Abstract
The modern obesogenic environment contains an abundance of food cues (e.g., sight, smell of food) as well as cues that are associated with food through learning and memory processes. Food cue exposure can lead to food seeking and excessive consumption in otherwise food-sated individuals, and a high level of food cue responsivity is a risk factor for overweight and obesity. Similar food cue responses are observed in experimental rodent models, and these models are therefore useful for mechanistically identifying the neural circuits mediating food cue responsivity. This review draws from both experimental rodent models and human data to characterize the behavioral and biological processes through which food-associated stimuli contribute to overeating and weight gain. Two rodent models are emphasized – cue-potentiated feeding and Pavlovian-instrumental transfer – that provide insight in the neural circuits and peptide systems underlying food cue responsivity. Data from humans are highlighted that reveal physiological, psychological, and neural mechanisms that connect food cue responsivity with overeating and weight gain. The collective literature identifies connections between heightened food cue responsivity and obesity in both rodents and humans, and identifies underlying brain regions (nucleus accumbens, amygdala, orbitofrontal cortex, hippocampus) and endocrine systems (ghrelin) that regulate food cue responsivity in both species. These species similarities are encouraging for the possibility of mechanistic rodent model research and further human research leading to novel treatments for excessive food cue responsivity in humans.
Keywords: Obesity, overeating, Pavlovian, food cue, conditioning
Obesity is a significant public health concern, as more than 70% of American adults[1,2] and over 40% of children[3] have overweight or obesity. Obesity-related conditions (i.e., stroke, hypertension, diabetes, heart disease) are some of the leading causes of preventable death, and overconsumption of calorically dense foods is one of the most proximal causes of the elevated overweight and obesity rates.[4]
Today’s environment encourages excess energy intake and discourages energy expenditure [5–8] and has been implicated as one of the drivers of the obesity epidemic.[9,10] An individual’s level of food cue responsivity (FCR) is a result of genetic risk factors interacting with the environment, through learning, neural changes, and memory.[11] Food cues include visual, auditory, olfactory, emotions, situations and any other cues (e.g., time) that are associated food-related memories.[4] Specifically, FCR is defined as responses to these cues that ultimately drive overeating and weight gain.[12] Responses to food cues include psychological responses (e.g., craving, urge), physiological changes (salivation, hormone secretion), and neurocognitive responses (brain activation and allocation of attentional resources).[13] Thus, it is important to understand the psychological, behavioral, and neurobiological mechanisms that underly FCR.
Beyond genetic susceptibility, overeating develops through basic learning processes, including Pavlovian and operant conditioning.[14,15] In today’s food environment, there are multiple opportunities to associate cues in the environment with food and overeating. Through Pavlovian conditioning, these food cues become directly associated with food intake and can elicit arousal, urges to eat, cravings, expectancies, thoughts, drives and motivations to eat. [16] Operant conditioning also occurs, where the association of food seeking actions or eating are paired with the reinforcing effects of eating.[17] These two learning processes act in concert [18] and the presentation of Pavlovian food cues can increase operant responding for palatable food (e.g., Pavlovian-instrumental transfer, described in more detail below). [19,20] Food cues can also acquire secondary reinforcing properties through their association with food-directed actions [21] and can eventually elicit the operant behavior. [22–24] Food cues that are present when operant actions are reinforced can influence operant responding by “setting the occasion” for the action–outcome relationship rather than eliciting or motivating behavior through their simple direct association with food. [25] Once established, FCR also provides opportunities for higher-order cognitive processes to take place, including planning to consume food in the future. [26] Additionally, food cues can grab attention resulting in a bias in attentional resources for food cues (attentional bias), which is shown to be associated with FCR. [27,28] This increased attention to food cues may provide more opportunities for both basic and complex learning processes to take place, thereby perpetually increasing the strength of FCR.
A primary goal of this review is to draw from preclinical work to understand neuronal circuit-level mechanisms driving two key behavioral phenomena that specifically relate to FCR, cue-potentiated feeding and Pavlovian-instrumental transfer. Next, we review the human data on FCR, overeating and weight gain. Finally, we conclude with recommendations for future research based on gaps in the literature.
Insights from preclinical models
Preclinical animal models have proven to be invaluable for gaining mechanistic understanding of the neurobiological controls of food intake and energy balance. In this section we describe two rodent models, cue-potentiated feeding (CPF) and Pavlovian-instrumental transfer (PIT), and review literature derived from these models that contribute to the current understanding of neurobiological systems that regulate stimulus-driven food seeking and consumption. We note that while various other rodent appetitive paradigms provide additional mechanistic insight into stimulus-induced eating (e.g., sign- and goal-tracking, incentive learning, US devaluation; see[22,29] for review on these topics), our focus is on CPF and PIT as these procedures provide a direct window into the capacity of food-associated cues to promote excessive food seeking and/or consumption. Moreover, we emphasize these models as their underlying neural substrates have been systematically investigated for decades, thus offering a rich literature to draw from.
Cue-potentiated feeding
Neural Pathways:
FCR, in pre-clinical models, is commonly referred to as “cue-potentiated feeding” (CPF) or “stimulus-induced eating” and is based on associative learning mechanisms through which external cues that have previously been paired with access to and consumption of highly palatable food gain stimulus control over behavior.[30,31] These models involve a training phase, typically conducted under conditions of food restriction to facilitate conditioning, in which the presentation of discrete cues (e.g., light, tone; CS+) reliably predicts the delivery of palatable food (the US) and the presentation of a control stimulus is not associated with food delivery (CS-). During a test session, food-sated animals are typically given free access to the US while being exposed to various CS+ and/or CS- presentations. Evidence for CPF is based on increased consumption during (or after) CS+ presentations compared to either comparable CS- presentations or a no stimulus condition (Figure 1A). Studies have demonstrated that contextual cues can also function as a CS+ and stimulate consumption in sated rats without any discrete cues present.[32–34] Evidence suggests that CS+ exposure in rodent CPF models does not induce a general state of hunger, but rather, is selective to the specific food/US used during training,[35] although this specificity, at least for contextual-based CPF, can be overcome when a variety of foods are used as USs.[34] Thus, CPF in rodents can be considered a direct analog to FCR.
Figure 1.
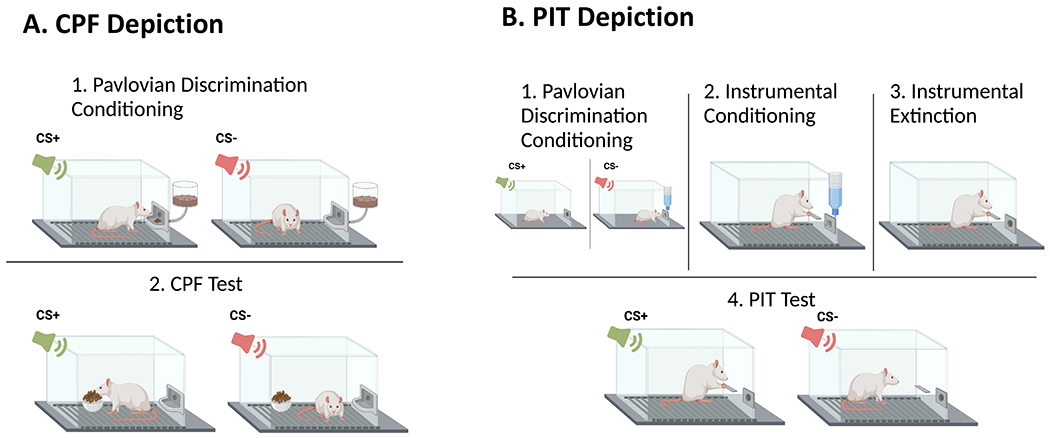
A. CPF Depiction, B PIT Depiction.
CPF research has used a combination of bilateral neurotoxic lesions, lesion-based disconnection between brain regions (unilateral and contralateral lesions of two brain regions with exclusively ipsilateral connections), behavioral, neural tract tracing, and immediate early gene mapping approaches to identify brain regions and connections that are necessary for CPF in rats. Results show that lesions to the basolateral amygdala (BLA), but not the amygdala central nucleus (CeA), eliminated the CPF effect to discrete food-conditioned cues.[36] Furthermore, a disconnection between the BLA and the lateral hypothalamic area (LHA), while having no effect on baseline eating or body weight gain, blocked the discrete cue CPF effect observed in control animals.[37] Presumably this outcome is based on ablation of a BLA to LHA pathway, although possibility of an LHA to BLA pathway cannot be ruled out given that this approach completely eliminates communication between the two brain regions. Additionally, the medial prefrontal cortex (mPFC) is a critical brain region mediating the capacity of contextual food cues to trigger excessive eating, as bilateral mPFC lesions eliminated CPF induced by contextual cues associated with food reward.[32]
Neuropeptides:
More recent work has extended these findings and provides a more complete neural circuit-level understanding of CPF control, including connections to hypothalamic neuropeptide systems. Using a systemic administration of an antagonist for the receptor for orexin (aka, hypocretin), a neuropeptide produced in the LHA, reduced discrete cue-induced CPF in rats yet had no effect on baseline food intake.[38] Further, the orexin receptor antagonist treatment increased food cue-induced c-Fos induction (a marker of neuronal activation) in the mPFC and in the paraventricular nucleus of the thalamus (PVT). A role for mPFC orexin signaling in CPF is further supported by their subsequent work revealing that mPFC-LHA disconnection reduced CPF induced by discrete food cues without influencing food-cue learning, and that CPF was also blocked with mPFC-specific orexin receptor blockade.[39] These findings collectively support that the mPFC is functionally associated with CPF for both discrete and contextual food cues, and that the neuropeptide orexin is an important neurochemical signal for CPF.
Like orexin, melanin-concentrating hormone (MCH) is an orexigenic neuropeptide produced predominantly within the LHA [although in different neurons than orexin].[40] Genetic deletion of MCH in mice significantly impairs discrete cue-induced CPF expression in food-sated mice.[41] This suggests that two distinct LHA-derived neuropeptide systems, orexin and MCH, are involved in cue-potentiated feeding, thus providing neurochemical specificity to early work identifying a role for the LHA in CPF.
Peripheral Signals:
In addition to the LHA-derived neuropeptides discussed above, emerging evidence suggests that the stomach-derived orexigenic hormone, ghrelin, is critical in the induction of CPF. Circulating levels of ghrelin are largely determined by levels of energy restriction, with higher levels observed following a fast. However, ghrelin is also released from the stomach as an anticipatory feeding signal in response to conditioned circadian cues,[42] and potentially in response to visual and other discrete food cues [43,44]. Evidence for ghrelin’s role in CPF comes from data in mice where genetic deletion of the ghrelin receptor (GHSR1a) inhibits the capacity of discrete conditioned food cues to stimulate CPF.[45] Similarly in rats, systemic administration of a GHSR1a antagonist also blocks CPF effects in response to discrete cues.[46] The ventral hippocampus (field CA1; HPCv) is a likely candidate brain region mediating these effects as pharmacological HPCv GHSR1a activation enhances CPF relative to vehicle/control treatment.[47] Ghrelin’s role in CPF may be stimulated by the capacity of palatable food-associated cues to trigger the physiological release of ghrelin, as recent findings show that olfactory detection of a familiar, palatable food caused both an increase in active ghrelin release and a persistent overconsumption of chow.[48]
Summary:
In summary, these findings identify the LHA, BLA, PVT, mPFC, and HPCv as brain regions of importance in the mediation of CPF. Interestingly, the HPCv has monosynaptic projections to all of these other regions associated with CPF control [49]. While HPCv (field CA1) projections to LHA[50] and mPFC[51] have been identified as relevant to feeding behavior, the function of these connections with regards to CPF remains to be explored. Given that palatable food-associated olfactory cues stimulate ghrelin release,[48] that HPCv GHSR1a-to-LHA signaling functionally targets LHA orexin neurons to enhance eating,[52] and that both mPFC orexin receptor signaling and LHA-mPFC signaling are necessary for CPF,[39] a putative model emerges in which exposure to cues associated with palatable food stimulates peripheral ghrelin release, which crosses the blood-brain-barrier to engage a [HPCv GHSR1a]-to-[LHA orexin neurons]-to-[mPFC] pathway to promote CPF. More research is required to understand the neural pathways through which MCH mediates CPF. [53,54]
Pavlovian-instrumental transfer
Animals and humans must be able to flexibly obtain desired outcomes while also avoiding aversive outcomes. Critical to these fundamental complementary behavioral drives is the ability to learn contingent relationships between actions and outcomes via a process known as instrumental conditioning (aka, operant conditioning). In addition to action-outcome learning, Pavlovian conditioning, including the type of stimulus-outcome (CS-US) training described above for CPF procedures, can also have a powerful influence over instrumental response performance. A classic example of this in rodent models is the Pavlovian-instrumental transfer (PIT) set of procedures.[20] This behavioral paradigm typically involves an initial Pavlovian training stage in which a stimulus/CS (e.g., light, tone, or multiple stimuli) is paired with an outcome/US, which for the focus of this review is palatable food. In the next stage, one or more instrumental actions (e.g., lever press, nose poke) are trained to yield the same US (or a different US) used in the Pavlovian training stage, but absent any Pavlovian stimuli. In the final stage, a PIT test is performed where the instrumental action(s) is available, and the Pavlovian-trained stimulus/stimuli are presented periodically such that their influence on instrumental actions can be assessed. This test usually occurs following extinction of the instrumental response, and under extinction conditions, such that no US is present during PIT testing regardless of the instrumental responses made or the stimuli presented. Evidence for PIT, for example, would be a reinvigoration of an extinguished instrumental response upon presentation(s) of the CS (Figure 1B). PIT in rodent models demonstrates food-seeking behavior that occurs after exposure to omnipresent palatable food-associated cues. Indeed, the translational relevance of PIT is strongly supported by recent findings showing that selectively-bred obesity-prone rats show heightened PIT (w/ palatable food as US) relative to obesity-resistant rats,[55] and that PIT magnitude in outbred rats is positively associated with susceptibility to diet-induced obesity.[56]
PIT procedures can be dissociated into two different subcategories that, as described in more detail below, appear to differ with regards to the underlying neurobiological substrates. “US-specific PIT” can be evaluated by comparing the effects of a Pavlovian CS on two distinct instrumental responses; one that shares the US with the CS, and another that does not. Alternatively, US-specific PIT can also be assessed with two CS+s associated with two different USs, and two distinct instrumental responses (e.g., lever press, chain pull) each yielding one of the USs used in Pavlovian training. “General PIT”, in contrast, is when stimulus control of instrumental behavior is triggered by the general motivational properties shared by the Pavlovian and instrumental training, as evidenced by a PIT effect (CS presentation enhances instrumental responding) when the Pavlovian and instrumental training phases are conducted with distinct USs (e.g., sucrose or high-fat pellets). While changes in energy status do not appear to substantially enhance or disrupt US-specific PIT, General PIT is enhanced or reduced with energy restriction or satiation, respectively, prior to testing.[57]
Mesostriatal Control:
The ACB is critical for PIT, as lesions to the nucleus accumbens shell (ACBsh) but not core (ACBc) impairs US-specific PIT.[58] In subsequent work complementing the lesion approach with pharmacological inactivation of the ACB subregions (via targeted muscimol infusions), data shows that ACBsh is required for the expression of US-specific, but not General PIT, whereas the opposite is true for the ACBc.[59] These findings collectively indicate that the ACBc mediates the general excitatory effects of food-associated cues, whereas the ACBsh mediates outcome-specific reward predictions on instrumental performance.
Recent studies identify a role for glutamate, dopamine, and acetylcholine signaling in the ACB in mediating PIT. For example, in studies using a Single US PIT design, the PIT effect is blocked by ACBc administration of an glutamatergic AMPA receptor antagonist,[55] a dopamine 1/2 receptor antagonist,[60] or a cholinergic muscarinic receptor antagonist.[61] Interestingly, ACBc blockade of cholinergic nicotinic receptors augmented PIT,[61] suggesting a complex bidirectional modulation of cue-driven food seeking behavior by ACB acetylcholine signaling. A functional role for ACBc dopamine signaling in mediating PIT is further supported by data showing that the magnitude of food cue-evoked dopamine release in the ACBc (using fast-scan cyclic voltammetry) correlated with the magnitude of US-specific PIT behavioral effect.[62] There is an intriguing yet incompletely understood interaction between ACBc acetylcholine and dopamine signaling in mediating PIT, as blockade of ACBc muscarinic receptors not only reduced PIT (as indicated above), but also suppressed the ACBc cue-evoked DA response.
Emerging findings indicate that the source of dopaminergic input to ACBc mediating PIT comes from the midbrain ventral tegmental area (VTA). For example, inactivation of the VTA disrupts Single US PIT.[63] Subsequent work using a PIT design that distinguished between US-specific and General PIT revealed that VTA inactivation attenuated these two PIT effects equally.[57] A specific role for VTA dopamine signaling in mediating these effects comes from findings showing that chemogenetic inhibition of VTA dopamine neurons blocks Single US PIT, likely through downstream ACB signaling as the same study showed similar results following chemogenetic inhibition of VTA-originating dopaminergic inputs to the ACBc but not the mPFC.[64] This pathway likely involves dopamine 1 (D1R), and not 2 receptor (D2R) signaling in the ACB, as D1R, but not D2R pharmacological blockade in the ACBsh abolished US-specific PIT without influencing General PIT.[65] Interestingly, in the same study blockade of either D1R or D2R in the ACBc had no effect on either US-specific or General PIT. While these results are consistent with the lesion studies described above, they are not consistent with results showing that blockade of D1R+D2R in the ACBc reduced Single US PIT,[60] although the former study blocked either D1R or D2R and the latter blocked both receptors, which may explain the discrepancy.
Recent work supports a model in which ventral pallidum (VP) to mediodorsal thalamus (MD) signaling acts downstream of VTA dopamine -> ACB signaling to mediate PIT. For example, the VP is a major downstream target of the ACB, [66]and either pharmacological inactivation of the VP or lesion-based disconnection of the VP and ACBsh blocked US-specific PIT.[67] Further, the MD receives substantial input from the VP,[68] and either MD lesions[69] or lesion-based VP-MD disconnection[70] blocked the US-specificity of PIT. More research is needed to determine whether the VP->MD mediation of PIT involves downstream signaling from VTA->ACB signaling, as hypothesized,[71] vs. functioning as a separate parallel neural network.
Cortical and Limbic Control:
Similar to the CPF results discussed, the amygdala appears to also play a key role in PIT when palatable food is used as reinforcement. There was some controversy, however in early reports examining the influence of different amygdala subregions on PIT, with some studies showing BLA involvement,[72,73] and others showing CeA but no BLA involvement.[74,75] These differences are likely based on differential PIT procedures between the studies, an issue that was at least partially resolved in a study that shows that BLA lesions abolished the US-specific but spared General PIT.[76] In contrast, CeA lesions abolished General but not US-specific PIT, suggesting that the BLA mediates palatable food outcome-specific incentive processes, whereas CeA is involved in controlling general motivational influence of food reward-related events.
Another study identified the lateral orbitofrontal cortex (lOFC) as a downstream target of BLA mediation of US-specific PIT, as chemogenetic-mediated inactivation of BLA terminals in the OFC blocked US-specific PIT, whereas inactivating the reverse pathway (OFC->BLA) had no effect.[77] Interestingly, however, subsequent work revealed that the lOFC and mOFC inputs to BLA involve distinct connections, and that while lOFC->BLA signaling does not appear to influence PIT, US-specific PIT is indeed mediated by mOFC->BLA signaling.[78] Additional support for a role for the OFC in PIT comes from electrophysiological recordings from OFC neurons in awake behaving rats, where it was found that the neural representation of PIT correlated with the strength of the PIT behavioral effect.[79]
Future Directions:
Similar to CPF, ghrelin signaling appears to influence PIT, although in the opposite direction. While ghrelin signaling enhances CPF in both mice and rats, peripheral administration of a ghrelin receptor antagonist in rats enhanced Single US PIT.[46] While more research is needed to understand the underlying neural substrates mediating these effects, the VTA is unlikely to be involved as, while VTA administration of ghrelin increased motivated lever pressing for palatable food under a progressive ratio schedule, it had no effect on Single US-PIT.[80]
Conclusions
The ACB and amygdala are key centers for palatable food-based PIT mediation, with the ACBsh and BLA being more linked with US-specific PIT, and the ACBc and CeA being tied to General PIT. Key upstream neural targets of these regions include the VTA dopamine neurons for modulation of ACB contributions to PIT, and the mOFC for the BLA contributions to PIT. Likely downstream targets include VP->MD signaling from the ACB, and lOFC signaling from the BLA. Evidence for peptide system contributions to PIT thus far are predominantly from research targeting the ACB, with glutamatergic, cholinergic (bidirectionally), and dopamine signaling being functionally linked with PIT mediation. Ghrelin signaling appears to have a surprising influence on PIT, as blockade of this orexigenic system increases PIT, an outcome opposite to that predicted from CPF literature. More research is needed to understand the neural loci mediating ghrelin’s influence on PIT, as well the neural circuit-level mechanisms through which midbrain basal ganglia pathways (VTA->ACB, VP->MD signaling) interact and converge with telencephalic pathways (amygdala-cortical interactions) to modulate FCR.
Research on the control of feeding behavior and energy balance has largely focused on peripherally-derived hormone systems that are modulated by energy status and function to potently regulate metabolism, food intake control, and energy expenditure. Such systems include: leptin, ghrelin, cholecystokinin, glucagon-like peptide-1, amylin, and insulin. Aside from ghrelin, the contribution of these systems to palatable food cue responsivity in preclinical animal models is poorly understood. Moreover, in addition to orexin and MCH, a number of hypothalamic-derived neuropeptides potently regulate energy balance, including agouti-related peptide, pro-opiomelanocortin, neuropeptide Y, cocaine-and-amphetamine-regulated transcript, and oxytocin. While central oxytocin signaling was recently shown to not influence Single US PIT,[81] its role in CPF has not been systematically investigated. Moreover, to our knowledge the role of these hypothalamic neuropeptide systems in mediating PIT is unknown.
Insights from human studies
In humans, a variety of measures exist to assess FCR, including self-reported cravings, questionnaires, tasks, physiological measures, and magnetic resonance imaging (MRI). Each of these measures will be described, and data are reported among individuals with overweight and obesity, binge eating, and healthy weight, as well as associations with overeating and weight gain when available.
Assessment of FCR in humans using self-report, psychophysiological measurements, or behavioral assessments:
When assessed through self-reported cravings, wanting and urges to eat, FCR is typically measured on a Likert or VAS scale. Other self-report questionnaires that measure FCR concepts, include the Power of Food scale, Eating in the Absence of Hunger questionnaire, Food Cravings Questionnaire, Child Eating Behavior Questionnaire, Adult Eating Behavior Questionnaire, Reward-Based Eating Drive Scale and the Food Cue Sensitivity Questionnaire. The Power of Food scale (PFS) [82] assesses appetite for high-palatable foods, and includes three subscales; Food Available, Food Present, and Food Tasted. The Eating in the Absence of Hunger questionnaire [83,84] assesses eating when exposed to food when physically satiated, and has three subscales; Negative Affect, External, and Fatigue/Boredom. The Food Craving Questionnaire State Version (FCQ-S) [85] assesses cravings using a multidimensional approach, and includes five subscales; an Intense Desire to Eat, Anticipation of Positive Reinforcement, Relief from Negative States, Lack of Control over Eating, and Hunger. The Child Eating Behavior Questionnaire (CEBQ) [86] includes a food responsiveness subscale that assesses overeating and desires to eat outside of typical hunger. This questionnaire has been adapted for adults [87] and babies. [88] The Reward-Based Eating Drive Scale (RED) includes questions evaluating lack of control over eating, lack of satiation, and preoccupation with food. [89] The Food Cue Sensitivity Questionnaire (FCSQ) is a newly validated questionnaire that assessed uncontrolled eating and food cue rumination. [90]
There are also several tasks that can be used to assess FCR, including psychophysiological tasks, attentional bias assessments as well as the eating in the absence of hunger (EAH) paradigm.[91] Psychophysiological assessments of FCR include cephalic phase responses (salivation, blood pressure, heart rate, heart rate variability among others)[92] that prepare the gastrointestinal tract for the optimal processing of food.[93] Attentional bias, or how individual’s attention is drawn toward or away from food cues, can be measured by reaction time, eye movements, or event related potentials.[94] The EAH paradigm typically includes a meal in which a child eats until physically full, and then is left alone with multiple snacks for a period of time (i.e. 10 min), and the amount of food consumed is measured. Those who eat more in the EAH paradigm could be considered to have high FCR, since they overeat when exposed to food cues when physically full. The EAH paradigm could be considered as similar to CPF in rodents.
Data show that exposure to food cues can increase cravings in both healthy individuals and those with overweight, obesity or binge eating. Research shows that exposure to real food is associated with increased self-reported cravings in individuals with overweight or obesity and those of healthy weight.[95] Interestingly, both real life and virtual reality exposure to food cues elicit cravings compared to neutral cues.[96] Among college females, food exposure was associated with changes in heart rate, heart rate variability (HRV), salivation, blood pressure, skin conductance and gastric activity, with significant correlations between blood pressure and cravings.[97] Another study showed that food craving intensity (as measured by the FCQ-S) significantly increased in individuals with binge eating and controls after watching a 5-minute video clip showing food and nonfood advertisements.[98]
Importantly, higher levels of FCR are associated with changes in physiology. Data shows that exposure to real food is associated with anticipatory increased heart rate, blood pressure (BP), skin response, [97,99,100] salivation,[96] and decreased heart rate variability.[95,97,101] Several studies show that these food-induced physiological responses are altered in individuals with overweight, obesity, or binge-eating. For example, after viewing and smelling pizza, individuals with overweight or obesity have increased salivation and enhanced desire for food compared to those with a healthy weight. [102] Another study found that after repeated exposure to food cues, women with obesity, compared to those with healthy weight, showed delayed decline of salivation response, suggesting a reduction of extinction of the salivary response to food cues.[103] Similarly, children with obesity have greater cue-related salivation compared to children who are healthy weight, which was associated with increased food consumption.[104] Individuals with higher levels of FCR may experience increased salivation and a delay in decline of salivary response suggesting increased level and duration of arousal in response to food cues.
These findings are mirrored by the data on attentional resources. Using EEG, data show that viewing pictures of high-calorie food elicits enhanced LPP amplitudes compared with pictures of non-food and low-calorie food.[105,106] Research shows that both women with obesity and those with a healthy weight show increased attention to food images in a fasted state, however, only women with obesity show increased attention to food images in a satiated state.[107] A recent review reports that individuals who engage in binge eating behavior exhibit an attentional bias toward food cue, in the automatic facilitated attentional engagement and purposeful attentional disengagement stages.[108] Thus, food cues capture attention, and in individuals with higher FCR, food cues may capture attention faster and there may be difficulties in disengaging their attention. This is consistent with emerging data on associations between food preoccupation and emotional eating. [109,110]
Neural understandings of FCR in humans:
Neural FCR can be assessed using MRI and is typically seen in brain regions associated with reward, motivation, learning, and inhibitory control systems. These fMRI paradigms use either pictures of food or tastes to measure FCR among individuals with overweight or obesity or those with healthy weight. This appetitive network includes the hippocampus,[111] the amygdala,[112,113] the insula,[113] the striatum,[114,115] anterior cingulate cortex (Acc),[116] the orbitofrontal cortex (OFC) and prefrontal cortex (PFC) (see Figure 2).[113,117] FCR also recruits brain regions known to underlie object recognition, gustatory, and somatosensory processing like the lateral occipital gyrus, primary gustatory cortex (comprised of the anterior insula and frontal operculum), and primary somatosensory cortex, respectively.[118,119]
Figure 2.
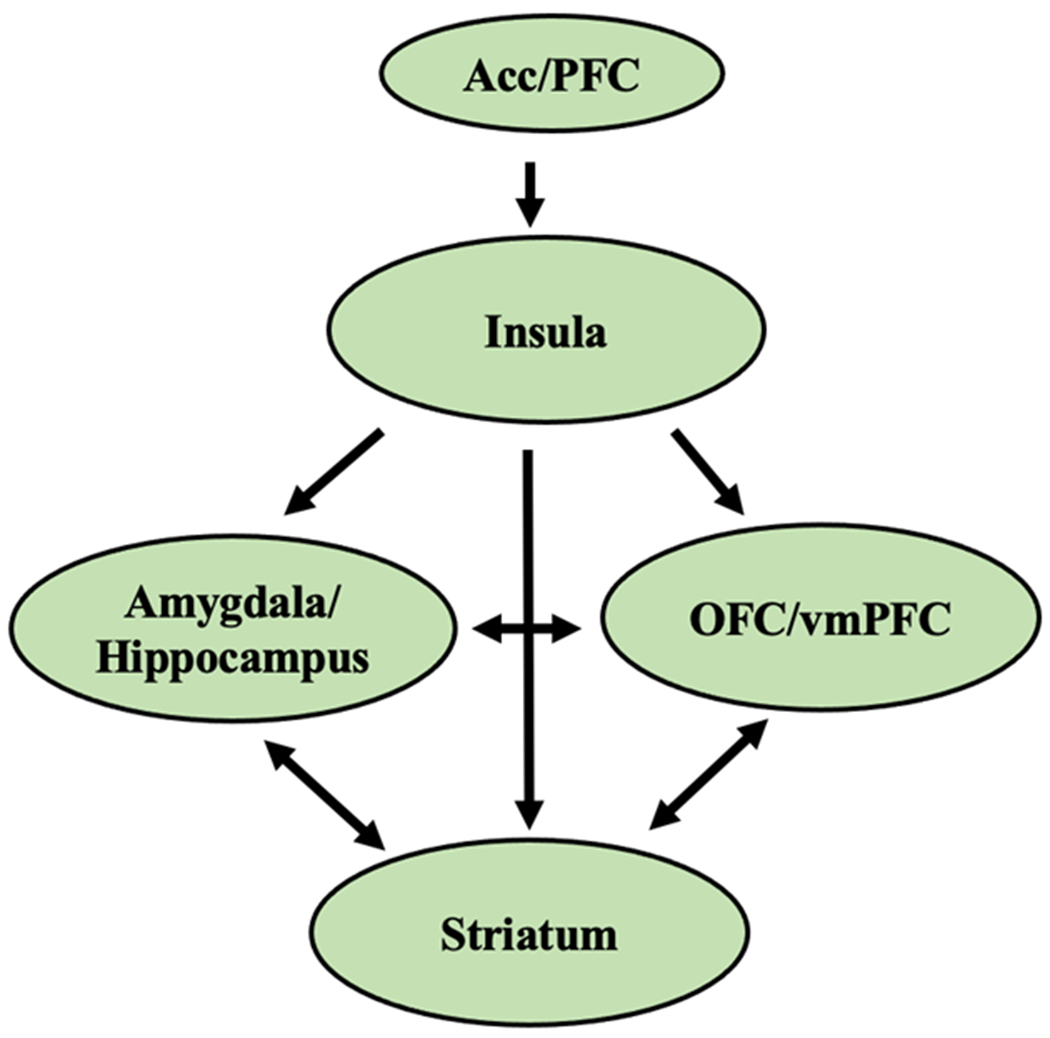
Neural regions implicated in food cue reactivity
When evaluating FCR to pictures of food, adults with obesity compared to those with a healthy weight show increased BOLD activation in the insula, caudate, orbitofrontal cortex, amygdala, nucleus accumbens, anterior cingulate cortex, pallidum, putamen, hippocampus and prefrontal cortex.[113,116,120–124] However, In contrast to these findings, there is decreased brain activation in individuals with obesity compared to those with healthy weight in response to food pictures is found in the anterior cingulate, lingual and superior occipital gyri, superior frontal gyrus, precentral gyrus, cingulate gyrus, dlPFC and the temporal lobe .[116,122,125,126] When evaluating FCR to tastes of food, results show that individuals with obesity, compared to those with a healthy weight, show greater activation in somatosensory (Rolandic operculum and parietal operculum), gustatory (insula and frontal operculum), and reward valuation regions (amgydala, ventramedial prefrontal cortex (vmPFC)) in response to intake of milkshake or chocolate milk versus tasteless solution.[114,127–129] Additionally research shows that individuals with obesity, compared to those with a healthy weight, show decreased activity in the striatum in response to receipt of palatable food relative to a tasteless solution.[128,130] Some studies also show a lack of relationship between FCR and BMI,[131–135] however, these mixed results may be due to mixed stimuli (pictures and tastes), small sample sizes, control conditions, and methods of analyses.
More recently, the hippocampus is being recognized as an important substrate in appetitive control (also summarized above).[136] A growing body of research highlights the importance of hippocampal-dependent learning mechanisms in integrating external food cues with the internal/interoceptive experience which can ultimately influence FCR.[49] In humans, inflammation and reductions in gray matter in the hippocampus are associated with having obesity.[137,138] Both adults and children with obesity show smaller hippocampal volumes, relative to those with healthy weight.[137,139,140] A large study among adolescents across the weight spectrum showed that BMI was not associated with hippocampal volume but was associated with measures of tissue integrity.[141]
Neural responding to food cues is consistently associated with eating behavior and weight change.[135] Exposure to pictures of food and changes in the appetitive network are associated with preference for high calorie foods, changes in caloric intake[142,143] and weight gain.[144,145] Responses to chocolate cues in the dorsal striatum predicted later chocolate consumption among a group of participants who were exposed to chocolate as part of a “taste test” prior to the scan, compared to a control group.[146] Similarly, activity in the medial OFC, amygdala, insula, and nucleus accumbens while viewing high-calorie foods predicted higher-fat food choices after an fMRI scan.[142] In one study, midbrain and medial OFC activity related to milkshake tastes during an fMRI scan positively predicted later ad libitum milkshake consumption among adolescents.[147] Another found that variability in nucleus accumbens activity to milkshake consumption was related to dietary disinhibition and variability in ad libitum food intake.[148,149] FCR in the nucleus accumbens, significantly predicted strength of food desires, enactment of those desires, and the amount eaten.[150] In children, activation in the hippocampus was associated with increased in the eating in the absence of hunger paradigm.[139] Higher activity in the nucleus accumbens in response to food pictures predicts weight change over 6-months.[144] A more recent study showed that increases in the motor processing areas, but not in the striatum, predicts BMI gain over 3 years.[151] Finally, a growing body of work focuses on identifying individual patterns of brain activity that predict weight change.[13,144,152] In summary, these studies point to a strong association between widespread neural activation, overeating and obesity risk, confirming that neural FCR is an important factor in weight gain in humans.
As discussed in the preclinical studies, the appetite-promoting hormone, ghrelin, plays an important role in FCR and can influence neurogenesis in the hippocampus. While leptin is also considered a hormone that influences appetite (in an opposite direction as ghrelin), ghrelin seems to activate areas associated with visual processing and attention while leptin is associated with activation of areas associated with anticipation of higher levels of reward.[153] Specifically, higher circulating levels of ghrelin are associated with activity in neural areas associated with visual processing (middle occipital gyrus, fusiform gyrus), reward (caudate) and the limbic system (amygdala, thalamus),[154,155] and reduction in ghrelin levels is associated with dorsolateral prefrontal cortex activation to food cues and reduction in craving ratings for food.[156] Among individuals with healthy weight, both fasting and subcutaneously injected ghrelin in a fed state increases hippocampus activation in response to pictures of high and low calorie foods, and orbitofrontal cortex activation in response to high calorie foods.[153] Interestingly, ghrelin and leptin are not associated with increased neural activity in response to food cues in the fed state.[153] A food-cue reactivity study in humans revealed that fasting ghrelin concentrations were associated with the hedonic effects of food pictures and with enhanced subjective craving when confronted with reward cues.[154] In summary, results show that similar to the preclinical work, ghrelin seems to play a significant role in FCR in humans.
Cue reward learning in obesity
As mentioned earlier, FCR is dependent on learning the relationship between a “cue” and food. Initially, the food elicits responding directly, but over time, the responding shifts from the food to the cue predicting food. Theorists suggest that this shift during cue-reward learning acts to update knowledge regarding the predictive cues or attribute reward value to the cues which guides behavior[157–159] and induces motivational states (e.g. salivation, cravings, expectations to eat) that can oppose the existing physiological drive. Analogous to the US-specific PIT described above, the drive in these circumstances is selective and specific and as such, is similar to induction of appetite, or even craving, rather than induction of a more general state of hunger.[160] Initial studies evaluated food cue reward learning among humans pairing fractal images with a taste of glucose, tasteless saliva or no cue among healthy adult volunteers.[161,162] These studies demonstrated learning as predicted, and there was a shift in the peak of the hemodynamic curve in the ventral striatum and orbitofrontal cortex from the taste itself to the cue that predicted the taste.
A behavioral study evaluated Pavlovian learning to innocuous cues associated with a hedonic and non-hedonic stimulus among young adults with overweight or obesity and those with healthy weight.[163] The conditioning paradigm presented innocuous visual cues (square, triangle) on a computer screen which were associated with a taste of chocolate milk or water, and swallowing frequency was measured by EMG recordings as a non-invasive estimate of salivation[164] for two minutes at baseline and after the acquisition trials.[164] Results showed a significant difference between chocolate and water swallowing at acquisition compared to baseline for individuals with obesity. Conversely, for healthy weight participants, there was no significant difference between chocolate and water swallowing at acquisition compared to baseline. These results suggest that participants with overweight or obesity learned the relationship between innocuous cues and hedonic vs. non-hedonic liquids faster than lean participants.
To our knowledge there have only been two published fMRI studies to date that link Pavlovian cue reward learning to weight, and both have used different stimuli and methods. The first study evaluated 35 adolescent girls who viewed cues (diamond, square, circle) that predicted a taste of milkshake or tasteless solution in the MRI.[165] Results showed that individual slopes of cue-reward learning in the ventral pallidum were significantly associated with BMI over a 2-year follow-up. The second study among 153 adolescents used real life cues (glasses of milkshake and water) that signaled impending taste of milkshake or tasteless solution.[166] Results showed increased BOLD activation in the orbitofrontal cortex predicted future body fat gain over three years, but not BMI change. Lower BOLD activity to the cue contrast in the bilateral superior visual cortex, lingual gyrus, and ventromedial prefrontal cortex also predicted body fat gain over three years. Since this study used pictures of glasses of milkshake and water as cues, the participants already had associations with the outcome from other learning experiences, and thus this last study did not purely test cue-reward learning. Cue-reward learning could be another individual difference that could be used to identify individuals at high risk for increased FCR.
Conclusions
FCR can be measured using several different methods in humans, including self-report, questionnaires, psychophysiological measures, and MRI. Emerging research demonstrates the relationship between FCR, eating and weight. Food pictures and tastes activate the appetitive network, which includes the hippocampus, amygdala, insula, striatum, anterior cingulate cortex, orbitofrontal cortex and prefrontal cortex. Emerging research suggests that ghrelin is an important hormone linked to attention and visual processing contributing to FCR which can also impact hippocampal neurogenesis. Finally, food cue-reward learning seems to be implicated in overeating and obesity, however understanding which individuals may be at risk for increased food cue-reward learning and how to intervene has yet to be elucidated.
Species Parallels
Comparisons between the preclinical and human study literature reviewed above identify several parallels in FCR underlying mechanisms. At the behavioral level, FCR is reliable and robust in both humans and rodents and can be triggered by both primary food cues (cues directly associated with food, e.g., food pictures, odors) and cues that are associated with palatable food via Pavlovian conditioning (e.g., fast food logos, otherwise neutral discrete lights and tones). In both species, such cues can not only stimulate elevated food consumption, but also increase appetitive operant responses that are conditioned to lead to palatable food access. FCR responses, both biological and behavioral, are present in individuals with healthy weight and lean rodents, but are heightened in humans with overweight, obesity, or binge eating, as well as in rodents that are either obese or particularly susceptible to obesity development. At the neuronal level, several common brain regions have been associated with FCR in both animal models and human studies, including the nucleus accumbens, the amygdala, the orbitofrontal cortex, and the hippocampus. Finally, the orexigenic stomach-derived hormone ghrelin is linked with elevated FCR in both humans and rats, as both species increase physiological ghrelin release in response to food-associated cues, show increased behavioral FCR with either physiological or pharmacological increases in ghrelin signaling, and show functional connections between FCR and ghrelin action in the hippocampus. That such strong behavioral, neural, and endocrine parallels exist between FCR preclinical and human studies is encouraging in the sense that mechanistic rodent models may lead to scientific advances in curbing FCR that will be relevant for human obesity prevention and treatment.
Acknowledgments
This work was funded by the National Institute of Health; DK123423(SK), DK104897(SK), DK118402 (SK), DK122504 (KB), DK111106 (KB), DK114794 (KB) and the Department of Defense; W81XWH1810220 (KB)
Funding:
This work was funded by the National Institute of Health; DK123423(SK), DK104897(SK), DK118402 (SK), DK122504 (KB), DK111106 (KB), DK114794 (KB) and the Department of Defense; W81XWH1810220 (KB)
Footnotes
Conflict of Interest: The authors have no conflict of interest
Ethical approval: NA – this is a review paper
Informed consent: NA – this is a review paper
References Cited
- 1.Wang Y, Beydoun M, Min J, et al. Has the prevalence of overweight, obesity and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol 2020; 49:810–823. doi: 10.1093/ije/dyz273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fryar C, Carroll M, Afful J, et al. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 through 2017–2018. US National Center for Health Statistics 2020; [Google Scholar]
- 3.Fryar C, Carroll M, Ogden C. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2 – 19 years: United States, 1963 – 1965 through 2015 – 2016. National Center for Health Statistics Health E-Stats 2018; doi: 10.15585/mmwr.mm6706a3 [DOI] [Google Scholar]
- 4.Belfort-DeAguiar R, Seo D. Food cues and obesity: Overpowering hormones and energy balance regulation. Curr Obes Rep 2018; 7:122–129. doi: 10.1007/s13679-018-0303-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brownell K. Fast food and obesity in children. Pediatrics 2004; 113:132. doi: 10.1542/peds.113.1.132 [DOI] [PubMed] [Google Scholar]
- 6.Lowe M. Self-regulation of energy intake in the prevention and treatment of obesity: is it feasible? Obes Res 2003; 11:Suppl 44S–59S. doi: 10.1038/oby.2003.223 [DOI] [PubMed] [Google Scholar]
- 7.Kessler D. The end of overeating: Taking control of the insatiable American appetite. New York, NY: Rodale. . 2009; [Google Scholar]
- 8.Berthoud H. Neural control of appetite: cross-talk between homeostatic and non-homeostatic systems. Appetite 2004; 43:315–317. doi: 10.1016/j.appet.2004.04.009 [DOI] [PubMed] [Google Scholar]
- 9.Swinburn B, Sacks G, Lo S, et al. Estimating the changes in energy flux that characterize the rise in obesity prevalence. Am J Clin Nutr 2009; 89:1723–1728. doi: 10.3945/ajcn.2008.27061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeffery R, Harnack L. Evidence implicating eating as a primary driver for the obesity epidemic. Diabetes 2007; 56:2673–2676. doi: 10.2337/db07-1029 [DOI] [PubMed] [Google Scholar]
- 11.Boutelle KN, Manzano M, Eichen D. Appetitive traits as targets for weight loss: The role of food cue responsiveness and satiety responsiveness. Physiol Behav 2020; 244:113018. doi: 10.1016/j.physbeh.2020.113018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carnell S, Benson L, Pryor K, et al. Appetitive traits from infancy to adolescence: using behavioral and neural measures to investigate obesity risk. Physiol Behav 2013; 121:79–88. doi: 10.1016/j.physbeh.2013.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokum S, Ng J, Stice E. Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity 2011; 19:1775–1783. doi: 10.1038/oby.2011.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berridge K, Ho C, Richard J, et al. The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Res 2010; 1350:43–64. doi: 10.1016/j.brainres.2010.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boutelle KN, Bouton M. Implications of learning theory for developing programs to decrease overeating. Appetite 2015; 93:62–74. doi: 10.1016/j.appet.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson P, Wiers R, Hommel B, et al. Working for food you don’t desire. Cues interfere with goal-directed food-seeking. Appetite 2014; 79:139–148. doi: 10.1016/j.appet.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 17.Bouton M. Learning and the persistence of appetite: extinction and the motivation to eat and overeat. Physiol Behav 2011; 103:51–58. doi: 10.1016/j.physbeh.2010.11.025 [DOI] [PubMed] [Google Scholar]
- 18.Bouton M. Learning theory. In: Sadock BJ, Sadock VA, Ruiz P, editors. Kaplan & Sadock’s Comprehensive Textbook of Psychiatry. New York: Lippincott Williams & Wilkins; 2009:647–658. [Google Scholar]
- 19.Holmes N, Marchand A, Coutureau E. Pavlovian to instrumental transfer: a neurobehavioural perspective. Neurosci Biobehav Rev 2010; 34:1277–1295. doi: 10.1016/j.neubiorev.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 20.Rescorla R, Solomon R. Two-process learning theory: Relationships between Pavlovian conditioning and instrumental learning. Psychol Rev 1967; 74:151–182. doi: 10.1037/h0024475 [DOI] [PubMed] [Google Scholar]
- 21.Williams B. Conditioned reinforcement: Experimental and theoretical issues. Behav Anal 1994; 17:261–285. doi: 10.1007/bf03392675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balleine B, O’Doherty J. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharm 2010; 35:48–69. doi: 10.1038/npp.2009.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thrailkill E, Bouton M. Contextual control of instrumental actions and habits. J Exp Psychol Anim Learn Cogn 2015; 41:69–80. doi: 10.1037/xan0000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tricomi E, Balleine B, O’Doherty J. A specific role for posterior dorsolateral striatum in human habit learning. Eur J Neurosci 2009; 29:2225–2232. doi: 10.1111/j.1460-9568.2009.06796.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogarth L, Retzler C, Munafo M, et al. Extinction of cue-evoked drug-seeking relies on degrading hierarchical instrumental expectancies. Behav Res & Ther 2014; 59:61–70. doi: 10.1016/j.brat.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferriday D, Brunstrom J. How does food-cue exposure lead to larger meal sizes? Br J Nutr 2008; 100:1325–1332. doi: 10.1017/S0007114508978296 [DOI] [PubMed] [Google Scholar]
- 27.Hou R, Mogg K, Bradley B, et al. External eating, impulsivity and attentional bias to food cues. Appetite 2011; 56:424–427. doi: 10.1016/j.appet.2011.01.019 [DOI] [PubMed] [Google Scholar]
- 28.Hardman C, Rogers P, Etchells K, et al. The effects of food-related attentional bias training on appetite and food intake. Appetite 2013; 71:295–300. doi: 10.1016/j.appet.2013.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reichelt A, Westbrook R, Morris MJ. Integration of reward signalling and appetite regulating peptide systems in the control of food-cue responses. Br J Pharmacol 2015; 172:5225–5238. doi: 10.1111/bph.13321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birch L, McPhee L, Sullivan S, et al. Conditioned meal initiation in young children. Appetite 1989; 13:105–113. doi: 10.1016/0195-6663(89)90108-6 [DOI] [PubMed] [Google Scholar]
- 31.Cornell CE. Rodin J, Weingarten H. Stimulus-induced eating when satiated. Physiol Behav 1989; 45:695–704. doi: 10.1016/0031-9384(89)90281-3 [DOI] [PubMed] [Google Scholar]
- 32.Petrovich G, Ross C, Gallagher M, et al. Learned contextual cue potentiates eating in rats. Physiol Behav 2007; 90:362–367. doi: 10.1016/j.physbeh.2006.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boggiano M, Dorsey J, Thomas J, et al. The Pavlovian power of palatable food: lessons for weight-loss adherence from a new rodent model of cue-induced overeating. Int J Obes 2009; 33:693–701. doi: 10.1038/ijo.2009.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kendig MD, Boakes RA, Corbit LH. Variety overcomes the specificity of cue-potentiated feeding in rats. J Exp Psychol Anim Learn Cogn 2018; 44:56–66. doi: 10.1037/xan0000159 [DOI] [PubMed] [Google Scholar]
- 35.Weingarten H. Stimulus control of eating: implications for a two-factor theory of hunger. Appetite 1985; 6:387–401. doi: 10.1016/s0195-6663(85)80006-4 [DOI] [PubMed] [Google Scholar]
- 36.Holland P, Petrovich G, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiol Behav 2002; 76:117–129. doi: 10.1016/s0031-9384(02)00688-1 [DOI] [PubMed] [Google Scholar]
- 37.Petrovich G, Setlow B, Holland P, et al. Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. J Neurosci 2002; 22:8748–8753. doi: 10.1523/JNEUROSCI.22-19-08748.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cole S, Mayer H, Petrovich G. Orexin/hypocretin-1 receptor antagonism selectively reduces cue-Induced feeding in sated rats and recruits medial prefrontal cortex and thalamus. Sci Rep 2015; 5:16143. doi: 10.1038/srep16143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cole S, Keefer S, Anderson LC, et al. Medial prefrontal cortex neural plasticity, orexin receptor 1 signaling, and connectivity with the lateral hypothalamus are necessary in cue-potentiated feeding. J Neurosci 2020; 40:1744–1755. doi: 10.1523/JNEUROSCI.1803-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swanson L, Sanchez-Watts G, Watts A. Comparison of melanin-concentrating hormone and hypocretin/orexin mRNA expression patterns in a new parceling scheme of the lateral hypothalamic zone. Neurosci Lett 2005; 387:80–84. doi: 10.1016/j.neulet.2005.06.066 [DOI] [PubMed] [Google Scholar]
- 41.Sherwood A, Holland P, Adamantidis A, et al. Deletion of melanin concentrating hormone receptor-1 disrupts overeating in the presence of food cues. Physiol Behav 2015; 152:402–407. doi: 10.1016/j.physbeh.2015.05.037 [DOI] [PubMed] [Google Scholar]
- 42.Drazen D, Vahl T, D’Alessio D, et al. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology 2006; 147:23–30. doi: 10.1210/en.2005-0973 [DOI] [PubMed] [Google Scholar]
- 43.Hsu T, Suarez A, Kanoski S. Ghrelin: A link between memory and ingestive behavior. Physiol Behav 2016; 162:10–17. doi: 10.1016/j.physbeh.2016.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watts A, Kanoski S, Sanchez-Watts G, et al. The physiological control of eating: Signals, neurons, and networks. Physiol Rev 2021. Epub ahead of print; doi: 10.1152/physrev.00028.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker A, Ibia I, Zigman J. Disruption of cue-potentiated feeding in mice with blocked ghrelin signaling. Physiol Behav 2012; 108 doi: 10.1016/j.physbeh.2012.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dailey M, Moran TH, Holland P, et al. The antagonism of ghrelin alters the appetitive response to learned cues associated with food. Behav Brain Res 2016; 303:191–200. doi: 10.1016/j.bbr.2016.01.040 [DOI] [PubMed] [Google Scholar]
- 47.Kanoski S, Fortin SM, Ricks K, et al. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol Psychiatry 2013; 73:915–923. doi: 10.1016/j.biopsych.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Preis-Sampedro F, Stoltenborg I, Le May M, et al. The orexigenic force of olfactory palatable food cues in rats. Nutrients 2021; 13:3101. doi: 10.3390/nu13093101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanoski S, Grill H. Hippocampus contributions to food intake control: Mnemonic, neuroanatomical, and endocrine mechanisms. Biol Psychiatry 2017; 81:748–756. doi: 10.1016/j.biopsych.2015.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsu T, Hahn J, Konanur V, et al. Hippocampus ghrelin signaling mediates appetite through lateral hypothalamic orexin pathways. Elife 2015; 4:e11190. doi: 10.7554/eLife.11190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu T, Noble E, Liu C, et al. A hippocampus to prefrontal cortex neural pathway inhibits food motivation through glucagon-like peptide-1 signaling. Mol Psychiatry 2018; 23:1555–1565. doi: 10.1038/mp.2017.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suarez A, Liu C, Cortella A, et al. Ghrelin and orexin interact to increase meal size through a descending hippocampus to hindbrain signaling pathway. Biol Psychiatry 2020; 87:1001–1011. doi: 10.1016/j.biopsych.2019.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Terrill S, Subramanian K, Lan R, et al. Nucleus accumbens melanin-concentrating hormone signaling promotes feeding in a sex-specific manner. Neuropharmacology 2020; 178:108270. doi: 10.1016/j.neuropharm.2020.108270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noble E, Wang Z, Liu C, et al. Hypothalamus-hippocampus circuitry regulates impulsivity via melanin-concentrating hormone. Nat Commun 2019; 10:4923. doi: 10.1038/s41467-019-12895-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Derman R, Ferrario C. Enhanced incentive motivation in obesity-prone rats is mediated by NAc core CP-AMPARs. Neuropharmacology 2018; 131:326–336. doi: 10.1016/j.neuropharm.2017.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Derman R, Ferrario C. Affective Pavlovian motivation is enhanced in obesity susceptible populations: Implications for incentive motivation in obesity. Behav Brain Res 2020; 380:112318. doi: 10.1016/j.bbr.2019.112318 [DOI] [PubMed] [Google Scholar]
- 57.Corbit L, Janak PH, Balleine B. General and outcome-specific forms of Pavlovian-instrumental transfer: the effect of shifts in motivational state and inactivation of the ventral tegmental area. Eur J Neurosci 2007; 26:3141–3149. doi: 10.1111/j.1460-9568.2007.05934.x [DOI] [PubMed] [Google Scholar]
- 58.Corbit L, Muir J, Balleine B. The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. J Neurosci 2001; 21:3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corbit L, Balleine B. The general and outcome-specific forms of Pavlovian-instrumental transfer are differentially mediated by the nucleus accumbens core and shell. J Neurosci 2011; 31:11786–11794. doi: 10.1523/JNEUROSCI.2711-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lex A, Hauber W. Dopamine D1 and D2 receptors in the nucleus accumbens core and shell mediate Pavlovian-instrumental transfer. Learn Mem 2008; 15:483–491. doi: 10.1101/lm.978708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Collins A, Aitken T, Greenfield V, et al. Nucleus accumbens acetylcholine receptors modulate dopamine and motivation. Neuropsychopharm 2016; 41:2830–2838. doi: 10.1038/npp.2016.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aitken T, Greenfield V, Wassum K. Nucleus accumbens core dopamine signaling tracks the need-based motivational value of food-paired cues. J Neurochem 2016; 136:1026–1036. doi: 10.1111/jnc.13494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murschall A, Hauber W. Inactivation of the ventral tegmental area abolished the general excitatory influence of Pavlovian cues on instrumental performance. Learn Mem 2006; 13:123–126. doi: 10.1101/lm.127106 [DOI] [PubMed] [Google Scholar]
- 64.Halbout B, Marshall A, Azimi A, et al. Mesolimbic dopamine projections mediate cue-motivated reward seeking but not reward retrieval in rats. Elife 2019; 8:e43551. doi: 10.7554/eLife.43551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laurent V, Bertran-Gonzalez J, Chieng B, et al. δ-opioid and dopaminergic processes in accumbens shell modulate the cholinergic control of predictive learning and choice. J Neurosci 2014; 34:1358–1369. doi: 10.1523/JNEUROSCI.4592-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zahm D, Heimer L. Two transpallidal pathways originating in the rat nucleus accumbens. J Comp Neurol 1990; 302:437–446. doi: 10.1002/cne.903020302 [DOI] [PubMed] [Google Scholar]
- 67.Leung B, Balleine B. The ventral striato-pallidal pathway mediates the effect of predictive learning on choice between goal-directed actions. J Neurosci 2013; 33:13848–13860. doi: 10.1523/JNEUROSCI.1697-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Churchill L, Kalivas P. The involvement of the mediodorsal nucleus of the thalamus and the midbrain extrapyramidal area in locomotion elicited from the ventral pallidum. Behav Brain Res 1999; 104:63–71. doi: 10.1016/s0166-4328(99)00051-0 [DOI] [PubMed] [Google Scholar]
- 69.Ostlund S, Balleine B. Differential involvement of the basolateral amygdala and mediodorsal thalamus in instrumental action selection. J Neurosci 2008; 28:4398–4405. doi: 10.1523/JNEUROSCI.5472-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leung B, Balleine B. Ventral pallidal projections to mediodorsal thalamus and ventral tegmental area play distinct roles in outcome-specific Pavlovian-instrumental transfer. J Neurosci 2015; 35:4953–4964. doi: 10.1523/JNEUROSCI.4837-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Corbit L, Balleine B. Learning and motivational processes contributing to Pavlovian-Instrumental Transfer and their neural bases: Dopamine and beyond. Curr Top Behav Neurosci 2016; 27:259–289. doi: 10.1007/7854_2015_388 [DOI] [PubMed] [Google Scholar]
- 72.Balleine B, Killcross A, Dickinson A. The effect of lesions of the basolateral amygdala on instrumental conditioning. J Neurosci 2003; 23:666–675. doi: 10.1523/JNEUROSCI.23-02-00666.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blundell P, Hall G, Killcross S. Lesions of the basolateral amygdala disrupt selective aspects of reinforcer representation in rats. J Neurosci 2001; 21:9018–9026. doi: 10.1523/JNEUROSCI.21-22-09018.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hall J, Parkinson J, Connor T, et al. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur J Neurosci 2001; 13:1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x [DOI] [PubMed] [Google Scholar]
- 75.Holland P, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. Eur J Neurosci 2003; 17:1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x [DOI] [PubMed] [Google Scholar]
- 76.Corbit L, Balleine B. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J Neurosci 2005; 25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lichtenberg N, Pennington Z, Holley S, et al. Basolateral amygdala to orbitofrontal cortex projections enable cue-triggered reward expectations. J Neurosci 2017; 37:8374–8384. doi: 10.1523/JNEUROSCI.0486-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lichtenberg N, Sepe-Forrest L, Pennington Z, et al. The medial orbitofrontal cortex-basolateral amygdala circuit regulates the influence of reward cues on adaptive behavior and choice. J Neurosci 2021; 41:7267–7277. doi: 10.1523/JNEUROSCI.0901-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Homayoun H, Moghaddam B. Differential representation of Pavlovian-instrumental transfer by prefrontal cortex subregions and striatum. Eur J Neurosci 2009; 29:1461–1476. doi: 10.1111/j.1460-9568.2009.06679.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sommer S, Hauber W. Ghrelin receptor activation in the ventral tegmental area amplified instrumental responding but not the excitatory influence of Pavlovian stimuli on instrumental responding. Neurobiol Learn Mem 2016; 134:210–215. doi: 10.1016/j.nlm.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 81.Liu C, Hsu T, Suarez A, et al. Central oxytocin signaling inhibits food reward-motivated behaviors and VTA dopamine responses to food-predictive cues in male rats. Horm Behav 2020; 126:104855. doi: 10.1016/j.yhbeh.2020.104855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lowe M, Butryn M, Didie E, et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite 2009; 53:114–118. doi: 10.1016/j.appet.2009.05.016 [DOI] [PubMed] [Google Scholar]
- 83.Arnold T, Johnston C, Lee C, et al. Eating in the absence of hunger in college students. Appetite 2015; 92:51–56. doi: 10.1016/j.appet.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 84.Tanofsky-Kraff M, Ranzenhofer L, Yanovski S, et al. Psychometric properties of a new questionnaire to assess eating in the absence of hunger in children and adolescents. Appetite 2008; 51:148–155. doi: 10.1016/j.appet.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nijs I, Franken IH, Muris P. The modified Trait and State Food Cravings Questionnaires: Development and validation of a general index of food craving. Appetite 2007; 49:38–46. doi: 10.1016/j.appet.2006.11.001 [DOI] [PubMed] [Google Scholar]
- 86.Wardle J, Guthrie C, Sanderson S, et al. Development of the Children’s Eating Behaviour Questionnaire. J Child Adol Psychiatry 2001; 42:963–970. doi: 10.1111/1469-7610.00792 [DOI] [PubMed] [Google Scholar]
- 87.Hunot C, Fildes A, Croker H, et al. Appetitive traits and relationships with BMI in adults: Development of the Adult Eating Behaviour Questionnaire. Appetite 2016; 105:356–363. doi: 10.1016/j.appet.2016.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Llewellyn C, van Jaarsveld C, Johnson L, et al. Development and factor structure of the Baby Eating Behaviour Questionnaire in the Gemini birth cohort. Appetite 2011; 57:388–396. doi: 10.1016/j.appet.2011.05.324 [DOI] [PubMed] [Google Scholar]
- 89.Epel E, Tomiyama A, Mason A, et al. The Reward-based Eating Drive Scale: a self-report index of reward-based eating. Plos One 2014; 9:e101350. doi: 10.1371/journal.pone.0101350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kang Sim D, Manzano M, Strong D, et al. Development of the Food Cue Sensitivity Questionnaire: A unidimensional measure of behavioral and cognitive cue reactivity. Under Review;
- 91.Fisher J, Birch L. Eating in the absence of hunger and overweight in girls from 5 to 7 y of age. Am J Clin Nutr 2002; 76:226–231. doi: 10.1093/ajcn/76.1.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zafra M, Molina F, Puerto A. The neural/cephalic phase reflexes in the physiology of nutrition. Neurosci Biobehav Rev 2006; 30:1032–1044. doi: 10.1016/j.neubiorev.2006.03.005 [DOI] [PubMed] [Google Scholar]
- 93.van den Akker K. Learned desires: the acquisition and extinction of appetitive responses to food cues in humans Doctoral Thesis. Maastricht University, Maastricht. 2017; [Google Scholar]
- 94.Hagan K, Alsasmar A, Exum A, et al. A systematic review and meta-analysis of attentional bias toward food in individuals with overweight and obesity. Appetite 2020; 151:104710. doi: 10.1016/j.appet.2020.104710 [DOI] [PubMed] [Google Scholar]
- 95.Camacho C, Mackinnon S, Ampolos L, et al. Food exposure, cravings, and physiological reactivity in normal-weight subjects. Appetite 2011; 57:S8. doi: 10.1016/j.appet.2011.05.138 [DOI] [Google Scholar]
- 96.van der Waal N, Janssen L, van Meirlo P, et al. The appeal of virtual chocolate: A systematic comparison of psychological and physiological food cue responses to virtual and real food. Food Quality and Preference 2021; 90:104167. doi: 10.1016/j.foodqual.2020.104167 [DOI] [Google Scholar]
- 97.Nederkoorn C, Smulders F, Jansen A. Cephalic phase responses, craving and food intake in normal subjects. Appetite 2000; 35:45–55. doi: 10.1006/appe.2000.0328 [DOI] [PubMed] [Google Scholar]
- 98.Meule A, Kuppers C, Harms L, et al. Food cue-induced craving in individuals with bulimia nervosa and binge-eating disorder. PLoS One 2018; 13:e0204151. doi: 10.1371/journal.pone.0204151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nederkoorn C, Jansen A. Cue reactivity and regulation of food intake. Eat Behav 2002; 3:61–72. doi: 10.1016/s1471-0153(01)00045-9 [DOI] [PubMed] [Google Scholar]
- 100.Vögele C, Florin I. Psychophysiological responses to food exposure: an experimental study in binge eaters. Int J Eat Disord 1997; 21:147–157. [DOI] [PubMed] [Google Scholar]
- 101.Mattes R. Physiologic responses to sensory stimulation by food: nutritional implications. J Am Diet Assoc 1997; 97:406–413. doi: 10.1016/S0002-8223(97)00101-6 [DOI] [PubMed] [Google Scholar]
- 102.Ferriday D, Brunstrom JM. ‘I just can’t help myself’: effects of food-cue exposure in overweight and lean individuals. Int J Obes 2011; 35:142–149. doi: 10.1038/ijo.2010.117 [DOI] [PubMed] [Google Scholar]
- 103.Epstein L, Paluch R, Coleman K. Differences in salivation to repeated food cues in obese and nonobese women. Psychosom Med 1996; 58:160–164. doi: 10.1097/00006842-199603000-00011 [DOI] [PubMed] [Google Scholar]
- 104.Jansen A, Theunissen N, Slechten K, et al. Overweight children overeat after exposure to food cues. Eat Behav 2003; 4:197–209. doi: 10.1016/S1471-0153(03)00011-4 [DOI] [PubMed] [Google Scholar]
- 105.Nijs I, Muris P, Euser A, et al. Differences in attention to food and food intake between overweight/obese and normal-weight females under conditions of hunger and satiety. Appetite 2010; 54:243–254. doi: 10.1016/j.appet.2009.11.004 [DOI] [PubMed] [Google Scholar]
- 106.Schwab D, Giraldo M, Spiegl B, et al. Disgust evoked by strong wormwood bitterness influences the processing of visual food cues in women: An ERP study. Appetite 2017; 108:51–56. doi: 10.1016/j.appet.2016.09.023 [DOI] [PubMed] [Google Scholar]
- 107.Castellanos E, Charboneau E, Dietrich M, et al. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. Int J Obes 2009; 33:1063–1073. doi: 10.1038/ijo.2009.138 [DOI] [PubMed] [Google Scholar]
- 108.Stojek M, Shank L, Vannucci A, et al. A systematic review of attentional biases in disorders involving binge eating. Appetite 2018; 123:367–389. doi: 0.1016/j.appet.2018.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tan C, Ruhl H, Chow C, et al. Retrospective reports of parental feeding practices and emotional eating in adulthood: The role of food preoccupation. Appetite 2016; 105:410–415. doi: 10.1016/j.appet.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 110.Williams N, Dev D, Hankey M, et al. Role of food preoccupation and current dieting in the associations of parental feeding practices to emotional eating in young adults: A moderated mediation study. Appetite 2017; 111:195–202. doi: 10.1016/j.appet.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 111.Wallner-Liebmann S, Koschutnig K, Reishofer G, et al. Insulin and hippocampus activation in response to images of high-calorie food in normal weight and obese adolescents. Obesity 2010; 18:1552–1557. doi: 10.1038/oby.2010.26 [DOI] [PubMed] [Google Scholar]
- 112.Connolly L, Coveleskie K, Kilpatrick L, et al. Differences in brain responses between lean and obese women to a sweetened drink. Neurogastroenterol Motil 2013; 25:579–e460. doi: 10.1111/nmo.12125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stoeckel L, Weller R, Cook E, et al. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 2008; 41:636–647. doi: 10.1016/j.neuroimage.2008.02.031 [DOI] [PubMed] [Google Scholar]
- 114.Ng J, Stice E, Yokum S, et al. An fMRI study of obesity, food reward, and perceived caloric density. Does a low-fat label make food less appealing? Appetite 2011; 57:65–72. doi: 10.1016/j.appet.2011.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rothemund Y, Preuschhof C, Bohner G, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 2007; 37:410–421. doi: 10.1016/j.neuroimage.2007.05.008 [DOI] [PubMed] [Google Scholar]
- 116.Dimitropoulos A, Tkach J, Ho A, et al. Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. Appetite 2012; 58:303–312. doi: 10.1016/j.appet.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dagher A. Functional brain imaging of appetite. Trends Endocrinol Metab 2012; 23:250–260. 10.1016/j.tem.2012.02.009 [DOI] [PubMed] [Google Scholar]
- 118.van der Laan L, de Ridder D, Viergever M, et al. The first taste is always with the eyes: A meta-analysis on the neural correlates of processing visual food cues. Neuroimage 2011; 55:296–303. doi: 10.1016/j.neuroimage.2010.11.055 [DOI] [PubMed] [Google Scholar]
- 119.Smeets P, Charbonnier L, van Meer F, et al. Food-induced brain responses and eating behaviour. Proc Nutr Soc 2012; 71:511–520. doi: 10.1017/S0029665112000808 [DOI] [PubMed] [Google Scholar]
- 120.Oltmanns K, Heldmann M, Daul S, et al. Sibutramine promotes amygdala activity under fasting conditions in obese women. Psychopharmacol (Berl) 2012; 221:693–700. doi: 10.1007/s00213-011-2615-7 [DOI] [PubMed] [Google Scholar]
- 121.Scharmuller W, Ubel S, Ebner F, et al. Appetite regulation during food cue exposure: a comparison of normal-weight and obese women. Neurosci Lett 2012; 518:106–110. doi: 10.1016/j.neulet.2012.04.063 [DOI] [PubMed] [Google Scholar]
- 122.Nummenmaa L, Hirvonen J, Hannukainen J, et al. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PLoS One 2012; 7:e31089. doi: 10.1371/journal.pone.0031089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pursey K, Stanwell P, Callister RJ, et al. Neural responses to visual food cues according to weight status: a systematic review of functional magnetic resonance imaging studies. Front Nutr 2014; 1:7. doi: 10.3389/fnut.2014.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nummenmaa L, Hietanen J, Calvo M, et al. Food catches the eye but not for everyone: a BMI-contingent attentional bias in rapid detection of nutriments. PloS One 2011; 6:e19215. doi: 10.1371/journal.pone.0019215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Heni M, Kullmann S, Ketterer C, et al. Differential effect of glucose ingestion on the neural processing of food stimuli in lean and overweight adults. Hum Brain Mapp 2014; 35:918–928. doi: 10.1002/hbm.22223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martin L, Holsen L, Chambers R, et al. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity 2010; 18:254–260. doi: 10.1038/oby.2009.220 [DOI] [PubMed] [Google Scholar]
- 127.Boutelle KN, Wierenga C, Bischoff-Grethe A, et al. Increased brain response to appetitive tastes in the insula and amygdala in obese compared with healthy weight children when sated. Int J Obes 2015; 39:620–628. doi: 10.1038/ijo.2014.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stice E, Spoor S, Bohon C, et al. Relation of reward from food intake and anticipated intake to obesity: A functional magnetic resonance imaging study. J Abnorm Psychol 2008; 117:924–935. doi: 10.1037/a0013600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bohon C. Brain response to taste in overweight children: A pilot feasibility study. PLoS One 2017; 12:e0172604. doi: 10.1371/journal.pone.0172604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stice E, Spoor S, Bohon C, et al. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 2008; 322:449–452. doi: 10.1126/science.1161550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Murdaugh D, Cox J, Cook E 3rd, et al. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage 2012; 59:2709–2721. doi: 10.1016/j.neuroimage.2011.10.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Garcia-Garcia I, Narberhaus A, Marques-Iturria I, et al. Neural responses to visual food cues: insights from functional magnetic resonance imaging. Eur Eat Disord Rev 2013; 21:89–98. 10.1002/erv.2216 [DOI] [PubMed] [Google Scholar]
- 133.Doornweerd S, De Geus E, Barkhof F, et al. Brain reward responses to food stimuli among female monozygotic twins discordant for BMI. Brain Imaging Behav 2018; 12:718–727. doi: 10.1007/s11682-017-9711-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Morys F, Bode S, Horstmann A. Dorsolateral and medial prefrontal cortex mediate the influence of incidental priming on economic decision making in obesity. Sci Rep 2018; 8:17595. doi: 10.1038/s41598-018-35834-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Boswell R, Kober H. Food cue reactivity and craving predict eating and weight gain: a meta-analytic review. Obes Rev 2016; 17:159–177. doi: 10.1111/obr.12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stevenson R, Francis H. Attuquayefio T, et al. Hippocampal-dependent appetitive control is impaired by experimental exposure to a Western-style diet. R Soc Open Sci 2020; 7:191338. doi: 10.1098/rsos.191338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Carnell S, Gibson C, Benson L, et al. Neuroimaging and obesity: current knowledge and future directions. Obes Res 2012; 13:43–56. doi: 10.1111/j.1467-789X.2011.00927.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Raji C, Ho A, Parikshak N, et al. Brain structure and obesity. Hum Brain Mapp 2010; 31:353–364. doi: 10.1002/hbm.20870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mestre Z, Bischoff-Grethe A, Eichen D, et al. Hippocampal atrophy and altered brain responses to pleasant tastes among obese compared with healthy weight children. Int J Obes 2017; 41:1496–1502. doi: 10.1038/ijo.2017.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Taki Y, Kinomura S, Sato K, et al. Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity 2008; 16:119–124. doi: 10.1038/oby.2007.4 [DOI] [PubMed] [Google Scholar]
- 141.Mestre Z, Bischoff-Grethe A, Wierenga C, et al. Associations between body weight, hippocampal volume, and tissue signal intensity in 12- to 18-year-olds. Obesity 2020; 28:1325–1331. doi: 10.1002/oby.22841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mehta S, Melhorn S, Smeraglio A, et al. Regional brain response to visual food cues is a marker of satiety that predicts food choice. Am J Clin Nutr 2012; 96:989–999. doi: 10.3945/ajcn.112.042341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tryon M, Carter C, Decant R, et al. Chronic stress exposure may affect the brain’s response to high calorie food cues and predispose to obesogenic eating habits. Physiol Behav 2013; 120:233–242. doi: 10.1016/j.physbeh.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 144.Demos K, Heatherton T, Kelley W. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci 2012; 32:5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lawrence N, Hinton E, Parkinson J, et al. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. Neuroimage 2012; 63:415–522. doi: 10.1016/j.neuroimage.2012.06.070 [DOI] [PubMed] [Google Scholar]
- 146.Frankort A, Roefs A, Siep N, et al. Neural predictors of chocolate intake following chocolate exposure. Appetite 2015; 87:98–107. doi: 10.1016/j.appet.2014.12.204 [DOI] [PubMed] [Google Scholar]
- 147.Nolan-Poupart S, Veldhuizen M, Geha P, et al. Midbrain response to milkshake correlates with ad libitum milkshake intake in the absence of hunger. Appetite 2013; 60:168–174. doi: 10.1016/j.appet.2012.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kroemer N, Sun X, Veldhuizen M, et al. Weighing the evidence: Variance in brain responses to milkshake receipt is predictive of eating behavior. Neuroimage 2016; 128:273–283. doi: 10.1016/j.neuroimage.2015.12.031 [DOI] [PubMed] [Google Scholar]
- 149.Giuliani N, Merchant J, Cosme D, et al. Neural predictors of eating behavior and dietary change. Ann NY Acad Sci 2018; 1428:208–220. doi: 10.1111/nyas.13637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lopez R, Hofmann W, Wagner D, et al. Neural predictors of giving in to temptation in daily life. Psychol Sci 2014; 25:1337–1344. doi: 10.1177/0956797614531492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stice E, Yokum S. Relation of neural response to palatable food tastes and images to future weight gain: Using bootstrap sampling to examine replicability of neuroimaging findings. Neuroimage 2018; 183:522–531. doi: 10.1016/j.neuroimage.2018.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Stice E, Yokum S, Blum K, et al. Weight gain is associated with reduced striatal response to palatable food. J Neurosci 2010; 30:13105–13109. doi: 10.1523/JNEUROSCI.2105-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Goldstone A, Prechtl C, Scholtz S, et al. Ghrelin mimics fasting to enhance human hedonic, orbitofrontal cortex, and hippocampal responses to food. Am J Clin Nutr 2014; 99:1319–1330. doi: 10.3945/ajcn.113.075291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kroemer N, Krebs L, Kobiella A, et al. Fasting levels of ghrelin covary with the brain response to food pictures. Addict Biol 2013; 18:855–862. doi: 10.1111/j.1369-1600.2012.00489.x [DOI] [PubMed] [Google Scholar]
- 155.Malik S, McGlone F, Bedrossian D, et al. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab 2008; 7:400–409. 10.1016/j.cmet.2008.03.007 [DOI] [PubMed] [Google Scholar]
- 156.Li G, Ji F, Hu Y, et al. Reduced plasma ghrelin concentrations are associated with decreased brain reactivity to food cues after laparoscopic sleeve gastrectomy. Psychoneuroendocrin 2019; 100:229–236. doi: 10.1016/j.psyneuen.2018.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Robinson T, Berridge K. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 1993; 18:247–291. doi: 10.1016/0165-0173(93)90013-p [DOI] [PubMed] [Google Scholar]
- 158.Flagel S, Robinson T, Clark J, et al. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharm 2010; 35:388–400. doi: 10.1038/npp.2009.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Balleine B. Taste, disgust and value: taste aversion learning and outcome encoding in instrumental conditioning. In: Reilly S, Schachtman TR, (eds), Conditioned taste aversion: Behavioral and neural processes. New York: Oxford University Press; 2008:262–280. [Google Scholar]
- 160.Weingarten H. Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science 1983; 220:431–433. doi: 10.1126/science.6836286 [DOI] [PubMed] [Google Scholar]
- 161.O’Doherty JP, Dayan P, Friston K, et al. Temporal difference models and reward-related learning in the human brain. Neuron 2003; 38:329–337. doi: 10.1016/s0896-6273(03)00169-7 [DOI] [PubMed] [Google Scholar]
- 162.O’Doherty J, Buchanan T, Seymour B, et al. Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron 2006; 49:157–166. doi: 10.1016/j.neuron.2005.11.014 [DOI] [PubMed] [Google Scholar]
- 163.Meyer M, Risbrough V, Liang J, et al. Pavlovian conditioning to hedonic food cues in overweight and lean individuals. Appetite 2015; 87:56–61. doi: 0.1016/j.appet.2014.12.002 [DOI] [PubMed] [Google Scholar]
- 164.Nederkoorn C, Smulders F, Jansen A. Recording of swallowing events using electromyography as a non-invasive measurement of salivation. Appetite 1999; 33:361–369. 10.1006/appe.1999.0268 [DOI] [PubMed] [Google Scholar]
- 165.Burger K, Stice E. Greater striatopallidal adaptive coding during cue-reward learning and food reward habituation predict future weight gain. Neuroimage 2014; 99:122–128. doi: 10.1016/j.neuroimage.2014.05.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Stice E, Burger K, Yokum S. Reward region responsivity predicts future weight gain and moderating effects of the TaqIA allele. J Neurosci 2015; 35:10316–10324. doi: 10.1523/JNEUROSCI.3607-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]


