Abstract
Aims:
SGLT2 inhibitors have been shown to reduce risk of hospitalization for heart failure (HHF) and composite kidney outcomes, but the mediators underlying these benefits are unknown.
Materials and methods:
Among participants from VERTIS CV, a trial of patients with type 2 diabetes and atherosclerotic cardiovascular disease randomized to ertugliflozin versus placebo, Cox proportional hazards regression models were used to evaluate the percent mediation of ertugliflozin efficacy on first HHF and kidney composite outcome between changes during the trial in 26 potential mediators. Time-dependent approaches were used to evaluate associations between early (change from baseline to the first post-baseline measurement) and average (weighted average of change from baseline using all post-baseline measurements) changes in covariates with clinical outcomes.
Results:
For the HHF analyses, early changes in four biomarkers (haemoglobin, haematocrit, serum albumin, and urate) and average changes in seven biomarkers (early biomarkers + weight, chloride, and serum protein) were identified as fulfilling the criteria for mediators of ertugliflozin HHF efficacy. Similar results were observed for the composite kidney outcome, with early changes in four biomarkers (HbA1c, haemoglobin, haematocrit, and urate), and average changes in five biomarkers (early biomarkers [not HbA1c] + weight, serum albumin) mediating the effects of ertugliflozin on the kidney outcome.
Conclusions:
In these analyses from the VERTIS CV trial, markers of volume status and haemoconcentration and/or haematopoiesis were the strongest mediators of the effect of ertugliflozin on reducing risk of HHF and composite kidney outcomes in the early and average change periods.
ClinicalTrials.gov identifier:
Keywords: Ertugliflozin, hospitalization for heart failure, kidney outcomes, mediation analyses, SGLT2 inhibitor, type 2 diabetes mellitus, VERTIS CV
1. INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a growing public health concern affecting over 30 million adults in the United States alone and an estimated 462 million individuals worldwide.1,2 Notably, cardiovascular (CV) complications are a major cause of hospitalizations and mortality among patients with T2DM.3,4 Sodium-glucose cotransporter 2 (SGLT2) inhibitors have demonstrated cardio-protective benefits in a wide spectrum of patients, including those with T2DM as well as heart failure, regardless of T2DM status.5–12 Additional reno-protective benefits, as shown by slowing the decline of estimated glomerular filtration rate (eGFR) and progression to end-stage kidney disease in both diabetic and non-diabetic kidney disease, have also been demonstrated.13,14
In the VERTIS CV trial,15 ertugliflozin, an SGLT2 inhibitor, was non-inferior to placebo for the primary composite outcome of CV death, myocardial infarction, and stroke; and it reduced the risk of hospitalization for heart failure (HHF). For kidney outcomes, in prespecified, exploratory analyses of kidney-related outcomes (sustained 40% reduction in eGFR, chronic kidney dialysis/transplant, or renal death), ertugliflozin was associated with a significantly reduced risk of the composite kidney outcome.16–18
CV outcome trials are primarily designed to assess the effects of respective interventions on specific outcomes, and it is usually not possible to elucidate the underlying mechanisms responsible for the impact of these interventions. Mediation analyses aim to identify potential mediators of such observed effects. Prior mediation analyses from the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) trial reported changes in haemoglobin and haematocrit as the strongest mediators of reduced cardiac death risk with empagliflozin when compared with placebo.19 Subsequent mediation analyses from the CANVAS Trials Program demonstrated similar findings where changes in erythrocyte/haemoglobin concentration and serum urate were identified as the strongest mediators of canagliflozin on reducing risk of HHF.20,21 Similarly, changes in erythrocyte/haemoglobin concentration, urate, and urine albumin-to-creatinine ratio (UACR) were the strongest mediators on the kidney composite including 40% reduction in eGFR.18,21 Accordingly, the goal of the present study was to perform mediation analyses using data from the VERTIS CV trial to assess the potential mediators of the effect of ertugliflozin on reducing HHF and composite kidney including 40% reduction in eGFR outcomes.
2. MATERIALS AND METHODS
2.1. Study population
The study used participant-level data from the VERTIS CV trial. The trial design, methodology, and primary results of VERTIS CV have been previously described.15,22 Briefly, VERTIS CV was a randomized, multicentre, double-blind trial that enrolled patients with T2DM and established atherosclerotic CV disease (ASCVD). Enrolment criteria included patients ≥40 years of age with glycated haemoglobin (HbA1c) between 7% and 10.5% and prevalent ASCVD involving the coronary, cerebrovascular, or peripheral arterial systems. Patients were randomized in a 1:1:1 ratio to ertugliflozin 5 mg, ertugliflozin 15 mg, or placebo in addition to standard-of-care treatment. Patients with type 1 diabetes, history of ketoacidosis, eGFR <30 mL/min/1.73 m2, or New York Heart Association (NYHA) class III and IV were excluded, with NYHA class III heart failure included by the protocol amendment which doubled the size of the trial and established the alpha-protected secondary endpoints based on EMPA-REG OUTCOME results. In total, 8246 patients were randomized to either ertugliflozin dose (n = 5499) or placebo (n = 2747). Informed consent was obtained from all individuals. The study was conducted in accordance with principles of the Declaration of Helsinki and the ICH Good Clinical Practice Guidelines and was approved by the appropriate institutional review boards and regulatory agencies.
2.2. Follow-up
Patients were randomized and administered the study drug in the clinic on Day 1. Routine face-to-face clinic visits occurred at Weeks 6, 12, 18, 26, 39, and 52 during the first year of participation. Thereafter, patients had routine clinic visits every 4 months.
2.3. Outcomes of interest
The primary CV outcome of interest in the present study was time to first occurrence of HHF, which was a prespecified secondary outcome of the trial that was not hierarchically tested. This outcome was 1) a component of the first alpha-protected secondary endpoint, 2) a protocol secondary endpoint, and 3) the most consistently improved outcome of SGLT2 inhibitors across completed CV outcomes trials.17 Briefly, HHF was defined as a hospitalization for at least 24 hours with a primary diagnosis of heart failure and new or worsening symptoms.15 A centralized blinded committee adjudicated heart failure events based on review of medical records including relevant heart failure signs, symptoms, diagnostics, and medications.
The kidney outcome of interest for the present analyses was a prespecified composite of sustained 40% reduction in eGFR, chronic kidney dialysis/transplant, or renal death.16 We used this composite definition since it demonstrates the most consistent effect—with zero heterogeneity—in pooled analyses across the SGLT inhibitors class.17,18,23 Cause of death was an adjudicated outcome of the primary trial. Sustained eGFR reduction required the occurrence of an eGFR value that met the cutoff criterion and was followed by a subsequent value more than 30 days later that also met the cutoff criterion.
2.4. Clinical covariates and potential mediators
Covariates were ascertained using standard protocols as described previously.22 Systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate were assessed as the average of three seated readings using an automated oscillometric device. Weight was recorded using a standardized, digital scale provided by the study sponsor. Blood and urine samples were collected and analysed in a central laboratory. eGFR was calculated using the Modification of Diet in Renal Disease equation.24
Potential mediators selected for analyses were based on biological considerations, results from prior mediation analyses of SGLT2 inhibitor trials, and consensus within the author group.19,20 The covariates chosen for mediation assessment represented several general mechanistic categories including glycaemia (HbA1c, fasting plasma glucose [FPG]), haemodynamics (SBP, DBP, heart rate), lipids (low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], LDL/HDL ratio, triglycerides), kidney function (UACR, eGFR), adiposity (weight), volume status/haematopoiesis (haematocrit, haemoglobin, serum albumin, red blood cell [RBC] count), indicators of acidosis/alkalosis (serum bicarbonate), and other (urate, sodium, chloride, phosphate, magnesium, serum protein, blood urea nitrogen [BUN], alanine aminotransferase [ALT], aspartate aminotransferase [AST]). UACR values were log-transformed due to non-normal distribution. Given eGFR was included in the composite kidney outcome, the measure was only assessed in the HHF analyses.
2.5. Statistical analysis
The nomination of mediators was based on the approach from Baron and Kenny.25 A potential mediator was considered eligible if it satisfied two conditions. First, there had to be a significant (p < .05) effect of ertugliflozin compared with placebo on the potential mediator, and second, post-randomization levels of the potential mediator had to be significantly (p < .05) associated with the outcome of interest and adjusting for the change in the mediator attenuated the magnitude of the estimated treatment effect of ertugliflozin versus placebo on this outcome due to the proportional mediation (i.e. hazard ratio [HR] increased toward unity when change in the mediator was added to the model as covariate). For each potential mediator, (1) the early change at the first measurement after randomization and (2) the average change of all post-randomization measurements between treatment groups were each assessed using mixed-effects models with random patient intercepts. In analyses of average change, each measured value was such that measurements at the late time points carried more weight in the analyses than the measurements at earlier time points. Eligible measurements were assessed as changes from baseline and all measurements collected prior to the outcome of interest or, in those not experiencing an outcome event, prior to final follow-up were included in the analyses. An unstructured covariance matrix was used to model the correlation among repeated measurements for average change from baseline. Models were adjusted for treatment arm, time points, the baseline value of the potential mediator, and baseline values of HbA1c, eGFR, BMI, and SBP and chosen a priori based on consensus from the writing group. The baseline value was not duplicated if it had been included as the baseline of the potential mediator. Differences in longitudinal changes between treatment groups were assessed by residual restricted maximum likelihood tests. All variables had < 10% missingness with missing data handled by linear mixed-effect models. Missing observations were attributed based on the maximum likelihood estimation.
The second condition was based on stratified Cox proportional hazards models which were used to assess the mediation of the post-randomization change of each potential mediator on the treatment effect of ertugliflozin versus placebo for each outcome of interest. Models were adjusted for the treatment arm, change from baseline (either early or average change), the baseline value of the potential mediator, and enrolment cohort as a stratification factor (VERTIS CV designated patients into cohorts 1 and 2 based on the respective date of enrolment into the trial before or after March 2016—a date that marked protocol amendments as described previously).15 For each potential mediator, the resultant percentage mediation was estimated by using the equation:
where HR is the unadjusted hazard ratio and HRC is the hazard ratio after adjustment for the potential mediator.26 Bootstrap resampling of 10 000 iterations was used to estimate the 95% confidence intervals (CI) of percentage mediation for each mediator.
Additional analyses were performed to assess the collective mediation of multiple mediators contributing to the effect of ertugliflozin. Specifically, both a forward stepwise selection and a backward stepwise selection were performed to assemble a multivariable model. In the forward stepwise selection, the mediators with the largest percentage mediation were sequentially added until the joint percentage mediation was near 100%. Conversely, the backward stepwise selection started with all potential mediators. The mediators with the smallest percentage mediation were sequentially removed until the joint percentage mediation was near 100%. Given the significant correlation between multiple potential mediators, multicollinearity was evaluated using variance proportions. Potential mediators with a variance proportion >0.70 were excluded, retaining the marker with the largest percentage mediation.
All analyses were performed using SAS Version 9.4 (The SAS Institute, Cary, NC, USA) with a two-sided p value < .05 indicating significance.
3. RESULTS
3.1. Effects of ertugliflozin on potential mediators
A total of 26 markers were considered as potential mediators. Compared with placebo, AST, HDL-C/LDL-C ratio, and triglycerides did not meet the first criterion demonstrating an ertugliflozin effect by either early or average change. However, ertugliflozin was associated with significant reductions in HbA1c, FPG, SBP, DBP, UACR, eGFR, weight, serum bicarbonate, and urate in the early and average time periods (Table 1). Ertugliflozin was associated with significant reductions in heart rate and ALT levels only during the average time period. Conversely, ertugliflozin was associated with significant elevations in haematocrit, haemoglobin, RBC count, serum albumin, serum protein, sodium, chloride, magnesium, phosphate, LDL-C, HDL-C, and BUN levels in both the early and average time periods (Table 1). Overall, by the first criterion, 21 of 26 markers were considered potential mediators in the early time period compared with 23 of 26 in the average time period.
TABLE 1.
Effects of ertugliflozin on biomarkers that may mediate the effects of ertugliflozin on hospitalization for heart failure or composite kidney outcomes
| Mean (SD) at baseline | Placebo-adjusted change from baseline (least squares mean [95% CI]) | |||
|---|---|---|---|---|
| Placebo (n = 2747) |
Ertugliflozin (n = 5499) |
Early | Average | |
| Glycaemia | ||||
| HbA1c, % | 8.2 (0.9) | 8.2 (1.0) | −0.46 (−0.49, −0.42) | −0.44 (−0.48. −0.40) |
| Fasting plasma glucose, mmol/L | 9.6 (2.7) | 9.7 (2.9) | −1.31 (−1.43, −1.19) | −1.07 (−1.16, −0.99) |
| Vascular | ||||
| SBP, mmHg | 133.1 (13.9) | 133.5 (13.7) | −2.85 (−3.47, −2.23) | −2.60 (−3.03, −2.16) |
| DBP, mmHg | 76.4 (8.7) | 76.8 (8.3) | −0.94 (−1.31, −0.56) | −0.86 (−1.13, −0.59) |
| Heart rate, bpm | 70.6 (10.1) | 70.8 (10.1) | −0.07 (−0.45, 0.31) | −0.38 (−0.67, −0.10) |
| Lipids | ||||
| LDL-C, mmol/L | 2.3 (1.0) | 2.3 (1.0) | 0.06 (0.02, 0.10) | 0.07 (0.03, 0.10) |
| HDL-C, mmol/L | 1.1 (0.3) | 1.1 (0.3) | 0.03 (0.02, 0.04) | 0.04 (0.03, 0.05) |
| LDL/HDL ratio | 2.1 (1.0) | 2.1 (1.0) | −0.01 (−0.05, 0.03) | −0.01 (−0.04, 0.02) |
| Triglycerides, mg/dL | 178.9 (104.7) | 181.4 (119.2) | −1.98 (−6.98, 3.03) | 0.51 (−3.30, 4.32) |
| Renal | ||||
| UACR, mg/g (median [IQR])† | 19.0 (6.0–66.5) | 18.0 (6.0–69.0) | −0.08 (−0.10, −0.05) | −0.09 (−0.11, −0.07) |
| eGFR, mL/min/1.73 m2 (MDRD) | 75.7 (20.8) | 76.1 (20.9) | −2.77 (−3.33, −2.21) | −1.32 (−1.79, −0.86) |
| Adiposity | ||||
| Weight, kg | 91.9 (18.3) | 91.7 (18.5) | −1.10 (−1.21, −1.00) | −2.07 (−2.25, −1.89) |
| Volume status and haematopoiesis | ||||
| Haematocrit, % | 42.9 (4.1) | 43.1 (4.1) | 1.70 (1.57, 1.83) | 2.14 (2.03, 2.26) |
| Haemoglobin, g/dL | 14.0 (1.4) | 14.0 (1.4) | 0.45 (0.41, 0.49) | 0.58 (0.54, 0.61) |
| Serum albumin, g/dL | 4.4 (0.3) | 4.4 (0.3) | 0.06 (0.05, 0.07) | 0.05 (0.04, 0.06) |
| Red blood cell count (106/μL) | 4.7 (0.5) | 4.8 (0.5) | 0.17 (0.15, 0.18) | 0.23 (0.21, 0.24) |
| Indicators of acidosis/alkalosis | ||||
| Serum bicarbonate, mEq/L | 24.4 (2.6) | 24.5 (2.5) | −0.59 (−0.71, −0.47) | −0.38 (−0.46, −0.30) |
| Other | ||||
| Urate, mg/dL | 5.7 (1.6) | 5.6 (1.6) | −0.34 (−0.39, −0.29) | −0.37 (−0.41, −0.32) |
| Sodium, mEq/L | 141.2 (2.7) | 141.1 (2.7) | 0.43 (0.31, 0.55) | 0.39 (0.31, 0.47) |
| Chloride, mmol/L | 100.4 (3.0) | 100.3 (3.0) | 0.57 (0.43, 0.71) | 0.40 (0.31, 0.49) |
| Magnesium, mEq/L | 1.5 (0.2) | 1.5 (0.2) | 0.15 (0.15, 0.16) | 0.15 (0.14, 0.15) |
| Phosphate, mg/dL | 3.5 (0.5) | 3.5 (0.5) | 0.18 (0.15, 0.20) | 0.14 (0.12, 0.15) |
| Protein, g/dL | 7.1 (0.4) | 7.1 (0.5) | 0.09 (0.08, 0.11) | 0.08 (0.06, 0.09) |
| BUN, mg/dL | 18.4 (6.7) | 18.2 (6.4) | 1.19 (0.93, 1.45) | 1.11 (0.93, 1.29) |
| ALT, IU/L | 25.1 (18.6) | 25.2 (17.5) | −0.64 (−1.28, 0.00) | −1.22 (−1.65, −0.79) |
| AST, IU/L | 21.4 (11.1) | 21.5 (19.9) | 0.09 (−0.46, 0.64) | −0.62 (−171.61, 170.38) |
Values in bold indicate a significant treatment effect (P < .05). Early change model adjusted by treatment, baseline value of outcome variable, baseline UACR, baseline eGFR, baseline BMI, and baseline SBP. Average change model is a mixed-model repeated-measures analysis using all available data from patients who had baseline and follow-up measurement for the respective outcome. The model adjusted for baseline of outcome variable, baseline UACR, baseline eGFR, baseline BMI, and baseline SBP.
The placebo-adjusted changes from baseline values are based on log-transformed data.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; HDL-C, high-density lipoprotein cholesterol; IQR, inter-quartile range; LDL-C, low-density lipoprotein cholesterol; MDRD, Modification of Diet in Renal Disease; SBP, systolic blood pressure, UACR, urine albumin-to-creatinine ratio.
3.2. Mediation analyses for HHF
3.2.1. Associations between change in potential mediators and risk of HHF
In the early change regression analyses, 9 of the 21 potential mediators that met the first criterion also had a significant association with risk of HHF (Supplemental Table 1). Specifically, adjusted Cox models showed that increases in HDL-C, haematocrit, haemoglobin, serum albumin, sodium, chloride, and serum protein and decreases in UACR and urate were associated with lower HHF risk. In the average change regression analyses, 13 of the 23 potential mediators (that met the first criterion) had a significant association with risk of HHF (Supplemental Table 1). In addition to the significant potential mediators in the early change analyses (all except HDL-C and sodium), SBP, heart rate, eGFR, weight, BUN, and ALT were significantly associated with risk of HHF. Notably, HDL-C and sodium were only associated with risk of HHF in the early time period analyses.
3.2.2. Estimated percentage mediation of ertugliflozin on HHF
Of the nine potential mediators meeting the first criterion and associated with HHF, only four (haematocrit, haemoglobin, serum albumin, and urate) significantly shifted the HR in the direction of unity and had a significant mediation effect, thereby fulfilling both criteria to be a mediator of the benefit of ertugliflozin on the risk of HHF (Figure 1, Supplemental Table 1). The largest percentage mediation was observed for haematocrit mediating 40% (95% CI 10.61–151.17) of the treatment group differences in early time period changes. When the average post-randomization levels were assessed, 7 of the potential 13 mediators fulfilled both criteria nominating them for mediation of the benefit of ertugliflozin on the risk of HHF. Changes in haemoglobin levels mediated the largest effect of ertugliflozin (63.33%; 95% CI 26.08–231.35) and risk of HHF (Figure 1, Supplemental Table 1).
FIGURE 1.
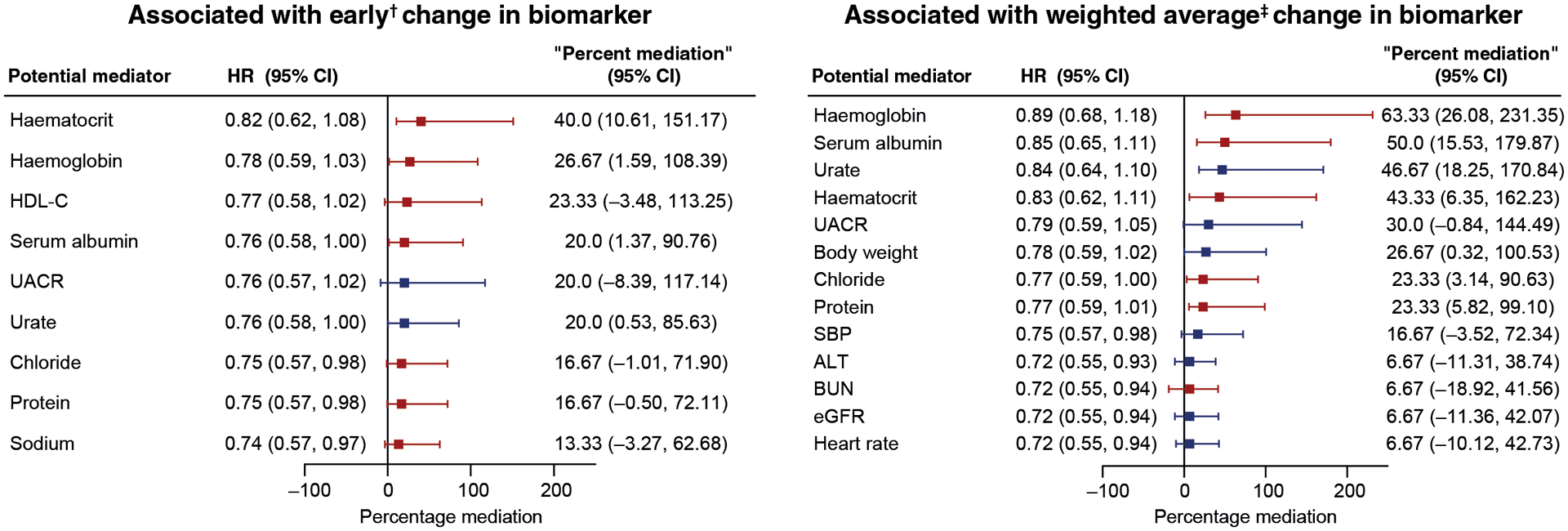
Percentage of mediation by biomarkers on hospitalization for heart failure HR (95% CI) for the unweighted prespecified HHF.
†First change from baseline measurement.
‡Weighted average of change from baseline from all post-baseline measurements.
Mediators in blue were associated with decreases in placebo-adjusted changes from baseline with ertugliflozin; mediators in red were associated with increases in placebo-adjusted changes from baseline.
ALT, alanine aminotransferase; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; HHF, hospitalization for heart failure; HR, hazard ratio; SBP, systolic blood pressure; UACR, urine albumin-to-creatinine ratio.
3.3. Mediation analyses for the composite kidney outcome
3.3.1. Associations between change in potential mediators and risk of composite kidney outcomes
A total of 25 markers were considered as potential mediators (eGFR was excluded due to its inclusion in the composite outcome). In the early change time period, 9 of the 20 potential mediators meeting the first criterion (HbA1c, UACR, haematocrit, haemoglobin, RBC count, serum bicarbonate, urate, phosphate, and BUN) were associated with the risk of the composite kidney outcome (Supplemental Table 2). For the average post-randomization levels, there were significant associations observed for 11 of the 22 markers (meeting the first criterion).
3.3.2. Estimated percentage mediation of ertugliflozin on composite kidney outcomes
Of the nine potential mediators meeting the first criterion and associated with the kidney endpoint risk, early changes in only four biomarkers significantly satisfied both mediator criteria and percent mediation for risk of the composite kidney outcome (HbA1c, haematocrit, haemoglobin, and urate) (Figure 2, Supplemental Table 2). Changes in HbA1c levels were associated with the largest effect of ertugliflozin (50.0%; 95% CI 13.76–197.18) on the risk of the composite kidney outcome. When the average post-randomization levels were assessed, five mediators significantly satisfied both mediator criteria and percent mediation for risk of the composite kidney outcome. Changes in haemoglobin levels were associated with the largest effect of ertugliflozin (61.76%; 95% CI 21.93–213.71) and risk of the composite kidney outcome (Figure 2, Supplemental Table 2).
FIGURE 2.
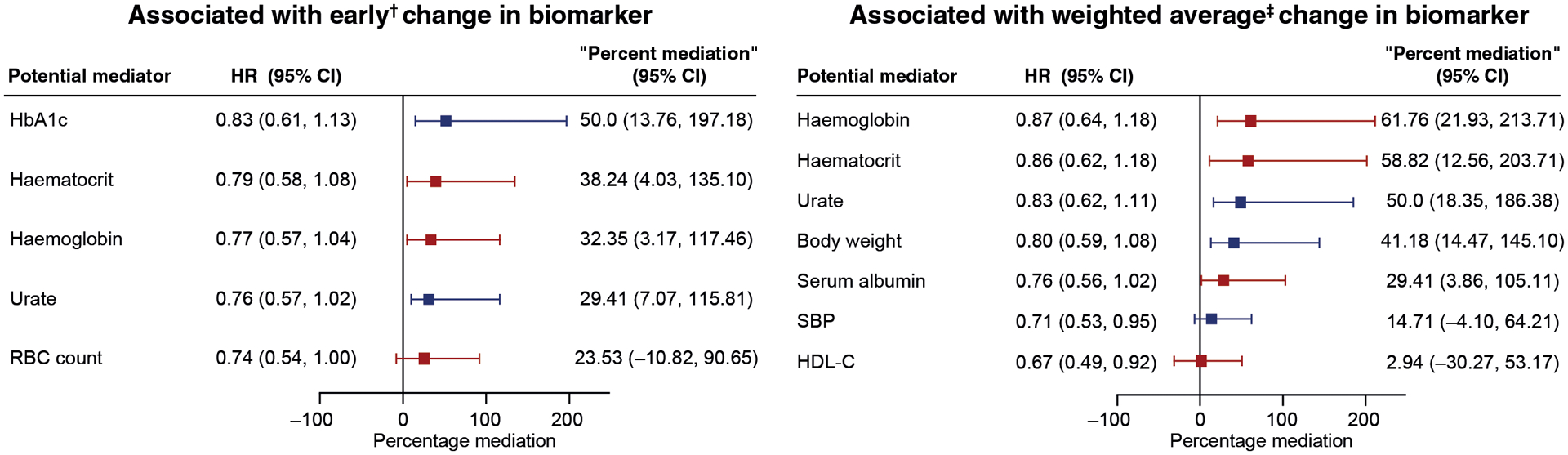
Percentage of mediation by biomarkers on a prespecified exploratory kidney composite outcome HR (95% CI) for the unweighted prespecified exploratory kidney composite outcome comprising sustained 40% decrease from baseline in estimated glomerular filtration rate, chronic kidney replacement therapy, or kidney death was 0.66 (0.50–0.88).
†First change from baseline measurement.
‡Weighted average of change from baseline from all post-baseline measurements.
Mediators in blue were associated with decreases in placebo-adjusted changes from baseline with ertugliflozin; mediators in red were associated with increases in placebo-adjusted changes from baseline.
HbA1c, glycated haemoglobin; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; RBC, red blood cell; SBP, systolic blood pressure.
3.4. Multi-variable mediation effects of biomarkers on risk of HHF and composite kidney outcome
In joint mediation analyses, the same mediators were identified in both forward and backward stepwise-selection approaches. Haemoglobin and haematocrit were noted to be collinear for which the mediator with a lower percent mediation in univariate analyses was removed from the selection process. In the early change from baseline analyses for risk of HHF, addition of HDL-C to haematocrit (the strongest percentage mediator) resulted in a proportion mediation of 67% (data not shown). Further addition of all nominated biomarkers (albumin, chloride, haematocrit, HDL-C, UACR, urate, and protein) increased the proportion mediated to 83% (Table 2). In the average post-randomization time period, haemoglobin, albumin, and urate produced a combined proportion mediation of 110%.
TABLE 2.
Summary of multivariate mediation analyses of hospitalization for heart failure and composite kidney outcome using forward selection approaches
| Time Point | HHF | Composite kidney outcome | ||||
|---|---|---|---|---|---|---|
| Mediator (by adding factors sequentially by larger percent mediation) | % mediation | (95% CI) | Mediator (by adding factors sequentially by larger percent mediation) | % mediation | (95% CI) | |
| Early change | ALB, CL, HCT, HDL-C, log (UACR), urate, protein | 83 | (22.81, 303.30) | HbA1c, HCT, urate | 118 | (47.43, 405.81) |
| Weighted average change | ALB, urate, haemoglobin | 110 | (43.48, 378.78) | Haemoglobin, urate | 121 | (51.49, 422.62) |
ALB, albumin; CL, chloride; HbA1c, glycated haemoglobin; HCT, haematocrit; HDL-C, high-density lipoprotein cholesterol; HHF, hospitalization for heart failure; UACR, urine albumin-to-creatinine ratio.
For the risk of the composite kidney outcome, the strongest mediator of early changes in biomarker levels was HbA1c. Addition of haematocrit led to a proportion mediated of 79% (data not shown). Further addition of urate increased the percentage mediation to 118% (Table 2). In the average post-randomization time period, haemoglobin and urate produced the largest combined proportion mediation of 121%.
4. DISCUSSION
In these post-hoc analyses among participants from the VERTIS CV trial, several potential mediators were identified for the effect of ertugliflozin on reducing the risk of HHF and the composite kidney outcome, accounting for both early and weighted average change of biomarkers. For HHF, the strongest mediators were those of erythropoiesis/plasma volume markers, namely haemoglobin, haematocrit, and serum albumin, as well as serum urate. HDL-C was only noted to have a significant mediation in the early change but not in the average change analyses. For the kidney composite outcome, in addition to the same plasma volume/erythropoiesis markers, the greatest association in the early stage was that of HbA1c. This association was not observed with the average change, whereas haemoglobin, haematocrit, serum urate, and body weight had significant mediation in the average time period. A summary of the study findings is displayed in Figure 3.
FIGURE 3.
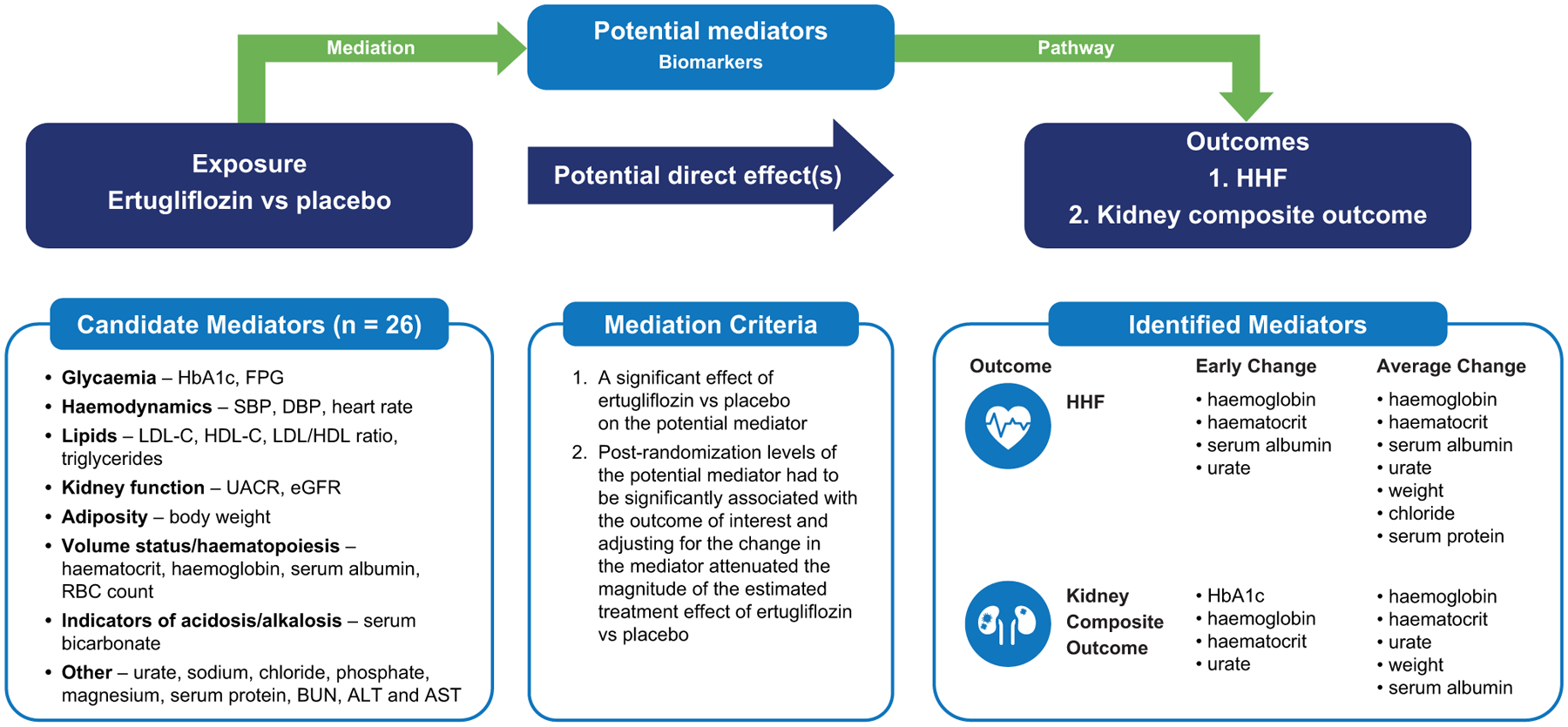
Criteria for mediation and possible mediators for effect of ertugliflozin on HHF and composite renal outcomes.
Adapted from JACC Heart Failure, Vol 8(1), Li, JW et al. Mediators of the Effects of Canagliflozin on Heart Failure in Patients with Type 2 Diabetes, pages 57–66, Copyright © 2020 with permission from Elsevier on behalf of the American College of Cardiology Foundation.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; HDL-C, high-density lipoprotein cholesterol; HHF, hospitalization for heart failure; LDL-C, low-density lipoprotein cholesterol; RBC, red blood cell; SBP, systolic blood pressure; UACR, urine albumin-to-creatinine ratio
4.1. Mediators of HHF
The present findings are largely consistent with existing literature. In prior mediation analyses from the CANVAS Trials Program, erythrocyte concentration, haemoglobin, and serum urate had the greatest modelled proportional mediation of effect for the SGLT2 inhibitor, canagliflozin, on HHF.20 The associations observed between changes in markers of intravascular volume status and/or haematopoiesis—including haemoglobin, haematocrit, and serum albumin—and improved HHF risk are consistent with prior observations.19,20,27 Additionally, there is evolving evidence that some of these biomarkers (such as haemoglobin and haematocrit) are not only markers of plasma volume, but also that of active erythropoiesis.28 Specifically, there is evidence that, as a consequence of SGLT2 inhibition, there is ensuing reduction in the highly active, oxygen-consuming Na+/K+ ATPase pump, which translates into reversal of the relative tissue hypoxia surrounding the proximal convoluted tubules.29 This is thought to restore the erythropoietin-producing capacity of the neighbouring fibroblasts and is supported by the findings of dapagliflozin dose-dependent increases in haematocrit, erythrocyte count, and reticulocyte count, contrasting with the lack of similar effects with other diuretics.28,30 Nonetheless, it remains unclear whether incremental RBC mass expansion across a “normal” range of haemoglobin would significantly alter the oxygen-carrying capacity of RBCs at the tissue level to achieve a clinically meaningful benefit, and long-term impact on erythropoeisis.19,31
The same markers (haemoglobin and haematocrit) were the strongest mediators of the effect of empagliflozin on cardiac death, based on the mediation analyses from the EMPA-REG OUTCOME trial.19 It is worth noting that death from worsening heart failure was only one of the components in the definition of cardiac death in the trial, which also included cardiac ischaemia, arrhythmias, sudden cardiac death, or if cause of death was unknown. This further suggests that the SGLT2 inhibitor class benefit may be derived from pleotropic effects with an interplay of different mechanisms. While there is evidence of association between hypoalbuminemia and worse heart failure outcomes32,33 and, conceptually, it seems plausible that haemoglobin/haematocrit and albumin might both reflect vascular volume, collinearity was not evident in the present analyses between the measurements, and changes in albumin remained statistically significant as a mediator in models adjusting for haemoglobin/haematocrit. Other potential mechanisms of SGLT2 inhibitors include improved myocardial energetics, as seen by ertugliflozin improving mitochondrial function, and resultant cardioprotection.34,35 Thus, the improvement in clinical outcomes with increased haemoglobin/haematocrit may be a consequence of improved cardiac performance, less congestion, and a more stable HF phenotype. Other hypotheses include SGLT2 inhibitors preventing hyperkalaemia thus allowing for higher doses of guideline-directed medical therapy (namely ACE-inhibitors, angiotensin receptor blockers, and mineralocorticoid receptor antagonists).36 Elevated serum urate levels have also been associated with heart failure and low ejection fraction,37–39 yet without a clear underlying causal relationship. One hypothesis is that serum urate is associated with increased oxidative stress that may result in myocardial fibrosis and left ventricular remodelling.40 However, the evidence is inconclusive on whether reductions in serum urate reduce heart failure events.41 It also remains unclear how changes in lipids, such as HDL-C, may mediate the effect of ertugliflozin on HHF, particularly because the early change in HDL-C had a robust association with HHF outcomes. This could be yet another marker of volume status and haemoconcentration, consistent with analyses from the CANVAS Program.20
4.2. Mediators of composite kidney outcomes
Similar to mediation effects of plasma volume markers on HHF, beneficial mechanisms can be extrapolated for the composite kidney outcome. In the present study, haemoglobin and haematocrit were the strongest mediators in the early and average change periods (plus albumin in the average change period). Another plausible mechanism of kidney benefit is the attenuation of kidney cortical hypoxia and improved trans-organ handling as a result of SGLT2 inhibition.17,29 Urate has also been implicated in the progression of kidney disease secondary to its pro-inflammatory effects as well as activation of the renin-angiotensin-aldosterone system, but there is no clear evidence on whether pharmacological reductions in serum urate can slow the progression of kidney disease.42–44 Notably, the largest mediating effect of the composite kidney outcome observed with the early change was improvement in HbA1c. However, beyond glycaemic effects, SGLT2 inhibitors have been shown to reduce the decline of eGFR in both diabetic and non-diabetic kidney disease, suggesting that the underlying effect extends beyond glucose control, and this remains an area for future research.13
4.3. Comparison of ertugliflozin versus canagliflozin mediators and renal composite outcomes
In contrast with what was observed in the CANVAS Program mediation analyses where UACR mediated 23.9% of the effect of canagliflozin on the composite kidney outcome, no mediation effect for UACR was observed in the present analyses on the kidney composite outcome.21 Notably, in the CANVAS Program analyses, the effect of UACR strongly differed based on baseline UACR, where it mediated 42% and 7% of the effect in those with UACR of ≥30 mg/g and <30 mg/g, respectively. These findings were limited by the low event rates in the low albuminuria subgroup. The lack of mediating effect in the present analyses cannot be fully explained, particularly in light of the fact that 40% of the VERTIS CV population had microalbuminuria or macroalbuminuria and previous observations of reduced UACR with ertugliflozin with larger reductions in albuminuria in those with baseline UACR ≥30 mg/g.16 The present findings of body weight being a mediator for improved kidney outcomes are consistent with prior evidence that non-surgical weight loss may be associated with stabilization of eGFR in patients with chronic kidney disease, albeit weight loss in the VERTIS CV trial was modest.45
4.4. Study strengths and limitations
The present analyses have several strengths including the well-conducted and large sample of the VERTIS CV trial, a large number of available biomarkers, and the standard statistical methods used for mediation assessment. However, the study is not without limitations. First, mediation analyses do not assess causation/mechanistic relations; rather, they demonstrate associations between changes in parameters of interest with treatment effects generating mechanistic hypotheses. Second, mediation could only be assessed for measured biomarkers, and inclusion of some unmeasured biomarkers, such as beta-hydroxybutyrate or natriuretic peptide levels, may have resulted in varying results. Additionally, direct effects, such as changes in mitochondrial function, may remain silent. Third, as ejection fraction was not systematically assessed and only available in a select subset with ejection fraction assessment prior to trial enrolment, we were unable to assess the difference in mediators between HF subtypes. Finally, the interaction between different mediators could not be fully assessed to evaluate the exact interplay that contributed to improved HHF and composite kidney outcomes. In the present study, the test for collinearity to reduce biological pathway overlap is likely conservative and, as evident by joint effects of mediators resulting in more than 100% of the explained effect, these mediators may have more mechanistic identity than we have accounted for. Additionally, despite a favourable overall hazard ratio, some biomarkers may be affected in an unfavourable direction. For example, in this study phosphate was identified as a biomarker that was significantly changed by the study intervention but is associated with worse HHF outcomes. The presence of such confounding effects makes it possible for the hazard ratio to cross unity when adjusted for mediators of benefit, which in turn makes it possible for the percent mediation of a given collection of mediators to exceed 100% (per equation in Statistical Analysis). Such an effect is also observed clinically with phosphate binders routinely used in those with decreased kidney function.
In conclusion, several potential mediators were identified that could mediate the effect of ertugliflozin on both HHF and composite kidney outcomes from the VERTIS CV trial. These findings are largely in agreement with prior mediation analyses and add to the body of evidence in an era of expanding indications for SGLT2 inhibitors. Further research is needed to better elucidate the underlying mechanistic processes that contributed to improved outcomes.
Supplementary Material
ACKNOWLEDGMENTS
A.A.K was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL125247. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. Editorial support was provided by Diane Hoffman, PhD, of Engage Scientific Solutions and was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and Pfizer Inc., New York, NY, USA. These analyses were presented at the European Society of Cardiology Congress 2021, The Digital Experience, August 27-30, 2021.
SOURCES OF FUNDING
Sponsored by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and Pfizer Inc., New York, NY, USA.
CONFLICTS OF INTEREST
M.W.S. and A.A.K declare no conflict of interest. C.P.C. has received research grants from Amgen, Better Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Janssen, Merck, Novo Nordisk, and Pfizer, as well as fees from Aegerion/Amryt, Alnylam, Amarin, Amgen, Applied Therapeutics, Ascendia, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Janssen, Lexicon, Merck, Pfizer, Rhoshan, and Sanofi; and serves on Data and Safety Monitoring Boards for the Veteran’s Administration, Applied Therapeutics, and Novo Nordisk. F.C. has received research grants from the Swedish Research Council, Swedish Heart & Lung Foundation, and King Gustav V and Queen Victoria Foundation; and has received consulting fees from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Merck Sharp & Dohme, Mundipharma, Novo Nordisk, and Pfizer Inc. S.D.-J has led clinical trials for AstraZeneca, Boehringer Ingelheim, and Novo Nordisk Inc.; has received consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Janssen, Merck & Co., Inc., and Sanofi; and has equity interests in Jana Care Inc. and Aerami Therapeutics. D.K.M. has received consulting fees from Afimmune, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Esperion, Lexicon, Lilly USA, Merck Sharp & Dohme, Metavant, Novo Nordisk, Pfizer Inc., and Sanofi US; has received honoraria from Boehringer Ingelheim; has received payment for expert testimony from Kirkland & Ellis on behalf of Boehringer Ingelheim; and has participated on data safety monitoring boards/advisory boards for CSL Behring, AbbVie, Eidos, Otsuka, Arena, and Akebia. R.E.P. has received grants (directed to his institution) from Hanmi Pharmaceutical Co., Ltd, Janssen, Metavention, Novo Nordisk, Poxel SA, and Sanofi; has received consulting fees (directed to his institution) from AstraZeneca, Corcept Therapeutics Incorporated, Glytec LLC, Hanmi Pharmaceutical Co., Ltd, Janssen, Merck & Co., Inc., Mundipharma, Novo Nordisk, Pfizer Inc., Sanofi, Scohia Pharma Inc., and Sun Pharmaceutical Industries; and has received support for attending meetings/travel (directed to his institution or to the travel provider) from AstraZeneca, Glytec LLC, Merck & Co., Inc., Mundipharma, Novo Nordisk, and Pfizer Inc. Am.P. has served on the advisory board of Roche Diagnostics and has received research support from the Texas Health Resources Clinical Scholarship, the Gilead Sciences Research Scholar Program, the National Institute of Aging GEMSSTAR Grant (1R03AG067960-01), and Applied Therapeutics. D.Z.I.C. has received consulting fees and/or speaking honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Merck & Co., Inc., Mitsubishi-Tanabe, Novo Nordisk, Prometic, and Sanofi; and has received operating funds from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck & Co., Inc., Novo Nordisk, and Sanofi. R.F. and N.B.C. are employees and shareholders of Pfizer Inc. J.L., C.L., and An.P. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may own stock and/or stock options in Merck & Co., Inc., Kenilworth, NJ, USA. M.M. is an employee of MSD Limited, London, UK, who owns stock in Merck & Co., Inc., Kenilworth, NJ, USA.
DATA ACCESSIBILITY
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information) Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programmes that have been terminated (i.e. development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
REFERENCES
- 1.Cavender MA, Steg PG, Smith SC Jr., et al. Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death: outcomes at 4 years from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Circulation. 2015;132(10):923–931. [DOI] [PubMed] [Google Scholar]
- 2.Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10(1):107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: a report From the American Heart Association. Circulation. 2020;141(9):e139–e596. [DOI] [PubMed] [Google Scholar]
- 4.Rawshani A, Rawshani A, Franzen S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379(7):633–644. [DOI] [PubMed] [Google Scholar]
- 5.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. [DOI] [PubMed] [Google Scholar]
- 6.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. [DOI] [PubMed] [Google Scholar]
- 7.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. [DOI] [PubMed] [Google Scholar]
- 8.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–1424. [DOI] [PubMed] [Google Scholar]
- 9.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. [DOI] [PubMed] [Google Scholar]
- 10.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–1461. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(Supplement 1):S111–S124. [DOI] [PubMed] [Google Scholar]
- 12.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur Heart J. 2019;41(2):255–323. [DOI] [PubMed] [Google Scholar]
- 13.Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. [DOI] [PubMed] [Google Scholar]
- 14.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. [DOI] [PubMed] [Google Scholar]
- 15.Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425–1435. [DOI] [PubMed] [Google Scholar]
- 16.Cherney DZI, Charbonnel B, Cosentino F, et al. Effects of ertugliflozin on kidney composite outcomes, renal function and albuminuria in patients with type 2 diabetes mellitus: an analysis from the randomised VERTIS CV trial. Diabetologia. 2021;64(6):1256–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6(2):148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7(11):845–854. [DOI] [PubMed] [Google Scholar]
- 19.Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care. 2018;41(2):356–363. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Woodward M, Perkovic V, et al. Mediators of the effects of canagliflozin on heart failure in patients with type 2 diabetes. JACC Heart Fail. 2020;8(1):57–66. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Neal B, Perkovic V, et al. Mediators of the effects of canagliflozin on kidney protection in patients with type 2 diabetes. Kidney Int. 2020;98(3):769–777. [DOI] [PubMed] [Google Scholar]
- 22.Cannon CP, McGuire DK, Pratley R, et al. Design and baseline characteristics of the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS-CV). Am Heart J. 2018;206:11–23. [DOI] [PubMed] [Google Scholar]
- 23.Cherney DZI, Dagogo-Jack S, McGuire DK, et al. Kidney outcomes using a sustained ≥40% decline in eGFR: A meta-analysis of SGLT2 inhibitor trials. Clin Cardiol. 2021;44(8):1139–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. [DOI] [PubMed] [Google Scholar]
- 25.Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. [DOI] [PubMed] [Google Scholar]
- 26.Hafeman DM. “Proportion explained”: a causal interpretation for standard measures of indirect effect? Am J Epidemiol. 2009;170(11):1443–1448. [DOI] [PubMed] [Google Scholar]
- 27.Packer M, Anker SD, Butler J, Filippatos G, Zannad F. Effects of sodium-glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA Cardiol. 2017;2(9):1025–1029. [DOI] [PubMed] [Google Scholar]
- 28.Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15(9):853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sano M, Goto S. Possible mechanism of hematocrit elevation by sodium glucose cotransporter 2 inhibitors and associated beneficial renal and cardiovascular effects. Circulation. 2019;139(17):1985–1987. [DOI] [PubMed] [Google Scholar]
- 30.Aberle J, Menzen M, Schmid SM, et al. Dapagliflozin effects on haematocrit, red blood cell count and reticulocytes in insulin-treated patients with type 2 diabetes. Sci Rep. 2020;10(1):22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawler PR, Liu H, Frankfurter C, et al. Changes in cardiovascular biomarkers associated with the sodium-glucose cotransporter 2 (SGLT2) inhibitor ertugliflozin in patients with chronic kidney disease and type 2 diabetes. Diabetes Care. 2021;44(3):e45–e47. [DOI] [PubMed] [Google Scholar]
- 32.El Iskandarani M, El Kurdi B, Murtaza G, Paul TK, Refaat MM. Prognostic role of albumin level in heart failure: A systematic review and meta-analysis. Medicine (Baltimore). 2021;100(10):e24785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, et al. Serum albumin concentration and heart failure risk The Health, Aging, and Body Composition Study. Am Heart J. 2010;160(2):279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Ropero A, Vargas-Delgado AP, Santos-Gallego CG, Badimon JJ. Inhibition of Sodium Glucose Cotransporters Improves Cardiac Performance. Int J Mol Sci. 2019;20(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Croteau D, Luptak I, Chambers JM, et al. Effects of Sodium-Glucose Linked Transporter 2 Inhibition With Ertugliflozin on Mitochondrial Function, Energetics, and Metabolic Gene Expression in the Presence and Absence of Diabetes Mellitus in Mice. J Am Heart Assoc. 2021;10(13):e019995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neuen BL, Oshima M, Perkovic V, et al. Effects of canagliflozin on serum potassium in people with diabetes and chronic kidney disease: the CREDENCE trial. Eur Heart J. 2021;42(48):4891–4901. [DOI] [PubMed] [Google Scholar]
- 37.Bhole V, Krishnan E. Gout and the heart. Rheum Dis Clin North Am. 2014;40(1):125–143. [DOI] [PubMed] [Google Scholar]
- 38.Huang H, Huang B, Li Y, et al. Uric acid and risk of heart failure: a systematic review and meta-analysis. Eur J Heart Fail. 2014;16(1):15–24. [DOI] [PubMed] [Google Scholar]
- 39.Borghi C, Cosentino ER, Rinaldi ER, Cicero AF. Uricaemia and ejection fraction in elderly heart failure outpatients. Eur J Clin Invest. 2014;44(6):573–578. [DOI] [PubMed] [Google Scholar]
- 40.Muiesan ML, Agabiti-Rosei C, Paini A, Salvetti M. Uric acid and cardiovascular disease: an update. Eur Cardiol. 2016;11(1):54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Givertz MM, Anstrom KJ, Redfield MM, et al. Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: the Xanthine Oxidase Inhibition for Hyperuricemic Heart Failure Patients (EXACT-HF) study. Circulation. 2015;131(20):1763–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang YH, Lei CC, Lin KC, Chang DM, Hsieh CH, Lee YJ. Serum uric acid level as an indicator for CKD regression and progression in patients with type 2 diabetes mellitus-a 4.6-year cohort study. Diabetes Metab Res Rev. 2016;32(6):557–564. [DOI] [PubMed] [Google Scholar]
- 43.Badve SV, Pascoe EM, Tiku A, et al. Effects of allopurinol on the progression of chronic kidney disease. N Engl J Med. 2020;382(26):2504–2513. [DOI] [PubMed] [Google Scholar]
- 44.Doria A, Galecki AT, Spino C, et al. Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N Engl J Med. 2020;382(26):2493–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Navaneethan SD, Yehnert H, Moustarah F, Schreiber MJ, Schauer PR, Beddhu S. Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4(10):1565–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information) Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programmes that have been terminated (i.e. development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.


