Abstract
Purpose:
The abnormal function of tumor blood vessels causes tissue hypoxia, promoting disease progression and treatment resistance. Although tumor microenvironment normalization strategies can alleviate hypoxia globally, how local oxygen levels change is not known because of inability to longitudinally assess vascular and interstitial oxygen in tumors with sufficient resolution. Understanding the spatial and temporal heterogeneity should help improve the outcome of various normalization strategies.
Experimental Design:
We developed a multiphoton phosphorescence quenching microscopy system using a low-molecular weight palladium porphyrin probe to measure perfused vessels, oxygen tension and their spatial correlations in vivo in mouse skin, bone marrow, and four different tumor models. Further, we measured the temporal and spatial changes in oxygen and vessel perfusion in tumors in response to anti-VEGFR2 antibody (DC101) and an angiotensin-receptor blocker (losartan).
Results:
We found that vessel function was highly dependent on tumor type. Although some tumors had vessels with greater oxygen carrying ability than those of normal skin, most tumors had inefficient vessels. Further, inter-vessel heterogeneity in tumors associated with heterogeneous response to DC101 and losartan. Using both vascular and stromal normalizing agents, we show that spatial heterogeneity in oxygen levels persist, even with reductions in mean extravascular hypoxia.
Conclusions:
High-resolution spatial and temporal responses of tumor vessels to two agents known to improve vascular perfusion globally reveal spatially heterogeneous changes in vessel structure and function. These dynamic vascular changes should be considered in optimizing the dose and schedule of vascular and stromal normalizing strategies to improve the therapeutic outcome.
Introduction
Abnormal blood vasculature and the resulting tissue hypoxia constitute hallmarks of malignant tumors and correlate with poor survival in patients with cancer (1, 2). Hypoxia drives tumor progression and promotes/mediates resistance to various treatments including radiation, most chemotherapies and immunotherapies (1, 2). The imbalance between pro- and anti-angiogenic signaling leads to tortuous, dilated and highly permeable blood vessels, resulting in reduced perfusion and insufficient oxygen delivery (1). Additionally, expansion of tumors due to uncontrolled cell proliferation and extracellular matrix production within confining surrounding tissue generates a compressive mechanical force (solid stress), which causes the collapse of tumor vessels (3, 4). Vessel compression compromises blood flow (5), leaving intratumoral regions up to 1 mm or larger without blood perfusion (6) and minimal oxygen supply. However, the extent of spatial heterogeneity in the function of tumor vessels and local oxygen concentrations and how they affect treatment response are not well characterized.
When judiciously applied, anti-angiogenic therapies (AATs) can prune immature, inefficient tumor vessels and fortify remaining vessels (7, 8). The net result is “normalization” of vessel permeability and architecture through pericyte recruitment via Ang1/Tie2 signaling as early as 6 hours after AAT administration (9–11). Vascular normalization transiently increases perfusion (12–15), oxygen delivery (9, 16) and immune cell infiltration in both murine and human tumors (12, 17). Therefore, increases in tissue perfusion and oxygenation are potential dynamic biomarkers of response to AATs (2, 13, 14, 16). Conversely, AATs administered at high doses and/or for extended duration can prune vessels excessively and exacerbate hypoxia (1, 2, 9, 18, 19). Separately, AAT cannot reach collapsed, and thus not perfused, vessels in highly desmoplastic tumors (1, 20, 21). In contrast, drugs that kill cancer cells and/or alleviate desmoplasia can decompress vessels in these hypo-perfused tumors (5, 22, 23). For instance, angiotensin receptor blockers (ARBs), such as losartan, have anti-fibrotic properties in tumors by reducing transforming growth factor (TGF)-β1 activation (24) by antagonizing the angiotensin-II-receptor-1 on cancer-associated fibroblasts (5). As a result, ARBs decompress and normalize blood vessels in a variety of tumor models (5, 25, 26). However, the spatiotemporal dynamics and heterogeneity of these processes at cellular resolution are unknown.
To address this unmet need, we developed a multiphoton (MP) phosphorescence quenching microscopy (PQM) method (MP-PQM). MP microscopy enables visualization of perfused vessels in tumor models propagated in mice with micron resolution (27). Meanwhile, PQM allows direct measurements of tissue oxygenation based on the interaction of surrounding molecular oxygen with the light-excited triplet state of a phosphorescent reporter molecule. We have previously used intravital PQM with conventional and confocal fluorescence microscopy to measure tissue oxygen levels (28, 29). However, these approaches can cause photodamage and have limited three-dimensional resolution (28). Existing PQM for MP microscopy techniques use high molecular weight probes that are confined to the vasculature following intravenous administration (30). These nano-sized probes might have difficulty distributing uniformly throughout tumor extracellular space even with an intratumor injection. Here, we use a low molecular weight palladium porphyrin probe that is able to (a) distribute throughout the tumor and (b) extravasate into tissues from sinusoidal, reticuloendothelial vessels, such as those in bone marrow, when injected intravenously (31). This probe along with MP-PQM allowed us to measure oxygen tension (pO2) in normal tissues and breast cancers in mice as well as the spatial heterogeneity in the vessel and pO2 response to AAT and ARBs. Our measurements reveal spatially heterogeneous changes in vessel structure and function in response to AAT, presumably due to the heterogeneities in the local imbalance of pro- and anti-angiogenic signaling. In contrast, losartan, an ARB, increased perfused vessel density—as expected—as well as increased oxygen delivery.
Materials and Methods
Multiphoton Phosphorescence Quenching Microscopy System:
We custom-modified a multiphoton microscope based on the Fluoview 300 laser scanner (Olympus FV300, Optical Analysis, Center Valley, PA) and upright microscope (Olympus BX61WI Optical Analysis, Center Valley, PA) incorporating a broadband (710 – 1020 nm) Ti:Sapphire femtosecond laser (MaiTai HP, Spectraphysics, Mountain View, CA) as the excitation source. The output power of the laser was adjusted by a zero‐order half‐wave plate (10RP52‐2, Newport Corp., Irvine, CA) and Glan‐Laser polarizer (10GL08AR.16, Newport Corp., Irvine, CA). For phosphorescence quenching microscopy, we employed a KD*P Pockels cell (Model: 350‐50; Conoptics, Inc., Danbury, CT) electro-optic modulator driven by custom-built electronics to amplify triggering pulses from a digital delay generator (DG535, SRS, Sunnyvale, CA) as a temporal gate. Excitation pulse selection was placed immediately prior to beam expansion which fed the laser into the galvanometer-based scanner. Based on the in vitro or biologic system (tissue) in which pO2 measurements were performed, the excitation pulse for MP-PQM ranged from 1.28 to 15.36 μs. Focusing and collecting light was performed with a 20×, 0.95 NA water immersion objective lens (Olympus XLUMPlanFl, Optical Analysis, Center Valley, PA). Given the short spectral separation between the long emission of the phosphorescent sensors (~690 nm) and the excitation laser wavelength, a short‐pass (< 750 nm) AR‐coated filter was used in the detector path (750SP‐2P, Chroma Technology Corp., Rockingham, VT) to limit background counts from the laser. A 690/90 bandpass filter (690/90M, Chroma Technology Corp., Rockingham, VT) and focusing lens were used before the detectors (GaAs photomultiplier tube for in vivo measurements: H7421‐50, Hamamatsu, Inc., Bridgewater, NJ; or a silicon avalanche photodiode detector for in vitro measurements: SPCM-AQR-12-FC, PerkinElmer, Fremont, CA). For time domain photon counting, we used a multichannel scaler (SR430, SRS, Sunnyvale, CA). PQM measurements were performed using a stationary beam with sample scanning performed by an automated mechanized XY stage (H101; Prior Scientific, Rockland, MA). A custom designed graphical user interface programmed in LabView (National Instruments, Austin, TX, RRID:SCR_014325) was used for data acquisition and processing with embedded MATLAB fitting algorithms (MathWorks, Natick, MA, RRID:SCR_001622).
Characterization and Calibration of Oxyphor R2 for MP-PQM
We obtained the soluble phosphorescent oxygen sensor Pd-meso-tetra-(4-carboxyphenyl) porphyrin dendrimer from commercial sources (OxyphorR2, Oxygen Enterprises, Ltd., Philadelphia, PA). We determined the ideal multiphoton excitation wavelength by measuring the single-photon excitation spectrum using a spectrophotometer (Beckham Instruments Inc., Fullerton, CA) with continuous illumination from 400–1000 nm and the two-photon excitation spectrum by measuring the photon counts after time-domain pulsed excitation from 800 – 1020 nm at constant laser power. We confirmed that phosphorescence saturation did not occur within our selected excitation pulse duration. For calibration of Oxyphor R2, we custom designed a sealed recirculating system employing a fiber oxygenator to equilibrate the circulating fluid with gas mixtures of known oxygen concentration balanced with nitrogen. The oxygen tension in the calibration solution was independently measured using a fiber optic ruthenium-based sensor (FOXY, Ocean Optics, Inc., Dunedin, FL). All tubing in the system was impermeable to oxygen (Tygon PVC, McMaster‐Carr Supply Co., Robbinsville, NJ). A solution of 1 mg/ml (3.6 × 10‐4 M) OxyphorR2 in PBS at pH 7.4 was equilibrated with known nitrogen gas mixtures of 0, 1, 2, 5, 10% oxygen (0 – 80 mmHg) and various concentrations of bovine serum albumin (BSA) from 0 – 10% using the fiber oxygenator (Hemophan® Membrane Fiber Oxygenator, Harvard Apparatus, Holliston, MA). After equilibration with the applied gas mixture, the solution was passed through an oxygen impermeable spectrophotometer flow cell with 500 µm thick walls (45-Q-2, Starna Cells, Inc., Atascadero, CA). MP‐PQM calibration measurements were performed within the flow cell at 37°C. To measure the anoxic phosphorescence lifetime (τ0) measurements, we used a Schlenk-line freeze-pump thaw method to create deoxygenated solutions in 10mm pathlength quartz cuvettes with a vapor pressure of 10−6 Torr. Using an unconstrained nonlinear optimization in MATLAB, we fit the phosphorescence decays to a two-component Stern-Volmer model due to binding of the oxygen sensor to proteins creating two species with different luminescent lifetimes.
Animal Studies:
All animal experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee. The dorsal skinfold chamber, mammary fat pad window and chronic bone marrow chamber models were prepared in SCID mice as previously described (32, 33). For the breast tumor experiments, the appropriate tumor type was implanted as a single cell suspension or homogenized tumor tissue from an existing xenograft in the center of the mammary fat pad chamber 2–3 days after the initial surgery. The murine mammary adenocarcinomas MCaIV and E0771 (CH3 BioSystems) were transplanted from subcutaneous tumors grown in syngeneic mice. Cranial window chamber models were prepared in nude mice as previously described (34). The mice were allowed to recover for one week following the procedure. U87 (ATCC) fragments up to 0.3 mm in diameter dissected from tumors excised growing in a source mouse were implanted in the left cerebral hemisphere 0.5 to 1.0 mm deep. Animals were anesthetized with Ketamine/Xylazine (90/9 mg/kg) for surgeries and isoflurane (1–3% in medical grade air) for all imaging experiments. The cells were tested and authenticated by mycoplasma testing, morphology using microscopy and binding of antibodies for common viral contaminations (VRL Diagnostics) after thawing cells to inoculate into source mice. Tumors were implanted usually after three but no more than 5 passages.
Fifteen minutes prior to MP-PQM measurements, the animal was anesthetized by isoflurane (3% for the first 4 minutes) in medical air carrier gas. This method of anesthesia was selected given its neutrality towards heart rate, blood pressure, and oxygen tension (35–37), particularly compared to other methods that alter oxygen tension (28, 35). Immediately following isoflurane induction, a mixture (200 μl) of 10 mg/ml Oxyphor R2 with 8 mg/ml 2M MW FITC-dextran was injected intravenously through retro-orbital route. With animals under isoflurane anesthesia, we secured the frame of the transparent window or secured the skull to prevent motion artifact during imaging. This allows both real-time measurements of pO2 and intravital high-resolution fluorescent angiography. For oxygen tension maps, we manually selected a rectangular region of interest and collected an automated series of mosaic multiphoton microscopy images covering the ROI. Each image contained an axial series up to 400 μm deep in the tissue at a 5 μm z-step size. Following angiography imaging, MP-PQM measurements were performed as a series of 144 stationary points based on a 12×12 grid covering the ROI at four equally spaced depth planes. Total data collection times ranged from two to three hours depending upon the size of the ROI. Anesthesia was maintained at 1% isoflurane in a normoxic (21% FiO2) atmospheric mixture with a circulating water heating pad maintaining the animal’s body temperature at 37 °C. Animals were monitored for any movement using a remote digital camera. To account for evaporation of water at the interface of the water immersion objective lens with the window chamber, a syringe pump was utilized to slowly apply distilled water to the top of the chamber.
Antiangiogenic VEGF-R2 blockade treatments with DC101, a monoclonal antibody against murine VEGFR-2, (Eli Lilly and Company, Indianapolis, IN) or nonspecific rat IgG were administered intraperitoneally at 40 mg/kg as prescribed in previous studies (24) or at 5 mg/kg for low AAT dose studies (33). We gave 3 doses of DC101 at three-day intervals (day 0, 3, 6) with MP-PQM performed the day immediately prior to each treatment. Imaging was performed through day 8. Therapeutic initiation was determined through monitoring of tumor growth by visual inspection of the enface tumor diameter (≥ 4 mm) and angiogenesis of the tumor (vascularized throughout).
To replicate the conditions of our previous work (5), losartan was obtained as pills intended for clinical use from the Massachusetts General Hospital Pharmacy, crushed and dissolved in PBS for 24 h. The solutions were then sterile filtered for injection. The concentration of active ingredient was not confirmed before administration, and it was assumed no active pharmaceutical ingredient was lost in this process when calculating doses administered. The mice were treated with 40 mg/kg losartan or an equal volume of PBS intraperitoneally every day for 6 days.
Data Analysis:
We developed a custom code in MATLAB to process and analyze the in vivo MPM angiography images. Colorized depth projections were created to display the vascular networks in conjunction with the oxygen tension measurements. A semi-automated vascular tracing method previously reported by our group (25, 26) was applied to the mosaic image of the entire ROI. Briefly, this method uses an algorithm that models vascular segments as superellipsoids to obtain three-dimensional trajectories and morphology of the individual segments of the vascular network. The output of the tracing algorithm was correlated with each individual oxygen measurement to quantify distance to nearest vessel and diameter of that vessel. Phosphorescence lifetimes were determined by fitting a two-component exponential decay model with oxygen tension values calculated using the Stern-Volmer relationship and the quenching constant and anoxic lifetime determined from in vitro calibrations. Oxygen tension maps were created in MATLAB using custom code to overlay the mesh grid of pO2 values with outlines of the vascular tracing. Mean oxygen tension was calculated from the pool of all the measurements for each individual tumor. We determined tumor oxygen profiles using a sliding averaging procedure. For each tumor type, the distributions are a collection of all tumors imaged at that time-point or within a specific treatment group. Measurements classified as interstitial or extravascular oxygenation occurred between 40 and 60 µm from the nearest vessel.
Statistical Analysis:
Data are reported as mean ± standard error of the mean (SEM). For experiments involving ensemble statistics, significant differences between groups were determined using a Kruskal-Wallis test correcting for multiple comparisons using Dunn’s correction (Prism, GraphPad Software, LLC., RRID:SCR_002798), because the distributions were not normally distributed. To rule out type I error inflation in statistically significant comparisons between control and treatment groups from the same study day (e.g., DC101 treatment on day 2), Bonferroni’s correction was applied, which involved multiplying the Mann-Whitney U test (obtained in Prism) P-value by the number of comparisons in the Kruskal-Wallis test (i.e. 15) and setting P < 0.05 as the threshold for statistical significance. For experiments involving ensemble statistics that investigated treatment response over time, general linear models (obtained in Prism) were applied to assess the time × treatment interaction on intravascular and extravascular oxygenation. In experiments involving comparisons between two groups, statistical significance was tested using an unpaired, two-sided Student’s t-test. All correlations were determined using the Pearson product-moment correlation. P < 0.05 was considered statistically significant for all comparisons except for the test with Bonferroni’s correction, in which P < 0.05 was used.
Data Availability:
The data generated in this study are available within the article and its supplementary data files.
Results
Oxygen probe and system characterization
We constructed a custom MP-PQM system for quantification of pO2 in vitro and in vivo (Figure 1A). To maximize signal to noise ratio in vivo, we measured phosphorescence lifetime in the time-domain (38) utilizing an electro-optic modulator to gate pulses of the femtosecond excitation laser at a stationary location with sample scanning performed by a programmable XY stage. Palladium porphyrin has longer lifetimes than platinum porphyrin thereby making the signal easier to detect (39). The two-photon action cross-section of Pd-porphyrin molecules, similar to the one we employed here, is known to be below 6 Goppart-Meyer (40). Thus, we measured the single- and two-photon action cross-sections in order to determine the ideal excitation wavelength balancing i) minimal overlap with the single photon excitation or phosphorescence emission and ii) maximal excitation/emission efficiency (Figure 1B). We selected the non-linear excitation at 1020 nm rather than 800 nm to limit cellular damage, reduce tissue scattering allowing for deeper measurements, and avoid overlap with the phosphorescence emission, which extends to 780 nm. Non-linear, two-photon excitation of the Pd-porphyrin probe was confirmed at the selected excitation wavelength of 1020 nm and was independent of oxygen tension (Figure 1C). This relationship held in vivo, which confirmed that excitation of the Pd-porphyrin probe at 1020 nm is a two-photon excitation process in our system (Figure 1D). Finally, we calibrated the phosphorescence lifetime of the Pd-porphyrin probe at various levels of pO2 in solutions with differing concentrations of bovine serum albumin using a customized calibration system (Figure 1E). Given two states of the probe (free in solution or bound to protein), the best fit was to a two-component Stern-Volmer model (41) (Figure 1F). Adding fluorescent quantum dots to the solution did not affect the calibration indicating that adding other fluorescent species into the same sample does not affect the measurements (Supplementary Figure 1). Variability increased with oxygenation because the phosphorescence lifetime is shorter with higher oxygen tension (Supplementary Figure 2). Measuring in the time domain, the technical maximum oxygen measurement limit of the system is a pO2 of 122 mmHg because the minimum phosphorescence signal collection time of our system is 2.56 µs.
Figure 1 – Multiphoton Phosphorescence Quenching Microscopy (MP-PQM).
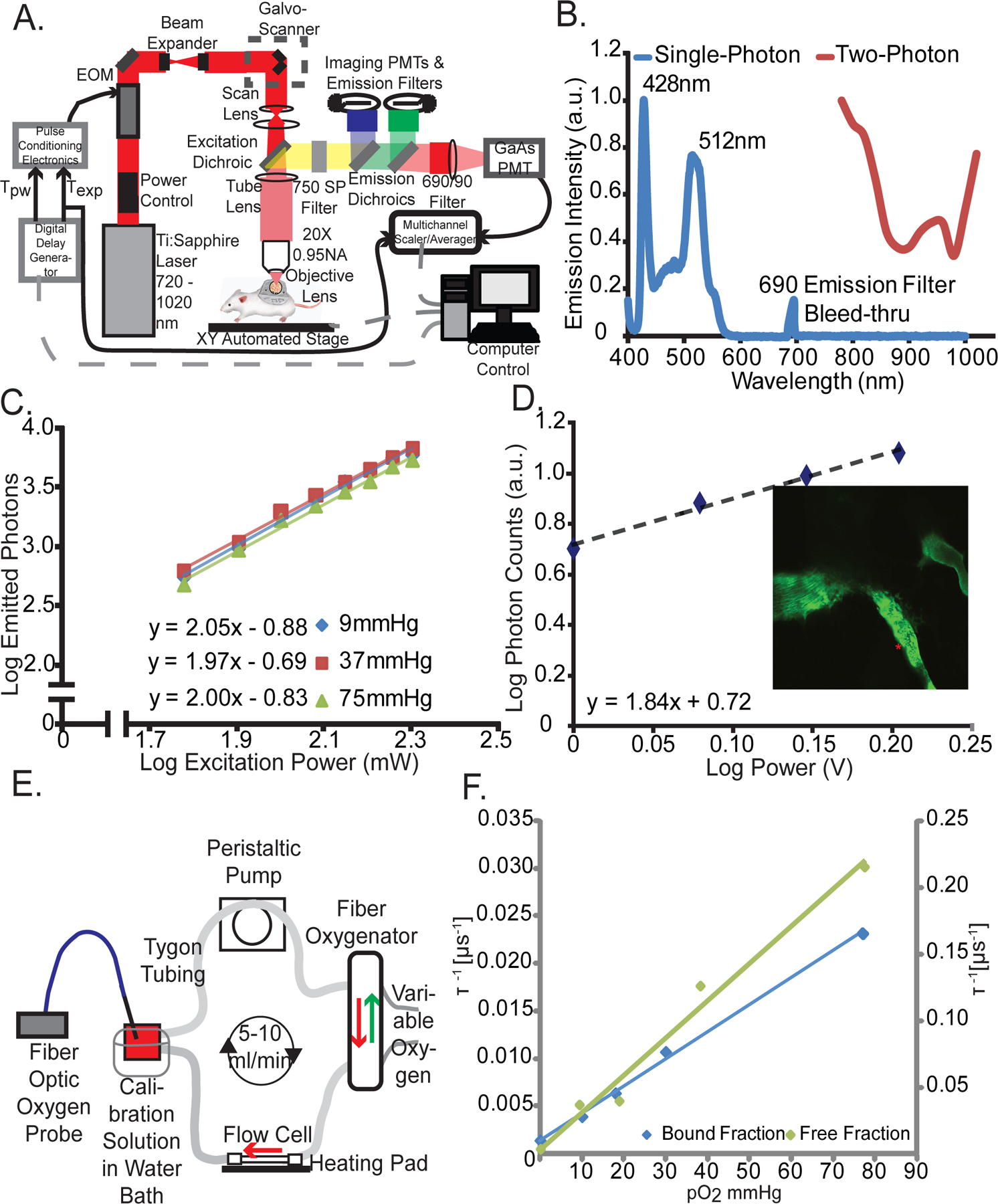
(A) Schematic of the experimental setup. EOM, electro-optic multiplier; PMTs, photomultiplier tubes; GaAs, Gallium arsenide; Tpw, temporal duration of the excitation pulse width; Texp, duration of the experimental triggering time. Arrows indicate direction of communication. Dashed grey lines indicate two-way communication. (B) Single- and two-photon action cross-sections for Pd-porphyrin soluble oxygen sensor. The single-photon excitation spectrum (blue line) does not show absorption or emission in the wavelength range of two-photon (red line). The lone peak at 690 nm is due to excitation light at the same wavelength as the emission band-pass filter in the spectrophotometer. (C) Two-photon excitation at 1,020 nm of Pd-porphyrin phosphorescent oxygen sensor relating the emitted photons to the excitation power on a log-log scale. The experiment was repeated at oxygen tensions of 9 mmHg, 37 mmHg, and 75 mmHg (blue, red, and green lines, respectively). (D) In vivo two-photon excitation of Pd-porphyrin oxygen sensor. A series of time domain lifetime measurements performed in tumor vasculature (red asterisk, inset) shows the non-linear excitation of Pd porphyrin sensor. (E) Diagram of the recirculating, sealed calibration system employing a fiber oxygenator indicating the direction of fluid and gas flow. An independent pO2 measurement is made with a calibrated optical fiber ruthenium-based probe. (F) Two-photon calibration of the albumin-bound (blue line) and single-component (green line) fits of the Pd-porphyrin oxygen sensor. The left vertical axis applies to the bound fraction. The right vertical axis applies to the free fraction. The vertical axes are the phosphorescence lifetime in units of μ s−1 and the horizontal axis is oxygen tension in units of mmHg. The Stern-Volmer constant is 3,115 mmHg−1s−1 for the free fraction and 285 mmHg−1s−1 for the bound fraction.
Oxygen delivery by blood vessels in normal skin and bone marrow
We next measured perfused vessel morphology and interstitial oxygen in the skin of mice breathing air (Figure 2A, B), the skin of mice breathing 100% oxygen (Figure 2C, D), and the bone marrow of mice breathing air (Supplementary Figure 3). Throughout the skin, extravascular regions were an average of 25 μm from the nearest perfused vessel and at most 84 μm away (Supplementary Table 1). Throughout the bone marrow, which has sinusoidal vessels hyperpermeable to bovine serum albumin (Supplementary Figure 4), extravascular regions were an average of 13 μm from the nearest perfused vessel and at most 58 μm away (Supplementary Table 1). These maximum distances are less than 150 μm from perfused vessels, which is considered the maximum diffusible distance of oxygen due to oxygen consumption (6, 29).
Figure 2 – Measurement of pO2 in mouse tissues.
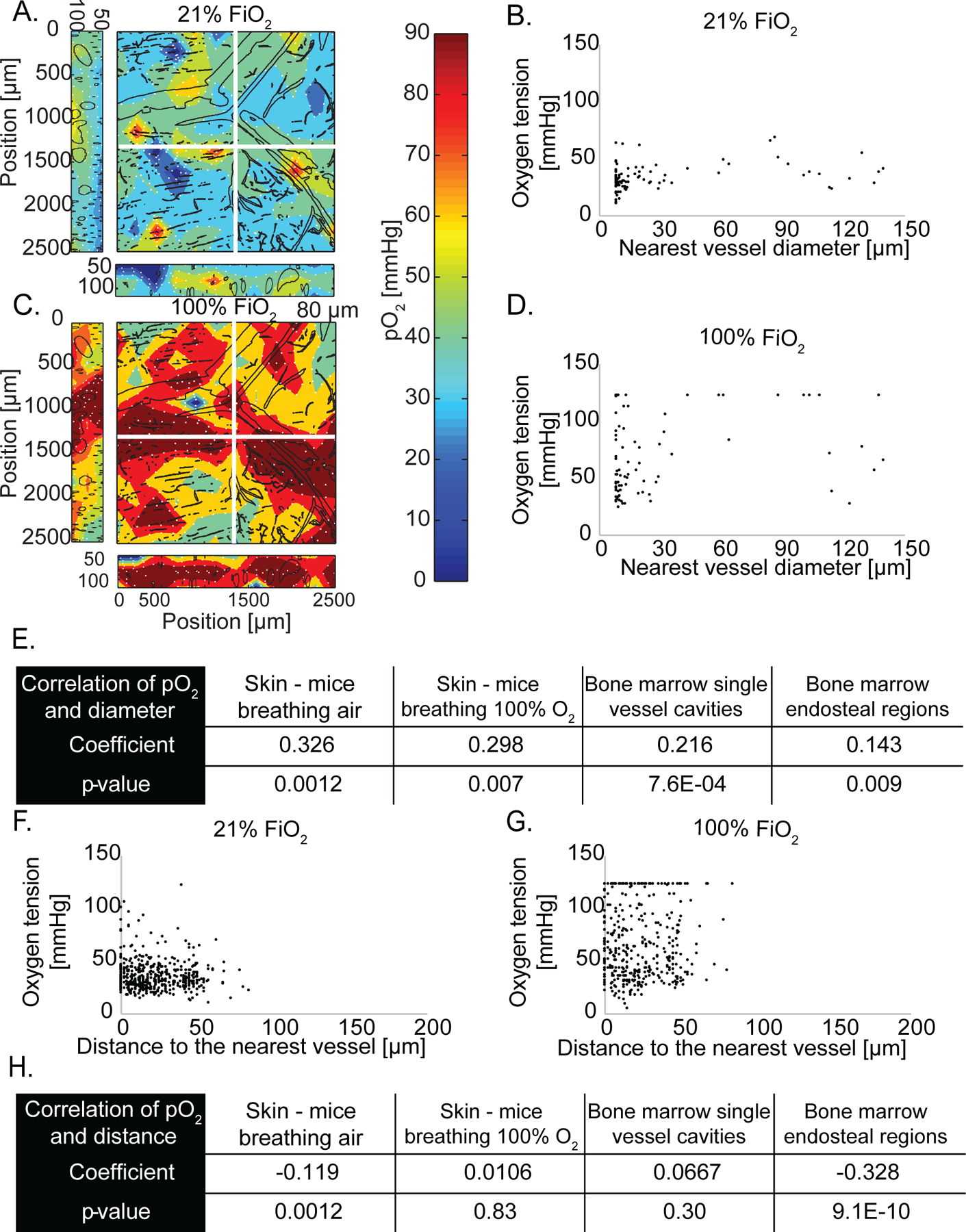
(A) Oxygen tension map in normal murine skin of a mouse breathing air (21% oxygen). The depth projection of vasculature (black lines) is overlaid. (B) Scatter plot of interstitial oxygen tension [mmHg] versus the diameter of the nearest vessel [μm] in mice breathing air. (C) Oxygen tension depth projection map in normal murine skin of a mouse breathing 100% oxygen. (D) Scatter plot of interstitial oxygen tension versus the diameter of the nearest vessel in mice breathing 100% oxygen. The detection limit of 122 mmHg is apparent in the data. (E) Table of Pearson correlation coefficients and the p-value of the correlation of oxygen tension and nearest vessel diameter. (F) Scatter plot of measured oxygen tension versus the distance to the nearest vessel in the skin of mice breathing 21% oxygen. (G) Scatter plot of measured oxygen tension versus the distance to the nearest vessel in the skin of mice breathing 100% oxygen. The detection limit of 122 mmHg is apparent in the data. (H) Table of Pearson correlation coefficients and the p-value of the correlation of oxygen tension and distance to the nearest vessel.
Typically, larger diameter vessels have higher blood flow rates than smaller vessels (e.g. capillaries < 10 μm, arteries and venules > 10 μm) and thus, we hypothesized that larger vessels can deliver oxygen at a higher rate to surrounding tissues (42). To test this hypothesis, we imaged perfused vessels using a fluorescent vascular tracer and measured oxygen content over a large region of tissue (2.5 × 2.5 mm). Within the dorsal skinfold chamber, there was a direct and statistically significant Pearson product-moment correlation (i.e., coefficient greater than 0.00 and p-value < 0.05) of 0.326 between vessel diameter and interstitial oxygenation (i.e., measured at 40 to 60 μm from the nearest vessel), indicating that larger vessels supply more oxygen to the extravascular space (Figure 2A, B, and E). Some large vessels above 90 μm diameter – likely venules - had low surrounding interstitial oxygen, which resulted in no correlation between vessel diameter and oxygen when considering the largest vessels only (Figure 2B). When the same mice were subsequently placed under hyperoxic conditions (100% inspired oxygen), the average intravascular oxygen tension increased from 42 mmHg to 80 mmHg, and the correlation between vessel diameter and interstitial oxygenation remained statistically significant (Figure 2C-E). Again, we found no correlation for large vessels with diameters larger than 90 μm (Figure 2D). In the bone marrow cavities of the mouse calvarium that contained multiple vessels (Supplementary Figures 3A, B) and in the cellular areas surrounding single endosteal vessels (Supplementary Figures 3C, D), there was also a correlation between vessel diameter and interstitial oxygen (Figure 2E). Thus, in murine skin and bone marrow, there is a direct correlation of interstitial oxygenation and the diameter of the nearest perfused capillary.
As oxygen diffuses radially through the vessel wall and away from vessels, it is consumed by cells and the concentration falls with increasing distance from the vessel (29). Nonetheless, oxygen tension levels are highly regulated to ensure that even cells farthest from blood vessels have sufficient oxygenation. As such, we observed in the skin a weak although statistically significant inverse correlation (i.e., coefficient less than 0.00 and p-value < 0.05) of –0.119 between extravascular oxygenation and distance from the nearest vessel (Figure 2F). However, we were unable to analyze our data for a correlation between extravascular oxygenation and distance from the nearest vessel when the same mice were subsequently placed under hyperoxic conditions because the measurement technique is constrained by an upper limit of 122 mmHg oxygen tension (Figure 2G, H). In the bone marrow cavities of the mouse calvarium containing multiple vessels, no correlation between oxygen and distance from the nearest vessel was found (Figure 2H and Supplementary Figure 3). This is because there are many vessels near each other, even tissue areas relatively far from a given vessel still have other vessels in close proximity (Supplementary Table 1) that could contribute to the oxygen supply. In contrast, the cellular areas surrounding single endosteal vessels surrounded by bone maintained a statistically significant inverse relationship between oxygenation and distance from the vessel (Figure 2H), consistent with a previous report (31). Thus, in murine skin and bone, there is a weak inverse correlation between oxygenation and distance to the nearest vessel, except in certain highly vascularized tissues.
Oxygen delivery by tumor vessels
While the vessels in normal tissues are well-organized and function effectively (Supplementary Figure 5A), tumor vessels are disorganized and dysfunctional (Supplementary Figure 5B). We next sought to compare tumor blood vessels from four different tumor models with those in normal tissues using MP angiography (Supplementary Figure 6) and PQM at four tissue depths (Figure 3A, B). Unlike vessels in normal tissue, the diameters of tumor vessels do not usually correlate with their function (42). Indeed, of all tumors assessed (Supplementary Figure 7A), only MCaIV, which is a highly vascularized breast tumor with some large avascular spaces (Figure 3C), exhibited a weak yet statistically significant correlation between vessel diameter and interstitial oxygen tension (Figure 3D, Supplementary Figure 7A, and Supplementary Discussion). This correlation was not seen in hypoperfused tumors, including E0771 murine mammary carcinomas that contain large un-perfused regions (Figure 3E), U87 human gliomas and LS174T human colon carcinomas (Figure 3F and Supplementary Figure 7A). Thus, in most tumors the size of the nearest blood vessel did not correlate with the interstitial oxygen tension.
Figure 3 – Simultaneous angiography and oxygen tension measurements reveal abnormal function of tumor vessels and the dependence of oxygen tension on distance to the nearest vessel.
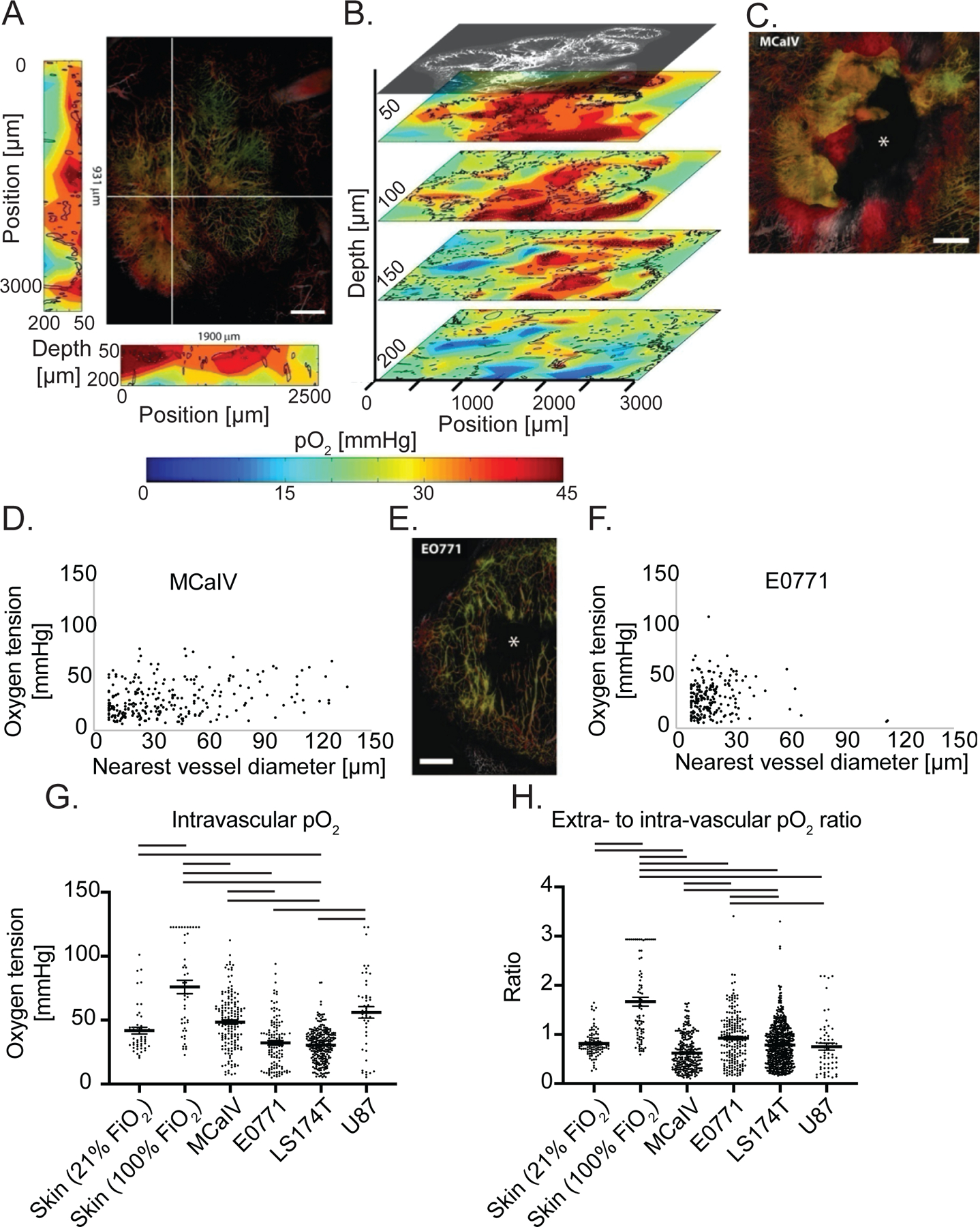
(A) The large panel shows a maximum intensity depth projection of MCaIV tumor angiography with an oxygen tension heat map to the left and bottom of the angiography image. The depths of the heat maps go from 50 μm to 200 μm and are taken from the planes noted by the white lines in the angiography image. In the angiography, color denotes depth (from deep to shallow: green, red, white). The color scale of the oxygen heat maps is shown in the bottom panel (blue indicates 0 mmHg oxygen tension while red indicates 45 mmHg). Black outlines on the oxygen heat maps indicate the morphometry of the tumor vessels. Scale bar 500 μm in all panels. (B) Oxygen tension maps from 50 μm, 100 μm, 150 μm, and 200 μm deep within the tumor shown in (A). The angiographic depth slice at 50 μm is shown above the 50 μm deep oxygen heat map for comparison. Black lines on the oxygen heat maps indicate the morphometry of the tumor vessels. (C) Angiography of a MCaIV tumor. Asterisk (*) indicates area lacking perfused vessels. Color denotes depth (from deep to shallow: green, red, white). (D) Scatter plot of extravascular (interstitial, i.e., measurements 40 to 60 μm from the nearest vessel) oxygen tension [mmHg] versus the diameter of the nearest vessel [μm] in MCaIV tumors. Data pooled from four mice. (E) Angiography of an E0771 tumor. (F) Scatter plot of extravascular (interstitial, i.e., measurements 40 to 60 μm from the nearest vessel) oxygen tension versus the diameter of the nearest vessel in E0771 tumors. Data pooled from five mice. (G) Measurements of oxygen tension in mmHg in blood vessels in skin and tumor models. Lines indicate statistically significant differences between multiple comparisons after a Kruskal-Wallis test with Dunn’s correction. (H) Measurements of extravascular oxygen tension in mmHg normalized to the intravascular oxygen tension of blood vessels in skin and tumor tissues.
The tumor microenvironment is highly heterogeneous and varies from one tumor to next. Thus, we used four tumor models to compare intravascular oxygen tension measurements. In terms of supply, only LS174T blood vessels had a lower mean intravascular pO2 than normal skin at room air (Figure 3G). In contrast, MCaIV and U87 tumor blood vessels had 31% and 8% higher intravascular pO2 than vessels in normal skin, which differed in range but not mean from E0771 (Figure 3G). While tumors did not consistently have limited supply of oxygen, highly oxygenated MCaIV and U87 tumor blood vessels did not result in elevated oxygen in the interstitial space (i.e., 40 μm through 60 μm from the nearest perfused vessel) compared to normal skin, which we observed as a lower ratio of extravascular (i.e., measurements 40 to 60 μm from the nearest vessel) to intravascular oxygen tension (Figure 3H). The lower ratio could be a result of high oxygen consumption in tumors by stromal and cancer cells. Our data further show that the oxygen supply in tumor blood vessels is heterogeneous across tumor types and within tumors. Similarly, the efficiency of vessels—defined here as the ratio of extravascular to intravascular oxygen tension—is heterogeneous across tumor types and within a single tumor type compared to vessels of the normal skin.
Next, we investigated how oxygen diffuses from vessels and is consumed by creating scatter plots of oxygen tension as a function of distance to the nearest vessel (Supplementary Figure 7B, C). Like in normal skin, all tumors except the orthotopic glioblastoma U87, which was imaged through an implanted cranial window, had a weak yet statistically significant inverse correlation between distance from the nearest vessel and tissue oxygenation (Supplementary Figure 7D). This inverse correlation is consistent with our previous report of other tumor models (29). Unlike the hierarchical vascular networks in normal tissues (arteriole to capillary to venule), tumors often have large avascular regions that are too far from vessels for oxygen to diffuse (6). Indeed, in these tumor models, the mean and maximum distances to nearest vessels was around 2–4 times larger than that in normal skin and bone marrow, respectively (Supplementary Figure 7B, C and Supplementary Tables 1 and 2). Orthotopic U87 glioblastoma model, although hypoxic (9), was an exception in regard to distance to nearest vessel, with high vascularization (43) leaving little extravascular space, as evidenced by the lowest average distance to a vessel among the tumors studied (Supplementary Table 2). Oxygen thus has short distances to diffuse and tissue oxygen could be supplied by multiple vessels, allowing oxygen to distribute relatively evenly, which is indicated by the lack of a correlation between oxygen tension and distance to the nearest vessel (Supplementary Figure 7D). This constant level of oxygenation in U87 gliomas mirrors that of the normal brain. Thus, like in skin, oxygen tension fell as a function of distance from the nearest perfused vessels in the tumor models assayed except for orthotopic glioblastoma.
AAT enhances individual vessel function in MCaIV and restores perfusion
Next, we longitudinally measured the density of perfused vessels (Supplementary Figure 8) and oxygenation (Supplementary Figure 9) in growing tumors. We then investigated the effect of AAT on tumor vessel pruning and function in two different orthotopic models of murine breast cancer, MCaIV and E0771. Although both tumor models have similar vascular permeabilities (44), MCaIV vessels have larger diameter, inter-endothelial gaps and intra-endothelial holes (~1.5 µm) (45). MCaIV blood vessels have greater intravascular oxygen tension yet are less efficient in delivering oxygen to the interstitial space compared to vessels in normal skin (Figure 3G, H). In contrast, E0771 vessels are compressed and hypoperfused owing to increased desmoplasia (5, 46). The oxygen delivery efficiency of E0771 vessels is similar to normal skin albeit with more variability (Figure 3H).
We first monitored oxygenation in MCaIV tumors during two cycles of DC101—a blocking antibody against murine VEGFR2—given i.p. at 40 mg/kg every three days (Figure 4A). We found the intravascular oxygenation in treated tumors decreased with the growth of tumors and there was no difference between DC101 treated tumors and control IgG treated ones (Figure 4B). However, extravascular oxygenation—which also decreased with tumor growth (Figure 4C)—was 35% higher on day 2 with DC101 treatment compared to control IgG treatment. This is not surprising as anti-VEGF antibody treatment decreases vessel permeability within six hours thereby causing a transient increase in blood flow and oxygenation (11). The extravascular oxygenation of DC101 treated tumors fell to levels in IgG treated tumors by day 5 (Figure 4C). Multiple linear regression analysis revealed that extravascular oxygenation in both groups decreased over time (p < 0.0001), and the treatment did not affect the overall oxygenation average. However, the rate of decrease per day varied, with the control group’s extravascular oxygenation reducing at a rate of 2.8 mmHg/day (p < 0.0001 compared to no change over time) while that of the treatment group reduced at a smaller rate of 1.7 mmHg/day (p < 0.0241 compared to the control group’s rate, Supplementary Table 3). Consistent with the greater extravascular oxygenation on day 2 in DC101 tumors compared to controls, the efficiency of vessels as measured by the ratio of extravascular oxygen tension to intravascular tension was 23% greater (Figure 4D). At this dose, DC101 reduced the mean extravascular distance to the nearest vessel compared to control on day 2 by 38% and on day 5 by 65% (Figure 4E), suggesting restored perfusion resulting from increased efficiency of blood flow in MCaIV tumors. Thus, in MCaIV, DC101 at 40mg/kg normalized tumor vessel function on day 2 and reduced the distance between perfused vessels on days 2 and 5.
Figure 4 – Anti-VEGFR2 antibody treatment at 40 mg/kg transiently increases interstitial pO2 on day 2 and restores perfusion in MCaIV.
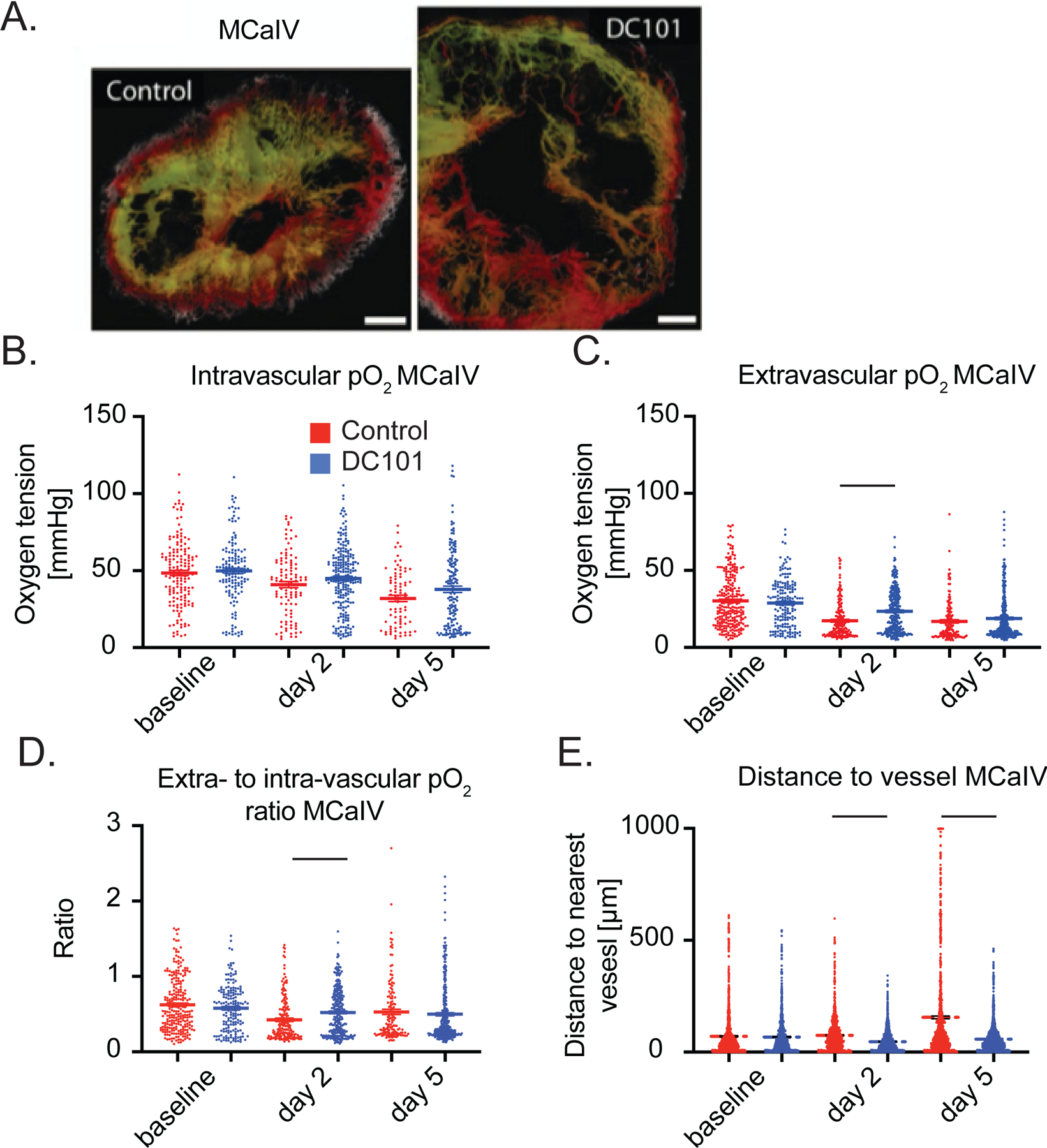
(A) Representative images of angiography of murine mammary carcinoma (MCaIV) tumors treated with control IgG (left panel) and murine anti-VEGFR2 antibody DC101 (right panel) (both 40 mg/kg i.p. every 3 days) on day 5 after two cycles of treatment. Tumor vasculature is presented as a colorized maximum intensity depth projection (superficial to deep: green, red, white). Scale bars, 500 μm. (B) Measurements of oxygen tension in mmHg in vessels in control IgG and DC101 antibody treated mice at baseline, day 2 and day 5. n = 81 – 226 measurements per timepoint per group. N = 4 mice per group. Horizontal black lines indicate statistically significant differences between multiple comparisons after a Kruskal-Wallis test with Dunn’s correction, with differences only depicted for comparisons between control and treatment groups on the same day. (C) Extravascular (interstitial, i.e., measurements 40 to 60 μm from the nearest vessel) oxygen measurements in the same tumors. n = 138 – 328 measurements per timepoint per group. (D) Measurements of extravascular oxygen tension in mmHg normalized to the intravascular oxygen tension of blood vessels. (E) Distance to the nearest vessel measurements in the same tumors. n = 1,117 – 1,851 measurements per group.
AAT induces widespread pruning in E0771
We next investigated DC101 at the same dose (40 mg/kg every 3 days) using the E0771 breast tumor model (Figure 5A), which only requires a lower dose of DC101 to normalize vessel function as measured by an increased rate of extravasation of small nanoparticles (33) or a homogenous distribution of perfused vessels (12). As with MCaIV, oxygenation in control IgG treated E0771 tumors decreased with tumor growth (Figure 5B, C). Unlike MCaIV, neither intravascular nor extravascular oxygenation in 40 mg/kg DC101 treated tumors was different compared to controls on day 2 (Figures 5B, C). The intravascular oxygen tension on day 2 was only 50% of that of vessels in skin in IgG treated E0771 tumor vessels, and 67% of that in skin vessels in DC101 treated tumor vessels.
Figure 5 – Anti-VEGFR2 antibody treatment at 40 mg/kg does not affect pO2 but extensively prunes the smallest microvessels by day 5 in E0771.
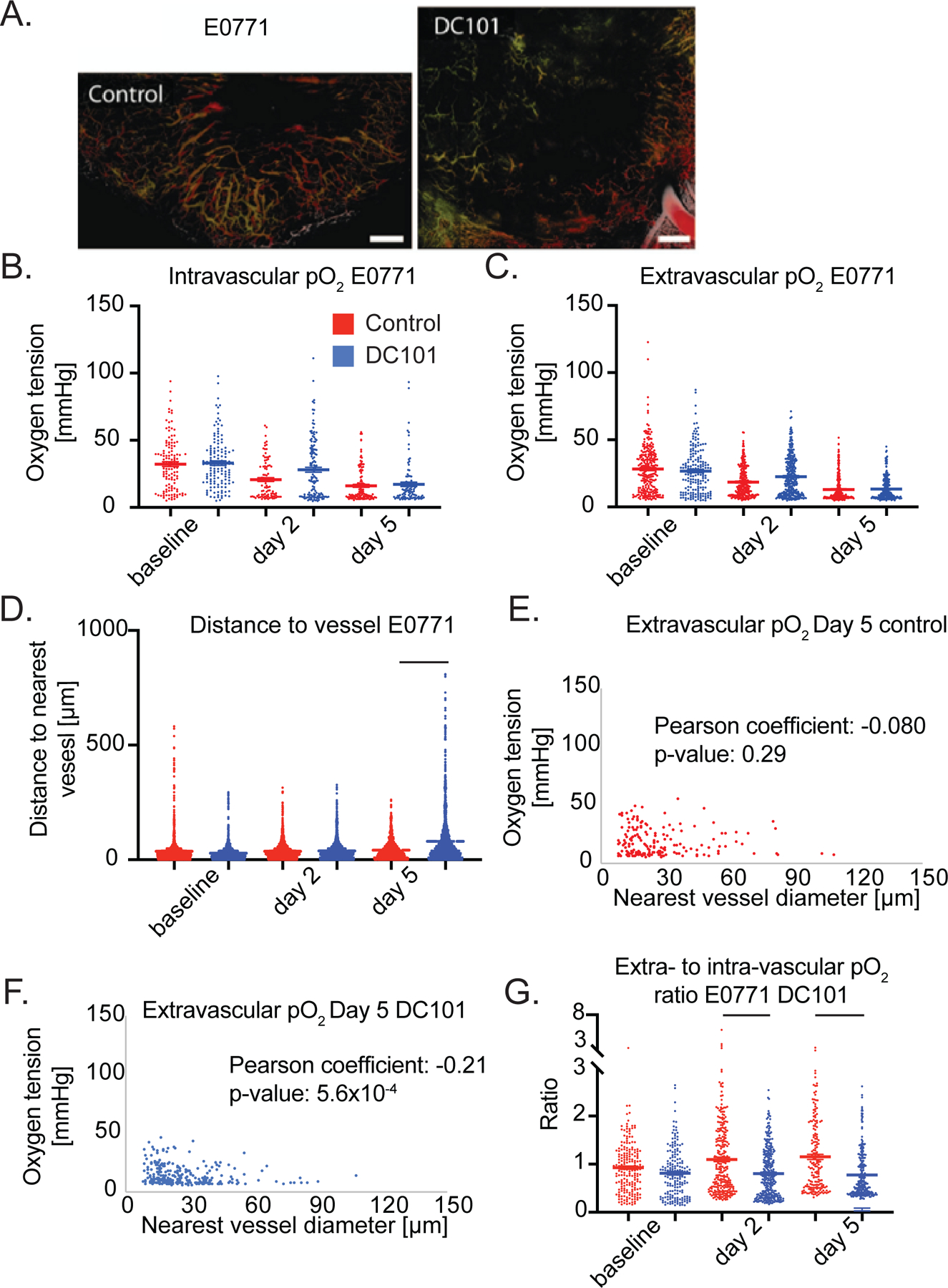
(A) Representative images of angiographies of murine mammary carcinoma (E0771) tumors treated with control IgG antibody (left panel) or the murine anti-VEGFR2 antibody DC101 (right panel) (both 40 mg/kg i.p. every 3 days) on day 5 after two cycles of treatment. Tumor vasculature is presented as a colorized maximum intensity depth projection (superficial to deep: green, red, white). Scale Bars 500 μm. (B) Measurements of oxygen tension in mmHg in vessels in control IgG and 40 mg/kg DC101 antibody treated mice at baseline, day 2 and day 5. n = 92 – 139 measurements per timepoint per group. N = 5 mice per group. Lines indicate statistically significant differences between multiple comparisons after a Kruskal-Wallis test with Dunn’s correction, with differences only depicted for comparisons between control and treatment groups on the same day. (C) Extravascular (interstitial, i.e., measurements 40 to 60 μm from the nearest vessel) oxygen measurements in the same tumors. n = 179 – 307 measurements per timepoint per group. (D) Distance to the nearest vessel measurements in the same tumors. n = 1,103 – 1961 measurements per timepoint per group. (E) Scatter plot of extravascular oxygen tension versus the diameter of the nearest vessel in control treated E0771 tumors on day 5. Correlation coefficient and p-value are denoted on graph. (F) Scatter plot of extravascular oxygen tension versus the diameter of the nearest vessel in DC101 treated E0771 tumors on day 5. Correlation coefficient and p-value are denoted on graph. (G) Measurements of extravascular oxygen tension in mmHg normalized to the intravascular oxygen tension of blood vessels.
In addition to negligible effects on oxygen tension, the 40 mg/kg dose of DC101 also caused detrimental effects on the distribution of perfused vessels in E0771 tumors as shown by larger avascular regions (Figure 5A). DC101 increased the mean and maximum distance to the nearest vessel on day 5 by 91% and 210%, respectively, compared to these distances in IgG treated tumors (Figure 5D). DC101 treatment pruned vessels as indicated by reduced total perfused microvascular density (MVD, Supplementary Figure 10), which is consistent with the larger distances to the nearest vessel in the 40 mg/kg group. To understand which sub-population of vessels was pruned, we examined how DC101 changes the distribution of vessel diameters between baseline and day 5 by plotting the frequencies of diameters in a histogram and found that there are fewer vessels less than 20 µm in diameter and more vessels greater than 20 µm in diameter on day 5 (Supplementary Figure 11) compared to control (Supplementary Figure 12). Indeed, the fraction of vessels less than 20 µm in diameter on day 5 was 25% in DC101 treated E0771 tumors compared to 44% in control. This small vessel pruning effect matched that of bevacizumab in breast cancer patients, as small vessels were selectively pruned in a representative patient (Supplementary Figure 13) (20). Thus, a 40 mg/kg dose of DC101 prunes small vessels resulting in large avascular regions in E0771 tumors.
To gain insight into the effect of DC101 on vessel function, we correlated the size of the nearest vessel to interstitial oxygenation (i.e., measurements 40 to 60 μm from the nearest vessel) on day 5 (Figure 5E, F). In normal tissues, the diameter of a vessel is positively correlated to the flow rate (34) and thus the amount of oxygen delivered (Figure 2E). Surprisingly, in DC101 treated E0771 tumors, smaller tumor vessels were associated with greater extravascular oxygen than larger tumor vessels as indicated by a statistically significant negative correlation coefficient (Figure 5F). Thus, the small vessels remaining after pruning deliver oxygen efficiently compared to the large vessels.
Interestingly, DC101-treated tumors had lower vessel efficiency than control tumors on days 2 and 5 (Figure 5G). The reduced vessel efficiency we observed could be a result of enhanced consumption of oxygen or the large distance between perfused vessels reducing the contribution to extravascular oxygen by nearby vessels. Thus, DC101 induced negligible effects on intravascular and extravascular oxygenation in desmoplastic E0771 breast tumors, and through pruning of small and inefficient vessels, eventually resulted in an insufficient network of perfused vessels.
A lower dose of AAT enhances individual vessel function without excessive vessel pruning in E0771
As DC101 resulted in excessive vessel pruning in E0771 tumors, we investigated the response to lower dose DC101, previously shown to normalize vessel function (nanoparticle extravasation and reduced heterogeneity) in murine models of breast cancer (12, 33). However, it remained unknown to what extent lower doses of AAT, which may not cause pruning, can enhance individual vessel function. Using an eighth of the dose of DC101 (5 mg/kg) (33) to treat E0771 tumors, we measured increased intravascular (Figure 6A) and extravascular (Figure 6B) oxygenation on day 2 compared to IgG treatment. There was no difference in lower dose DC101 and IgG treatment on Day 5. Multiple linear regression analysis revealed that intravascular oxygenation in the control group decreased between day 0 and day 2 at a rate of 2.8 mmHg/day (p = 0.048 compared to no change over time), and it increased in the lower dose DC101 group at a rate of 3.7 mmHg/day (p = 0.0012 compared to the control group’s rate, Supplementary Table 4). Extravascular oxygenation in the control group decreased between day 0 and day 2 at a rate of 4.4 mmHg/day (p < 0.0001 compared to no change over time), and it increased in the DC101 group at a rate of 1.5 mmHg/day (p < 0.0001 compared to the control group’s rate, Supplementary Table 5). The intravascular oxygen tension in IgG treated E0771 tumors on day 2 was 71% of that of vessels in skin, while the oxygen tension reached 98% in 5 mg/kg DC101 treated tumor vessels. Compared to the 40 mg/kg dose DC101, 5mg/kg treatment in E0771 postponed the onset of lesser vessel efficiency compared to controls, which was affected by the low intravascular oxygenation of control tumor blood vessels, from day 2 to day 5 (Figure 6C).
Figure 6 – Anti-VEGFR2 antibody treatment at 5 mg/kg increases intravascular and interstitial pO2 while restoring perfusion (A-D), while Losartan increases interstitial pO2 on day 5 after re-perfusing vessels on day 2 in E0771 (E-J).
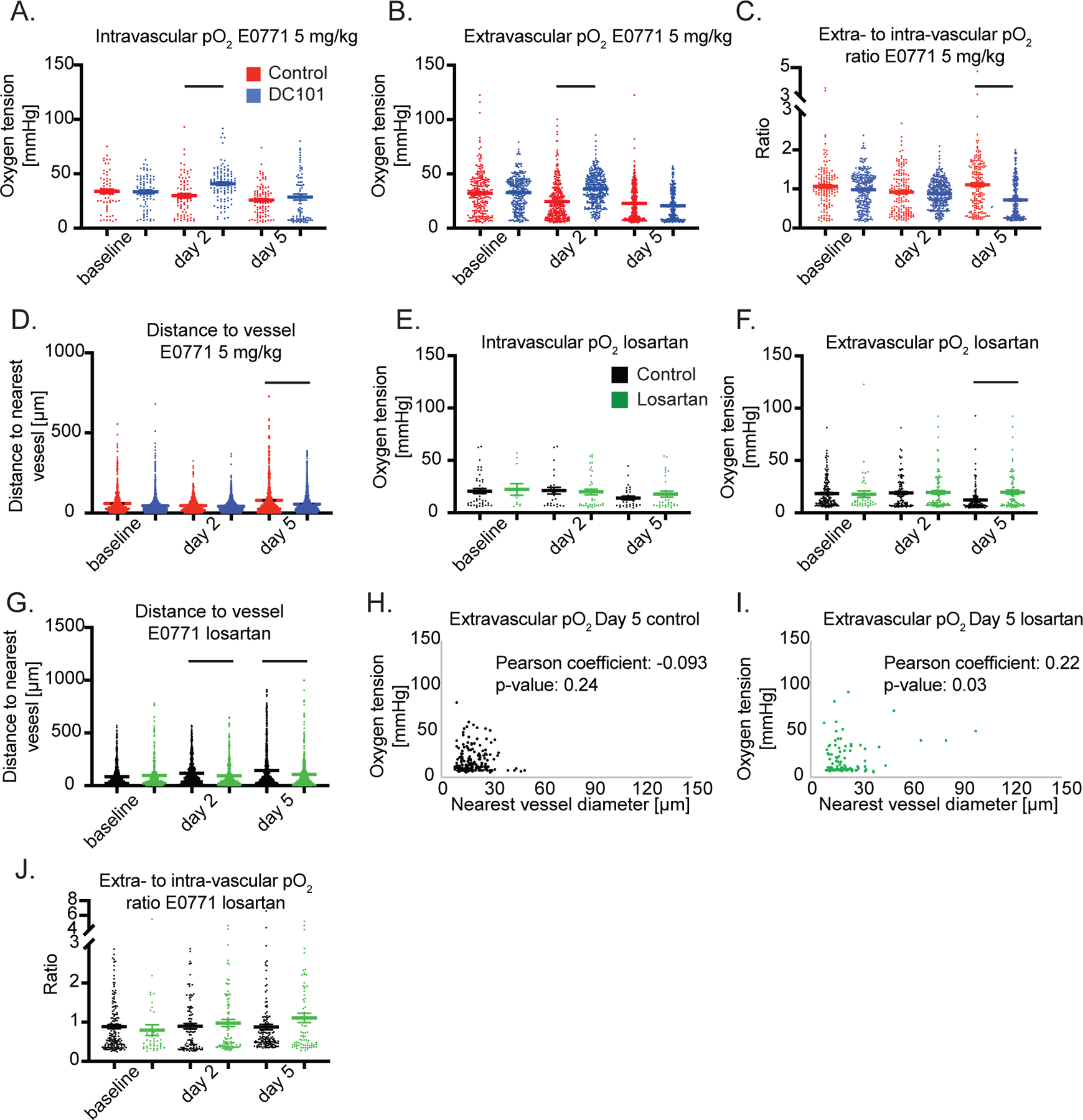
(A) Measurements of intravascular oxygen tension in mmHg in control IgG and anti-VEGFR2 (DC101) (both 5 mg/kg i.p. every 3 days) antibody treated mice at baseline, Day 2 and Day 5. n = 64 – 103 measurements per timepoint per group. N = 5–8 mice per group in the DC101 studies. Lines indicate statistically significant differences between multiple comparisons after a Kruskal-Wallis test with Dunn’s correction, with differences only depicted for comparisons between control and treatment groups on the same day. (B) Extravascular (interstitial, i.e., measurements 40 to 60 μm from the nearest vessel) oxygen measurements in the same tumors. n = 246 – 367 measurements per timepoint per group. (C) Measurements of extravascular oxygen tension in mmHg normalized to the intravascular oxygen tension of blood vessels. (D) Distance to the nearest vessel measurements in the same tumors. n = 870 – 1,962 measurements per timepoint per group. (E) Measurements of intravascular oxygen tension in mmHg in control PBS and losartan treated mice at baseline, day 2 and day 5. n = 12 – 38 measurements per timepoint per group. N = 4–6 mice per group. (F) Extravascular (interstitial, i.e., measurements 40 to 60 μm from the nearest vessel) oxygen measurements in the same tumors. n = 42 – 161 measurements per timepoint per group. N = 4–6 mice per group. (G) Distance to the nearest vessel measurements in the same tumors. n = 412 – 1,279 measurements per timepoint per group. (H) Scatter plot of interstitial oxygen tension versus the diameter of the nearest vessel in E0771 control treated tumors on day 5. Correlation coefficient and p-value are denoted on graph. (I) Scatter plot of interstitial oxygen tension versus the diameter of the nearest vessel in E0771 losartan treated tumors on day 5. Correlation coefficient and p-value are denoted on graph. (J) Measurements of extravascular oxygen tension in mmHg normalized to the intravascular oxygen tension of blood vessels.
We next investigated the effect of lower dose DC101 on vessel pruning. Unlike the 40 mg/kg dose, the 5mg/kg dose DC101 decreased the average and maximum distance to a vessel on day 5 by 36% and 48%, respectively, compared to control (Figure 6D). Thus, 5 mg/kg DC101 induces superior transient normalization of individual vessels. By avoiding the detrimental pruning observed after higher dose treatment, lower dose treatment restored perfusion to hypoperfused regions.
Angiotensin system inhibition increases extravascular oxygenation after re-perfusing small, nonfunctional vessels in E0771
Experimental and theoretical studies indicate that reversing vessel compression—by reducing the compressive force generated by the growing tumor—using cancer-associated fibroblast reprogramming agents, such as the ARB losartan, can enhance oxygen delivery to desmoplastic tumors by converting nonfunctional vessels to perfused vessels (5, 25, 26). However, the effects of cancer-associated fibroblast reprogramming through angiotensin system inhibition on oxygen delivery from individual vessels is not known. Thus, we investigated how losartan affects oxygenation and the distribution of perfused vessels in E0771 breast tumors. While losartan did not affect intravascular oxygenation (Figure 6E), it led to 61% greater extravascular oxygenation compared to controls on day 5 after treatment initiation (Figure 6F). Multiple linear regression analysis revealed that extravascular oxygenation in the control group decreased between day 2 and day 5 at a rate of 1.9 mmHg/day (p = 0.0049 compared to no change over time), and it increased in the losartan group at a rate of 2.0 mmHg/day (p = 0.0002 compared to the control group’s rate, Supplementary Table 6).
The perfused vessels in losartan treated tumors were closer to each other by day 2, as there were shorter mean distances to the nearest vessels (Figure 6G). On days 2 and 5, the mean distance was 19% and 38% less than controls, respectively. Because losartan may decompress vessels without affecting vessel fortification (5), we next investigated the correlation between nearest vessel diameter and interstitial oxygenation at the individual vessel level (Figure 6H, I). Losartan-treated tumors exhibited a positive correlation between extravascular oxygen tension and the diameter of the nearest vessel (Figure 6I) similar to normal tissues, with larger vessels supplying more oxygen. Losartan increased perfused MVD (5) and plotting the frequencies of vessel diameters in a histogram revealed that by day 5 losartan increased the number of vessels with diameters between 12–30 µm and maintained the number of small vessels less than 12 µm (Supplementary Figure 14). The latter are the vessel type pruned by DC101 (Supplementary Figure 11). The greater extravascular oxygenation on day 5 was likely caused by the reperfused vessels, as vascular efficiency was unaffected with losartan treatment (Figure 6J). Thus, losartan decreases the size of avascular regions by day 2, which leads to greater extravascular oxygenation relative to control tumors by day 5.
Discussion
We report here a new approach to dynamically evaluate the effects of treatment on the function and distribution of blood vessels. Capillaries in normal tissues supply more oxygen as they are densely and evenly distributed in tissues. This pattern is lost in most tumors, as there are spatial and temporal heterogeneities within a tumor, between lesions in the same patient and across patients with the same cancer type (2). Indeed, we observed different responses in two murine breast cancer models that represent different sub-types of breast cancer. In MCaIV, a 40 mg/kg dose of DC101 transiently increased extravascular oxygen delivery by day 2 and restored perfusion, whereas in E0771, 40 mg/kg DC101 did not affect oxygen delivery yet pruned vessels. In contrast, a lower dose of DC101 treatment of E0771 tumors increased intravascular and extravascular oxygen delivery on day 2 and restored perfusion on day 5. These results are consistent with various clinical studies highlighting the importance of AAT dose and disparate responses of patients with the same tumor type (1, 2, 15).
While AAT is known to prune immature vessels in mice after a couple of days, AAT improves single vessel function within 6 hours (11) and alleviates hypoxia within two days by inducing pericyte recruitment via Ang1/Tie2 signaling (9). In the E0771 breast tumor model, 40 mg/kg AAT reduced the number of small vessels without affecting larger functional vessels on day 5. One potential strategy to avoid too much vessel pruning—resulting in increased hypoperfusion—would be to induce vessel fortification by non-pruning AAT (47).
We also found that losartan enhances oxygen delivery by maintaining the density of small diameter vessels in desmoplastic E0771 breast tumors, which reduces over time in untreated controls. These data provide additional mechanistic insights into multiple retrospective studies indicating that drugs like losartan correlate with longer survival in cancer patients (26, 48–50). The kinetics of functional vessel remodeling with losartan differ from AAT. In the tumor models we examined, losartan re-perfuses small, poorly functioning vessels by day 2—consistent with data from glioblastoma patients1—while AAT can cause pruning of similar vessels in patients with lower baseline tumor VEGF. In our phase II trial in GBM patients, we observed increased blood flow rate in some patients and decreased blood flow in other in response to identical dose and schedule of VEGF inhibition (16). Our hypothesis is that these differential changes in blood flow rate were dependent on the baseline levels of VEGF in these tumors. However, better outcomes in patients were consistently correlated with the extent of functional normalization (13–16, 20).
Losartan also improves the oxygen delivery function of individual vessels compared to control later than AAT (i.e., on day 5 versus transiently on day 2) in mice. The normalization window can last more than a month in humans (14, 16). The finding that losartan improves extravascular oxygen tension compared to control on day 5 suggests that decompression allows blood to flow in more vessels thereby providing more vessels to supply extravascular oxygen to tissue. These results likely are dependent on the tumor model. The mechanisms associated with the rapid increase of perfused vessel density followed by greater single vessel function needs to be investigated in future studies.
Our data indicate small, re-perfused vessels after losartan treatment are less efficient in delivering oxygen to the interstitial space than the larger microvessels, even though they have a positive effect on increasing tissue oxygen compared to no vessel perfusion. These findings support the notion that vessels newly reperfused by agents like losartan are immature functionally. Our hypothesis that losartan treatment should be combined with normalizing doses of AAT to fortify newly re-perfused, immature vessels needs to be tested in independent studies. Of note, in moderately desmoplastic breast tumor types, our mathematical model predicts enhanced vascular function by combining AAT and decompressing agents (21, 51), while experimental studies have demonstrated that agents that simultaneously act as AAT and decompressing agents increase vessel function (52, 53). Importantly, vessel decompressing agents, including losartan, often have pleiotropic effects including directly and indirectly affecting immune cells (49). The latter may also contribute to vascular normalization resulting in increased perfusion and efficacy of immune checkpoint blockers (54).
The system of MP-PQM we developed is subject to several limitations. The maximum oxygen detectable is 122 mmHg. However, we rarely encountered measurements approaching this value in tumors, and this technique is more accurate at lower oxygen tensions because of the longer quenching time. The maximum tissue depth that can be imaged is 300 µm, but the tumors we imaged had diameters at least 3.0 mm. Thus, the tumor center cannot be entirely probed. Fluorescence imaging and phosphorescence quenching microscopy are performed serially rather than in parallel thereby increasing experiment time.
Our study provided new insights into the kinetics of increased vascular function with AAT and losartan at single vessel resolution. Depending on the tumor model and dose, AAT enhances vascular function compared to control on day 2 and restores perfusion or does not affect vascular function. In the latter “unresponsive” model (i.e., E0771), a lower dose of AAT enhanced vascular function and improved perfusion. In the same “unresponsive” model, losartan enhances vascular function compared to control on day 5 and had greater perfused vessel density compared to control on day 2. AAT prunes poorly functioning small vessels, while vessels re-perfused with losartan are small, poorly functioning vessels. Thus, combining these two tumor microenvironmental normalizing strategies has the potential to enhance treatment further (21, 51). In fact, a recent retrospective analysis showed survival benefit in patients who received a combination of anti-VEGF agents and angiotensin system inhibitors with immune-checkpoint blockers (50). Thus, future work is needed to identify optimal dose and sequence of these two normalization strategies.
Supplementary Material
Statement of Translational Relevance.
(Clinical Cancer Research – Imaging)
Abnormal tumor vessels promote tumor progression and treatment resistance. We have developed two approaches to repair tumor blood vessels and improve their function. One approach employs VEGF pathway inhibition, which can restore the balance between pro- and anti-angiogenic signaling and transiently “normalize” vascular structure and function resulting in improved tumor blood flow. The second approach utilizes angiotensin system inhibitors (ASIs), which can alleviate the compressive forces generated by growing tumors that collapse tumor blood vessels. ASIs decompress tumor blood vessels thereby restoring blood flow towards alleviating hypoxia and its downstream effects. Using multiphoton microscopy, here we show that although mean extravascular hypoxia in tumors is alleviated with judicious application of an anti-VEGFR2 antibody or losartan (an ASI), spatial and temporal heterogeneities persist. Moreover, the complementary vascular changes in response to these two approaches suggests potential for combining them to improve treatment outcomes dependent on oxygenation.
Acknowledgements:
The authors thank Julia Kahn, Sylvie Roberge, and Elisabeth Niemeyer for their technical assistance. RKJ’s research is supported by grants from the Jane’s Trust Foundation, the Niles Albright Research Foundation, the National Foundation for Cancer Research, the Ludwig Center at Harvard, the US National Cancer Institute grants R35CA197743, R01CA259253, R01269672, U01CA261842, and U01CA224348. RKJ’s and DF’s research is supported by US National Cancer Institute grant R01-CA208205. DF’s work is also supported by NIH grant R01NS118929. DGD’s work is supported by NIH grant R01CA260872, R01CA260857, R01CA247441, and R03CA256764, and by Department of Defense grants W81XWH-19–1-0284 and W81XWH-21–1-0738. TPP’s research is supported by US National Institute of Health grants R01CA214913, R01HL128168, R21AI135092 and R21AG072205 and by the Rullo Family MGH Research Scholar Award.
Footnotes
Conflict of Interest Statement:
JDM is an employee of NanoCarrier Co., Ltd. ASM is an employee of, and owns equity in, Pieris Pharmaceuticals. WSK is an employee of Takeda Pharmaceuticals. DGD received consultant fees from Innocoll and research grants from Bayer, Exelixis, Surface Oncology and BMS. RKJ received consulting fees from Innocoll, SPARC, and SynDevRx, owns equity in Accurius, Enlight and SynDevRx, serves on the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund and Tekla World Healthcare Fund and received a grant from Boehringer-Ingelheim. TPP received consultant fees from PureTech Health. No reagents from these companies were used in this study.
K. E. Emblem et al., Matrix-depleting anti-hypertensives decompress tumor blood vessels and improve perfusion in patients with glioblastomas receiving anti-angiogenic therapy. [abstract]. In: Proceedings of the 107th Annual Meeting of the American Association for Cancer Research; 2016 Apr 16–20; New Orleans, LA. Philadelphia (PA): AACR; Cancer Res 2016;76(14 Suppl): Abstract nr 3975.
References
- 1.Jain RK, Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell 26, 605–622 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin JD, Seano G, Jain RK, Normalizing Function of Tumor Vessels: Progress, Opportunities and Challenges. Annual Review of Physiology 81, 505–534 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain RK, Martin JD, Stylianopoulos T, The role of mechanical forces in tumor growth and therapy. Annual Review of Biomedical Engineering 16, 321–346 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nia HT, Munn LL, Jain RK, Physical traits of cancer. Science 370 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chauhan VP et al. , Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nature communications 4, 2516 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baish JW et al. , Scaling rules for diffusive drug delivery in tumor and normal tissues. Proceedings of the National Academy of Sciences 108, 1799–1803 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain RK, Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nature Medicine 7, 987–989 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Jain RK, Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Winkler F et al. , Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6, 553–563 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Tong RT et al. , Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer research 64, 3731–3736 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Yuan F et al. , Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proceedings of the National Academy of Sciences 93, 14765–14770 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y et al. , Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proceedings of the National Academy of Sciences 109, 17561–17566 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heist RS et al. , Improved tumor vascularization after anti-VEGF therapy with carboplatin and nab-paclitaxel associates with survival in lung cancer. Proceedings of the National Academy of Sciences 112, 1547–1552 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorensen AG et al. , Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Research 72, 402–407 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerstner E et al. , Bevacizumab reduces permeability and concurrent temozolomide delivery in a subset of patients with recurrent glioblastoma. Clinical Cancer Research 26, 206–212 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batchelor TT et al. , Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proceedings of the National Academy of Sciences 110, 19059–19064 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boucher Y et al. , Bevacizumab improves tumor infiltration of mature dendritic cells and effector T-cells in triple-negative breast cancer patients. NPJ Precision Oncology 5, 62 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain RK, Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. Journal of Clinical Oncology 31, 2205–2218 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vakoc BJ et al. , Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nature Medicine 15, 1219–1223 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tolaney SM et al. , Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. Proceedings of the National Academy of Sciences 112, 14325–14330 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stylianopoulos T, Jain RK, Combining two strategies to improve perfusion and drug delivery in solid tumors. Proceedings of the National Academy of Sciences 110, 18632–18637 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffon-Etienne G, Boucher Y, Brekken C, Suit HD, Jain RK, Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: clinical implications. Cancer Research 59, 3776–3782 (1999). [PubMed] [Google Scholar]
- 23.Padera TP et al. , Pathology: cancer cells compress intratumour vessels. Nature 427, 695 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK, Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proceedings of the National Academy of Sciences 108, 2909–2914 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chauhan VP et al. , Reprogramming the microenvironment with tumor-selective angiotensin blockers enhances cancer immunotherapy. Proceedings of the National Academy of Sciences 116, 10674–10680 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y et al. , Losartan treatment enhances chemotherapy efficacy and reduces ascites in ovarian cancer models by normalizing the tumor stroma. Proceedings of the National Academy of Sciences 116, 2210–2219 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown EB et al. , In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nature Medicine 7, 864–868 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Torres Filho IP, Leunig M, Yuan F, Intaglietta M, Jain RK, Noninvasive measurement of microvascular and interstitial oxygen profiles in a human tumor in SCID mice. Proceedings of the National Academy of Sciences 91, 2081–2085 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helmlinger G, Yuan F, Dellian M, Jain RK, Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nature Medicine 3, 177–182 (1997). [DOI] [PubMed] [Google Scholar]
- 30.Sakadžić S et al. , Two-photon high-resolution measurement of partial pressure of oxygen in cerebral vasculature and tissue. Nature Methods 7, 755–759 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spencer JA et al. , Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508, 269–273 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han H-S et al. , Quantum dot/antibody conjugates for in vivo cytometric imaging in mice. Proceedings of the National Academy of Sciences 112, 1350–1355 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chauhan VP et al. , Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nature Nanotechnology 7, 383–388 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan F et al. , Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Research 54, 4564–4568 (1994). [PubMed] [Google Scholar]
- 35.Matsuda Y et al. , Comparison of newly developed inhalation anesthesia system and intraperitoneal anesthesia on the hemodynamic state in mice. Biological and Phamaceutical Bulletin 30, 1716–1720 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Ziemer LS, Lee WM, Vinogradov SA, Sehgal C, Wilson DF, Oxygen distribution in murine tumors: characterization using oxygen-dependent quenching of phosphorescence. Journal of Applied Physiology 98, 1503–1510 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Wen B, Urano M, O’Donoghue JA, Ling CC, Measurements of partial oxygen pressure using the OxyLite system in R3327-AT tumors under isoflurane anesthesia. Radiation Research 166, 512–518 (2006). [DOI] [PubMed] [Google Scholar]
- 38.McGraw CM, Khalil G, Callis JB, Comparison of time and frequency Domain methods for luminescence lifetime measurements. The Journal of Physical Chemistry C 112, 8079–8084 (2008). [Google Scholar]
- 39.O’Donovan C, Hynes J, Yashunshki D, Papkovsky DB, Phosphorescent oxygen-sensitive materials for biological applications. Journal of Materials Chemistry 15, 2946–2951 (2005). [Google Scholar]
- 40.Kruk M et al. , Two-photon absorption of tetraphenylporphin free base. Journal of luminescence 105, 45–55 (2003). [Google Scholar]
- 41.Lakowicz J (1999) Principles of fluorescence microscopy (Kluwer Academic, New York: ). [Google Scholar]
- 42.Jain RK, Determinants of tumor blood flow: a review. Cancer Research 48, 2641–2658 (1988). [PubMed] [Google Scholar]
- 43.Ricard C et al. , Dynamic quantitative intravital imaging of glioblastoma progression reveals a lack of correlation between tumor growth and blood vessel density. PloS one 8, e72655 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han HS et al. , Spatial charge configuration regulates nanoparticle transport and binding behavior in vivo. Angewandte Chemie International Edition 52, 1414–1419 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hashizume H et al. , Openings between defective endothelial cells explain tumor vessel leakiness. The American journal of pathology 156, 1363–1380 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Panagi M et al. , TGF-β inhibition combined with cytotoxic nanomedicine normalizes triple negative breast cancer microenvironment towards anti-tumor immunity. Theranostics 10, 1910–1922 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goel S et al. , Effects of vascular-endothelial protein tyrosine phosphatase inhibition on breast cancer vasculature and metastatic progression. Journal of the National Cancer Institute 105, 1188–1201 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin JD, Cabral H, Stylianopoulos T, Jain RK, Improving cancer immunotherapy using nanomedicine: progress, opportunities and challenges. Nature Reviews Clinical Oncology 17, 251–266 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinter M, Jain RK, Targeting the renin-angiotensin system to improve cancer treatment: Implications for immunotherapy. Science Translational Medicine 9, eaan5616 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drobni ZD et al. , Renin–angiotensin–aldosterone system inhibitors and survival in patients with hypertension treated with immune checkpoint inhibitors. European Journal of Cancer 163, 108–118 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mpekris F et al. , Combining microenvironment normalization strategies to improve cancer immunotherapy. Proceedings of the National Academy of Sciences 117, 3728–3737 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J et al. , TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proceedings of the National Academy of Sciences 109, 16618–16623 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin JD et al. , Dexamethasone Increases Cisplatin-Loaded Nanocarrier Delivery and Efficacy in Metastatic Breast Cancer by Normalizing the Tumor Microenvironment. ACS Nano 13, 6396–6408 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Zheng X et al. , Increased vessel perfusion predicts the efficacy of immune checkpoint blockade. The Journal of Clinical Investigation 128, 2104–2115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available within the article and its supplementary data files.


