Abstract
The Neuronal Membrane Glycoprotein M6B (Gpm6b) gene encodes a membrane glycoprotein that belongs to the proteolipid protein family, and is enriched in neurons, oligodendrocytes, and subset of astrocytes in the central nervous system. GPM6B is thought to play a role in neuronal differentiation, myelination, and inactivation of the serotonin transporter via internalization. Recent human genome-wide association studies (GWAS) have implicated membrane glycoproteins (both GPM6B and GPM6A) in the regulation of traits relevant to psychiatric disorders, including neuroticism, depressed affect, and delay discounting. Mouse studies have implicated Gpm6b in sensorimotor gating and regulation of serotonergic signaling. We used CRISPR to create a mutant Glycoprotein M6B (Gpm6b) allele on a C57BL/6J mouse background. Because Gpm6b is located on the X chromosome, we focused on male Gpm6b mutant mice and their wild-type littermates (WT) in two behavioral tests that measured aspects of impulsive or flexible decision-making. We found that Gpm6b deletion caused deficits in delayed discounting, as measured by the delay discounting task. In contrast, reward sensitivity was enhanced thereby facilitating behavioral flexibility and improving performance in the probabilistic reversal learning task. Taken together these data further delineate the role of Gpm6b in decision making behaviors that are relevant to multiple psychiatric disorders.
INTRODUCTION
Glycoprotein M6B (GPM6B) is a transmembrane protein that is crucial for regulating numerous brain functions (Yan et al., 1993; Möbius et al., 2008). Gpm6b is ubiquitously expressed throughout the brain and is most abundant in neurons, oligodendrocytes, and a subset of astrocytes of the central nervous system (Choi et al., 2013). GPM6B belongs to the proteolipid protein (PLP) family (Yan et al., 1993; Möbius et al., 2008), and is thought to be involved in myelination and cellular housekeeping functions, such as membrane trafficking, cell-to-cell communication (Drabek et al., 2011), axon growth and guidance (Mita et al., 2015) and stress response (Fernández et al., 2010). GPM6B has also been reported to reduce the activity of the serotonin transporter (SERT) by down-regulation of transporter surface expression (Fjorback et al., 2009).
Perturbations of GPM6B have been associated with a variety of phenotypes and disorders, all of which have well-established links to serotonergic signaling. For example, Gpm6b mutant mice exhibited reduced prepulse inhibition (PPI) and an altered response to the 5-HT2A/C agonist DOI (Dere et al., 2015). GPM6B was one of the most strongly downregulated genes identified in the hippocampus and the prefrontal cortex from post-mortem brains of suicide victims compared to controls (Fuchsova et al., 2015). More recently, a genome-wide association study (GWAS) of delay discounting identified a significant association between GPM6B and delay discounting, a measure of impulsive decision-making (Sanchez-Roige et al., 2018). Delay discounting has been implicated in various aspects of suicidal ideation and family history of suicide behavior (McHugh et al., 2019; Gifuni et al., 2020).
Despite the evidence implicating Gpm6b in impulsive tendencies and psychopathology that is associated with deficits in inhibition or decision-making in humans, the influence of Gpm6b on these behavior remains unknown. To follow up on these observations, we created a mutant allele of Gpm6b on a C57BL/6J background and assessed measures of impulsive or flexible decision-making. In particular, we evaluated both delay discounting (DD, (Mitchell, 2014) and probabilistic reversal learning (PRL, (Ineichen et al., 2012). Both of these behaviors are modulated by serotonergic signalling in mice (e.g., (Brigman et al., 2010; Ineichen et al., 2012; Mori et al., 2018; Phillips et al., 2018) and have been associated with suicide in humans (Dombrovski et al., 2010; McHugh et al., 2019; Gifuni et al., 2020).
MATERIALS AND METHODS
Establishment of a Gpm6b mutant mouse line using CRISPR/Cas9
All animal procedures were consistent with guidelines from the National Institutes of Health and the Association for the Assessment and Accreditation of Laboratory Animal Care and approved by the University of California San Diego Institutional Animal Care and Use Committee.
As previously described (Zhou et al., 2019), we followed the Jackson Labs (JAX) protocol for microinjection of clustered regularly interspaced short palindromic repeats (CRISPR) mix using sgRNA and Cas9 mRNA (https://www.jax.org/news-and-insights/1998/july/superovulation-technique). We designed a sgRNA targeting exon 3 (out of 11) of Gpm6b (Vector Name: pRP[CRISPR]-hCas9-U6>{20nt_GCCACCATCCTATGTTTCTC}; Supplementary Figure 1). Exon 3, which is present in RefSeq supported transcripts for both coding and non-coding protein variants, contains coding regions for transmembrane domains and/or a coiled coil structural motif, both of which are important in cellular interaction/trafficking.
The CRISPR microinjection procedures were performed at the University of California San Diego, Moores Cancer Center, Transgenic Mouse Core. We ordered five C57BL/6J stud males (7–8 weeks old) and five C57BL/6J females (3–4 weeks old) from the Jackson Laboratory (Bar Harbor, ME). We selected 6J mice because they are more impulsive than other strains. Upon arrival at the vivarium, the stud males were single-housed and the females were housed in groups of four. On Day 1 of the microinjection week, all five females were super-ovulated via 0.1ml pregnant mare serum intraperitoneal injection per animal. On Day 3, all females were super-ovulated via 0.1ml human chorionic gonadotropin intraperitoneal injection per animal. After hormonal priming, each female was placed into the home cage of one stud male for mating. On Day 4, fecundation was expected to occur, and females were separated from the stud males. The fallopian tubes were dissected out from the mated females and were collected in M2 medium. Zygotes were harvested and microinjected with the CRISPR mix (625ng = 3.1ul×200ng/ul of Gpm6b sgRNA + 1250ng = 5ul×250ng/ul of Cas9 mRNA + 17.6ul ph7.5 IDTE; total volume 25ul). Injected zygotes were surgically transplanted to pseudopregnant female C57BL/6JOlaHsd (Harlan) mice. Pregnant surrogate dams were singly caged one week before the expected birth date of the pups. Cesarian sections were carried out when necessary.
Gpm6b mutant line breeding, genotyping scheme and qRT-PCR
For ease of propagating mutant alleles, we focused on male founder offspring. We obtained 7 Gpm6b CRISPR male founders. The founders were genotyped via Sanger sequencing to verify the presence of deletions. The male founders were then backcrossed to wildtype C57BL/6J mice to minimize the effect of off-targeting. F1/F2 mutant mice were genotyped via Sanger/NGS to ensure the transmission of the mutant allele. Heterozygous F1s were paired to produce F2s, which were genotyped via next-generation sequencing.
We identified a number of ‘founder’ mutations in the offspring, including one individual with a 79bp deletion. Genotyping was performed using the following primers CGGAGCCTATGGAAAAACGC (forward) and CAGATCCGGTTCTTCCGTCT (reverse; all sequences are shown in the 5’ to 3’ orientation); amplicons were separated on a 1% agarose gel, stained with ethidium bromide and scored based on their length.
We measured Gpm6b expression levels in brain tissue using quantitative RT-PCR (N=8; 4 mice/group). RNA was extracted from prefrontal cortex tissue using RNAEasy kit (Qiagen, Cat: 74904). cDNA was prepared from 1 microgram of RNA using Invitrogen™ SuperScript™ VILO™ cDNA Synthesis Kit (Invitrogen, Cat: 11754050) according to the manufacturer’s protocol. Gpm6b expression levels of exons 4–6, which are downstream of the 79 bp deletion, were measured using Taqman probes spanning exons 4–5 (ThermoFisher, Assay ID: Mm00499158_m1) and exons 5–6 (ThermoFisher, Assay ID: Mm00499159_m1). The probe for housekeeping gene beta-actin, Actb (ThermoFisher, Assay ID: Mm04394036_g1) was used for normalization. The Taqman reaction was carried out using TaqMan™ Fast Advanced Master Mix (Invitrogen, Cat: 4444556) according to the manufacturer’s protocol, using StepOnePlus instrument (ThermoFisher, RRID:SCR_015805). Expression levels were calculated as deltaCt between Gpm6b and Actb thresholds. The expression of Gpm6b at exons 2–4 was measured using custom-designed primers. The amplification was carried out using PowerTrack™ SYBR Green Master Mix (Applied Biosystems, Cat: A4601).
Experimental timeline
Three separate cohorts of male mice were used for these studies; the first cohort (n=16) was used to examine delay discounting and reversal learning behaviors; the second cohort (n=18) was used to examine general locomotion and anxiety-like behavior; the final cohort was used to measure PPI (n=21). Mutant and WT littermates were used for each cohort. The mice in the first cohort were food restricted to reduce their body weights to 90% of their free-feeding weight and kept under food restriction until the end of the experiments. The second and third cohorts were not food restricted. All three cohorts had ad libitum access to water throughout the study. Behavioral testing took place between 8:00 and 4:00 PM, 5 days a week, on a 12h/12h light-dark cycle with lights on at 0600 h. The animals were between 3–5 months at the time of the experiments.
DD and PRL: Apparatus
Training and testing for both DD and PRL was conducted in eight mouse operant boxes (Med Associates, St Albans, VT) contained within light- and sound-attenuating chambers. Retractable response levers were located on the front wall on either side of a recessed reward port. A peristaltic pump (Lafayette Instrument, IN) was used to deliver liquid reinforcement (strawberry Nesquik©) which was presented along with the illumination of an LED. The rear wall contained an array of 5 nose-poke response apertures but were inactive. The apparatus was controlled by a PC running MedPC software (Med Associates).
Basic Training Sessions
The same Basic Training session was used for both DD and PRL, as described below. The training procedure was adapted from Isles et al (2003, 2004). Mice were habituated to the operant chamber for two days, where liquid reinforcement was non-contingently delivered every 20 sec over the course of a 20-min session. Reward delivery was paired with the illumination of the reward port light, which was extinguished when the mouse collected the reward. Mice were then trained to lever press for a reward. A single lever was extended into the chamber and a single lever press resulted in the lever retracting, illumination of the reward port light, and delivery of the liquid reward. Levers were presented in a pseudo-random order. Mice were trained in this procedure until they made >90 responses over two consecutive days.
Delayed Discounting
We used the DD task to examine impulsive choice behavior by determining the preference for a smaller yet immediately available reward versus a larger reward that was delivered after a delay. One lever was associated with a large reward (strawberry Nesquik©, 120 uL), while the remaining lever was associated with a smaller reward (strawberry Nesquik©, 40 uL). The lever associated with the large reward was assigned at the start of testing (counterbalanced between subjects) and remained consistent for the duration of the experiment. The session began with six forced trials, where a single lever was presented in a pseudo-random order. After the forced trials, there were ten choice trials where both levers were presented, and mice could choose between the lever associated with smaller vs. larger reward. This block of 16 trials (6 forced and 10 choice) was presented twice for each delay. A response on either lever resulted in both levers immediately retracting. If the small reward lever was chosen, the reward is immediately delivered. Selecting the larger reward lever resulted in the reward being delivered after a delay period had elapsed. For the first block of 32 trials, the delay associated with the larger reward was 0 s but increased with subsequent trial blocks (1, 4, 8, 16 s). Mice were tested in the DD task daily until the preference for the large lever in the initial block of trials was at least 80% (approximately 7 days). Each session ended after 160 trials (e.g., 5 × 32 trial blocks) or 60 mins, whichever occurred first. Each trial was initiated automatically after a variable inter-trial interval (ITI, 4.5, 5, or 5.5 s), and mice could respond within a 45 s limited hold period. Failure to respond during the limited hold period was recorded as an omission and resulted in both levers retracting and the illumination of the house light for an 8 s timeout period.
The primary outcome variable for this task was the preference for the large reward lever for each trial block (the number of large lever presses divided by total responses multiplied by 100). Additional measures relating to general motoric responses and motivation were collected, including the number of omitted trials (no lever response during the limited hold period) or the latency to make a response after lever presentation.
Probabilistic reversal learning
After DD, mice were moved back to the basic training session for several days; in those sessions alternating levers were presented, which delivered a single, identical reward. Mice were then tested in the probabilistic reversal learning (PRL) task to examine flexible decision-making. At the start of the PRL session, one lever was randomly assigned as the target lever and the other lever was the non-target lever. Target responses were mostly (80% of trials) rewarded but occasionally (20% of trials) the target response delivered misleading negative feedback. In contrast, non-target responses were mostly (80%) non-rewarded but occasionally (20%) resulted in the delivery of misleading rewards. Trials were interspersed by a variable 4.5–5-5.5 sec ITI and non-rewarded outcomes resulted in a 4 sec time-out period during which the house-light was illuminated. Failure to respond within the limited hold period (5 sec) was considered an omission and resulted in a time-out period. After 120 trials, the reward contingencies reversed such that the lever that was previously the target lever became the non-target lever and vice versa. The session consisted of a total of 240 trials or 60 min, whichever came first. Primary outcomes included in the analysis were total number of target responses; accuracy (target vs. non-target); number of trials to discrimination; win-stay responses (proportion of rewarded responses repeated on next trial); and lose-shift responses (proportion of non-rewarded responses avoided on next trial).
Open field testing and apparatus
The OF test was administered to measure locomotor activity, as previously described (Williams et al., 2009; Distler et al., 2012; McMurray et al., 2016; Barkley-Levenson et al., 2018). Mice were moved into the testing room and allowed to acclimate for at least 30 minutes prior to testing. Testing took place between 10:00 and 12:00 h. Mice were placed in the center of a square chamber (43 × 43 × 33 cm; the size of the center was 26 × 16 cm), with dim overhead lighting inside of sound- and light-attenuating boxes, and allowed to freely explore for 30 minutes. A grid of infrared detection beams in each chamber and Versamax software was used to track animal location and locomotor activity (distance traveled) during the test. We also recorded the time spent in the center zone as a measure of anxiety-like behavior. The chambers were wiped down with a solution containing 30% ethanol between each animal to eliminate odors.
Measures taken were entries into the center, total locomotor activity (number of line crossings) and time spent in the center square (central zone). Number of rearings and defecations were also measured (data not shown).
Elevated Plus Maze, Light-dark box and apparatus
In order to assess the role of Gpm6b in anxiety, we tested WT and mutant mice in two well-established tests of anxiety-like behavior, the elevated plus maze (EPM) test (Dawson and Tricklebank, 1995) and the light-dark (LD) box test (Bourin and Hascoët, 2003). We have previously described the testing apparatus and procedures for LD and EPM (Distler et al 2012).
Immediately after the OF testing, each animal was placed in the centre of the EPM facing one of the open arms and allowed to explore the apparatus freely for 5 minutes. The EMP (Stoelting) consisted of 2 open arms (35 cm long × 5 cm wide) and 2 closed arms (35 cm long × 5 cm wide × 15 cm high) forming the shape of a cross. The apparatus was made from black Perspex and was elevated 40 cm above the ground. The room was illuminated with dim light and the open arms of the maze were under illumination of 15 lux. Measures taken included time spent in the open and closed arms and entries into the open and closed arms. An entry was defined as placing all four paws within the given arm.
One day after EPM, each animal was placed in the LD box. The LD boxes were white plastic testing chambers (40 × 40 × 30 cm) containing a black plastic box insert (20 × 20 × 30 cm) connected by a shuttle door located in the centre of the partition at floor level (Sittig et al., 2016). The light box was open at the top and illuminated with bright fluorescent light (~500 lux). The dark box had a removable black lid at the top that blocked all light from entering. Mice were placed at the center of the illuminated compartment, and the animal was allowed to freely explore both compartments for 5 minutes. Total number of crossings between the two compartments (defined the placement of all four paws in a given compartment), latency to enter into the dark compartment, latency to enter the illuminated compartment after the first entry into the dark box, time spent in both compartments and total defecations in the apparatus were measured. Number of head entries towards both compartments (attempts to enter into the adjacent compartment) and rearings in the illuminated compartment were also measured (data not shown).
Both the EPM and LD box were cleaned with a solution containing 30% ethanol after each 5 minute run and wiped dry before the next test.
In the EPM and LD the movement of each animal was recorded using a video camera (Sony SPT- M108CE) connected to a recorder to allow subsequent analysis, and scored by a trained human observer.
PPI
The PPI apparatus, testing procedures, and data analysis were conducted as previously described (Samocha et al., 2010; Sittig et al., 2016). Animals were placed in a 5 cm diameter plexiglas cylinder on a platform contained within a lighted, ventilated chamber (San Diego Instruments, San Diego, CA, USA). The cylinder was connected to a piezoelectric accelerometer which measured the mouse’s startle responses. Mice experienced 5 min of 70 dB white noise followed by 62 trials which occurred with the 70 dB noise in the background. Testing consisted of pulse-alone trials (40 ms, 120 dB burst); no-stimulus trials; and prepulse trials (20 ms prepulse, 3, 6, or 12 dB above background noise) followed 100 ms later by a 40 ms, 120 db pulse. Trials were arranged into four blocks. Blocks 1 and 4 were pulse alone trials; blocks 2 and 3 contained pseudo-random combinations of pulse alone, no stimulus, and each type of prepulse trial (3, 6, and 12 dB). Responses were recorded for 65 ms after the beginning of the 120 dB stimulus. The ITI was 9–20 s (average 15 s) throughout the test. The acoustic startle response was the average startle amplitude (SA) measured in the pulse-alone trials in testing blocks 2 and 3.The prepulse inhibition (PPI) phenotype was defined as the difference of the average startle amplitude during the 6-db prepulse trials and the average startle amplitude during the pulse-alone trials, normalized by the pulse-alone startle amplitude: PPI = (SApulse – SAprepulse) / SApulse.
Statistical analyses
Statistical analysis was performed using the ‘Statistical Package for Social Sciences’ (SPSS, version 14.0). OFT, EPM, LD data were assessed for normality and then analyzed by Student’s t-test. Data from the DD and PPI was analyzed using mixed analysis of variance (ANOVA), with between-subject factors of genotype (mutant vs. WT), and within-subjects factor of delay (0, 1, 2, 8, and 16 sec) for DD, and within-subjects factor of PP (3, 6, 12) for PPI. Data from the PRL task were analyzed with ANOVA with genotype as a between-subject factor and stage (pre- or post-reversal) or trial block as a within-subject factor. Post-hoc comparisons were conducted using Fisher’s-LSD test to adjust for multiple comparisons. Mice that differed by more than 2 standard deviations from the mean were excluded (2 WT outliers were removed from the PPI analyses). Paired t-tests were used for post hoc comparisons. A p<0.05 was required for results to be considered statistically significant. All testing and analyses were performed with the researchers blind to the condition.
RESULTS
Creation and characterization of mutant Gpm6b allele using CRISPR/Cas9
We generated the mutant alleles on a C57BL/6J background. We selected a mutant mouse line that carried a 79bp frameshifting deletion in exon 3 of Gpm6b (Figure S1).
We characterized expression levels of the Gpm6b gene in prefrontal cortex tissue using quantitative RT-PCR (Supplementary Figure S2). Our results showed that deletion of 79 bp in exon 3 did not alter expression of the remaining transcript. Expression measured by a Taqman probe spanning exons 4 and 5, and by a probe spanning exons 5 and 6, was similar in WT and KO animals (Supplementary Table S1). The transcripts NM_001177956.1, NM_001177962.1, NM_023122.3 that contain exons 4–6 also contain exon 3. We also demonstrated that mRNA from KO mice had a deletion in exon 3, as expected. Primers in exons 2 and 4 produced the expected shortened product in KO mice, compared with WT mice, consistent with the deletion in exon 3. A second set of primers in exon 3 downstream of 79 bp deletion, and in exon 4, produced identical products in KO and WT mice, as expected (data not shown). Because part of exon 3 downstream of the deletion was not missing, the 79bp deletion in KO mice will cause a frameshift and will not produce a functional protein.
Gpm6b mutant mice make more impulsive choices
Across all mice, increasing the delay to the larger reward decreased the likelihood of choosing that reward (Figure 1B; main effect of delay, F(4,64)=211.399, p<0.0001). Mutant mice preferred the immediate reward relative to the WT littermate mice (main effect of genotype, F(1,16)=5.788, p=0.028). However, we did not detect a significant interaction between genotype and delay (F(4,64)=1.140, p=0.34), indicating that mutant mice did not increasingly diverge from WT mice as the delay increased. However, analysis of the latency to choose a lever did reveal a significant genotype by delay interaction (Figure 1C; F(4,64)=3.771, p<0.01). This interaction was driven by the increased response latency evident in WT but not mutant mice during the 4, 8, or 16 s delay relative to the 0 s delay (p<0.05 for WT; p>0.39 for mutant). There was no difference in the number of omissions between genotypes (F(1,16)=0.557, p=0.466). Moreover, omissions were not affected by delay (F(1,16)=0.994, p=0.417) or influenced by an interaction between genotype and delay (F(4,64)=0.906, p=0.465).
Figure 1. Effects of a mutant allele of Gpm6b on delay discounting.
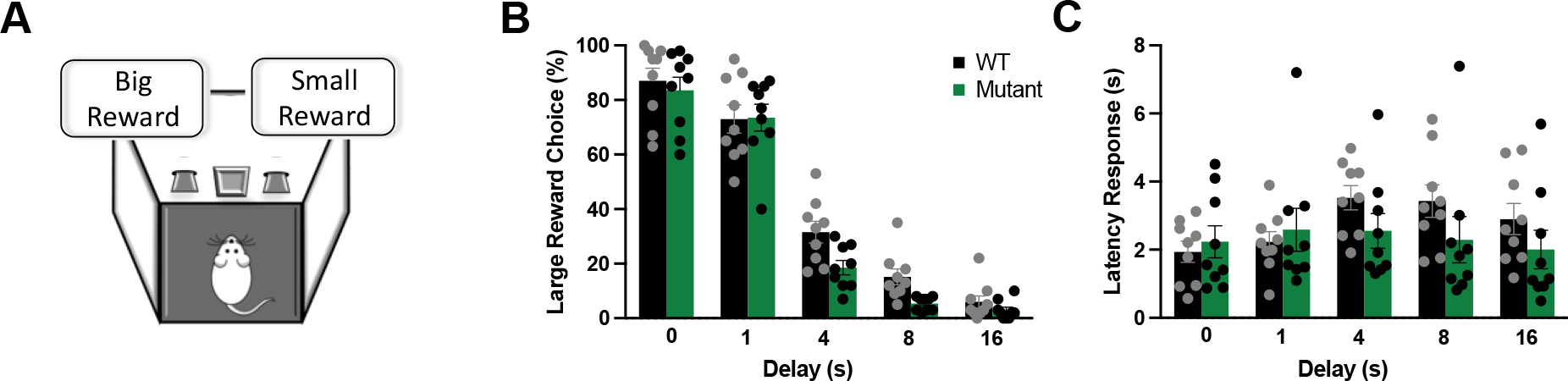
(A). Mutant mice were more intolerant to delayed discounting, as revealed by the preference towards choosing smaller but immediate rewards (B), and decreased latency to make a response across delays (C). Data expressed as mean ± SEM (n=16, 8/group), along with individual values.
Gpm6b mutant mice are less sensitive to reversal-induced performance impairments
Male WT and mutant mice were tested in a PRL task. Plotting the cumulative number of target or non-target responses made throughout the session revealed that, during the first stage of the session (i.e., pre-reversal), both groups of mice readily developed a preference for the target lever and the cumulative number of target responses increased. After the reversal, mice made fewer target responses and consequently the cumulative number of non-target responses began to increase in both genotypes. However, approximately 60 trials after the reversal occurred, MUTANT mice began selecting the new target lever more frequently, relative to WT littermates (Figure 2B). Analysis of the cumulative target responses averaged across blocks of 60 trials revealed a Genotype × Trial Block interaction [F(3,48)=3.92, p<0.05] (Figure 2C). While there was no difference in cumulative target responses between genotypes in block 1, 2, or 3 (all ps >0.84), this measure was significantly reduced in WT mice during the last block of 60 trials (p<0.05).
Figure 2. Mutant allele of Gpm6b decreased sensitivity to reversal-induced performance impairments in the probability reversal learning task.
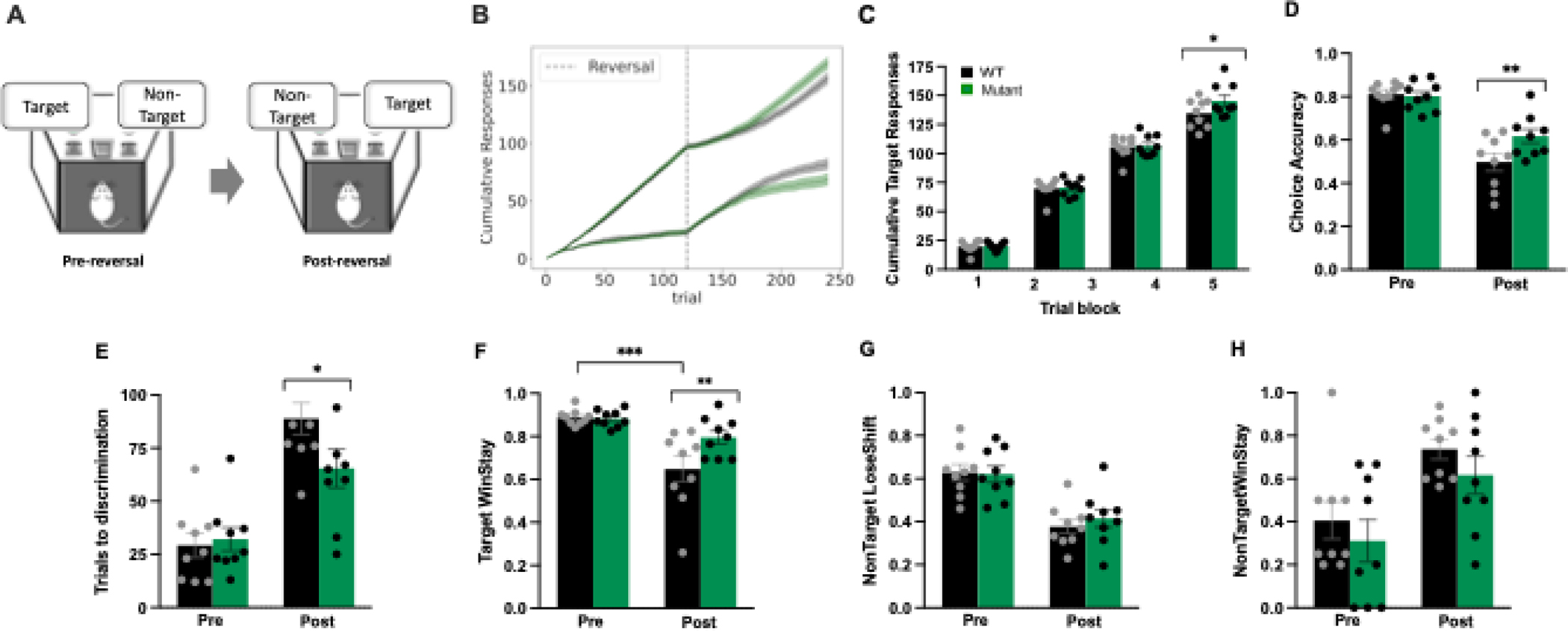
(A). After the reversal occurred, mutant mice selected the target lever more frequently, relative to WT littermates (B). Cumulative target responses post reversal were higher in mutant mice, WT mice showing significantly reduced responses during the last block of 60 trials (C). During post-reversal, reduction in accuracy was more pronounced in WT mice, relative to mutant mice (D). Higher number of trials were required by the WT mice to shift their preference to the new target lever after the reversal had occurred (E). After the reversal, mutant mice had a greater tendency to repeat rewarded target responses compared to WT mice (F). While the reversal had a significant impact on target lose-shift, non-target lose-shift, and non-target win-stay responding, there was no main effect of genotype for any of these measures (G-H). *** p>0.001, ** p>0.01, * p>0.05
Analysis of the choice accuracy before and after the reversal revealed a Genotype × Stage interaction [F(1,16)=5.19, p<0.05] (Figure 2D). There was no difference in accuracy between genotypes before the reversal (p=0.83). Relative to the first stage of the session, the reversal of the reward contingencies significantly reduced choice accuracy in both WT (p<0.001) and mutant mice (p<0.001). However, the post-reversal reduction in accuracy was more pronounced in WT mice, relative to mutant mice (p<0.01). Next, we determined how many trials were required to make eight consecutive target responses (often used as an index of target vs. non-target discrimination). The Genotype × Stage interaction [F(1,16)=4.35, p=0.05] (Figure 2E) was also mediated by no difference between genotypes in trials required to identify the target response before the reversal (p=0.77) but a significantly greater number of trials required by the WT mice to shift their preference to the new target lever after the reversal had occurred (p<0.05).
To delineate the behavioral mechanisms underlying these effects, we calculated the win-stay and lose-shift ratios for both target and non-target responses. A Genotype × Stage interaction emerged when target win-stay responding was analyzed [F(1,16)=4.35, p=0.05] (Figure 2F). Consistent with the effects we observed with choice accuracy and trials to discrimination, there was no difference in target win-stay responses between genotypes before the reversal (p=0.90). However, after the reversal target win-stay responses were increased in mutant mice, relative to WT mice (p<0.01). Indeed, in response to the reversal of reward contingencies, this measure was unaffected in mutant mice (pre vs. post; p=0.12) but significantly reduced in WT mice (pre vs. post; p<0.001). While the reversal had a significant impact on target lose-shift [F(1,16)=22.41, p<0.001], non-target lose-shift [F(1,16)=30.21, p<0.001], and non-target win-stay responding [F(1,16)=15.01, p<0.001], there was no main effect of Genotype (all ps >0.21) or interaction between Genotype and Stage (all ps>0.3) for any of these measures (Figure 2G–H). Finally, these behaviors were not associated with motivational changes or non-specific performance alterations as the number of omissions made within a session was minimal (WT, 1.67 ± 0.37; mutant, 1.44 ± 0.56) and was no different between Genotype [F(1,16)=0.11, p=0.74].
Locomotion and anxiety-like behaviors
Figure 3 shows that there were no differences between the WT and mutant mice in the OF, EPM or LD tests. For the OF, the total amount of distance travelled in the boxes was similar across the groups [t(15)=0.951, p>0.05; Figure 3A], indicating normal locomotor activity. The distance travelled in the center, sometimes taken as a measure of anxiety-like behavior, was also consistent across the two groups [t(15)=0.671, p>0.05; Figure 3B]. The number of defecations did not differ [t(15)=−0.277, p>0.05; Figure 3C].
Figure 3. Mutant allele of Gpm6b did not modify locomotor activity in the Open Field (A-C), anxiety-like behavior in the elevated plus maze (D-F), or the light-dark box (G-J).
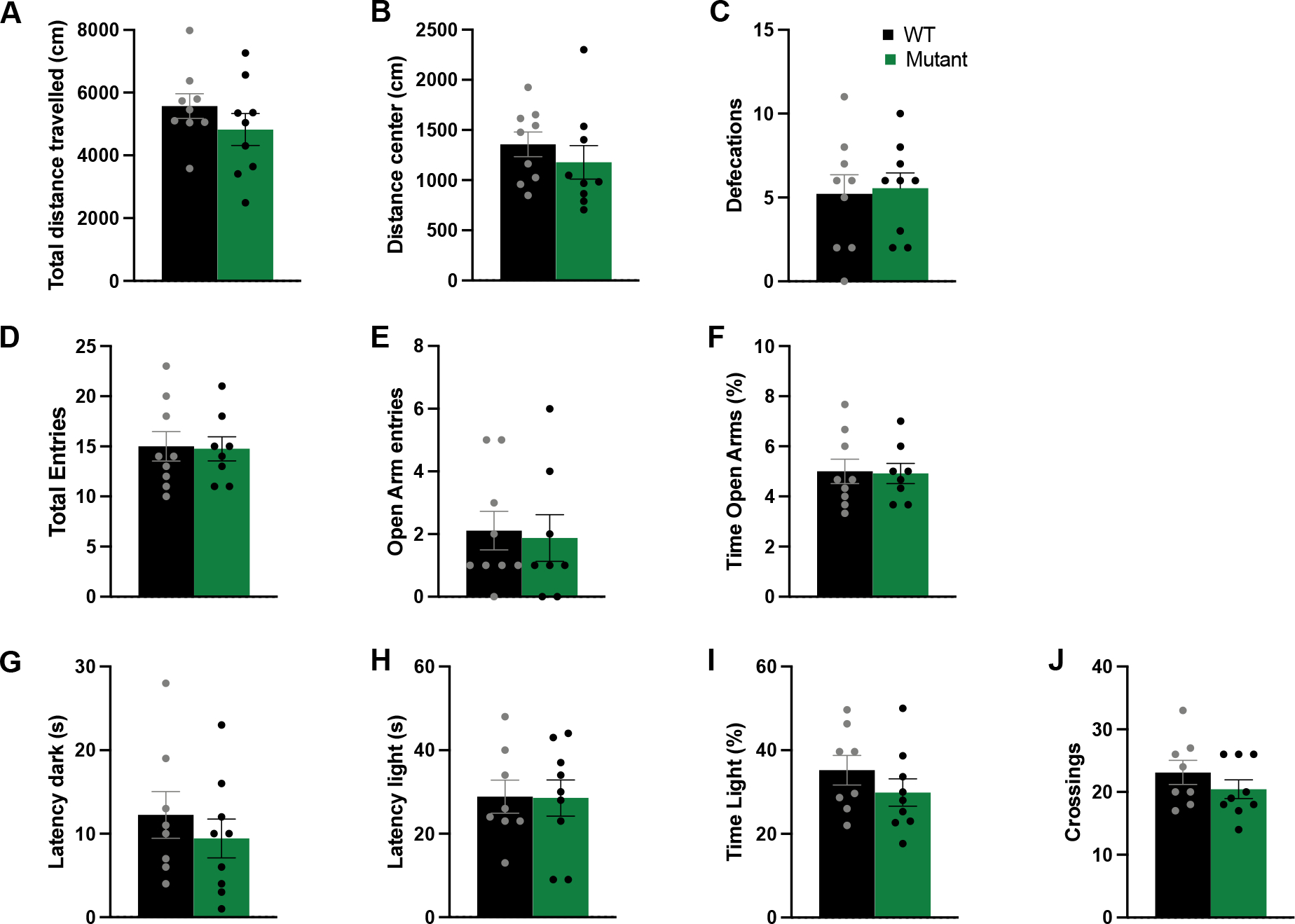
Both WT and mutant mice travelled a similar total distance in the apparatus (A), spent equal time in the center (B) and displayed a similar number of defecations (C) during the Open Field task. Similarly, both WT and mutant mice displayed similar numbers of total (D) and open arm entries (E), and spent an equal percentage of time in the open arms (F) as measured in the Elevated Plus Maze. Lastly, latency to enter into the dark compartment (G) or to re-emerge to the light compartment (H) was equal between WT and mutant mice, as it was the percentage of time spent in the light (I) and the total number of crossings between compartments (J) in the Light-Dark box. Data expressed as mean ± SEM (n=18, 8–9/group), along with individual values.
In the EPM, WT and mutant mice did not differ in the total number of arm entries [t(15)=−0.253, p>0.05], again indicating normal locomotor activity (Figure 3D). Mutant mice made an equal number of entries into the open arms [t(15)=−0.498, p>0.05], and spent the same amount in the open arms [t(15)=−0.253, p>0.05], compared with WT mice (Figure 3E–F).
In the LD box test, mutant mice spent an equal amount of time in the light compartment compared with WT mice [t(15)=1.104, p>0.05; Figure 3I]; mutant mice consistently performed the same as WT mice across all the other measures in this task [t(15)<1.118, p>0.05; Figure 3G–J]. Together, these data demonstrate that suppression of Gpm6b does not modify anxiety-like behavior in these tasks.
Prepulse inhibition
As shown in Figure 4, PPI response increased over prepulses across all mice [main effect of PPI: F(2,38)=50.406, p<0.001]. Although there were no significant genotype by PPI intensity interactions [F(2,38)=0.891, p>0.05], or main genotype effects [F(2,38)=1.912, p>0.05], we observed a non-significant tendency for mutant mice to have lower in prepulse inhibition at pp6 [t(19)=1.65, p=0.069; pp3, pp12, p>0.05].
Figure 4. Effects of mutant allele of Gpm6b on sensoriomotor gating.
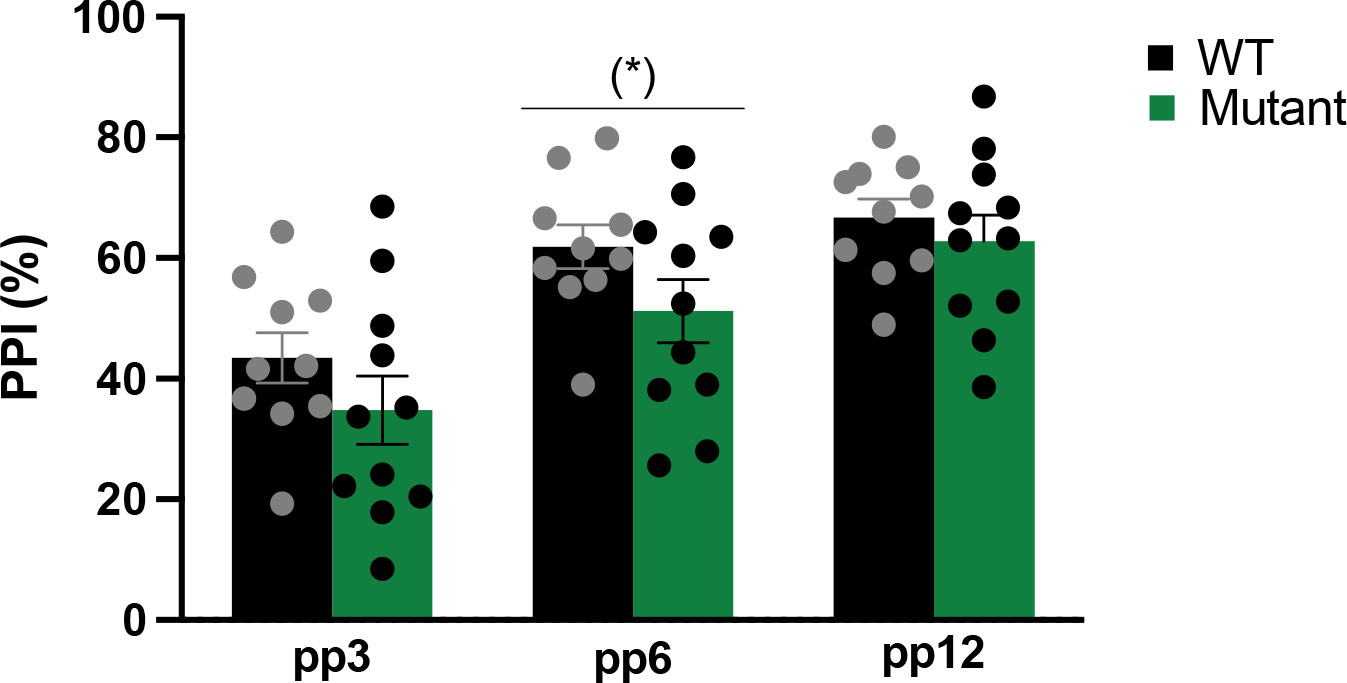
Mutant mice showed a tendency for decreased PPI of the acoustic startle reflex (pp6). Prepulse inhibition (PPI) response increased over pp across all mice. Data expressed as mean ± SEM (n=21, 10–11/group, 2 outliers were removed from the WT group), along with individual values. (*) p=0.069, pp prepulse intensity.
DISCUSSION
Glycoprotein M6B (GPM6B), which is known to be involved in serotonergic neurotransmission, has been identified as a candidate gene for traits that involve aspects of impulse control in humans. In the present study, we used two behavioral paradigms to assess the role of Gpm6b on impulsive or flexible decision-making in mice. We found that mutant mice exhibited an elevation in impulsive-like behaviors in the DD task, exemplified by an increased preference for the smaller reward which was immediately available. In contrast, mutant mice exhibited an enhancement in flexible decision-making. Performance in the PRL task was unaffected prior to the reversal. However, after the reward contingencies reversed, mutant mice were better able to adjust their responding to the correct response lever; an effect that was predominantly driven by increased win-stay responding.
GPM6B has been suggested to regulate SERT by affecting cellular trafficking of the protein away from the cell membrane in a HEK-293 cell system, resulting in a reduction in SERT-mediated removal of 5HT from the synaptic cleft (Fjorback et al., 2009). It is therefore possible that Gpm6b deletion reduces extracellular 5HT levels via increased SERT-mediated clearance, though this requires experimental confirmation. Deletion of Gpm6b has been reported to diminish the sensitivity of 5HT2A/C receptors (Dere et al., 2015). Both impulsive and flexible decision-making are, at least in part, mediated by serotonergic transmission (Dalley and Robbins, 2017). Hence, the behavioral effects observed here are potentially the consequence of changes in extracellular serotonin levels or secondary disruptions in 5HT2A/C receptor signaling. Considering that impulsive decision-making and flexible decision-making are modulated by a complex and distinct neuronal circuit including several brain regions (Dalley and Robbins, 2017), the task-specific effects reflect a novel mechanism whereby impulsive decision-making is impaired but flexible decision-making is enhanced.
We found that mutant mice were impaired in the DD task and choose the smaller, but immediately available reward, relative to WT littermates. This phenotype is suggested to result from an intolerance to the delay period and is consistent with increases in impulsive-like behavior. Serotonergic manipulations clearly have the ability to disrupt impulsive choice. For example, reductions in 5HT content via tryptophan depletion increased the preference for the lower-valued yet immediate reward (Schweighofer et al., 2008; Crockett et al., 2010). In rodents, increasing extracellular 5HT levels via genetic reduction of SERT expression (Zoratto et al., 2013), pharmacological inhibition of the SERT (Darna et al., 2015), or viral-mediated silencing of SERT (Zoratto et al., 2013), resulted in a reduction in impulsive decision-making. These reductions in impulsive choice behavior mirror the increase in impulsive decision-making evident in mutant mice, suggesting that this phenotype may be driven by an increase in SERT-mediated 5HT clearance. On the other hand, as noted earlier, Gpm6b deletion reduces the sensitivity of 5HT2A/C receptors (Dere et al., 2015). Antagonism of the 5HT2C receptor (Fletcher et al., 2007) or genetic knockdown of the 5HT2C receptor (Anastasio et al., 2015) increased motoric impulsivity in the 5-choice serial reaction time task, although motoric impulsivity is a functionally distinct aspect of impulsive behavior (Dalley and Robbins, 2017).
In addition to impulsivity, serotonergic neurotransmission is a central mechanism underlying reversal learning and flexible decision-making (Winstanley et al., 2005). Here, we found that mutant mice displayed improvements in the PRL task. Notably, performance was no different to WT littermates during the first stage of the task, demonstrating that discrimination learning was unaffected. However, improvements in task performance emerged once the contingencies had reversed. These improvements were most apparent towards the later stage of the reversal phase and were driven by an increased tendency to repeat rewarded actions (i.e., increased win-stay responding). The increase in win-stay responses evident in mutant mice is consistent with that observed after repeated treatment with the serotonin-selective reuptake inhibitor (SSRI) citalopram (Bari et al., 2010). Interestingly, however, repeated SSRI treatment is thought to increase extracellular levels of 5HT (Baudry et al., 2019), whereas deletion of Gpm6b is likely to decrease extracellular 5HT levels. Moreover, reversal learning improvements are evident in 5HT-transporter mutant mice (Brigman et al., 2010), consistent with the notion that increased extracellular 5HT content improves flexible decision-making. However, the behavioral mechanisms underlying this improvement (reduced perseveration immediately after the reversal) is different to that observed here in mutant mice. Currently, it is unclear whether the PRL improvement observed in mutant mice seen here are the result of alterations in extracellular 5HT content.
Deletion of Gpm6b resulted in a blunted response to 5HT2A/C activation (Dere et al., 2015). Interestingly, dissociable effects of 5HT2A vs. 5HT2C antagonists have been reported, either impairing or improving reversal learning, respectively (Boulougouris et al., 2008). Therefore, an alternative explanation for the improvement in PRL observed in mutant mice is potentially through a reduced sensitivity of the 5HT2C receptor.
The previous association between Gpm6b suppression and impairment in PPI of the acoustic startle reflex (Dere et al., 2015) motivated us to include PPI in this study. Our sample size (n=21) was much lower than in Dere et al (n=72). Nevertheless, we detected a non-significant trend towards an impairment in PPI in mutant mice. Similarly, mutant mice showed no alterations in the OF, EPM or LD tests, which replicates similar findings from Dere et al (2015).
The current study has several limitations. First of all, we only examined male mice; therefore, it is possible that a mutant allele of Gpm6b could have very different effects in females. Furthermore, we have limited our studies to a single inbred strain, even though there is ample evidence that the effect of a mutant allele can be influenced by genetic background (Sittig et al 2016). Another limitation is that it is impossible to conclude at this point that the DD task deficits or PRL enhancements are a direct consequence of serotoninergic disruptions. Although prior reports have identified a connection between Gpm6b and SERT, we did not directly measure those parameters in this study. Changes in SERT can have a broad impact on the 5HT system, including compensatory mechanisms by either affecting 5HT synthesis in presynaptic neurons, alter the metabolization rate of 5HT in intra- or extra-cellular space, modify post-synaptic 5HT receptors or presynaptic autoreceptors that control 5HT transmission (Meneses et al., 2011). Even though an interaction of Gpm6b with the brain serotonin system is possible, it remains to be determined how exactly Gpm6b deficiency affects this system and identify the precise neurobiological mechanisms for which Gpm6b deletion influences decision-making. Additionally, Gpm6b is also implicated in deficits in neuronal differentiation and myelination. It is therefore plausible that the DD or PRL effects can be explained by alterations in mechanisms other than the serotonergic system. Furthermore, we have examined a constitutively expressed mutant allele of Gpm6b, meaning we did not assess the relative importance of this gene in developmental processes versus in adulthood, nor did we assess neuroanatomical specificity. Another limitation is that while our mutant allele produced a truncated mRNA that was missing much of exon 3, including the start codon, the truncated transcript was present at normal levels in the KO mice and could have retained some residual gene function. The double dissociation of effects on DD and PRL tasks highlights the importance for more brain or even cell-type models in the future.
In summary, we examined the effect of a mutant allele of Gpm6b on two tasks measuring impulsive decision-making or flexible decision-making. Our results indicate that disrupting Gpm6b expression increases impulsive-like behavior yet enhances behavioral flexibility, which is consistent with and extends upon our prior publication (Sanchez-Roige et al., 2018). Although we have not directly assessed serotonergic signaling in this paper, we hypothesize that alterations in serotonergic reuptake and subsequent changes to post-synaptic 5HT2C-mediated neurotransmission may be the most likely mechanisms for these behavioral alterations. Given the well-established clinical utility of serotonin specific reuptake inhibitors and related drugs, it is possible that pharmacological manipulation of Gpm6b might also be clinically useful and could have a favorable side-effect profile.
Supplementary Material
ACKNOWLEDGEMENTS AND FUNDING
We thank Drs. Suzanne Mitchel and Anthony Isles for the insightful discussions on conducting delay discounting in mice. We are grateful to Jin Yi Wu, Kaiqi (Cathy) Zhang, Rebecca Tsai and Frederick Martinez for helping maintain the mouse colony.
SSR and JM were funded by the Brain and Behavior Foundation (grant 27676). SSR was also supported by the California Tobacco-Related Disease Research Program (grants 28IR-0070, T29KT0526) and a pilot grant from P50DA037844. SSR was also supported by NIDA DP1DA054394. SAB and RW were supported by a grant from NIMH (R01MH108653). AAP was supported by TRDRP (28IR-0070). Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Institutes of Health.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding authors upon request.
REFERENCES
- Anastasio NC, Stutz SJ, Fink LHL, Swinford-Jackson SE, Sears RM, DiLeone RJ, Rice KC, Moeller FG & Cunningham KA (2015) Serotonin (5-HT) 5-HT2A Receptor (5-HT2AR):5-HT2CR Imbalance in Medial Prefrontal Cortex Associates with Motor Impulsivity. ACS Chem Neurosci 6, 1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW & Robbins TW (2010) Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology 35, 1290–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley-Levenson AM, Lagarda FA & Palmer AA (2018) Glyoxalase (GLO1) inhibition or genetic overexpression does not alter ethanol’s locomotor effects: implications for GLO1 as a therapeutic target in alcohol use disorders. Alcohol Clin Exp Res 42, 869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry A, Pietri M, Launay J-M, Kellermann O & Schneider B (2019) Multifaceted Regulations of the Serotonin Transporter: Impact on Antidepressant Response. Front Neurosci 13, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulougouris V, Glennon JC & Robbins TW (2008) Dissociable effects of selective 5-HT2A and 5-HT2C receptor antagonists on serial spatial reversal learning in rats. Neuropsychopharmacology 33, 2007–2019. [DOI] [PubMed] [Google Scholar]
- Bourin M & Hascoët M (2003) The mouse light/dark box test. Eur J Pharmacol 463, 55–65. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Mathur P, Harvey-White J, Izquierdo A, Saksida LM, Bussey TJ, Fox S, Deneris E, Murphy DL & Holmes A (2010) Pharmacological or genetic inactivation of the serotonin transporter improves reversal learning in mice. Cereb Cortex 20, 1955–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charfi C, Edouard E & Rassart E (2014) Identification of GPM6A and GPM6B as potential new human lymphoid leukemia-associated oncogenes. Cell Oncol 37, 179–191. [DOI] [PubMed] [Google Scholar]
- Choi KM, Kim JY & Kim Y (2013) Distribution of the Immunoreactivity for Glycoprotein M6B in the Neurogenic Niche and Reactive Glia in the Injury Penumbra Following Traumatic Brain Injury in Mice. Exp Neurobiol 22, 277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett MJ, Clark L, Lieberman MD, Tabibnia G & Robbins TW (2010) Impulsive choice and altruistic punishment are correlated and increase in tandem with serotonin depletion. Emotion 10, 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW & Robbins TW (2017) Fractionating impulsivity: neuropsychiatric implications. Nat Rev Neurosci 18, 158–171. [DOI] [PubMed] [Google Scholar]
- Darna M, Chow JJ, Yates JR, Charnigo RJ, Beckmann JS, Bardo MT & Dwoskin LP (2015) Role of serotonin transporter function in rat orbitofrontal cortex in impulsive choice. Behav Brain Res 293, 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson GR & Tricklebank MD (1995) Use of the elevated plus maze in the search for novel anxiolytic agents. Trends Pharmacol Sci 16, 33–36. [DOI] [PubMed] [Google Scholar]
- Dere E, Winkler D, Ritter C, Ronnenberg A, Poggi G, Patzig J, Gernert M, Müller C, Nave K-A, Ehrenreich H & Werner HB (2015) Gpm6b deficiency impairs sensorimotor gating and modulates the behavioral response to a 5-HT2A/C receptor agonist. Behavioural Brain Research 277, 254–263. [DOI] [PubMed] [Google Scholar]
- Distler MG, Plant LD, Somutantloff G, Hawk AJ, Aneas I, Wuenschell GE, Termini J, Meredith SC, Nobrega MA & Palmer AA (2012) Glyoxalase 1 increases anxiety by reducing GABAA receptor agonist methylglyoxal. J Clin Invest 122, 2306–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Clark L, Siegle GJ, Butters MA, Ichikawa N, Sahakian BJ & Szanto K (2010) Reward/Punishment reversal learning in older suicide attempters. Am J Psychiatry 167, 699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabek K, van de Peppel J, Eijken M & van Leeuwen JP (2011) GPM6B regulates osteoblast function and induction of mineralization by controlling cytoskeleton and matrix vesicle release. J Bone Miner Res 26, 2045–2051. [DOI] [PubMed] [Google Scholar]
- Fernández ME, Alfonso J, Brocco MA & Frasch AC (2010) Conserved cellular function and stress-mediated regulation among members of the proteolipid protein family. J Neurosci Res 88, 1298–308. [DOI] [PubMed] [Google Scholar]
- Fjorback AW, Müller HK & Wiborg O (2009) Membrane Glycoprotein M6B Interacts with the Human Serotonin Transporter. J Mol Neurosci 37, 191–200. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J & Higgins GA (2007) Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl) 195, 223–234. [DOI] [PubMed] [Google Scholar]
- Fuchsova B, Alvarez Juliá A, Rizavi HS, Frasch AC & Pandey GN (2015) Altered expression of neuroplasticity-related genes in the brain of depressed suicides. Neuroscience 299, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifuni AJ, Perret LC, Lacourse E, Geoffroy M-C, Mbemutantu V, Jollant F & Renaud J (2020) Decision-making and cognitive control in adolescent suicidal behaviors: a qualitative systematic review of the literature. Eur Child Adolesc Psychiatry. [DOI] [PubMed] [Google Scholar]
- Ineichen C, Sigrist H, Spinelli S, Lesch K-P, Sautter E, Seifritz E & Pryce CR (2012) Establishing a probabilistic reversal learning test in mice: evidence for the processes mediating reward-stay and punishment-shift behaviour and for their modulation by serotonin. Neuropharmacology 63, 1012–1021. [DOI] [PubMed] [Google Scholar]
- McHugh CM, Chun Lee RS, Hermens DF, Corderoy A, Large M & Hickie IB (2019) Impulsivity in the self-harm and suicidal behavior of young people: A systematic review and meta-analysis. Journal of Psychiatric Research 116, 51–60. [DOI] [PubMed] [Google Scholar]
- McMurray KMJ, Du X, Brownlee M & Palmer AA (2016) Neuronal overexpression of Glo1 or amygdalar microinjection of methylglyoxal is sufficient to regulate anxiety-like behavior in mice. Behav Brain Res 301, 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneses A, Perez-Garcia G, Ponce-Lopez T, Tellez R & Castillo C (2011) Serotonin transporter and memory. Neuropharmacology 61, 355–363. [DOI] [PubMed] [Google Scholar]
- Mita S, de Monasterio-Schrader P, Fünfschilling U, Kawasaki T, Mizuno H, Iwasato T, Nave K, Werner HB & Hirata T (2015) Transcallosal Projections Require Glycoprotein M6-Dependent Neurite Growth and Guidance. Cereb Cortex 25, 4111–25. [DOI] [PubMed] [Google Scholar]
- Mitchell SH (2014) Assessing delay discounting in mice. Curr Protoc Neurosci 66, 8.30.1–8.30.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möbius W, Patzig J, Nave K-A & Werner HB (2008) Phylogeny of proteolipid proteins: divergence, constraints, and the evolution of novel functions in myelination and neuroprotection. Neuron Glia Biol 4, 111–127. [DOI] [PubMed] [Google Scholar]
- Mori M, Tsutsui-Kimura I, Mimura M & Tanaka KF (2018) 5-HT3 antagonists decrease discounting rate without affecting sensitivity to reward magnitude in the delay discounting task in mice. Psychopharmacology (Berl) 235, 2619–2629. [DOI] [PubMed] [Google Scholar]
- Phillips BU, Dewan S, Nilsson SRO, Robbins TW, Heath CJ, Saksida LM, Bussey TJ & Alsiö J (2018) Selective effects of 5-HT2C receptor modulation on performance of a novel valence-probe visual discrimination task and probabilistic reversal learning in mice. Psychopharmacology (Berl) 235, 2101–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighofer N, Bertin M, Shishida K, Okamoto Y, Tanaka SC, Yamawaki S & Doya K (2008) Low-serotonin levels increase delayed reward discounting in humans. J Neurosci 28, 4528–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Roige S, Fontanillas P, Elson SL, the 23andMe Research Team, Pandit A, Schmidt EM, Foerster JR, Abecasis GR, Gray JC, de Wit H, Davis LK, MacKillop J & Palmer AA (2018) Genome-wide association study of delay discounting in 23,217 adult research participants of European ancestry. Nat Neurosci 21, 16–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R, Lim JE, Harr B, Wing C, Walters R, Distler MG, Teschke M, Wu C, Wiltshire T, Su AI, Somutantloff G, Tarantino LM, Borevitz JO & Palmer AA (2009) A common and unstable copy number variant is associated with differences in Glo1 expression and anxiety-like behavior. PLoS One 4, e4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DEH, Dalley JW & Robbins TW (2005) Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacology 30, 669–682. [DOI] [PubMed] [Google Scholar]
- Yan Y, Lagenaur C & Narayanan V (1993) Molecular cloning of M6: identification of a PLP/DM20 gene family. Neuron 11, 423–431. [DOI] [PubMed] [Google Scholar]
- Zhang X, Xie H, Chang P, Zhao H, Xia Y, Zhang L, Guo X, Huang C, Yan F, Hu L, Lin C, Li Y, Xiong Z, Wang X, Li G, Deng L, Wang S & Tao L (2019) Glycoprotein M6B Interacts with TβRI to Activate TGF-β-Smad2/3 Signaling and Promote Smooth Muscle Cell Differentiation: Glycoprotein M6B Interacts with TβRI. Stem Cells 37, 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Barkley-Levenson A, Montilla-Perez P, Telese F & Palmer AA (2020) Functional validation of a finding from a mouse genome-wide association study demonstrates that a mutant allele of Azi2 alters sensitivity to methamphetamine (preprint). Animal Behavior and Cognition. [DOI] [PubMed] [Google Scholar]
- Zoratto F, Tringle AL, Bellenchi G, Speranza L, Travaglini D, di Porzio U, Perrone-Capano C, Laviola G, Dreyer J-L & Adriani W (2013) Impulsivity and home-cage activity are decreased by lentivirus-mediated silencing of serotonin transporter in the rat hippocampus. Neurosci Lett 548, 38–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon request.


