Rapid development of vaccines against SARS-CoV-2 led to substantial reductions in morbidity and mortality early in the pandemic. Concerns regarding waning immunity and the risk of emerging new variants, including Omicron, prompted recommendations for a third ‘booster’ vaccine dose after completion of a two-dose mRNA vaccine regimen, given its efficacy at further reducing risk for severe illness by up to 70%.1 However, a proportion of individuals who received three mRNA vaccine doses still required hospitalization for COVID-19 during the Omicron surge. We sought to understand the characteristics associated with severe Omicron infection, necessitating hospitalization, despite having completed a full three-dose mRNA vaccine regimen.
We conducted a retrospective cohort study of adults who received at least three mRNA vaccine doses but were subsequently treated for confirmed COVID-19 infection in our academic healthcare system during the Omicron surge onset in our region, and had at least two outpatient visits within the preceeding two years. All laboratory testing for COVID-19 was performed using rtPCR of extracted RNA from nasopharyngeal swabs. We obtained demographic (age, sex, and race/ethnicity), clinical, and outcomes data from the electronic health record (EHR) and manually confirmed the validity of key variables. We used ICD-10 diagnoses to identify specific clinical characteristics previously associated with COVID-19 severity, including: diabetes mellitus, chronic kidney disease (CKD), prior myocardial infarction (MI) or heart failure (HF), and prior chronic obstructive pulmonary disease (COPD) or asthma. Hypertension was defined by ICD-10 code or the prescription of antihypertensive pharmacotherapy. Obesity was defined as a calculated body mass index (BMI) of ≥30 kg/m2. Patients with missing data on key variables were excluded. We also curated EHR data on ACE inhibitor, ARB, and statin use, and days from most recent SARS-CoV-2 vaccine dose to confirmed infection. In statistical analyses, we used multivariable logistic regression to assess for associations between each of the characteristics listed above and risk of hospitalization. To minimize confounding by indication for ACEi/ARB use, we performed two separate sensitivity analyses: first, we removed ACEi/ARB from the multivariable; second, we excluded individuals with a history of CKD, MI, or HF. All analyses were conducted using R v4.0.2, with a two-tailed P<0.05 considered significant.
Overall, we identified a total of 912 individuals who received ≥3 mRNA vaccine doses and were subsequently diagnosed with COVID-19 during the Omicron surge, of whom 145 (15.9%) required hospitalization. Demographic and clinical characteristics of the cohort are shown in the Figure. In multivariable analyses, factors significantly associated with risk of hospitalization for Omicron infection included older age, hypertension, CKD, and MI or HF, as well as longer duration between last vaccination and infection (Figure). Notably, presence of hypertension was associated with the greatest magnitude of risk, which remained significant in sensitivity analyses excluding patients with a history of CKD, MI, or HF. Results were similar when ACEi/ARB use was removed from the model.
Figure. Risk factors for Omicron infection requiring hospitalization, despite receiving prior booster vaccination.
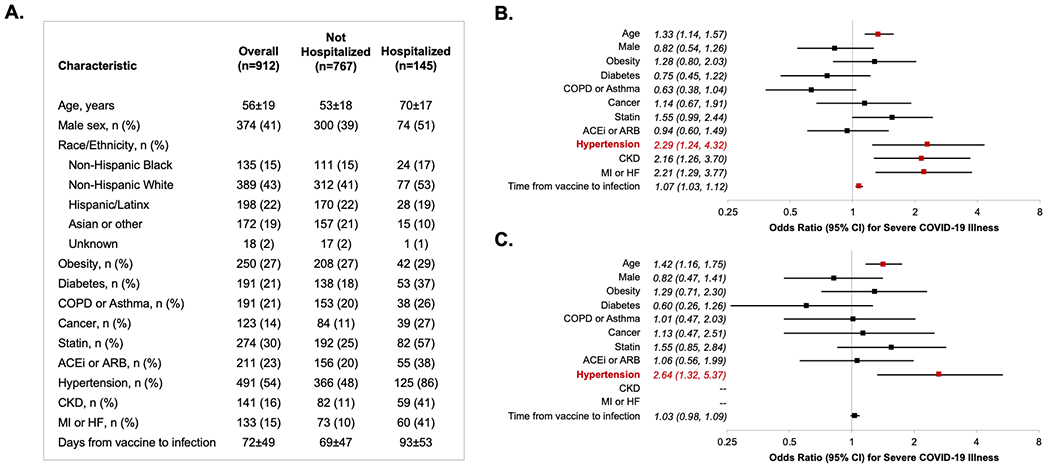
Demographic and clinical characteristics are shown in Panel A. Multivariable-adjusted risk factors for hospitalization in the total cohort are shown in Panel B, and risk factors for hospitalization in the cohort without CKD, MI, or HF are shown in Panel C. All multivariable analyses are adjusted for the covariates displayed in addition to race/ethnicity. Age estimates shown are per 10 years of age. Time from vaccine to infection represents the interval (per 10 days) between the date of last vaccine dose received (i.e. ‘booster’) and the date of COVID-19 infection diagnosed during the Omicron surge period.
ACEi (ACE inhibitor); ARB (angiotensive receptor blocker); COPD (chronic obstructive pulmonary disease); CKD (chronic kidney disease); HF (heart failure); MI (myocardial infarction); CI (confidence interval).
Our findings reveal a persistent and marked association between hypertension and risk for severe COVID-19 illness, even among a fully vaccinated patient population. The Omicron variant of SARS-CoV-2 has led to overall less severe COVID-19 illness in most affected individuals when compared to prior variants – with morbidity and mortality even further reduced by receiving three doses of vaccine. Our findings were consistent with prior studies demonstrating greater hospitalization risk with advanced age and time since last vaccine dose.2 Even when controlling for these and other clinical variables, the risk of hospitalization related to breakthrough Omicron infection was more than doubled by the presence of hypertension. Reconizing that hypertension is quite prevalent in the setting of high-risk conditions such as CKD, MI, and HF, we repeated our analyses exluding patients with these diagnoses and found still substantial and significant risks associated with hypertension. Our findings extend from prior reports of equivocal or potentially confounded associations of hypertension with COVID-19 illness severity that were based on analyzing early-pandemic and particularly pre-Omicron outcomes data.3 In the context of shifts in the risk factors associated with more severe forms of COVID-19 over the course of the pandemic,4 our results indicate persistence and even accentuation of hypertension related risk in the setting of a more transmissible albeit generally less virulent strain of SARS-CoV-2 and in the era of multi-dose vaccination. Although the mechanism for hypertension associated COVID-19 risk remains unclear, prior studies have identified delayed SARS-CoV-2 viral clearance and prolonged inflammatory response among hypertensive patients, which may contribute to greater disease severity.5 Additional studies in separate cohorts are also needed to validate and assess the generalizability of our results. Given that hypertension is one of the most prevalent chronic medical conditions, affecting individuals across the age spectrum, concordant findings would suggest the need for further investigations focused on understanding the hypertension specific risks from SARS-CoV-2 and on identifying individual and population level strategies for mitigating these risks as the pandemic transitions to an endemic.
Acknowledgements.
We are grateful to all the front-line healthcare workers in our healthcare system who continue to be dedicated to delivering the highest quality care for all patients, as well as the invaluable contributions of the CORALE and EMBARC study investigators and staff.
Funding.
This work was supported in part by Cedars-Sinai Medical Center, the Erika J Glazer Family Foundation and NIH grants R01-HL131532 and K23-HL153888.
Footnotes
Ethics Approval. This study was approved by the Cedars-Sinai Institutional Review Board (Study 00000603), with a waiver for informed consent.
Consent for Publication. Not applicable.
Competing Interests. The authors declare that they have no competing interests.
Availability of Data and Materials.
Due to the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in protocols on the protection of human subjects may be sent to Cedars-Sinai Medical Center at biodatacore@cshs.org.
REFERENCES
- 1.Barda N, Dagan N, Cohen C, Hernán MA, Lipsitch M, Kohane IS, Reis BY and Balicer RD. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398:2093–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, Doolman R, Asraf K, Mendelson E, Ziv A, et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. New England Journal of Medicine. 2021;385:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dessie ZG and Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infectious Diseases. 2021;21:855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yek C, Warner S, Wiltz JL, Sun J, Adjei S, Mancera A, Silk BJ, Gundlapalli AV, Harris AM, Boehmer TK, et al. Risk Factors for Severe COVID-19 Outcomes Among Persons Aged ≥18 Years Who Completed a Primary COVID-19 Vaccination Series - 465 Health Care Facilities, United States, December 2020-October 2021. MMWR Morb Mortal Wkly Rep. 2022;71:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trump S, Lukassen S, Anker MS, Chua RL, Liebig J, Thurmann L, Corman VM, Binder M, Loske J, Klasa C, et al. Hypertension delays viral clearance and exacerbates airway hyperinflammation in patients with COVID-19. Nat Biotechnol. 2021;39:705–716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in protocols on the protection of human subjects may be sent to Cedars-Sinai Medical Center at biodatacore@cshs.org.


