screened of whom 26 patients (3.6%) were enrolled into the study and included in the FAS; 11 (1.5%) and 15 (2.1%) patients, respectively, were enrolled before and after the protocol amendment that excluded patients with FGFR amplifications only. At the cut-off date (December 4, 2018), all patients had completed study treatment (progressive disease, n = 22; withdrawal by subject/guardian, n = 2; physician decision, n = 1; adverse event, n = 1).
Patients had a median age of 55 years (range, 20–76), 16 (61.5%) were male, and 18 (69.2%) had an ECOG performance status of ≤1 (Table 1). FGFR1 amplifications, mutations or fusions were identified in 1 (3.8%), 3 (11.5%) and 1 (3.8%) patients, respectively, and FGFR3 amplifications, mutations or fusions were identified in 11 (42.3%), 2 (7.7%) and 10 (38.5%) patients, respectively (Supplementary Fig. S2 and S3). Three patients (11.5%) had evidence of both FGFR3 amplifications and FGFR3-TACC3 fusions. No FGFR2 or FGFR4 alterations were identified.
Table 1.
Baseline characteristics (Full analysis set)
| Variable | Infigratinib (N = 26) |
|---|---|
| Age, years | |
| Median (range) | 55 (20–76) |
| Sex, n (%) | |
| Male | 16 (61.5) |
| Female | 10 (38.5) |
| Race, n (%) | |
| Caucasian | 26 (100) |
| ECOG performance status, n (%) | |
| 0 | 6 (23.1) |
| 1 | 12 (46.2) |
| 2 | 8 (30.8) |
| Histology, n (%)a | |
| Glioblastoma | 19 (73.1) |
| Anaplastic astrocytoma | 5 (19.2) |
| Other glioma | 2 (7.7) |
| Measurable disease at baseline, n (%) | 22 (84.6) |
| IDH1/IDH2 status, n (%) | |
| IDH1 mutation (R132H) | 2 (7.7) |
| FGFR1 status, n (%)b | |
| Amplification | 1 (3.8) |
| Fusion (ARHGEF18) | 1 (3.8) |
| Mutation | 3 (11.5) |
| K656E | 2 (7.7) |
| N546K | 1 (3.8) |
| FGFR3 status, n (%)bc | |
| Amplification | 11 (42.3) |
| Fusion (TACC3) | 10 (38.5) |
| Mutation | 2 (7.7) |
| K650E | 1 (3.8) |
| S249C | 1 (3.8) |
| Prior treatment, n (%) | |
| Radiotherapy | 26 (100.0) |
| Anti-neoplastic therapy | 25 (96.2) |
| Temozolomide | 23 (88.5) |
| Bevacizumab | 10 (38.5) |
| Other | 1 (3.8) |
| Prior anti-neoplastic regimens, n (%) | |
| 0 | 1 (3.8) |
| 1 | 11 (42.3) |
| 2 | 9 (34.6) |
| ≥3 | 5 (19.2) |
Both diagnoses of “glioma” were subsequently clarified post-hoc as glioblastoma and one of “anaplastic astrocytoma” would be currently defined as a molecular glioblastoma (IDH-wild type, TERT-mutant diffuse astrocytoma; Supplementary Fig. S6 with references therein).
FGFR alterations for enrollment by local CLIA-accredited or central laboratory during screening, as reported by the investigator.
Three patients had more than 1 FGFR3 alteration.
Abbreviations: CLIA, Clinical Laboratory Improvement Amendments; ECOG, Eastern Cooperative Oncology Group; FGFR, fibroblast growth factor receptor; IDH, isocitrate dehydrogenase.
Treatment exposure
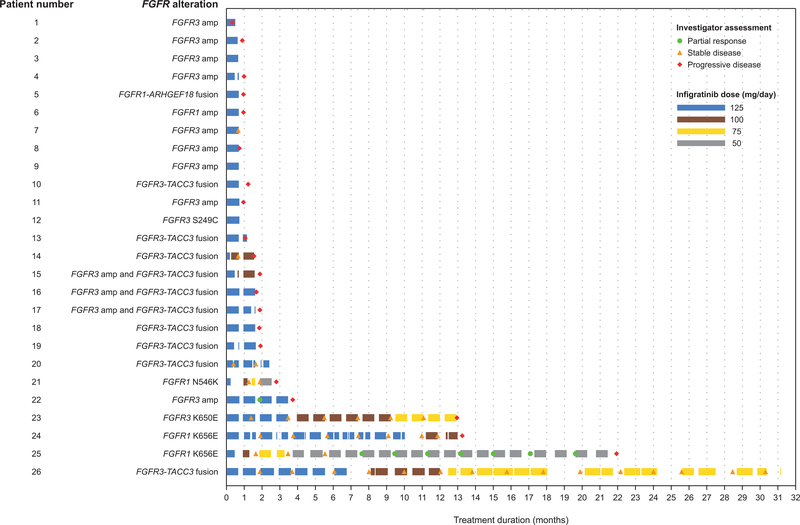
The median duration of infigratinib therapy was 1.4 months (range, 0.5–31.1). Nineteen patients (73.1%) received infigratinib for ≤2 months, and 4 patients (15.4%) for >12 months. Mean relative dose intensity, defined as actual cumulative dose divided by planned cumulative dose for actual treatment duration, was 89.4% (SD ±17.9).
Efficacy
Efficacy findings are presented in Table 2. In the FAS, the 6-month PFS rate, the primary endpoint, was 16.0% (95% CI 5.0–32.5) and median PFS was 1.7 months (95% CI 1.1–2.8) [Supplementary Fig. S4; Supplementary Table S1].
Table 2.
Efficacy
| Variable | Outcome |
|---|---|
| Primary endpoint | |
| Progression-free survival (n = 26) | |
| 6-month progression-free survival rate (95% CI), % | 16.0 (5.0–32.5) |
| Median (95% CI), months | 1.7 (1.1–2.8) |
| Secondary endpoints | |
| Best response | |
| Efficacy analysis set (n = 21), n (%) a | |
| Objective response rate | 1 (4.8) |
| (95% CI) | (0.1–23.8) |
| Partial response | 1 (4.8) |
| Stable disease | 6 (28.6) |
| Progressive disease | 13 (61.9) |
| Full analysis set (n= 26), n (%) | |
| Objective response rate | 1 (3.8) |
| (95% CI) | (0.1–19.6) |
| Partial response | 1 (3.8) |
| Stable disease | 6 (23.1) |
| Progressive disease | 15 (57.7) |
| Unknown/missing | 4 (15.4) |
| Overall survival (n = 26) | |
| Median (95% CI), months | 6.7 (4.2–11.7) |
Patients with measurable lesions at baseline and who were re-assessed during treatment.
Abbreviation: CI, confidence interval.
Note: Full analysis set (n = 26) unless otherwise stated.
A swimmer plot showing outcomes for individual patients at each assessment is presented in Fig. 1. Four patients had durable disease control with infigratinib lasting >1 year; one patient had a partial response (FGFR1 K656E mutation) with a PFS of 21.9 months, and three patients had stable disease [FGFR1 K656E mutation, PFS, 13.2 months; FGFR3-TACC3 fusion, PFS, 30.2 months; FGFR3 K650E mutation, PFS, 12.9 months]. Two of the patients with stable disease (FGFR1 K656E and FGFR3-TACC3 fusion) had received two prior lines of systemic treatment including bevacizumab. In both cases treated with bevacizumab before infigratinib, the timing of progression for which infigratinib was started (approximately 8 years after radiotherapy and 10 months after radiotherapy in one case each) makes enrollment for pseudoprogression extremely unlikely; moreover, in one of these cases, there was histologically proven recurrence before starting infigratinib. In one patient with partial response, the pattern of progression after chemoradiotherapy (newly enhancing, leptomeningeal, outside the high dose radiotherapy field) and biomarker pattern [H3 K27M mutation which is nearly always mutually exclusive with MGMT promoter (20,21)] also makes it unlikely the patient was treated with infigratinib for pseudoprogression (22) rather than disease refractory to standard treatment. Magnetic resonance images of all 4 patients with durable stable disease or partial response are shown in Supplementary Figs. S5–S8.
Figure 1.
Swimmer plot of time on infigratinib therapy and response (per local investigator) at each assessment (n = 26)
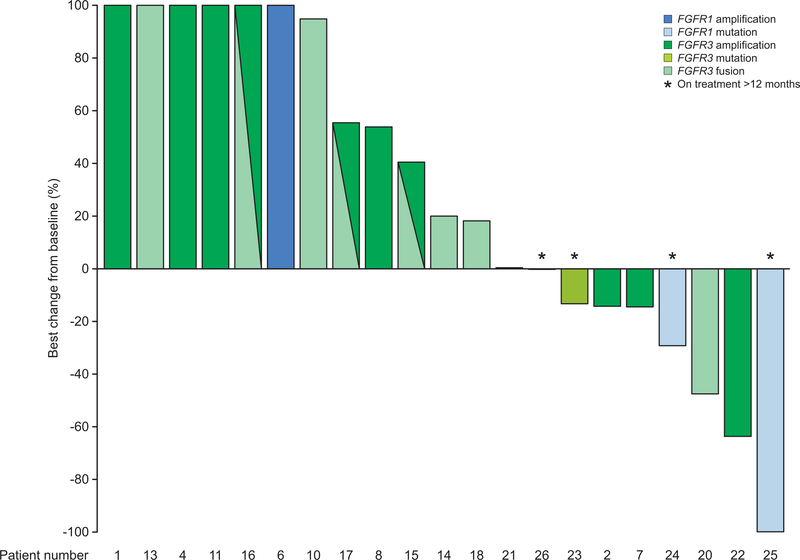
In patients with measurable disease at baseline and who were re-assessed during treatment (n = 21), ORR was 4.8% (1 partial response; Table 2). Seven patients experienced tumor shrinkage, with best percent change ranging from −13% to −100%. In addition to one RANO-confirmed partial response, one patient had a decrease of −64% that was not confirmed on follow-up assessment, and another had a decrease of −48%. The single patient with a RANO-confirmed partial response harbored an FGFR1 K656E mutation, whereas a further 6 patients (33.3%) had stable disease (FGFR1: mutation, n = 2; FGFR3: mutation, n = 1; FGFR3-TACC3 fusion, n = 2; amplification, n = 1). A waterfall plot of the percentage change in tumor size in patients with measurable disease is presented in Fig. 2.
Figure 2.
Best percentage change from baseline in tumor size in patients with measurable lesions at baseline and who were re-assessed post-baseline (n = 21)
Note: The percentage changes of 6 patients on left of the waterfall plot were greater than 100% but are truncated at 100% for presentation purposes. FGFR1 mutation: N546 (n = 1) and K656E (n = 2); FGFR3 mutation: K650E (n = 1); FGFR3-TACC3 fusion (n = 9).
FGFR, fibroblast growth factor receptor.
OS analysis is mature. In the FAS, 24 patients (88.5%) had died at the time of data cut-off. Median OS was 6.7 months (95% CI 4.2–11.7) [Supplementary Fig. S4]. The 6-month and 12-month OS rates were 53.8% (95% CI 33.3–70.6) and 29.6% (95% CI 13.5–47.7), respectively (Supplementary Table S2).
Exploratory biomarker analyses
Sufficient tumor tissue for comprehensive NGS was available for 16 of 26 patients (Supplementary Figs. S1 and S3). The number of deleterious genetic alterations in each tumor ranged from 2 to 25, and there were too few tumors with any single alteration to correlate formally with efficacy. Testing encompassed analysis for other molecular abnormalities common in glioma. For example, IDH1 R132H, common in lower grade gliomas and associated with a favorable prognosis independent of treatment, was identified in two patients with FGFR3 amplification, one patient with progressive disease, and one patient with stable disease as best response, respectively; however, IDH mutation was mutually exclusive with FGFR fusions, consistent with prior publications (9). IDH mutation was also mutually exclusive with FGFR point mutations. Mutations in the TERT promoter, known to activate telomerase expression, were most prevalent (11/16, 69%), followed by alterations in CDKN2A (7/16, 44%), amplification of CDK4 (5/16, 31%), CDKN2B deletions (5/16, 31%), alterations in PTEN (5/16, 31%), and known mutations in TP53 (4/16, 25%). Mutations in the H3K27 methyltransferase KMT2C genes, which are associated with poor prognosis, were identified in 6/16 patients (37%) including one patient with a partial response. Known deleterious mutations in ATRX (3/16, 19%) and NF1 (3/16, 19%), that are commonly mutated in gliomas, were also identified (Supplementary Fig. S3). Discordance between NGS and another assay occurred in 6 of 8 cases with detailed response and molecular data (Supplementary Fig. S3, and Table S3 with additional case-level detail).
Safety
Hyperphosphatemia was the most frequently reported treatment-related adverse event [all-grade, 20 patients (76.9%); grade 3, 1 patient (3.8%)]. For the management of hyperphosphatemia, 22 patients (84.6%) used a phosphate-lowering agent, primarily sevelamer, and 5 patients (19.2%) required infigratinib dose reductions or interruptions. Other common all-grade treatment-related adverse events were fatigue in 7 patients (26.9%) and diarrhea in 5 patients (19.2%). Other grade 3 treatment-related adverse events were hyperlipasemia in 2 patients (7.7%), hypophosphatemia in 2 patients (7.7%), and diarrhea, fatigue, stomatitis and nail disorder in 1 patient each (3.8%). The most common treatment-related adverse events are reported in Table 3, and a safety summary is presented in Supplementary Table S4.
Table 3.
Most common treatment-related adverse events occurring in at least 5% of patients (Safety set)
| Infigratinib (N = 26) |
||
|---|---|---|
| Adverse event, n (%) | All grades | Grade 3a |
| Total | 22 (84.6) | 7 (26.9) |
| Hyperphosphatemia | 20 (76.9) | 1 (3.8) |
| Fatigue | 7 (26.9) | 1 (3.8) |
| Diarrhea | 5 (19.2) | 0 |
| Hyperlipasemia | 4 (15.4) | 2 (7.7) |
| Stomatitis | 4 (15.4) | 1 (3.8) |
| Dry skin | 4 (15.4) | 0 |
| Hypophosphatemia | 3 (11.5) | 2 (7.7) |
| Alopecia | 3 (11.5) | 0 |
| Decreased appetite | 3 (11.5) | 0 |
| Dyspepsia | 3 (11.5) | 0 |
| Onycholysis | 3 (11.5) | 0 |
| Palmar-plantar erythrodysesthesia | 3 (11.5) | 0 |
| Nail disorder | 2 (7.7) | 1 (3.8) |
| Constipation | 2 (7.7) | 0 |
| Dermatitis acneiform | 2 (7.7) | 0 |
| Dry eye | 2 (7.7) | 0 |
| Mucosal inflammation | 2 (7.7) | 0 |
No grade 4 or 5 treatment-related toxicities were reported.
There were no grade 4 or 5 treatment-related toxicities. One patient experienced a serious adverse event which was suspected to be treatment-related (hyperphosphatemia). No patients had treatment-related adverse events which required discontinuation of infigratinib. There was no apparent cumulative toxicity reported for subjects receiving infigratinib for >1 year.
Discussion
We tested targeted therapy with an FGFR1–3 inhibitor, infigratinib, in a multicenter phase II study in patients with recurrent gliomas and FGFR alterations. At the outset, the study was designed to include patients with any FGFR abnormality (i.e. amplification, fusions, or mutations) in the hope that broad selection criteria would enrich the population of responders. The inclusion criteria were subsequently restricted to FGFR mutations and fusions after it became apparent that FGFR amplification alone did not predict benefit pre-clinically or serve as a surrogate for fusion (10). Our data further support this observation, as most patients with FGFR1/3 amplification had progressive disease and the only RANO-confirmed response was observed among the 16 patients with FGFR fusions or mutations. One patient with an FGFR3 amplification that achieved a 64% decrease in tumor size from baseline had a concurrent IDH1 R132H mutation that may have contributed to the patient’s best response of stable disease. This absence of response for amplification-positive patients is consistent with other cancers where point mutations/deletions rather than amplification alone, predict response to tyrosine kinase inhibitors, for example EGFR mutations in non-small cell lung cancer (23). Perhaps impacted by the inclusion of amplification-only patients, our study showed that FGFR inhibitor monotherapy had limited efficacy, with a 6-month PFS rate of 16% and ORR <5%. Although this technically met the pre-specified threshold for 6-month PFS rate as meriting further study, treatment of patients with broad FGFR selection criteria is not warranted in this disease setting.
Our study was also limited by lack of consistent or mandated central confirmation of the FGFR alterations used for eligibility, with discordance for FGFR3 amplification between the screening assays and post-hoc NGS observed commonly (Supplementary Table S3 and Fig. S3). We view NGS as a “gold standard” for the detection of FGFR alterations absent a suitable and reliable alternative, and we recommend that future studies use a single, central assay for molecular screening to homogenize the biomarker-selected population for FGFR3 fusions or activating point mutations in determining eligibility.
Regardless of the small ORR, durable disease control lasting >1 year was observed in 4 patients, one of whom continues to receive infigratinib on a compassionate-use basis with stable disease lasting 46.5 months (as of January 17, 2020). It is also notable that 2 of the 4 patients with prolonged benefit had received prior bevacizumab, a patient profile with a particularly poor prognosis. Molecular profiling in these cases with durable response or stabilization revealed the presence of FGFR3-TACC3 fusions (n = 1) or activating mutations in FGFR1 K656E (n = 2) or FGFR3 K650E (n = 1) which occur at analogous positions in these two receptors (Supplementary Fig. S9). While it has been reported previously that the presence of FGFR-TACC3 fusions in patients with glioma confers sensitivity to FGFR tyrosine kinase inhibitors (10), we are unaware of any reports describing clinical activity through FGFR inhibition in glioma tumors with FGFR point mutations. Therefore, our study provides additional information about genetic lesions that sensitize gliomas to FGFR inhibition. Of note, the patient with a durable partial response had a FGFR1 K565E-positive midline glioma with a H3K27M mutation, a condition that is associated with a poor prognosis and is not sensitive to standard treatment options (24). Collectively, our data suggest that a future trial of infigratinib, either alone or in combination with another targeted agent and/or radiotherapy, in patients with gliomas harboring FGFR point mutations and/or FGFR3 fusions or FGFR-mutated midline gliomas would be of interest. Coincident alterations in cell cycle genes (CDK4, CDKN2A, and CDKN2B) or H3F3A observed in our study population provide insight into possible combination partners with targeted inhibitors of CDKs and histone deacetylase enzymes.
Unlike the more common activating mutations that occur in the extracellular IgG-like domains in FGFR2 in cholangiocarcinoma (16) and FGFR3 in urothelial carcinoma (17), the activating mutations that were detected in this glioma cohort occurred within the intracellular kinase domains in both FGFR1 (N546K and K656E) and FGFR3 (K650E). Of the four patients with mutations in the intracellular kinase domain, 3 had durable benefit as described above and the fourth patient, with an FGFR1 N546K mutation, had a decrease of 32%. The stable disease control observed for patients with kinase domain mutations is supported by in vitro cell growth inhibition assays, which showed that the growth of Ba/F3 cells transformed by FGFR1-N546K, FGFR1-K656E, FGFR3b-K652E and FGFR3c-K650E could be inhibited efficiently by infigratinib (25) (Supplementary Fig. S10). These observations lend further support to the importance of activating point mutations at FGFR1/3 as both are transforming and adequately targeted by infigratinib. Infigratinib also showed clinical activity in two patients with recurrent glioblastomas and FGFR3 fusions, the same as we found here, in a separate basket trial (Supplementary Table S5) (Clinicaltrials.gov ID NCT02160041) (26).
Our findings are particularly encouraging in a disease setting where there is currently no established therapy beyond radiotherapy and alkylating chemotherapy for patients with recurrent gliomas (27), and outcomes with available treatment options are generally poor. For example, nitrosoureas (lomustine or carmustine), recommended for patients with recurrent disease after standard radiotherapy and temozolomide (27), provide only short-lived disease control (median PFS, 1–4 months) (28–30). Future clinical investigation in glioma should consider further refinements to the FGFR biomarker criteria and glioma subtypes that are most likely to benefit from FGFR inhibition. Combinatorial trials with an FGFR inhibitor and existing treatments may help to identify specific biomarker cohorts that show increased sensitivity to radiotherapy and alkylating chemotherapy. Of note, FGFR-mediated phosphorylation of PTEN (pY240-PTEN) has been identified as a mechanism of radiation resistance and actionable target for improving radiotherapy efficacy (31).
Similar to most anticancer drugs, FGFR inhibitors were not developed specifically for CNS tumors, and the distribution of these agents within intracranial tumors is largely unknown because of a lack of supporting pharmacokinetic studies (13). Preclinical studies in Wistar rats suggest that infigratinib penetrates into the CNS: infigratinib was detectable in the brain for up to 12 hours with a brain-to-plasma under the time-concentration curve ratio of 0.68 after a single oral 10 mg/kg dose (data on file, QED Therapeutics). The protocol design encompassed a surgical arm to explore tissue pharmacokinetics, but it did not accrue any patients which is a limitation of our study. Additional preclinical and clinical studies to investigate the CNS penetration of infigratinib are currently in progress and will be reported separately.
The most commonly reported treatment-related adverse events with infigratinib at the oncologic doses used in this study included hyperphosphatemia, fatigue, and diarrhea, a safety profile that is consistent with previous clinical trials (16,17,32). Hyperphosphatemia, a class effect thought to be due to FGFR inhibition of FGF-23-mediated renal phosphate homeostasis (33), was the most common toxicity. A proactive strategy, including an intermittent dosing schedule, active monitoring, early intervention with dose interruptions or dose reductions, dietary restrictions, and prophylactic use of a phosphate-lowering agent (32), appeared to be effective for the management of hyperphosphatemia in our study.
In conclusion, single-agent infigratinib had limited efficacy in a population of patients with recurrent gliomas without robust molecular selection other than harboring any FGFR alteration. However, durable disease control lasting >1 year was observed in a patient subset with activating FGFR1 or FGFR3 point mutations or FGFR3 fusions. Further trials with refined biomarker inclusion criteria and centrally conducted molecular analyses are under development.
Supplementary Material
Translational relevance.
This study highlights that FGFR inhibitor monotherapy has limited efficacy in patients with recurrent gliomas harboring any FGFR alteration. However, durable disease control was apparent in 4 patients with tumors with activating FGFR1 or FGFR3 mutations or FGFR3-TACC3 fusions. Further studies of FGFR inhibitors with refined biomarker inclusion criteria are warranted.
Acknowledgments
The field is indebted to Drs. Antonio Iavarone, Anna Lasorella, and their colleagues and collaborators for seminal discoveries regarding FGFR fusions. Medical writing support was provided by Harriet Lamb and Lee Miller of Miller Medical Communications, and funded by QED Therapeutics Inc. Randi Isaacs (formerly Clinical Site Head, Translational Clinical Oncology, Novartis Institutes for Biomedical Research, East Hanover, NJ) provided historical perspective and critical review of the manuscript.
Outside of the reported study, A.B.L. was supported in part by The William Rhodes and Louise Tilzer-Rhodes Center for Glioblastoma at New York-Presbyterian Hospital, The Michael Weiner Glioblastoma Research Into Treatment Fund, Voices Against Brain Cancer, and the National Institutes of Health (NIH)/ National Cancer Institute NCI grants P30CA013696 and UG1CA189960. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH/NCI.
Financial support:
The study was sponsored by Novartis and QED Therapeutics, an affiliate of BridgeBio Pharma.
Footnotes
Conflict of interest disclosure statement
Andrew Lassman: received study-relevant honoraria, travel support, and research funding from QED and Novartis, and (in the last 12-months) also received research funding and/or other support from Bayer, Orbus, Agios, Kadmon, VBI Vaccines, Beigene, Oncoceutics, Pfizer, Genentech/Roche, Millenium, Celldex, Novartis, BMS, AbbVie. Novocure, Northwest Biotherapeutics, Celgene, Aeterna Zentaris, Abbott Molecular; and honoraria from Novocure, Orbus, Karyopharm, Sapience, Vivacitas Oncology, and Bioclinica as a blinded independent reader of clinical and imaging data for a BMS-sponsored trial.
Juan Manuel Sepúlveda-Sánchez: Research grants from IDC Pharma and Pfizer, and honoraria from Abbvie, Celgene, and Pfizer, and travel support from Abbvie.
Timothy Cloughesy is cofounder, major stock holder, consultant and board member of Katmai Pharmaceuticals, member of the board for the 501c3 Global Coalition for Adaptive Research, holds stock option of Notable Labs, holds stock in Chimerix and receives milestone payments and possible future royalties, member of the scientific advisory board for Break Through Cancer, member of the scientific advisory board for Cure Brain Cancer Foundation, has provided paid consulting services to GCAR, Gan & Lee, BrainStorm, Katmai, Sapience, Inovio, Vigeo Therapeutics, DNATrix, Tyme, SDP, Novartis, Roche, Kintara, Bayer, Merck, Boehinger Ingelheim, VBL, Amgen, Kiyatec, Odonate Therapeutics QED, Medefield, Pascal Biosciences, Bayer, Tocagen, Karyopharm, GW Pharma, Abbvie, VBI, Deciphera, VBL, Agios, Genocea, Celgene, Puma, Lilly, BMS, Cortice, Wellcome Trust, Novocure, Novogen, Boston Biomedical, Sunovion, Human Longevity, Insys, ProNai, Pfizer, Notable labs, Medqia Trizel, Medscape and has contracts with UCLA for the Brain Tumor Program with Oncovir, Merck, Oncoceutics, Novartis, Amgen, Abbvie, DNAtrix, Beigene, BMS, AstraZeneca, Kazia, Agios, Boston Biomedical, Deciphera, Tocagen, Orbus, AstraZenica, Karyopharm.
Miguel J. Gil-Gil: Honoraria from Daiichi-Sankyo, Novartis, Pfizer and Roche, and Advisory board for Genomic Health.
Vinay K. Puduvalli: Research funding from Abbvie, Beigene, Bexion, Celldex, DNAtrix, and Novartis, advisory or data safety monitoring board honoraria from Novocure, Orbus Therapeutics, SK Lifesciences, and Ziopharm, and equity in Gilead and Amarin.
Jeffrey Raizer: Employment: Astellas
Filip Y. F. De Vos: Research grants from AbbVie, BioClin Therapeutics, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Octimed Oncology BV, and Vaximm, and travel costs from Roche.
Patrick Y. Wen: Research support from Agios, Astra Zeneca/Medimmune, Beigene, Celgene, Eli Lily, Genentech/Roche, Kazia, MediciNova, Merck, Novartis, NuvationBio, Oncoceutics, Vascular Biogenics, and VBI Vaccines, and advisory board for Agios, AstraZeneca, Bayer, Boston Pharmaceuticals, CNS Pharmaceuticals, ElevateBio, ImmunomicTherapeutics, Imvax, Karyopharm, Merck, Novartis, NuvationBio, QED Therapeutics, Vascular Biogenics, VBI Vaccines, and Voyager.
Nicolas Butowski: Research support from Agios, Beigene, Eli Lily, Genentech/Roche, Kiyatec, Medicenna, Merck, Oncoceutics, and Vascular Biogenics, and advisory board for Boston Pharmaceuticals, Cellinta, CNS Pharmaceuticals, Delmar, Karyopharm, Lynx Group, QED Therapeutics and Vascular Biogenics.
Paul M. Clement: Research grants from AstraZeneca, and consultancy/advisory board for Abbvie, BMS, Daiichi-Sankyo, Merck, MSD, Leo Pharma, and Vifor Pharma.
Morris Groves: None.
Cristobal Belda-Iniesta: None.
Pierre Giglio: Research support from Agios, Denovo Biopharma, Prelude Therapeutics, Novocure.
Harris S. Soifer: Employment, stock & other ownership interests for QED Therapeutics.
Steven Rowsey: Employment for QED Therapeutics.
Cindy Xu: Employment, stock & other ownership interests for QED Therapeutics.
Francesca Avogadri: Employment, stock & other ownership interests for QED Therapeutics.
Susan Moran: Employment, stock & other ownership interests for QED Therapeutics.
Ge Wei: Employment, stock & other ownership interests for QED Therapeutics.
Patrick Roth: Research grants from MSD and Novocure, and honoraria for advisory board participation or lectures from Bristol-Myers Squibb, Covagen, Debiopharm, Medac, MSD, Novartis, Novocure, QED Therapeutics, Roche, and Virometix.
Publisher's Disclaimer: Presented in part at the 14th European Association of Neuro-Oncology (EANO) Annual Meeting held in Lyon, France from 19–22 September 2019, and the Society for Neuro-Oncology (SNO) 2019 Annual Meeting held in Phoenix, Arizona from 20–24 November 2019.
References
- 1.Molinaro AM, Taylor JW, Wiencke JK, Wrensch MR. Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol 2019;15:405–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol 2019;21:v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wen PY, Weller M, Lee EQ, Alexander BM, Barnholtz-Sloan JS, Barthel FP, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol 2020;22:1073–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 2016;131:803–20. [DOI] [PubMed] [Google Scholar]
- 5.Bush NA, Butowski N. The effect of molecular diagnostics on the treatment of glioma. Curr Oncol Rep 2017;19:26. [DOI] [PubMed] [Google Scholar]
- 6.Diamandis P, Aldape KD. Insights from molecular profiling of adult glioma. J Clin Oncol 2017;35:2386–93. [DOI] [PubMed] [Google Scholar]
- 7.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005;352:997–1003. [DOI] [PubMed] [Google Scholar]
- 8.Helsten T, Elkin S, Arthur E, Tomson BN, Carter J, Kurzrock R. The FGFR landscape in cancer: analysis of 4,853 tumors by next-generation sequencing. Clin Cancer Res 2016;22:259–67. [DOI] [PubMed] [Google Scholar]
- 9.Singh D, Chan JM, Zoppoli P, Niola F, Sullivan R, Castano A, et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science 2012;337:1231–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Stefano AL, Fucci A, Frattini V, Labussiere M, Mokhtari K, Zoppoli P, et al. Detection, characterization, and inhibition of FGFR-TACC fusions in IDH wild-type glioma. Clin Cancer Res 2015;21:3307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonsson P, Lin AL, Young RJ, DiStefano NM, Hyman DM, Li BT, et al. Genomic correlates of disease progression and treatment response in prospectively characterized gliomas. Clin Cancer Res 2019;25:5537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jimenez-Pascual A, Siebzehnrubl FA. Fibroblast growth factor receptor functions in glioblastoma. Cells 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lasorella A, Sanson M, Iavarone A. FGFR-TACC gene fusions in human glioma. Neuro Oncol 2017;19:475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rand V, Huang J, Stockwell T, Ferriera S, Buzko O, Levy S, et al. Sequence survey of receptor tyrosine kinases reveals mutations in glioblastomas. Proc Natl Acad Sci U S A 2005;102:14344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guagnano V, Kauffmann A, Wohrle S, Stamm C, Ito M, Barys L, et al. FGFR genetic alterations predict for sensitivity to NVP-BGJ398, a selective pan-FGFR inhibitor. Cancer Discov 2012;2:1118–33. [DOI] [PubMed] [Google Scholar]
- 16.Javle M, Roychowdhury S, Kelley RK, Sadeghi S, Macarulla T, Weiss KH, et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol Hepatol 2021;6:803–15. [DOI] [PubMed] [Google Scholar]
- 17.Pal SK, Rosenberg JE, Hoffman-Censits JH, Berger R, Quinn DI, Galsky MD, et al. Efficacy of BGJ398, a fibroblast growth factor receptor 1–3 inhibitor, in patients with previously treated advanced urothelial carcinoma with FGFR3 alterations. Cancer Discov 2018;8:812–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol 2013;31:1023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 2010;28:1963–72. [DOI] [PubMed] [Google Scholar]
- 20.Picart T, Barritault M, Poncet D, Berner LP, Izquierdo C, Tabouret E, et al. Characteristics of diffuse hemispheric gliomas, H3 G34-mutant in adults. Neurooncol Adv 2021;3:vdab061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banan R, Christians A, Bartels S, Lehmann U, Hartmann C. Absence of MGMT promoter methylation in diffuse midline glioma, H3 K27M-mutant. Acta Neuropathol Commun 2017;5:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 2008;26:2192–7. [DOI] [PubMed] [Google Scholar]
- 23.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129–39. [DOI] [PubMed] [Google Scholar]
- 24.Daoud EV, Rajaram V, Cai C, Oberle RJ, Martin GR, Raisanen JM, et al. Adult brainstem gliomas with H3K27M mutation: radiology, pathology, and prognosis. J Neuropathol Exp Neurol 2018;77:302–11. [DOI] [PubMed] [Google Scholar]
- 25.Guagnano V, Furet P, Spanka C, Bordas V, Le Douget M, Stamm C, et al. Discovery of 3-(2,6-dichloro-3,5-dimethoxy-phenyl)-1-{6-[4-(4-ethyl-piperazin-1-yl)-phenylamin o]-pyrimidin-4-yl}−1-methyl-urea (NVP-BGJ398), a potent and selective inhibitor of the fibroblast growth factor receptor family of receptor tyrosine kinase. J Med Chem 2011;54:7066–83. [DOI] [PubMed] [Google Scholar]
- 26.Slosberg ED, Kang BP, Peguero J, Taylor M, Bauer TM, Berry DA, et al. Signature program: a platform of basket trials. Oncotarget 2018;9:21383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NCCN. National Comprehensive Cancer Network (NCCN) guidelines central nervous system cancers, version 2, 2021. 2021.
- 28.Brandes AA, Tosoni A, Basso U, Reni M, Valduga F, Monfardini S, et al. Second-line chemotherapy with irinotecan plus carmustine in glioblastoma recurrent or progressive after first-line temozolomide chemotherapy: a phase II study of the Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). J Clin Oncol 2004;22:4779–86. [DOI] [PubMed] [Google Scholar]
- 29.Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med 2017;377:1954–63. [DOI] [PubMed] [Google Scholar]
- 30.Wick W, Puduvalli VK, Chamberlain MC, van den Bent MJ, Carpentier AF, Cher LM, et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol 2010;28:1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma J, Benitez JA, Li J, Miki S, Ponte de Albuquerque C, Galatro T, et al. Inhibition of nuclear PTEN tyrosine phosphorylation enhances glioma radiation sensitivity through attenuated DNA repair. Cancer Cell 2019;35:504–18 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nogova L, Sequist LV, Perez Garcia JM, Andre F, Delord JP, Hidalgo M, et al. Evaluation of BGJ398, a fibroblast growth factor receptor 1–3 kinase inhibitor, in patients with advanced solid tumors harboring genetic alterations in fibroblast growth factor receptors: results of a global phase I, dose-escalation and dose-expansion study. J Clin Oncol 2017;35:157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gattineni J, Alphonse P, Zhang Q, Mathews N, Bates CM, Baum M. Regulation of renal phosphate transport by FGF23 is mediated by FGFR1 and FGFR4. Am J Physiol Renal Physiol 2014;306:F351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available within the article and its supplementary data files.