Abstract
Objective:
Little is known regarding the reactogenicity and related SARS-CoV-2 vaccine response in patients with chronic inflammatory disease (CID). Our objective was to characterize the adverse event (AE) profile of CID patients following SARS-CoV-2 vaccination and understand the relationship between reactogenicity and immunogenicity of SARS-CoV-2 vaccines.
Methods:
CID patients and healthy controls eligible to receive mRNA SARS-CoV-2 vaccines participated in 3 study visits (pre-vaccine, after dose 1, after dose 2) where blood and clinical data were collected. Assessment of AEs were solicited within 7 days of receiving each dose. Serum anti-SARS-CoV-2 spike IgG± antibody titers were quantified following vaccination. Statistical analysis was performed utilizing mixed models and tobit regressions, adjusting for covariates.
Results:
441 participants (322 CID patients and 119 controls) were included. Compared to controls, CID patients reported greater symptom severity after dose 1 (p=0.0001), including more myalgia and fatigue (p<0.05). For immunogenicity, a higher symptom severity after dose 1 and higher number of symptoms after dose 2 was associated with higher antibody titers (p≤0.05). Each increase of one symptom was associated with 15.1% increase in antibody titer. Symptom association was strongest with site pain after dose 1 (105%, p=0.03) and fatigue after dose 2 (113%, p=0.004).
Conclusions:
CID patients have a distinct reactogenicity profile following SARS-CoV-2 vaccination compared to controls. Furthermore, there is an association between increased reactogenicity and increased vaccine response. This finding may speak to the more variable immunogenicity in CID patients and may be an important indicator of vaccine response to the novel SARS-CoV-2 vaccines.
The COVID-19 pandemic caused by the SARS-COV-2 virus is a global health emergency that has affected tens of millions of individuals worldwide. To address this crisis, the Food and Drug Administration has approved several vaccines for emergency use, including novel mRNA-based and adenovirus-based approaches.1–3 Patients with chronic inflammatory diseases (CID) have dysregulation of their immune system and often require long-term use of immunosuppressive medications that may increase their risk of developing severe illness from SARS-CoV-2 infection.4,5 Therefore, the importance of immunization in this population is particularly high.
Concerns regarding the side effect profile and novelty of the mRNA SARS-CoV-2 vaccines have been shown to influence attitudes toward the vaccines and contribute to vaccine hesitancy in the general population.6,7 The clinical trials of two mRNA-based vaccines and one adenovirus-based vaccine identified most participants reported at least one local or systemic reaction, with very few reactions characterized as severe.1–3 Follow up data from the CDC V-safe active surveillance system have confirmed these initial findings and helped provide reassurance to providers and patients regarding vaccine safety.8,9 Yet while side effect profile of the general population to the SARS-CoV-2 vaccines continues to be studied, little is known regarding the reactogenicity of patients with CID following SARS-CoV-2 vaccination.10,11
While rheumatic disease-specific reactogenicity studies are limited, the safety profile of the several vaccines in immunocompromised patients including HIV and renal transplant patients have been examined.12–15 Given the presence of immune dysregulation and use of chronic immunosuppression, it is plausible that patients with CID may have a unique reactogenicity profile to the novel SARS-CoV-2 vaccines. In addition, many of the reported adverse events to the SARS-CoV-2 vaccine including arthralgias, myalgias, fatigue and even fever mimic symptoms of CID flare.9 It is therefore crucial to better understand the reactogenicity of CID patients following SARS-CoV-2 vaccination to better inform physicians and patients regarding expectations.
Another pressing question has been the relationship between immunogenicity and reactogenicity to the SARS-COV-2 vaccines. It has been hypothesized increased symptomatology following vaccine would be indicative of a more robust vaccine response, however, this has yet to be demonstrated in the general population.16 One explanation could be the robustness of the vaccine response in the immunocompetent host that has been demonstrated to occur in nearly all individuals. However, in patients with CID and other immunocompromised states, it has been shown that immune response may be blunted in certain groups.17,18 Therefore, understanding the relationship of immunogenicity and reactogenicity in the CID patient population may be of particular clinical importance.
The primary objective of this study was to characterize the adverse event profile of patients with CID after receiving SARS-CoV-2 vaccines and to better understand the relationship between reactogenicity and immunogenicity of the SARS-CoV-2 vaccines in patients with CID. We were interested in understanding the degree of severity and individual symptoms experienced by CID patients, as well as the impact of immunosuppressive medications. We hypothesized patients with CID would have a reactogenicity profile unique to that of the general population and that increased symptomatology would be associated with increased vaccine response.
Methods
Study Design and Participants
This sub-study within COVID-19 Vaccine Responses in Patients with Autoimmune Disease (COVaRiPAD) examined the magnitude and quality of immune response to the SARS-COV-2 vaccines. COVARIPAD is a longitudinal, prospective, observational study taking place at two large academic centers, Washington University of Saint Louis (WUSTL) and University of California San Francisco (UCSF). This study was approved by WUSTL and UCSF Institutional Review Board.
Participants with confirmed CID and healthy controls who were eligible to receive the SARS-CoV-2 vaccine were recruited for this study from the faculty, staff, employees and patients at Washington University School of Medicine and BJC Healthcare system (St. Louis, MO) and UCSF, UCSF Health, and Zuckerberg San Francisco General Hospital (San Francisco, CA). All participants provided informed consent. As part of the COVaRiPAD study, participants were assessed in standardized intervals (pre-vaccine, post-vaccine dose 1, post-vaccine dose 2) to answer questionnaires and provide blood samples. Prior to first vaccine dose, demographic data and clinical data including disease classification, and current and historic medications were collected. When enrolling, control participants had the option to only complete questionnaires and not provide blood for immunogenicity studies; these participants provided demographic, verification of the absence of CID and reactogenicity information only.
Assessment of SARS-CoV-2 Vaccine Response
Humoral response quantification was performed for patients and controls who had completed baseline and post-vaccination blood draws. As previously described, anti-spike IgG quantification was performed utilizing enzyme-linked immunosorbent assay (ELISA) and direct ex-vivo enzyme-linked immunosorbent spot (ELISpot) assays were performed to quantify recombinant S protein-binding IgG secreting cells.17
Assessment of Reactogenicity
For assessment of adverse events, the outcomes of interest included overall severity of symptoms (0–3 ordinal scale), number of symptoms, and each individual symptom present (yes/no) following both doses of vaccine. We utilized an online administered survey consisting of severity on an ordinal scale (0=no symptoms, 1=mild, 2=moderate, and 3=severe) and solicited symptoms presented in list format (injection site pain, injection site redness, headache, fever, rash, myalgia, arthralgia, fatigue, nausea, and diarrhea). Due to differences in initial protocol, healthy controls from the WUSTL site did not provide graded severity. Participants were asked to respond with their symptoms up to 7 days post vaccination.
Statistical Analysis
Demographic differences between CID patients and healthy subjects were assessed using t-tests and chi-square tests. Differences in reactogenicity outcomes between CID patients and healthy controls were analyzed using mixed models, with site as a random effect and adjusting for age, gender, and vaccine (Stata meologit for ordinal regression of severity; mixed for linear regression of number of symptoms; and melogit for logistic regression of symptom presence/absence). Within CID patients, differences in reactogenicity were examined among A) different CID disease states and B) medications of interest by comparing exposed and nonexposed groups (for example, IBD versus non-IBD CID, and TNF inhibitor medication versus no TNF inhibitor). We did not stratify or adjust for medications within disease states or vice-versa due to small sample sizes. Severity and number of symptoms were evaluated using mixed models as above; diseases and medications for each individual symptom were assessed using chi-square tests. Study site was incorporated as a random effect in our mixed models as there were site differences in adverse events and correlated demographics (e.g. age) (Supplementary Table 1). To further account for these study site differences, sensitivity analysis of reactogenicity in CID patients versus healthy controls was performed using a subset of patients in homogeneous matched groups. Non-overlapping groups were selected so that they contained one or more CID subjects, one or more healthy subjects, and all group members had the same vaccine exposure, gender, and age within 5 years. Some subjects were excluded when there were no corresponding CID/healthy with the match criteria. A total of 397 patients were included in 31 groups. These were analyzed using the mixed model functions above but with the matched group as the random effect.
For assessment of reactogenicity impact on vaccine antibody response, the outcome of interest was anti-SARS-CoV-2 spike IgG± antibody titer. Differences in antibody titers were examined with reactogenicity as a predictor, using graded symptom severity, number of symptom and individual symptoms among CID patients and healthy controls. Tobit regressions adjusting for participant status, age, gender, and vaccine type were utilized to account for left-censoring below the response detect limit (1:30).19,20 Vaccine type and site were not significant and removed from these models.
Results
Study Participants and Clinical Characteristics
A total of 441 participants were included in the study, including 322 patients with CID and 119 healthy controls. The mean age was 47.3 ± 15.9 years with 18% of participants ≥65 years old. The majority of participants were female (68%) and white (82%). Vaccine distribution included 74% receiving BNT162b2 and 26% receiving mRNA-1273. The most common diagnoses among CID patients included inflammatory bowel disease (32%) and rheumatoid arthritis (23%). Demographic and clinical characteristics are shown in Table 1.
Table 1:
Demographic and Clinical Characteristics of Participants.
| Demographic Data | CID (n=322) | Control (n=119) | P-value |
|
| |||
| Age [years], mean (SD) | 48.5 ± 15.7 | 44.2 ± 15.8 | 0.01 |
| <65 (%) | 80.4 | 85.9 | 0.18 |
| 65+ (%) | 19.6 | 15.1 | |
|
| |||
| Gender (%female) | 71.4 | 59.9 | 0.02 |
|
| |||
| Race (%white) | 85.7 | 73.9 | 0.01 |
|
| |||
| Vaccine | |||
| BNT162b2 (%) | 73.9 | 73.1 | 0.86 |
| mRNA-1273 (%) | 26.1 | 26.9 | |
|
| |||
| Site | |||
| WUSTL (%) | 65.5 | 39.5 | <0.001 |
| UCSF (%) | 35.5 | 60.5 | |
|
| |||
| Immunologic Diagnosis | N (%) | ||
|
| |||
| Inflammatory Bowel Disease | 105 (32.6) | ||
| Rheumatoid Arthritis | 74 (23.0) | ||
| Spondyloarthritis | 46 (14.3) | ||
| Systemic Lupus Erythematosus | 36 (11.2) | ||
| Sjogren’s Syndrome | 18 (5.6) | ||
| Other Connective Tissue Disease | 18 (5.6) | ||
| Uveitis | 22 (6.8) | ||
| Multiple Sclerosis | 20 (6.2) | ||
| Hidradenitis Suppurativa | 16 (5.0) | ||
| Vasculitis | 7 (2.2) | ||
| Autoinflammatory Syndrome | 3 (1.0) | ||
| IgG4-Related Disease | 3 (1.0) | ||
| NMO | 2 (0.6) | ||
| Other | 8 (2.5) | ||
|
| |||
| Medications | N (%) | ||
|
| |||
| Prednisone | 41 (12.7) | ||
| Disease Modifying Antirheumatic Drug | |||
| Methotrexate | 58 (18.0) | ||
| Hydroxychloroquine | 60 (18.6) | ||
| Azathioprine | 20 (6.2) | ||
| Sulfasalazine | 19 (5.9) | ||
| Mycophenolate Mofetil | 17 (5.3) | ||
| Leflunomide | 11 (3.4) | ||
| Janus Kinase inhibitors | 18 (5.6) | ||
| Biological therapies | |||
| Tumor Necrosis Factor inhibitors | 99 (30.7) | ||
| B cell depleting therapies | 29 (9.0) | ||
| Belimumab | 4 (1.2) | ||
| Vedolizumab | 26 (8.1) | ||
| Interleukin 12/23 or 23 inhibitors | 22 (6.8) | ||
| Abatacept | 5 (1.6) | ||
| Other | 4 (1.2) | ||
| Nonsteroidal Anti-inflammatory Drugs | 59 (18.3) | ||
CID = Chronic Inflammatory Disease, WUSTL = Washington University Saint Louis, UCSF = University of California San Francisco, NMO = Neuromyelitis Optica.
Adverse Event Profile
Solicited adverse events occurred frequently in both the CID and control groups following the first and second dose of the SARS-CoV-2 vaccine. In CID patients, the most common symptoms following both first and second dose of vaccine included injection site pain, fatigue, headaches and myalgias (Figure 1). When compared to healthy controls, CID patients had significantly more severe symptoms following the first dose of vaccine after adjusting for age, gender, and vaccine type (OR=3.7 [95% CI 1.91–7.12] per severity level, p=0.0001, i.e., the odds of a CID patient having severity 1 level higher is 3.7 times that of a healthy patient with other covariates equal). With random effect mixed model of the combined sites, there was more fatigue (OR=1.85, [1.01–3.37], p=0.045) and myalgias (OR=1.95 [1.03–3.67], p=0.04) in patients with CID following the first dose compared to healthy controls. Following the second dose, CID patients had more headache compared to healthy controls (OR = 1.68 [1.06–2.66], p=0.03) but other differences in symptoms severity and number of symptoms did not vary significantly between CID patients and controls. A sensitivity analysis with matched groups for gender, age, and vaccine type yielded similar results to original combined analysis (Supplementary Table 1).
Figure 1. Severity and Solicited Symptoms among Controls vs. CID patients.
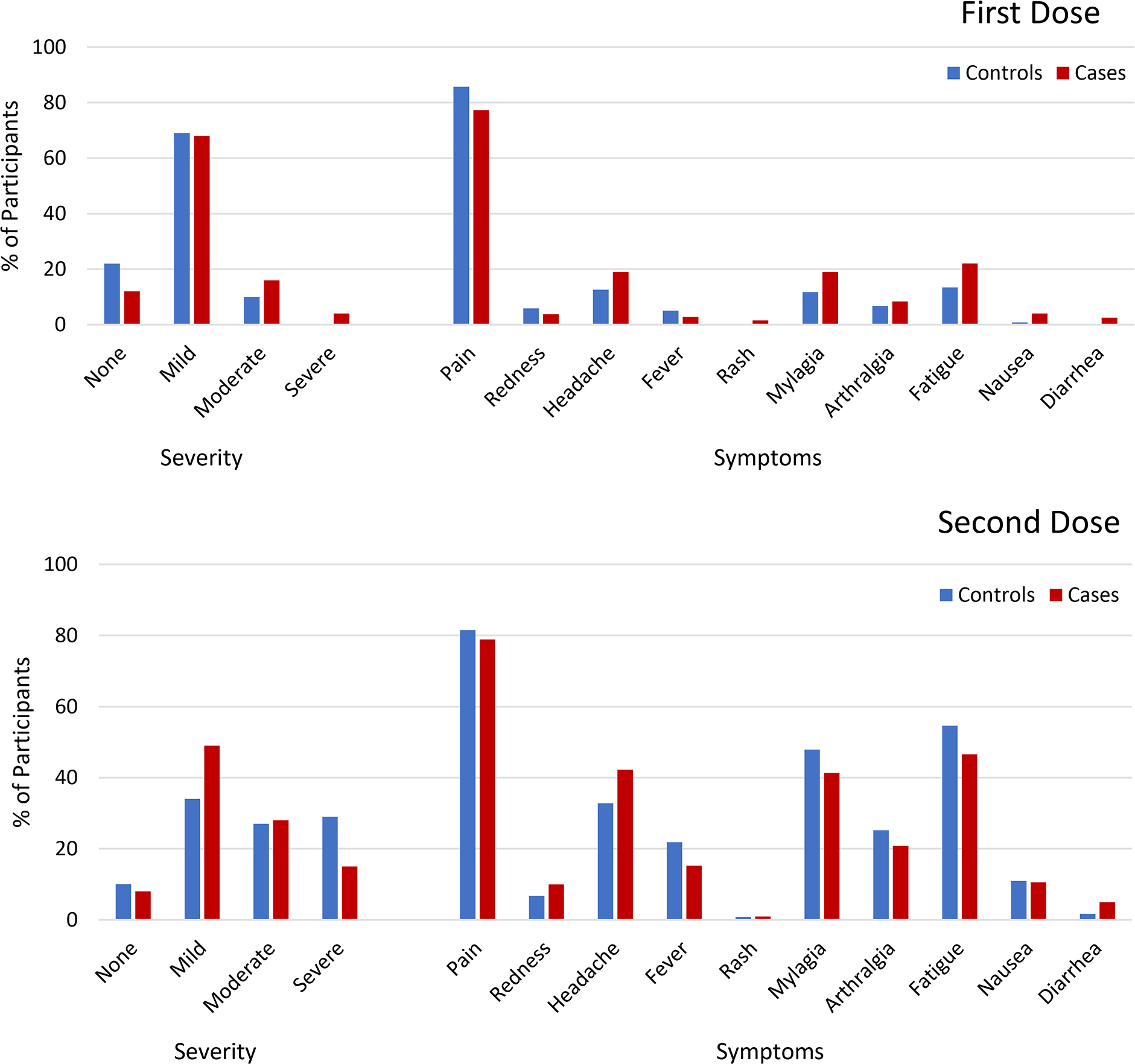
Depicted is the percentage of participants from each group who had endorsed symptom severity and solicited adverse events within 7 days after dose 1 and dose 2 of SARS-CoV-2 vaccine.
Within CID patients, no specific disease group was associated with increased symptomatology following mixed-model regressions adjusting for age, gender, and vaccine. There was suggestive evidence of increased reactogenicity in spondyloarthritis (SpA) patients with increased severity following the second dose (OR=1.98 [95% CI 1.08–3.64], p=0.03). The strongest individual symptom associations included SpA patients with increased fatigue following the second (OR=2.35 [1.24–4.51], p=0.005) and the first (OR=2.27 [1.14–4.15], p=0.009) dose and connective tissue disease (CTD) patients with increased nausea following the first dose (OR = 4.69 [1.23–16.99], p=0.004). Among medications used in CID patients, methotrexate (OR 1.54 [0.89–2.68], p=0.13) had the strongest adjusted association with increased symptom severity following the second dose of vaccine. B cell depleting agents including rituximab and ocrelizumab had suggestive protective effects for site pain following the first dose (OR=0.39 [0.16–0.97], p=0.02). Among other more prevalent medications, hydroxychloroquine was most associated with arthralgia after the second dose (OR 2.03 [1.01–3.95], p=0.03) and TNFi was most associated with less redness after dose one (OR=0.19 [0.004–1.37], p=0.08).
When examining additional factors influencing reactogenicity, we found age and gender influenced reactogenicity (Figure 2). In multivariable models, age >65 was associated with significantly less symptom severity and fewer symptoms reported following the second dose of vaccine (OR=0.51 [0.31–0.83] p=0.006, OR=−0.81 [−1.27- −0.34] per symptom p=0.001, respectively). We also found female gender was associated with increased symptom severity when compared to their male counterparts (second dose OR=2.028 [1.33–3.10] p=0.001). Those receiving BNT162b2 mRNA vaccine demonstrated a trend towards less severe symptoms following second dose compared to those receiving mRNA-1273 vaccine however this was not statistically significant (OR 0.66 [0.43–1.01] p=0.06). Complete results are shown in Supplementary Table 2.
Figure 2. Symptom Severity among Participants by Age and Gender.
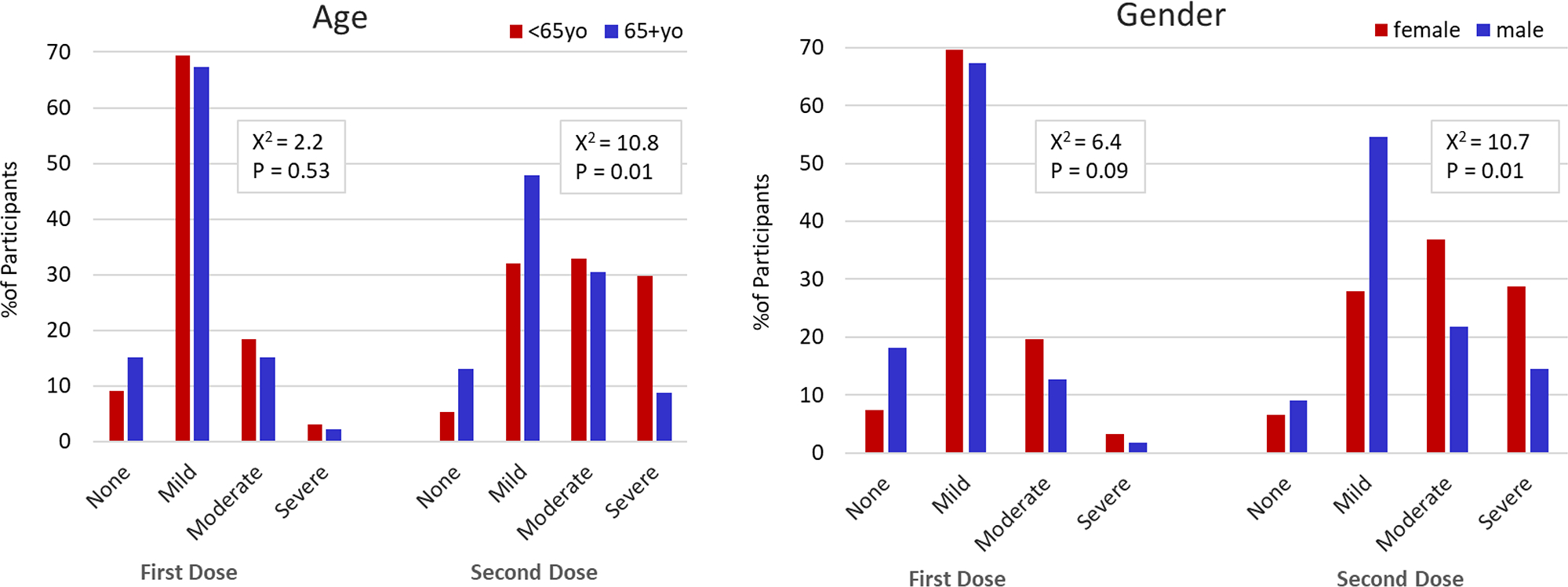
Comparison of endorsed symptom severity following dose 1 and dose 2 of SARS-CoV-2 vaccine by age groups (left) and gender (right).
Reactogenicity and Immunogenicity
Regarding reactogenicity and its relationship to vaccine response, we found certain adverse event characteristics were associated with higher spike protein antibody titer to the SARS-CoV-2 vaccine in both CID patients and controls. Specifically, after adjustment for covariates, increased symptom severity following the first dose of vaccine and a higher number of reported symptoms after the second dose of vaccine was associated with higher antibody titers (Table 2). Each increase in degree of severity following the first dose was associated with 68% increase of antibody titer [4.6%–170%] (p=0.03). Each increase of one endorsed symptom following the second dose of vaccine was associated with 15.1% increase in antibody titer [0%–32.4%] (p=0.05) in our regression model (Figure 3). Complete results are shown in Supplementary Table 3.
Table 2.
Top Reactogenicity Predictors (p≤0.05) of Increase in Antibody Titer.
| Reactogenicity predictors | Beta (log10titer) | %Change increase | %Change lower 95% CI | %Change upper 95% CI | P value |
|---|---|---|---|---|---|
| 1st dose severity per level | 0.226 | 68.1% | 4.6% | 170.2% | 0.03 |
| 2nd dose N symptoms per symptom | 0.061 | 15.1% | 0.0% | 32.4% | 0.05 |
| 1st dose site pain | 0.313 | 105.6% | 7.9% | 291.8% | 0.03 |
| 2nd dose fever | 0.309 | 103.6% | 3.1% | 301.9% | 0.04 |
| 2nd dose fatigue | 0.329 | 113.5% | 27.8% | 256.8% | 0.004 |
Shown are the reactogenicity attributes, and their associated changes in anti-SARS-CoV-2 spike IgG± antibody titers, that when present predicted the largest increase in antibody titers compared to when attribute was not present using tobit regression for each predictor, adjusting for CID/healthy, age, gender, and vaccine type.
Figure 3. Predicted Antibody Titers following 2nd dose by N# Symptoms in CID Patients and Healthy Controls.
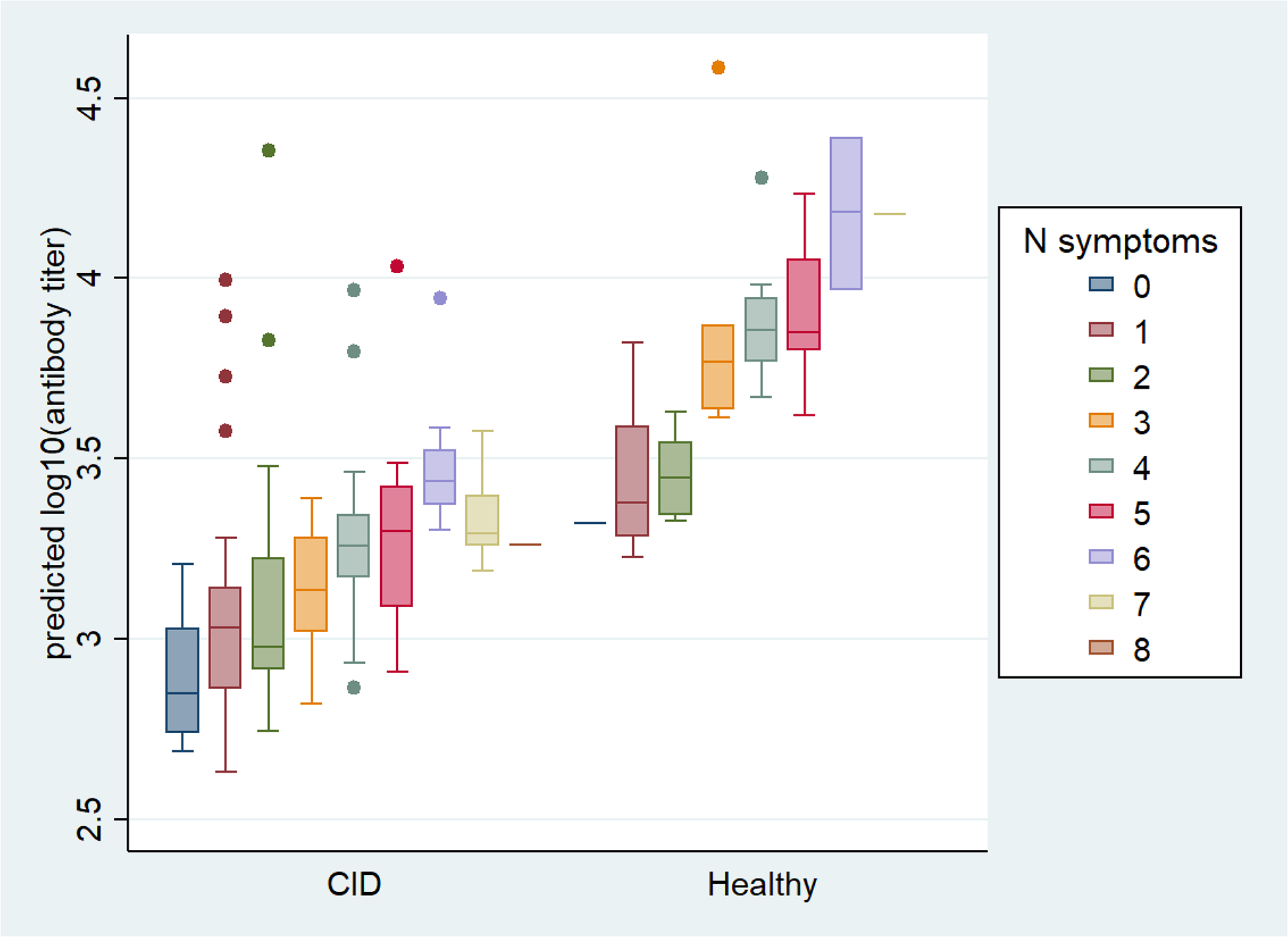
The regression model depicts change in anti-SARS-CoV-2 spike IgG± antibody titer with each increase of 1 endorsed symptom following 2nd dose of vaccine in CID patients and healthy controls.
Among all individual symptoms, the most strongly associated after each dose of vaccine included site pain after first dose and fatigue after second dose. The presence of site pain following the first dose of vaccine demonstrated an increase of 105% [7.9%–292%] (p=0.03) increase in antibody titer compared to when absent and presence of fatigue after the second dose demonstrated an increase of 114% [27.8%–257%] (p=0.004) in antibody titer. Notably in CID patients, the presence of fatigue following the second dose of vaccine was associated with the greatest increase in antibody titer compared to other symptoms of 138% [27%- 346%] (p=0.007).
Discussion
This study is one of the first to examine reactogenicity of the novel mRNA SARS-CoV-2 vaccines among patients with CID and the relationship between reactogenicity and vaccine response. In this study, we found patients with CID had more numerous and more severe AEs following the first dose of the novel SARs-COV-2 mRNA vaccines but overall similar AE profile as healthy controls following the second dose. Most notably we found number of AEs reported was associated with increased antibody titer, demonstrating a link between reactogenicity and immunogenicity in patients with CID as well as healthy controls. The findings from this study will help provide the much-needed information on the adverse event profile of the novel SARS-CoV-2 vaccines and help to better inform patients and providers.
Concerns regarding side effect profile of the novel mRNA vaccines have been shown to be a contributor to vaccine hesitancy in not only the general population, but also in patients with CID.21–23 In this study, we demonstrate differences in reactogenicity profile between the general population and CID patients following the first dose of vaccine. This finding could be potentially due to the existing underlying immune dysregulation in patients with CID or possible alterations in disease activity. Overall, however, when compared to healthy controls, there were not significant differences following the second dose of vaccine, which has been associated with more severe AEs than the first dose. Also reassuringly, burden of severe AEs experienced by patients with CID following vaccination mirrored those reported on a national level.8,9
While we were unable to determine significant differences in reactogenicity among specific CID diseases or medications due to small, stratified sample sizes, the data did show a trend suggesting increased reactogenicity in SpA patients, specifically with increased severity of symptoms following 2nd dose of vaccine and increased fatigue following both doses. This finding may be attributed to SpA patients holding their NSAIDs around vaccination, which is first line treatment for axial SpA.11 This in turn, could lead to increased disease activity including fatigue. In both CID patients and controls, similar to prior studies, we demonstrate that younger patients and female patients had higher AE burden than their older and male counterparts.15,24,25 Differences in immune system competency, hormone status, as well as recognition and reporting of symptoms may explain some of these variations among age and gender.16,25
It has been previously hypothesized that increased symptomatology following vaccination may be indicative of increased immune response, however, that has yet to be demonstrated in vaccine studies to date. In this study we demonstrate a link between reactogenicity and immunogenicity with increased AEs and certain specific AEs associated with increased antibody titers. This finding, present among CID patients and controls, mechanistically supports the underlying physiology of vaccination with immunity coming about via stimulation of the immune system.26 While it is still unclear what clinical significance these differences in antibody titers may have, reactogenicity may be a signal for robustness of immune response in certain populations.
There were both strengths and limitations to this study. This was one of the first studies to examine reactogenicity and its link with immunogenicity of the novel mRNA SARS-COV-2 vaccines in the patients with CID. We applied prospective data to a relatively large and well characterized cohort of patients from two different centers with a diverse number of chronic inflammatory diseases. Limitations of this study include our control population, which was notably different in age and gender between study sites and distinct from our CID population. While we attempted to control for these variables utilizing multivariable models, residual confounding cannot be excluded. There were also differences with regards to reactogenicity between the two study sites, which may be reflective of the population eligible for vaccination at each site. Additionally given differences in initial protocol between sites, there was a reduction in sample size for symptom severity, which could imply less power for analyses. However, given there was a highly significant difference at dose 1, and an effect size close to one for dose 2, sample size alone is unlikely to affect these conclusions. We did lack the ability to compare across cohorts and therefore rule out latent site-specific effects for severity. Overall, it was important to include these two sites to include a more representative study population. Additionally, given our CID patients included multiple disease states and varying medication regimens including combination therapy, it was difficult to isolate differences based on specific disease states or medication exposures.
In conclusion, we examined the reactogenicity and subsequent immunogenicity of patients with CID and found that although CID patients had more numerous symptoms following the first dose of SARS-COV-2 vaccine, their reactogenicity following full vaccination was similar to healthy controls and the general public. The presence of more numerous symptoms and select AEs following vaccine was associated with increased SARS-COV-2 antibody titers. Overall, this study serves to provide much needed safety data for patients with CID and as an initial step to better understand the link between reactogenicity and immunogenicity following SARS-COV-2 vaccines.
Supplementary Material
Significance and Innovation:
CID patients have a distinct reactogenicity profile compared to healthy controls following SARS-CoV-2 vaccination and demonstrate an association between reactogenicity and immunogenicity.
The unique relationship of reactogenicity and immunogenicity in CID patients following vaccination with the mRNA SARS-CoV-2 vaccines, has not been demonstrated in the general population.
Funding:
This research was primarily funded/supported by The Leona M. and Harry B. Helmsley Charitable Trust, Marcus Program in Precision Medicine Innovation, National Institutes of Health (NIH)/National Center for Advancing Translational Sciences (NCATS) grant UL1 TR002345, and NIH/National Institutes of Arthritis and Musculoskeletal and Skin Diseases (UCSF) grant P30AR070155 and T32AR079068.
Footnotes
Competing Interests: P.D. participated in consulting, advisory board, or speaker’s bureau for Janssen, Pfizer, Prometheus Biosciences, Boehringer Ingelheim, AbbVie, and Arena Pharmaceuticals and received funding under sponsored research agreement unrelated to the data in the paper from Takeda Pharmaceutical, Arena Pharmaceuticals, Bristol Myers Squibb-Celgene, and Boehringer Ingelheim. M.A.C. participated in consulting, advisory board, or speaker’s bureau for AbbVie, Pfizer, Bristol Myers Squibb, and Theravance, and received funding under a sponsored research agreement unrelated to the data in the paper from Incyte, Pfizer, Janssen, and the Crohn’s and Colitis Foundation. The A.H.E. laboratory received funding under a sponsored research agreement unrelated to the data in the paper from Emergent BioSolutions and Abbvie. A.H.J.K. participated in consulting, advisory board, or speaker’s bureau for Alexion Pharmaceuticals, Aurinia Pharmaceuticals, Annexon Biosciences, Exagen Diagnostics, Inc., and GlaxoSmithKilne and received funding under a sponsored research agreement unrelated to the data in the paper from GlaxoSmithKline. M.A.P. receives honoraria for consulting or presentations from AbbVie and JK Market Research and sponsored research agreement unrelated to the data in the paper from Eli Lilly. M.C.N. owns stock in Moderna. L.S.G. received honoraria for consulting for AbbVie, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, and UCB received funding under a sponsored research agreement unrelated to the data in the paper from Pfizer and UCB. All other authors declare no competing interests.
References:
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. December 2020:NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadoff J, Gray G, Vandebosch A, et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao Y, Chen Y, Liu M, Shi S, Tian J. Impacts of immunosuppression and immunodeficiency on COVID-19: A systematic review and meta-analysis. J Infect. 2020;81(2):e93–e95. doi: 10.1016/j.jinf.2020.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80(7):930–942. doi: 10.1136/annrheumdis-2020-219498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pogue K, Jensen JL, Stancil CK, et al. Influences on Attitudes Regarding Potential COVID-19 Vaccination in the United States. Vaccines. 2020;8(4):582. doi: 10.3390/vaccines8040582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreps S, Prasad S, Brownstein JS, et al. Factors Associated With US Adults’ Likelihood of Accepting COVID-19 Vaccination. JAMA Netw Open. 2020;3(10):e2025594. doi: 10.1001/jamanetworkopen.2020.25594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gee J, Marquez P, Su J, et al. First Month of COVID-19 Vaccine Safety Monitoring — United States, December 14, 2020–January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283–288. doi: 10.15585/mmwr.mm7008e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapin-Bardales J, Gee J, Myers T. Reactogenicity Following Receipt of mRNA-Based COVID-19 Vaccines. JAMA. April 2021. doi: 10.1001/jama.2021.5374 [DOI] [PubMed] [Google Scholar]
- 10.Connolly CM, Ruddy JA, Boyarsky BJ, et al. Disease Flare and Reactogenicity in Patients with Rheumatic and Musculoskeletal Diseases Following Two-Dose SARS-CoV-2 Messenger RNA Vaccination. Arthritis Rheumatol. August 2021:art.41924. doi: 10.1002/art.41924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Botwin GJ, Li D, Figueiredo J, et al. Adverse Events Following SARS-CoV-2 MRNA Vaccination Among Patients with Inflammatory Bowel Disease. Gastroenterology; 2021. doi: 10.1101/2021.03.30.21254607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berkowitz EM, Moyle G, Stellbrink HJ, et al. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis. 2015;211(8):1279–1287. doi: 10.1093/infdis/jiu606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vink P, Ramon Torrell JM, Sanchez Fructuoso A, et al. Immunogenicity and Safety of the Adjuvanted Recombinant Zoster Vaccine in Chronically Immunosuppressed Adults Following Renal Transplant: a Phase III, Randomized Clinical Trial. Clin Infect Dis. March 2019. doi: 10.1093/cid/ciz177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ou MT, Boyarsky BJ, Chiang TPY, et al. Immunogenicity and Reactogenicity After SARS-CoV-2 mRNA Vaccination in Kidney Transplant Recipients Taking Belatacept. Transplantation. 2021;105(9):2119–2123. doi: 10.1097/TP.0000000000003824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miraglia JL, Abdala E, Hoff PM, et al. Immunogenicity and reactogenicity of 2009 influenza A (H1N1) inactivated monovalent non-adjuvanted vaccine in elderly and immunocompromised patients. PloS One. 2011;6(11):e27214. doi: 10.1371/journal.pone.0027214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hervé C, Laupèze B, Del Giudice G, Didierlaurent AM, Tavares Da Silva F. The how’s and what’s of vaccine reactogenicity. Npj Vaccines. 2019;4(1):39. doi: 10.1038/s41541-019-0132-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deepak P, Kim W, Paley MA, et al. Effect of Immunosuppression on the Immunogenicity of mRNA Vaccines to SARS-CoV-2: A Prospective Cohort Study. Ann Intern Med. August 2021:M21–1757. doi: 10.7326/M21-1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA. 2021;325(21):2204–2206. doi: 10.1001/jama.2021.7489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tobin J Estimation of Relationships for Limited Dependent Variables. Econometrica. 1958;26(1):24. doi: 10.2307/1907382 [DOI] [Google Scholar]
- 20.Amemiya T Tobit models: A survey. J Econom. 1984;24(1–2):3–61. doi: 10.1016/0304-4076(84)90074-5 [DOI] [Google Scholar]
- 21.Priori R, Pellegrino G, Colafrancesco S, et al. SARS-CoV-2 vaccine hesitancy among patients with rheumatic and musculoskeletal diseases: a message for rheumatologists. Ann Rheum Dis. 2021;80(7):953–954. doi: 10.1136/annrheumdis-2021-220059 [DOI] [PubMed] [Google Scholar]
- 22.Gaur P, Agrawat H, Shukla A. COVID-19 vaccine hesitancy in patients with systemic autoimmune rheumatic disease: an interview-based survey. Rheumatol Int. 2021;41(9):1601–1605. doi: 10.1007/s00296-021-04938-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko T, Dendle C, Woolley I, Morand E, Antony A. SARS-COV-2 vaccine acceptance in patients with rheumatic diseases: a cross-sectional study. Hum Vaccines Immunother. August 2021:1–9. doi: 10.1080/21645515.2021.1958611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook IF. Sex differences in injection site reactions with human vaccines. Hum Vaccin. 2009;5(7):441–449. doi: 10.4161/hv.8476 [DOI] [PubMed] [Google Scholar]
- 25.Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10(5):338–349. doi: 10.1016/S1473-3099(10)70049-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moser M, Leo O. Key concepts in immunology. Vaccine. 2010;28 Suppl 3:C2–13. doi: 10.1016/j.vaccine.2010.07.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


