Abstract
BACKGROUND:
Children from economically distressed families and neighborhoods are at risk for stress and pollution exposure and potential neurotoxic sequelae. We examine dimensions of early-life stress affecting hippocampal volumes, how prenatal exposure to air pollution might magnify these effects, and associations between hippocampal volumes and visuospatial reasoning.
METHODS:
Fifty-three Hispanic/Latinx and/or Black children of ages 7 to 9 years were recruited from a longitudinal birth cohort for magnetic resonance imaging and cognitive assessment. Exposure to airborne polycyclic aromatic hydrocarbons was measured during the third trimester of pregnancy. Maternal report of psychosocial stress was collected at child age 5 and served as measures of early-life stress. Whole hippocampus and subfield volumes were extracted using FreeSurfer. Wechsler performance IQ measured visuospatial reasoning.
RESULTS:
Maternal perceived stress associated with smaller right hippocampal volume among their children (B = −0.57, t34 = −3.05, 95% CI, −0.95 to −0.19). Prenatal polycyclic aromatic hydrocarbon moderated the association between maternal perceived stress and right CA1, CA3, and CA4/dentate gyrus volumes (B ≥ 0.68, t33 ≥ 2.17) such that higher prenatal polycyclic aromatic hydrocarbon exposure magnified negative associations between stress and volume, whereas this was buffered at lower exposure. Right CA3 and CA4/dentate gyrus volumes (B ≥ 0.35, t33 > 2.16) were associated with greater performance IQ.
CONCLUSIONS:
Prenatal and early-life exposures to chemical and social stressors are likely compounding. Socioeconomic deprivation and disparities increase risk of these exposures that exert critical neurobiological effects. Developing deeper understandings of these complex interactions will facilitate more focused public health strategies to protect and foster the development of children at greatest risk of mental and physical effects associated with poverty.
Socioeconomic disparities place children from economically distressed families and neighborhoods at disproportionate risk for exposure to stressful life events and to air pollution. Economic disadvantage increases the likelihood of experiencing physical and social stressors, such as inadequate access to basic resources or living in neighborhoods with increased violence (1,2). In addition, air pollution is often higher in these neighborhoods, placing children at increased risk for exposure both in utero and during development (2,3). Exposures to early-life stress (ELS) (4–6) and prenatal air pollution (7–9) have been separately linked to increased risk for behavioral, psychological, and cognitive problems, which may derive from the neurobiological effects of exposure.
The hippocampus is a key region involved in stress regulation and, critically, is vulnerable to the neurotoxic effects of stress and cortisol, which can lead to deficits in hippocampal structure and volume in both animal and human research (10,11). In addition, psychological stress differentially alters subfields of the hippocampus with the CA1, CA3, and dentate gyrus generally found to be most vulnerable (12). Animal models have shown that psychological stress (e.g., restraint stress, housing in dominance hierarchies, maternal deprivation) can result in shortened dendrites, neurogenesis suppression, and the loss of spine synapses in subregions of the hippocampus (10). More specifically, chronic restraint stress results in neuronal atrophy in CA3 and CA1 (13,14). Other forms of chronic and acute stress have been shown to decrease neurogenesis in the dentate gyrus (10,13) and impair long-term potentiation and synaptic plasticity in CA1 and dentate gyrus (15). This neuronal remodeling is plausibly mediated by changes in cortisol and glutamate, both of which are involved in the stress response and have been linked to hippocampal structure and function (10). These changes likely give rise to the gross morphological differences commonly observed in human research (16). Socioeconomic disadvantage and its associated stressors, at both the household level (17–19) and neighborhood level (20), have been consistently linked to reductions in hippocampal structure, as have maternal anxiety (21,22) and the lack of maternal support (23,24). Herein, we examine potentially distinct contributions of early-life exposure to different types of maternal psychosocial and socioeconomic stress on hippocampal volumes, specifically, maternal perceived stress, maternal psychological distress, material hardship, neighborhood quality, and maternal intimate partner violence.
Exposure to air pollution also alters the hippocampus and exhibits differential effects on hippocampal subfields. Decreased structural integrity across the hippocampus (25), decreased neurogenesis in the dentate gyrus, and activated microglia in the dentate gyrus and CA1 subfields (26) have all been observed following perinatal air pollutant exposure in animal models. Decreased dendritic branching in CA1 and CA3 subregions (27) and reductions in hippocampal brain-derived neurotrophic factor (BDNF) expression (28) have been shown to follow prenatal particulate matter (PM) 2.5 (PM2.5) exposure. Prior human research has also focused on exposure to polycyclic aromatic hydrocarbons (PAHs)—a group of air pollutants resulting from the incomplete combustion of organic matter and tobacco smoke, grilling, oil- and coal-burning power plants, and vehicular emissions (29). Prenatal PAH exposure—estimated by land use regression models or directly measured personal exposure—has been associated with structural alterations in the brain, specifically cortical thinning in brain regions in both hemispheres (30), volume reductions in the corpus callosum (31), and reduced left hemisphere white matter (32). In addition, consistent with findings from animal models, infants born after the closing of a coal-burning factory in China had decreased markers of exposure to PAH and increased level of mature BDNF (33). Yet, few human studies have focused on the effects of air pollution exposure on specific neuroanatomical regions implicated in cognitive development and, of particular interest here, the hippocampus. Such findings would enhance our understanding of the etiologic mechanisms by which air pollution affects neurodevelopment. Consistent with the findings from animal models, extant human research demonstrates an association between concurrent air pollution exposure in adulthood and smaller hippocampal volume, while controlling for socioeconomic status (29,34–37), which was further supported in a meta-analysis (38). Similarly, prenatal exposure to air pollution (estimated by land use regression models) was shown to associate with smaller left hippocampal volumes in school-age children (39). The effects of air pollution on hippocampal subfields remain understudied.
Overall, the hippocampus may represent a common site of the neurotoxic effects of air pollution and stress, particularly experienced prenatally and in early life, which can lead to deleterious downstream cognitive and neurodevelopmental outcomes. Conceptual work suggests that models describing threats to neurodevelopment must account for the amplified effects of multiple exposures (40). Specifically, recent findings indicate that the effects of exposure to ELS and air pollution on brain and behavior may be compounding (41–43). Herein, we investigate potential interactions between prenatal exposure to PAH and ELS on the hippocampal structure in school-age children. Understanding these aggravated effects may facilitate a comprehensive public health campaign that targets distinct patterns of risk for child development.
Finally, structural and functional neuroimaging studies have demonstrated an integral role for the hippocampus in learning, remembering, and flexibly navigating spatial information (44–48). This relationship has been observed bilaterally (49) and across different subfields (47). Larger hippocampal volume has been associated with greater navigational experience in taxi drivers (50,51), and both severe hippocampal damage (45) and CA1 atrophy in patients with multiple sclerosis (52) are correlated with impaired spatial recall. Structural differences have been similarly identified in children with nonverbal learning disorder, which is characterized by visuospatial deficits (53). These visuospatial deficits are independently associated with differences in stress and air pollution exposure in early life (54–56), with 1 study reporting that higher PAH DNA-adducts, a biological marker of exposure, were associated with lower visuospatial intelligence among children who experienced material hardship (41). Importantly, additional animal research has begun to place hippocampal subfields as potential mediators in the relationship between adversity and visuospatial cognitive skills (57,58).
Herein, we use structural magnetic resonance imaging (MRI) data acquired from Black and/or Latinx children from economically disadvantaged families enrolled in a prospective longitudinal birth cohort to examine several hypotheses. Though these longitudinal data are powerful, our sample size was limited, and thus we opted for analyses to minimize multiple testing. First, we hypothesized that individual differences in ELS, measured by maternal report at child age 5, would associate with reduced children’s hippocampal volumes at ages 7 to 9. We aimed to parse which of the 5 measured domains of ELS would have the most potent influence. Next, given prior work showing interactions between prenatal exposure to air pollution and ELS on children’s neurodevelopmental outcomes (42), we hypothesized that greater prenatal PAH exposure would potentiate stress-related reductions in hippocampal volumes. Last, given the effects of air pollution and stress on cognitive development and the role of the hippocampus in visuospatial processing (44–49), we explored the extent to which hippocampal volume associated with performance IQ (PIQ) scores, as a measure of visuospatial abilities.
METHODS AND MATERIALS
Participants
As described previously (59), we recruited N = 53 children of ages 7 years and older from the Sibling-Hermanos birth cohort at Columbia University for a neuroimaging visit (see Supplemental Methods). Mothers were nonsmokers. Five children opted out of the study, 5 were unable to complete the MRI scan, and 3 were excluded during quality control checks. In total, n = 40 children had available hippocampal subfield volumes and ELS data, n = 37 of those participants had prenatal air pollution exposure data, and n = 35 had visuospatial ability data (n = 64 children had available PAH, ELS, and IQ data). The Institutional Review Boards at Columbia University and New York State Psychiatric Institute approved the study; children and guardians provided written informed assent and consent.
Early-Life Stress
Each participant’s mother completed validated scales measuring 5 aspects of ELS at child age 5: maternal perceived stress over the past month (60), maternal psychological distress (61), material hardship (62), neighborhood quality (63–65), and intimate partner violence (66). All items were rescaled 0–1 and averaged for each scale so that each scale yields a continuous score, with higher scores indicating more stress. These scales have good reported reliability and psychometric properties and have been reported on extensively in our prior work (42). Our primary analyses used maternal reports of stress to measure 5 distinct domains of ELS.
Air Pollution
Maternal personal exposure to air pollution was measured and analyzed as previously described (67,68). Briefly, during the third trimester of pregnancy, mothers wore an air monitoring backpack for 48 continuous hours. The backpack contained a filter that collected airborne vapors, aerosols, and PM2.5 from which 8 PAHs (benz[a]anthracene, benzo[a]pyrene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, indeno-[1,2,3-cd] pyrene, disbenz[a,h]anthracene, and benzo[g,h,i]perylene) were extracted and measured at Southwest Research Institute. Exposure was computed as the sum of these 8 carcinogenic PAHs (see Supplemental Methods). The distribution of exposure was assessed, and participants were subsequently grouped by a median split of total PAH exposure (median = 1.60 ng/m3) (Figure S1) as our predictor of interest, aligned with previous work (59,69). The median split separates higher and lower exposure groups minimizing measurement error in the estimate of exposure, i.e., how well the 48-hour assessment captures cumulative exposure. Furthermore, we did not expect the individual variance at the low end, reflecting minimal to no exposure, to systematically relate to biological/psychological outcomes. Exploratory analyses using PAH as a continuous variable are reported in Supplemental Results.
Visuospatial Reasoning
As part of the cohort study, participants completed neuropsychological assessment at age 7 to 9, including the Wechsler Abbreviated Scale of Intelligence, Second Edition (70). The Wechsler Abbreviated Scale of Intelligence assesses verbal IQ (VIQ) reflecting verbal reasoning and PIQ reflecting visuospatial reasoning, such that VIQ and PIQ can be combined into a full-scale IQ score. VIQ is composed of the Vocabulary subtest, measuring word knowledge, and the Similarities subtest, measuring analogical reasoning. PIQ is composed of the Matrix Reasoning and Block Design subtests and is considered a gold standard measure of visuospatial abilities (70). Each Index score is a continuous variable with a mean of 100 and SD of 15 points. Two participants were missing PIQ data.
MRI Acquisition and Processing
Participants completed MRI scanning on the same day as the neuropsychological assessment. Data were acquired on a 3T GE MR750 scanner (GE Healthcare) with a 32-channel head coil. Two structural T1 images were collected for each participant using a 3D-FSPGR sequence (flip angle = 11°, echo time = 2.588 ms, repetition time = 6.412 ms, 180 slices, 1-mm isotropic resolution). Structural data were processed using the standard FreeSurfer version 6.0 pipeline (recon-all) (71,72) including hippocampal subfield segmentation (73). Based on prior animal model findings on the effects of stress, we focused on the CA1, CA3, and CA4/dentate gyrus subfields.
Statistical Analyses
Distributions of continuous variables were examined (Figures S2 and S3). Cases with missing data were excluded per analysis. We report standardized regression coefficient estimates, significance tests were two-tailed, and α was set at p < .05. Tables S1 and S2 show simple correlations between prenatal PAH and postnatal socioeconomic and stress-related factors (child age 5): maternal perceived stress over the past month, maternal psychological distress, material hardship, neighborhood quality, intimate partner violence, and income-to-needs ratio. One participant exhibited right hippocampal total and subfield volumetric outliers (z > 3), which were winsorized to the next most extreme nonoutlier value. A sensitivity analysis examined whether removing this participant had any effect on the results (Supplemental Results).
Given our sample size and interest in parsing the distinct contributions of different types of maternal psychosocial and socioeconomic stress, our first analysis tested which ELS variables were associated with left or right hippocampal volume. We sought to delineate the domains of ELS at age 5 years (maternal perceived stress, maternal psychological distress, material hardship, intimate partner violence, or neighborhood instability) that were associated with children’s hippocampal volumes. To do this, we entered these ELS variables into 2 multiple linear regression models (n = 40) predicting either left or right whole hippocampal volumes (residualized for age, sex assigned at birth, and intracranial volume to preserve degrees of freedom). We corrected for multiple comparisons using false discovery rate (FDR) across the 10 tested terms (5 domains; 2 hemispheres).
Our second analysis evaluated whether prenatal PAH exposure potentiated stress-related reductions in hippocampal volumes. We used multiple linear regression (n = 37) to test whether ELS, PAH, and the ELS × prenatal air pollution interaction associated with whole or subregional hippocampal volumes of that hemisphere (residualized for age, sex assigned at birth, and intracranial volume). To limit multiple testing given our sample size, we only examined domains of ELS that significantly related to whole hippocampal volumes rather than testing the interaction between prenatal PAH and all 5 ELS variables. We corrected for multiple comparisons using FDR across the number of tested interaction terms (4 volumes [whole, CA4/DG, CA3, and CA1] 3 number of domain-hemisphere associations identified in analysis 1).
Finally, in a third analysis, we explored whether visuospatial reasoning as measured by PIQ was associated with hippocampal subregions (n = 35). We restricted planned regression analyses to testing associations between PIQ and hippocampal subregions that were associated with the PAH × ELS interaction term in analysis 2. Control analyses evaluated the specificity of any observed associations between subfield volumes and PIQ versus VIQ. For completeness, we also present Supplemental Results from models testing associations between left hippocampal subregions and VIQ and PIQ. Furthermore, because brain structure and cognitive outcomes were measured at a single time point, we did not conduct a formal test of mediation PAH and ELS effects on PIQ by hippocampal subregions but did test the effect of the PAH × ELS interaction term on PIQ in a subset of children with available data (n = 64).
RESULTS
Participants
Hispanic/Latinx and/or Black children of ages 7 to 9 years participated in this study. Table 1 presents demographic, psychosocial, and cognitive data that describe the sample.
Table 1.
Demographic, Psychosocial, and Cognitive Characteristics of the Sample (n = 40)
| Characteristics | n (%) or Mean (SD) [Median] |
|---|---|
| Sex Assigned at Birth, Male | 17 (42.5%) |
| Race/Ethnicity | |
| Black | 18 (45%) |
| Hispanic/Latinx | 22 (55%) |
| Age, Months | 103.62 (9.03) [106.00] |
| VIQ Score | 94.73 (14.44) [92.00] |
| PIQ Score | 89.14 (11.82) [90.00] |
| Maternal Education, Years | 12.25 (1.6) [12.00] |
| Total PAH, ng/m3 | 1.98 (1.22) [1.60] |
n = 2 missing PIQ. n = 2 missing VIQ. n = 3 missing total PAH.
PAH, polycyclic aromatic hydrocarbon; PIQ, performance IQ; VIQ, verbal IQ.
Domains of ELS and Hippocampal Volume
Greater maternal perceived stress at child age 5 associated with smaller right hippocampal volume at child age 7 to 9 (B = −0.57, t34 = −3.05, 95% CI, −0.95 to −0.19, pFDR = .04). Maternal psychological distress, material hardship, neighborhood quality, and intimate partner violence were not significantly associated with hippocampal volume after FDR correction was applied (pFDRs > .14) (see Table S3). Variance inflation factors were sufficiently low to determine that collinearity was not a concern, and simple linear regressions confirmed our findings (variance inflation factor ≤ 1.75) (Table S4). We tested hemispheric specificity in a mixed-effect model and found a main effect of maternal perceived stress on smaller hippocampal volumes across hemispheres (B = −0.30, t76 = −1.98, p = .05) and no significant maternal perceived stress × hemisphere interaction (p = .71) (Table S5).
Joint Effects of Air Pollution and ELS on Hippocampal Volume
The maternal perceived stress × prenatal PAH interaction was associated with right CA1 (B = 0.68, t33 = 2.24, 95% CI, 0.06 to 1.29, pFDR = .049), CA3 (B = 0.82, t33 = 2.86, 95% CI, 0.23 to 1.40, pFDR = .029), and CA4/dentate gyrus volumes (B = 0.68, t33 = 2.17, 95% CI, 0.04 to 1.32, pFDR = .049). Right whole hippocampus volume was not associated with the perceived stress × prenatal PAH interaction term (B = 0.49, t33 = 1.57, 95% CI, −0.15 to 1.13, pFDR = .126), although there was still a significant main effect for maternal perceived stress (B = −0.66, t33 = −2.64, 95% CI, −1.17 to −0.15). Specifically, among individuals with higher prenatal PAH exposure, greater stress was associated significantly with smaller hippocampal subfield volumes; among individuals with lower prenatal PAH exposure, stress and hippocampal subfield volumes were not significantly associated (Figure 1 and Table 2). Results from all 4 models, including nonsignificant findings, are reported in Table S6.
Figure 1.
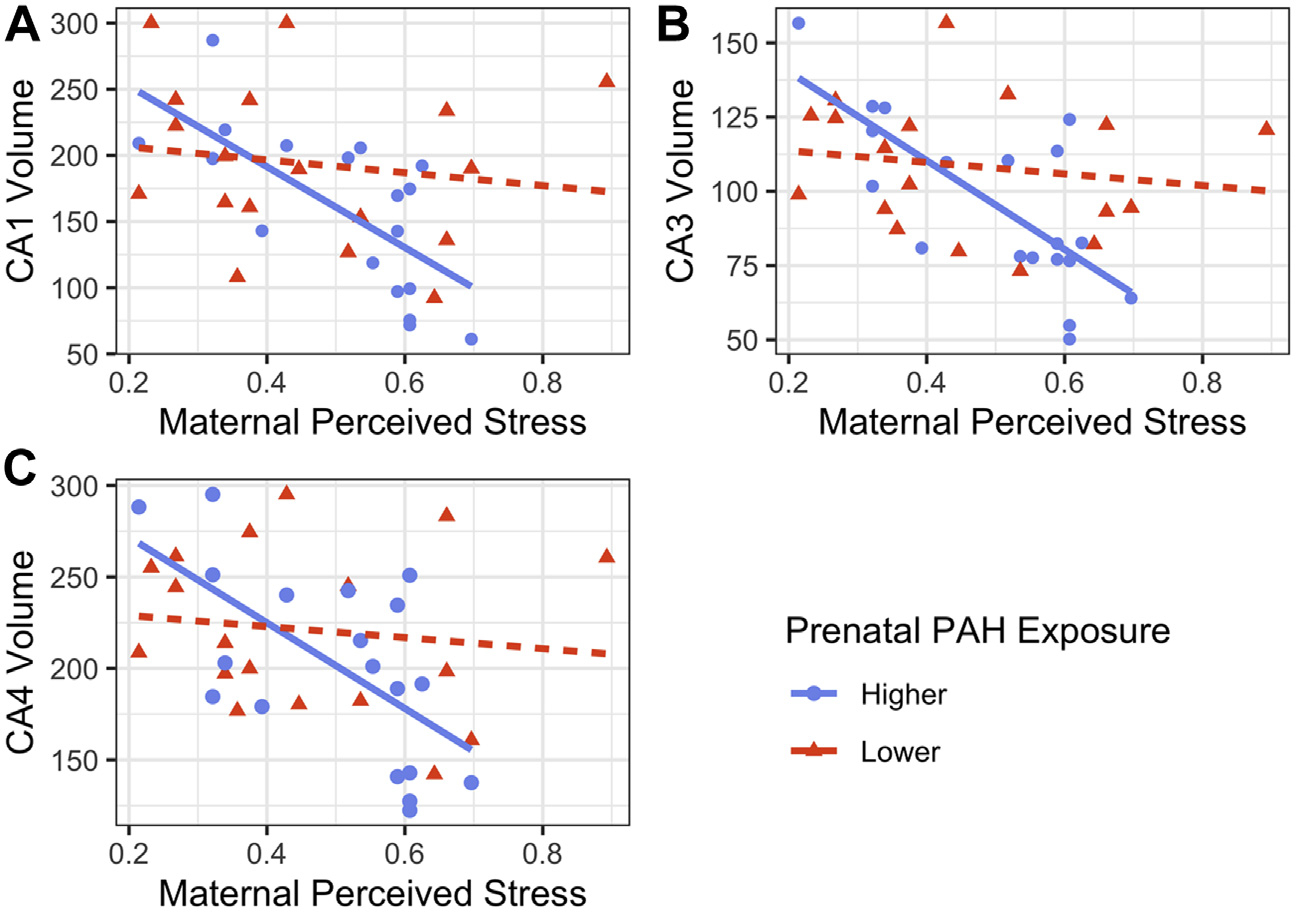
Prenatal polycyclic aromatic hydrocarbon (PAH) exposure moderates the association between maternal perceived stress (0–1 scale) at age 5 and right hippocampal (A) CA1, (B) CA3, and (C) CA4/dentate gyrus subfield volumes (mm3), winsorized and residualized for age, sex assigned at birth, and intracranial volume (n = 37: nhigher = 19, nlower = 18).
Table 2.
Simple Slopes Analysis for the Maternal Perceived Stress × Prenatal PAH Exposure Interaction Effect on CA1, CA3, and CA4/Dentate Gyrus Subfield Volumes
| Subfields | PAH Exposure | B | SE | t | p | 95% CI |
|---|---|---|---|---|---|---|
| CA1 | Lower | −0.13 | 0.18 | −0.70 | .487 | −0.50 to 0.24 |
| Higher | −0.81 | 0.24 | −3.34 | .002 | −1.30 to −0.32 | |
| CA3 | Lower | −0.12 | 0.17 | −0.71 | .484 | −0.47 to 0.23 |
| Higher | −0.94 | 0.23 | −4.12 | <.001 | −1.40 to −0.47 | |
| CA4/Dentate Gyrus | Lower | −0.10 | 0.19 | −0.53 | .598 | −0.48 to 0.28 |
| Higher | −0.78 | 0.25 | −3.13 | .004 | −1.29 to −0.27 |
Each simple slope was independently tested for significance using a t test (n = 37: nhigher = 19, nlower = 18).
PAH, polycyclic aromatic hydrocarbon.
Hippocampal Volume and Visuospatial Reasoning
PIQ was significantly associated with right hippocampal subfield volumes CA3 (B = 0.38, t33 = 2.39, 95% CI, 0.06 to 0.71, p = .02) and CA4/dentate gyrus (B = 0.35, t33 = 2.16, 95% CI, 0.02 to 0.68, p = .04) (Figure 2) but not with CA1 (B = 0.16, t33 = 0.95, 95% CI, −0.19 to 0.51, p = .35) (Table S7 and Figure S4). Right hippocampal subfield volumes did not predict VIQ in simple linear regressions (Table S7), nor did left hippocampal subfield volumes predict PIQ or VIQ (Table S8). For completeness, a model containing PAH, ELS, and their interaction term (PAH × ELS) did not significantly predict PIQ or VIQ (Table S9).
Figure 2.
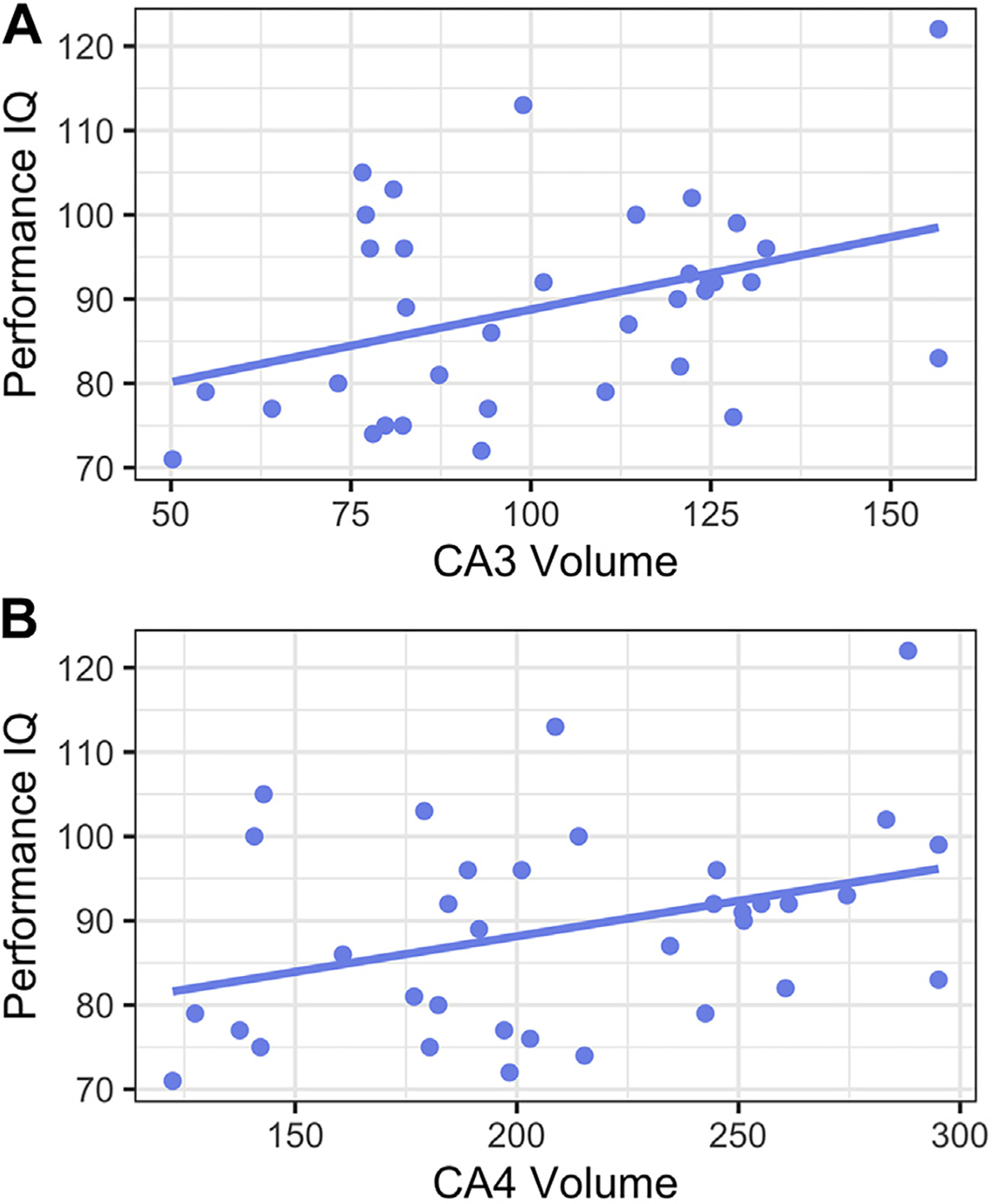
Hippocampal subfield volumes CA3 (A) and CA4/dentate gyrus (B), winsorized and residualized for age, sex assigned at birth, and intracranial volume, predict visuospatial reasoning (performance IQ).
DISCUSSION
Our study reports on the effects of different aspects of ELS on children’s hippocampal volumes, as well as the moderating effects of prenatal exposure to PAH in a community sample of Black or Latinx children from economically disadvantaged backgrounds. Of the 5 aspects of ELS that we have previously investigated (42), maternal perceived stress was negatively associated with right hippocampal volume. Over and above the effects of perceived stress, maternal psychological distress, material hardship, neighborhood quality, and intimate partner violence were not significantly associated with hippocampal volumes. Critically, maternal perceived stress was associated with reduced right hippocampal subfield volumes (CA1, CA3, and CA4/dentate gyrus) when children had higher levels of prenatal exposure to air pollution. Finally, in exploratory analyses, hippocampal CA3 and CA4/dentate gyrus volumes were positively associated with visuospatial abilities. Overall, our results point to the need to measure and consider multiple exposures when studying threats to neurodevelopment, particularly in the context of socioeconomic deprivation.
Prior studies have examined associations between household (17–19) and neighborhood (20) socioeconomic status and hippocampal volumes; here, we extend this work with a preliminary study of the contributions of different aspects of socioeconomic status–related stress and stressors on the hippocampus. In our study, maternal perceived stress was associated with smaller right hippocampal volume. Effects were not significant for maternal psychological distress, material hardship, neighborhood quality, or intimate partner violence (though some were trend-level significant). Note that maternal stress effects on the left hemisphere were significant but did not pass FDR correction; we also did not see significant differences in the strength of this effect in the left versus right hemisphere. Our findings are consistent with prior work showing inverse associations between maternal anxiety or psychosocial stress and hippocampal volume in animal models (10,12–15) and in newborns and children (21,22). Our measure may reflect the effect of maternal stress on children’s neurodevelopment or may act as a proxy for stress in the home environment, each of which could have separate biological mechanisms; we are limited in our ability to discern this as the cohort study did not include direct report of children’s experience of stress. Our findings are further limited by the measurement of maternal stress at child age 5, as we have recently shown that the effects of poverty on hippocampal volumes in these children were most salient prior to age 4.5 (74). Timing-specific effects on the hippocampus have additionally been found for general adverse experiences and maternal support (24,75,76). Future research should interrogate the timing for critical periods of vulnerability, neuroanatomical change, and the manifestation of symptoms.
Consistent with prior studies showing that prenatal PAH exposure moderates effects of ELS on neurodevelopmental outcomes (41,42), we detected a significant interaction between prenatal PAH exposure and maternal perceived stress on the right CA1, CA3, and CA4/dentate gyrus volumes. Our analyses focused on these hippocampal subregions because of the converging evidence in animal models linking both air pollution and ELS to these regions. A full multivariate model would better address the aims of the project but would be underpowered with n = 40 participants. Neurobiological findings converging on hippocampal subregions provide a coherent framework to understand the mutual effects of ELS to maternal perceived stress and air pollution, which may give rise to the cognitive and behavioral deficits observed in hippocampus-dependent tasks. Animal models and human epidemiological findings point to a potential biological mechanism underlying the associations observed herein. We hypothesize that effects of prenatal exposure to air pollution on hippocampal BDNF may cause increased vulnerability to stress via reductions in hippocampal BDNF, which buffers against the deleterious effects of stress (77–79). This proposed mechanism requires further study and may offer future directions for both pharmacological and public health intervention/prevention strategies. In addition, we did not detect a significant interaction between prenatal PAH exposure and maternal perceived stress on the whole hippocampal volume, suggesting that those subfields not included in our analyses are not implicated in the PAH × stress interaction.
Finally, we observed associations between hippocampal subfield volumes and visuospatial abilities, as measured by PIQ. The contemporaneous measurement of hippocampal volume and PIQ limits our ability to establish a directional mechanism by which the compounding effects of social and physical stressors impact visuospatial abilities. Still, animal models have shown effects of stress and air pollution on visuospatial learning and memory and on hippocampal structure (57,58). Future studies should examine whether alterations to specific hippocampal subfields mediate the effects of these exposures on visuospatial processing.
Effects of prenatal exposure to air pollution can be transmitted across generations. In animal models, maternal exposure to PM2.5 during pregnancy induced deficits in spatial learning and memory for a subsequent second generation not exposed to PM2.5 in utero. Such findings suggest that early-life exposure to PM2.5 may shape the intergenerational risk for neurobehavioral alterations (28). Multiple potential mechanisms may account for this intergenerational effect, e.g., maternal stress may influence the child’s development via parenting or other home environment factors and/or via direct effects of maternal stress on the placenta during critical periods of brain development.
Although we account for certain factors that could have confounded our findings, there may be additional unmeasured factors that associate with living near pollution sources, such as noise or vibrations near roadways (80–82), that could contribute to the observed effects of prenatal PAH on brain structure. Prior animal and human work also suggests the potential for sex-specific effects (55), but our study was limited in sample size, and thus we could not test sex-specific effects. Future studies should investigate sex-specific effects of prenatal exposure to air pollution on visuospatial processes and their intergenerational transmission. Current findings may also inform potential etiologic pathways to nonverbal learning disability [alternatively called developmental visuospatial disorder (83)] or other neurodevelopmental disorders that are sometimes characterized by visuospatial deficits, such as autism spectrum disorder (84). Future studies should investigate this potential underlying mechanism and should attempt to measure and control for additional factors such as roadway noise and vibrations to isolate the specificity of observed effects. Given that we conducted our study in a birth cohort of Black and Latinx children from economically disadvantaged backgrounds, our results may not generalize to children from more diverse socioeconomic (parent education, occupation, income), ethnic, or racial backgrounds.
Prenatal and ELS to chemical and social stressors are likely compounding, acting through complex biological mechanisms causing the increased risk of these exposures to exert critical biological effects. These interactions likely extend beyond exposures studied herein. For example, maternal anxiety or psychosocial stress is associated with reductions in hippocampal volumes (21,22), but maternal support associated with increased hippocampal volumes (23), perhaps indicating a buffering effect of support. These effects have additionally been shown to interact with prenatal risk factors at the hippocampus such as preterm birth (85). By developing a deeper understanding of these complex interactions, we may develop more focused public health prevention strategies that will help protect the developmental trajectory of those children at greatest risk for mental and physical health effects associated with socioeconomic disadvantage.
Supplementary Material
KEY RESOURCES TABLE.
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | none | |||
| Bacterial or Viral Strain | none | |||
| Biological Sample | none | |||
| Cell Line | none | |||
| Chemical Compound or Drug | none | |||
| Commercial Assay Or Kit | none | |||
| Deposited Data; Public Database | none | |||
| Genetic Reagent | none | |||
| Organism/Strain | none | |||
| Peptide, Recombinant Protein | none | |||
| Recombinant DNA | none | |||
| Sequence-Based Reagent | none | |||
| Software; Algorithm | R software package, FreeSurfer v6.0 pipeline (recon-all) | |||
| Transfected Construct | none | |||
| Other | none |
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by the National Institute of Environmental Health Sciences (Grant Nos. R01ES030950, R01ES032296, and K23026239 [to AEM]) and the National Institute of Mental Health (Grant No. R01MH126181 [to DP]).
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Amy E. Margolis, New York State Psychiatric Institute and Department of Psychiatry, Vagelos College of Physicians and Surgeons
Jacob W. Cohen, New York State Psychiatric Institute and Department of Psychiatry, Vagelos College of Physicians and Surgeons
Bruce Ramphal, New York State Psychiatric Institute and Department of Psychiatry, Vagelos College of Physicians and Surgeons.
Lauren Thomas, New York State Psychiatric Institute and Department of Psychiatry, Vagelos College of Physicians and Surgeons.
Virginia Rauh, Department of Population and Family Health, Mailman School of Public Health.
Julie Herbstman, Columbia Center for Children’s Environmental Health, Department of Environmental Health Sciences, Mailman School of Public Health, Columbia University, New York, New York..
David Pagliaccio, New York State Psychiatric Institute and Department of Psychiatry, Vagelos College of Physicians and Surgeons.
REFERENCES
- 1.Evans GW, Kim P (2013): Childhood poverty, chronic stress, self-regulation, and coping. Child Dev Perspect 7:43–48. [Google Scholar]
- 2.Evans GW (2004): The environment of childhood poverty. Am Psychol 59:77–92. [DOI] [PubMed] [Google Scholar]
- 3.Hajat A, Hsia C, O’Neill MS (2015): Socioeconomic disparities and air pollution exposure: A global review. Curr Environ Health Rep 2:440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith KE, Pollak SD (2020): Early life stress and development: Potential mechanisms for adverse outcomes. J Neurodev Disord 12:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pechtel P, Pizzagalli DA (2011): Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology 214:55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLaughlin K (2020): Early life stress and psychopathology. In: Harkness KL, Hayden EP, editors. The Oxford Handbook of Stress and Mental Health. Oxford: Oxford University Press, 44–74. [Google Scholar]
- 7.Volk HE, Perera F, Braun JM, Kingsley SL, Gray K, Buckley J, et al. (2021): Prenatal air pollution exposure and neurodevelopment: A review and blueprint for a harmonized approach within ECHO. Environ Res 196:110320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Margolis AE, Ramphal B, Pagliaccio D, Banker S, Selmanovic E, Thomas LV, et al. (2021): Prenatal exposure to air pollution is associated with childhood inhibitory control and adolescent academic achievement. Environ Res 202:111570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bansal E, Hsu HH, de Water E, Martínez-Medina S, Schnaas L, Just AC, et al. (2021): Prenatal PM2.5 exposure in the second and third trimesters predicts neurocognitive performance at age 9–10 years: A cohort study of Mexico City children. Environ Res 202:111651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McEwen BS, Gianaros PJ (2010): Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann N Y Acad Sci 1186:190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barch D, Pagliaccio D (2018): Stress and the Brain. Oxford: Oxford University Press. [Google Scholar]
- 12.Xiao L, Sharma VK, Toulabi L, Yang X, Lee C, Abebe D, et al. (2021): Neurotrophic factor-α1, a novel tropin is critical for the prevention of stress-induced hippocampal CA3 cell death and cognitive dysfunction in mice: Comparison to BDNF. Transl Psychiatry 11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEwen BS (1999): Stress and hippocampal plasticity. Annu Rev Neurosci 22:105–122. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe Y, Gould E, McEwen BS (1992): Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res 588:341–345. [DOI] [PubMed] [Google Scholar]
- 15.Alfarez DN, Joëls M, Krugers HJ (2003): Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur J Neurosci 17:1928–1934. [DOI] [PubMed] [Google Scholar]
- 16.Kassem MS, Lagopoulos J, Stait-Gardner T, Price WS, Chohan TW, Arnold JC, et al. (2013): Stress-induced grey matter loss determined by MRI is primarily due to loss of dendrites and their synapses. Mol Neurobiol 47:645–661. [DOI] [PubMed] [Google Scholar]
- 17.Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, et al. (2013): The effects of poverty on childhood brain development: The mediating effect of caregiving and stressful life events. JAMA Pediatr 167:1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanson JL, Chandra A, Wolfe BL, Pollak SD (2011): Association between income and the hippocampus. PLoS One 6:e18712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noble KG, Houston SM, Kan E, Sowell ER (2012): Neural correlates of socioeconomic status in the developing human brain. Dev Sci 15:516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor RL, Cooper SR, Jackson JJ, Barch DM (2020): Assessment of neighborhood poverty, cognitive function, and prefrontal and hippocampal volumes in children. JAMA Netw Open 3:e2023774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu A, Rifkin-Graboi A, Chen H, Chong YS, Kwek K, Gluckman PD, et al. (2013): Maternal anxiety and infants’ hippocampal development: Timing matters. Transl Psychiatry 3:e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moog NK, Nolvi S, Kleih TS, Styner M, Gilmore JH, Rasmussen JM, et al. (2021): Prospective association of maternal psychosocial stress in pregnancy with newborn hippocampal volume and implications for infant social-emotional development. Neurobiol Stress 15:100368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luby JL, Barch DM, Belden A, Gaffrey MS, Tillman R, Babb C, et al. (2012): Maternal support in early childhood predicts larger hippocampal volumes at school age. Proc Natl Acad Sci U S A 109:2854–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luby JL, Belden A, Harms MP, Tillman R, Barch DM (2016): Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development. Proc Natl Acad Sci U S A 113:5742–5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nephew BC, Nemeth A, Hudda N, Beamer G, Mann P, Petitto J, et al. (2020): Traffic-related particulate matter affects behavior, inflammation, and neural integrity in a developmental rodent model. Environ Res 183:109242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodward NC, Haghani A, Johnson RG, Hsu TM, Saffari A, Sioutas C, et al. (2018): Prenatal and early life exposure to air pollution induced hippocampal vascular leakage and impaired neurogenesis in association with behavioral deficits. Transl Psychiatry 8:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tseng CY, Yu JY, Chuang YC, Lin CY, Wu CH, Liao CW, et al. (2019): The Effect of Ganoderma microsporum immunomodulatory proteins on alleviating PM2.5-induced inflammatory responses in pregnant rats and fine particulate matter-induced neurological damage in the offsprings. Sci Rep 9:6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y, Zhang M, Liu W, Li Y, Qin Y, Xu Y (2020): Transgenerational transmission of neurodevelopmental disorders induced by maternal exposure to PM2.5. Chemosphere 255:126920. [DOI] [PubMed] [Google Scholar]
- 29.Cho J, Noh Y, Kim SY, Sohn J, Noh J, Kim W, et al. (2020): Long-term ambient air pollution exposures and brain imaging markers in Korean adults: The environmental pollution-induced neurological EFfects (EPINEF) study. Environ Health Perspect 128:117006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guxens M, Lubczyńska MJ, Muetzel RL, Dalmau-Bueno A, Jaddoe VWV, Hoek G, et al. (2018): Air pollution exposure during fetal life, brain morphology, and cognitive function in school-age children. Biol Psychiatry 84:295–303. [DOI] [PubMed] [Google Scholar]
- 31.Mortamais M, Pujol J, Martínez-Vilavella G, Fenoll R, Reynes C, Sabatier R, et al. (2019): Effects of prenatal exposure to particulate matter air pollution on corpus callosum and behavioral problems in children. Environ Res 178:108734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson BS, Rauh VA, Bansal R, Hao X, Toth Z, Nati G, et al. (2015): Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry 72:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang D, Lee J, Muirhead L, Li TY, Qu L, Yu J, Perera F (2014): Molecular and neurodevelopmental benefits to children of closure of a coal burning power plant in China. PLoS One 9:e91966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Power MC, Lamichhane AP, Liao D, Xu X, Jack CR, Gottesman RF, et al. (2018): The association of long-term exposure to particulate matter air pollution with brain MRI findings: The ARIC study. Environ Health Perspect 126:027009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crous-Bou M, Gascon M, Gispert JD, Cirach M, Sánchez-Benavides G, Falcon C, et al. (2020): Impact of urban environmental exposures on cognitive performance and brain structure of healthy individuals at risk for Alzheimer’s dementia. Environ Int 138:105546. [DOI] [PubMed] [Google Scholar]
- 36.Hedges DW, Erickson LD, Kunzelman J, Brown BL, Gale SD (2019): Association between exposure to air pollution and hippocampal volume in adults in the UK Biobank. Neurotoxicology 74:108–120. [DOI] [PubMed] [Google Scholar]
- 37.Cserbik D, Chen JC, McConnell R, Berhane K, Sowell ER, Schwartz J, et al. (2020): Fine particulate matter exposure during childhood relates to hemispheric-specific differences in brain structure. Environ Int 143: 105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balboni E, Filippini T, Crous-Bou M, Guxens M, Erickson LD, Vinceti M (2022): The association between air pollutants and hippocampal volume from magnetic resonance imaging: A systematic review and meta-analysis. Environ Res 204:111976. [DOI] [PubMed] [Google Scholar]
- 39.Lubczyńska MJ, Muetzel RL, El Marroun H, Hoek G, Kooter IM, Thomson EM, et al. (2021): Air pollution exposure during pregnancy and childhood and brain morphology in preadolescents. Environ Res 198:110446. [DOI] [PubMed] [Google Scholar]
- 40.Padula AM, Monk C, Brennan PA, Borders A, Barrett ES, McEvoy CT, et al. (2020): A review of maternal prenatal exposures to environmental chemicals and psychosocial stressors-Implications for research on perinatal outcomes in the ECHO program. J Perinatol 40:10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vishnevetsky J, Tang D, Chang HW, Roen EL, Wang Y, Rauh V, et al. (2015): Combined effects of prenatal polycyclic aromatic hydrocarbons and material hardship on child IQ. Neurotoxicol Teratol 49:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pagliaccio D, Herbstman JB, Perera F, Tang D, Goldsmith J, Peterson BS, et al. (2020): Prenatal exposure to polycyclic aromatic hydrocarbons modifies the effects of early life stress on attention and thought problems in late childhood. J Child Psychol Psychiatry 61:1253–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller JG, Dennis EL, Heft-Neal S, Jo B, Gotlib IH (2022): Fine particulate air pollution, early life stress, and their interactive effects on adolescent structural brain development: A longitudinal tensor-based morphometry study. Cereb Cortex 32:2156–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee ACH, Brodersen KH, Rudebeck SR (2013): Disentangling spatial perception and spatial memory in the hippocampus: A univariate and multivariate pattern analysis fMRI study. J Cogn Neurosci 25:534–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guderian S, Dzieciol AM, Gadian DG, Jentschke S, Doeller CF, Burgess N, et al. (2015): Hippocampal volume reduction in humans predicts impaired allocentric spatial memory in virtual-reality navigation. J Neurosci 35:14123–14131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown TI, Whiteman AS, Aselcioglu I, Stern CE (2014): Structural differences in hippocampal and prefrontal gray matter volume support flexible context-dependent navigation ability. J Neurosci 34:2314–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Travis SG, Huang Y, Fujiwara E, Radomski A, Olsen F, Carter R, et al. (2014): High field structural MRI reveals specific episodic memory correlates in the subfields of the hippocampus. Neuropsychologia 53:233–245. [DOI] [PubMed] [Google Scholar]
- 48.Morgan LK, Macevoy SP, Aguirre GK, Epstein RA (2011): Distances between real-world locations are represented in the human hippocampus. J Neurosci 31:1238–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shavitt T, Johnson INS, Batistuzzo MC (2020): Hippocampal formation volume, its subregions, and its specific contributions to visuospatial memory tasks. Braz J Med Biol Res 53:e9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD (2000): Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A 97:4398–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maguire EA, Woollett K, Spiers HJ (2006): London taxi drivers and bus drivers: A structural MRI and neuropsychological analysis. Hippocampus 16:1091–1101. [DOI] [PubMed] [Google Scholar]
- 52.Longoni G, Rocca MA, Pagani E, Riccitelli GC, Colombo B, Rodegher M, et al. (2015): Deficits in memory and visuospatial learning correlate with regional hippocampal atrophy in MS. Brain Struct Funct 220:435–444. [DOI] [PubMed] [Google Scholar]
- 53.Banker SM, Pagliaccio D, Ramphal B, Thomas L, Dranovsky A, Margolis AE (2021): Altered structure and functional connectivity of the hippocampus are associated with social and mathematical difficulties in nonverbal learning disability. Hippocampus 31:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suglia SF, Gryparis A, Wright RO, Schwartz J, Wright RJ (2008): Association of black carbon with cognition among children in a prospective birth cohort study. Am J Epidemiol 167:280–286. [DOI] [PubMed] [Google Scholar]
- 55.Chiu YH, Hsu HH, Coull BA, Bellinger DC, Kloog I, Schwartz J, et al. (2016): Prenatal particulate air pollution and neurodevelopment in urban children: Examining sensitive windows and sex-specific associations. Environ Int 87:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harris MH, Gold DR, Rifas-Shiman SL, Melly SJ, Zanobetti A, Coull BA, et al. (2015): Prenatal and childhood traffic-related pollution exposure and childhood cognition in the project viva cohort (Massachusetts, USA). Environ Health Perspect 123:1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM (2000): Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience 97:253–266. [DOI] [PubMed] [Google Scholar]
- 58.Zheng X, Wang X, Wang T, Zhang H, Wu H, Zhang C, et al. (2018): Gestational exposure to particulate matter 2.5 (PM2.5) leads to spatial memory dysfunction and neurodevelopmental impairment in hippocampus of mice offspring. Front Neurosci 12:1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Margolis AE, Pagliaccio D, Ramphal B, Banker S, Thomas L, Robinson M, et al. (2021): Prenatal environmental tobacco smoke exposure alters children’s cognitive control circuitry: A preliminary study. Environ Int 155:106516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen S, Kamarck T, Mermelstein R (1994): Perceived stress scale. Measuring Stress: A Guide for Health and Social Scientists 10:1–2. [Google Scholar]
- 61.Dohrenwend BP, Shrout PE, Egri G, Mendelsohn FS (1980): Nonspecific psychological distress and other dimensions of psychopathology. Measures for use in the general population. Arch Gen Psychiatry 37:1229–1236. [DOI] [PubMed] [Google Scholar]
- 62.Mayer SE, Jencks C (1989): Poverty and the distribution of material hardship. J Hum Resour 24:88–114. [Google Scholar]
- 63.Kim SY, Nair R, Knight GP, Roosa MW, Updegraff KA (2008): Measurement equivalence of neighborhood quality measures for European American and Mexican American families. J Community Psychol 37:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sampson RJ, Raudenbush SW, Earls F (1997): Neighborhoods and violent crime: A multilevel study of collective efficacy. Science 277:918–924. [DOI] [PubMed] [Google Scholar]
- 65.Sampson RJ, Raudenbush SW (1999): Systematic social observation of public spaces: A new look at disorder in urban neighborhoods. Am J Sociol 105:603–651. [Google Scholar]
- 66.Straus MA, Hamby SL, Boney-McCoy S, Sugarman DB (1996): The revised conflict tactics scales (CTS2): Development and preliminary psychometric data. J Fam Issues 17:283–316. [Google Scholar]
- 67.Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, et al. (2006): Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect 114:1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tonne CC, Whyatt RM, Camann DE, Perera FP, Kinney PL (2004): Predictors of personal polycyclic aromatic hydrocarbon exposures among pregnant minority women in New York City. Environ Health Perspect 112:754–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lovasi GS, Eldred-Skemp N, Quinn JW, Chang HW, Rauh VA, Rundle A, et al. (2014): Neighborhood social context and individual polycyclic aromatic hydrocarbon exposures associated with child cognitive test scores. J Child Fam Stud 23:785–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.David Wechsler XZ (2011): WASI-II: Wechsler Abbreviated Scale of Intelligence. San Antonio: Psychological Corporation. [Google Scholar]
- 71.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. (2002): Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. [DOI] [PubMed] [Google Scholar]
- 72.Fischl B, Salat DH, van der Kouwe AJW, Makris N, Ségonne F, Quinn BT, Dale AM (2004): Sequence-independent segmentation of magnetic resonance images. Neuroimage 23(suppl 1):S69–S84. [DOI] [PubMed] [Google Scholar]
- 73.Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M, et al. (2015): A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage 115:117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramphal B, Pagliaccio D, Dworkin JD, Herbstman J, Noble KG, Margolis AE (2021): Timing-specific associations between income-to-needs ratio and hippocampal and amygdala volumes in middle childhood: A preliminary study. Dev Psychobiol 63:e22153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tottenham N, Sheridan MA (2009): A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Front Hum Neurosci 3:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luby JL, Tillman R, Barch DM (2019): Association of timing of adverse childhood experiences and caregiver support with regionally specific brain development in adolescents. JAMA Netw Open 2: e1911426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taliaz D, Loya A, Gersner R, Haramati S, Chen A, Zangen A (2011): Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J Neurosci 31:4475–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Radecki DT, Brown LM, Martinez J, Teyler TJ (2005): BDNF protects against stress-induced impairments in spatial learning and memory and LTP. Hippocampus 15:246–253. [DOI] [PubMed] [Google Scholar]
- 79.Miranda M, Morici JF, Zanoni MB, Bekinschtein P (2019): Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci 13:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Berg EL, Pedersen LR, Pride MC, Petkova SP, Patten KT, Valenzuela AE, et al. (2020): Developmental exposure to near roadway pollution produces behavioral phenotypes relevant to neurodevelopmental disorders in juvenile rats. Transl Psychiatry 10:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patten KT, González EA, Valenzuela A, Berg E, Wallis C, Garbow JR, et al. (2020): Effects of early life exposure to traffic-related air pollution on brain development in juvenile Sprague-Dawley rats. Transl Psychiatry 10:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R (2011): Residential proximity to freeways and autism in the CHARGE study. Environ Health Perspect 119:873–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Broitman J, Melcher M, Margolis A, Davis JM (2020): NVLD and Developmental Visual-Spatial Disorder in Children: Clinical Guide to Assessment and Treatment. Cham, Switzerland: Springer. [Google Scholar]
- 84.Feczko E, Balba NM, Miranda-Dominguez O, Cordova M, Karalunas SL, Irwin L, et al. (2018): Subtyping cognitive profiles in autism spectrum disorder using a Functional Random Forest algorithm. Neuroimage 172:674–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buss C, Lord C, Wadiwalla M, Hellhammer DH, Lupien SJ, Meaney MJ, Pruessner JC (2007): Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. J Neurosci 27:2592–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


