Abstract
While the adoption of multimodal therapy including surgery, radiation, and aggressive combination-chemotherapy has improved outcomes for many children with high-risk neuroblastoma, we appear to have reached a plateau in what can be achieved with cytotoxic therapies alone. Most children with cancer, including high-risk neuroblastoma, do not benefit from treatment with immune-checkpoint-inhibitors (ICI) that have revolutionized the treatment of many highly immunogenic adult solid tumors. This likely reflects the low tumor mutation burden as well as the downregulated MHC-I that characterizes most high-risk neuroblastomas. For these reasons, neuroblastoma represents an immunotherapeutic challenge that may be a model for the creation of effective immunotherapy for other “cold” tumors in children and adults that do not respond to ICI. The identification of strong expression of the disialoganglioside, GD2, on the surface of nearly all neuroblastoma cells provided a target for immune recognition by anti-GD2 mAbs which recruit Fc-receptor-expressing innate immune cells that mediate cytotoxicity or phagocytosis. Adoption of anti-GD2 antibodies into both upfront and relapse treatment protocols has dramatically increased survival rates and altered the landscape for children with high-risk neuroblastoma. This review describes how these approaches have been expanded to additional combinations and forms of immunotherapy that have already demonstrated clear clinical benefit. We also describe the efforts to identify additional immune targets for neuroblastoma. Finally we summarize newer approaches being pursued that may well help both innate and adaptive immune cells, endogenous or genetically engineered, to more effectively destroy neuroblastoma cells, in order to better induce complete remission and prevent recurrence.
Introduction
The cancer immunotherapy revolution is exemplified by the outstanding success of checkpoint inhibitors in melanoma(1) and certain adult carcinomas(2) and CAR T cells in both adult and pediatric hematologic malignancies(3,4). In contrast, the majority of childhood solid cancers have seen few clinical successes from immunotherapy, with especially disappointing response rates to checkpoint inhibitors(5,6).
Neuroblastoma, a cancer of the sympathetic nervous system that derives from neural crest cells, is the most common extra-cranial solid malignancy occurring in children and accounts for approximately 10% of pediatric cancer deaths(7,8). Like many childhood malignancies, neuroblastoma is a disease of disordered development, meaning that the malignancy is driven by aberrant expression and regulation of developmental proteins(9). The core regulatory circuitry driving neuroblastoma consists of normal human proteins that are expressed during embryonic development but largely turned off postnatally in normal tissues. The adaptive immune system is thought to be unable to target these so called oncofetal antigens because high affinity, self-reactive T cells are deleted during thymopoesis to prevent autoimmunity. It is therefore tempting to adopt a reductionist viewpoint that childhood cancer is not immunogenic, and thus alternate therapeutic strategies should be prioritized. However, neuroblastoma stands out amongst pediatric solid cancers as the exemplar where immunotherapy (with anti-GD2 antibodies) has been incorporated into both frontline and relapse treatment protocols to significantly improve patient outcomes and increase cure rates.
THE FACTS:
Immunotherapy for Neuroblastoma
The relative success of immunotherapy with anti-GD2 mAbs raises the question of whether neuroblastoma is an immunogenic tumor. Here, care must be taken in the definition of terms. If immunogenicity refers to a cancer’s rejection by an adaptive immune response, there is scant clinical evidence for this phenomenon in neuroblastoma patients. Lack of consistent clinical responses to checkpoint inhibition(6,10) or vaccination approaches is consistent with histopathological evidence of a general lack of tumor reactive infiltrating T cells in a majority of cases(11–14), although high risk neuroblastoma with higher T cell infiltrate has been associated with improved survival(15). Tumor mutational burden estimations place primary neuroblastoma amongst the least mutated of human cancers, consistent with an “immunologically cold” classification(16).
Despite the lack of evidence of an adaptive immune response, there is also ample evidence that neuroblastoma, in common with most human cancers, has immune evasion hardwired into its biology(12) through mechanisms such as downregulation of MHC class-I(17), infiltration by suppressive myeloid cells(13,18–20), and production of inhibitory factors such as arginase-2(21) and TGF-beta(22). This raises the intriguing possibility that the relative coldness of neuroblastoma may reflect immune evasion as much as a lack of inherent danger, providing some encouragement for immunotherapeutic strategies.
While harnessing a native immune response in neuroblastoma has largely been unsuccessful, researchers have instead focused on engineering synthetic immune recognition to help activate a response by endogenous or laboratory-manipulated immune cells (Figure 1 and Table 1). By engineering therapeutics that can recognize neuroblastoma cells, scientists have been able to create a new immune response in tumors that otherwise appear impervious to native immune recognition. Such immunotherapies that use synthetic immune recognition are typically based on monoclonal antibodies (mAb). mAbs recognizing the disialoganglioside GD2, overexpressed on most neuroblastoma cells, have revolutionized the care of neuroblastoma, increasing event free survival by up to 20% (23). Other synthetic recognition agents can be further engineered from antibody derivatives, including chimeric antigen receptor (CAR) T cells and antibody drug conjugates. These therapeutics have begun to demonstrate signs of preclinical and early clinical efficacy, indicating that the immunotherapy revolution is poised to further alter the neuroblastoma treatment landscape.
Figure 1: Endogenous and synthetic recognition involved in immunotherapies for neuroblastoma.
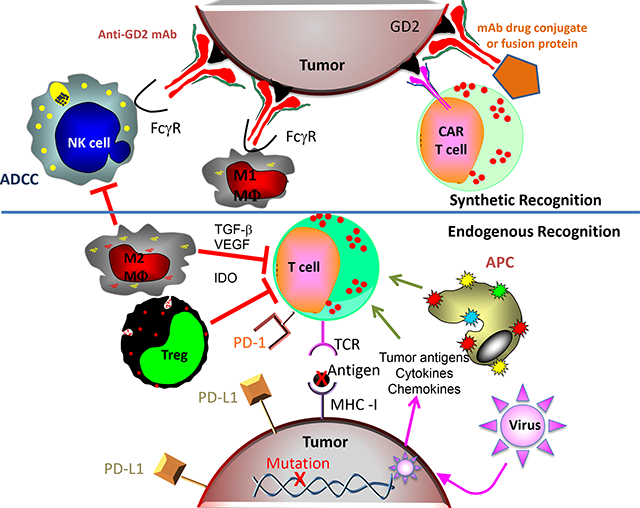
This simplified schematic shows some of the relationships between immune cells, molecules and cancer cells involved in current and developing immunotherapies for neuroblastoma. The synthetic recognition pathways are shown above the horizontal line, and are all shown here as mediated via mAb induced tumor recognition. The mAb-based tumor-recognition components, shown at the top, include an intact anti-GD2 mAb (at top left) binding to GD2 on the tumor engaging the Fcγ-receptor (FcγR) on the NK cell or on the M1 macrophage (MΦ) to activate antibody dependent cell-mediated cytotoxicity (ADCC). At the top right is that same anti-GD2 mAb, now carrying a “payload”. This payload can be a drug, as an antibody drug conjugate, an immune activator as in a fusion protein, such as an IL-2 linked immunocytokine, a radionuclide, or a toxin. To its left is a chimeric antigen receptor (CAR)-T cell that utilizes the ScFv of the anti-GD2 mAb to provide anti-GD2 recognition for the genetically modified T cell. Below the horizontal line are the pathways involved in endogenous recognition, with a central role given to effector T cells. At the bottom of the endogenous T cell is its T-cell antigen receptor (TCR), which on clonally derived T cells can recognize tumor associated peptides presented by the MHC molecules on the tumor surface. This recognition and T cell activation can induce effector functions, that include cytokine release, activation of innate immune anti-tumor cells, and direct T-cell mediated tumor cell lysis. Those tumor associated peptides can be mutation-driven neo-antigens (shown here) or germ-line controlled proteins that have restricted expression to tumor cells, with little or no expression on normal post-natal tissues. To the left of the T cell are endogenous cells that can interfere with T cell function. One such inhibitory cell is an M2 macrophage (MΦ), that can interfere with anti-tumor immunotherapy via many pathways, including release of transforming growth factor β, (TGF- β), vascular-endothelial growth factor (VEGF) and indoleamine 2,3-dioxygenase (IDO). Other myeloid elements, like myeloid-derived suppressor cells (MDSCs, not shown) can also interfere with effector immune function. T-regulatory (Treg) cells are normally FoxP3+ CD4+ T cells that can directly kill or inhibit the functions of effector T cells. These inhibitory cells can also interfere with NK cell function (not shown). To the right of the T cell is an antigen presenting cell (APC), normally a dendritic cell, that picks up and processes tumor antigens and then presents them to T cells to induce an endogenous adaptive immune response. Cytokines and chemokines can help recruit immune cells into the tumor. Certain oncolytic tumor viruses are being injected in some trials to infect the tumor, release more chemokines, and recruit additional immune cells to the tumor micro-environment. The immunosuppressive PD-L1 ligand, is one of several checkpoint molecules expressed by tumor cells (and shown here). PD-L1 activates the immune-inhibitory PD-1 receptor on the T cell (shown) and some NK cells (not shown). Not depicted is how anti-PD1 or anti-PD-L1 mAbs (forms of immune checkpoint blockade) can block these inhibitory interactions, enabling T and NK cells functionality in the suppressive tumor microenvironment.
Table1:
Types of immune recognition potentially relevant to neuroblastoma immunotherapy
| Immune recognition | Type | Caveat (requires) | Example | Clinical relevance and application |
|---|---|---|---|---|
| Endogenous | T-cell Receptor | Peptide presented by MHC on cell surface | NY-ESO-1 presented by MHC on NBL cells to autologous T cells(71) | Endogenous T cell responses of HR-NBL patients are weak, due to the substantial immunosuppressive chemotherapy received. No effective vaccine to stimulate yet tested in NBL. |
| Antibody | Cell surface molecule | 1.Antibody seen in OMS sees neuroblastoma(111) 2.GD2/GD3 |
1. Patients with NBL and opsoclonus-myoclonus syndrome (OMS) have induced an endogenous antibody against their neuroblastoma and also to normal CNS, causing this auto-immune syndrome. 2. Vaccination to these gangliosides is inducing antibody to them that may delay/prevent relapse |
|
| Synthetic | mAb | Cell surface molecule | GD2(23) | Three separate mAbs to GD2 ganglioside have been approved for clinical use and have shown antitumor benefit in preventing relapse for patients in remission, for inducing responses for relapsed disease, and for anti-tumor effects when combined with chemotherapy for relapse, with early data indicating benefit when included with chemotherapy during induction. |
| CAR | Cell surface molecule | GD2 (recognized by mAb ScFv)(49,50,80) | Several trials are testing CAR-T cells with CARs directed at GD2 through mAb technology; with some showing early signs of anti-tumor benefit. | |
| CAR | Cell surface MHC-presenting a tumor peptide | PHOX2B peptide presented by HLA-I(95) | Even though PHOX2B is a NBL “driver” expressed only in cytoplasm and nucleus, its peptides are presented on the surface by MHC-I. A mAb against the PHOX2B peptide/MHC-I complex has been put into CAR-T cells and mediates potent tumor destruction in vitro and in vivo in PDX models. | |
| T-cell Receptor | Peptide presented by MHC on cell surface | NY-ESO-1(69)(112–115) | Using in vitro binding and selection processes, T cell receptors specific for the NY-ESO-1 antigen (seen on some neuroblastomas, and several tumors in adults) can be cloned from lymphocytes and transfected into cells of a cancer patient to get autologous tumor killing in vitro. Clinical testing in other diseases is proceeding. |
Different Immune recognition mechanisms, of different types, each with separate caveats for translation are indicated for the antigens exemplifying their use, and with mechanistic clinical considerations for each.
Anti-GD2 Antibodies
Evidence based therapy for high risk neuroblastoma prior to ~ 2009 relied on combining surgery, local radiation therapy and gradually more aggressive combination chemotherapy regimens, supplemented with supralethal chemotherapy-based “consolidation” regimens requiring autologous hematopoietic stem cell rescue. While this approach prolonged survival for some, fewer than 40% of patients survived for more than 5 years without relapse; relapsed disease could only rarely be cured(24). Only a decade after the original description of monoclonal antibody (mAb) selection and production, separate studies led by Reisfeld and by Cheung identified murine neuroblastoma-reactive mAbs 14.18 (later class switched to generate 14G2a) and 3F8 respectively, shown to recognize disialoganglioside, GD2(25,26). These mAbs could recognize GD2 on a variety of cancers, including some melanomas, small cell lung cancers, osteosarcomas. They were particularly able to recognize neuroblastomas, which appeared to show relatively uniform, high level expression on virtually all tumor cells from nearly all patients. Preclinical studies demonstrated that anti-GD2 antibodies were effective and that their major mechanism of activity was via antibody-dependent cell-mediated cytotoxicity (ADCC)(27).
ADCC is mediated via FCR-bearing cells: NK cells that can be activated with IL-2 stimulation, and macrophages and other myeloid cells whose production can be stimulated with GM-CSF. Preclinical data suggested in vivo antitumor efficacy was better realized in the face of microscopic, rather than bulky disease(28). The Children’s Oncology Group (COG) ran a large randomized trial for patients in remission or partial remission from standard upfront chemotherapy, treating them with dinutuximab (a murine-human chimeric version of 14G2a bearing a human IgG1 Fc region) in combination with IL-2 and GM-CSF, added to the standard of isotretinoin (23). Patients receiving the immunotherapy showed improved event-free survival (EFS) and overall survival (OS) initially, and after nearly a decade of follow-up(23,29). This led to the FDA approval of dinutuximab, as the the first mAb approved specifically for a pediatric cancer indication and the first effective mAb recognizing a lipid-based cancer molecule. A similar antibody, dinutuximab-beta (produced in CHO cells), is approved in Europe, although it was not tested in a randomized fashion(30,31). A third anti-GD2 antibody, naxitamab, a humanized version of 3F8, was recently FDA approved based on its activity in regressing neuroblastoma in patients with relapsed or refractory disease limited to bone or bone marrow(32,33).
Based in part on preclinical data, and on the clinical development of anti-HER2 mAb in combination with chemotherapy as breast cancer treatment, COG and the St. Jude Children’s Research Hospital each independently began testing anti-GD2 mAb in combination with conventional chemotherapy, for relapsed and refractory neuroblastoma, including bulky disease(34,35). The randomized COG trial ANBL1221 compared a combination of dinutuximab with chemotherapy (irinotecan/temozolomide, I/T) vs. temsirolimus (a targeted agent) with I/T and found that chemoimmunotherapy was highly effective in patients, especially those with chemorefractory disease. Remarkably, patients with bulky disease experienced significant regressions of otherwise chemorefractory soft tissue masses. A number of responses appeared durable(34,36). For this reason, this anti-GD2 mAb + chemotherapy approach has become standard for patients with relapsed or refractory disease and was also incorporated into induction chemotherapy for newly diagnosed NBL patients in a recent St. Jude study with promising efficacy(37,38). COG is now also pursuing this strategy in larger trials (including the recently completed, but not yet published ANBL17P1 trial of dinutuximab incorporated into induction as well as maintenance phases).
The impressive progress with anti-GD2 antibody has also uncovered important challenges. First, many patients relapse despite having received anti-GD2 during upfront therapy, and the combination of anti-GD2 and chemotherapy induces responses in <50% of patients with relapse(23,29,34,36). Second, some patients have decreased GD2 expression at relapse, suggesting in vivo antigen remodeling in response to anti-GD2 (39,40). Third, the administration of anti-GD2 mAbs is associated with substantial neuropathic pain, driven by mAb binding to GD2+ myelin sheaths of nerve fibers(41,42); this restricts the maximum tolerated dose of anti-GD2 far below the doses used (approximately 1/10 on a mg/M2 basis) for other approved tumor-reactive mAbs (23,43–45), and requires substantial administration of narcotics and other analgesics even at these low doses. These three challenges emphasize the need to identify additional cell-surface antigens, other than GD2, that are selectively over-expressed on NBL and can be targeted with mAbs to overcome antigen-loss escape, devise additional anti-GD2 strategies that can overcome tumor cell resistance, and reduce the neuropathic pain associated with current anti-GD2 mAb based therapy.
Anti-GD2 CAR T cells
Building on their successes in leukemia, chimeric antigen receptor (CAR) T cells have emerged as a promising approach for immunotherapy based on synthetic immune recognition for so-called ‘immune cold’ solid cancers(46). The clinical validation of GD2 as a target antigen drove early adoption of CAR T cells in neuroblastoma. In fact, neuroblastoma was the first pediatric cancer to be targeted with CAR T cells in a clinical trial. A trial of first generation CAR-T cells, containing the same antigen recognition domain as dinutuximab (but no embedded costimulatory domain), mediated several clinical responses, and demonstrated no signs of on-target, off-tumor neurotoxicity despite known expression of GD2 on peripheral nerves (47,48). A subsequent trial with an altered GD2 CAR T cell design was disappointing due to lack of clinical responses, even when combined with checkpoint inhibition(49). However, recently published work employing a CAR with an alternate anti-GD2 binder(50) and an abstract from a trial with a next-generation 14G2a based CAR T cell(51) have both demonstrated signs of clinical efficacy, including multiple complete responses in the second trial. GD2 CAR T cells have similarly demonstrated clinical efficacy in patients with the universally fatal brainstem tumor, diffuse intrinsic pontine glioma(52).
Of note, despite GD2 expression on neural tissues and a high incidence of infusion related pain in patient receiving monoclonal antibodies targeting GD2, patients have not experienced on-target, off-tumor toxicity in trials of GD2 CAR T cells. The precise mechanistic reasons for the different off-tumor toxicity of GD2 CAR-T cells versus anti-GD2 antibody remain yet to be fully elucidated. However some important conclusions can be made: a) CAR T cells demonstrate a therapeutic window when targeting antigens expressed at low levels on normal tissue(52,53) and b) the mechanism of anti-GD2 antibody associated allodynia/neuropathy may be specific to antibody based therapeutics, with evidence of the role of complement recruitment potentially playing a role (42). In neuroblastoma patients, reported toxicities of GD2-targeting CAR-T have so far been related to immune activation and cytokine release syndrome(47–51). As these and other ongoing GD2 CAR trials continue to mature, it is likely that further clinical advances will be achieved.
Other immunotherapy targets in neuroblastoma
While the disialoganglioside GD2 is the most well-known and most highly expressed target in neuroblastoma, several other molecules have been identified as overexpressed on the surface of neuroblastoma cells for use in antibody based immunotherapies (naked antibodies, antibody conjugates, bispecific antibodies, and CAR T cells). Many of these have been targeted in preclinical models and early phase clinical trials. B7-H3 (CD276) is a checkpoint molecule (from the same family as PD-L1) that is broadly overexpressed on neuroblastoma and most other pediatric solid tumors, but has highly restricted expression on normal tissues. This molecule was first targeted by researchers at Memorial Sloan Kettering with an antibody named 8H9 before its exact target was even identified(54,55). This antibody is currently being developed as a radioconjugate (omburtomab) for use in patients with neuroblastoma that has spread to the central nervous system (CNS) and other primary CNS malignancies(56–58). Another B7-H3 targeted antibody (MGA271(59), enoblituzumab) has been tested in children with solid tumors including neuroblastoma (NCT02982941, results not published). B7-H3 targeted CAR T cells have shown promise in preclinical models of pediatric cancer(60,61), and have recently reached the clinic for patients with neuroblastoma (NCT04483778).
Anaplastic Lymphoma Kinase (ALK) is a receptor tyrosine kinase that is mutated or amplified in ~14% of neuroblastoma patients(62) and is often expressed on the surface of neuroblastoma cells(63). Both antibody drug-conjugates (ADC) and CAR T cells targeting ALK have been described in preclinical studies(64,65). GPC2 was recently discovered to be expressed on neuroblastoma tumors, particularly those harboring MYCN amplification(66). Both ADC and CAR targeting approaches for GPC2 have also been described(66,67). In the case of both ALK and GPC2, the limited expression density on neuroblastoma (compared to the highly expressed GD2) may limit the efficacy of these therapeutics with the current generation of CAR T cells(64,68). Other targets identified in neuroblastoma include NCAM (preclinical studies describing an ADC(69)) and L1CAM(70) (L1CAM CAR is currently in clinical trials (NCT02311621)).
While early phase clinical trials are ongoing for several antibody based therapeutics, other preclinical research has focused on targeting intracellular proteins that are specific to neuroblastoma. So called cancer testes antigens, including NY-ESO-1(71) and PRAME(72), have been identified as immunotherapy targets for neuroblastoma. These antigens, which are overexpressed in cancer but not in most normal tissues other than testes, have been safely and effectively targeted in patients with other malignancies using engineered T cell receptors(73,74), but these are yet to be clinically deployed in the context of neuroblastoma.
HOPES:
As anti-GD2 antibodies have already proven an essential part of the anti-neuroblastoma armament, we anticipate that the role of immunotherapy in neuroblastoma will continue to grow as new targets are identified and newer targeting technologies are developed. Here, we explore the emerging data that we believe is poised to alter the trajectory of immunotherapy for neuroblastoma.
Innate immunity
While neuroblastomas demonstrate little evidence of T cell infiltration (11–13) and T cell checkpoint inhibition has thus far not worked well in the clinic (6), other cell types in the tumor microenvironment may also be harnessed for anti-tumor activity. Neuroblastoma tumors are well known to be infiltrated by innate immune cells, including natural killer (NK) cells and macrophages(20,75). These cells are thought to be the major effectors involved in the efficacy of anti-GD2 antibody. In fact, patients inheriting certain NK cell receptors (KIR) and their ligands are more likely to derive benefit from anti-GD2 antibody than those patients lacking their expression (76,77). To further harness the activity of NK cells, researchers have attempted to administer ex vivo expanded NK cells with anti-GD2 antibody to patients with neuroblastoma. While some responses have been seen, it is unclear how much of this is attributable to the adoptive transfer of NK cells as opposed to the anti-GD2 antibody itself(78). More work is needed to understand if administration of unmanipulated, or in vivo activated, NK cells can improve outcomes of patients with neuroblastoma. NK-cell mediated ADCC can be augmented via PD1 blockade, and this approach is now being tested with anti-GD2(79). Researchers have also attempted to enhance the efficacy of NK cells by endowing them with CARs, including those recognizing GD2. Researchers at Baylor recently reported that endowing NKT cells (an innate immune cell type that shares features of NK cells and T cells) with a GD2 CAR resulted in NK cell expansion and early signs of clinical activity(80).
Macrophages can be similarly harnessed for anti-tumor effects in neuroblastoma. Tumor cells express CD47, a macrophage checkpoint that suppresses tumor cell phagocytosis by macrophages(81). Recent clinical trials of anti-CD47 and anti-CD20 (rituximab) monoclonal antibodies in patients with non-Hodgkins Lymphoma indicate that the addition of anti-CD47 can overcome rituximab resistance(82). Preclinical studies have now demonstrated that anti-CD47 can similarly enhance the efficacy of anti-GD2, with potent synergy for the combination of these two antibodies. This synergy is driven by a newly uncovered role for GD2, a sialoglycan that was found to directly interact with Siglec-7, an inhibitory immunoreceptor expressed on both macrophages and NK cells(83). This approach has reached the clinic with a first-in-child/first-in-human clinical trial of combined anti-GD2/anti-CD47 for children with relapsed neuroblastoma (NCT04751383).
Next generation anti-tumor mAb-based therapy
Recent advances in protein engineering have enabled creation of next generation antibody-based off-the-shelf agents. Antibody drug conjugates are showing strong preclinical activity, including in neuroblasoma(66,84), and clinical testing is moving forward for some in a variety of cancers. Bispecific T-cell Engager (BiTE) antibodies link a tumor specific mAb or mAb fragment to an anti-CD3 mAb or mAb fragment. This architecture enables selective binding and bridging of tumor cells to a T cell and then subsequent activation of the T-cell to kill the tumor. Blinatumomab is a bispecific CD19 × CD3 antibody that FDA approved for B cell acute lymphoblastic leukemia, that can be effective even in the face of lymphopenia or immunedeficiancy(85,86). Multiple analogous or similar constructs are being studied for various solid tumors. While BiTEs activate T cells, other mAb-based constructs bind to and activate other effectors cells such as NK cells, (so-called BiKEs) and can also be engineered to incorporate cytokines (so-called TriKEs) (87). However, despite potent in vitro destruction of tumor cells by these bi- and tri-functional agents, their potency in mice or patients bearing solid tumors has not yet matched their potency against leukemia; possibly implicating the immune-excluded/immune-suppressive tumor microenvironment of many solid tumors, including neuroblastoma(20). Unique engineering strategies are also being deployed to reduce or avoid pain associated with anti-GD2 mAb, including use of alternative or mutated mAb isotypes to avoid pain-inducing complement activation(88,89), or mAb strategies that require co-recognition of two separate tumor antigens that are co-expressed simultaneously on the same tumor cells, but not co-expressed on cells from normal tissues(90). In addition, refinement of the antigen-binding component of the Fab (or ScFv) of the anti-tumor antibody, can identify more advantageous binding kinetics to facilitate improved interactions with the tumor cell surface for any antibody-based therapeutic modality (mAbs, antibody drug conjugates or CARs).
The potential for endogenous immune-mediated destruction of neuroblastoma
In contrast to the “synthetic” immune recognition of anti-tumor-based mAbs and their engineered derivatives, the activity of T cell checkpoint blockade depends entirely on the ability of endogenous immune cells to recognize and destroy autochthonous cancer, without a need for synthetic immune recognition. For the most part, this involves an adaptive T-cell response recognizing immunogenic tumor neoantigens (91,92).
As most pediatric cancers have a very low tumor mutation burden, children likely have few if any actionable, mutation-generated, immunogenic tumor neoantigens(16,93). Even so, they may still have some targetable MHC associated tumor antigens that are expressed only at very low levels on a restricted number of normal tissues. These could include cancer-testis antigens, and other embryonic or differentiation antigens expressed during development and on pediatric cancers but not on normal post-natal tissues(94). Recently, researchers identified that members of the core regulatory circuitry driving neuroblastoma, such as PHOX2B, have peptides that are displayed on the surface of neuroblastoma cells by thier MHC molecules. These so called oncofetal proteins, expressed during embryonic development but then silenced in normal tissue after birth, may be ideal targets for T cell based immunotherapies because of their restricted expression outside of the tumor. Proof of concept preclinical studies utilizing a CAR recognizing PHOX2B as presented by the MHC demonstrate the potential power of this approach(95).
Thus, while there may be limited neoantigen expression in neuroblastoma due to its relatively low tumor mutational burden, developmental antigens may instead be a focus for T cell based immunotherapy. Because high affinity TCRs against self-antigens are generally deleted during thymic development, engineering of high affinity TCRs or CARs recognizing peptide as displayed in MHC may have to be employed. Investigators will need to be wary of the potential for antigen cross reactivity given the high sensitivity and potency of some of these receptors as has been previously observed with certain engineered TCRs(96,97). It may also be possible to activate endogenous tumor reactive T cells in a patient by giving agents that augment the immunogenicity of the tumor, activate antigen presentation, expand the endogenous tumor reactive T cells, and block the immunosuppressive tumor microenvironment. This experimental approach seeks to immunize the tumor-bearing individual with their own tumor, functioning as an in situ vaccine (98–101).
For these approaches to be effective, NBL cells will require at least some low level of surface MHC expression. For patients with low MHC expression due to “soft” (namely reversible, epigenetic) downregulation, this may be possible via epigenetic modificiation. For patients with no MHC expression due to “hard” genetic mutations in MHC or other antigen presentation machinery, strategies that rely on MHC recognition are not applicable.
Engineering CAR-T cells
Although anti-GD2 antibodies engage NK cells and macrophages, they do not recruit T cells, which have shown themselves to be highly potent in regressing solid cancers in certain adult malignancies(102). Thus, researchers have focused on engineering CARs, synthetic receptors that harness the cytolytic capacity of T cells in a genetically unrestricted manner by employing an antibody fragment as the antigen binding domain. Although many preclinical studies have demonstrated the promise of CAR T cells to treat neuroblastoma(61,68,70) and some clinical data provide proof that these can translate(47,48,50–52), obstacles remain. Some major roadblocks that need to be overcome are insufficient CAR T cell expansion, and persistence and reduced functionality in the suppressive tumor microenvironment. Engineering CAR T cells to overcome these barriers must be balanced against potential for causing toxicity; promotion of enhanced functionality can increase the risk immune overactivation (e.g. cytokine release syndrome) or on-target, off-tumor recognition of normal tissues. For instance, because GD2 is expressed on peripheral nerves and normal neurons in the CNS(41,42), there has long been concern for GD2 CAR mediated neurotoxicity, contributing to conservative design in terms of dose and even CAR T cell potency. However, GD2 CAR T cells have now mediated significant clinical responses without evidence of on-target, off-tumor neurotoxicity, indicating that the CAR constructs being used fall within a therapeutic window in which they recognize high GD2 expression on tumor but not lower GD2 expression on normal tissue(47,48,50–52,68). As next generation CAR T cells (Figure 2 ) are being engineered to contain additional modules to enhance functionality through transcriptional reprogramming(103), avoidance of inhibitory molecules(104), and provision of cytokine signaling(105), it is possible that they may also recognize lower levels of antigen expressed by normal tissues and potentially cause significant on-target, off-tumor toxicity. Thus, as our ability to engineer and manufacture highly functional CAR T cells matures, researchers may also need to deploy so-called Boolean logic gating strategies that can further improve specificity and prevent immune attack of normal tissues(106,107), as is also being done for the improvement of mAb-based therapy (noted above).
Figure 2. Enhancing CAR-T cell sensitivity and specificity.
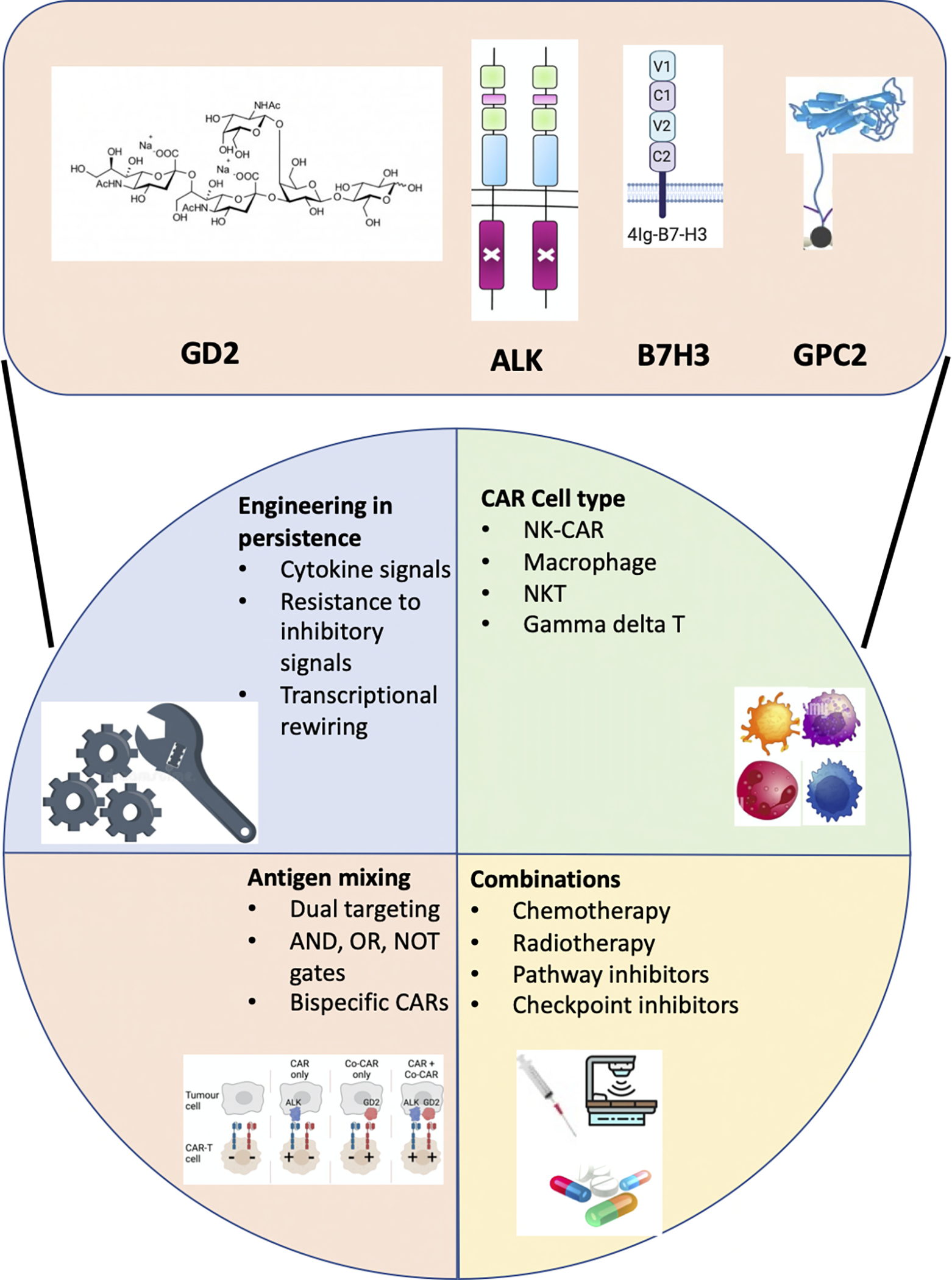
Schematic of technological solutions to enhance CAR-T cell sensitivity and specifity. Representative neuroblastoma tumor antigens that are currently under clinical and preclinical evaluation are indicated at the top. At the bottom are the categories of approaches for enhancing functionality of CAR-T cells that are relevant to neuroblastoma.
Conclusion
Neuroblastoma is a childhood malignancy that is marked by aberrant development and, as opposed to the majority of adult malignancies, does not usually harbor a high mutational burden. The limited number of somatic, actionable mutations severely restricts the de novo immune responses that might be unleashed using checkpoint blockade(92,93). Therefore, approaches to immunotherapy for neuroblastoma must differ significantly from those being successfully employed for many adult solid tumors. To date, virtually all active immunotherapies for neuroblastoma have relied on targeting GD2, a glycolipid overexpressed on the surface of neuroblastoma cells with only low level expression on normal tissue. Such differentially expressed antigens may represent the best classes of immunotherapy targets in pediatric oncology and thus there has been an intense research focus on identifying similar targets. Those studies have begun to bear fruit with the identification of targets including GPC2, B7-H3, and ALK. Researchers have used synthetic immune recognition to engineer antibody based immunotherapies which are now reaching clinical trials. Additionally, scientists have recently discovered that the developmental origins of neuroblastoma may also serve as an Achilles heel because many developmental proteins are expressed in neuroblastoma cells but not healthy post-natal tissues and therefore may serve as unique and specific targets for immunotherapy.
Despite these exciting emerging approaches, the neuroblastoma tumor microenvironment remains hostile to endogenous immune elements, containing immune cells such as M2 polarized macrophages and myeloid derived suppressor cells that can interfere with immunotherapeutic strategies. A focus on reprogramming the tumor microenvironment and reversing its suppressive activity with therapies such as radiation, chemotherapy, or CD47 blockade may improve the anti-tumor efficacy of endogenous immune cells or of genetically modified immune cells. This is perhaps the reason a chemoimmunotherapy approach combining cytotoxic agents with anti-GD2 has been successful for some children with neuroblastoma, and is currently considered the standard of care for children with relapsed or refractory disease (34–37).
In addition to identifying new immunotherapy targets and engineering therapeutics, researchers will also need to focus on developing additional rational combinations of traditional cytotoxic agents and radiotherapy, small molecule inhibitors of oncogenic pathways, and immunotherapies. As next generation small molecules, such as ALK inhibitors, aurora-A inhibitors and CDK9/2 inhibitors (108–110) are being integrated into treatment for appropriate patients, their ability to potentially synergize with (or antagonize) combination immunotherapy regimens will require careful analyses in preclinical studies and clinical trials. Once these approaches have established clinical efficacy, it will become an important focus to reduce the reliance on high-dose radio-chemo-therapy in order to minimize acute treatment-associated toxicity and long term late effects. Eventually, correlative lab testing may enable selection of somewhat personalized combination therapy regimens, based on analyses of tumor or host/immune factors measured at the time of diagnosis or relapse(75,76).
The hope of the basic, translational, and clinical neuroblastoma research community is for efficacious treatment regimens that employ novel immunotherapies which enable effective cancer eradication while relying less on high-dose genotoxic radio-chemotherapy; the goal is to minimize the long-term morbidity and mortality of the disease, and its therapy. Furthermore, the vast majority of children with other high-risk solid tumors are similarly plagued by 1) poor responses to current “conventional” immunotherapy being used to treat some cancers of adults, due to few actionable mutations/neo-antigens, and an immunosuppressive tumor microenvironment and 2) substantial acute and long term treatment-induced toxicity due to high-dose radio-chemo-therapy. As such, the efforts of the neuroblastoma research community to address these hurdles in a combinatorial, rather than sequential, manner may translate into hope for the creation of similar strategies for other high-risk cancers. We hope that through greater collaboration and rapid adoption of technology, novel immunotherapies will move quickly towards the clinic and rapidly alter the treatment paradigm for children with neuroblastoma.
Grant Support Acknowledgment:
J. Anderson is supported by The NIHR Great Ormond Street Biomedical Research Centre.
R. G. Majzner is supported by Alex’s Lemonade Stand Foundation, Doris Duke Charitable Foundation, V Foundation, Solving Kids’ Cancer, National Institutes of Health (DP2- CA272092), and the Parker Institute for Cancer Immunotherapy.
P. M. Sondel is supported by Midwest Athletes Against Childhood Cancer; a St. Baldrick’s Foundation - Stand Up To Cancer Pediatric Dream Team Translational Research Grant (SU2C-AACR-DT-27–17); the Crawdaddy Foundation; the University of Wisconsin Carbone Cancer Center; and the Children’s Neuroblastoma Cancer Foundation. This research was also supported in part by public health service grants U54-CA232568, R35-CA197078, U01-CA233102, P01-CA250972, and P30-CA014520 from the National Cancer Institute; and UL1TR002373 from the National Institutes of Health. Stand Up to Cancer is a division of the Entertainment Industry Foundation. The indicated SU2C research grant is administered by the American Association for Cancer Research, the Scientific Partner of SU2C.
Footnotes
Conflict of Interest Statement:
JA has founder shares in Autolus Ltd. And share options in TC Biopharm, holds patents in Chimeric Antigen Receptor technologies and has received consultancy fees from Roche.
RGM is an inventor on several patents related to chimeric antigen receptors. He is a cofounder of and holds equity in Syncopation Life Sciences. He is a consultant for Lyell Immunopharma, Syncopation Life Sciences, NKarta, Gamma Delta Therapeutics, Aptorum Group, Illumina Radiopharmaceuticals, Arovella Therapeutics, Zai Lab, and ImmunAI.
PMS holds patents regarding radio-immunotherapy and novel mAbs administered for the University of Wisconsin through its Wisconsin Alumni Research Foundation, including patents held jointly by Invenra Inc. where he is an unpaid, volunteer medical consultant.
These bodies had no role in the design of the article; in the writing of the manuscript, or in the decision to publish the article.
References
- 1.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372(21):2006–17 doi 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375(19):1823–33 doi 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 3.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med 2018;378(5):439–48 doi 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med 2017;377(26):2531–44 doi 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merchant MS, Wright M, Baird K, Wexler LH, Rodriguez-Galindo C, Bernstein D, et al. Phase I Clinical Trial of Ipilimumab in Pediatric Patients with Advanced Solid Tumors. Clin Cancer Res 2016;22(6):1364–70 doi 10.1158/1078-0432.CCR-15-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis KL, Fox E, Merchant MS, Reid JM, Kudgus RA, Liu X, et al. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): a multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol 2020;21(4):541–50 doi 10.1016/S1470-2045(20)30023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith MA, Altekruse SF, Adamson PC, Reaman GH, Seibel NL. Declining childhood and adolescent cancer mortality. Cancer 2014;120(16):2497–506 doi 10.1002/cncr.28748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthay KK, Maris JM, Schleiermacher G, Nakagawara A, Mackall CL, Diller L, et al. Neuroblastoma. Nat Rev Dis Primers 2016;2:16078 doi 10.1038/nrdp.2016.78. [DOI] [PubMed] [Google Scholar]
- 9.Scotting PJ, Walker DA, Perilongo G. Childhood solid tumours: a developmental disorder. Nat Rev Cancer 2005;5(6):481–8 doi 10.1038/nrc1633. [DOI] [PubMed] [Google Scholar]
- 10.Pearson ADJ, Rossig C, Lesa G, Diede SJ, Weiner S, Anderson J, et al. ACCELERATE and European Medicines Agency Paediatric Strategy Forum for medicinal product development of checkpoint inhibitors for use in combination therapy in paediatric patients. Eur J Cancer 2020;127:52–66 doi 10.1016/j.ejca.2019.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Apps JR, Hasan F, Campus O, Behjati S, Jacques TS, N JS, et al. The immune environment of paediatric solid malignancies: evidence from an immunohistochemical study of clinical cases. Fetal Pediatr Pathol 2013;32(4):298–307 doi 10.3109/15513815.2012.754527. [DOI] [PubMed] [Google Scholar]
- 12.Wienke J, Dierselhuis MP, Tytgat GAM, Kunkele A, Nierkens S, Molenaar JJ. The immune landscape of neuroblastoma: Challenges and opportunities for novel therapeutic strategies in pediatric oncology. Eur J Cancer 2021;144:123–50 doi 10.1016/j.ejca.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Majzner RG, Simon JS, Grosso JF, Martinez D, Pawel BR, Santi M, et al. Assessment of programmed death-ligand 1 expression and tumor-associated immune cells in pediatric cancer tissues. Cancer 2017;123(19):3807–15 doi 10.1002/cncr.30724. [DOI] [PubMed] [Google Scholar]
- 14.Cheung IY, Cheung NV, Modak S, Mauguen A, Feng Y, Basu E, et al. Survival Impact of Anti-GD2 Antibody Response in a Phase II Ganglioside Vaccine Trial Among Patients With High-Risk Neuroblastoma With Prior Disease Progression. J Clin Oncol 2021;39(3):215–26 doi 10.1200/JCO.20.01892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bao R, Spranger S, Hernandez K, Zha Y, Pytel P, Luke JJ, et al. Immunogenomic determinants of tumor microenvironment correlate with superior survival in high-risk neuroblastoma. J Immunother Cancer 2021;9(7) doi 10.1136/jitc-2021-002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grobner SN, Worst BC, Weischenfeldt J, Buchhalter I, Kleinheinz K, Rudneva VA, et al. The landscape of genomic alterations across childhood cancers. Nature 2018;555(7696):321–7 doi 10.1038/nature25480. [DOI] [PubMed] [Google Scholar]
- 17.Raffaghello L, Prigione I, Bocca P, Morandi F, Camoriano M, Gambini C, et al. Multiple defects of the antigen-processing machinery components in human neuroblastoma: immunotherapeutic implications. Oncogene 2005;24(29):4634–44 doi 10.1038/sj.onc.1208594. [DOI] [PubMed] [Google Scholar]
- 18.Frosch J, Leontari I, Anderson J. Combined Effects of Myeloid Cells in the Neuroblastoma Tumor Microenvironment. Cancers (Basel) 2021;13(7) doi 10.3390/cancers13071743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song L, Asgharzadeh S, Salo J, Engell K, Wu HW, Sposto R, et al. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest 2009;119(6):1524–36 doi 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asgharzadeh S, Salo JA, Ji L, Oberthuer A, Fischer M, Berthold F, et al. Clinical significance of tumor-associated inflammatory cells in metastatic neuroblastoma. J Clin Oncol 2012;30(28):3525–32 doi 10.1200/JCO.2011.40.9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mussai F, Egan S, Hunter S, Webber H, Fisher J, Wheat R, et al. Neuroblastoma Arginase Activity Creates an Immunosuppressive Microenvironment That Impairs Autologous and Engineered Immunity. Cancer Res 2015;75(15):3043–53 doi 10.1158/0008-5472.CAN-14-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iolascon A, Giordani L, Borriello A, Carbone R, Izzo A, Tonini GP, et al. Reduced expression of transforming growth factor-beta receptor type III in high stage neuroblastomas. Br J Cancer 2000;82(6):1171–6 doi 10.1054/bjoc.1999.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010;363(14):1324–34 doi 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med 1999;341(16):1165–73 doi 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 25.Mujoo K, Cheresh DA, Yang HM, Reisfeld RA. Disialoganglioside GD2 on human neuroblastoma cells: target antigen for monoclonal antibody-mediated cytolysis and suppression of tumor growth. Cancer Res 1987;47(4):1098–104. [PubMed] [Google Scholar]
- 26.Suzuki M, Cheung NK. Disialoganglioside GD2 as a therapeutic target for human diseases. Expert Opin Ther Targets 2015;19(3):349–62 doi 10.1517/14728222.2014.986459. [DOI] [PubMed] [Google Scholar]
- 27.Hank JA, Robinson RR, Surfus J, Mueller BM, Reisfeld RA, Cheung NK, et al. Augmentation of antibody dependent cell mediated cytotoxicity following in vivo therapy with recombinant interleukin 2. Cancer Res 1990;50(17):5234–9. [PubMed] [Google Scholar]
- 28.Neal ZC, Yang JC, Rakhmilevich AL, Buhtoiarov IN, Lum HE, Imboden M, et al. Enhanced activity of hu14.18-IL2 immunocytokine against murine NXS2 neuroblastoma when combined with interleukin 2 therapy. Clin Cancer Res 2004;10(14):4839–47 doi 10.1158/1078-0432.CCR-03-0799. [DOI] [PubMed] [Google Scholar]
- 29.Yu AL, Gilman AL, Ozkaynak MF, Naranjo A, Diccianni MB, Gan J, et al. Long-Term Follow-up of a Phase III Study of ch14.18 (Dinutuximab) + Cytokine Immunotherapy in Children with High-Risk Neuroblastoma: COG Study ANBL0032. Clin Cancer Res 2021;27(8):2179–89 doi 10.1158/1078-0432.CCR-20-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ladenstein R, Potschger U, Valteau-Couanet D, Luksch R, Castel V, Ash S, et al. Investigation of the Role of Dinutuximab Beta-Based Immunotherapy in the SIOPEN High-Risk Neuroblastoma 1 Trial (HR-NBL1). Cancers (Basel) 2020;12(2) doi 10.3390/cancers12020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ladenstein R, Potschger U, Valteau-Couanet D, Luksch R, Castel V, Yaniv I, et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): a multicentre, randomised, phase 3 trial. Lancet Oncol 2018;19(12):1617–29 doi 10.1016/S1470-2045(18)30578-3. [DOI] [PubMed] [Google Scholar]
- 32.Park JA, Cheung NV. Targets and Antibody Formats for Immunotherapy of Neuroblastoma. J Clin Oncol 2020;38(16):1836–48 doi 10.1200/JCO.19.01410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kushner BH, Cheung IY, Modak S, Basu EM, Roberts SS, Cheung NK. Humanized 3F8 Anti-GD2 Monoclonal Antibody Dosing With Granulocyte-Macrophage Colony-Stimulating Factor in Patients With Resistant Neuroblastoma: A Phase 1 Clinical Trial. JAMA Oncol 2018;4(12):1729–35 doi 10.1001/jamaoncol.2018.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mody R, Naranjo A, Van Ryn C, Yu AL, London WB, Shulkin BL, et al. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): an open-label, randomised, phase 2 trial. Lancet Oncol 2017;18(7):946–57 doi 10.1016/S1470-2045(17)30355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Federico SM, McCarville MB, Shulkin BL, Sondel PM, Hank JA, Hutson P, et al. A Pilot Trial of Humanized Anti-GD2 Monoclonal Antibody (hu14.18K322A) with Chemotherapy and Natural Killer Cells in Children with Recurrent/Refractory Neuroblastoma. Clin Cancer Res 2017;23(21):6441–9 doi 10.1158/1078-0432.CCR-17-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mody R, Yu AL, Naranjo A, Zhang FF, London WB, Shulkin BL, et al. Irinotecan, Temozolomide, and Dinutuximab With GM-CSF in Children With Refractory or Relapsed Neuroblastoma: A Report From the Children’s Oncology Group. J Clin Oncol 2020;38(19):2160–9 doi 10.1200/JCO.20.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furman WL, Federico SM, McCarville MB, Shulkin BL, Davidoff AM, Krasin MJ, et al. A Phase II Trial of Hu14.18K322A in Combination with Induction Chemotherapy in Children with Newly Diagnosed High-Risk Neuroblastoma. Clin Cancer Res 2019;25(21):6320–8 doi 10.1158/1078-0432.CCR-19-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furman WL, McCarville B, Shulkin BL, Davidoff A, Krasin M, Hsu C-W, et al. Improved Outcome in Children With Newly Diagnosed High-Risk Neuroblastoma Treated With Chemoimmunotherapy: Updated Results of a Phase II Study Using hu14.18K322A. Journal of Clinical Oncology;0(0):JCO.21.01375 doi 10.1200/jco.21.01375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schumacher-Kuckelkorn R, Volland R, Gradehandt A, Hero B, Simon T, Berthold F. Lack of immunocytological GD2 expression on neuroblastoma cells in bone marrow at diagnosis, during treatment, and at recurrence. Pediatr Blood Cancer 2017;64(1):46–56 doi 10.1002/pbc.26184. [DOI] [PubMed] [Google Scholar]
- 40.Keyel M DH, Nguyen T, Reynolds CP. Association of Anti-tumor Activity in Neurobllastoma Patient-derived Xenografts with Levels of GD2 Expression. Advances in Neuroblastoma Research, Published Abstract Book, Abstract 101, page 98, 2018. https://wwwanrmeetingorg/dl/ANR2018/ANR_Abstract_Book_5-3-18pdf 2018. [Google Scholar]
- 41.Xiao WH, Yu AL, Sorkin LS. Electrophysiological characteristics of primary afferent fibers after systemic administration of anti-GD2 ganglioside antibody. Pain 1997;69(1–2):145–51 doi 10.1016/s0304-3959(96)03280-0. [DOI] [PubMed] [Google Scholar]
- 42.Sorkin LS, Otto M, Baldwin WM 3rd, Vail E, Gillies SD, Handgretinger R, et al. Anti-GD(2) with an FC point mutation reduces complement fixation and decreases antibody-induced allodynia. Pain 2010;149(1):135–42 doi 10.1016/j.pain.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunleavy K, Fanale MA, Abramson JS, Noy A, Caimi PF, Pittaluga S, et al. Dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in untreated aggressive diffuse large B-cell lymphoma with MYC rearrangement: a prospective, multicentre, single-arm phase 2 study. Lancet Haematol 2018;5(12):e609–e17 doi 10.1016/S2352-3026(18)30177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017;389(10075):1195–205 doi 10.1016/S0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trivedi S, Ferris RL. Epidermal Growth Factor Receptor-Targeted Therapy for Head and Neck Cancer. Otolaryngol Clin North Am 2021;54(4):743–9 doi 10.1016/j.otc.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Majzner RG, Heitzeneder S, Mackall CL. Harnessing the Immunotherapy Revolution for the Treatment of Childhood Cancers. Cancer Cell 2017;31(4):476–85 doi 10.1016/j.ccell.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 2011;118(23):6050–6 doi 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 2008;14(11):1264–70 doi 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heczey A, Louis CU, Savoldo B, Dakhova O, Durett A, Grilley B, et al. CAR T Cells Administered in Combination with Lymphodepletion and PD-1 Inhibition to Patients with Neuroblastoma. Mol Ther 2017;25(9):2214–24 doi 10.1016/j.ymthe.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Straathof K, Flutter B, Wallace R, Jain N, Loka T, Depani S, et al. Antitumor activity without on-target off-tumor toxicity of GD2-chimeric antigen receptor T cells in patients with neuroblastoma. Sci Transl Med 2020;12(571) doi 10.1126/scitranslmed.abd6169. [DOI] [PubMed] [Google Scholar]
- 51.Del Bufalo F QC, De Angelis B, Pagliara D, Caruana I, Li Pira G, Sinibaldi M, Bertaina V, Merli P, Serra A, Del Baldo G, Di Cecca S, Leone L, Garganese MC, Locatelli F. Academic, Phase I/II Trial on T Cells Expressing a Third-Generation GD2 Chimeric Antigen Receptor and Inducible Caspase-9 Safety Switch for Treatment of Relapsed/Refractory High-Risk Neuroblastoma. Advances in Neuroblastoma Research, Published Abstract Book, Abstract MS71, page 57, 2021. https://wwwanr2022org/resources/uploads/sites/26/2021/01/Abstract-book-Oral-presentations-1pdf 2021. [Google Scholar]
- 52.Majzner RG, Ramakrishna S, Yeom KW, Patel S, Chinnasamy H, Schultz LM, et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 2022. doi 10.1038/s41586-022-04489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Majzner RG, Rietberg SP, Sotillo E, Dong R, Vachharajani VT, Labanieh L, et al. Tuning the Antigen Density Requirement for CAR T-cell Activity. Cancer Discov 2020;10(5):702–23 doi 10.1158/2159-8290.CD-19-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Modak S, Kramer K, Gultekin SH, Guo HF, Cheung NK. Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Res 2001;61(10):4048–54. [PubMed] [Google Scholar]
- 55.Ahmed M, Cheng M, Zhao Q, Goldgur Y, Cheal SM, Guo HF, et al. Humanized Affinity-matured Monoclonal Antibody 8H9 Has Potent Antitumor Activity and Binds to FG Loop of Tumor Antigen B7-H3. J Biol Chem 2015;290(50):30018–29 doi 10.1074/jbc.M115.679852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kramer K, Kushner BH, Modak S, Pandit-Taskar N, Smith-Jones P, Zanzonico P, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neurooncol 2010;97(3):409–18 doi 10.1007/s11060-009-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kramer K, Smith M, Souweidane MM. Safety profile of long-term intraventricular access devices in pediatric patients receiving radioimmunotherapy for central nervous system malignancies. Pediatr Blood Cancer 2014;61(9):1590–2 doi 10.1002/pbc.25080. [DOI] [PubMed] [Google Scholar]
- 58.Souweidane MM, Kramer K, Pandit-Taskar N, Zhou Z, Haque S, Zanzonico P, et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: a single-centre, dose-escalation, phase 1 trial. Lancet Oncol 2018;19(8):1040–50 doi 10.1016/S1470-2045(18)30322-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loo D, Alderson RF, Chen FZ, Huang L, Zhang W, Gorlatov S, et al. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin Cancer Res 2012;18(14):3834–45 doi 10.1158/1078-0432.CCR-12-0715. [DOI] [PubMed] [Google Scholar]
- 60.Majzner RG, Theruvath JL, Nellan A, Heitzeneder S, Cui Y, Mount CW, et al. CAR T Cells Targeting B7-H3, a Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity Against Pediatric Solid Tumors and Brain Tumors. Clin Cancer Res 2019. doi 10.1158/1078-0432.CCR-18-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Du H, Hirabayashi K, Ahn S, Kren NP, Montgomery SA, Wang X, et al. Antitumor Responses in the Absence of Toxicity in Solid Tumors by Targeting B7-H3 via Chimeric Antigen Receptor T Cells. Cancer Cell 2019;35(2):221–37 e8 doi 10.1016/j.ccell.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bresler SC, Weiser DA, Huwe PJ, Park JH, Krytska K, Ryles H, et al. ALK mutations confer differential oncogenic activation and sensitivity to ALK inhibition therapy in neuroblastoma. Cancer Cell 2014;26(5):682–94 doi 10.1016/j.ccell.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carpenter EL, Haglund EA, Mace EM, Deng D, Martinez D, Wood AC, et al. Antibody targeting of anaplastic lymphoma kinase induces cytotoxicity of human neuroblastoma. Oncogene 2012;31(46):4859–67 doi 10.1038/onc.2011.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walker AJ, Majzner RG, Zhang L, Wanhainen K, Long AH, Nguyen SM, et al. Tumor Antigen and Receptor Densities Regulate Efficacy of a Chimeric Antigen Receptor Targeting Anaplastic Lymphoma Kinase. Mol Ther 2017;25(9):2189–201 doi 10.1016/j.ymthe.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sano R, Krytska K, Larmour CE, Raman P, Martinez D, Ligon GF, et al. An antibody-drug conjugate directed to the ALK receptor demonstrates efficacy in preclinical models of neuroblastoma. Sci Transl Med 2019;11(483) doi 10.1126/scitranslmed.aau9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bosse KR, Raman P, Zhu Z, Lane M, Martinez D, Heitzeneder S, et al. Identification of GPC2 as an Oncoprotein and Candidate Immunotherapeutic Target in High-Risk Neuroblastoma. Cancer Cell 2017;32(3):295–309 e12 doi 10.1016/j.ccell.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li N, Torres MB, Spetz MR, Wang R, Peng L, Tian M, et al. CAR T cells targeting tumor-associated exons of glypican 2 regress neuroblastoma in mice. Cell Rep Med 2021;2(6):100297 doi 10.1016/j.xcrm.2021.100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heitzeneder S, Bosse KR, Zhu Z, Zhelev D, Majzner RG, Radosevich MT, et al. GPC2-CAR T cells tuned for low antigen density mediate potent activity against neuroblastoma without toxicity. Cancer Cell 2022;40(1):53–69 e9 doi 10.1016/j.ccell.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feng Y, Wang Y, Zhu Z, Li W, Sussman RT, Randall M, et al. Differential killing of CD56-expressing cells by drug-conjugated human antibodies targeting membrane-distal and membrane-proximal non-overlapping epitopes. MAbs 2016;8(4):799–810 doi 10.1080/19420862.2016.1155014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kunkele A, Taraseviciute A, Finn LS, Johnson AJ, Berger C, Finney O, et al. Preclinical Assessment of CD171-Directed CAR T-cell Adoptive Therapy for Childhood Neuroblastoma: CE7 Epitope Target Safety and Product Manufacturing Feasibility. Clin Cancer Res 2017;23(2):466–77 doi 10.1158/1078-0432.CCR-16-0354. [DOI] [PubMed] [Google Scholar]
- 71.Rodolfo M, Luksch R, Stockert E, Chen YT, Collini P, Ranzani T, et al. Antigen-specific immunity in neuroblastoma patients: antibody and T-cell recognition of NY-ESO-1 tumor antigen. Cancer Res 2003;63(20):6948–55. [PubMed] [Google Scholar]
- 72.Oberthuer A, Hero B, Spitz R, Berthold F, Fischer M. The tumor-associated antigen PRAME is universally expressed in high-stage neuroblastoma and associated with poor outcome. Clin Cancer Res 2004;10(13):4307–13 doi 10.1158/1078-0432.CCR-03-0813. [DOI] [PubMed] [Google Scholar]
- 73.D’Angelo SP, Melchiori L, Merchant MS, Bernstein D, Glod J, Kaplan R, et al. Antitumor Activity Associated with Prolonged Persistence of Adoptively Transferred NY-ESO-1 (c259)T Cells in Synovial Sarcoma. Cancer Discov 2018;8(8):944–57 doi 10.1158/2159-8290.CD-17-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vasileiou S, Lulla PD, Tzannou I, Watanabe A, Kuvalekar M, Callejas WL, et al. T-Cell Therapy for Lymphoma Using Nonengineered Multiantigen-Targeted T Cells Is Safe and Produces Durable Clinical Effects. J Clin Oncol 2021;39(13):1415–25 doi 10.1200/JCO.20.02224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wei JS, Kuznetsov IB, Zhang S, Song YK, Asgharzadeh S, Sindiri S, et al. Clinically Relevant Cytotoxic Immune Cell Signatures and Clonal Expansion of T-Cell Receptors in High-Risk MYCN-Not-Amplified Human Neuroblastoma. Clin Cancer Res 2018;24(22):5673–84 doi 10.1158/1078-0432.CCR-18-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Erbe AK, Wang W, Carmichael L, Kim K, Mendonca EA, Song Y, et al. Neuroblastoma Patients’ KIR and KIR-Ligand Genotypes Influence Clinical Outcome for Dinutuximab-based Immunotherapy: A Report from the Children’s Oncology Group. Clin Cancer Res 2018;24(1):189–96 doi 10.1158/1078-0432.CCR-17-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Forlenza CJ, Boudreau JE, Zheng J, Le Luduec JB, Chamberlain E, Heller G, et al. KIR3DL1 Allelic Polymorphism and HLA-B Epitopes Modulate Response to Anti-GD2 Monoclonal Antibody in Patients With Neuroblastoma. J Clin Oncol 2016;34(21):2443–51 doi 10.1200/JCO.2015.64.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Modak S, Le Luduec JB, Cheung IY, Goldman DA, Ostrovnaya I, Doubrovina E, et al. Adoptive immunotherapy with haploidentical natural killer cells and Anti-GD2 monoclonal antibody m3F8 for resistant neuroblastoma: Results of a phase I study. Oncoimmunology 2018;7(8):e1461305 doi 10.1080/2162402X.2018.1461305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Siebert N, Zumpe M, Juttner M, Troschke-Meurer S, Lode HN. PD-1 blockade augments anti-neuroblastoma immune response induced by anti-GD2 antibody ch14.18/CHO. Oncoimmunology 2017;6(10):e1343775 doi 10.1080/2162402X.2017.1343775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heczey A, Courtney AN, Montalbano A, Robinson S, Liu K, Li M, et al. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat Med 2020;26(11):1686–90 doi 10.1038/s41591-020-1074-2. [DOI] [PubMed] [Google Scholar]
- 81.Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009;138(2):271–85 doi 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N Engl J Med 2018;379(18):1711–21 doi 10.1056/NEJMoa1807315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Theruvath J, Menard M, Smith BAH, Linde MH, Coles GL, Dalton GN, et al. Anti-GD2 synergizes with CD47 blockade to mediate tumor eradication. Nat Med 2022. doi 10.1038/s41591-021-01625-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raman S, Buongervino SN, Lane MV, Zhelev DV, Zhu Z, Cui H, et al. A GPC2 antibody-drug conjugate is efficacious against neuroblastoma and small-cell lung cancer via binding a conformational epitope. Cell Rep Med 2021;2(7):100344 doi 10.1016/j.xcrm.2021.100344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, et al. Phase I/Phase II Study of Blinatumomab in Pediatric Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia. J Clin Oncol 2016;34(36):4381–9 doi 10.1200/JCO.2016.67.3301. [DOI] [PubMed] [Google Scholar]
- 86.Ramdeny S, Chaudhary A, Worth A, Ghorashian S, Slatter M, Lum SH, et al. Activity of blinatumomab in lymphoblastic leukemia with impaired T-cell immunity due to congenital immunodeficiency. Blood Adv 2021;5(8):2153–5 doi 10.1182/bloodadvances.2021004284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vallera DA, Ferrone S, Kodal B, Hinderlie P, Bendzick L, Ettestad B, et al. NK-Cell-Mediated Targeting of Various Solid Tumors Using a B7-H3 Tri-Specific Killer Engager In Vitro and In Vivo. Cancers (Basel) 2020;12(9) doi 10.3390/cancers12092659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anghelescu DL, Goldberg JL, Faughnan LG, Wu J, Mao S, Furman WL, et al. Comparison of pain outcomes between two anti-GD2 antibodies in patients with neuroblastoma. Pediatr Blood Cancer 2015;62(2):224–8 doi 10.1002/pbc.25280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Evers M, Stip M, Keller K, Willemen H, Nederend M, Jansen M, et al. Anti-GD2 IgA kills tumors by neutrophils without antibody-associated pain in the preclinical treatment of high-risk neuroblastoma. J Immunother Cancer 2021;9(10) doi 10.1136/jitc-2021-003163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Erbe AK, Hernandez R, Gerhardt D, Hammer B, Felder M, Bercher M, et al. Improving Specific Targeting of Tumors Through Bispecific SNIPER Antibodies. The Journal of Immunology 2020;204(1 Supplement):91.2–.2. [Google Scholar]
- 91.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015;348(6230):69–74 doi 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 92.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348(6230):124–8 doi 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pugh TJ, Morozova O, Attiyeh EF, Asgharzadeh S, Wei JS, Auclair D, et al. The genetic landscape of high-risk neuroblastoma. Nat Genet 2013;45(3):279–84 doi 10.1038/ng.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haen SP, Loffler MW, Rammensee HG, Brossart P. Towards new horizons: characterization, classification and implications of the tumour antigenic repertoire. Nat Rev Clin Oncol 2020;17(10):595–610 doi 10.1038/s41571-020-0387-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yarmarkovich M, Marshall QF, Warrington JM, Premaratne R, Farrel A, Groff D, et al. Cross-HLA targeting of intracellular oncoproteins with peptide-centric CARs. Nature 2021;599(7885):477–84 doi 10.1038/s41586-021-04061-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96.Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother 2013;36(2):133–51 doi 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013;122(6):863–71 doi 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marabelle A, Kohrt H, Caux C, Levy R. Intratumoral immunization: a new paradigm for cancer therapy. Clin Cancer Res 2014;20(7):1747–56 doi 10.1158/1078-0432.CCR-13-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sagiv-Barfi I, Czerwinski DK, Levy S, Alam IS, Mayer AT, Gambhir SS, et al. Eradication of spontaneous malignancy by local immunotherapy. Sci Transl Med 2018;10(426) doi 10.1126/scitranslmed.aan4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morris ZS, Guy EI, Francis DM, Gressett MM, Werner LR, Carmichael LL, et al. In Situ Tumor Vaccination by Combining Local Radiation and Tumor-Specific Antibody or Immunocytokine Treatments. Cancer Res 2016;76(13):3929–41 doi 10.1158/0008-5472.CAN-15-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Voeller J, Erbe AK, Slowinski J, Rasmussen K, Carlson PM, Hoefges A, et al. Combined innate and adaptive immunotherapy overcomes resistance of immunologically cold syngeneic murine neuroblastoma to checkpoint inhibition. J Immunother Cancer 2019;7(1):344 doi 10.1186/s40425-019-0823-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006;314(5796):126–9 doi 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lynn RC, Weber EW, Sotillo E, Gennert D, Xu P, Good Z, et al. c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature 2019;576(7786):293–300 doi 10.1038/s41586-019-1805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kloss CC, Lee J, Zhang A, Chen F, Melenhorst JJ, Lacey SF, et al. Dominant-Negative TGF-beta Receptor Enhances PSMA-Targeted Human CAR T Cell Proliferation And Augments Prostate Cancer Eradication. Mol Ther 2018;26(7):1855–66 doi 10.1016/j.ymthe.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shum T, Omer B, Tashiro H, Kruse RL, Wagner DL, Parikh K, et al. Constitutive Signaling from an Engineered IL7 Receptor Promotes Durable Tumor Elimination by Tumor-Redirected T Cells. Cancer Discov 2017;7(11):1238–47 doi 10.1158/2159-8290.CD-17-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roybal KT, Williams JZ, Morsut L, Rupp LJ, Kolinko I, Choe JH, et al. Engineering T Cells with Customized Therapeutic Response Programs Using Synthetic Notch Receptors. Cell 2016;167(2):419–32 e16 doi 10.1016/j.cell.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med 2013;5(215):215ra172 doi 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Poon E, Liang T, Jamin Y, Walz S, Kwok C, Hakkert A, et al. Orally bioavailable CDK9/2 inhibitor shows mechanism-based therapeutic potential in MYCN-driven neuroblastoma. J Clin Invest 2020;130(11):5875–92 doi 10.1172/JCI134132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wood AC, Krytska K, Ryles HT, Infarinato NR, Sano R, Hansel TD, et al. Dual ALK and CDK4/6 Inhibition Demonstrates Synergy against Neuroblastoma. Clin Cancer Res 2017;23(11):2856–68 doi 10.1158/1078-0432.CCR-16-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Roeschert I, Poon E, Henssen AG, Garcia HD, Gatti M, Giansanti C, et al. Combined inhibition of Aurora-A and ATR kinase results in regression of MYCN-amplified neuroblastoma. Nat Cancer 2021;2(3):312–26 doi 10.1038/s43018-020-00171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Berridge G, Menassa DA, Moloney T, Waters PJ, Welding I, Thomsen S, et al. Glutamate receptor delta2 serum antibodies in pediatric opsoclonus myoclonus ataxia syndrome. Neurology 2018;91(8):e714–e23 doi 10.1212/WNL.0000000000006035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Camisaschi C, Renne SL, Beretta V, Rini F, Spagnuolo RD, Tuccitto A, et al. Immune landscape and in vivo immunogenicity of NY-ESO-1 tumor antigen in advanced neuroblastoma patients. BMC Cancer 2018;18(1):983 doi 10.1186/s12885-018-4910-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jones HF, Molvi Z, Klatt MG, Dao T, Scheinberg DA. Empirical and Rational Design of T Cell Receptor-Based Immunotherapies. Front Immunol 2020;11:585385 doi 10.3389/fimmu.2020.585385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao L, Cao YJ. Engineered T Cell Therapy for Cancer in the Clinic. Front Immunol 2019;10:2250 doi 10.3389/fimmu.2019.02250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bethune MT, Li XH, Yu J, McLaughlin J, Cheng D, Mathis C, et al. Isolation and characterization of NY-ESO-1-specific T cell receptors restricted on various MHC molecules. Proc Natl Acad Sci U S A 2018;115(45):E10702–E11 doi 10.1073/pnas.1810653115. [DOI] [PMC free article] [PubMed] [Google Scholar]


