Abstract
The global pandemic of coronavirus disease 2019 (COVID-19) affected many aspects of randomized controlled trials, including recruiting and screening participants. The purpose of this paper is to (a) describe adjustments to recruitment and screening due to COVID-19, (b) compare the proportional recruitment outcomes (not completed, ineligible, eligible) at three screening stages (telephone, health assessment, physical activity assessment) pre- and post-COVID-19 onset, and (c) compare baseline demographic characteristics pre- and post-COVID-19 onset in the Working Women Walking program. The design is a cross-sectional descriptive analysis of recruitment and screening data from a 52-week sequential multiple assignment randomized trial (SMART). Participants were women 18–70 years employed at a large urban medical center. Recruitment strategies shifted from in-person and electronic to electronic only post-COVID-19 onset. In-person eligibility screening for health and physical activity assessments continued post-COVID-19 onset with Centers for Disease Control and Prevention precautions. Of those who expressed interest in the study pre- and post-COVID-19 onset (n=485 & n=269 respectively), 40% (n=194) met all eligibility criteria pre-COVID-19 onset and 45.7% (n=123) post-COVID-19 onset. Although there were differences in the proportions of participants who completed or were eligible for some of the screening stages, the final eligibility rates did not differ significantly pre-COVID-19 versus post-COVID-19 onset. Examination of differences in participant demographics between pre- and post-COVID-19 onset revealed a significant decrease in the percentage of Black women recruited into the study from pre- to post-COVID-19 onset. Studies recruiting participants into physical activity studies should explore the impact of historical factors on recruitment.
Keywords: Clinical trial, COVID-19, physical activity, recruitment, Working Women Walking
Background
Launched in March 2018, the Working Women Walking program is a 52-week sequential multiple assignment randomized trial (SMART) that tests interventions to increase physical activity and improve cardiovascular health in working women (Buchholz et al., 2020). The interventions all emphasize a lifestyle physical activity approach that builds in opportunities for success throughout the day through leisure, household, work, and transportation. The study targeted to recruit 312 women employees for intervention delivery at a large urban medical center. Initially, women were randomized to either an enhanced physical activity monitor (a Fitbit activity monitor and mobile app with goal setting and physical activity prescription) or an enhanced physical activity monitor plus text messaging for eight months. Non-responders to the initial intervention at the two-month assessment were further randomized to either personal calls or groups meetings for the next six months. At the eight-month assessment, all participants returned to only an enhanced physical activity monitor until their final 12-month assessment.
We planned to use multiple recruitment strategies for a successful and timely recruitment (Daniel et al., 2013; Gibbs et al., 2021; Rodríguez-Larrad et al., 2021; United Nations, 2020; Wilbur et al., 2006; Wilbur et al., 2013), relying heavily on an in-person recruitment approach with distribution of study flyers and presentations at workplace settings to promote study awareness. However, in-person recruitment sometimes can create inconveniences related to time, location (Wilbur et al., 2013), and accessibility (Wilbur et al., 2006) that could become a barrier for potential participants. Therefore, to facilitate recruitment of potential participants with added flexibility and convenience (Carroll et al., 2011; Foster et al., 2011; Wilbur et al., 2006) we planned to incorporate electronic recruitment strategies. We used emails associated with women’s employment at the institution, which has been found to be an effective recruitment strategy at workplace settings (Audrey et al., 2019). We collaborated with key stakeholders (e.g., human resources, clinicians, staff) to receive input regarding wording for study description, color, logos, and photographs used for recruitment (Foster et al., 2011; Wilbur et al., 2006; Wilbur et al., 2013).
To assure participants are safe to participate in a physical activity trial, a screening health assessment (history and physical) is frequently required to identify risk factors. However, in-person health assessment screening can also create inconveniences related to time, location (Wilbur et al., 2013), accessibility, and distance to travel (Wilbur et al., 2006) that could become barriers for potential participants. Therefore, we planned for a three-stage screening starting with telephone screening and followed by an in-person health assessment and physical activity assessment. Telephone screening stage has been found to be the most effective approach after in-person screening to obtain a high response (Gould et al., 2021; Heerman et al., 2017; Villarosa et al., 2021). The telephone screening was used to provide an introductory overview of the study and assess eligibility for general inclusion criteria (Buchholz et al., 2020; Wilbur et al., 2013). The second stage was an in-person health assessment (Buchholz et al., 2020; Wilbur et al., 2013; Canadian Society for Exercise Physiology, 2002a). To be less restrictive (Foster et al; 2011; Wilbur et al., 2006; Wilbur et al., 2013) and to allow flexibility in the timeline for receipt of medical clearance, if needed, and to prevent delays in starting the intervention, we added the option of receiving medical clearance electronically (i.e., via email). Finally, a physical activity assessment was done, based on objective information, to determine if a woman was insufficiently active (Buchholz et al., 2020; Wilbur et al., 2013).
In March 2020, approximately two years after the launch of the Working Women’s Walking program, the coronavirus disease 2019 pandemic (COVID-19) (World Health Organization [WHO], 2021) began to affect many aspects of recruitment and screening in randomized controlled trials, including those conducted to improve physical activity. Preventive practices related to the COVID-19 pandemic, including social distancing, quarantine, shelter-in-place orders, and other protective regulatory restrictions (Centers for Disease Control and Prevention [CDC], 2021) were put into place by the federal and state governments. These precautions were critical for participant safety and community spread of COVID-19 from close person-to-person contact outside the household. However, these mandates caused delays in study trial recruitment timelines and decreased enrollment numbers (Röhr et al., 2021; Sathian et al., 2020).
Globally, as of February 4, 2022, there have been 386,548,962 confirmed cases of COVID-19, including 5,705,754 deaths (WHO, 2021). A recent systematic review (Sathian et al., 2020) that examined the global impact of the COVID-19 pandemic on the conduction of clinical trials and research, reported findings from a Medidata survey (a global platform that is monitoring worldwide impact of COVID-19 on conducting clinical trials). The Medidata survey, which included 1,030 research staff members representing North America, South America, Asia, Europe, and Middle East and North Africa, reported difficulties in enrolling subjects as the primary concern followed by financial concerns related to either study cancellation or from delayed endpoints (Medidata, 2020). A trial in the review that was conducted in the U.S. reported that the primary impact of COVID-19 on clinical trials was the participants’ decreased willingness to come to the study site (Waterhouse et al., 2020). Additional knowledge regarding how the COVID-19 pandemic has impacted study recruitment, including screening, ineligibility, and eligibility status is needed.
Purpose
To address the national call for innovative strategies to safely conduct clinical trials during the COVID-19 pandemic (National Institute of Environmental Health Sciences, 2021), modifications were made to the Working Women Walking program’s recruitment strategies and screening protocol after the onset of the COVID-19 pandemic. Therefore, the purpose of this paper is to: (a) describe adjustments to participant recruitment/screening, (b) compare the proportional recruitment outcomes for “not completed,” “completed,” “ineligible,” and “eligible” at the three screening stages (telephone screening, health assessment, physical activity assessment) pre- and post-COVID-19 onset, and (c) compare participant baseline demographic characteristics (ethnicity, age, income, education) pre- and post-COVID-19 onset, in a large physical activity study with 312 working women.
Methods
Design
The parent study was a 52-week SMART (Buchholz et al., 2020) that aimed to determine the most effective adaptive intervention combining four efficacious treatments (Fitbit physical activity monitor, motivational text messages, motivational personal calls, and group meetings) to increase physical activity and improve cardiovascular health. Details of the design of the parent study are published elsewhere (Buchholz et al., 2020) This is a cross-sectional descriptive analysis of recruitment and screening data from SMART. Recruitment and screening was scheduled to be staggered in five waves over the course of two years. Recruitment and screening for each wave was scheduled to last approximately two months with a planned break for three to five months before the start of recruitment and screening for the next wave.
Setting and Sample
The recruitment site was a workplace setting, specifically a large urban academic medical center in the Midwest. Given d = .41, a α2-tailed = .01, pre-post correlation of r = .60 and over-sampling by 10% to account for attrition, the target sample size was 312 working women (Buchholz et al., 2020). According to the proposed timeline and recruitment goals, dividing 312 participants into five waves, the target enrollment for each wave was 62 or more participants (Buchholz et al., 2020). In-person screening was done at a designated clinical space in the recruitment site.
Measures
For each of the three screening stages (telephone, health assessment, physical activity), potential participants were classified as “not completed,” “completed,” “ineligible,” or “eligible,” resulting in four possible categories for each of the screening stages. Potential participants classified as “not completed” were lost to the study prior to establishing eligibility at any time from the point of first contact through the three screening stages (Wilbur et al., 2013). Within the “not completed” category, potential participants could have been “active” or “passive.” Potential “active not completed” potential participants were those who said they were not interested or provided some other reason for not being able to participate in a screening stage (Wilbur et al., 2013). Potential “passive not completed” participants were those whom either study staff were unable to contact to schedule the screening visits or who missed the recruitment window. Those who missed two scheduled appointments for telephone, health assessment, or physical activity screening without making contact were also classified as “passive not completed” (Wilbur et al., 2013). Participants who were classified as “ineligible” were those who failed to meet the screening criteria for telephone, health assessment, or physical activity screening (Buchholz et al., 2020). Participants who were classified as “eligible” met the screening criteria for telephone, health assessment, and physical activity screening.
Pre- and Post-COVID-19-Onset Recruitment Strategies
Recruitment strategies were approved by the Institution Review Board pre-COVID-19 onset. Pre-COVID-19 onset, the recruitment strategies included both in-person (cafeteria outreach by Working Women’s Walking program staff and distributing flyers by directly handing to employees) and electronic (institutional email announcements). Paper flyers were posted on departmental bulletin boards and recruitment information was posted on electronic bulletin boards in common areas in the medical center. All recruitment materials provided information regarding the study, eligibility criteria and the program email address and telephone number to contact study staff for those interested in learning more and in being screened for eligibility. Post-COVID-19 onset, in-person recruitment strategies were discontinued due to social distancing precautions, limiting recruitment to electronic (email) announcements and posted flyers (i.e. paper and electronic).
Pre- and Post-COVID-19-Onset Screening Protocol
The screening protocol consisted of three stages: (a) telephone, (b) health assessment, and (c) physical activity assessment (Figure 1). The protocol called for each stage of the screening to occur within seven days after completion of the prior stage. If potential participants did not complete all three stages of screening by the predetermined screening period of a given wave, they were notified that screening for that wave was closed. The study staff would then offer to contact them prior to the start of the next wave when applicable.
Figure 1.
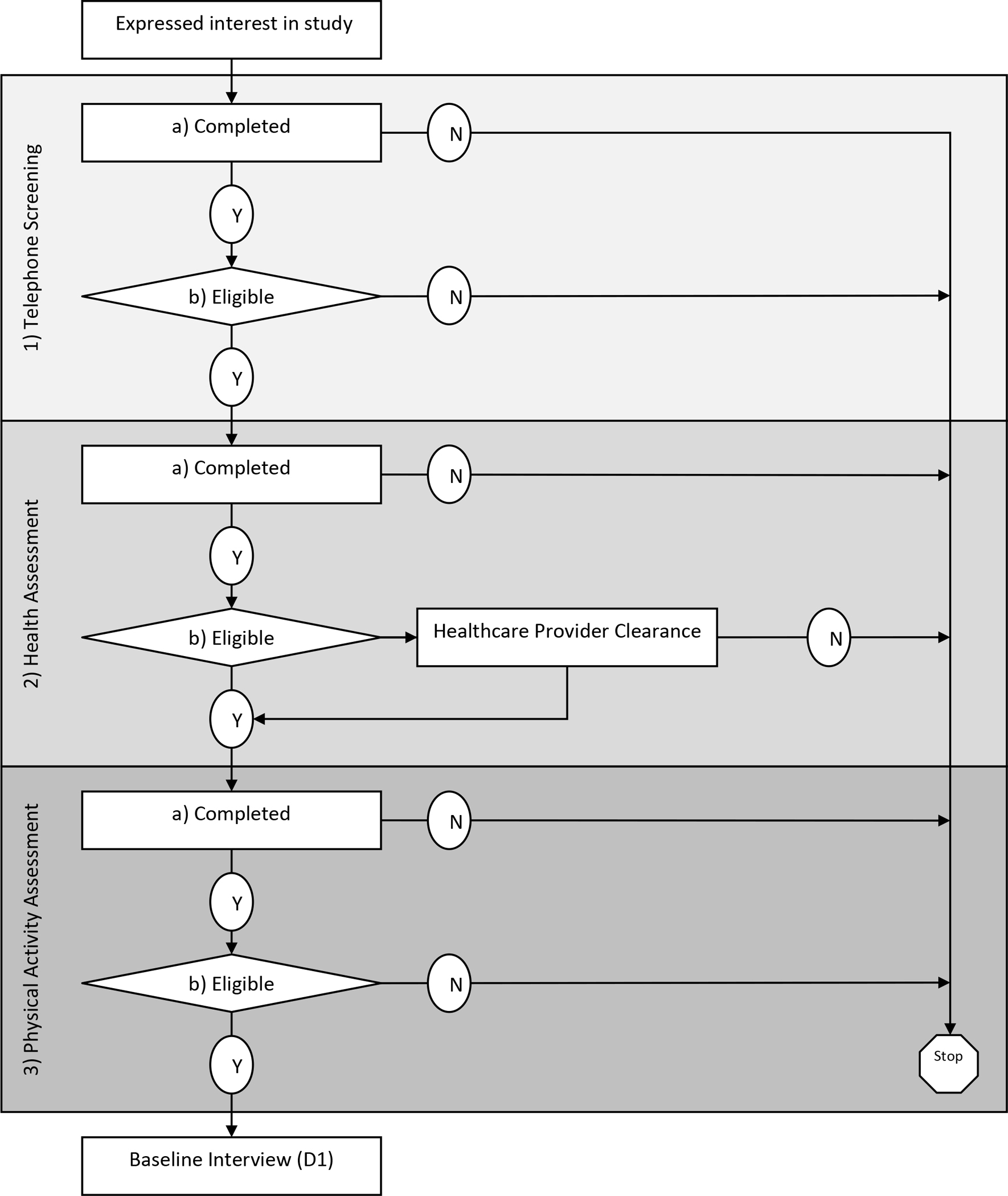
Three Screening Stages of Eligibility
Stage 1: Telephone Screening
Throughout the study, study staff responded to inquiry telephone calls and emails from potential participants within 24 hours (Monday through Friday). Eight contact attempts were made across three different modes of contact (four emails, two texts, and two telephone calls) for three consecutive weeks (Wilbur et al., 2013). When interested potential participants were reached, they were asked for a convenient 20-minute block of time to receive a brief study overview and screening for eligibility. The introductory overview of the study included the screening and study protocol, benefits, risks, and expectations (Wilbur et al., 2013). In addition, they were also asked how they heard about the study (e.g., institutional emails, Working Women Walking program staff, or flyers). Participants could also report hearing about the study through word of mouth from another employee or an “other” method.
The eligibility criteria for telephone screening included employment at the recruitment site institution (clinical and non-clinical staff), self-identification as a female between the ages of 18 and 70, ability to speak and read English, and owning a smartphone with text messaging capability (Buchholz et al., 2020). All women pre-and post-Covid-19-onset who were found to be “eligible” at the telephone screening, were given an appointment for in-person Stage-2 health assessment screening within one week at the recruitment site. Their email addresses and alternative telephone numbers were obtained (Wilbur et al., 2013). Potential participants were offered an electronic copy of the consent and a HIPAA form to allow them time to read the material prior to their health assessment screening visit. Because telephone screening did not involve any in-person contact pre-COVID-19 onset, no changes were made to the telephone screening protocol post-COVID-19 onset.
Stage 2: Health Assessment Screening
Potential pre- and post- COVID-19-onset participants received two person-administered telephone reminder calls, one a week prior to and one the day before the in-person health assessment screening. Throughout the study, the CDC biohazard guidelines were followed for HbA1c (CDC, 2015). For in-person screening for health assessments post-COVID-19 onset, COVID 19 precautions were put in place, including COVID-19 screening of staff and potential participants (i.e., temperature check, symptoms, exposure, if tested positive, and quarantine status) strict hand hygiene, social distancing, use of personal protective equipment, and disinfecting the surfaces and equipment in the assessment room. The institution had implemented ambient temperature scanners at main entrances and provided masks to participants if needed. Prior to beginning the health assessment, a study staff member read the detailed 30-minute informed consent to the potential participants as they read along.
Health assessment screening included subjective and objective health information. To ensure it was safe for the potential participant to be involved in the study, eligibility was determined subjectively by answering the Physical Activity Readiness Questionnaire (Canadian Society for Exercise Physiology, 2002a) and a brief health questionnaire in RedCap. The eligibility criteria obtained from the Physical Activity Readiness Questionnaire included absence of physical activity restriction based on a heart condition, chest pain with and without physical activity, balance impairment, and a bone or joint problem that could be made worse by a change in physical activity. Additional eligibility criteria required the absence of any prescription medications for blood pressure or a heart condition.
Eligibility was determined objectively by assessing blood pressure and HbA1c. Eligibility criteria for blood pressure was a systolic blood pressure less than 160 and a diastolic less than 100 (Buchholz et al., 2020; U.S. DHHS, 2004; Weber et al., 2014). Eligibility criteria for HbA1c was less than 9% with known diabetes and less than 6.5% without a prior diabetes diagnosis (American Diabetes Association, 2016; Buchholz et al., 2020) unless given clearance by their healthcare provider.
If a potential participant did not meet all the subjective and objective health assessment screening eligibility criteria, they were given the opportunity to sign an Authorization for Disclosure of Research Record Information. This authorized the study staff to release health findings to the participant’s designated healthcare provider. Potential participants were asked to contact their healthcare providers to determine if it was safe to participate by completing a PARmed-X form (Buchholz et al., 2020; Canadian Society for Exercise Physiology, 2002b).
Potential participants who met all the health assessment screening criteria were given two physical activity monitors to be used for the physical activity screening (see below). Pre- and post-COVID-19 onset potential participants who required provider clearance were scheduled for an in-person return visit to receive the monitors upon receipt of their signed PARmed-X. For both pre- and post-COVID-19-onset participants, an in-person appointment was scheduled one week later to return the activity monitors.
Stage 3: Physical Activity Assessment Screening
To complete the physical activity assessment, potential participants wore two activity monitors for 7 days (ActiGraph accelerometer, Fitbit) during waking hours for a minimum of 10 hours. The ActiGraph accelerometer (ActiGraph, 2019; McClain et al., 2007) was used to determine eligibility based on their average steps/day. Both pre- and post- COVID-19-onset potential participants received one person-administered reminder telephone call to come to their appointment at the recruitment site for the physical activity assessment screening.
The eligibility criterion for physical activity was less than 7,500 average steps/day (sedentary to low active; (Tudor-Locke et al., 2011) from the ActiGraph accelerometer which had to be worn ≥ 10 hours a day on ≥ 4 days per week with at least one weekend day (Buchholz et al., 2020; Wilbur et al., 2016). Based on their Actigraph step-count data, all “eligible” women were enrolled and scheduled for their baseline assessment visit. The Fitbit Charge 2 or Fitbit Charge 3 (Diaz et al., 2015) was used for determining a baseline physical activity prescription for eligible women.
Data Analysis
Analyses compared differences between pre- and post-COVID-19 onset for recruitment process and characteristics of the final, recruited sample. Chi-square tests of independence were used to examine differences pre-and post-COVID-19 onset for aspects of the recruitment process, including (a) recruitment methods, (b) completion of each recruitment step (telephone, health status assessment, physical activity assessment), (c) eligibility at each recruitment step, and (d) final eligibility status (i.e., among all those who completed the telephone screening, Independent sample t-tests, and Chi-square tests of independence were used to compare differences pre-and post-COVID-19 onset in demographic characteristics of the final sample. For multinomial data (race/ethnicity), significant overall tests were followed by tests of differences in proportions for each race/ethnicity individually. All analyses were conducted with SPSS v26.
Results
Recruitment Timeline
The study began recruiting in March 2018 and all three screening stages for the first three waves were completed by December 2019 (pre-COVID-19 onset). Recruitment for wave 4 was scheduled to begin in May 2020, five months after completion of wave 3. However, due to federal- and state-mandated COVID-19 precautions initiated in March 2020, the in-person activities for the trial were put on hold. Once human subject research recruitment was permitted at the study institution, in July 2020 recruitment for wave 4 began. This resulted in an overall two-month delay in our recruitment plan. Once recruitment began for each of the waves, it was completed without further delay.
Pre-and Post-COVID-19-Onset Recruitment Strategies
We looked at those who completed the telephone screening pre-COVID-19 onset (n=329) and post-COVID-19 onset (n=202) and identified how potential participants heard about the study (Table 1). The recruitment methods reported were consistent with the methods being used pre- COVID onset (electronic institutional emails, Working Women Walking program staff, flyers, word of mouth from another employee, and other) and post-COVID-19 onset (electronic institutional emails, flyers, word of mouth from another employee, and other), X2(4, N=531) = 157.7, p < .001. Follow-up tests revealed significant changes in report of every method except word of mouth. For potential participants in both pre- and post-COVID-19-onset groups, approximately one in six reported hearing about the study by word of mouth
Table 1.
Differences in Recruitment Method Reported Pre- and Post-COVID-19 Among Those Who Completed a Telephone Screening (n=531)
| Recruitment Method | Pre-COVID-19 onset (n = 329) |
Post-COVID-19 onset (n =202) |
||
|---|---|---|---|---|
|
| ||||
| n | % | n | % | |
|
| ||||
| Electronic: institutional email announcements | 83 | 25.2 | 153 | 75.7 |
| Cafeteria outreach by study staff | 92 | 28.0 | 4a | 2.0 |
| Flyer | 65 | 19.8 | 4 | 2.0 |
| Word of mouth from another employee | 53 | 16.1 | 34 | 16.8 |
| Other/Unknown | 36 | 10.9 | 7 | 3.5 |
X2(4, N=531) = 157.7, p < .001
Note - all row percentages are significantly different except word of mouth
These cases initially heard about the study before the onset of COVID-19 but completed the telephone screening after the onset of COVID-19
Post-COVID-19-Onset Recruitment Screening Protocol
Telephone Screening
Of those who responded to invitations to participate in the study, completion of telephone screening occurred at a significantly higher rate during the post-COVID-19-onset phase compared to pre-COVID19 onset (Table 2). Of those who did not complete the telephone screening pre-COVID-19 onset and were categorized as “active not completed” (76/156, 48.7%), the primary reasons were loss of interest (47.4%), left the employment site (22.4%), and too busy (11.8%). Of those who did not complete the telephone screening post-COVID-19 onset and were categorized as “active not completed” (30/67 44.8%), the primary reasons were loss of interest (50%) and too busy (10.0%). For those who were categorized as “passive not completed” for pre-COVID-19 onset (80/156, 51.3%) and for post-COVID-19 onset (37/67, 55.2%), the primary reason was study staff’s inability to contact them (98.8% & 97.3% respectively). Among those who completed telephone screening, there were no significant differences between pre- and post-COVID-19 onset in the eligibility rates. The primary reason for ineligibility pre- and post-COVID-19 onset was not being employed at the target institution (90.9% & 66.7%, respectively). For instance, non-employees contacted the Principal Investigator to express their interest in the study, after seeing the study on clinicaltrials.gov.
Table 2.
Impact of Pre- and Post-COVID-19 Recruitment Screening Among Those Who Expressed Interest in Study (n=754)
| Pre-COVID-19 onset (n = 485) |
Post-COVID-19 onset (n =269) |
|||||
|---|---|---|---|---|---|---|
|
| ||||||
| Screening Phases | n | %* | n | %* | χ2 | P |
|
| ||||||
| 1) Telephone | ||||||
| a) Completed | 329 | 67.8 | 202 | 75.1 | 4.38 | .04 |
| b) Eligible | 318 | 96.7 | 199 | 98.5 | 1.68 | .19 |
| 2) Health Assessment | ||||||
| a) Completed | 264 | 83.0 | 159 | 79.9 | 0.80 | .37 |
| b) Eligible | 254 | 96.2 | 143 | 89.9 | 6.77 | .009 |
| 3) Physical Activity Assessment | ||||||
| a) Completed | 229 | 90.2 | 138 | 96.5 | 5.27 | .02 |
| b) Eligible | 194 | 84.7 | 123 | 89.1 | 1.43 | .23 |
Note: Percentages use the sample size from the prior step as the denominator.
Health Assessment Screening
Among those eligible following the telephone screening, there were no significant differences in rates of completing the health assessment (Table 2). Of those who did not complete the screening and were categorized as “active not completed” pre- COVID-19 onset (33/54, 61.1%), the primary reasons were reported loss of interest (51.5%) and being too busy (21.2%). Of those who did not complete the screening and were categorized as “active not completed” post- COVID-19 onset (33/40, 82.5%), the primary reasons reported were too busy (54.5%), loss of interest (18.2%) and health concerns (12.1%). For those who were categorized as “passive not completed” for pre- and post-COVID-19 onset (21/54, 38.9% & 7/40,17.5% respectively), the reason for all cases was study staff’s inability to contact them. Among those who completed the health assessment, eligibility rates were significantly higher pre-COVID-19 onset compared to those recruited post-COVID-19 onset (96.2% & 89.9%, respectively). A need for medical clearance based on the Physical Activity Readiness Questionnaire was the primary reason for ineligibility for pre- and post-COVID-19 onset (60.0% & 87.8%, respectively).
Physical Activity Assessment Screening
Among those potential participants eligible following the health assessment, completion of the physical activity screening occurred at a significantly higher rate during the post-COVID-19-onset phase compared to pre-COVID-19 onset (Table 2). Of those who did not complete the screening and were categorized as “active not completed” for pre- COVID 19 onset (15/25, 60%), the primary reasons reported were too busy (33.3%), loss of interest (26.7%), and did not agree to wear the physical activity devices (26.7%). Of those who did not complete the screening and were categorized as “active not completed” for post-COVID-19 onset (1/5, 20%), the only reason reported was health concerns. For those who were categorized as “passive not completed” for pre-COVID-19 onset (10/25, 40%), the primary reasons were inability of study staff to contact them (50%) and missing the recruitment window (50%). For those who were categorized as “passive not completed” for pre-COVID-19 onset (4/5, 80%), the reason for all was missing the recruitment window. Among those who completed the physical activity assessment screening, there were no significant differences in the eligibility rates pre-and post-COVID-19 onset. The primary reason for ineligibility pre- and post-COVID-19 onset was being too active based on their physical activity assessment with an Actigraph (35/35, 100% & 12/15, 80% respectively).
Pre- and Post-COVID-19-Onset Demographic Characteristics
Of those who were eligible (n=317; 194 pre-COVID onset; 123 post-COVID-19 onset), 16 (5.0%) did not complete the baseline assessment. Of the 301 participants who completed the baseline assessment, 181 (60.1%) were enrolled pre-COVID-19 onset and 120 (39.9%) were enrolled post-COVID-19 onset. Examination of demographic characteristics of those who completed the baseline assessment by pre- and post-COVID-19 onset revealed no differences in age, income, education. or marital status (Table 3). There was a significant difference, however, in racial/ethnic distribution overall. Follow-up tests showed that a lower proportion of Black women participated post-COVID-19 onset compared to pre-COVID-19 onset (36.5% vs. 21.7% respectively, p = < 0.05). No significant differences were found for participation rates of Whites (49.2% vs. 51.7%), Asians (6.1% vs. 10.8%), and Latinas (2.8% vs. 5%).
Table 3.
Characteristics of Participants (Demographics)
| Pre-COVID-19 onset n = 181a |
Post-COVID-19 onset n = 120a |
t, χ2 b | p | |
|---|---|---|---|---|
|
| ||||
| Age M (SD) | 45.4 (11.3) | 44.5 (11.5) | 0.65 | .52 |
| Personal Income n (%) | 0.75 | .38 | ||
| - <50,000 | 51 (34.0) | 28 (28.3) | ||
| - 50,000–99,000 | 76 (50.7) | 54 (54.5) | ||
| - >100, 000 | 23 (15.3) | 17 (17.2) | ||
| Household Income n (%) | 1.90 | .17 | ||
| - <50,000 | 33 (22.0) | 13 (13.8) | ||
| - 50,000–99,000 | 55 (36.7) | 37 (39.4) | ||
| - >100,000 | 62 (41.3) | 44 (46.8) | ||
| Education n (%) | 1.34 | .25 | ||
| - Less than college degree | 42 (23.2) | 21 (17.6) | ||
| - College degree or higher | 139 (76.8) | 98 (82.4) | ||
| Race/ethnicity n (%) | 10.96 | .03 | ||
| - White | 89 (49.2) | 62 (51.7) | ||
| - Blackc | 66 (36.5) | 26 (21.7) | ||
| - Latina | 5 (2.8) | 6 (5.0) | ||
| - Asian | 11 (6.1) | 13 (10.8) | ||
| - Not reported | 10 (5.5) | 13 (10.8) | ||
| Married/living with a partner n (%) | 103 (57.2) | 74 (61.7) | 0.59 | .44 |
Sample sizes may not total column sample sizes due to missing data.
Tests statistics were t-tests for means (age), and Chi-Square for proportions.
Follow-up pair-wise tests determined significant differences for the proportion of Black participants only; X2(1, N=301) =7.45, p=.006.
Discussion
To address the impact of the COVID-19 pandemic on participation in clinical trials, adjustments in study recruitment strategies and screening were necessary. Researchers needed to pivot contact strategies and screening to address concerns for safety of potential participants in behavioral, physical activity intervention trials. This paper described how recruitment and screening of potential participants for a physical activity study with working women, begun prior to COVID-19 onset, continued successfully post-COVID-19 onset with only a two-month delay.
Consistent with prior studies (Audrey et al., 2019; Carriedo et al., 2020; Foster et al., 2011; Shaw et al., 2004; Wilbur et al., 2006; Wilbur et al., 2013), our study provided for multiple recruitment strategies, in-person and electronic, that were developed with thorough planning prior to the onset of the COVID-19 pandemic. Not surprisingly, the percentage of potential participants who heard about the study in-person from program staff went down while institutional emails went up post-COVID-19 onset. As with prior studies (Shaw et al., 2004; Thomas & Williams, 2006) conducted in the workplace settings, we found electronic institutional emails for study announcements to be a viable recruitment strategy, particularly post-COVID-19 onset. We speculate that women who were working remotely during the pandemic may have become even more attentive to email announcements of research opportunities. No difference was seen in the percentage of potential participants who heard about the study via word of mouth, pre-or post-COVID-19 onset. Although we did not distinguish between in-person versus electronic means of relaying information about the study by word of mouth, it is possible that word of mouth among potential participants was facilitated electronically post-COVID-19 onset (Carriedo et al., 2020; Venegas-Vera et al., 2020).
Telephone screening was recommended as an alternative approach to face-to-face recruitment during the COVID-19 pandemic. (Gould et al., 2021; Sathian et al., 2020; Villarosa et al., 2021). We put telephone screening in-place pre-COVID-19 onset; therefore, no changes in the initial stage of screening were required post-COVID-19 onset. Examination of completion rates for the telephone screening revealed it to be significantly higher post-COVID-19 onset compared to pre-COVID19 onset. Women working at home post-COVID-19 onset may have been easier to reach. Further, due to the closure or reduced occupancy at exercise facilities post-COVID-19 onset (CDC, 2020), some women may have been more motivated to learn about a lifestyle physical activity intervention that did not require access to facilities outside the home. However, this would need further exploration since the pandemic could have affected women differently with some women having fewer competing activities and, therefore, more time available to participate in a physical activity program.
A health assessment screening is necessary to ensure women’s safe participation in physical activity trials (Canadian Society for Exercise Physiology, 2002b). We required screening blood pressures and HbA1c; thus we continued with in-person health assessments at the recruitment site post-COVID-19 onset. The recruitment site was a large academic medical center, where most of the clinical and non-clinical staff were expected to work in person for patient care. Therefore, in-person screening may not have placed additional burden on some women post-COVID-19 onset. Recruiting potential participants from a large medical center facilitated the continued recruitment and completion of screening even post-COVID-19-onset. Further, similar to an earlier study (Foster et al., 2011), the research team adopted the use of virtual training to learn the required CDC COVID-19-onset-related changes necessary for in-person assessments. This virtual training facilitated the timely implementation of the updated screening protocols at the recruitment site for the study waves that were scheduled to begin in the months post-COVID-19 onset. As a result, the start of recruitment for wave 4, originally scheduled to begin five months after wave 3, was only delayed by two months. A relatively small number of women did not complete the third stage of screening (physical activity assessment). Therefore, it is difficult to discern why a larger proportion completed the physical assessment post-COVID-19 onset.
Examination of the demographics of eligible participants pre- and post-COVID-19 onset revealed significant differences in racial/ethnic distribution. Black women comprised 36.5% of the eligible participants pre-COVID-19 onset, declining to 21.7% post-COVID-19 onset. However, the overall post-COVID-19-onset recruitment of Black women closely reflected the proportion of Black women employed at the recruitment site (24%) (Buchholz et al., 2020). Although we do not have the data to confirm it, we speculate that the higher proportion of COVID-19-related hospitalizations and deaths among Blacks compared to non-Hispanic Whites (CDC, 2022) may have contributed to a decrease in interest among Black women in study participation post-COVID-19 onset. Also, social unrest taking place during post-COVID19 onset, due to the murder of George Floyd by Minneapolis police, may have contributed to Black women being reluctant to participate in a study at that time (Sullivana et al., 2021). Declines in recruitment of Black women post-COVID-19 onset suggest the importance of monitoring the impact of concurrent historical environmental, social, and political events on recruitment efforts. Alterations in recruitment strategies may be necessary following historical events.
There were several study limitations to recruitment and screening. Since recruitment strategies primarily transitioned away from in-person recruitment interaction to electronic modalities post-COVID-19 onset, a sampling bias could have occurred in obtaining a diverse sample. Potential participants whose job responsibilities did not require the use of computers likely had fewer opportunities to learn about the study post-COVID-19 onset. Also, potential participants who transitioned to working remotely post-COVID-19 onset may have seen electronic notices but did not respond due to the need for in-person screening at the recruitment site for the health and physical activity assessments.
Conclusions and Implications
We attribute our success in recruitment to having multiple recruitment strategies from which to choose, including in-person and electronic, that were in place pre-COVID-19 onset and required only minimal adjustments in order to meet recruitment goals. In addition, learning and implementing in-person COVID-19 preventive screening protocols was done quickly using virtual training at the beginning of the post-COVID-19 onset. Despite these efforts, we did find a significant decrease in the percentage of Black women recruited into the study from pre- to post-COVID-19 onset. Future physical activity studies would benefit from exploring how historical events occurring concurrently may differentially impact potential participants in clinical trials.
Funding
This work is funded by the National Institutes of Health, National Institute of Nursing Research R01 NR017635
Footnotes
Declaration of Conflicting Interests
The authors declare no potential conflicts of interest
References
- ActiGraph. (2019). ActiGraph wgt3x-bt. Retrieved from https://actigraphcorp.com/actigraphwgt3x-bt/
- American Diabetes Association. (2016). Position statement 2. Classification and diagnosis of diabetes. Diabetes Care, 39(1), 13. Retrieved from 10.2337/dc16-S005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrey S, Fisher H, Cooper A, Gaunt D, Metcalfe C, Garfield K, Hollingworth W, Procter S, Gave-Walters M, Rodgers S, Gillison F, Davis A & Insall P (2019). A workplace-based intervention to increase levels of daily physical activity: the Travel to Work cluster RCT. Public Health Research, 7(11). doi: 10.3310/phr07110 [DOI] [PubMed] [Google Scholar]
- Buchholz SW; Wilbur J; Halloway S; Schoeny M; Johnson T; Vispute S; Kitsiou S. (2020). Study protocol for a sequential multiple assignment randomized trial (SMART) to improve physical activity in employed women. Contemporary Clinical Trials, 89(12), 105921. doi: 10.1016/j.cct.2019.105921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Society for Exercise Physiology. (2002a). Par-Q & you. Retrieved from https://geriatrictoolkit.missouri.edu/his/Par-Q.pdf
- Canadian Society for Exercise Physiology. (2002b). PARmed-X (revised 2002). Physical activity readiness medical examination. Retrieved from https://www.chp.gov.hk/archive/epp/files/PARmed-X.pdf [Google Scholar]
- Carriedo A, Cecchini J, Fernandez-Rio J, Mendez-Gimenez A (2020). COVID-19, psychological well-being and physical activity levels in older adults during the nationwide lockdown in Spain. American Journal of Geriatric Psychiatry, 28(11), 1146–1155. doi: 10.1016/j.jagp.2020.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J, Yancey K, Spring B, Figueroa-Moseley C, Mohr D, Mustian K, Fiscella K (2011). What are successful recruitment and retention strategies for underserved populations? Examining physical activity interventions in primary care and community settings. Translational Behavioral Medicine, 1(2), 234–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2015). Glycohemoglobin NHANES 2015–2016. Retrieved from https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/GHB_I_MET.pdf
- Centers for Disease Control and Prevention. (2021, August 13). Coronavirus disease 2019 (COVID-19): Social distancing. Retrieved from https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/social-distancing.html
- Centers for Disease Control and Prevention (2022, Jan 25). Health Equity Considerations and Racial and Ethnic Minority Groups. Retrieved from https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html
- Daniel M, Wilbur J, Marquez D, & Farran C (2013). Lifestyle physical activity behavior among South Asian Indian immigrants. Journal of Immigrant and Minority Health, 15(6), 1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz K, Krupka D, Chang M, Peacock J, Ma Y, Goldsmith J, Schwartz J, & Davidson K (2015). Fitbit(R): An accurate and reliable device for wireless physical activity tracking. Int. J. Cardiol, 185, 138–140. doi: 10.1016/j.ijcard.2015.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster C, Bennan G, Matthews A, McAdam C, Fitzsimons C, & Mutrie N (2011). Recruiting participants to walking intervention studies: A systematic review. The International Journal of Behavioral Nutrition and Physical Activity, 8(137). doi: 10.1186/1479-5868-8-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs B, Conroy M, Huber K, Muldoon M, Perera S, Jakicic J (2021). Effect of reducing sedentary behavior on blood pressure (RESET BP): Rationale, design, and methods. Contemporary Clinical Trials, 106(106428). doi: 10.1016/j.cct.2021.106428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould J, Best K, Netting M, Gibson R, & Makrides M (2021). New Methodologies for Conducting Maternal, Infant, and Child Nutrition Research in the Era of COVID-19. Nutrients, 13(3), 941. doi: 10.3390/nu13030941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerman W, Jackson N, Roumie C, Harris P, Rosenbloom S, Pulley J, Wilkins C, Williams N, Crenshaw D, Leak C, Scherdin J, Muñoz D, Bachmann J, Rothman R & Kripalani S (2017). Recruitment methods for survey research: Findings from the Mid-South Clinical Data Research Network. Contemporary Clinical Trials, 62, 50–55. doi: 10.1016/j.cct.2017.08.006 [DOI] [PubMed] [Google Scholar]
- McClain J, Craig C, Sisson S, & Tudor-Locke C (2007). Comparison of Lifecorder EX and ActiGraph accelerometers under free-living conditions. Appl. Physiol. Nutr Metab, 32(4), 753–61. doi: 10.1139/H07-060 [DOI] [PubMed] [Google Scholar]
- Medidata. (2020, June 10). COVID-19 and Clinical Trials: The Medidata Perspective. Release 5.0. Retrieved from https://www.medidata.com/wp-content/uploads/2020/05/COVID19-Response5.0_Clinical-Trials_20200518_v2.2.pdf6.
- National Institute of Environmental Health Sciences. (2021). Disaster Research Response (DR2) Program. Retrieved from https://www.niehs.nih.gov/research/programs/disaster/index.cfm
- Rodríguez-Larrad A, Mañas A, Labayen I, González-Gross M, Espin A, Aznar S, Serrano-Sánchez JA, Vera-Garcia FJ, González-Lamuño D, Ara I, Carrasco-Páez L, Castro-Piñero J, Gómez-Cabrera MC, Márquez S, Tur JA, Gusi N, Benito PJ, Moliner-Urdiales D, Ruiz JR, . . . Irazusta J (2021). Impact of COVID-19 confinement on physical activity and sedentary behaviour in Spanish university students: Role of gender. Int J Environ Res Public Health. 18(2), 369. doi: 10.3390/ijerph18020369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhr S, Arai H, Mangialasche F, Matsumoto N, Peltonen M, Raman R, Riedel-Heller S, Sakurai T, Snyder H, Sugimoto T, Carrilo M, Kivipelto M, Espeland M, & World-Wide FINGERS Study Group. (2021). Impact of the COVID-19 pandemic on statistical design and analysis plans for multidomain intervention clinical trials: Experience from World-Wide FINGERS. Alzheimer’s & Dementia, 7(1): e12143. doi: 10.1002/trc2.12143. eCollection 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathian B, Asim M, Banerjee I, Pizarro AB, Roy B, Teijlingen E, Nascimento I, & Alhamad H (2020). Impact of COVID-19 on clinical trials and clinical research: A systematic review. Nepal Journal of Epidemiology, 10(3), 878–887. doi: 10.3126/nje.v10i3.31622. eCollection 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G, Alfonso H, Howat P, & Corben K (2007). Use of pedometers in a workplace physical activity program. Australasian Journal of Podiatric Medicine, 41(2), 23–28. [Google Scholar]
- Sullivana JN, Eberhardta J.l., & Robertsa SO (2021). Conversations about race in Black and White US families: before and after George Floyd’s death. Proceedings of the National Academy of Sciences of the United States of America, 118(38), e2106366118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L & Williams M (2006). Promoting physical activity in the workplace: using pedometers to increase daily activity levels. Health Promotion Journal of Australia, 17(2), 97–102. doi: 10.1071/he06097 [DOI] [PubMed] [Google Scholar]
- Tudor- Tudor Locke Catrine; Craig Cora; Brown Wendy; Clemes Stacy; De Cocker Katrien; Giles Corti Billie; Hatano Yoshiro; Inoue Shigeru; Matsudo Sandra; Mutrie Nanette and 8 others (2011). How many steps are enough. The international Journal of Behavioral Nutrition and Physical Activity, 8 (ISSN 1479–5868), pages 75. doi 10.1186/1479-5868-8-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations, Department of Economic and Social Affairs, Statistics, COVID-19 Response. (2020). Carrying out a telephone survey under the impact of COVID-19—What to consider. Retrieved from https://covid-19-response.unstatshub.org/posts/carrying-out-a-telephone-survey-under-the-impact-of-covid-19/ [Google Scholar]
- U.S. department of health and human services. (2004). National High Blood Pressure Education Program. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Retrieved from https://www.nhlbi.nih.gov/sites/default/files/media/docs/jnc7full.pdf.
- Venegas-Vera V, Colbert G, & Lerma E (2020). Positive and negative impact of social media in the COVID-19 era. Rev Cardiovascular Med, 21(4), 561–564. [DOI] [PubMed] [Google Scholar]
- Villarosa A, Ramjan L, Maneze D, & George A (2021). Conducting population health research during the COVID-19 pandemic: Impacts and recommendations. Sustainability, 13(3320). Retrieved from 10.3390/su13063320 [DOI] [Google Scholar]
- Waterhouse D, Harvey R, Hurley P, Levit L, Kim E, Klepin H, Mileham K, Nowakowski G, Schenkel C, Davis C, Bruinooge S, & Schilsky R (2020). Early impact of COVID-19 on the conduct of oncology clinical trials and long-term opportunities for transformation: Findings from an American Society of Clinical Oncology Survey. JCO Oncol Pract, 16(7), 417–421. [DOI] [PubMed] [Google Scholar]
- Weber M, Schiffrin E, White W, Mann S, Lindholm L, Kenerson J, Flack J, Carter B, Matterson B, Ram C, Cohen D, Cadet J, Jean-Charles R, Taler S, Kountz D, Townsend R, Chalmers J, Ramirez A, Bakris G, . . . Harrap S (2014). Clinical practice guidelines for the management of hypertension in the community: A statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich), 16(1), 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur J, McDevitt J, Wang E, Dancy B, Briller J, Ingram D, & Zenk S (2006). Recruitment of African American women to a walking program: Eligibility, ineligibility and attrition during screening. Research in Nursing & Health, 29(3), 176–189. [DOI] [PubMed] [Google Scholar]
- Wilbur J, Miller A, Fogg L, McDevitt J, Castro C, Schoeny M, Buchholz S, Braun L, Ingram D, Volgman A & Dancy B (2016). Randomized clinical trial of the women’s lifestyle physical activity program for African-American women: 24- and 48-week outcomes. American Journal of Health Promotion, 30(5), 335–45. [DOI] [PubMed] [Google Scholar]
- Wilbur J, Braun L, Buchholz S, Ingram D, Fogg L, Miller A, Johnson T, Volgman A, & McDevitt J (2013). Effectiveness, efficiency, durations, and costs of recruiting for an African American women’s lifestyle physical activity program. Research in Nursing & Health, 36(5), 487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2021). Naming the coronavirus disease (COVID-19) and the virus that causes it. Retrieved from https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it


