Abstract
Pediatric oncology patients are at risk for poor outcomes with respiratory viral infections. Outcome data for COVID-19 in children and young adults with cancer are needed; data are sparse for obese/overweight and adolescent and young adult subgroups. We conducted a single center cohort study of COVID-19 outcomes in patients younger than 25 years with cancer. Candidate hospitalization risk factors were analyzed via univariable and multivariable analyses. 87 patients with cancer and COVID-19 were identified. Most were Hispanic/Latinx (n=63, 72%). 42 (48%) were overweight/obese. Anti-cancer therapy included chemotherapy only (n=64, 74%), chimeric antigen receptor T-cells (CAR-T, n=7), hematopoietic stem cell transplantation (HSCT, n=12), or CAR-T and HSCT (n=4). There was no COVID-19 related mortality. 26 patients (30%) required COVID-19 related hospitalization; 4 required multiple hospitalizations. 9 (10%) had severe/critical infection; 6 needed intensive care. COVID-19 resulted in anti-cancer therapy delays in 22 (34%) of 64 patients on active therapy (median delay=14 days). Factors associated with hospitalization included steroids within 2 weeks prior to infection, lymphopenia, previous significant non-COVID infection, and low COVID-19 PCR cycle threshold value. CAR-T recipients with B-cell aplasia tended to have severe/critical infection (3 of 7 patients). A COVID-19 antibody response was detected in 14 of 32 patients (44%). A substantial proportion of COVID-19 infected children and young adults with cancer require inpatient management; morbidity may be high in B-cell immunodeficiency. However, a majority of patients can be taken through chemotherapy without prolonged therapy delays. Viral load is a potential outcome predictor in COVID-19 in pediatric cancer.
Keywords: Pediatric cancer, Adolescents and Young Adults, COVID-19
Graphical Abstract
Introduction
Understanding outcomes of COVID-19 in pediatric patients with cancer is essential for the treatment of COVID-19 and the optimal modification of cancer therapy to minimize both the risk of severe infection due to immunosuppressive cancer therapy and the risk of cancer progression due to interruption of cancer therapy. COVID-19 in adults with cancer results in high severe disease (26%) and death (13%) rates1. Multicenter registry and several small single center studies have provided valuable insights regarding COVID-19 in pediatric cancer2–8. Two multicenter registry studies showed low rates of mortality (0–1%) and severe infection (6–8%) in children with cancer in high income countries2,3,4. In contrast, another multi-national registry reported substantial morbidity including a 37% hospitalization rate5.
Several gaps remain in the knowledge of COVID-19 in pediatric cancer. The rate of hospitalization for COVID-19 has not been well defined. Pediatric oncology patients require hospitalization for anti-cancer therapy or management of toxicities. In published studies, COVID-19 related and unrelated hospitalizations were not separately reported4 or the criteria used to distinguish the two were unclear 2,3,5. Lack of uniform criteria for COVID-19 testing across centers, inherent limitations of multicenter registry studies related to the depth of data, differences in management practices between centers, and the use of an infection severity classification system that did not include oxygen saturation create challenges in the extrapolation of morbidity data from published registry studies2–5,8 to clinical practice. Furthermore, data regarding details of anti-cancer therapy modification in the context of specific intensive anti-cancer therapy regimens remain scarce.
Obesity and Hispanic/Latinx ethnicity are risk factors for adverse outcomes in COVID-19 in adults without cancer9–11. There is a paucity of data for COVID-19 outcomes in pediatric cancer patients with obesity, a subgroup that has inferior cancer survival outcomes relative to non-obese patients12. One multicenter study reported a similar hospitalization rate but a higher rate of anti-cancer therapy modification for Hispanic/Latinx pediatric cancer patients relative to their peers8. The adolescent/ young adult age group (AYA, 15–29 years) has lower cancer cure rates and higher rates of anti-cancer therapy induced toxicities relative to younger children13, raising the possibility of worse COVID-19 outcomes in this age group. However, AYA have either been fully or partly excluded in several pediatric studies of COVID-19 and cancer2–5. Chimeric antigen receptor T-cell therapy (CAR-T) recipients have unique immunodeficiencies (B-cell aplasia), and a recent study reported high mortality (33%) in COVID-19 infected adult CAR-T recipients14. Little data has been reported for outcomes of COVID-19 in pediatric CAR-T recipients, a group of patients with lower prevalence of organ dysfunction than adults.
In this study, we report COVID-19 morbidity outcomes, risk factors associated with COVID-19 related hospitalization including viral load, and detailed data about anti-cancer therapy modifications in a cohort of children and AYA with cancer receiving chemotherapy, hematopoietic stem cell transplantation (HSCT), or CAR-T and comprised of substantial proportions of Hispanic/Latinx and overweight/obese patients.
Methods
Data extraction
Since March 2020, patients with cancer at Children’s Hospital Los Angeles (CHLA) have been universally screened using reverse-transcriptase-PCR (RT-PCR) for the detection of SARS-CoV-2 in nasopharyngeal specimens. Patients were screened prior to any sedation, hospitalization, CAR-T, or HSCT. In addition, RT-PCR was performed in patients with symptoms suspicious for COVID-19. CHLA’s Children’s Center for Cancer and Blood Disease COVID-19 database was used to identify patients with cancer aged 25 years and younger who were treated with chemotherapy, HSCT, or CAR-T, and diagnosed with COVID-19 by RT-PCR. Inclusion and exclusion criteria are shown in Fig.S1. In the case of patients who received chemotherapy only, patients who developed COVID-19 while receiving chemotherapy or within 6 months of completion of chemotherapy were included. There were no post-therapy time-based exclusion criteria for HSCT and CAR-T patients. We retrospectively reviewed medical records of patients treated at CHLA for a cancer and diagnosed with COVID-19 between March 1, 2020 and March 1, 2021. In addition, we prospectively enrolled patients with COVID-19 between March 2, 2021 and August 5, 2021. We used a data extraction form consisting of fields covering patient demographics, cancer diagnosis, anti-cancer therapy, co-morbidities, laboratory values, clinical course of COVID-19 infection, anti-COVID-19 therapies, and COVID-19 related modifications of anti-cancer therapy. Data was stored in a REDCap database (version 11.3.2). The study was approved by the CHLA Institutional Review Board (IRB). The IRB granted a consent waiver for retrospective data extraction. Informed consent was obtained for prospectively enrolled patients. Following March 1, 2021, patients were enrolled onto a prospective trial that collected clinical data, and obtained blood samples at serial time-points to assess the immune response to COVID-19 infection. Due to a sharp decline in new cases, accrual for the prospective study was low; thus, these patients are aggregated here with the retrospective patients.
Obesity/overweight status was defined based on CDC criteria15, 16. Comorbidities were defined as coexisting organ dysfunction, health conditions that required ongoing therapeutic intervention, or developmental delay. Neutropenia and lymphopenia were both defined as <500 based on CTCAE version 5.0 criteria for grade 3 or higher neutropenia and lymphopenia17. Metrics used to assess COVID-19 infection severity included WHO severity class (mild, moderate, severe, or critical)18 and the need for hospitalization for management of COVID-19 related manifestations. All pediatric oncology patients with febrile neutropenia at our center are hospitalized for sepsis work-up regardless of clinical symptoms and remain inpatient until count recovery due to the risk of life-threatening bacterial infection. Hence, COVID-19 related hospitalizations were defined as hospital stays where COVID-19 manifestations necessitated inpatient management (respiratory support, intravenous fluids, or vasopressors) and excluded hospitalizations of COVID-19 infected patients that were solely for febrile neutropenia. While it is possible that fever in patients admitted solely for febrile neutropenia may be due to COVID-19, the need for hospitalization is not reflective of COVID-19 infection severity in these patients. Hospitalizations were classified as COVID-19 related based on a consensus of two reviewers (RP and CP). A hospitalization was deemed to be COVID-19 related if both reviewers determined the hospitalization to be COVID-19 related.
A WHO COVID-19 severity classification based categorization was used to classify COVID-19 infections into mild, moderate, severe, and critical disease (Table S1). Mild disease was defined as COVID-19 infection without evidence of pneumonia. Moderate disease included infections with evidence of pneumonia that did not meet the definition of severe disease. Severe disease was defined as an oxygen saturation <90% on room air or signs of severe respiratory distress (accessory muscle use or grunting). Critical disease included cases requiring life-sustaining treatment (non-invasive or invasive ventilation or vasopressors) for acute respiratory distress syndrome (ARDS), septic shock, or Multi-system Inflammatory syndrome of children. We assessed each intensive care admission through a detailed review of the medical records including a search for confounding etiologies (e.g. bacterial sepsis or hyperleukocytosis) to determine whether the admission was attributable to the need for intensive care support for COVID-19 manifestations.
The control cohort consisted of all healthy individuals younger than 25 years of age without cancer who were seen at the CHLA emergency room with RT-PCR confirmed COVID-19 between March 1, 2020 and March 15, 2021. In order to identify patients seen with COVID-19 in the emergency room, we searched the CHLA Electronic Medical Records system using the ICD-10 code for COVID-19 (U07.1) as the query. Only those patients with a documented positive COVID-19 RT-PCR result were retained. Patients with a history of hematology/oncology, pulmonology, or rheumatology clinic encounters were excluded to eliminate those with cancer, those on immunosuppressive medications, or those with pulmonary co-morbidities. In addition, medical records for individual patients were reviewed to exclude those with cancer, immunodeficiencies, or pulmonary co-morbidities. Fig.S2 depicts the approach used to generate the control cohort.
Antibody assay
Antibody data for retrospective patients were extracted from the results of clinical antibody testing obtained by the treating physician. The anti-COVID-19 IgG antibody assay was done on plasma or serum using the EUROIMMUN Anti-SARS-CoV-2 ELISA kit19. In the case of prospective patients, the assay was done on plasma samples using the same kit. The test has been validated for serum and plasma samples. A sample : internal control ratio >1.2 was considered a positive result.
COVID-19 PCR
Nasopharyngeal swabs were sent to the Clinical Virology Laboratory at CHLA for testing by RT-PCR. The CDC20, Taqpath (Thermo Fisher, Walham, MA)21, Xpert Xpress (Cepheid, Sunnyvale, CA)22, or Simplexa (Diasorin Molecular, Cypress, CA)23 SARS-CoV-2 RT-PCR assays were used. Cycle threshold (Ct values) were extracted from the Ct value data generated during the clinical RT-PCR assay. These Ct values were not available to the treating physician and were not used for clinical decision making.
Statistical analysis
Statistical analysis was done using R version 3.6.1 and the R package epitools24. Fishers’ exact (categorical variables) or t-test (PCR Ct value) were used for univariable analysis. Logistic regression was used for multivariable analysis. Two sided tests with p<0.05 were considered significant. The primary endpoints were COVID-19 related hospitalization and WHO severity grade (severe/critical vs non-severe/critical). To identify risk factors associated with COVID-19 related hospitalization in patients with cancer, we first screened candidate clinical and laboratory predictors in univariable analyses. All categorical variables with Fisher’s exact p-value<0.05 were considered in a multivariable (MV) analysis. This initial MV model included data from all patients with cancer who had COVID-19 infection. We then built a MV model that included PCR Ct value and all the categorical variables that showed a Wald’s test p-values <0.05 in the initial MV model. Patients with missing PCR Ct values (8 patients) were excluded in this final MV model. MV analysis was done using the R function glm (family = binomial), which generates Wald’s test p-values.
Results
Baseline characteristics
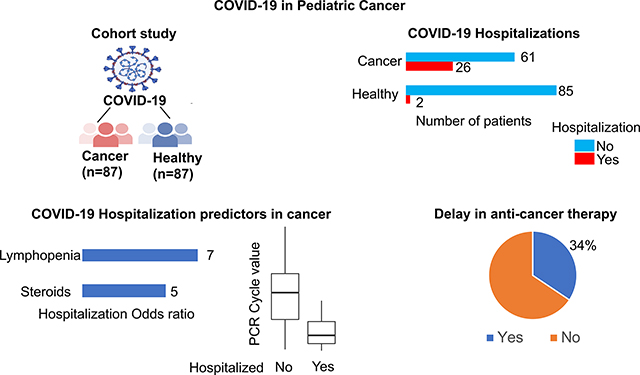
We identified 87 patients with cancer and RT-PCR confirmed COVID-19 infection. Eight four patients were identified through retrospective review while the remaining 3 were prospectively identified at the time of COVID-19 diagnosis. Tables 1 and S2 (detailed data for individual subjects) list the baseline characteristics for the cohort. The median age was 12 years. A majority of the cohort (74%) was of Hispanic/Latinx ethnicity. Almost half the patients (n= 42, 48%) were either obese (n=28) or overweight (n=14). The most common cancer was acute lymphoblastic leukemia (ALL, 61%). Anti-cancer therapy regimens included chemotherapy alone (74%), HSCT (n =12), anti CD19 CAR-T (n=7), and both HSCT and CAR-T (n=4). Among those who received chemotherapy alone, 29 (45%) had COVID-19 during an intensive phase of chemotherapy (ALL induction, ALL delayed intensification, acute myeloid leukemia therapy, and therapy phases that are expected to drop ANC to less than 500 for more than one week). Over half the patients (59%) had at least one comorbidity.
Table 1.
Baseline patient, cancer, immune status, and anti-cancer therapy characteristics
| N=87 | ||
|---|---|---|
|
| ||
| Age (years) | median (range) | 12 (0–24) |
| Sex, n (%) | ||
| Male | 48 (55) | |
| Female | 39 (45) | |
| Ethnicity, n (%) | ||
| Hispanic | 64 (74) | |
| Non-Hispanic | 23 (26) | |
| Weight status | ||
| Overweight | 14 (16) | |
| Obese | 28 (32) | |
| Lean | 45 (52) | |
| Cancer, n (%) | ||
| Hem n (%) | 63 (72) | |
| ALL | 53 | |
| AML | 2 | |
| Hodgkin | 2 | |
| NHL | 2 | |
| Other | 4 | |
| Non-Hem n (%) | 24 (28) | |
| Brain | 3 | |
| Sarcoma | 7 | |
| Neuroblastoma | 8 | |
| Wilms | 3 | |
| Retinoblastoma | 1 | |
| Other | 2 | |
| Therapy, n (%) | ||
| Chemo | 64 (74) | |
| HSCT | 12 (14) | |
| CAR | 7 (8) | |
| CAR & HSCT | 4 (4) | |
| Chemotherapy intensity (n=64) a | ||
| Intense | 29 | |
| Non-intense | 35 | |
| HSCT type (n=16) | ||
| Allogeneic | 10 | |
| Autologous | 6 | |
| Days since therapy, median(range) b | ||
| HSCT | 305 (−39–3939) | |
| CAR | 629 (21–2142) | |
| Steroids c , n (%) | 18 (21) | |
| Anthracyclines, n (%) | 65 (75) | |
| Neutropenia d , n (%) | 14 (16) | |
| Lymphopenia e , n (%) | 35 (40) | |
| Any Comorbidity, n (%) | 51 (59) | |
| Neurological | 14 | |
| Cardiac | 9 | |
| Pulmonary | 5 | |
| Renal | 4 | |
| Endocrine | 4 | |
| Infectionf | 8 | |
| Gastrointestinal | 10 | |
| Other | 19 | |
| GVHD | ||
| Acute | 1 | |
| Chronic | 2 | |
| Immune status (allo-HSCT, n=9) g | ||
| Lymphopenia | 0 | |
| Neutropenia | 0 | |
| CD4<200 | 0 | |
| Immune status (CAR, n=8) g | ||
| Lymphopenia | 3 | |
| Neutropenia | 0 | |
| CD4<200 | 2 | |
| B-cell aplasia | 7 | |
Intense: patients in the midst of a highly myelosuppressive phase of chemotherapy at the time of COVID-19 infection.
Days: time interval between the date of last anti-cancer therapy (HSCT or CAR) and the date of the first positive COVID-19 PCR.
Received systemic steroids (not including steroids given as part of COVID-19 management) within two weeks prior to the date of COVID-19 diagnosis.
Absolute neutrophil count <500.
Absolute lymphocyte count <500.
Includes 4 patients with non-COVID viral infection and 4 patients with fungal infection.
Patients who had received both HSCT and CAR were classified as HSCT or CAR based on the last anti-cancer therapy prior to COVID-19 infection.
COVID-19 outcomes
Clinical presentation:
61 patients (70%) had symptomatic infection. The most common clinical presentations were fever and upper respiratory infection symptoms (Table 2).
Table 2.
Clinical presentation and outcomes of COVID-19 infection.
| N=87 | ||
|---|---|---|
|
| ||
| Clinical presentation, n(%) | ||
| Asymptomatic | 26 (30) | |
|
| ||
| Fever | 38 (44) | |
| Myalgia | 16 (18) | |
|
| ||
| URI | 47 (54) | |
| LRI | 18 (21) | |
|
| ||
| GI | 25 (29) | |
| Neurological | 9 (10) | |
|
| ||
| Cardiac | 5 (6) | |
| Skin | 6 (7) | |
| Hospitalization, n(%) | ||
| COVID related | 26 (30) | |
| -Multiple hospitalizations | 4 | |
| COVID unrelated | 14 (16) | |
| Not hospitalized | 47 (54) | |
| Severity * | ||
| Mild | 69 | |
| Moderate | 10 | |
| Severe | 2 | |
| Critical | 6 | |
| Hypoxia, n(%) | 12 (14) | |
| Intensive care, n (%) | 6(7) | |
| Invasive ventilation# | 2 | |
| Organ failure | 6 | |
| Any Treatment, n (%) | 19 (22) | |
| Remdesivir | 14 | |
| Steroids | 11 | |
| Monoclonal | 2 | |
| Convalescent Plasma | 4 | |
| Hydroxychloroquine | 1 | |
| Azithromycin | 2 | |
| Other | 1 | |
|
Therapy delay
(n=64 on active therapy) |
||
| Delayed | 22 | |
|
Duration of delay
(days, median (range)) |
14(2–39)@ | |
URI: Upper respiratory infection, LRI: lower respiratory infection
Severity was graded using the World Health Organization scale. Mild: no pneumonia or hypoxia. Moderate: clinical signs of pneumonia, chest radiography may help in diagnosis. Severe: oxygen saturation <90% on room air or signs of severe respiratory distress (accessory muscle use, grunting.) Critical: life-sustaining treatment for conditions such as acute respiratory distress syndrome (ARDS), sepsis, or septic shock.
Intubation and ventilation.
radiotherapy was omitted in one patient due to COVID-19.
Mortality and morbidity outcomes:
There were no COVID-19 related deaths; two patients died from progression of cancer. Twenty-six (30%) patients required hospitalization for the management (respiratory support, intravenous fluids, or vasopressors) of COVID-19 manifestations (COVID-19 related hospitalizations). Four patients (Table S3) needed multiple hospitalizations due to recurrent fever and respiratory symptoms (data for three of these four have been previously reported25). COVID-19 PCR positivity was seen at all these recurrent hospitalizations. Nine patients (10%) had severe or critical infection (as defined by the WHO COVID-19 severity classification); 6 (7%) needed intensive care management. The median duration following COVID-19 for which data was available was 98 days (19–351 days). COVID-19 outcomes are described in Tables 2 and S2 (detailed data for individual subjects).
COVID-19 directed therapy:
19 (22%) patients required treatment for COVID-19; the most common treatments were Remdesivir and steroids (Table 2).
COVID-19 related complications:
Two patients (one CAR-T recipient and one autologous HSCT recipient) developed multisystem inflammatory syndrome of children (treated with IVIG and Anakinra in the CAR-T recipient). One patient developed a new thrombus. Two patients experienced COVID-19 symptoms of intermittent fever and respiratory symptoms for greater than two months.
Changes to anti-cancer therapy:
Among the 64 patients who were in the midst of anti-cancer therapy, 22 (34%) experienced modifications or delay in anti-cancer therapy as a result of COVID-19 infection (median delay = 14 days, range = 2–39 days, radiation therapy discontinued for one patient). Anti-cancer therapy was delayed or modified in 13 of 31 patients and 9 of 33 patients in the midst of intensive and non-intensive anti-cancer therapy respectively. Among the eight patients who presented with COVID-19 at the time of cancer diagnosis, chemotherapy was delayed or modified up to 2 weeks in two (both with acute leukemia), and there was no worsening of COVID-19 with intensive chemotherapy in the 7 patients who required an intensive regimen (Table S4). The three patients with COVID-19 at the time of leukemia diagnosis showed absence of minimal residual disease at the end of induction.
Duration of PCR positivity:
Repeat RT-PCR data were available for 50 patients. In 14 patients, a positive PCR result was seen in at least 2 successive tests. The time interval between the first and last positive tests was > 3 weeks for 12 of these patients and > 6 weeks for 4 of these patients. Two patients (one CAR-T recipient with B-cell aplasia, one with ALL) showed PCR positivity for >150 days25.
Comparison to healthy cohort:
Children and AYA with cancer and COVID-19 showed a significantly higher rate of hospitalization and severe/critical infection relative to a cohort of COVID-19 infected patients without cancer that were seen at our center (Fig.1). The two cohorts did not significantly differ in gender, ethnicity, proportion of AYA, or time period of COVID-19 diagnosis (Table S5).
Figure 1:
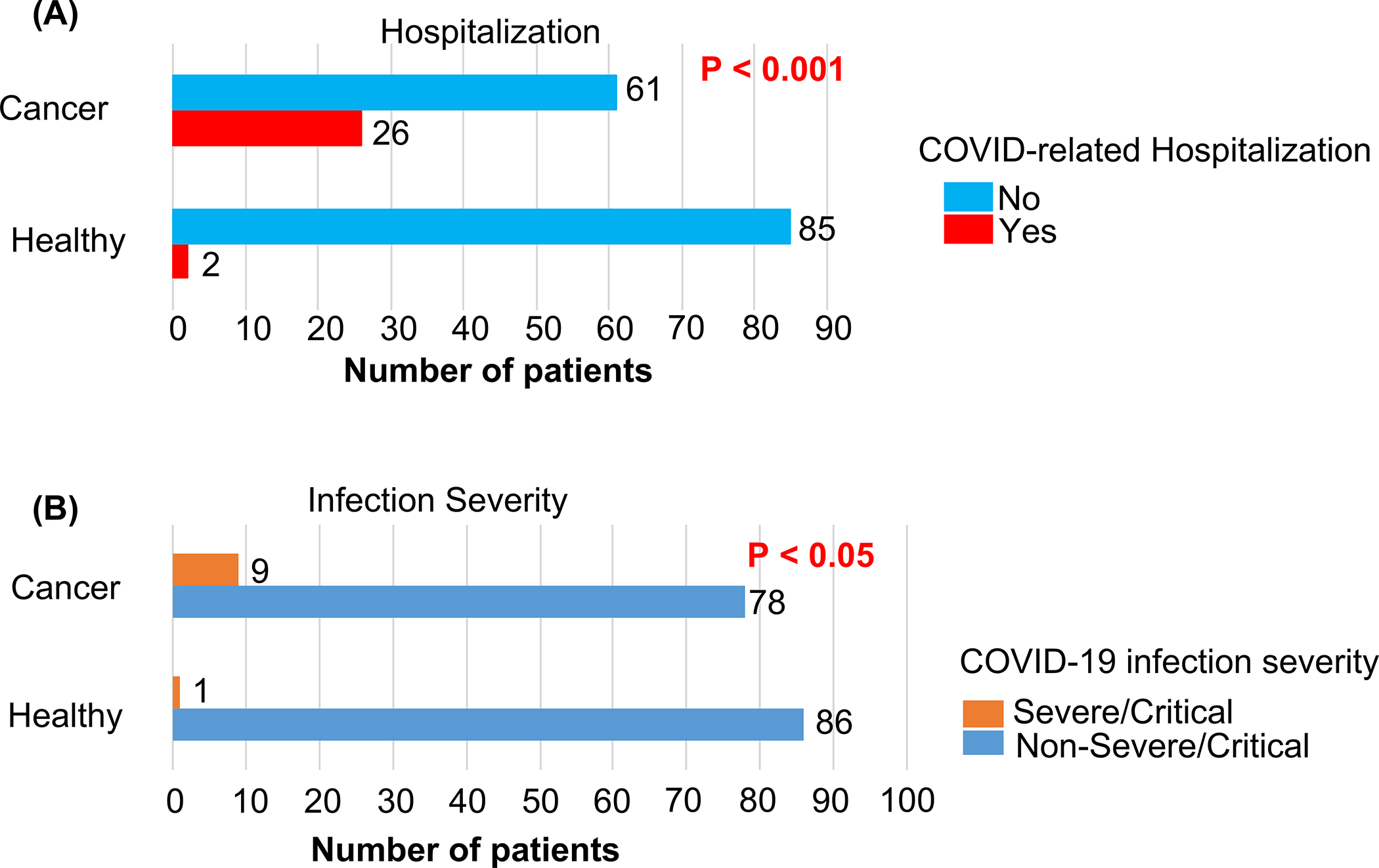
Hospitalization (A) and infection severity (B) outcomes in children, adolescents and young adults (AYA) with cancer and COVID-19 infection (Cancer, N=87 patients) and children and AYA without cancer who had COVID-19 infection (Healthy, n=87). Two-sided Fisher exact test p-values for Cancer vs Healthy shown.
Risk factors associated with COVID-19 outcomes
Risk factors significantly associated with COVID-related hospitalization in a univariable analysis included neutropenia (<500 cells per mm3), lymphopenia (<500 cells per mm3), steroid exposure within two weeks prior to the diagnosis of COVID-19, and history of a prior significant non-COVID infection (defined as past infections for which patients were receiving ongoing secondary prophylaxis medications at the time of COVID-19 infection.) Four patients had a past history of invasive fungal infection, and four patients had a past history of viral infection (CMV, HHV-6, and HSV). Conversely, age in the AYA range (age ≥15), body mass index (BMI) in the obese/overweight range (BMI >25), and Hispanic/Latinx ethnicity were not significantly associated with hospitalization. The RT-PCR cycle threshold value (Ct) for virus detection is an inverse measure of viral load. Patients who required hospitalization had a significantly lower Ct (Ct data available for 79 patients, mean Ct = 20.2 vs 27.2, hospitalized (n=24) vs not hospitalized (n=55), p<0.05, Fig.2a). The low number of patients with severe/critical COVID-19 infection limited the ability to analyze associations with disease severity. However, CAR-T (as the last therapy prior to COVID-19) tended to be associated with severe/critical infection (p=0.04, Fig.2b); three of 8 CAR-T recipients developed severe/critical infection; all three had B-cell aplasia.
Figure 2.
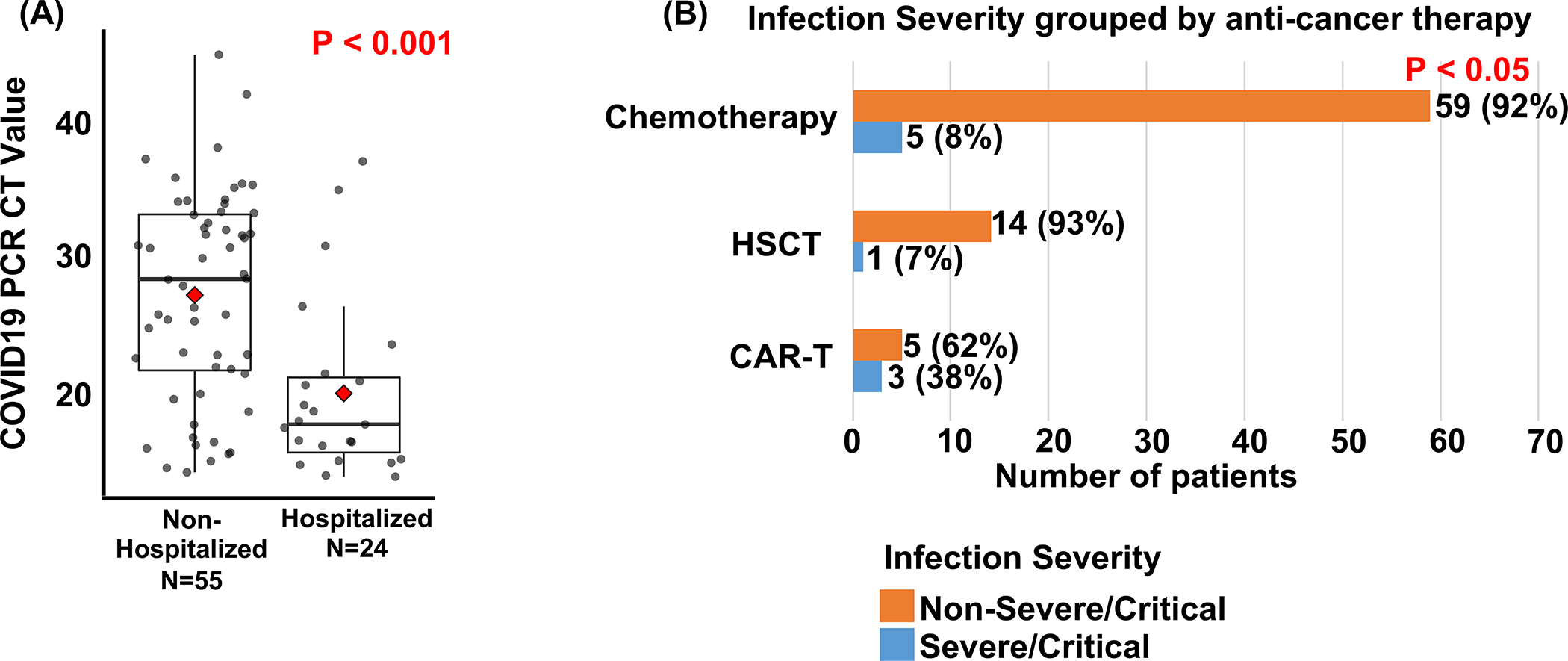
Associations between COVID-19 outcomes and COVID-19 PCR cycle threshold value or type of anti-cancer therapy. (A) cycle threshold value for diagnostic COVID-19 PCR in children, adolescents and young adults (AYA) with cancer and COVID-19 infection grouped by need for COVID-19 related hospitalization (PCR cycle threshold value data available for 79 patients). Red diamond: mean. Two-sided t test p value shown. Each data-point represents an individual patient (B) COVID-19 infection severity grouped by type of anti-cancer therapy. P value: Two-sided Fisher exact test p value for a comparison of the three groups testing for a difference between at least two of the three groups.
In a multivariable analysis of categorical variables that were significant in the univariable analysis (Table 3), only lymphopenia, prior steroids, and a history of non-COVID infection retained a significant association with hospitalization. The multivariable analysis was then repeated with the above mentioned three variables along with the continuous variable, Ct. Lymphopenia, prior steroids, a history of non-COVID infection, and lower Ct retained a significant association with hospitalization in the multivariable model (Table 3), indicating that these factors are independent potential risk factors for COVID-19 related hospitalization in children and AYA with cancer.
Table 3.
Associations between risk factors and COVID related hospitalization.
| Variables | Hospitalized N=26 |
Not hospitalized N=61 |
Univariable | Multivariable* (n= 79; 24 Hospitalized and 55 Not hospitalized) | ||||
|---|---|---|---|---|---|---|---|---|
| OR | P value | OR | P value | |||||
| Age (years) | ||||||||
| ≥15 years | 11 | 18 | 1.8 (0.7–4.5) | 0.31 | ND | ND | ||
| <15 years | 15 | 43 | ||||||
| Sex | ||||||||
| Male | 17 | 31 | 1.8 (0.7–4.7) | 0·25 | ND | ND | ||
| Female | 9 | 30 | ||||||
| Ethnicity | ||||||||
| Hispanic | 22 | 42 | 2.5 (0.8–8.2) | 0·18 | ND | ND | ||
| Non-Hispanic | 4 | 19 | ||||||
| Weight statusa | ||||||||
| Obese/OW | 14 | 28 | 1.4 (0.5–3.5) | 0.64 | ND | ND | ||
| Lean | 12 | 33 | ||||||
| Cancer | ||||||||
| Hem | 22 | 41 | 2.7 (0.8–8.8) | 0.12 | ND | ND | ||
| Non-hem | 4 | 20 | ||||||
| Comorbidities | ||||||||
| Yes | 18 | 33 | 1.9 (0.7–5.1) | 0.24 | ND | ND | ||
| No | 8 | 28 | ||||||
| Co_pulm | ||||||||
| Yes | 3 | 2 | 3.9 (0.6–24.5) | 0.16 | ND | ND | ||
| No | 23 | 59 | ||||||
| Co_cardiac | ||||||||
| Yes | 4 | 5 | 2 (0.5–8.3) | 0·44 | ND | ND | ||
| No | 22 | 56 | ||||||
| Co_renal | ||||||||
| Yes | 1 | 3 | 0.8 (0.1–7.8) | 1 | ND | ND | ||
| No | 25 | 58 | ||||||
| Co_neuro | ||||||||
| Yes | 4 | 10 | 0.9 (0.3–3.3) | 1 | ND | ND | ||
| No | 22 | 51 | ||||||
| Co_GI | ||||||||
| Yes | 4 | 6 | 1.7 (0.4–6.5) | 0·48 | ND | ND | ||
| No | 22 | 55 | ||||||
| Co_endocrine | ||||||||
| Yes | 1 | 3 | 0.8 (0.1–7.8) | 1 | ND | ND | ||
| No | 25 | 58 | ||||||
| Co_infection | ||||||||
| Yes | 5 | 3 | 4.6 (1–21) | 0.048 | 6.6 (1.02–42.7) | 0.046 | ||
| No | 21 | 58 | ||||||
| Co_other | ||||||||
| Yes | 9 | 10 | 2.7 (0.9–7.8) | 0.15 | ND | ND | ||
| No | 17 | 51 | ||||||
| Therapy | ||||||||
| Chemo | 21 | 43 | 0.37 | ND | ND | |||
| HSCT | 2 | 13 | 0.3 (0.1–1.5) | 0.21 | ||||
| CAR | 3 | 5 | 1.2 (0.3–5.6) | 1 | ||||
| Steroids | ||||||||
| Yes | 12 | 6 | 7.9 (2.5–24.6) | 0.003 | 5.1 (1.3–20.1) | 0.02 | ||
| No | 14 | 55 | ||||||
| Anthracycline | ||||||||
| Yes | 22 | 43 | 2.3 (0.7–7.6) | 0.19 | ND | ND | ||
| No | 4 | 18 | ||||||
| Neutropenia | ||||||||
| Yes | 9 | 5 | 5.9 (1.7–20.1) | 0.004 | NS | |||
| No | 17 | 56 | ||||||
| Lymphopenia | ||||||||
| Yes | 17 | 18 | 4.5 (1.7–12) | 0.004 | 7.1 (1.8–28) | 0.006 | ||
| No | 9 | 43 | ||||||
| PCR Ct (mean) | 20.2 (n=24) |
27.2 (n=55) |
0.0002 | 0.9(0.83–0.97) | 0.01 | |||
All p values are two sided. Univariable p values (Fisher exact test for all categorial variables and t-test for Ct) were not adjusted for multiple comparisons and should be interpreted with caution. Multivariable analysis was done using logistic regression. ND: not done. NS: not significant. Co: comorbidity. GI: gastrointestinal. Pulm: pulmonary. Neur: neurological. Steroids: exposure to steroids within 14 days prior to COVID-19 diagnosis (does not include steroids administered for COVID-19 management). Neutropenia: ANC <500. Lymphopenia: ALC < 500.
Multivariable analysis p values and odds ratios shown are from a logistic regression analysis of data from the 79 patients for whom PCR Ct data were available. Lymphopenia, Steroids, and Co-Infection were significantly associated with hospitalization, and neutropenia was not significantly associated with hospitalization in a separate multivariable logistic regression analysis of data from all 87 patients that did not include PCR Ct as a variable.
Antibody response to COVID-19 infection
Anti-COVID-19 IgG antibody data were available for 32 patients (Table 4). Antibodies were detected in 18 patients. Among these 18, two were CAR-T recipients receiving IVIG for B-cell aplasia (one had received monoclonal antibody), one was a patient in the midst of ALL chemotherapy who had received monoclonal COVID-19 antibody therapy, and one was undergoing AML chemotherapy and had received convalescent plasma. Antibody positivity in these four patients was most likely from the IVIG, monoclonal antibody or convalescent plasma. One of the patients who received monoclonal COVID-19 antibody therapy showed high antibody levels > 90 days following monoclonal antibody therapy. Among the remaining 14 patients who showed antibodies, 5 were HSCT recipients (all >150 days post-HSCT), and four other patients were diagnosed with COVID-19 at the time of cancer diagnosis or within two weeks of initiation of chemotherapy for a new diagnosis of cancer or relapsed cancer. Two of these 14 patients showed antibodies for > 90 days.
Table 4.
Data for anti-COVID-19 IgG antibodies
| Subject ID | Diagnosis | Therapy | Antibody ratio* | Phase of therapy at COVID-19 diagnosis | Antibody testing timepoint (Days)# | Comment |
|---|---|---|---|---|---|---|
| 2 | ALL | HSCT | 6.7 | 305 days post-HSCT | 38 | |
| 3 | ALL | CAR | Negative | 110 | B-cell aplasia | |
| 4 | ALL | HSCT | 7.5 | 165 days post-HSCT | 17 | |
| 5 | ALL | CAR | 6.9 | 27 | On IVIG, received monoclonal, B-cell aplasia | |
| 7 | ALL | HSCT | 4.9 | 923 days post-HSCT | 41 | |
| 8 | ALL | CAR | 7.7 | 28 | On IVIG, was negative at 16 days, B-cell aplasia | |
| 9 | ALL | HSCT | 4.8 | 258 days post-HSCT | 50 | |
| 10 | ALL | HSCT | 6.9 | 1075 days post-HSCT | 26 | |
| 12 | Neuroblastoma | HSCT | 1.5 | 17 | ||
| 13 | ALL | CAR | Negative | 52 | B-cell aplasia | |
| 16 | NHL | CAR | Negative | 39 | B-cell aplasia | |
| 18 | Soft tissue sarcoma | HSCT | Negative | 14 | ||
| 20 | ALL | Chemo | 2.3 | Delayed Intensification | 83 | |
| 22 | ALL | Chemo | Negative | maintenance | 27 | |
| 23 | Bone sarcoma | Chemo | 7.5 | Induction week 1 | 4 | |
| 25 | Neuroblastoma | Chemo | Negative | Induction cycle 2 | 31 | |
| 37 | AML | Chemo | 1.4 | At cancer diagnosis | 39 | Was negative at 21 days |
| 40 | ALL | Chemo | Negative | Consolidation | 86 | |
| 42 | ALL | Chemo | Negative | Induction day 27 | 3 | |
| 52 | Sarcoma | Chemo | Negative | Cycle 5 | 19 | |
| 53 | AML | Chemo | 2 | At cancer diagnosis | 2 | Received convalescent plasma |
| 58 | ALL | Chemo | 11.2 | Induction day 5 | 46 | Antibody ratio = 8.2 at day 117 |
| 59 | ALL | Chemo | Negative | Maintenance | 31 | |
| 60 | NHL | Chemo | Negative | COP-R prophase (week 1 of induction chemotherapy) | 149 | |
| 64 | Relapsed ALL | Chemo | 9.3 | Re-induction Day 10 |
38 | |
| 75 | ALL | Chemo | Negative | Delayed intensification | 31 | |
| 78 | ALL | Chemo | 4.4 | Maintenance | 23 | |
| 79 | Neuroblastoma | HSCT | Negative | 161 days post-HSCT, cycle 3 of post-HSCT anti-GD2 immunotherapy | 7 | |
| 84 | ALL | CAR | Negative | 2142 days post-CAR | 148 | B-cell aplasia |
| 85 | ALL | Chemo | 2.9 | Induction day 27 | 2 | Antibody titer 1.2 at day 92 |
| 86 | ALL | Chemo | 2.2 | Maintenance | 19 | |
| 87 | ALL | Chemo | 8.3 | Interim maintenance | 2 | received monoclonal, antibody titer 8.3 at day 98 |
Antibody ratio: ratio of sample to internal control17. Negative: Antibody ratio <1.2. Days post-infection: day 0 = day of PCR diagnosis of COVID-19.
Time interval between the date of the first positive COVID-19 RT-PCR and the date when the sample was obtained for antibody testing. COP-R: cyclophosphamide, vincristine, prednisone, and Rituxumab.
An IgG antibody response was not detected in 14 of the 32 patients with available Anti-COVID-19 IgG antibody data. Two of the patients with undetectable antibodies were tested within 7 days of diagnosis of COVID-19, a time-point that may have been too early for the detection of an antibody response. Ten of the remaining 12 negative patients were tested at ≥ 27 days after the diagnosis of COVID-19. Among these 10, 4 were CAR-T recipients with B-cell aplasia, and 4 were diagnosed with COVID-19 during an intensive phase of chemotherapy. Overall, these data suggest that at least some patients among those with active leukemia who have not yet or just initiated chemotherapy or those with a history of allogeneic HSCT > 6 months prior to COVID-19 can mount an immune response to COVID-19. Furthermore, the antibody response is sustained for several months in some patients.
Discussion
Our study provides comprehensive data for COVID-19 outcomes as well as the underlying cancer for a pediatric cohort with robust representation of Hispanic/Latinx and AYA individuals. Consistent with the low to no mortality reported in other studies of COVID-19 in pediatric cancer, there were no COVID-19 related deaths in our cohort. However, we observed a substantial rate of COVID-19 related hospitalization in our study; over one in four patients and over one in three symptomatic patients required hospitalization. A small minority required recurrent hospitalization, a finding not reported in other cohorts. Consistent with other studies2–4, the overall rate of severe/critical infection and need for intensive care were low. While the rate of severe/critical infection for the whole cohort was low, CAR-T recipients with COVID-19 tended to have severe/critical infection, results consistent with a high risk for adverse outcomes in COVID-19 in the setting of CAR-T induced B-cell immunodeficiency14. The need for inpatient management for several non-severe infections observed in both our cohort and a multinational registry study5 suggests that severity scales based largely on respiratory criteria do not fully capture the extent of morbidity in COVID-19 in pediatric cancer. Several aspects of our findings differ from those reported in studies of adults with COVID-19. In contrast to studies of the general adult population9–11, our data suggests that children and AYA with cancer who are overweight/obese or are of Hispanic/Latinx ethnicity do not seem to be at higher risk for adverse COVID-19 outcomes relative to other pediatric oncology patients. Up to a fifth of adults with COVID-19 infection have been reported to experience symptoms for months, a syndrome that has been termed long or post-COVID-19 condition26. However, prolonged COVID-19 symptoms were rarely observed in our cohort during a median observation time period of > 3 months. A multicenter study of 30 adult CAR-T recipients with COVID-19 reported a COVID-19 related mortality rate of 33%14. In comparison, we observed no deaths among 8 CAR-T recipients.
Defining clinical and biomarker predictors of hospitalization in COVID-19 in pediatric oncology is critical for informing decisions regarding early administration of monoclonal antibodies or antivirals. Consistent with other studies, lymphopenia and prior steroids were associated with higher COVID-19 morbidity. Of note, unlike other studies4,5, neutropenia was not associated with higher morbidity in our cohort when adjusted for other risk factors. Inclusion of CAR-T recipients, a subgroup that tended to have severe infection despite the absence of neutropenia, could account for this lack of association. The association between lymphopenia and morbidity seen in our study is consistent with the essential role of lymphocytes in mediating an effective anti-COVID-19 immune response 27,28. Given the need for prognostic biomarkers in COVID-19, several studies have investigated the value of PCR Ct, a surrogate measure of viral load, as an outcome predictor in COVID-19 in the general population. However, varying data ranging from no prognostic value to being a risk factor for mortality have been reported for PCR Ct in adults with COVID-19 29,30; Ct value criteria for demarcation between low and high viral loads remain to be defined26. To our knowledge, our study represents the first report of an association between low Ct and hospitalization in COVID-19 in pediatric cancer. Our results raise the need for further prospective studies investigating Ct as a potential biomarker for predicting outcomes in COVID-19 in pediatric cancer.
Given the risk of worsening of COVID-19 infection with immunosuppressive anti-cancer therapy, COVID-19 in patients with cancer can have significant ramifications for the delivery of effective anti-cancer therapy. Balancing interruption of anti-cancer therapy against the risk for malignancy progression can be challenging, especially in patients with newly diagnosed cancers. Chemotherapy was delayed or modified in a third of patients in our cohort, a proportion lower than the 63% rate reported in an international registry study4. The averaged nature of the rate across chemotherapy regimens that likely varied between different countries may account for the discrepancy. Our study also describes patients with COVID-19 at the time of the diagnosis of cancer. Two of 5 patients with newly diagnosed hematologic malignancies experienced delays and/or modifications in induction therapy. However, several patients with mild COVID-19 were able to tolerate intensive induction regimens without worsening of COVID-19. Overall, these results suggest that most children with cancer and COVID-19, including those who are newly diagnosed, can be taken through chemotherapy without prolonged delays.
Characterization of the immune response to COVID-19 in patients receiving anti-cancer therapy is needed to inform COVID-19 therapy and prophylaxis approaches in pediatric cancer. Antibody responses were detected in over a third of patients in our study for whom anti-COVID-19 IgG data were available, a result consistent with the notion that some subgroups of pediatric oncology patients including HSCT recipients could benefit from COVID-19 vaccination. In a few patients, we found antibodies that were most likely from monoclonal antibody therapy or IVIG including one patient who showed antibodies for >90 days. These results suggest a single dose of monoclonal antibody may provide immunity in pediatric cancer patients for several months, and that in some instances IVIG could provide antibodies.
A key strength of our study is the detailed nature of clinical, laboratory, and outcome data provided for the entire cohort, valuable data for informing management decisions in clinical practice. Our study has several limitations. The data were from a single center and largely retrospective. The number of HSCT and CAR-T-cell recipients was low, limiting the ability to make conclusions about outcomes in these patients. The number of patients with severe/ critical infection was low, limiting the ability to analyze predictors of severe/critical infection. The relatively small size of the study cohort limited the ability to analyze associations in the multivariable analysis especially for variables with a sparse distribution. Antibody data were available for just a third of the patients, and the timepoints for these data varied between patients. The time point in the infection course at which RT-PCR was obtained would have inevitably varied between patients, a caveat to the association between Ct and hospitalization. However, we observed lower Ct in patients who required hospitalization. Since these patients would be expected to have undergone PCR later in the course of infection relative to those who did not require hospitalization, it is unlikely that the temporal decline in viral load after the onset of symptoms that typically occurs in COVID-1931 majorly confounded our analysis. The comparison of hospitalization rates between COVID-19 infected children with or without cancer could be confounded by physician bias toward admitting children with cancer given their immunodeficiencies. Nevertheless, we also found a higher rate of severe/critical infection defined by objective WHO criteria in children with cancer relative to those without cancer, an outcome analysis unlikely to be confounded by such biases. Lastly, 18 of the HSCT or CAR-T recipients in our cohort were also included in the recently reported Pediatric Oncology COVID-19 Case (POCC) registry study8. Here, we include previously unreported data for these 18 patients including outcomes specifically in the CAR-T subgroup, immune responses, and RT-PCR Ct data.
Our study provides data that could inform the management of the infection and the anti-cancer therapy in COVID-19 in children and AYA receiving intensive cancer therapy regimens. Prospective multicenter studies are needed to define clinical as well as immune and viral biomarker prognostic factors in COVID-19 in pediatric cancer.
Supplementary Material
Novelty and Impact.
Pediatric cancer patients with COVID-19 have a substantial hospitalization rate with lymphopenia, recent steroids, CAR T-cell therapy, and high viral load being risk factors for adverse outcomes. A majority of patients with COVID-19 can be taken through chemotherapy without prolonged therapy delays.
Acknowledgements
We thank patients and their families for contributing data and blood samples, the CHLA Health information and management department for assistance with data extraction for the healthy cohort, and the CHLA clinical immunology laboratory staff for performing antibody assays.
Funding
This work was supported by the Concern (CP), Nautica (RP), and Children’s Cancer Research Fund Foundations. The Redcaps database was supported by the National Center for advancing translational Science of the US National Institutes of Health (UL1TR001855 and UL1TR000130).
Abbreviation List
- ALL
acute lymphoblastic leukemia
- AML
acute myeloid leukemia
- ARDS
acute respiratory distress syndrome
- AYA
adolescent/young adult
- BMI
body mass index
- CAR-T
Chimeric antigen receptor T-cell therapy
- CDC
Centers for Disease Control
- CMV
cytomegalovirus
- Ct
cycle threshold
- CTCAE
common terminology criteria for adverse events
- ELISA
enzyme-linked immunosorbent assay
- HHV-6
human herpesvirus 6
- HSCT
hematopoietic stem cell transplantation
- HSV
herpes simplex virus
- ICU
intensive care unit
- IRB
institutional review board
- IVIG
intravenous immune globulin
- LRI
lower respiratory infection
- POCC
Pediatric Oncology COVID-19 Case registry
- REDCap
research electronic data capture
- RT-PCR
reverse transcriptase-polymerase chain reaction
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2 (COVID-19)
- URI
Upper respiratory infection
- WHO
World Health Organization
Footnotes
Conflict of Interest
The authors have no competing financial interests. PSP has received consultant fees from Sanofi-Pasteur and Seqirus and receives funding from Pfizer and AstraZeneca for studies unrelated to this manuscript.
Ethics Statement
This study was approved by the Children’s Hospital Los Angeles (CHLA) Institutional Review Board (IRB). The IRB granted a consent waiver for retrospective data extraction. Informed consent was obtained for prospectively enrolled patients.
Data Availability Statement
Detailed data for individual subjects are provided in Table S2. Further data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. Jun 20 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Millen GC, Arnold R, Cazier JB, et al. Severity of COVID-19 in children with cancer: Report from the United Kingdom Paediatric Coronavirus Cancer Monitoring Project. Br J Cancer. Feb 2021;124(4):754–759. doi: 10.1038/s41416-020-01181-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukkada S, Bhakta N, Chantada GL, et al. Global characteristics and outcomes of SARS-CoV-2 infection in children and adolescents with cancer (GRCCC): a cohort study. Lancet Oncol. Oct 2021;22(10):1416–1426. doi: 10.1016/S1470-2045(21)00454-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Millen GC, Arnold R, Cazier JB, et al. COVID-19 in children with haematological malignancies. Arch Dis Child. Jul 22 2021;doi: 10.1136/archdischild-2021-322062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haeusler GM, Ammann RA, Carlesse F, et al. SARS-CoV-2 in children with cancer or after haematopoietic stem cell transplant: An analysis of 131 patients. Eur J Cancer. Oct 9 2021;159:78–86. doi: 10.1016/j.ejca.2021.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamdar KY, Kim TO, Doherty EE, et al. COVID-19 outcomes in a large pediatric hematology-oncology center in Houston, Texas. Pediatr Hematol Oncol. Nov 2021;38(8):695–706. doi: 10.1080/08880018.2021.1924327 [DOI] [PubMed] [Google Scholar]
- 7.Gampel B, Troullioud Lucas AG, Broglie L, et al. COVID-19 disease in New York City pediatric hematology and oncology patients. Pediatr Blood Cancer. Sep 2020;67(9):e28420. doi: 10.1002/pbc.28420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnston EE, Martinez I, Davis ES, Caudill C, Richman J, Brackett J, Dickens DS, Kahn A, Schwalm C, Sharma A, Patel PA, Bhatia S, Levine JM, Wolfson JA, & POCC Consortium (2021). SARS-CoV-2 in Childhood Cancer in 2020: A Disease of Disparities. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 39(34), 3778–3788. 10.1200/JCO.21.00702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmen Gao MP; Astbury Nerys; Hippisley-Cox Julia; O’Rahilly Stephen; Aveyard Paul; Jebb Susan. Associations between body-mass index and COVID-19 severity in 6·9 million people in England: a prospective, community-based, cohort study. Lancet. 2021;9:350–59. doi: 10.1016/S2213-8587(21)00089-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foldi M, Farkas N, Kiss S, et al. Obesity is a risk factor for developing critical condition in COVID-19 patients: A systematic review and meta-analysis. Obes Rev. Oct 2020;21(10):e13095. doi: 10.1111/obr.13095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nanchal R, Patel D, Guddati AK, et al. Outcomes of Covid 19 patients-Are Hispanics at greater risk? J Med Virol. Oct 11 2021;doi: 10.1002/jmv.27384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orgel E, Sposto R, Malvar J, et al. Impact on survival and toxicity by duration of weight extremes during treatment for pediatric acute lymphoblastic leukemia: A report from the Children’s Oncology Group. J Clin Oncol. May 1 2014;32(13):1331–7. doi: 10.1200/JCO.2013.52.6962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller KD, Fidler-Benaoudia M, Keegan TH, Hipp HS, Jemal A, Siegel RL. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin. Nov 2020;70(6):443–459. doi: 10.3322/caac.21637 [DOI] [PubMed] [Google Scholar]
- 14.Busca A, Salmanton-Garcia J, Corradini P, et al. COVID-19 and CAR-T cells: current challenges and future directions-a report from the EPICOVIDEHA survey by EHA-IDWP. Blood Adv. Nov 8 2021;doi: 10.1182/bloodadvances.2021005616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Force USPST, Barton M. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. Pediatrics. Feb 2010;125(2):361–7. doi: 10.1542/peds.2009-2037 [DOI] [PubMed] [Google Scholar]
- 16.Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 through 2017–2018 (2020). NCHS Health E-Stats. 2020. [Google Scholar]
- 17.Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0, November 2017, National Institutes of Health, National Cancer Institute. Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf [Google Scholar]
- 18.Organization WH. Country & Technical Guidance - Coronavirus disease (COVID-19). World Health Organization. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance-publications [Google Scholar]
- 19.Tilley K, Ayvazyan V, Martinez L, et al. A Cross-Sectional Study Examining the Seroprevalence of Severe Acute Respiratory Syndrome Coronavirus 2 Antibodies in a University Student Population. J Adolesc Health. Dec 2020;67(6):763–768. doi: 10.1016/j.jadohealth.2020.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandey U, Yee R, Shen L, et al. High Prevalence of SARS-CoV-2 Genetic Variation and D614G Mutation in Pediatric Patients With COVID-19. Open Forum Infect Dis. Jun 2021;8(6):ofaa551. doi: 10.1093/ofid/ofaa551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appliedbiosystems TaqPath COVID-19 Combi Kit and TaqPath COVID-19 Combo Kit Advanced Instructions for Use. https://www.fda.gov/media/136112/download
- 22.Cepheid. Xpert Xpress SARS-CoV-2 Instructions for Use. https://www.fda.gov/media/136314/download
- 23.DiaSorin Molecular. Simplexa COVID-19 Direct. https://www.fda.gov/media/136286/download
- 24.Aragon TJ, Fay MP, Daniel Wollschlaeger, Omidpanah A. epitools. https://cran.r-project.org/web/packages/epitools/epitools.pdf [Google Scholar]
- 25.Truong TT, Ryutov A, Pandey U, et al. Increased viral variants in children and young adults with impaired humoral immunity and persistent SARS-CoV-2 infection: A consecutive case series. EBioMedicine. May 2021;67:103355. doi: 10.1016/j.ebiom.2021.103355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus. World Health Organization. October 6, 2021. https://apps.who.int/iris/bitstream/handle/10665/345824/WHO-2019-nCoV-Post-COVID-19-condition-Clinical-case-definition-2021.1-eng.pdf [Google Scholar]
- 27.Scheid JF, Barnes CO, Eraslan B, Hudak A, Keeffe JR, Cosimi LA, Brown EM, Muecksch F, Weisblum Y, Zhang S, Delorey T, Woolley AE, Ghantous F, Park SM, Phillips D, Tusi B, Huey-Tubman KE, Cohen AA, Gnanapragasam P, Rzasa K, … Xavier RJ (2021). B cell genomics behind cross-neutralization of SARS-CoV-2 variants and SARS-CoV. Cell, 184(12), 3205–3221.e24. 10.1016/j.cell.2021.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, Rawlings SA, Sutherland A, Premkumar L, Jadi RS, Marrama D, de Silva AM, Frazier A, Carlin AF, Greenbaum JA, Peters B, Krammer F, Smith DM, Crotty S, & Sette A (2020). Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell, 181(7), 1489–1501.e15. 10.1016/j.cell.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salto-Alejandre S, Berastegui-Cabrera J, Camacho-Martinez P, et al. SARS-CoV-2 viral load in nasopharyngeal swabs is not an independent predictor of unfavorable outcome. Sci Rep. Jun 21 2021;11(1):12931. doi: 10.1038/s41598-021-92400-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fajnzylber J, Regan J, Coxen K, et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun. Oct 30 2020;11(1):5493. doi: 10.1038/s41467-020-19057-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Zein S, Chehab O, Kanj A, Akrawe S, Alkassis S, et al. (2021) SARS-CoV-2 infection: Initial viral load (iVL) predicts severity of illness/outcome, and declining trend of iVL in hospitalized patients corresponds with slowing of the pandemic. PLOS ONE 16(9): e0255981. 10.1371/journal.pone.0255981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. May 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196- [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Detailed data for individual subjects are provided in Table S2. Further data that support the findings of this study are available from the corresponding author upon reasonable request.


