Abstract
Purpose:
GEN-1 (phIL-12-005/PPC), an interleukin-12 (IL-12) plasmid formulated with polyethyleneglycol-polyethyleneimine cholesterol lipopolymer, has preclinical activity when combined with platinum-taxane intravenous (IV) chemotherapy and administered intraperitoneally (IP) in epithelial ovarian cancer (EOC) models. OVATION I was a multi-center, non-randomized, open-label phase IB trial to evaluate the safety, preliminary anti-tumor activity, and immunological response to GEN-1 in combination with neoadjuvant chemotherapy (NAC) carboplatin-paclitaxel in patients with advanced EOC.
Patients and Methods:
18 patients with newly diagnosed stage IIIC and IV EOC were enrolled. A standard 3+3 dose-escalation design tested four GEN-1 doses (36, 47, 61, 79 mg/m2) to determine the maximum tolerated dose and dose limiting toxicities (DLT). GEN-1 was administered in eight weekly IP infusions starting at cycle 1 week 2 in combination with three 21-day cycles of NAC carboplatin AUC 6 and weekly paclitaxel 80 mg/m2.
Results:
The most common treatment-emergent adverse events at least possibly related were nausea, fatigue, abdominal pain/cramping, anorexia, diarrhea, and vomiting. Eight patients experience grade 4 neutropenia attributed to NAC. No DLTs occurred. 14 patients were evaluable for response and 12 (85.7%) had radiological response (2CR + 10PR) prior to debulking; 9 were R0 at debulking and one patient had complete pathological response. IL-12 and its downstream cytokine, interferon-γ (IFN-γ), increased in peritoneal washings but not in blood. Increased levels of myeloid dendritic cells and T effector memory cells in peritoneal fluid, plus elevated CD8+ T cells and reduced immunosuppression within the tumor microenvironment were found. A median time to treatment failure (TTF) of 18.4 months (95% CI, 9.2, 24.5) was observed in the ITT population.
Conclusions:
Adding GEN-1 to standard NAC is safe, appears active and impacts the tumor microenvironment.
Keywords: Immunotherapy, IL-12, Ovarian Cancer, Intraperitoneal therapy
Introduction
Epithelial ovarian cancer (EOC) is the 5th deadliest malignancy among women in the United States. [1] There are approximately 22,000 new cases of ovarian cancer every year and the majority, approximately 70% of cases, are diagnosed in advanced stages III and IV. EOC is characterized by dissemination of tumor in the peritoneal cavity with a high risk of recurrence (75%, stage III and IV) after seemingly successful surgery and chemotherapy. [2] Maintenance therapy following a complete or partial remission for patients with a germline or somatic BRCA 1/2 mutation utilizing poly ADP -ribose polymerase inhibitors (PARPi), olaparib or niraparib prolongs time to recurrence. These maintenance therapies have not yet demonstrated improved overall survival. [3][4] Similarly, the angiogenesis inhibitor bevacizumab improves progression free survival as a primary treatment in EOC without improving overall survival. [5] Since the 5-year survival rates of patients with stages III and IV disease at diagnosis are poor, at 41% and 20% respectively, there remains a need for a therapy that not only reduces the recurrence rate but also meaningfully improves overall survival. [1][6]
Immunotherapy interventions are considered promising candidates for the treatment of ovarian cancer considering the immunogenic nature of the malignancy. [7] The evidence of immune activation in ovarian cancer has been demonstrated in the production of antibodies or anti-tumor T-cell lymphocytes in primary tumor, ascites, and blood. [8] [9] [10] Presence of tumor-infiltrating cytotoxic T-cell lymphocytes has been linked to better prognosis while presence of immunosuppressive regulatory T cells (Tregs) has been associated with poor prognosis survival in ovarian cancer. [11] [12] [13] The peritoneal cavity of advanced ovarian cancer patients contains the primary tumor environment and is an attractive target for a regional approach to immune modulation.
Interleukin-12 (IL-12) is a pluripotent cytokine associated with stimulation of innate and adaptive immune response against cancer. Clinical responses to recombinant IL-12 have been observed in multiple cancers. [14] [15] [16] For optimal effect, cytokines must be present over an extended period of time, which is not achievable with recombinant IL-12 due to its short half-life when a single dose is administered in humans. [16] [17] Toxicity is a serious dose limiting concern with systemic exposure, leading researchers to explore alternative means of IL-12 delivery. [18]
GEN-1 is a gene therapy that produces safe and durable local levels IL-12 to stimulate innate and adaptive components of the immune system. The GEN-1 nanoparticle comprises a DNA plasmid encoding IL-12 gene and a synthetic polymer facilitating plasmid delivery. [19] Intraperitoneal administration of GEN-1 in recurrent ovarian cancer patients and in preclinical models of the disease has been shown to elevate IL-12 and its downstream cytokines IFN-γ and TNF-α levels locally at tumor site for several days after a single injection and have resulted in encouraging efficacy results. [20] [21] [22] [23] In these studies GEN-1 was well-tolerated, and unlike previous studies of IL-12 no dose-limiting toxicities (DLT) were identified up to the highest evaluated dose of 36 mg/m2.
Recent preclinical and clinical studies evaluating the timing of immunotherapy suggest that the greatest opportunity for effectiveness is in the neoadjuvant setting. [24] In mouse models neoadjuvant immunotherapy generated persistent levels of tumor-specific CD8+ T cells in the blood even after the mice were tumor free and throughout life. [25] Furthermore, clinically, neoadjuvant chemotherapy (NAC) has been shown to be associated with increased concentrations of CD3+ and CD8+ T cells, CD8+ TIA-1+ T cells, and CD20+ B cells. Meanwhile, the immunosuppressive cells FoxP3+, PD-1+, IDO-1+ cells and CD68+ PD-L1 macrophages remain unchanged. [26]
The OVATION I study evaluated escalating doses of GEN-1 (36, 47, 61, and 79 mg/m2) in combination with a standard carboplatin/paclitaxel neoadjuvant regimen. This setting ensured the patient’s immune system had not been weakened by prior therapies and maximized the potential for complementary or synergistic effects of the immune stimulator and chemotherapy. Moreover, the neoadjuvant setting allowed for collection of treatment naïve and post-treatment primary tumor tissue at interval debulking for translational studies that was not possible in previous GEN-1 studies conducted in recurrent disease.
Patients & Methods
Study design and patient eligibility
The OVATION I Study was a multi-center open-label phase IB trial that enrolled newly diagnosed patients with advanced EOC who were candidates for NAC. The study used a standard 3+3 dose-escalation design to determine the safety, biological activity, and preliminary activity of GEN-1 in combination with a standard neoadjuvant dose dense paclitaxel with tri-weekly carboplatin regimen. A recommended Phase II dose would be based on a MTD or a maximum biological activity dose.
A histologic diagnosis of epithelial ovarian, fallopian tube, or primary peritoneal carcinoma with an epithelial cell type of either: high grade serous adenocarcinoma, endometrioid adenocarcinoma, undifferentiated carcinoma, clear cell adenocarcinoma, mixed epithelial carcinoma, or adenocarcinoma not otherwise specified (See Table 1) was required prior to enrollment. Patients were at least 18 years old, with adequate bone marrow, renal, hepatic, and neurologic functions and had to be free of active infection requiring parenteral antibiotics or a serious uncontrolled medical illness/disorder within four weeks of study entry. Patients were also required to have an ECOG performance score of 0, 1, or 2 and had to be free of any condition/anomaly that would interfere with the appropriate placement of the intraperitoneal (IP) catheter for GEN-1 administration.
Table 1.
Patient Characteristics (N=18)
| Characteristics | |
|---|---|
| Age median (range) | 63.3 (48 – 79) |
| Race/ethnicity | |
| White, non-Hispanic | 15 (83%) |
| Black | 3 (17%) |
| FIGO | |
| IIIC | 12 (67%) |
| IV | 6 (33%) |
| Staging laparoscopy Findings | |
| Omental disease | 14 (78%) |
| Carcinomatosis | 14 (78%) |
| Diaphragmatic carcinomatosis | 10 (56%) |
| Mesenteric retraction | 8 (44%) |
| Bowel infiltration | 7 (39%) |
| Stomach infiltration | 1 (6%) |
| Liver infiltration | 4 (22%) |
| Histology | |
| High grade, serous adenocarcinoma | 17 (95%) |
| Adenocarcinoma NOS | 1 (6%) |
| Homologous-recombination deficiency (HRD) | 5 (28%) |
| BRCA 1 | 1 (6%) |
| BRCA 2 | 1 (6%) |
| HRD Unknown | 1 (6%) |
Patients who were treated previously with GEN-1 or with chemotherapy or radiotherapy for any tumor of the abdominal cavity or pelvis were excluded, as were patients who had received oral or parenteral corticosteroids within 2 weeks of study entry or required ongoing systemic immunosuppressive therapy. Additional exclusion criteria included active autoimmune disease requiring treatment, active hepatitis, other invasive malignancies (other than non-melanoma skin cancer), a history or evidence of CNS disease, and a concurrent severe medical problem unrelated to the malignancy that would expose the patient to extreme risk or decreased life expectancy.
Four centers in the United States participated in OVATION I. This study was sponsored by Celsion Corporation and registered with ClinicalTrials.gov (NCT02480374). The protocol was approved by the institutional review board (IRB) or central IRB and biological safety committees of each institution, and all patients provided written informed consent before enrollment and performance of any study-related procedures. The study was conducted in compliance with the protocol, International Conference on Harmonization Good Clinical Practice Guidelines E6 (ICH-GCP), National Institute of Health (NIH) Guidelines for Research Involving Recombinant DNA Molecules (April 2002) and with the Declaration of Helsinki and its amendments. The study was monitored for safety and dose escalation decisions by an independent Data Safety Monitoring Board (DSMB).
Treatment
This study evaluated four dose levels of GEN-1 [36, 47, 61, and 79 mg/m2 q week intraperitoneal (IP) for up to 8 weeks] in combination with a standard NAC regimen, carboplatin [AUC of 6 mg/mL-min intravenously (IV) q 3 weeks for 3 cycles] and paclitaxel [80 mg/m2 q week IV for 9 weeks]. The starting dose of 36 mg/m2 was chosen since it was the highest dose examined in a previous study combining GEN-1 with pegylated liposomal doxorubicin without evidence of DLT in patients with platinum-resistant ovarian cancer. [22] Eighteen patients were enrolled, and an IP catheter with subcutaneous reservoir was implanted. GEN-1 dosing commenced on cycle one day eight in order to skip administration during dexamethasone pretreatment given on cycle one day one. Steroids may blunt the effects of immunotherapies such as GEN-1; therefore, concurrent administration was not permitted. [27] The DSMB met to review safety data for subjects in each cohort before the next highest dosage strength was assessed. Patients had to have at least four doses of GEN-1 in order to be evaluated for safety at each dose level. Treated patients were planned to undergo interval debulking surgery after three cycles of NAC with weekly GEN-1 followed by three additional cycles of adjuvant chemotherapy. The dose escalation was stopped at 79 mg/m2 due to limitations in the manufacturing of additional doses. These limitations were overcome for a subsequent trial of GEN-1 currently ongoing where the starting dose has been determined to be 100 mg/m2. [28]
Assessments
Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 and dose limiting toxicities were defined in the Data Safety Monitoring Board charter. [29] Clinical endpoints included objective response rate (RECIST version 1.1), R0 resection rate, pathological response, chemotherapy response score (CRS) and progression free survival (PFS) per physician assessment. [30] [31] [32] All enrolled patients were assessed for toxicity however patients had to complete four administrations of GEN-1 to be considered evaluable for a dose level.
Tumor, ascites, and blood samples were collected at time of initial staging and at interval debulking surgery. Venous blood and peritoneal fluid samples (via intraperitoneal port) prior to and 24 hours after each of the first four GEN-1 treatments. Cytokine ELISAs, flow cytometry, and immunohistochemical analyses were conducted at Roswell Park Cancer Institute (Buffalo, NY).
Immunohistochemistry (IHC)
IHC assays and automated digital pathology analysis were performed on FFPE tissue blocks. Tissue sections (4–5 μm) were prepared on slides loaded on a DAKO autostainer (Dako, Glostrup, Denmark) and after serum free protein block the respective primary antibodies for CD3, CD4, CD8, FoxP3, IDO-1, PD-1 and PD-L1 were applied separately. The EnVision+ horseradish peroxidase system (Dako) and DAB chromogen were used for visualization. Slides were digitally scanned using Aperio Scanscope (Aperio Technologies, Inc., Vista, CA) with 20× bright-field microscopy. These images were then accessible using Spectrum (Aperio Technologies, Inc., Vista, CA), a web-based digital pathology information management system. Slide images are automatically associated to a digital slide created in the Digital Slide table in Aperio eSlide Manager. Once slides are scanned, Aperio ImageScope version 11.2.0.780 (Aperio Technologies, Inc., Vista, CA) was used to view images for image analysis. An outline of the tumor and the size of the analysis area were defined, and the lymphocytes were counted using an optimized algorithm for each stain and results were normalized to number of lymphocytes per square millimeter.
ELISA
Cytokine levels in plasma and peritoneal fluid were measured using ELISA kits according to the manufacturer’s protocol. IL-12 ELISA kit was purchased from R&D Systems (Minneapolis, MN) and IFN-γ ELISA kit was purchased from Thermo Fisher Scientific (Waltham, MA). For peritoneal fluid or wash samples, the cytokine data was normalized with total protein concentration which was quantified with a Pierce BCA protein assay kit from Thermo Fisher (Waltham, MA). Optical Density of triplicate wells was read by BioTek Synergy HT microplate reader (Winooski, VT) and concentration was determined from standard curve. The cytokine values generated from each treatment were used to generate the overall mean for each cohort.
Flow Cytometry
Peripheral blood mononuclear cells (PBMC) and cells from peritoneal fluid were isolated by density gradient centrifugation and cryopreserved in liquid nitrogen freezer until analysis. The cells were stained with fixable viability stain 700 (BD Horizon) and incubated with FcR block (Myltenyi Biotech) followed by staining with various antibodies for T-cell phenotype (APC/H7-CD45, PerCP/Cy5.5-CD3, BUV395-CD4, BV650-CD45RA and BV421-CCR7) and myeloid dendritic cells (APC/H7-CD45, PE-CD11c and BV650-CD123). The stained cells were acquired by BD Fortessa flow cytometer and analyzed using FlowJo software.
Statistical design
This study utilized a standard 3 + 3 design to identify the MTD of GEN-1 with NAC in patients with newly diagnosed EOC. Dose escalation would be considered by the DSMB after a cohort of at least three patients who received at least 4 doses of GEN-1 were evaluated. If no patients out of three in a specific dose level demonstrated a DLT, then the study can proceed to the next higher dose level. If one patient out of three demonstrated a DLT, an additional three patients were to be enrolled at that dose level. If two or more patients experience a DLT then the GEN-1 dose must be dropped to the previous level. Less than 2 patients of six can experience a DLT before declaring a maximum tolerated dose. PFS is defined as the duration of time from start of treatment (cycle 1 day 1) to time of progression or death, whichever occurs first. The data was expressed as the median value for the intent to treat or per protocol population. Resection scores were expressed as a percentage of total patients for a defined group. Translational data was tabulated as mean ± standard deviation except for instances where average values were plotted for trend analysis and where comparisons are expressed as a percentage of baseline. Data were analyzed using Student’s t-test. For comparison of pre-treatment and post-treatment paired samples, Wilcoxon matched pairs signed rank test was used, after checking that for most differences in cell density, the normality assumption was violated.
Results
Four centers enrolled 18 patients between September 2015 and May 2017 (ITT). Six patients did not receive the full regimen of GEN-1 treatments due to: port related infection (0 GEN-1 doses), bowel perforation (1 dose of GEN-1), bowel obstruction (1 dose of GEN-1), dosing delays of >21 days due to myelotoxicity (6 doses of GEN-1), sepsis and congestive heart failure (5 doses of GEN-1) and altered taste (5 doses of GEN-1). One patient voluntarily withdrew from GEN-1 treatment due to altered taste attributed to GEN-1. In addition, one patient died within 40 days of her first dose of chemotherapy due to complications of the ovarian cancer. As a result, 15 patients were evaluable for safety of dose (received 4 administrations of GEN-1) and 14 underwent interval debulking thus were evaluable for RECIST, for resection status, and pathological response (per protocol population). Biological samples (i.e., blood, ascites/peritoneal washes, and tumor tissue) were collected from 12 patients who received the full complement of 8 doses of GEN-1 for translational data.
Safety
The DSMB reviewed the safety data at the completion of every dosing cohort which would comprise of at least three patients who completed at least 4 doses of GEN-1. Table 2 summarizes the adverse events that were at least possibly related to GEN-1 by frequency and severity for those 15 patients treated with at least 4 doses of GEN-1. In general, all dose levels were well tolerated. Most AEs were grade 1 or 2 in nature. The most commonly reported adverse events at least possibly attributed to GEN-1 in descending order include nausea (67%), fatigue (53%), abdominal pain/cramping (40%), anorexia (40%), diarrhea (40%), and vomiting (40%). Of the grade 3 and 4 reported AEs, the following were at least possibly attributed to GEN-1: nausea (n =2), fatigue (n=2), abdominal pain/cramping (n=1), anorexia (n=1), diarrhea (n=2), dehydration (n=2), vomiting (n=1), hypokalemia (n=1), sepsis (n=1), and vasovagal reaction (n=1). Hematological toxicities were also reported as possibly being attributed to GEN-1 which were also associated with chemotherapy. There were no dose limiting toxicities detected and a MTD was not reached at the doses evaluated in this study. Limiting dexamethasone premedication to cycle one day 1 did not result in any clinically significant hypersensitivity reactions at subsequent cycles of chemotherapy administration. A supplementary table is provided which presents all adverse events for all patients (N=18) regardless of attribution.
Table 2.
Frequency and Severity* of Adverse Events At Least Possibly Related to GEN-1 (N=15)
| Term | Frequency | Percentage | Grade 1 – 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|---|
| Nausea | 10 | 67% | 8 | 2 | 0 | 0 |
| Fatigue | 8 | 53% | 6 | 2 | 0 | 0 |
| Neutropenia** | 8 | 53% | 2 | 1 | 5 | 0 |
| Abdominal Pain / Cramping | 6 | 40% | 5 | 1 | 0 | 0 |
| Leukopenia** | 6 | 40% | 3 | 2 | 1 | 0 |
| Anorexia | 6 | 40% | 5 | 1 | 0 | 0 |
| Diarrhea | 6 | 40% | 4 | 2 | 0 | 0 |
| Vomiting | 6 | 40% | 5 | 1 | 0 | 0 |
| Anemia** | 6 | 40% | 2 | 4 | 0 | 0 |
| Thrombocytopenia** | 5 | 33% | 2 | 1 | 2 | 0 |
| Weakness | 5 | 33% | 5 | 0 | 0 | 0 |
| Chills | 4 | 27% | 4 | 0 | 0 | 0 |
| Fever | 4 | 27% | 4 | 0 | 0 | 0 |
| Dehydration | 3 | 20% | 1 | 2 | 0 | 0 |
| Hypomagnesemia | 3 | 20% | 3 | 0 | 0 | 0 |
| Constipation | 2 | 13% | 2 | 0 | 0 | 0 |
| Dysguesia | 2 | 13% | 2 | 0 | 0 | 0 |
| Hypokalemia | 2 | 13% | 1 | 1 | 0 | 0 |
| Alopecia | 1 | 7% | 1 | 0 | 0 | 0 |
| Creatinine Increased | 1 | 7% | 1 | 0 | 0 | 0 |
| Cytokine Release Syndrome | 1 | 7% | 1 | 0 | 0 | 0 |
| Dizziness | 1 | 7% | 1 | 0 | 0 | 0 |
| Dyspnea | 1 | 7% | 1 | 0 | 0 | 0 |
| Elevated Alkaline Phosphatase | 1 | 7% | 1 | 0 | 0 | 0 |
| Elevated ALT | 1 | 7% | 1 | 0 | 0 | 0 |
| Elevated AST | 1 | 7% | 1 | 0 | 0 | 0 |
| Erythema (port site) | 1 | 7% | 1 | 0 | 0 | 0 |
| Hot Flash | 1 | 7% | 1 | 0 | 0 | 0 |
| Hyperglycemia | 1 | 7% | 1 | 0 | 0 | 0 |
| Hypocalcemia | 1 | 7% | 1 | 0 | 0 | 0 |
| Nasal Congestion | 1 | 7% | 1 | 0 | 0 | 0 |
| Neuropathy | 1 | 7% | 1 | 0 | 0 | 0 |
| Pain | 1 | 7% | 1 | 0 | 0 | 0 |
| Port Infection | 1 | 7% | 1 | 0 | 0 | 0 |
| Sepsis | 1 | 7% | 0 | 0 | 1 | 0 |
| Sinus Disorder | 1 | 7% | 1 | 0 | 0 | 0 |
| Tremor | 1 | 7% | 1 | 0 | 0 | 0 |
| Vasovagal Reaction | 1 | 7% | 0 | 1 | 0 | 0 |
Common Terminology Criteria for Adverse Events (CTCAE) version 4.0
Hematological toxicities were also reported as possibly being attributed to GEN-1 which were also associated with chemotherapy
Clinical Response
All patients were evaluated for efficacy (ITT) and a per protocol assessment was conducted for patients who underwent interval debulking. Time to treatment failure (TTF) of 18.4 months with 95% CI (9.2, 24.5) (range: 0.1–48.4 months) was observed in the ITT population (N=18) while a TTF of 21 months with 95% CI (11.5, 33.8) (range: 9.3–48.4 months) in the per protocol population (n=14).
Table 3 presents radiographic tumor response, surgical outcome, pathological response, and chemotherapy response score by dose. Objective response rates (ORR) to NACT/GEN-1 appeared to favor higher doses of GEN-1 between the two high and two low dose cohorts as calculated for RECIST-evaluable patients, with 100% of the high-dose cohorts having a complete or partial response (1 CR and 7 PR) and 67% of the low-dose patients (1 CR and 3 PR). Patients in the high-dose cohort achieved 88% R0 resection, versus 33% in the low-dose cohorts. There was a single case of a complete pathological response at 36 mg/m2. Similarly, pathological response favored the higher dose cohort with 50% of subjects achieving the optimal chemotherapy response score (CRS) of 3 while only 17% of patients did so at the lower doses. In addition, one patient remains progression free at 4 years of follow up.
Table 3.
Tumor Response, Surgical Outcome, Pathological Response and Chemotherapy Response Score with NAC/GEN-1 escalating doses.
| Radiographic Response | Total (n) | Cohort 1 36 mg/m2 |
Cohort 2 47 mg/m2 |
Cohort 3 61 mg/m2 |
Cohort 4 79 mg/m2 |
|
|---|---|---|---|---|---|---|
| Tumor Response | CR | 2 | 1 | 0 | 0 | 1 |
| PR | 10 | 0 | 3 | 3 | 4 | |
| SD | 2 | 2 | 0 | 0 | 0 | |
| Objective Response Rate | 67% | 100% | ||||
| Surgical Outcome | R0 | 9 | 2 | 0 | 2 | 5 |
| R1 | 3 | 1 | 2 | 0 | 0 | |
| R2 | 2 | 0 | 1 | 1 | 0 | |
| R0 Resection Rate | 33% | 88% | ||||
| Pathological Response | cPR | 1 | 1 | 0 | 0 | 0 |
| Micro | 8 | 1 | 2 | 1 | 4 | |
| Macro | 5 | 1 | 1 | 2 | 1 | |
| cPR/Micro Rate | 60% | 63% | ||||
| Chemotherapy Response Score | CRS 3 | 5 | 1 | 0 | 2 | 2 |
| CRS 2 | 5 | 2 | 1 | 0 | 2 | |
| CRS 1 | 4 | 0 | 2 | 1 | 1 | |
| CRS 3 Rate | 17% | 50% | ||||
Translational Responses
GEN-1 intraperitoneal treatment increased IL-12 and IFN-γ levels in ascites in a dose-response manner, as shown in Figure 1. IL-12 levels in ascites increased 3.2.- and 23-fold at the lowest and highest GEN-1 doses (36 and 79 mg/m2) respectively, while IFN-γ levels rose 3.1- and 67-fold. The differences in IFN-γ increase between the various dose levels were statistically significant. The increase in IL-12 levels followed a similar dose pattern, but the differences between various dose levels were statistically not as significant as seen with IFN-γ. As expected, GEN-1 had a much smaller effect on IL-12 and IFN-γ expression in the blood, where both cytokines’ levels increased between 1.2- and 3.1-fold (Supplementary Figure 1). (The graphs present data from patients in RECIST-evaluable cohorts of Table 2 with one exception – a subject in the 79 mg/m2 cohort could not be assayed.)
Figure 1.
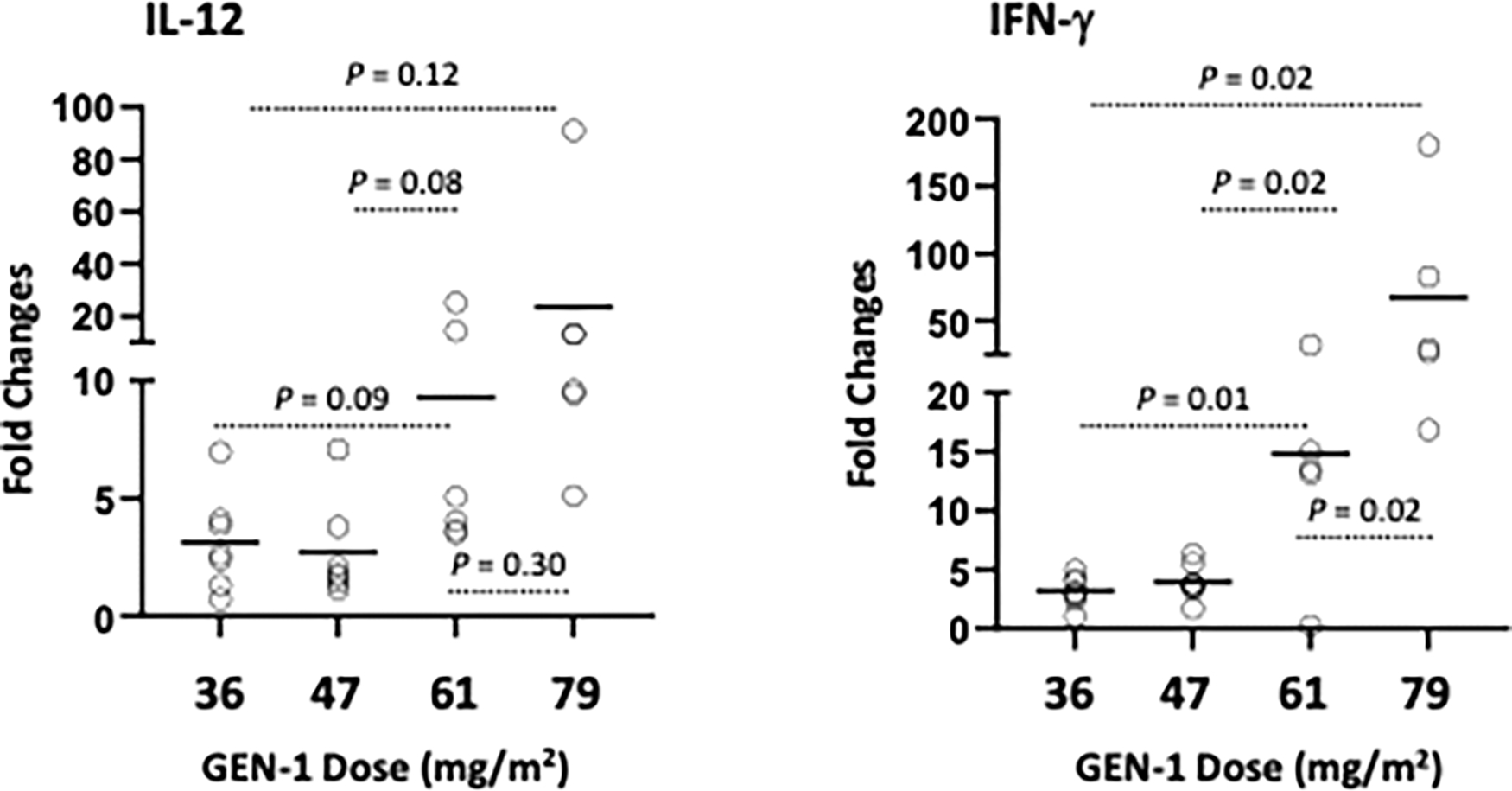
Changes in IL-12 and IFN-γ levels in ascites/peritoneal wash before and 24 hours after IP administration of GEN-1 and expressed relative to total protein in the sample (pg/mg protein). Fold changes over pre-treatment values for individual samples are represented by circles and the mean for each group is represented by the solid lines. The differences between the dose groups were determined by the Student t test.
The effects of GEN-1/NAC included reductions in four immunosuppressive markers in the tumor microenvironment: forkhead box p3 (Foxp3), indoleamine 2,3-dioxygenase-1 (IDO1), programmed cell death protein-1 (PD-1), and programmed death-ligand1 (PD-L1). The pre- and post-treatment density of cells with immunosuppressive markers on an individual patient basis are shown in Fig. 2A–D, and % changes from pre-treatment values for each patient are provided in Fig. 2E. The combination regimen reduced the cell density of all four indicators of immunosuppression between the initial biopsy sample and tumor resection in majority of patients. Reduction in FoxP3, IDO1, PD-1, and PD-L1 was observed in 67%, 67%, 83%, and 67% of patients, respectively. The range of inhibition in these patients varied from 8–95% (Foxp3), 33–94% (IDO1), 20–92% (PD-1) and 37–98% (PD-L1). Although from a small sample size, these trends are instructive since the Foxp3, IDO1, and PD-1 changes occurred generally in a GEN-1 dose-dependent manner (Fig. 2F) and are consistent with the changes in IL-12 and IFN-γ levels in ascites. The ratio of CD8+ cells to each of the four immunosuppressive markers also increased in majority of patients (Fig. 3 A–D). The increase in CD8+/Foxp3, CD8+/IDO1, CD8+/PD-1 and CD8+/PD-L1 ratio was observed in 91%, 75%, 67%, and 75% of patients, respectively. The increase in CD8+/Foxp3 and CD8+/PD-1 ratio was statistically significant with p-values of 0.016 and 0.03, respectively. The percent change in the ratio of CD8+ cells to immunosuppressive markers is plotted in Fig 3E, demonstrating a positive shift in CD8+ ratios in majority of patients.
Figure 2.

Changes in immunosuppressive markers in the tumor microenvironment following GEN-1 and NAC treatment. Changes in Foxp3 (A), PD-1 (B), IDO1 (C), and PD-L1 (D) immune cell markers before treatment (pre) and after treatment and at debulking surgery (post) for individual patients. E. Percent changes in each of the cell types. F. Percent changes between low dose group (36 mg/m2, 47 mg/m2) (n=4) and high dose group (61 mg/m2, 79 mg/m2) (n=8). The paired comparison of pre- and post-treatment values was done by using Wilcoxon matched pairs signed rank test.
Figure 3.
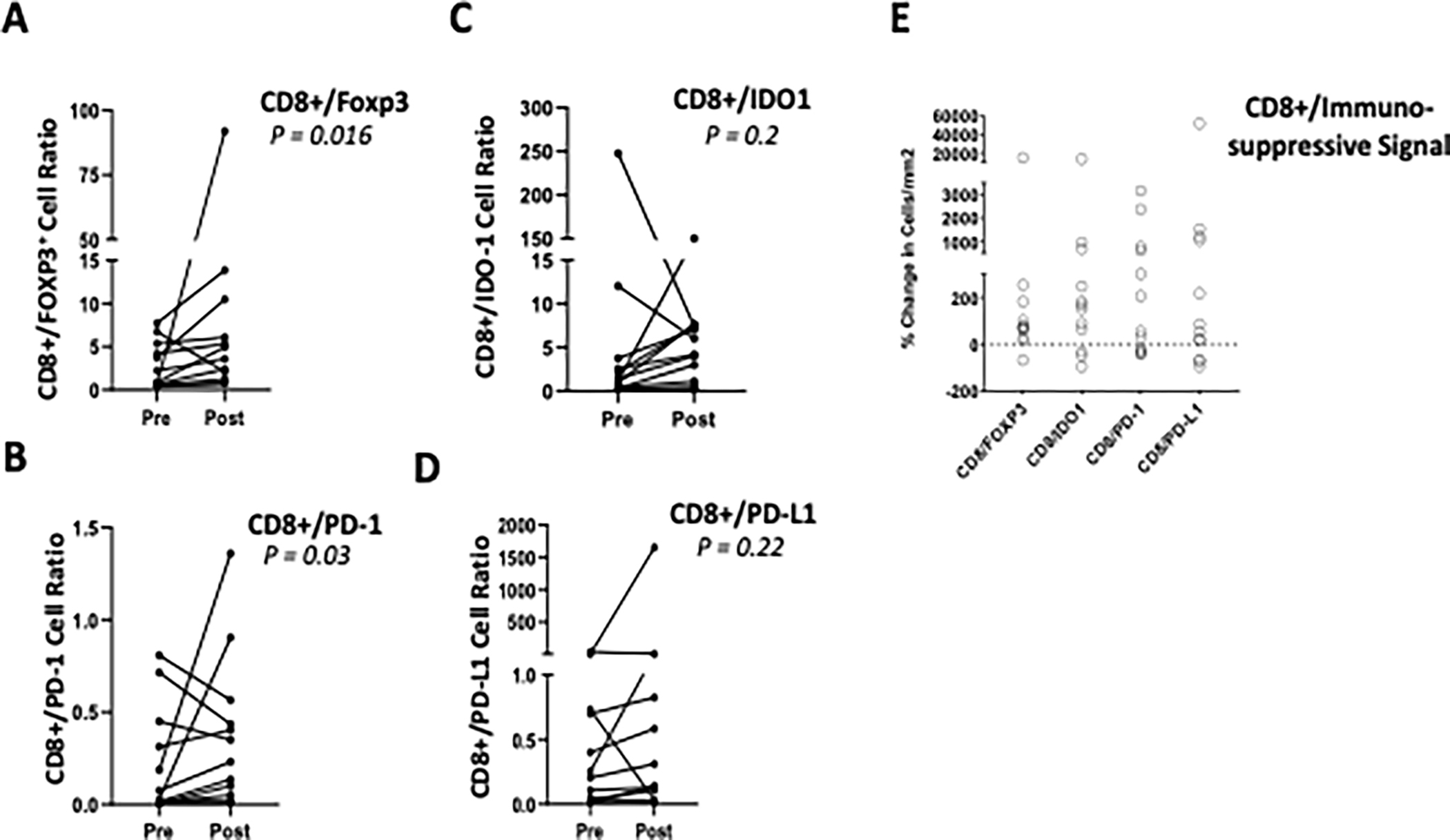
Changes in the ratio of CD8+ cells to immunosuppressive markers following GEN-1 and chemotherapy treatment. A-D. Changes in the ratio of CD8+ T cells to FoxP3, PD-1, IDO1, and PD-L1 markers in tumors of individual patients before treatment (pre) and after treatment and at debulking surgery (post). E. Percent change in CD8+/Foxp3, CD8+/PD-1, CD8+/IDO1, CD8+/PD-L1 ratio before (pre) and after treatment (post) for individual patients. The paired comparison of pre- and post-treatment values was done by using Wilcoxon matched pairs signed rank test.
GEN-1/NAC therapy also altered the densities of CD4+ and CD8+ T cells in tumor specimens collected at enrollment and during debulking surgery. The pre- and post-treatment density of CD4+ and CD8+ cells and the CD8+/CD4+ cell ratio on an individual patient basis and the mean values (n=11 for CD4+ and n=12 for CD8+) are shown in Fig. 4. The CD8+ cell density increased in 67% of patients, CD4+ cell density decreased in 72% of patients, and the CD8+/CD4+ ratio increased in 82% of patients (Fig. 4A–C). These changes noted on an individual patient basis are consistent with the mean values where the CD8+ T cell density increased 53% between pre- and post-treatment versus a decline of 73% in CD4+ T cells (Fig. 4D). Thus, the ratio of CD8+/CD4+ T cells increased 483% from 0.63 prior to GEN-1/NACT to 3.54 after. Again, these changes, although from a small sample size, are consistent with reports of an increased CD8+ T cell density after NAC. [26]
Figure 4.
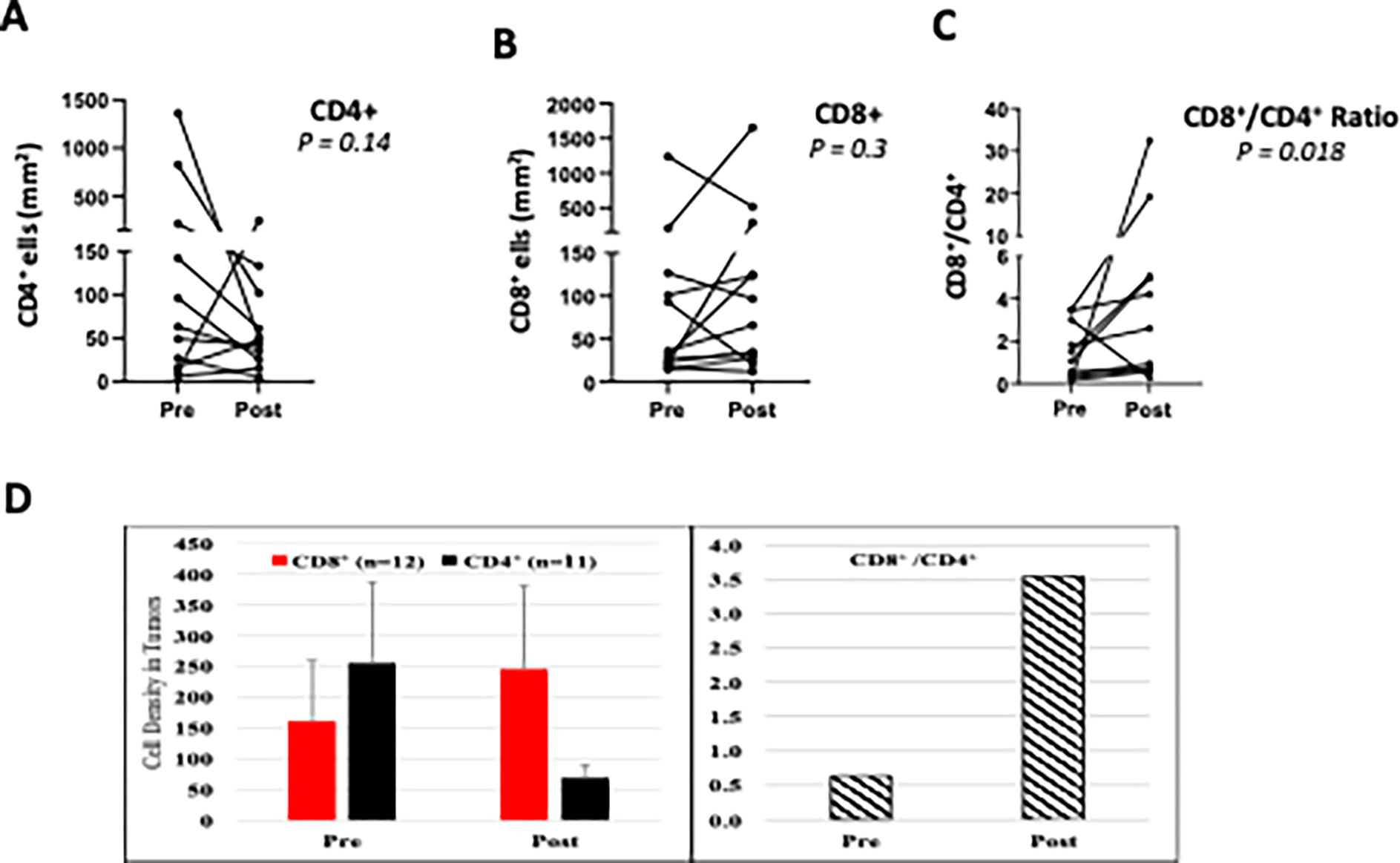
Changes in CD4+ and CD8+ cell density following GEN-1 and chemotherapy treatment. A-B. Changes in CD4+ and CD8+ cell density in individual patients before treatment (pre) and after treatment and at debulking surgery (post). C. CD8+/CD4+ cell ratio at pre- and post-treatment. D. The paired comparison of pre- and post-treatment values was done by using Wilcoxon matched pairs signed rank test.
An assessment of the effects of GEN-1/NAC on immune cells in ascites found favorable trends in myeloid dendritic cell (mDC) and effector memory T cell (TEM) populations. The pre- and post-treatment density of mDC and TEM cells (mDC: CD45+dump (CD3/CD56/CD19/CD14) −CD123− CD11c+ and TEM: CD45+CD3+CD4−CD8+CD45RA− CCR7−) on an individual patient basis are shown in Fig. 5 A–B. For both mDC and TEM the cell density increased in 4 out of 5 patients, and the percentage of total cells (mean ± SE, n=5) increased approximately 3-fold in response to therapy (Fig. 5C).
Figure 5.

The effect of GEN-1 on myeloid dendritic cells and CD8+ TEM cells in peritoneal fluid. A-B. Changes in myeloid dendritic cells and CD8+ TEM cell density in individual patients (n=5) before treatment (pre) and after treatment at debulking surgery (post). C. The average pre- and post-treatment data. The paired comparison of pre- and post-treatment values was done by using Wilcoxon matched pairs signed rank test.
Discussion
Escalating GEN-1 to doses up to 79 mg/m2 IP administered weekly for up to 8 dosages was safe and reasonably tolerated when administered in combination with neoadjuvant chemotherapy in newly diagnosed epithelial ovarian cancer patients. There were no dose-limiting toxicities detected and an MTD was not reached. The majority of adverse events attributed to GEN-1 were low grade, and manageable. These safety findings are consistent with previous studies of GEN-1 in patients with ovarian cancer. [20] [21] [22] Moreover, the OVATION I study data supports that GEN-1 may be safely administered with standard neoadjuvant chemotherapy.
The safety profile of GEN-1 is consistent with its local administration and appears to have a distinct advantage over recombinant IL-12 therapy which is associated with systemic dose limiting toxicity. The activity of intraperitoneally administered GEN-1 appears to be localized to the peritoneal cavity and draining lymph nodes and potentially in the resident B cells, macrophages and dendritic cells. [33] The increases detected in IL-12 and IFN-γ levels were primarily peritoneal with relatively little increases in systemic circulation resulting in this favorable safety profile.
There appears to be a suggestion of clinical activity with the addition of GEN-1 to NAC with the apparent high degree of R0 resection rates and the median times of progression free survival seen in the study subjects. Our population included five subjects that tested homologous-recombination deficient (Table 1) with three receiving niraparib during maintenance and a fourth was randomized to either niraparib or placebo in a subsequent clinical study. Historically, large, randomized studies of patients with EOC treated similarly with NAC have reported an R0 rate of about 50% and a median time to progression of 12 months. [34] [35]
The results from our translational studies show activation of a multitude of immune responses following GEN-1 + NAC treatment. First, there was a dose-dependent increase in powerful immunostimulatory cytokines IL-12 and its downstream cytokine IFN-γ in ascitic fluid. The anti-cancer effects of these cytokines have been widely recognized in human malignancies. [36] The dose dependence of the cytokine response to GEN-1 at a fixed NAC dose suggests that it is GEN-1 related. Additionally, given the accumulation of the IL-12 plasmid in mesenteric lymph nodes in an animal model, it is likely that GEN-1 had a similar effect on the stimulatory cytokines in those key secondary lymphoid organs where ovarian metastases commonly occur). [33] Second, the proportion of myeloid dendritic cells in the peritoneal fluid trended higher, by 3.1-fold, accompanied by a similar 3.0-fold rise in CD8+ TEM cells. Such concomitant changes in these cell types is noteworthy, given the important role of antigen-presenting cells in stimulating a cytotoxic T cell response and fostering immunological memory. Third, GEN-1 appeared to reduce four immunosuppressive signals (Foxp3, IDO1, PD-1 and PD-L1) within the tumor microenvironment, a trend not seen with NAC therapy. [9] [10] [11] [12] [13]
Finally, the GEN-1 gene therapy was associated with an apparent increase in the cytotoxic state of T cells within the tumor microenvironment as indicated by the increases in the ratios of CD8+/CD4+ and CD8+/Treg cells. Indeed, higher CD8+/CD4+ T cell and CD8+/Treg ratios have been considered prognostic for prolonged survival. [12] [13] [37] The OVATION I study results are consistent with the known activities of IL-12 and its related downstream cytokines IFN-γ including a reduction in the production of IL-2 which is required for proliferation of immunosuppressive Treg cells. As such, these cytokines render ovarian cancer cells more sensitive to platinum chemotherapy by inhibiting cancer-associated fibroblasts’ production of glutathione and cysteine, and conversion of tumor-associated macrophages from the immunosuppressive M2 to the anti-tumor M1 phenotype. [38] [39] [40].
The immune changes resulting from GEN-1 + NAC treatment are distinct from those reported with NAC therapy in epithelial ovarian cancer. First, the intraepithelial CD4+ and CD8+ T cell densities following neoadjuvant chemotherapy increased or remained unchanged, and the CD8+/CD4+ ratio remain unchanged. [13] [26] [41] [42] In comparison, our study shows an increase in CD8+ cell density, decrease in CD4+ cell density and increase in CD8+/CD4+ ratio in majority of patients, although the magnitude of the increase highly varied from patient to patient. These data suggest that the addition of GEN-1 to NAC produces a different pattern of immune response that is not typically associated with the neoadjuvant chemotherapy alone. Second, the neoadjuvant chemotherapy increased or produced no change in immunosuppressive markers. [13] [26] [41] [42] In one of the studies, NAC produced a doubling of CD8+ cell density but failed to relieve the immunosuppression markers including FoxP3+, IDO1, PD-1, PD-L1 in tumor tissue. [26] In another study, NAC-treated tumors had higher PD-L1 expression on tumor infiltrating immune cells and persistent high levels of PD-1- and CTLA-4- expressing cells. [41] In a separate study, NAC therapy increased tumor infiltrating lymphocytes but did not affect FoxP3 cells. [13] In another study, NAC increased PD-L1 positive cells from 30% to 53%. [42] These studies consistently demonstrate an increase or no change in the immunosuppressive signals following neoadjuvant chemotherapy in advanced epithelial ovarian cancer. In comparison, the present study demonstrates a reduction in FoxP3, IDO1, PD-1, and PD-L1 signals and an increase in the ratio of CD8+ cells to FoxP3, IDO1, PD-1 and PD-L1 cells in majority of patients suggesting GEN-1 may have a role in the observed immune changes in NAC combination setting. A decrease in immunosuppressive markers in this study is consistent with the inhibitory action of IL-12 on PD-1 expression in malignant melanoma and peripheral lymphocytes and on FoxP3 and other regulatory T cells in lymphocyte cultures. [43] [44] [45]
A parallel increase in IFN-γ and decrease in PD-1/PDL-1 after GEN-1/NAC therapy is interesting since an increase in IFN-γ has been associated with upregulation of PD-1/PD-L1 expression in the action of some immunotherapy agents. [46] The mechanism of a parallel increase in IFN-γ and inhibition of immunosuppressive markers following GEN-1 treatment in the present study warrants further investigation. However, there is some evidence in the literature to suggest that these parallel effects could be explained by dual action of IL-12 on immune cells. IL-12 increases IL-2 receptor expression on CD4+ and CD8+ cells to produce IFN-γ but diminishes its expression on immunosuppressive T cells resulting in the starvation of immunosuppressive T cells thereby favoring the outgrowth of non-Treg cells. [45] In another study, IL-12-stimulated IFN-γ production from CD8+ cells and countered IFN-γ-mediated PD-L1 expression by downregulating IFN-γ receptors. [47] The stimulation of IFN-γ and inhibition of immunosuppressive markers in our study may be explained by dual actions of IL-12 involving IFN-γ-independent mechanisms. [48]
The multifactorial nature of GEN-1 immune response built on a durable local production of IL-12 may be activating the innate and adaptive immune system creating a unique tumor microenvironment potentially favorable to anti-tumor responses and also conducive to other therapeutic drugs that may be suboptimal as single agents due to highly immunosuppressive tumor microenvironment in ovarian cancer. For example, checkpoint inhibitors despite having demonstrated activity in some cancer types are only of limited to modestly active in ovarian cancer. [49] [50] Combination with GEN-1 could potentiate CD8+ T cell infiltration and reduce immunosuppressive tumor microenvironment to improve the efficacy of checkpoint inhibitors and produce an overall better quality of clinical response against cancer. Similarly, the efficacy of adaptive T-cell therapies may also be improved by remodeling the peritoneal cavity with GEN-1 pre-treatment reducing the tumor immunosuppressive environment and improving the T-cell survival and clinical efficacy. Similarly, other novel combinations with GEN-1 may also be investigated to improve clinical outcome in ovarian cancer.
This study was limited by its small sample size and termination of dose escalation at 79 mg/m2 even though a MTD was not achieved. A maximum biological dose was not established as well. Another limitation is that a control arm was not employed to evaluate the full impact of GEN-1 on the immune response as well as therapeutic response when GEN-1 is combined with chemotherapy. Future studies will evaluate GEN-1 at higher doses in this patient population with a control group to address these limitations.
In conclusion, weekly intraperitoneal GEN-1 treatment in conjunction with standard NAC in advanced epithelial ovarian cancer patients is safe, well-tolerated and appears to be active. Repeated durable increases in lL-12 and IFN-γ levels at tumor site for an eight-week treatment period provides for an unprecedented pharmacology remodeling of the tumor microenvironment as evidenced by reduction in immunosuppressive signals Foxp3, IDO1, PD-1 and PD-L1 and potentiation of immunostimulatory signals including the increases in the ratios of CD8+/CD4+ and CD8+/Treg cells and increases in myeloid dendritic cells and CD8+ TEM cells. These immunomodulatory effects of GEN-1 may result in an increased sensitivity of tumor microenvironment to other anti-cancer agents including cytotoxic drugs and immunotherapies such as checkpoint inhibitors and adaptive T cell therapies. OVATION II (NCT03393884) is a phase I/II study of concurrent GEN-1 at a dose of 100 mg/m2 weekly for up to 17 doses administered during chemotherapy and is currently actively accruing.
Supplementary Material
Acknowledgements:
This work was partially conducted at Roswell Park’s Pathology Network and Immune Analysis Shared Resources, P30CA016056.
Footnotes
Conflict of Interest: P.H. Thaker, W.H. Bradley and C.A. Leath, III are advisory board members for Celsion. N. Borys, K. Anwer, and L. Musso are employees of Celsion Corporation. No potential conflicts of interest were disclosed by the other authors.
Presented as an Oral Presentation at the 2019 ASCO-SITC Clinical Immuno-Oncology Symposium, San Francisco, CA
References
- [1].Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ and Cronin KA, “SEER Cancer Statistics Review, 1975–2017, National Cancer Institute. Bethesda, MD,” [Online]. Available: https://seer.cancer.gov/csr/1975_2017. [Accessed 16 October 2020]. [Google Scholar]
- [2].du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I and Pfisterer J, “Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials,” Cancer, vol. 115, no. 6, pp. 1234–1244, 2009. [DOI] [PubMed] [Google Scholar]
- [3].Moore KN, Colombo N, Scambia G, Kim B-G, Oaknin A, Friedlander M, Lisyanskaya AS, Floquet A, Leary A, Sonke GS, Gourley C, Banerjee S, Oza AM, González-Martín A, Aghajanian C, Bradley WH, Mathews C, Liu JF, Lowe ES, Bloomfield R and DiSilvestro P, “Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer.,” The New England Journal of Medicine, vol. 379, no. 26, pp. 2495–2505, 2018. [DOI] [PubMed] [Google Scholar]
- [4].Pothuri B, Vergote I, Christensen RD, Gonzalez-Martin A, Greybill W, Mirza MR, McCormick C, Lorusso D, Hoskins P, Freyer G, Baumann K and Jardon K, “Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer,” The New England Journal of Medicine, no. 381, pp. 2391–2402, 2019. [DOI] [PubMed] [Google Scholar]
- [5].Tewari KS, Burger RA, Enserro D, Norquist BM, Swisher EM, Brady MF, Bookman MA, Fleming GF, Huang HQ, Homesley HD, Fowler JM, Greer BE, Boente MP, Liang SX, Ye C, Bais C, Randall LM, Chan JKC, Ferriss JS, Coleman RL, Aghajanian C, Herzog TJ, DiSaia PJ, Copeland LJ, Mannel RS, Birrer MJ and Monk BJ, “Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer,” Journal of Clinical Oncology, vol. 37, no. 26, pp. 2317–2328, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Siegel RL, DeSantis C, Virgo KS, Stein K, Mariotto AB, Smith T, Cooper DL, Gansler T, Lerro CC, Fedewa SA, Lin CC, Leach CR, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch RA, Jemal A and Ward EM, “Cancer treatment and survivorship statistics, 2012,” CA: A Cancer Journal for Clinicians, vol. 62, no. 4, pp. 220–241, 2012. [DOI] [PubMed] [Google Scholar]
- [7].Ghisoni E, Imbimbo M, Zimmermann S and Valabrega G, “Ovarian Cancer Immunotherapy: Turning up the Heat,” International Journal of Molecular Sciences, vol. 20, no. 12, p. 2927, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fortner RT, Damms-Machado A and Kaaks R, “Systematic review: Tumor-associated antigen autoantibodies and ovarian cancer early detection,” Gynecologic Oncology, vol. 147, no. 2, pp. 465–480, 2017. [DOI] [PubMed] [Google Scholar]
- [9].Singh M, Loftus T, Webb E and Benencia F, “Minireview: Regulatory T Cells and Ovarian Cancer,” Immunological Investigations, vol. 45, no. 8, pp. 712–720, 2016. [DOI] [PubMed] [Google Scholar]
- [10].Odunsi K, “Immunotherapy in Ovarian Cancer,” Annals Oncology, vol. 28, no. Supplement 8, pp. viii1–viii7, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Toker A, Nguyen LT, Stone SC, Yang SYC, Katz SR, Shaw P, Clarke BA, Ghazarian D, Al-Habeeb A, Easson AM, Leong WL, McCready DR, Reedijk M, Reedijk M, Reedijk M, Guidos CJ, Pugh TJ, Pugh TJ, Bernardini MQ, Ohashi PS and Ohashi PS, “Regulatory T Cells in Ovarian Cancer Are Characterized by a Highly Activated Phenotype Distinct from that in Melanoma,” Clinical Cancer Research, vol. 24, no. 22, pp. 5685–5696, 2018. [DOI] [PubMed] [Google Scholar]
- [12].Curiel TJ, Coukos G and Zhou L, “Specific Recruitment of Regulatory T-cells in Ovarian Carcinoma Foster Immune Privilege and Predicts Reduced Survival,” Nature Medicine, no. 10, pp. 942–947, 2004. [DOI] [PubMed] [Google Scholar]
- [13].Polcher M, Braun M and Friedrichs N, “Foxp3(+) cell infiltration and granzyme B(+)/Foxp3(+) cell ratio are associated with outcome in neoadjuvant chemotherapy-treated ovarian carcinoma,” Cancer Immunology and Immunotherapy, no. 59, pp. 909–916, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lu X, “Impact of IL-12 in Cancer,” Current Cancer Drug Targets, no. 17, pp. 682–697, 2017. [DOI] [PubMed] [Google Scholar]
- [15].Tugues S, Burkhard SH and Ohs I, “New insights into IL-12 mediated tumor suppression,” Cell Death Differ, no. 22, pp. 237–246, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lenzi R, Rosenblum MG, Verschraegen CF, Kudelka AP, Kavanagh JJ, Hicks ME, Lang EA, Nash M, Levy LB, Garcia ME, Platsoucas CD, Abbruzzese JL and Freedman RS, “Phase I Study of Intraperitoneal Recombinant Human Interleukin 12 in Patients with Müllerian Carcinoma, Gastrointestinal Primary Malignancies, and Mesothelioma,” Clinical Cancer Research, vol. 8, no. 12, pp. 3686–3695, 2002. [PubMed] [Google Scholar]
- [17].Bajetta E, Del Vecchio M and Mortarini R, “Pilot study of subcutaneous recombinant human interleukin 12 in metastatic melanoma,” Clinical Cancer Research, no. 8, pp. 3686–3695, 2002. [PubMed] [Google Scholar]
- [18].Marshall E, “Cancer Trial of Interleukin-12 Halted,” Science, vol. 268, p. 1555, 1995.17754594 [Google Scholar]
- [19].Fewell JG, Matar M, Slobodkin G, Han S.-o., Rice J, Hovanes B, Lewis DH and Anwer K, “Synthesis and application of a non-viral gene delivery system for immunogene therapy of cancer.,” Journal of Controlled Release, vol. 109, no. 1, pp. 288–298, 2005. [DOI] [PubMed] [Google Scholar]
- [20].Anwer K, Barnes MN, Fewell JG, Lewis DH and Alvarez RD, “Phase-I clinical trial of IL-12 plasmid/lipopolymer complexes for the treatment of recurrent ovarian cancer,” Gene Therapy, vol. 17, no. 3, pp. 360–369, 2010. [DOI] [PubMed] [Google Scholar]
- [21].Anwer K, Kelly FJ, Chu CS, Fewell JG, Lewis DH and Alvarez RD, “Phase I trial of a formulated IL-12 plasmid in combination with carboplatin and docetaxel chemotherapy in the treatment of platinum-sensitive recurrent ovarian cancer,” Gynecologic Oncology, vol. 131, no. 1, pp. 169–173, 2013. [DOI] [PubMed] [Google Scholar]
- [22].Thaker PH, Brady WE, Lankes HA, Odunsi K, Bradley WH, Moore KN, Muller CY, Anwer K, Schilder RJ, Alvarez RD and Fracasso PM, “A phase I trial of intraperitoneal GEN-1, an IL-12 plasmid formulated with PEG-PEI-cholesterol lipopolymer, administered with pegylated liposomal doxorubicin in patients with recurrent or persistent epithelial ovarian, fallopian tube or primary peritoneal cancers: An NRG Oncology/Gynecologic Oncology Group study,” Gynecologic Oncology, vol. 147, no. 2, pp. 283–290, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Thaker PH, Borys N, Fewell JG and Anwer K, “GEN-1 immunotherapy for the treatment of ovarian cancer.,” Future Oncology, vol. 15, no. 4, pp. 421–438, 2019. [DOI] [PubMed] [Google Scholar]
- [24].O’Donnell JS, Hoefsmit EP, Smyth MJ, Blank CU and Teng MWL, “The Promise of Neoadjuvant Immunotherapy and Surgery for Cancer Treatment,” Clinical Cancer Research, vol. 25, no. 19, pp. 5743–5751, 2019. [DOI] [PubMed] [Google Scholar]
- [25].Liu J, Blake SJ, Yong MC, Harjunpaa H, Ngiow SF and Takeda K, “Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease,” Cancer Discov, no. 6, pp. 1382–99, 2016. [DOI] [PubMed] [Google Scholar]
- [26].Lo CS, Sanii S, Kroeger DR, Milne K, Talhouk A, Chiu DS, Rahimi K, Shaw P, Clarke BA, Nelson BH and Nelson BH, “Neoadjuvant chemotherapy of ovarian cancer results in three patterns of tumor-infiltrating lymphocyte response with distinct implications for immunotherapy.,” Clinical Cancer Research, vol. 23, no. 4, pp. 925–934, 2017. [DOI] [PubMed] [Google Scholar]
- [27].Meriggi F and Zaniboni A, “Antibiotics and steroids, the double enemies of anticancer immunotherapy: a review of the literature,” Cancer Immunology Immunotherapy, no. 10.1007/s00262-020-02786-3, November 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Thaker PH, Holloway RW, Kuroki L, DePasquale SE, Bradley W, ElNagger A, Bell MC, Rocconi RP, Bregar A, Indermaur MD, Gunderson C, Pothuri B, Agajanian R, Warshal D, Provencher D, McHale M, John V, Bergman M, Lau S, Musso L, Anwer K, Borys N and Leath III CA, “A Phase I/II study evaluating intraperitoneal GEN-1 in combination with neoadjuvant chemotherapy in patients with newly diagnosed advanced epithelial ovarian cancer (EOC),” in SGO 2021 Virtual Annual Meeting on Women’s Cancer, Virtual, 2021. [Google Scholar]
- [29].“Common Terminology Criteria for Adverse Events (CTCAE) : Version 4.0,”,. [Online]. Available: http://www.acrin.org/Portals/0/Administration/Regulatory/CTCAE_4.02_2009-09-15_QuickReference_5x7.pdf. [Accessed 30 11 2020].
- [30].Rustin GJS, Vergote I, Eisenhauer EA, Pujade-Lauraine E, Quinn M, Thigpen T, Bois A. d., Kristensen GB, Jakobsen A, Sagae S, Greven KM, Parmar MKB, Friedlander M, Cervantes A and Vermorken JB, “Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG),” International Journal of Gynecological Cancer, vol. 21, no. 2, pp. 419–423, 2011. [DOI] [PubMed] [Google Scholar]
- [31].Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D and Verweij J, “New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1),” Eur. J. Cancer, vol. 45, no. 2, p. 228–47, 2009. [DOI] [PubMed] [Google Scholar]
- [32].Cohen PA, Cohen PA, Powell A, Böhm S, Gilks CB, Stewart CJ, Meniawy T, Meniawy T, Bulsara M, Avril S, Avril S, Brockbank E, Bosse T, Focchi G. R. d. A., Ganesan R, Glasspool RM, Howitt BE, Kim H-S, Lee J-Y, Le ND, Lockley M, Lockley M, Manchanda R, Mandalia T, McCluggage WG, McNeish IA, Midha D, Srinivasan R, Tan YY, Griend R. v. d., Yunokawa M, Zannoni GF, Aggarwal S, Bronger H, Brown EB, Buck M, Bukhari SA, Coghlan E, Cope N, Almeida M. S. d., Kroon CDD, Dean A, Devlin M-J, Ditzel HM, Drecoll E, Fagotti A, Faruqi A, Feeney L, Gupta K, Harley I, Inzani F, Jeyarajah AR, Koay ME, Kroep JR, Leung Y, Loft AR, MaGee D, McKenna S, Millan D, Millar J, Miller RE, Mohan GR, Mughal S, Nicolau SM, Nevin J, Oakley AS, Quigley M, Rai B, Rajwanshi A, Salfinger SG, Scambia G, Scatchard K, Schmalfeldt B, Simcock B, Singh P, Strickland KC, Suri V, Syed S, Sykes P, Tan A, Tan J, Thompson EA, Tinker AV, Trevisan G, Uyeda MGBK, Vaughan MM, Weichert W, Williams A, Williams S, Zorzato PC and Singh N, “Pathological chemotherapy response score is prognostic in tubo-ovarian high-grade serous carcinoma: A systematic review and meta-analysis of individual patient data,” Gynecologic Oncology, vol. 154, no. 2, pp. 441–448, 2019. [DOI] [PubMed] [Google Scholar]
- [33].Brunhoeber E, Matar M, Anwer K and Fewell JG, “286. Biodistribution and Cearance Following Intraperitoneal Injection of Murine Interleukin-12 Plasmid Formulated with a Novel Polymeric Delivery System,” Molecular Therapy, vol. 13, no., p., 2006. [Google Scholar]
- [34].Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener HC, Lopes T, Luesley D, Perren TJ, Bannoo S, Mascarenhas M, Dobbs S, Essapen S, Twigg J, Herod J, McCluggage G, Parmar MKB, Swart AM and Swart AM, “Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial.,” The Lancet, vol. 386, no. 9990, pp. 249–257, 2015. [DOI] [PubMed] [Google Scholar]
- [35].Vergote I, Tropé CG, Amant F, Ehlen T, Reed N and Casado A, “Neoadjuvant Chemotherapy Is the Better Treatment Option in Some Patients With Stage IIIc to IV Ovarian Cancer,” Journal of Clinical Oncology, vol. 29, no. 31, pp. 4076–4078, 2011. [DOI] [PubMed] [Google Scholar]
- [36].Conlon KC, Miljkovic MD and Waldmann TA, “Cytokines in the Treatment of Cancer,” Journal of Interferon & Cytokine Research, vol. 39, no. 1, pp. 6–21, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sato E, Olson SH, Ahn J, Bundy BN, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone CB, Kepner JL, Odunsi T, Ritter G, Lele S, Chen Y-T, Ohtani H, Old LJ and Odunsi K, “Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer.,” Proceedings of the National Academy of Sciences of the United States of America, vol. 102, no. 51, pp. 18538–18543, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cao X, Leonard K, Collins LI, Cai SF, Mayer JC, Payton JE, Walter MJ, Piwnica-Worms D, Schreiber RD and Ley TJ, “Interleukin 12 Stimulates IFN-γ–Mediated Inhibition of Tumor-Induced Regulatory T-Cell Proliferation and Enhances Tumor Clearance,” Cancer Research, vol. 69, no. 22, pp. 8700–8709, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang W, Kryczek I, Dostal L, Lin H, Tan L, Zhao L, Lu F, Wei S, Maj T, Peng D, He G, VAtan L, Kuick R, Szeliga W, Kotarski J, Tarkowski R, Dou Y, Rattan R and Mu, “Effector T Cells Abrogate Stroma-Mediated Chemoresistance in Ovarian Cancer,” Cell, no. 165, pp. 1092–1099, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Watkins SK, Egilmez NK, Suttles Jand Stout RD, “IL-12 Rapidly Alters the Functional Profile of Tumor-Associated and Tumor-Infiltrating Macrophages In Vitro and In Vivo,” Journal of Immunology, vol. 178, no. 3, pp. 1357–1362, 2007. [DOI] [PubMed] [Google Scholar]
- [41].Bohm S, Montfort A, Pearce OM, Topping J, Chakravarty P, Everitt GL, Clear A, McDermott JR, Ennis D, Dowe T, Fitzpatrick A, Brockbank EC, Lawrence AC, Jeyarajah A and Far, “Neoadjuvant Chemotherapy Modulates the Immune Microenvironment in Metastases of Tubo-Ovarian High-Grade Serous Carcinoma,” Clinical Cancer Research, vol. 22, pp. 3025–3036, 2016. [DOI] [PubMed] [Google Scholar]
- [42].Mesnage SJ, Auguste A, Genestie C, Dunant A, Pain E, Drusch F, Gouy S, Morice P, Bentivegna E, Lhomme C, Pautier P, Michels J, Le Formal A, Cheaib B, Adam J and Leary AF, “Neoadjuvant Chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PD-L1) expression in epithelial ovarian cancer (EOC),” Annals of Oncology, vol. 28, pp. 651–657, 2016. [DOI] [PubMed] [Google Scholar]
- [43].Liu Y, Xy H, Lai N, Yang Z and Kang S, “Interleukin-12 over-espressionin malignant melanoma B16 cells reduces programmed death-1 expression on T cells in mice with immune reconstitution,” J South Med Univ, vol. 40, no. 6, pp. 856–863, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Costa SF, Gomes VO, dos Santos Maciel MO, Melo LM, Venturin GL, Bragato JP, Rebech GT, de Oliveira Santos C and de Sa Oliveira GG, “Combined in vitro IL-12 and IL-15 stimulation promotes cellular immune resonse in dogs with visceral leishmaniasis,” PLoS Negl Trop, vol. 14, no. 1, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhao J, Zhao J and Perlman S, “Differential Effects of IL-12 on Tregs and Non-Treg Cells: Roles of IFN-g, IL-2 and IL-2R,” PLoS ONE, vol. 7, no. 9, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lai Q, Wang H, Li A, Xu Y, Tang L, Chen Q, Zhang C.-f., Gao Y, Song J, Du Z and Du Z, “Decitibine improve the efficiency of anti-PD-1 therapy via activating the response to IFN/PD-L1 signal of lung cancer cells,” Oncogene, vol. 37, no. 17, pp. 2302–2312, 2018. [DOI] [PubMed] [Google Scholar]
- [47].Lin L, Rayman P, Pavicic PG, Tannenbaum C, Hamilton T and Montero A, ““Ex vivio conditioning with IL-12 protects tumor infiltrating CD8+T cells from negative regulation by local IFN-y”,” Cancer Immunology Immunotherapy, vol. 68, no. 3, pp. 395–405,, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Eisenring M, vom Berg J, Kristiansen G, Saller E and Becher B, “IL-12 initiates tumor rejection via lymphoid tissue-inducer cells bearing the natural cytotoxicity receptor NKp46,” Nature Immunology, vol. 11, pp. 1030–1038, 2010. [DOI] [PubMed] [Google Scholar]
- [49].Moore KN, Bookman M, Sehouli J, Miller A, Anderson C, Scambia G, Myers T, Taskiran C, Robison K, Maenpaa J, Willmott LJ, Colombo N, Thomes-Pepin J, Gold MA, Aghajanian C, Wu F, Molinero L, Khor V, Lin YG and Pignata S, “Primary results from IMagyn050/GOG 3015/ENGOT-OV39, a double-blind placebo (pbo)-controlled randomised phase III trial of bevacizumab (bev)-containing therapy +/− atezolizumab (atezo) for newly diagnosed stage III/IV ovarian cancer (OC),” in Annals of Oncology, 2020. [Google Scholar]
- [50].González-Martín A and Sánchez-Lorenzo L, “Immunotherapy with checkpoint inhibitors in patients with ovarian cancer: Still promising?,” Cancer, vol. 125, pp. 4616–4622, 2019. [DOI] [PubMed] [Google Scholar]
- [51].Wright AA, Bohlke K, Armstrong DK, Bookman MA, Cliby WA, Coleman RL, Dizon DS, Kash JJ, Meyer LA, Moore KN, Olawaiye AB, Oldham J, Salani R and Spa, “Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of gynecologic oncology and American society of clinical oncology clinical practice guideline,” Obstetrical & Gynecological Survey, vol. 71, no. 12, pp. 717–718, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rodriguez-Freixinos V, Mackay HJ and Karakasis K, “Current and emerging treatment options in the management of advanced ovarian cancer,” Expert Opinion Pharmacotherapy, vol. 17, pp. 1063–1076, 2016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


