Abstract
As humans age, we lose skeletal muscle mass, even in the absence of disease (sarcopenia), increasing the risk of death. Low mitochondrial mass and activity contributes to sarcopenia. It is our hypothesis that, a ketogenic diet improves skeletal muscle mitochondrial mass and function when they have declined due to aging or disease, but not in athletes where mitochondrial quality is high.
Keywords: Exercise, Sarcopenia, Mitochondria, Dynopenia, Type IIa fibers
Summary for table of contents:
Ketogenic diets improve muscle mitochondrial mass and function with age or disease, but not in athletes with high mitochondrial quality.
Introduction
Humans progressively lose skeletal muscle mass with age in the absence of disease (sarcopenia). Sarcopenia increases the risk of all-cause mortality and is a reliable indicator of frailty and poor prognosis in clinical settings (1). In 2000, it was estimated that the direct cost of sarcopenia in America was $18.5 billion annually with this number rapidly increasing (2) — highlighting the importance of maintaining muscle mass throughout our lifespan. This loss of muscle mass in humans with advancing age is accompanied by a preferential loss of type II fiber area (3). This is an important distinction as type IIa fibers are able to sustain high force better than other fiber types (4). One way to ensure the preservation of skeletal muscle mass and strength with across the lifespan is exercise; however, many are unable or unwilling to exercise at the intensity needed to achieve the physiological adaptations required to maintain an enhanced quality of life.
One of many physiological factors that can initiate or accentuate sarcopenia in both humans and rodents is a decline in mitochondrial mass and/or activity within skeletal muscle (5). Beyond sarcopenia, mitochondrial health plays a role in a variety of skeletal muscle-based diseases. One of the major consequences of aging is a decline in mitochondrial bioenergetics, independent of changes in fat free mass (5). Studies using human muscle biopsies from healthy older people show an age related decline in mitochondrial mass, O2 consumption, mitochondrial quality control, and oxidative phosphorylation (OxPhos) activity when compared to young controls (6–8). These data suggest that the age-related changes in mitochondrial function are due to a decrease in both the quantity and quality of the mitochondria. Therefore, it is paramount to consider how to maintain mitochondrial health and activity as we age. In 2017, Roberts and colleagues studied the effect of a ketogenic diet (KD) on lifespan and health span in male mice (9); specifically measuring muscle and brain function as a function of age and diet. In this context, we demonstrated that a KD increased lifespan 13.6% with a concomitant increase in skeletal muscle mitochondrial mass and enzyme activity in late life, as well as maintained muscle strength (grip strength) and endurance (4-limb wire hang). As in mice, a KD in humans increases mitochondrial function (7), suggesting that the metabolic shift needed to adapt to a KD drives an increase in mitochondrial mass and function in skeletal muscle.
Since oxidative energy production is key to VO2max and endurance performance, high fat diets have similarly been proposed as a tool to increase athletic performance. However, the improved muscle function in old animals on a KD has not translated into improved performance for elite athletes. In fact, numerous studies have shown that there is either no benefit or possibly an impairment of elite performance on a KD (10, and references within).
It is our working hypothesis that a ketogenic diet improves skeletal muscle mitochondrial mass and function only when mitochondrial quality has declined due to aging or disease, but in athletes where mitochondrial quality is already high a ketogenic diet does not improve mitochondrial mass and activity further. In this review, we discuss the evidence for and against this hypothesis and how these effects of a ketogenic diet (KD) are mediated at a molecular level. For the purposes of this review, a KD is defined as a diet consisting of <50g of carbohydrate per day in humans, whereas in rodents the requirement is <5% and <10% of total calories coming from carbohydrates and protein, respectively. The lower protein intake is necessary due to the heightened capacity for gluconeogenesis in rats and mice. Further, all studies cited within the text include confirmation of elevated blood ketone levels, the primary determinant of a successful ketogenic diet.
Effect of KD on Skeletal Muscle:
Metabolism
Production of ketone bodies (KBs) such as acetoacetate (AcAc) and β-hydroxybutyrate (βHB), via ketogenesis is an evolutionary conserved process that plays a significant role in mammalian survival under the stress of limited food availability. KBs are lipid derived molecules that are primarily produced in the liver in response to a scarcity of glucose. In response to a KD, fasting, or prolonged physical exercise, KBs are distributed to skeletal muscle which, on average, comprises 41.3% and 33.1% of body mass in adult men and women, respectively (11). KBs, namely βHB, are imported into skeletal muscle mitochondria via monocarboxylate transporters, and oxidized into AcAc via D-β-hydroxybutyrate dehydrogenase which rapidly generates two molecules of acetyl-CoA (12). Together with the increase in βHB, skeletal muscle also becomes more reliant on fatty acids for the generation of ATP, resulting in a dramatic shift in metabolism (Figure 1).
Figure 1. Mitochondrial metabolism on (A) a high carbohydrate (CHO) versus (B) a ketogenic diet.
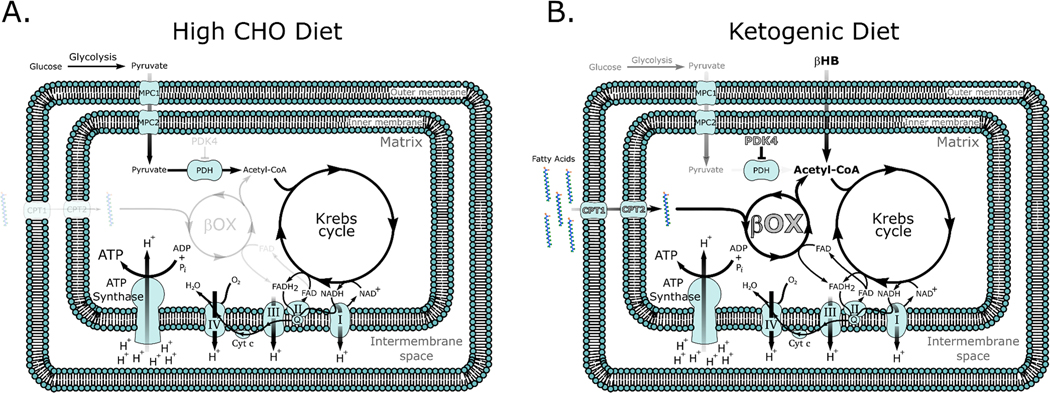
Note that on a high CHO diet acetyl-CoA is primarily synthesized from pyruvate generated from the breakdown of glucose in the cytoplasm. By contrast, on a ketogenic diet β-hydroxybutyrate (βHB) can directly enter the mitochondria and be converted into acetyl-CoA. The increased oxidation of fatty acids also activates PPAR transcription factors resulting in the upregulation (in grey with black outline) of key PPAR target genes such as fatty acid transporters (carnitine palmitoyl transferase (CPT)1 and 2), enzymes of β-oxidation, as well as pyruvate dehydrogenase kinase (PDK) 4, driving fatty acid oxidation and inhibiting CHO oxidation, respectively.
A consequence of a KD, the production of ketones via ketogenesis in the liver, and the rapid generation of acetyl-CoA is an abundance of intracellular acetyl-CoA. The rapid increase in acetyl-CoA likely explains the rise in acetylated lysine levels seen upon diet administration (9). Acetylation of lysine moieties is a post translational modification that affects both histones and other cellular proteins. Acetylation of histones removes the positive charge inherent in lysine residues, diminishing the electrostatic affinity between histone proteins and DNA; promoting the more open chromatin structure that is permissive to gene transcription (13). Acetylation of other cellular proteins alters their stability, activity, localization within the cell, and/or affinity to binding partners (14) in a way that mimics the effect of phosphorylation. Similar to a KD, an increase in acetylation is seen following exercise (15) – another stimulus that increases fat oxidation, and mitochondrial mass and activity (16). However, the relationship between acetylation and increases in mitochondrial biogenesis and activity in skeletal muscle remains largely unexplored.
A diet low in carbohydrates (CHOs), even one that does not increase circulating ketone levels like a strict ketogenic diet, results in fat adaptation and together with elevated levels of acetyl-CoA leads to greater activity of the peroxisome proliferator-activated receptor (PPAR) family of transcription factors. Higher PPAR activity increases key enzymes of fat oxidation such as lipoprotein lipase (LPL), fatty acid binding protein (FABP), cluster of differentiation 36 (CD36) and stearoyl-Coenzyme A desaturase (SCD)-1 (17). This stimulation of fatty acid oxidation through PPAR upregulation comes at the expense of glucose oxidation. PPAR∂, the most active PPAR isoform in skeletal muscle (18), also increases pyruvate dehydrogenase kinase-4 (PDK4) expression. PDK4 serves to inactivate pyruvate dehydrogenase (PDH), a key enzyme in the conversion of pyruvate into acetyl-CoA resulting in a decrease in CHO entering the mitochondria (19). Stellingwerff and colleagues showed that individuals cycling at 70% of VO2max in a fat adapted state showed significantly lower PDH activity and a concomitant increase in fat oxidation during exercise (20). Thus, elevated free fatty acid oxidation is in part driven by an increase in PPAR activity that upregulates fatty acid transport and oxidation enzymes and decreases the oxidation of CHO resulting in a shift to fat as the primary fuel in skeletal muscle (Figure 1).
Collectively, a KD increases levels of circulating fatty acids and KBs resulting in an increase in fat oxidation, acetyl-CoA generation, and acetylated lysine levels within muscle. With this shift in metabolism there is a beneficial effect on transcriptional availability of DNA, stability/localization/affinity of cellular proteins, and increased activity of PPARs.
Mitochondria Mass and Function
As previously mentioned there is growing evidence suggesting a KD is able to increase mitochondrial biogenesis and activity within skeletal muscle, resulting in greater muscle function with age (9,21). In mice, a KD increases the expression of the master mitochondrial biogenesis regulator PGC-1α (peroxisome proliferator activated receptor gamma) and proteins from each complex of the ETC (21). Interestingly, markers of mitochondrial mass and enzymatic activity increase in a tissue specific manner with only skeletal muscle showing a significantly greater mitochondrial:nuclear DNA (mtDNA:nDNA) ratio and improved complex I and IV activity when compared to their control diet fed counterparts, even after 14 months on diet. By contrast, brain and liver tissue showed no change or a decrease in mtDNA:nDNA ratio and limited improvements in activity (22). The increase in muscle mitochondrial mass and activity upon adoption of a KD occurs concomitantly with elevated levels of acetylated lysine protein levels. Manipulating acetylation in muscle using the histone deacetylase (HDAC) inhibitor, scriptaid, can similarly increase mitochondrial mass, lipid oxidation, and fatigue resistance (23). Having established in the previous section that a similar physiological response is seen following exercise, the increase in mitochondrial biogenesis in muscle observed with a KD may result from the activation of MEF2, a vital transcription factor in the control of PGC-1α expression, and subsequent increased transcription of the exercise-inducible form of PGC-1α, PGC-1α2/3 (24). In C2C12 cells, dosing with 5mM of AcAc resulted in an ∼4-fold increase in MEF2A binding capacity to transcriptional promoter regions (25). HDAC acetylation and activation of MEF2 could also be a direct effect of the primary ketone, βHB (26). Though the mechanism of action of βHB’s inhibition of class I HDACs has yet to be confirmed, the proposed mechanism of action is via competitive inhibition of HDAC catalytic sites. For the structurally similar butyrate, which differs from βHB only by its 3’carbon oxidation state, the carboxylic acid group binds to the catalytic zinc at the bottom of the HDACs active site effectively suppressing it’s activity (27). Additionally, to further increase fat oxidation in response to a KD, a rise in the phosphorylation and activity of the AMP-activated protein kinase at the threonine 172 (AMPK) site is observed (20). Active AMPK is then free to phosphorylate HDACs promoting the release of MEF2 and an increase in PGC-1α2/3 expression (28). Further, acetoacetate increases p38 mitogen activated protein kinase (p38 MAPK) activity (29). When activated, p38 MAPK phosphorylates and activates PGC-1α protein (30) and promotes PGC-1α2/3 expression through its target protein ATF2 (activating transcription factor 2). Lastly, the tumor suppressor p53 is a regulator of mitochondrial integrity, function, content, and biogenesis (31). Interestingly, acetylation of p53, which rises ∼10-fold on the KD (9), is fundamental for its activity, complex assembly and thereby, its cellular responses (32). Specifically, p53 is colocalized to the mitochondria where it acts to stabilize mtDNA expression, preventing DNA damage (33) — a hallmark of aging. When p53 is knocked out, there is a significant decline in mitochondrial content, mitochondrial aerobic capacity, and mtDNA depletion (34). Within the nuclear genome, p53 encourages mitochondrial biogenesis via upregulation of mitochondrial transcription factor A (Tfam), nuclear respiratory factor-1 (NRF1) and cytochrome C oxidase (35). However, it is important to note that there have been contrasting reports of p53 and its influence on mitochondrial content and enzymatic activity. Specifically, in 2017 Stocks and colleagues used a muscle specific KO of p53 to show that p53 was not required for mitochondrial biogenesis, morphology, or enzymatic activity (36). These data suggest that a KD has multiple, possibly redundant ways, through which it can increase mitochondrial mass and activity in skeletal muscle.
Within the realm of mitochondrial quality control, there is still much to be investigated concerning the effect of a KD on skeletal muscle. A progressive decline in muscle function and quality is hypothesized to result from an accumulation of damaged or dysfunctional mitochondria in humans (6,37). The accumulation of damaged or dysfunctional mitochondria is prevented through mitochondrial-specific autophagy (mitophagy). Since mitophagy is increased when AMPK is activated and mTORC1 (mechanistic target of rapamycin complex 1) activity is inhibited (38), similar to what is observed in muscle following a KD (9,21), there is support for the hypothesis that a KD increases mitophagy in skeletal muscle. However, to date there is little published evidence showing the effects of a KD on in skeletal muscle mitophagy. Understanding how this important aspect of mitochondrial quality control is modulated in response to a KD will provide key insight to the field.
In order to maintain a healthy mitochondrial network and population in rodents, mitochondria must also undergo continuous cycles of fission and fusion (39).Though exercise can promote mitochondrial fission and fusion in humans (40) there is limited evidence in the literature as to whether a KD might cause similar effects. In 2012, Sebastián and colleagues demonstrated that expression of mitochondrial fusion protein 2, Mitofusin 2 (Mfn2) was required for adaptation to a high fat diet in mice (41). Subsequently in 2015, Mishra and colleagues showed that mitochondrial dynamics in mice were regulated in a fiber type specific manner; with type IIa fibers requiring both Mitofusin1 (Mfn1) and Mitofusin2 (Mfn2) for mitochondrial elongation and fusion (42). With a preferential preservation of type IIa fibers on a KD (21), this preservation may be dependent on the maintenance of healthy skeletal muscle mitochondria. In cultured cells, βHB stimulates mitochondrial elongation (43) and dampens irregularities in mitochondrial morphology in skeletal muscle (21). Together, these data indicate that in response to a KD there is an increase in fatty acid mobilization, mitochondrial biogenesis and activity that is accompanied by improved mitochondrial dynamics in skeletal muscle. Though this has yet to be verified in humans, evidence from animal models suggest a potential benefit of a KD on mitochondrial dynamics sufficient to preserve mitochondrial viability when compared to those on a control diet.
Muscle Mass, Function, and Fiber Type
The importance of maintaining skeletal muscle mass and function through age is paramount in humans since it is a strong predictor of mortality, and of our ability to respond/receive/tolerate disease burden or standard of care therapies (1). One study suggested that a KD can drive severe skeletal muscle atrophy in female mice (44), suggesting that a KD may not be effective in females. However, it is important to note that the KD feed used in the study by Nakao and colleagues was either unpalatable or methionine deficient resulting in a starvation phenotype. When a palatable KD has been used in rodents, muscle mass and function are preserved or improved depending on the length of the study (9,22,45). In our hands, we have demonstrated that a KD results in a protective effect on skeletal muscle in an aged mouse model and significantly increases measures of strength (grip strength) and endurance (4-limb wire hang) late in life when compared to their control diet fed counterparts (9). One reason for this is the preservation of type IIa (fast-oxidative) fibers at the expense of IIb (fast-glycolytic) fibers (Figure 2). This shift in fiber type has been theorized to take place due to: 1) the shift in metabolism leading to a preferential atrophy of fibers that can’t generate sufficient energy aerobically (IIb (fast-glycolytic) fibers); 2) improved protein quality control; or 3) the ability of a KD to increase the rate of reinnervation through axonal sprouting of IIa nerves, since reinnervation normally diminishes with aging (21,46). The preservation of type IIa fibers with a KD is important since type IIa fibers are preferentially lost with advanced age; resulting in loss of lean body mass and skeletal muscle function in humans (3). Beyond aging, in a rat model of Duchenne’s muscular dystrophy (DMD) a medium chain triglyceride based ketogenic diet showed impressive benefits on skeletal muscle quality, mass, and grip strength (45). A possible explanation for these beneficial changes in muscle function is the ∼6-fold increase in acetylation of histone acetyltransferase p300 in KD fed mice (9). Acetylation of p300 on the 17 lysine residues within its regulatory domain stimulates acetyltransferase activity and interaction with other proteins (47). A skeletal muscle-specific knockout of p300 in mice results in rapid decreases grip strength and rotarod performance leading to death, even with minimal changes in fiber cross sectional area CSA (48), making p300 an attractive candidate for future studies regarding the KD and muscle function.
Figure 2. Skeletal muscle morphology in mice following 14 months on a control (CON) or ketogenic (KETO) diet.
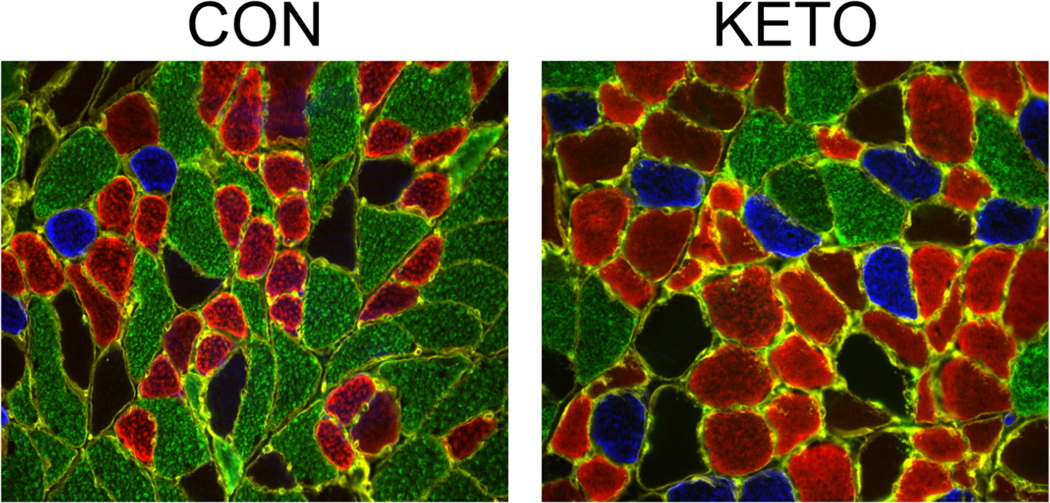
Muscle sections from 26-month-old mice were stained for type I (blue), IIa (red), IIx (black), and IIb (green) myosin heavy chain. Note the increase in oxidative (blue and red) fiber number and size and the concomitant decrease in glycolytic (green) fibers. [Adapted from (21). © 2021 The Authors. CC BY.]
The cellular basis for changes in muscle fiber cross-sectional area is more than just a shift in balance between muscle protein synthesis and muscle protein breakdown. A KD does little to alter the increase skeletal muscle mass in response to resistance exercise in men (49). This can potentially be explained by the fact that a KD causes a decrease in myofibrillar fractional synthetic rates—in male mice (21). The impaired rate of protein synthesis in mouse skeletal muscle can be explained in part by a ∼3-fold increase in phosphorylation of eIF2αser51, which over 30 years ago was shown to inhibit the initiation of translation (50). This decrease in translation initiation would be expected to dramatically slow protein synthesis rates which, while bad for muscle hypertrophy, could promote better protein quality control, decreasing misfolding and ER stress. It is also possible that the decrease in translation initiation can be partially overcome by an increase in acetylation of the ribosome. Acetylation of ribosomes effectively stabilizes them, increasing their translational efficiency and improving protein quality control at least in rat liver (51).
Since muscle mass reflects the balance between myofibrillar protein synthesis and degradation and a KD decreases myofibrillar protein synthesis it is important to understand how a KD affects breakdown. In 2018, Thomsen and colleagues demonstrated that βHB induces anticatabolic effects during LPS induced weight loss resulting in a 70% reduction in phenylalanine efflux from muscle in 10 healthy men (52). This, coupled with blunted proteosome activity seen on a KD in mice (21), suggests that there is less protein degradation in muscle on a KD. However, in our studies the decrease in degradation on a KD is only observed in older animals, suggesting that a KD may have a bigger effect on degradation when breakdown is increased by age or disease rather than in young healthy muscle.
Altogether, a KD presents a promising strategy to mitigate the age- or disease-related decline in skeletal muscle mass and function through increases in mitochondrial mass and function, the preservation of type IIa fibers, increased quality control of protein synthesis, and anticatabolic effects.
Effect of KD on Elite Athletic Performance
As described above, a KD can improve muscle size, strength, and mitochondrial mass and activity in old mice. Since muscle strength and endurance are essential components of athletic performance, many athletes, coaches, and sports scientists have hypothesized that a KD would increase performance in human athletes. Since the primary benefit of a KD is to enhance mitochondrial mass and function—we will focus our discussion on endurance performance since mitochondria play a greater role in this type of sport. The data to date in this area indicates that there is either no additional benefit or an impairment in elite performance when athletes train on a KD (10). Chief among these studies are Prof. Burke and colleague’s Supernova studies that demonstrated that a KD impairs elite athletic endurance performance. In this outstanding series of studies, the authors found an 8.5% decrease in race walking performance (10, and references within). To put this into perspective, an 8.5% reduction in performance in the 2020 Olympic 20km or 50km race walk final would result in a last place finish when compared to the top time. These performance deficiencies can be explained by the long-standing concept of substrate utilization efficiency (53). This shift in metabolism is potentially driven by the decrease in PDH activity following fat adaptation (20). As discussed above, fat adaptation, or a long term KD, leads to the activation of PPARs and the subsequent increase in PDK4 (19). The rise in PDK4 results in the phosphorylation and inhibition of PDH, decreasing the capacity to oxidize CHO and increasing the reliance on fat as a fuel. The generation of ATP from fat oxidation normally declines as exercise intensity increases (53) and this has been attributed to the inability to efficiently transport fatty acids into the mitochondria during higher levels of exercise intensity (greater than ∼65% VO2max). Even if fatty acid transport was not limiting, endurance athletes on a KD would require significantly more oxygen to perform at the same power or velocity when compared with athletes on a carbohydrate based diet (10). Since economy, the volume of oxygen needed to maintain a specific power or speed, is directly related to performance, this would suggest that a KD would limit high end performance. However, this is not to say that a KD cannot be useful to the elite athletic population. There have been reports that submaximal exercise performance (<60% VO2 max) can be improved in runners on a KD (54). Separately, semi-professional soccer players that adhered to a 30-day KD, benefited from a significant decrease in body fat, visceral adipose tissue and waist circumference, without changes in muscle strength (55). This implies that, athletes that would like to lose weight without losing muscle strength may benefit from adopting a KD during the offseason to improve lean mass:body weight ratio prior to building their aerobic fitness.
Another rapidly developing area is the use of ketone esters (KE) such as 1,3-butanediol acetoacetate diester, (R)-3-hydroxybutyl (R)-3-hydroxybutyl, (R)-1,3-hydroxybutyl (R)-3-hydroxybutyrate and ketone salts (KS) like β-hydroxybutyrate-mineral salts to improve performance (56–59). Currently, the suitability of ketone supplements for elite athletes on a high carbohydrate diet is equivocal. Cox and colleagues reported that supplementation with (R)-1,3-hydroxybutyl (R)-3-hydroxybutyrate, together with carbohydrate ingestion, increased cycling time trial performance after 1 hour of fatiguing exercise (56) while studies by Rodger et.al and Leckey et.al contradict this report (58,59). It is important to note that the contradicting studies used a β-hydroxybutyrate-mineral salt and 1,3-butanediol acetoacetate diester, respectively. This indicates that different forms of dietary ketone may modulate their effectiveness as a performance enhancer in athletes, and this is an area of study that has yet to be properly explored.
Conclusion
With limited strategies to mitigate the age-related loss of skeletal muscle in humans, a ketogenic diet and its concomitant increase in fatty acid uptake and oxidation as well as acetylation levels remains an exciting area of research with much of the exciting data to date coming from rodent studies needing to be translated into humans. With the potential to maintain muscle mass and function and mitochondrial biogenesis and quality control as we age, it will be important to elucidate further the mechanisms of action driving these beneficial changes seen in rodents. Since the behavioral change necessitated by a KD is significant, the role of other agents (KEs or HDAC inhibitor drugs) that can mimic a KD without the need for drastic changes in behavior must also be explored. However, even though it is already clear that KDs have been successful at improving muscle size and function in disease and aging animal models, a KD fails to improve elite athletic performance likely because one of the key molecular changes that underlies the muscle adaptation to the diet (PPAR activation) is the same thing that decreases high intensity performance. Until this paradox is addressed, we are unlikely to see KDs in elite athletes during competition, but supplemental ketone esters may gain in popularity with athletes if they can provide supplemental fuel to power long term endurance performance without slowing carbohydrate oxidation.
Key Points.
-
>
A ketogenic diet (KD) increases longevity 13.6% in mice
-
>
A KD increases skeletal muscle mitochondrial mass and activity as well as measures of muscle strength (grip force), and endurance (wire hang) in aged mice.
-
>
A ketogenic diet activates the peroxisome proliferator-activated receptor (PPAR) family of transcription factors resulting in an increase in the enzymes necessary to transport and oxidize fatty acids as a fuel.
-
>
The PPARs also increase pyruvate dehydrogenase kinases that limit the rate of carbohydrate oxidation, resulting in impaired glucose utilization and impaired elite performance.
Acknowledgements:
Funding received:
This work was supported by a Program Project Grant (PO1 AG062817) from the NIH (USA).
Footnotes
Conflicts of Interest: K. Baar has received funding to study ketogenic diets from NIH and is a Scientific Advisor to KetoKind. Prof Baar has also received grants and donations from other nutritional companies such as PepsiCo, Bergstrom Nutrition, Ynsect, and GelTor. S. Pathak has no conflicts of interest.
References
- 1.Liu P, Hao Q, Hai S, Wang H, Cao L, Dong B. Sarcopenia as a predictor of all-cause mortality among community-dwelling older people: A systematic review and meta-analysis. Maturitas. 2017;103:16–22. [DOI] [PubMed] [Google Scholar]
- 2.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The Healthcare Costs of Sarcopenia in the United States. J Am Geriatr Soc. 2004; 52(1):80–5. [DOI] [PubMed] [Google Scholar]
- 3.Nilwik R, Snijders T, Leenders M, Groen BBL, van Kranenburg J, Verdijk LB, et al. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol. 2013;48(5):492–8. [DOI] [PubMed] [Google Scholar]
- 4.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. Journals Gerontol - Ser A Biol Sci Med Sci. 2006;61(1):72–7. [DOI] [PubMed] [Google Scholar]
- 5.Safdar A, Hamadeh MJ, Kaczor JJ, Raha S, deBeer J, Tarnopolsky MA. Aberrant Mitochondrial Homeostasis in the Skeletal Muscle of Sedentary Older Adults. PLoS One [Internet]. 2010. May 24;5(5):e10778. Available from: 10.1371/journal.pone.0010778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crane JD, Devries MC, Safdar A, Hamadeh MJ, Tarnopolsky MA. The effect of aging on human skeletal muscle mitochondrial and intramyocellular lipid ultrastructure. Journals Gerontol - Ser A Biol Sci Med Sci. 2010;65(2):119–28. [DOI] [PubMed] [Google Scholar]
- 7.Miller VJ, LaFountain RA, Barnhart E, Sapper TS, Short J, Arnold WD, et al. A ketogenic diet combined with exercise alters mitochondrial function in human skeletal muscle while improving metabolic health. Am J Physiol Metab [Internet]. 2020. Sep 28;319(6):E995–1007. Available from: 10.1152/ajpendo.00305.2020 [DOI] [PubMed] [Google Scholar]
- 8.Luukkonen PK, Dufour S, Lyu K, Zhang X-M, Hakkarainen A, Lehtimäki TE, et al. Effect of a ketogenic diet on hepatic steatosis and hepatic mitochondrial metabolism in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A. 2020;117(13):7347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, et al. A Ketogenic Diet Extends Longevity and Healthspan in Adult Mice. Cell Metab. 2017;26(3):539–546.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke LM, Ross ML, Garvican-Lewis LA, Welvaert M, Heikura IA, Forbes SG, et al. Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J Physiol. 2017;595(9):2785–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee RC, Wang Z, Heo M, Ross R, Janssen I, Heymsfield SB. Total-body skeletal muscle mass: development and cross-validation of anthropometric prediction models. Am J Clin Nutr. 2000;72(3):796–803. [DOI] [PubMed] [Google Scholar]
- 12.Evans M, Cogan KE, Egan B. Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. J Physiol. 2017;595(9):2857–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandy M, Gutiérrez JL, Prochasson P, Workman JL. SWI/SNF displaces SAGA-acetylated nucleosomes. Eukaryot Cell. 2006;5(10):1738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen DG, Xie X, Basisty N, Byrnes J, McSweeney S, Schilling B, et al. Post-translational Protein Acetylation: An Elegant Mechanism for Bacteria to Dynamically Regulate Metabolic Functions. Frontiers in Microbiology. 2019;1604. doi: 10.3389/fmicb.2019.01604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGee SL, Fairlie E, Garnham AP, Hargreaves M. Exercise-induced histone modifications in human skeletal muscle. J Physiol. 2009;587(24):5951–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242(9):2278–82. [PubMed] [Google Scholar]
- 17.Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62(3):720–33. [DOI] [PubMed] [Google Scholar]
- 18.Ehrenborg E, Krook A. Regulation of Skeletal Muscle Physiology and Metabolism by Peroxisome Proliferator-Activated Receptor δ. Pharmacol Rev. 2009;61(3):373– 393. [DOI] [PubMed] [Google Scholar]
- 19.Peters SJ, St Amand TA, Howlett RA, Heigenhauser GJ, Spriet LL. Human skeletal muscle pyruvate dehydrogenase kinase activity increases after a low-carbohydrate diet. Am J Physiol. 1998;275(6):E980–6. [DOI] [PubMed] [Google Scholar]
- 20.Stellingwerff T, Spriet LL, Watt MJ, Kimber NE, Hargreaves M, Hawley JA, et al. Decreased PDH activation and glycogenolysis during exercise following fat adaptation with carbohydrate restoration. Am J Physiol Endocrinol Metab. 2006;290(2):E380–8. [DOI] [PubMed] [Google Scholar]
- 21.Wallace MA, Aguirre NW, Marcotte GR, Marshall AG, Baehr LM, Hughes DC, et al. The ketogenic diet preserves skeletal muscle with aging in mice. Aging Cell. 2021;20(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Z, Hagopian K, López-Domínguez JA, Kim K, Jasoliya M, Roberts MN, et al. A ketogenic diet impacts markers of mitochondrial mass in a tissue specific manner in aged mice. Aging (Albany NY). 2021;13(6):7914–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaur V, Connor T, Sanigorski A, Martin SD, Bruce CR, Henstridge DC, et al. Disruption of the Class IIa HDAC Corepressor Complex Increases Energy Expenditure and Lipid Oxidation. Cell Rep. 2016;16(11):2802–10. [DOI] [PubMed] [Google Scholar]
- 24.Akimoto T, Sorg BS, Yan Z. Real-time imaging of peroxisome proliferator-activated receptor-gamma coactivator-1alpha promoter activity in skeletal muscles of living mice. Am J Physiol Cell Physiol. 2004;287(3):C790–6. [DOI] [PubMed] [Google Scholar]
- 25.Zhong R, Miao R, Meng J, Wu R, Zhang Y, Zhu D. Acetoacetate promotes muscle cell proliferation via the miR-133b/SRF axis through the Mek-Erk-MEF2 pathway. Acta Biochim Biophys Sin (Shanghai)]. 2021. ;53(8):1009–16. [DOI] [PubMed] [Google Scholar]
- 26.Newman JC, Verdin E. β-Hydroxybutyrate: A Signaling Metabolite. Annu Rev Nutr. 2017;37:51–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D-F, Helquist P, Wiech NL, Wiest O. Toward Selective Histone Deacetylase Inhibitor Design: Homology Modeling, Docking Studies, and Molecular Dynamics Simulations of Human Class I Histone Deacetylases. J Med Chem. 2005;48(22):6936–47. [DOI] [PubMed] [Google Scholar]
- 28.Czubryt MP, McAnally J, Fishman GI, Olson EN. Regulation of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1 alpha ) and mitochondrial function by MEF2 and HDAC5. Proc Natl Acad Sci U S A. 2003;100(4):1711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdelmegeed MA, Kim SK, Woodcroft KJ, Novak RF. Acetoacetate activation of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase in primary cultured rat hepatocytes: role of oxidative stress. J Pharmacol Exp Ther. 2004;310(2):728–36. [DOI] [PubMed] [Google Scholar]
- 30.Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, et al. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell. 2001;8(5):971–82. [DOI] [PubMed] [Google Scholar]
- 31.Bartlett JD, Close GL, Drust B, Morton JP. The emerging role of p53 in exercise metabolism. Sports Med. 2014;44(3):303–9. [DOI] [PubMed] [Google Scholar]
- 32.Tang Y, Zhao W, Chen Y, Zhao Y, Gu W. Acetylation is indispensable for p53 activation. Cell [Internet]. 2008;133(4):612–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park J-H, Zhuang J, Li J, Hwang PM. p53 as guardian of the mitochondrial genome. FEBS Lett. 2016;590(7):924–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lebedeva MA, Eaton JS, Shadel GS. Loss of p53 causes mitochondrial DNA depletion and altered mitochondrial reactive oxygen species homeostasis. Biochim Biophys Acta - Bioenerg. 2009;1787(5):328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saleem A, Carter HN, Hood DA. p53 is necessary for the adaptive changes in cellular milieu subsequent to an acute bout of endurance exercise. Am J Physiol Cell Physiol. 2014;306(3):C241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stocks B, Dent JR, Joanisse S, McCurdy CE, Philp A. Skeletal Muscle Fibre-Specific Knockout of p53 Does Not Reduce Mitochondrial Content or Enzyme Activity. Frontiers in Physiology. 2017;8:941. doi: 10.3389/fphys.2017.00941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joseph AM, Adhihetty PJ, Buford TW, Wohlgemuth SE, Lees HA, Nguyen LMD, et al. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell. 2012;11(5):801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian W, Li W, Chen Y, Yan Z, Huang X, Zhuang H, et al. Phosphorylation of ULK1 by AMPK regulates translocation of ULK1 to mitochondria and mitophagy. FEBS Lett. 2015;589(15):1847–54. [DOI] [PubMed] [Google Scholar]
- 39.Archer SL. Mitochondrial Dynamics — Mitochondrial Fission and Fusion in Human Diseases. N Engl J Med. 2013;369(23):2236–51. [DOI] [PubMed] [Google Scholar]
- 40.Balan E, Schwalm C, Naslain D, Nielens H, Francaux M, Deldicque L. Regular Endurance Exercise Promotes Fission, Mitophagy, and Oxidative Phosphorylation in Human Skeletal Muscle Independently of Age. Frontiers in Physiology. 2019;10:1088. doi: 10.3389/fphys.2019.01088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sebastián D, Hernández-Alvarez MI, Segalés J, Sorianello E, Muñoz JP, Sala D, et al. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci. 2012;109(14):5523 – 5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mishra P, Varuzhanyan G, Pham AH, Chan DC. Mitochondrial Dynamics is a Distinguishing Feature of Skeletal Muscle Fiber Types and Regulates Organellar Compartmentalization. Cell Metab. 2015;22(6):1033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santra S, Gilkerson RW, Davidson M, Schon EA. Ketogenic treatment reduces deleted mitochondrial DNAs in cultured human cells. Ann Neurol. 2004;56(5):662–9. [DOI] [PubMed] [Google Scholar]
- 44.Nakao R, Abe T, Yamamoto S, Oishi K. Ketogenic diet induces skeletal muscle atrophy via reducing muscle protein synthesis and possibly activating proteolysis in mice. Sci Rep. 2019;9(1):19652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujikura Y, Sugihara H, Hatakeyama M, Oishi K, Yamanouchi K. Ketogenic diet with medium-chain triglycerides restores skeletal muscle function and pathology in a rat model of Duchenne muscular dystrophy. FASEB J Off Publ Fed Am Soc Exp Biol. 2021;35(9):e21861. [DOI] [PubMed] [Google Scholar]
- 46.Rudolf R, Khan MM, Labeit S, Deschenes MR. Degeneration of Neuromuscular Junction in Age and Dystrophy. Frontiers in Aging Neuroscience. 2014;6:99. doi: 10.3389/fnagi.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karukurichi KR, Wang L, Uzasci L, Manlandro CM, Wang Q, Cole PA. Analysis of p300/CBP histone acetyltransferase regulation using circular permutation and semisynthesis. J Am Chem Soc. 2010;132(4):1222–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Svensson K, LaBarge SA, Sathe A, Martins VF, Tahvilian S, Cunliffe JM, et al. p300 and cAMP response element-binding protein-binding protein in skeletal muscle homeostasis, contractile function, and survival. J Cachexia Sarcopenia Muscle. 2020;11(2):464–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vargas S, Romance R, Petro JL, Bonilla DA, Galancho I, Espinar S, et al. Efficacy of ketogenic diet on body composition during resistance training in trained men: a randomized controlled trial. J Int Soc Sports Nutr. 2018;15(1):31. DOI: 10.1186/s12970-018-0236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaufman RJ, Davies MV, Pathak VK, Hershey JW. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol Cell Biol. 1989;9(3):946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liew CC, Gornall AG. Acetylation of ribosomal proteins. I. Characterization and properties of rat liver ribosomal proteins. J Biol Chem. 1973;248(3):977–83. [PubMed] [Google Scholar]
- 52.Thomsen HH, Rittig N, Johannsen M, Møller AB, Jørgensen JO, Jessen N, et al. Effects of 3-hydroxybutyrate and free fatty acids on muscle protein kinetics and signaling during LPS-induced inflammation in humans: anticatabolic impact of ketone bodies. Am J Clin Nutr [Internet]. 2018;108(4):857–67. [DOI] [PubMed] [Google Scholar]
- 53.Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993;265(3 Pt 1):E380–91. [DOI] [PubMed] [Google Scholar]
- 54.SHAW DM, MERIEN F, BRAAKHUIS A, MAUNDER ED, DULSON DK Effect of a Ketogenic Diet on Submaximal Exercise Capacity and Efficiency in Runners. Med Sci Sport Exerc [Internet]. 2019;51(10):2135–2146. [DOI] [PubMed] [Google Scholar]
- 55.Antonio Paoli A, Mancin L, Caprio M, Monti E, Narici MV, Cenci L, et al. Effects of 30 days of ketogenic diet on body composition, muscle strength, muscle area, metabolism, and performance in semi-professional soccer players. J Int Soc Sports Nutr. 2021;18(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cox PJ, Kirk T, Ashmore T, Willerton K, Evans R, Smith A, et al. Nutritional Ketosis Alters Fuel Preference and Thereby Endurance Performance in Athletes. Cell Metab. 2016;24(2):256–68. [DOI] [PubMed] [Google Scholar]
- 57.Stubbs BJ, Cox PJ, Evans RD, Santer P, Miller JJ, Faull OK, et al. On the Metabolism of Exogenous Ketones in Humans. Frontiers in Physiology. 2017;5: 848. doi: 10.3389/fphys.2017.00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leckey JJ, Ross ML, Quod M, Hawley JA, Burke LM. Ketone Diester Ingestion Impairs Time-Trial Performance in Professional Cyclists. Frontiers in Physiology. 2017;8: 806. doi: 10.3389/fphys.2017.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodger S, Plews D, Laursen P, Driller M. The effects of an oral β-hydroxybutyrate supplement on exercise metabolism and cycling performance. J Sci Cycl. 2017:30;6(1 SE-):26–31. [Google Scholar]


