Abstract
OBJECTIVE
Broca’s aphasia is a syndrome of impaired fluency with retained comprehension. The authors used an unbiased algorithm to examine which neuroanatomical areas are most likely to result in Broca’s aphasia following surgical lesions.
METHODS
Patients were prospectively evaluated with standardized language batteries before and after surgery. Broca’s area was defined anatomically as the pars opercularis and triangularis of the inferior frontal gyrus. Broca’s aphasia was defined by the Western Aphasia Battery language assessment. Resections were outlined from MRI scans to construct 3D volumes of interest. These were aligned using a nonlinear transformation to Montreal Neurological Institute brain space. A voxel-based lesion-symptom mapping (VLSM) algorithm was used to test for areas statistically associated with Broca’s aphasia when incorporated into a resection, as well as areas associated with deficits in fluency independent of Western Aphasia Battery classification. Postoperative MRI scans were reviewed in blinded fashion to estimate the percentage resection of Broca’s area compared to areas identified using the VLSM algorithm.
RESULTS
A total of 289 patients had early language evaluations, of whom 19 had postoperative Broca’s aphasia. VLSM analysis revealed an area that was highly correlated (p < 0.001) with Broca’s aphasia, spanning ventral sensorimotor cortex and supramarginal gyri, as well as extending into subcortical white matter tracts. Reduced fluency scores were significantly associated with an overlapping region of interest. The fluency score was negatively correlated with fraction of resected precentral, postcentral, and supramarginal components of the VLSM area.
CONCLUSIONS
Broca’s aphasia does not typically arise from neurosurgical resections in Broca’s area. When Broca’s aphasia does occur after surgery, it is typically in the early postoperative period, improves by 1 month, and is associated with resections of ventral sensorimotor cortex and supramarginal gyri.
Keywords: Broca’s aphasia, Broca’s area, VLSM, voxel-based lesion-symptom mapping, neurosurgery, surgical technique
The neuroanatomical architecture supporting speech production has continued to capture the interest of the scientific community since 1861, when Paul Broca described his 2 index cases of a severe aphasia. One of these patients could only say a single syllable— “tan”—and the second patient’s speech was limited to 5 words. Both patients retained comprehension and intellectual faculties, and both were reported to have lesions to the posterior aspect of the inferior frontal gyrus.1,2 Broca’s dissemination of these case reports is credited with the idea that fluent speech can be localized to a defined area of the brain. The posterior aspect of the inferior frontal gyrus has since borne the eponym Broca’s area.
Anatomically, the classic Broca’s area encompasses the pars opercularis (anterior to the precentral sulcus) and pars triangularis (between the ascending and horizontal rami of the anterior sylvian fissure) of the inferior frontal gyrus, or Brodmann areas 44/453 (Fig. 1A).
FIG. 1.
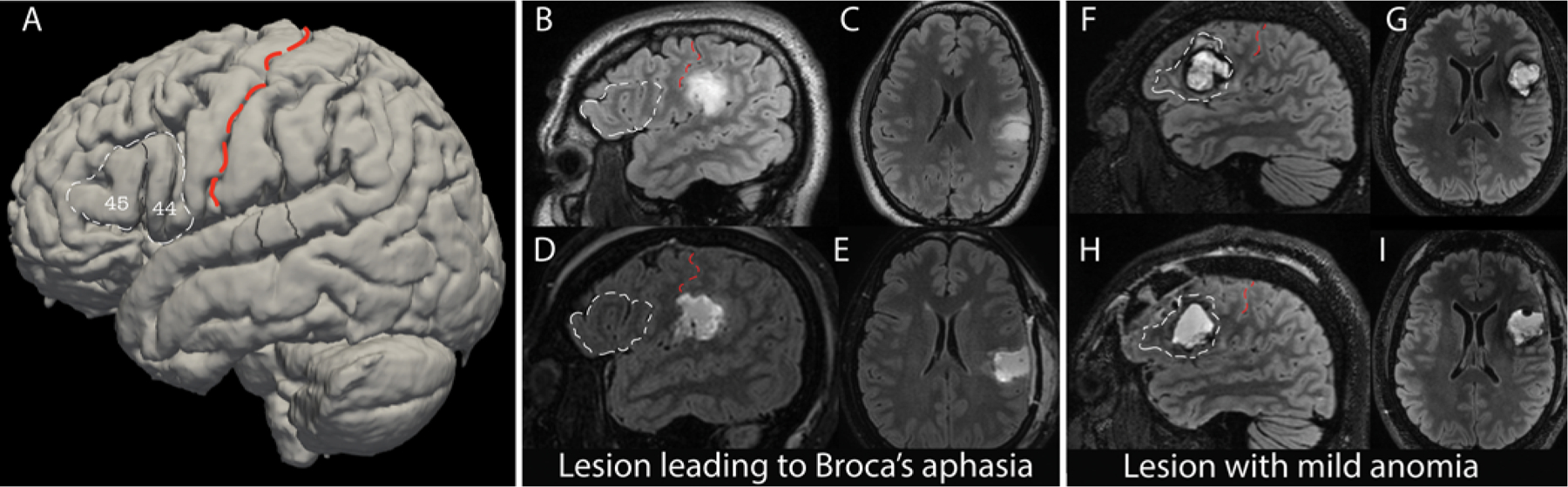
Broca’s aphasia after surgery. A: A 3D reconstruction of the MNI brain, with Brodmann areas 44/45 outlined in white dashed lines and labeled. These correspond to pars triangularis and pars opercularis of the inferior frontal gyrus—classic Broca’s area. The central sulcus is overlaid with a red dashed line. B–E: Example of a neurosurgical resection outside of Brodmann areas 44/45 that resulted in Broca’s aphasia. Preoperative sagittal (B) and axial (C) T2 FLAIR sequences (upper images) and postoperative sagittal (D) and axial (E) sequences (lower images), with Broca’s area outlined in white dashed lines. The central sulcus is overlaid with red dashed lines. F–I: MRI scans obtained in a patient with a resection in Broca’s area who had mild, nonspecific anomia on language testing postoperatively. Preoperative sagittal (F) and axial (G) T2 FLAIR sequences (upper images) and postoperative sagittal (H) and axial (I) sequences (lower images), with Broca’s area outlined in white dashed lines. The central sulcus is overlaid with red dashed lines.
Historically, standardizing the definitions for different types of aphasia was a challenge for the study of aphasia. The Western Aphasia Battery (WAB) is a widely used, standardized tool for assessing and categorizing aphasias, based on its 0- to 10-point rating scale in measures of fluency, comprehension, repetition, and naming.4–6 Broca’s aphasia syndrome encompasses a spectrum of deficits in fluent speech with relatively spared comprehension,1 and is defined quantitatively by the WAB as a fluency score of 0–4, comprehension score of 4–10, repetition score of 0–7.9, and naming score of 0–8 (out of 10).
Regarding the initial patients in Broca’s seminal report, a detailed examination1 suggests more extensive subcortical damage and undermines the specificity of the cortical localization to pars opercularis and triangularis. The work of neurosurgical electrostimulation studies7,8 and detailed outcomes of glioma resections from eloquent cortex9–11 have suggested that Broca’s area can be removed without causing Broca’s aphasia.12 Although there is evidence against anatomical localization of Broca’s aphasia to the inferior frontal gyrus, there remains controversy as to which anatomical area is most likely to cause this deficit when lesioned.
Many studies seeking to localize Broca’s aphasia come from the stroke literature.13–20 One hypothesis from this literature is an upper-division middle cerebral artery syndrome.13,14 It is relatively rare to have an isolated stroke of Broca’s area without affecting surrounding areas as well, given the stereotyped patterns of vascular flow of the middle cerebral artery.21,22 White matter tract disruption to the anterior limb of the arcuate fasciculus deep to both the posterior inferior frontal gyrus and ventral sensorimotor cortex (vSMC)16,18 have also been implicated.
Resections provide a complementary data source that does not have these same confounders and may therefore help refine our understanding of Broca’s area and Broca’s aphasia. Many patients are only offered biopsies or undergo subtotal resections when the lesion is located in Broca’s area. The present study asks which neuroanatomical area is most likely to lead to Broca’s aphasia when lesioned surgically, and presents a quantitative assessment of the anatomy associated with Broca’s aphasia.
Methods
Language Testing
Patients who underwent resective brain surgery in the language-dominant perisylvian areas were evaluated with standardized language batteries: either the WAB4 or Quick Aphasia Battery6 (QAB). A total of 198 patients were examined with the WAB and 91 patients with the QAB. The QAB is highly concordant with the WAB, and QAB scores were transformed to WAB scores based on equivalent constructs in the two batteries.6 Language evaluations were performed preoperatively, in the postoperative period within 48 hours (early), and at 1 month postoperatively, as follow-up allowed. This project was approved by the IRB at the University of California, San Francisco.
Definitions
It is important to define two distinct terms that will be used throughout this study: Broca’s area and Broca’s aphasia. In some literature the term Broca’s area is defined functionally through intraoperative stimulation;23 however, this is not the way Broca’s area is defined in the present study. We define Broca’s area by anatomy alone, as the pars opercularis and pars triangularis of the inferior frontal gyrus. Intraoperative stimulation is not a part of this definition. Broca’s aphasia is defined quantitatively by the WAB as a fluency score of 0–4, comprehension score of 4–10, repetition score of 0–7.9, and naming score of 0–8 (out of 10).
Anatomy
Broca’s area is defined here anatomically, as the pars opercularis and triangularis of the inferior frontal gyrus, coincident with Brodmann areas 44/45. The ventral segment of the inferior precentral sulcus is the caudal border of the pars opercularis, separating it from the precentral gyrus.24 The pars triangularis lies anterior to the anterior ascending ramus of the sylvian fissure, and is separated from the pars orbitalis rostrally by the anterior horizontal ramus of the sylvian fissure.24
Voxel-Based Lesion-Symptom Mapping
MRI scans of sufficient resolution for 3D imaging (approximately 1-mm slices) were obtained within 48 hours of resections. Resection cavities were outlined on these MRI scans to construct 3D volumes of interest. These were aligned using a nonlinear25 transformation to Montreal Neurological Institute (MNI) brain space. A voxel-based lesion-symptom mapping (VLSM26) algorithm determined areas associated with Broca’s aphasia when incorporated into a resection. These volumes of interest were overlaid to generate the schematics for lesion overlay depicted in Fig. 2. The power analysis for the images generated in Fig. 2 was performed by the VLSM algorithm, with an alpha of 0.01. Multiple comparisons were corrected for using a permutation method, with 1000 permutations used. VLSM was used with a binary comparison of either having Broca’s aphasia or not having Broca’s aphasia, as well as for fluency on a 10-point scale.
FIG. 2.
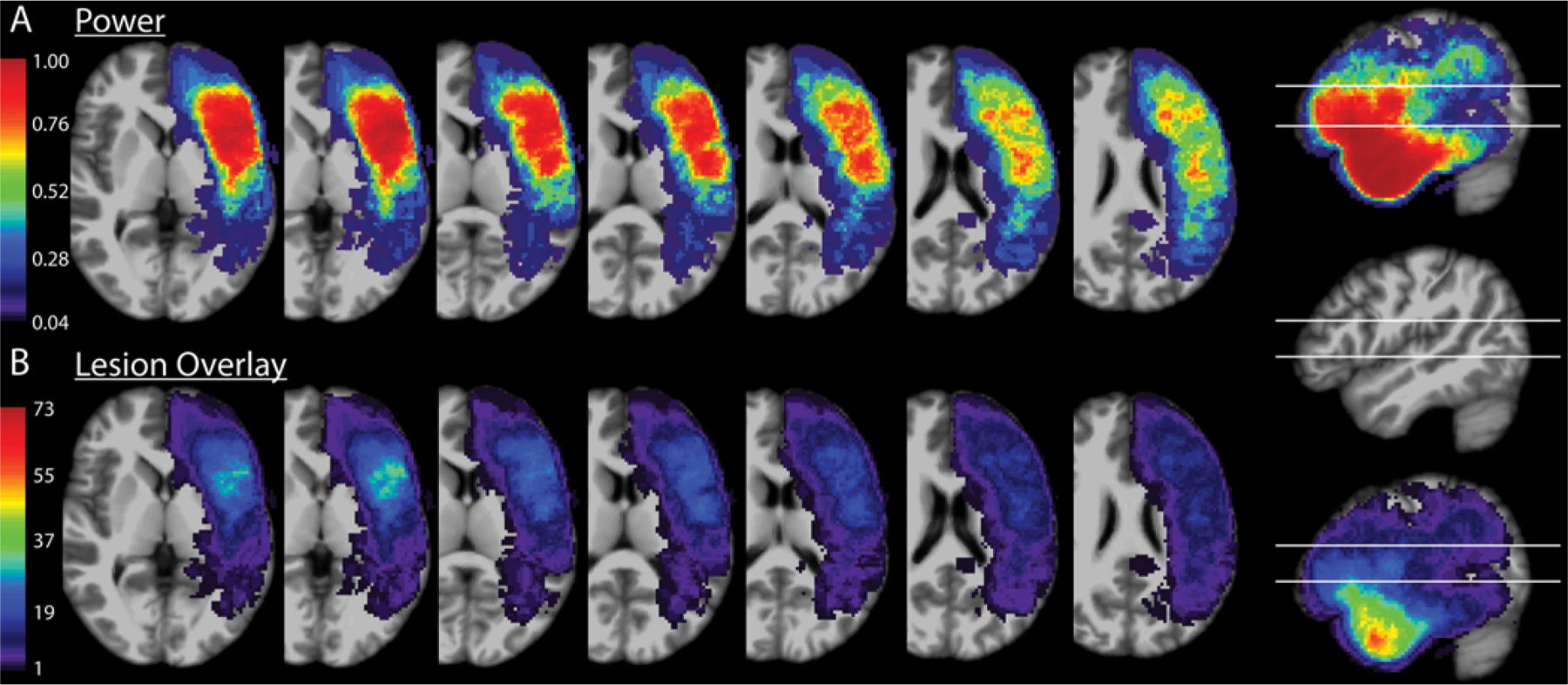
VLSM power and overlay. A: Axial cuts through areas of interest on the MNI brain, ranging from inferior (left) to superior (right), overlaid with the power calculated by the VLSM algorithm. The sagittal cut to the far right shows the inferior and superior margins of the axial slices depicted in the sagittal plane. B: Axial cuts of the MNI brain at the same levels as in panel A, overlaid with a lesion heat map with each pixel’s color corresponding to the number of resections overlapping with that area of space. The sagittal cut to the far right shows the inferior and superior margins of the axial slices depicted in the sagittal plane.
Resection Analysis
Postoperative MRI scans were read by reviewers who were blinded to the aphasia diagnosis. Resection cavities were evaluated for involvement of the pars opercularis or pars triangularis of the inferior frontal gyrus, as well as the fractional percentage of Broca’s area as a whole. For involvement of the area identified by VLSM (referred to as the VLSM area), resections were quantified for the component of the VLSM area in the precentral gyrus, the postcentral gyrus, and the supramarginal gyrus, as well as for the VLSM area treated as a singular whole. In patients with prior resections, only the areas of new resection were included in analyses. The fractional resection of each area (i.e., pars opercularis or triangularis) was estimated visually on a scale from 0 to 1.0, with 0 meaning no resection of that component, and 1.0 meaning complete resection. The same was done for the precentral, postcentral, and supramarginal components of the VLSM area. A linear regression was performed for each gyral subcomponent against the postoperative fluency scores for these patients.
Results
The study population included 289 patients with preoperative and postoperative language evaluations. The pathology and aphasia-type distribution of the cohort are shown in Table 1. Regarding prior treatment, 3.1% of patients had radiotherapy, 6.9% had resections, and 12.4% had a biopsy. The most common pathology was high-grade glioma (HGG), followed by low-grade glioma (LGG) and epilepsy. Nineteen patients had a diagnosis of Broca’s aphasia in the early postoperative period.
Table 1.
Aphasia diagnoses and underlying pathology for the cohort
| No. (%) | |
|---|---|
| Total no. of pts | 289 (100) |
| Pathology | |
| HGG | 142 (49.1) |
| LGG | 97 (33.6) |
| Epilepsy | 35 (12.1) |
| Brain metastases | 11 (3.8) |
| Other | 4 (1.4) |
| WAB classification on POD 2 | 289 |
| WNL | 106 (36.7) |
| Anomia | 102 (35.3) |
| Wernicke’s aphasia | 20 (6.9) |
| Broca’s aphasia | 19 (6.6) |
| Conduction | 14 (4.8) |
| Global aphasia | 14 (4.8) |
| Transcortical sensory | 7 (2.4) |
| Transcortical motor | 6 (2.1) |
| Isolation | 1 (0.3) |
| WAB classification at 1 mo | 178 |
| WNL | 136 (76.4) |
| Anomia | 38 (21.3) |
| Transcortical sensory | 2 (1.1) |
| Conduction | 2 (1.1) |
POD 2 = postoperative day 2; pts = patients; WNL = within normal limits. Epilepsy was defined as mesial temporal sclerosis, focal cortical dysplasia, gliosis, or nonspecific pathology in the clinical setting of epilepsy. HGGs were defined as WHO grade III or higher, and LGGs were defined as WHO grade II or lower.
Figure 1 presents an outline of Broca’s area (Brodmann areas 44/45) on a 3D reconstruction of the MNI brain (Fig. 1A), as well as illustrative cases of patients whose postoperative language testing showed Broca’s aphasia (Fig. 1B–E) versus mild anomia (Fig. 1F–I). The fraction of the full cohort with each WAB aphasia diagnosis is depicted in Supplemental Figure S1. Eighty-one percent of patients had normal speech preoperatively, whereas 15% had an anomia-spectrum WAB diagnosis, which is the mildest form of aphasia in the WAB classification. Only 2% had another type of preoperative aphasia.
The postoperative distribution of fluency, comprehension, repetition, and naming scores at postoperative day 2 is plotted grouped by WAB aphasia diagnosis in Supplemental Figure S2.
VLSM Analyses
Figure 2 shows the anatomical distribution of power for the VLSM analyses (Fig. 2A) as well as the resection overlay of all resections in the area of interest (Fig. 2B).
The results of the VLSM algorithm run on the complete cohort are depicted in Fig. 3. The voxels most highly correlated with acute postoperative Broca’s aphasia spanned the vSMC and supramarginal gyrus, as well as extending medially (deep) into perirolandic subcortical white matter (Fig. 3A and B).
FIG. 3.
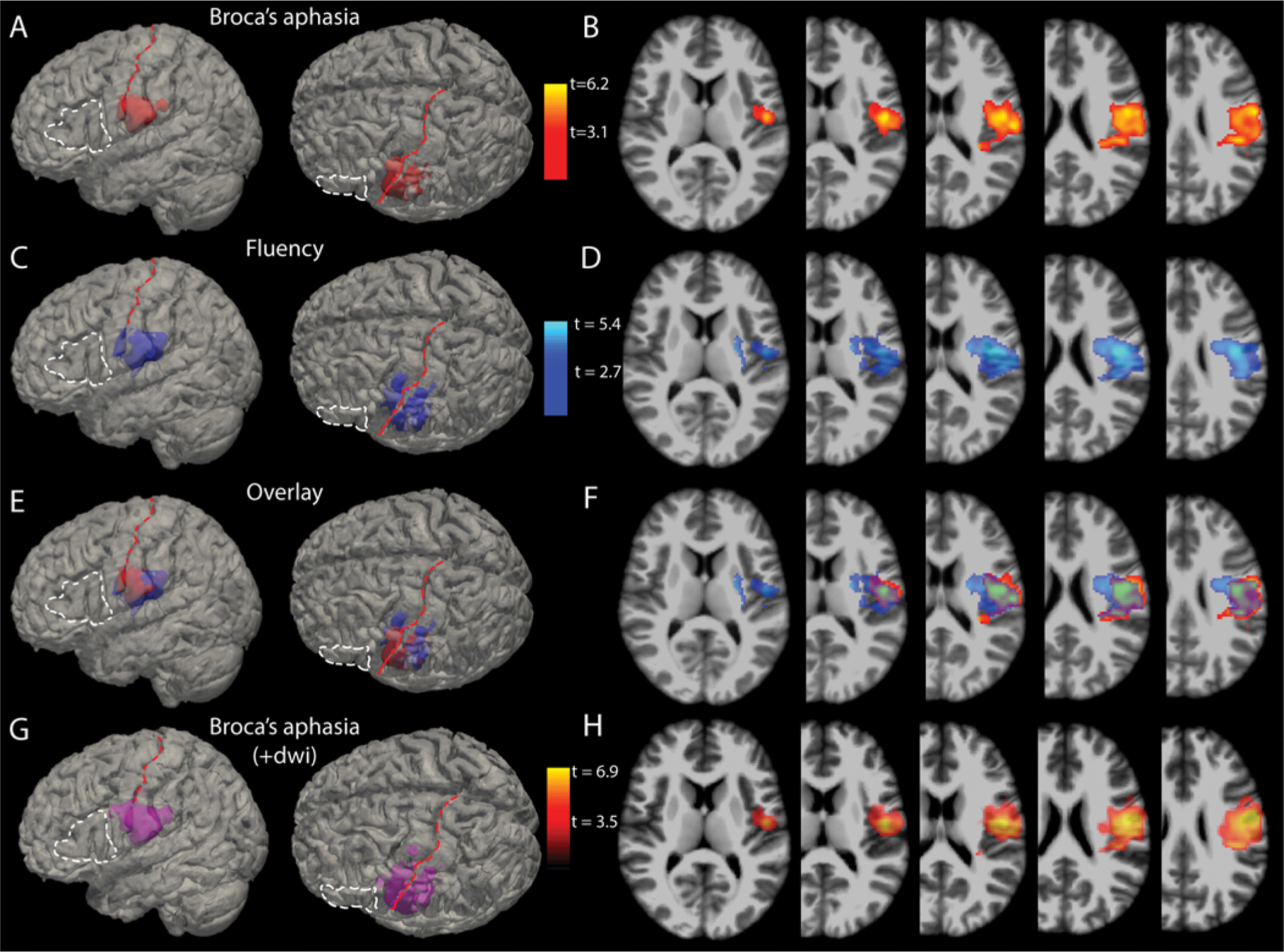
VLSM analysis. A: 3D reconstructions of the voxels (red) most highly associated with Broca’s aphasia (p < 0.001) overlaid on the MNI brain. For anatomical reference, the white dashed line outlines Broca’s area and the red dashed line lies over the central sulcus. B: Axial slices through the t-maps of the voxels depicted in panel A, windowed for t-scores corresponding to p values < 0.001. C and D: 3D reconstructions (C) and 2D cuts (D) through t-maps of voxels associated with reductions in fluency scores independent of WAB diagnosis (i.e., without regard to a diagnosis of Broca’s aphasia). E: Overlay of the voxels statistically associated with Broca’s aphasia and those associated with deficits in fluency. F: Axial slices with the t-maps of Broca’s aphasia and fluency deficits overlaid. G: 3D reconstructions of the voxels (violet) most highly associated with Broca’s aphasia (p < 0.001) when both resection area and diffusion-restricting areas are included in the volumes of interest. H: Axial slices through the t-maps of the voxels depicted in panel G. +dwi = diffusion-weighted imaging, shorthand referring to the inclusion of diffusion-restricting areas in resectional analyses.
The analysis shown in Fig. 3A and B is based on a dichotomous definition of Broca’s aphasia; thus, all aphasias that are not Broca’s aphasia and any other speech outcome are seen by the algorithm as not Broca’s aphasia. Given that every patient has a fluency score no matter the aphasia category, a second VLSM analysis was conducted in which only the fluency scores from language testing (Fig. 3C and D) were considered. This was done to address the spectrum of deficits in fluency that exist within and across diagnostic cutoffs of Broca’s aphasia. If the VLSM area associated with Broca’s aphasia is biologically valid, then resections involving these voxels should also be predictive of reduced fluency scores (on a 10-point scale) independent of the WAB diagnosis. Figure 3E and F shows the high degree of overlap for voxels associated with Broca’s aphasia and areas associated with reductions in fluency— namely posterior ventral precentral, ventral postcentral, and supramarginal gyri, with some extension into subcortical white matter. To rule out the contribution of perire-sectional areas of ischemia, the VLSM results depicted in Fig. 3G and H include diffusion-restricting areas in the volumes of interest analyzed with resectional volume.
As shown in Table 1, multiple pathologies are included in these analyses, but 83% of the surgeries were for resection of gliomas. The HGG and LGG resections were separated out and VLSM analyses were again run for both the dichotomous categorical association of Broca’s aphasia and the correlation with fluency score. The results are shown in Fig. 4, with panels A–D based on the HGG cohort and panels E–H based on the LGG cohort.
FIG. 4.
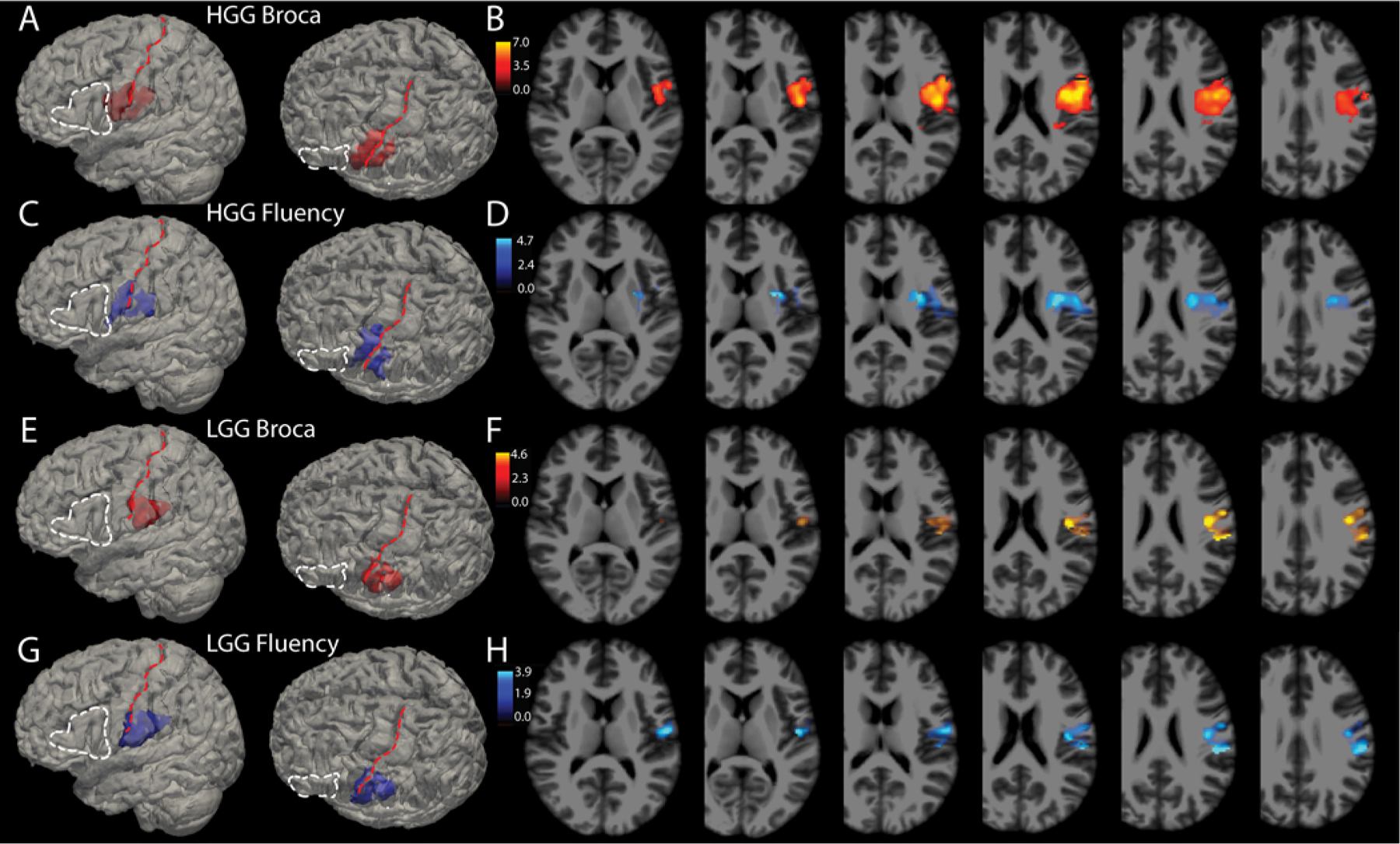
Broca’s aphasia and fluency deficits by pathology. Glioma resections made up 83% of this cohort and it is possible that intrinsic differences among these tumors, specifically HGG (WHO grade III or IV) versus LGG (WHO grade I or II), might influence the results. A: A total of 142 HGGs were analyzed using VLSM for association with Broca’s aphasia on a binary scale (yes or no). For anatomical reference, the white dashed line outlines Broca’s area and the red dashed line lies over the central sulcus. Voxels in red in this panel show the 3D reconstruction of voxels meeting the p < 0.001 threshold. B: Axial slices through the t-maps of the voxels depicted in panel A, windowed for t-scores corresponding to p values < 0.001. C and D: 3D reconstructions (C) and 2D cuts (D) through t-maps of voxels associated with reductions in fluency scores independent of WAB diagnosis (i.e., without regard to WAB diagnosis of Broca’s aphasia). E: A total of 97 LGGs were analyzed using VLSM for association with Broca’s aphasia on a binary scale. Voxels in red in this panel show the 3D reconstruction of voxels meeting the p < 0.001 threshold. F: Axial slices through the t-maps of the voxels depicted in panel A, windowed for t-scores corresponding to p values < 0.001. G and H: 3D reconstructions (G) and 2D cuts (H) through t-maps of voxels associated with reductions in fluency scores for a p < 0.005 in the LGG cohort.
Resection Area and Diagnosis
Figure 5 shows the postoperative aphasia distribution of the 75 patients with resections involving Brodmann areas 44/45 (Broca’s area) (Fig. 4A–D), and the 44 patients with resections involving the VLSM area. Of patients with Broca’s area resections, only 8% had Broca’s aphasia, and half of these overlapped with the VLSM area. For the 44 patients with resections involving the VLSM area, 25% had Broca’s aphasia after surgery. Figure 6 shows the correlation of fractional resection with fluency score for patients with Broca’s area or VLSM area resections. Resections of Broca’s area components showed no correlation with fluency scores postoperatively. Together, these data suggest the localization of Broca’s aphasia to an area distinct from Brodmann areas 44/45, spanning the vSMC and supramarginal gyri, as well as white matter deep to these cortical areas.
FIG. 5.
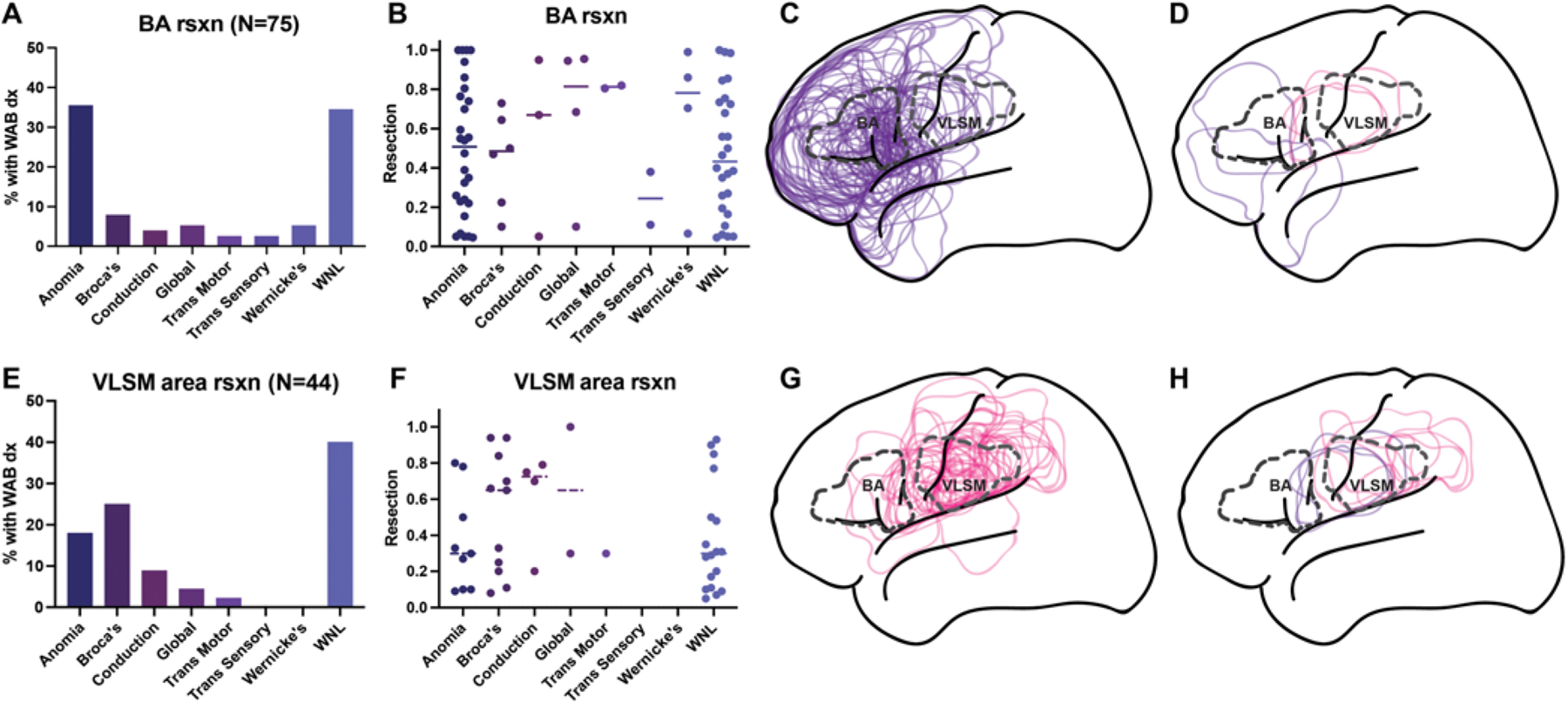
Aphasia diagnoses for Broca’s area versus VLSM area resections. A: The distribution of aphasia diagnoses (dx) for resections involving Broca’s area (BA rsxn). A total of 75 resections involved Broca’s area. At postoperative day 2, 34.7% of patients with Broca’s area resections had language testing within normal limits (WNL), 37.3% were associated with a WAB diagnosis of anomia (WAB score ranges: fluency 5–10, comprehension 7–10, repetition 7–10, naming 0–9), 8.0% had Broca’s aphasia, 5.3% had global aphasia, 5.3% had Wernicke’s aphasia, 4.0% had conduction aphasia, 2.7% had transcortical motor aphasia, and 2.7% had transcortical sensory aphasia. B: Fractional resections of Broca’s area on the y-axis and aphasia diagnosis on the x-axis, for each patient with a resection involving Broca’s area. C: Lateral diagrammatic overlay of all resections that involved Broca’s area, superimposed with dashed outlines of Broca’s area (labeled “BA”) and the area identified by the VLSM algorithm as significantly associated with Broca’s aphasia (labeled “VLSM”). D: Overlays of the 6/75 patients with Broca’s area resections who had Broca’s aphasia postoperatively, colored violet in the 3 whose resections did not overlap with the VLSM area, and colored pink in the 3 whose resections overlapped with the VLSM area. E: The distribution of aphasia diagnoses for resections involving the area identified by the VLSM algorithm as significantly associated with Broca’s aphasia (i.e., VLSM area). A total of 44 patients had resections in the VLSM area. Of these, 40.9% had language within normal limits. A total of 25.0% had Broca’s aphasia, 18.2% had anomia, 9.1% had conduction aphasia, 4.5% had global aphasia, 2.3% had transcortical motor aphasia, and none had transcortical sensory or Wernicke’s aphasia. F: Fractional resections of VLSM area on the y-axis and aphasia diagnosis on the x-axis, for each patient with a resection involving the VLSM area. G: Outlines of all 44 resections involving the VLSM area, superimposed with dashed outlines of Broca’s area and the VLSM area. H: The 11/44 resections involving the VLSM area that were associated with Broca’s aphasia. The 8 VLSM resections that did not involve Broca’s area are in pink, whereas the 3 that colocalized to Broca’s area are in violet.
FIG. 6.
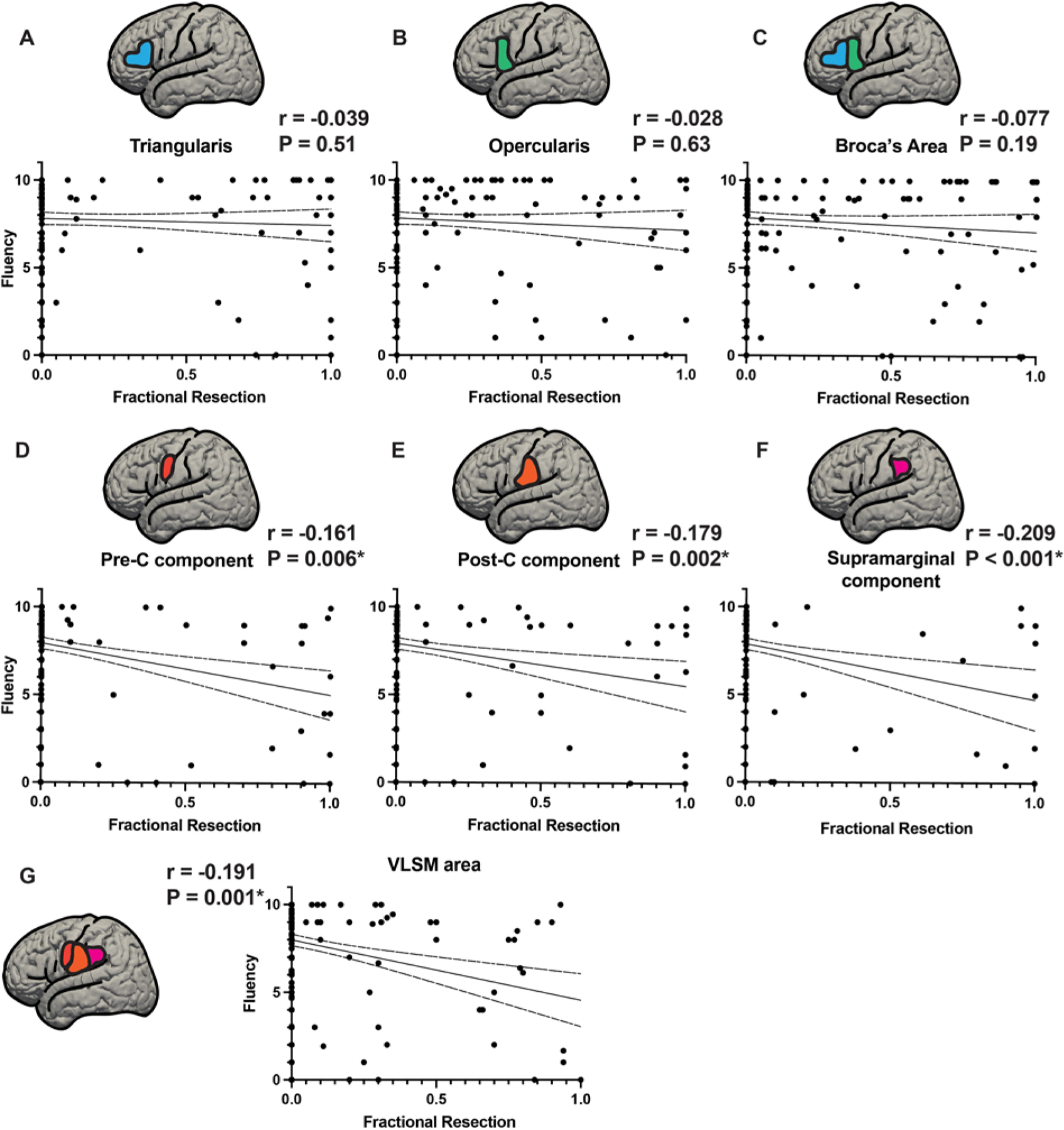
Fractional resections of Broca’s area and VLSM area gyral components versus fluency score. Resections of Broca’s area and the VLSM area were parsed into their component gyri and quantified based on the estimated fractional resection (from 0 = no involvement to 1 = total resection of the component in that gyrus) of each component. Broca’s area was divided into pars triangularis and pars opercularis and the VLSM area was divided into its components within precentral, postcentral, and supramarginal gyri. Fluency scores are plotted on the y-axis and fractional resections (0 = no resection, 1.0 = complete resection) on the x-axis. A–C: Components of Broca’s area, pars triangularis (A) and pars opercularis (B), with the fractional resections of Broca’s area as a whole (C). None of these showed significant correlations of fluency with fractional resection (A: p = 0.51, B: p = 0.63, C: p = 0.19). D–F: Fractional resections of the components of the VLSM area: precentral gyrus component (D), postcentral gyrus component (E), and supramarginal gyrus component (F). G: The VLSM area resection as a whole is shown. All 3 subcomponents of the VLSM area as well as the VLSM area treated as a whole were significantly correlated with reduced fluency (D: p = 0.006, E: p = 0.002, F: p < 0.001, G: p = 0.001). *Statistically significant at p < 0.05. Post-C = postcentral gyrus; Pre-C = precentral gyrus.
Discussion
These data provide insight into the anatomical etiology of Broca’s aphasia as a postoperative neurosurgical syndrome. This cohort suggests that permanent Broca’s aphasia is rare after neurosurgical resection. Broca’s aphasia was observed in the early postoperative period and was associated with resections posterior to the classic Broca’s area.
The most clinically distinctive aspect of Broca’s aphasia is the deficit in fluency. Broca’s aphasia diagnosed on the WAB scale involves a fluency score of 4 or less on a 10-point scale, with retained comprehension and variable deficits in repetition and naming. Although the categorical language/aphasia diagnoses are helpful from a diagnostic perspective by grouping patients into separate syndromes, speech fluency itself may be biologically or anatomically independent of these diagnostic criteria.
The overlap of VLSM areas associated with Broca’s aphasia (Fig. 3A and B) and the area associated with reduced fluency in general (Fig. 3C and D) is what would be expected if the anatomical region associated with Broca’s aphasia was biologically linked to fluent speech.
Ischemia and Neoplasia
Areas of ischemia, which show up in MRI as diffusion restriction on diffusion-weighted imaging, may occur around resection cavities in surgery. Although significant areas of diffusion restriction were not common in this cohort, in Fig. 3G and H we included all areas of diffusion restriction combined with resection in the volumes of interest analyzed by VLSM. These analyses show results very similar to the pure resectional analyses in the same figure, reinforcing the results, and again showing that the classic Broca’s area is not associated with Broca’s aphasia.
Neoplastic pathology in particular may play a role in reorganization of language areas, and it is possible that this may vary between slow-growing and fast-growing tumors.27,28 We ran VLSM analyses for LGGs and HGGs separately and found that the areas associated with Broca’s aphasia and reduced fluency are similar between these groups, with some variation in the amount of precentral gyrus that the regions overlap. The HGG group’s area was slightly more anterorostrally positioned compared to the LGG group. Both of these cohorts, however, still spanned vSMC and extended into the subcortical white matter.
These findings dissociate classic Broca’s area from Broca’s aphasia. They suggest that nonfluent aphasias in the postoperative period are associated with lesions to an area spanning vSMC and supramarginal gyri, as well as the subcortical white matter tracts deep to these areas.
Follow-Up
Whereas many patients did not have standardized testing at the 30-day postoperative time point, many of these individuals did nonetheless have follow-up neurological examinations in their charts that were accessible. Reviewing these charts revealed that the 178 patients with 30-day follow-up were representative of the remainder with neurological examinations documented near the 1-month time point.
Regarding longer-term follow-up of the cohort with Broca’s aphasia, 13/19 patients with Broca’s aphasia underwent standardized testing at 1 month, 9 of whom had speech within normal limits at that time, and the other 4 had WAB anomia (see comment regarding anomia below). The remaining 6 had neurological examinations in their charts in a comparable time frame. With a conservative interpretation of chart review, only 1 of all 19 patients with acute Broca’s aphasia had speech 1 month later that may have been worse than some mild naming deficits (WAB classification of anomia), and even this 1 patient had significantly improved per the notes. Thus, at 1 month postoperatively, 9 patients who previously had acute Broca’s aphasia had speech testing within normal limits, 9 were putatively anomic, and 1 was described as moderately to severely dyspractic but able to name high- and low-frequency words and able to repeat.
The anomia spectrum of WAB naming deficits is better described as dysnomia, because they involve some naming deficits with otherwise fluent speech, normal comprehension, and repetition. Patients with anomia in our cohort generally required a neurological examination to delineate that they had a language deficit—a sharp contrast to those with Broca’s aphasia whose language problem would be obvious in any interaction. This anomia/dysnomia deficit that does not preclude conversation and communication with one’s peers is not to be equated with the severity of Broca’s aphasia and the disability associated with a lack of fluent speech.
VLSM and Fractional Resection
When transforming abnormal brain volumes from multiple patients into a common brain space, there is a certain amount of error that is introduced into anatomical localization. To minimize error from a VLSM analytical perspective, we included total resection volume as a covariate to avoid highlighting spurious effects in voxels that were more likely to be involved in large resections, which was similar to strategies used in prior VLSM studies.29
The analysis in Fig. 6—fractional resections of Broca’s area and VLSM area gyral components versus fluency—is used as a buttress to the results of VLSM. We collected the Fig. 6 data by manually going through the scans of each of the patients individually and determining the location of resection, overlap of resections with Broca’s area and the VLSM area, and fractional resection of their component gyri. In this way we complement the algorithmic strength of VLSM with a low-tech, manual validation of resectional anatomy.
Defining Broca’s Area and Broca’s Aphasia
There are certain studies that use a functional definition of Broca’s area, defining it as the area of the brain that elicits speech arrest without a motor response intraoperatively on direct stimulation.23 This definition of the term “Broca’s area” is common parlance for many surgeons, so it is paramount that we emphasize that we are using a purely anatomical definition of the term “Broca’s area.” In the present study, we define Broca’s area anatomically, as the pars opercularis and triangularis of the inferior frontal gyrus, coincident with Brodmann areas 44/45. We define Broca’s aphasia independent of anatomy, based strictly on standardized language testing and the definitions laid out in the WAB. Thus, although stimulation mapping is the surgical technique used to perform these surgeries with the best outcome, the definitions of Broca’s area and Broca’s aphasia used in this study are not affected by intraoperative stimulation findings.
Broca’s Area and VLSM Resections
One question asked specifically was whether the pars opercularis and triangularis, the classic seat of Broca’s area, was associated with Broca’s aphasia or deficits in fluency more generally. The answer, for this large cohort, was no. Of 75 resections in Broca’s area, only 6 patients had Broca’s aphasia (Fig. 4A). Regarding all 19 patients with Broca’s aphasia in this 298-person cohort, 13 (68%) of patients with Broca’s aphasia did not have resections of pars opercularis or triangularis. Normal language (i.e., within normal limits [34.7%]) or nonspecific anomia (37.3%) were the most common outcomes for Broca’s area resections. This does not mean that the pars opercularis and triangularis lack any role in language, or that their resection is without risk. Notably, the majority of patients with resections involving Brodmann areas 44/45 had some detectable language abnormality after surgery (Fig. 5). However, these findings are consistent with accumulating evidence dissociating the syndrome of Broca’s aphasia from an anatomical basis in Broca’s area.13,14,16,18
If Broca’s aphasia is not associated with Brodmann areas 44/45, the next question is whether it is associated with another area. VLSM demonstrated that Broca’s aphasia was associated with vSMC and supramarginal gyri, plus subcortical white matter deep to these gyri (Fig. 3). This localization of fluency has been suggested in prior work,29 yet the area is traditionally thought to represent face and vocal tract articulator sensorimotor cortex.30–32 There are several resectional studies of smaller cohorts that point away from classic Broca’s area, and similar areas spanning sensorimotor cortex have been associated with fluency deficits previously.29 Resectional studies of the inferior frontal gyrus and classic Broca’s area10,11,33 have not shown high rates of Broca’s aphasia.
Intraoperative Stimulation
Awake speech mapping was used in 265 (92%) of the cases in this cohort. Of the 19 patients whose surgery led to acute Broca’s aphasia, awake mapping was used in 17. The two done while patients were asleep were because one patient became violent when awakened and was subsequently sedated for the rest of the surgery, and the other’s preoperative language baseline precluded the ability for the necessary participation. Although stimulation results were not used as part of the analyses in this study, intraoperative stimulation is critical for safely performing surgeries in the areas discussed here. Two hundred sixty-five (92%) of the patients included in this study had awake stimulation speech mapping surgery. In the operative notes, 133 of these reported an area that elicited speech arrest from stimulation. The most common area to elicit speech arrest was in the ventral precentral gyrus, which is notably part of the VLSM area. This was followed by the pars opercularis, with less than half the frequency of ventral precentral gyrus. There have been a number of major studies of speech arrest from direct cortical stimulation that have pointed to precentral gyrus and vSMC,9,34–36 including Penfield’s original work.37
Stroke Studies
Contemporary stroke studies16 point away from classic inferior frontal gyrus and instead point more posteriorly toward subcortical white matter tracts of the superior longitudinal fasciculus (i.e., SLF III16). Although distinguishing between the specific white matter bundles is beyond the scope of the current study, it should be noted that these subcortical divisions of the superior longitudinal fasciculus run just deep to vSMC and the area highlighted by the current study.16
Much of the literature on language deficits, including those implicating white matter tract etiology, is based on the study of vascular lesions.17,20,38–40 These studies may have a vascular bias given that they are more likely to implicate areas supplied by commonly affected vasculature.41 A key advantage of examining resections is that they do not share this vascular distribution bias. A surgical cohort such as this serves to complement the stroke literature’s anatomical insights.
There have been a number of studies of neurosurgical resections in classic Broca’s area and in the inferior frontal gyrus.10,11,29,33,42 In addition, neurosurgical intraoperative stimulation studies have greatly increased our understanding of human aphasia and language systems.8,31,37,43
As eloquently laid out in a recent article in the neuroscience literature,12 there remains a pervasive misunderstanding of the role of anatomical Broca’s area in language function that is still taught as dogma to students of neuroscience around the world. The present study builds on this foundational work with quantitative metrics, and is the largest neurosurgical study to date on this topic, showing dissociation of anatomical Broca’s area from postoperative Broca’s aphasia.
Acuity
Another contrast of this study with those preceding it is that Broca’s aphasia in this study was acute rather than chronic, and showed improvement over time. The timeline of these deficits in the early postoperative period is compatible with edema affecting the relevant language centers in addition to, or in lieu of, the resection. Regarding the central finding that Broca’s aphasia was associated with lesions outside Broca’s area, this work also found that lesions directly within Broca’s area lacked association with Broca’s aphasia or deficits in fluency. Whereas the etiology of the neurological deficit may not be clear, the anatomical localization is clear. Thus, whether it is due to adjacent edema or removal of tissue, resections in the VLSM area described here were more likely to be followed by Broca’s aphasia than resections of Brodmann areas 44/45.
VLSM Resections Without Broca’s Aphasia
It should also be noted that some patients with resections in the VLSM area do not have Broca’s aphasia postoperatively. However, 2 of the 33 patients with non-Broca’s aphasia diagnoses who underwent VLSM area resections had global aphasia and 1 with a non-Broca’s aphasia diagnosis had transcortical motor aphasia, which are notable for both being nonfluent aphasias. In addition, we observed a narrowing of the spectrum of aphasia diagnoses with respect to the VLSM area versus Broca’s area. No patients with VLSM area resections had Wernicke’s or transcortical sensory aphasias—both fluent aphasias. In this way the VLSM resection cohort skewed toward deficits in fluency. Individual variation in connectivity could also be a contributing factor to the spectrum of language deficits observed here, but is beyond the scope of the current study.
Limitations
Regarding limitations to our study, most of the patients in this cohort had intrinsic gliomas, which raises the possibility of plasticity of cortical language.28 Areas harboring critical language function may be different for patients with slow-growing LGGs versus fast-growing HGGs.44,45 Figure 4 shows that when sectioning the neoplastic lesions into HGGs and LGGs, the area remains posterior to classic Broca’s area, overlapping with the vSMC and supramarginal gyrus. It is, however, more posterior in the LGG cohort, with less precentral gyrus overlap. It is not clear whether this is a function of lower numbers when analyzing these cohorts, or whether perhaps the low-grade lesions do indeed reorganize language areas to be more posterior. This deserves further analyses, and conclusions at this point would be speculative.
Conclusions
Classically described Broca’s area did not show statistically significant associations with either Broca’s aphasia or fluency of speech in this cohort. This large cohort with prospective language evaluations suggests that permanent Broca’s aphasia after neurosurgical resection is rare. Broca’s aphasia was observed in the acute postoperative setting associated with lesions to the vSMC and supramarginal gyri, suggesting that cortical fluency centers are more posteriorly localized than the classic Broca’s area.
Supplementary Material
Acknowledgments
This study was supported by National Institute of Neurological Disorders and Stroke (NINDS) grant no. 5U01NS098971-03 (to E.F.C.).
ABBREVIATIONS
- HGG
high-grade glioma
- LGG
low-grade glioma
- MNI
Montreal Neurological Institute
- QAB
Quick Aphasia Battery
- VLSM
voxel-based lesion-symptom mapping
- vSMC
ventral sensorimotor cortex
- WAB
Western Aphasia Battery
Footnotes
Disclosures
Dr. Wilson received clinical or research support for the study described (includes equipment of material) from NIDCD.
Supplemental Information
Online-Only Content
Supplemental material is available with the online version of the article.
Supplemental Figures S1 and S2. https://thejns.org/doi/suppl/10.3171/2022.6.JNS2297.
References
- 1.Dronkers NF, Plaisant O, Iba-Zizen MT, Cabanis EA. Paul Broca’s historic cases: high resolution MR imaging of the brains of Leborgne and Lelong. Brain 2007;130(Pt 5):1432–1441. [DOI] [PubMed] [Google Scholar]
- 2.Broca P Remarques sur le siège de la faculté du langage articulé, suivies d’une observation d’aphémie (perte de la parole). Bull Mem Soc Anat Paris 1861;6:330–357. [Google Scholar]
- 3.Zilles K, Amunts K. Centenary of Brodmann’s map—conception and fate. Nat Rev Neurosci 2010;11(2):139–145. [DOI] [PubMed] [Google Scholar]
- 4.Kertesz A The Western Aphasia Battery Grune & Stratton; 1982. [Google Scholar]
- 5.Kertesz A Western Aphasia Battery: Revised Pearson; 2007. [Google Scholar]
- 6.Wilson SM, Eriksson DK, Schneck SM, Lucanie JM. A quick aphasia battery for efficient, reliable, and multidimensional assessment of language function. PLoS One 2018;13(2): e0192773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penfield W, Roberts L. Speech and Brain Mechanisms Princeton University Press; 1959. [Google Scholar]
- 8.Ojemann G, Mateer C. Human language cortex: localization of memory, syntax, and sequential motor-phoneme identification systems. Science 1979;205(4413):1401–1403. [DOI] [PubMed] [Google Scholar]
- 9.Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med 2008; 358(1):18–27. [DOI] [PubMed] [Google Scholar]
- 10.Benzagmout M, Gatignol P, Duffau H. Resection of World Health Organization Grade II gliomas involving Broca’s area: methodological and functional considerations. Neurosurgery 2007;61(4):741–753. [DOI] [PubMed] [Google Scholar]
- 11.Rolston JD, Englot DJ, Benet A, Li J, Cha S, Berger MS. Frontal operculum gliomas: language outcome following resection. J Neurosurg 2015;122(4):725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandonnet E, Duffau H. Broca’s area: why was neurosurgery neglected for so long when seeking to re-establish the scientific truth? Brain 2021;144(7):e60. [DOI] [PubMed] [Google Scholar]
- 13.Mohr JP, Pessin MS, Finkelstein S, Funkenstein HH, Duncan GW, Davis KR. Broca aphasia: pathologic and clinical. Neurology 1978;28(4):311–324. [DOI] [PubMed] [Google Scholar]
- 14.Mohr JP. Broca’s area and Broca’s aphasia. In: Whitaker H, Whitaker HA, eds. Studies in Neurolinguistics Vol 1. Academic Press; 1976:110–118. [Google Scholar]
- 15.Basilakos A, Fillmore PT, Rorden C, Guo D, Bonilha L, Fridriksson J. Regional white matter damage predicts speech fluency in chronic post-stroke aphasia. Front Hum Neurosci 2014;8:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gajardo-Vidal A, Lorca-Puls DL, Team P, et al. Damage to Broca’s area does not contribute to long-term speech production outcome after stroke. Brain 2021;144(3):817–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fridriksson J, Fillmore P, Guo D, Rorden C. Chronic Broca’s aphasia is caused by damage to Broca’s and Wernicke’s areas. Cereb Cortex 2015;25(12):4689–4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fridriksson J, Guo D, Fillmore P, Holland A, Rorden C. Damage to the anterior arcuate fasciculus predicts non-fluent speech production in aphasia. Brain 2013;136(Pt 11):3451–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fridriksson J, Hubbard HI, Hudspeth SG, et al. Speech entrainment enables patients with Broca’s aphasia to produce fluent speech. Brain 2012;135(Pt 12):3815–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochfeld E, Newhart M, Molitoris J, et al. Ischemia in Broca area is associated with Broca aphasia more reliably in acute than in chronic stroke. Stroke 2010;41(2):325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandra A, Li WA, Stone CR, Geng X, Ding Y. The cerebral circulation and cerebrovascular disease I: anatomy. Brain Circ 2017;3(2):45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibo H, Carver CC, Rhoton AL Jr, Lenkey C, Mitchell RJ. Microsurgical anatomy of the middle cerebral artery. J Neurosurg 1981;54(2):151–169. [DOI] [PubMed] [Google Scholar]
- 23.Quiñones-Hinojosa A, Ojemann SG, Sanai N, Dillon WP, Berger MS. Preoperative correlation of intraoperative cortical mapping with magnetic resonance imaging landmarks to predict localization of the Broca area. J Neurosurg 2003; 99(2):311–318. [DOI] [PubMed] [Google Scholar]
- 24.Keller SS, Highley JR, Garcia-Finana M, Sluming V, Rezaie R, Roberts N. Sulcal variability, stereological measurement and asymmetry of Broca’s area on MR images. J Anat 2007; 211(4):534–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005;26(3):839–851. [DOI] [PubMed] [Google Scholar]
- 26.Bates E, Wilson SM, Saygin AP, et al. Voxel-based lesion-symptom mapping. Nat Neurosci 2003;6(5):448–450. [DOI] [PubMed] [Google Scholar]
- 27.Duffau H Lessons from brain mapping in surgery for low-grade glioma: insights into associations between tumour and brain plasticity. Lancet Neurol 2005;4(8):476–486. [DOI] [PubMed] [Google Scholar]
- 28.Southwell DG, Hervey-Jumper SL, Perry DW, Berger MS. Intraoperative mapping during repeat awake craniotomy reveals the functional plasticity of adult cortex. J Neurosurg 2016;124(5):1460–1469. [DOI] [PubMed] [Google Scholar]
- 29.Wilson SM, Lam D, Babiak MC, et al. Transient aphasias after left hemisphere resective surgery. J Neurosurg 2015; 123(3):581–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conant D, Bouchard KE, Chang EF. Speech map in the human ventral sensory-motor cortex. Curr Opin Neurobiol 2014;24(1):63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouchard KE, Mesgarani N, Johnson K, Chang EF. Functional organization of human sensorimotor cortex for speech articulation. Nature 2013;495(7441):327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chartier J, Anumanchipalli GK, Johnson K, Chang EF. Encoding of articulatory kinematic trajectories in human speech sensorimotor cortex. Neuron 2018;98(5):1042–1054.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plaza M, Gatignol P, Leroy M, Duffau H. Speaking without Broca’s area after tumor resection. Neurocase 2009;15(4): 294–310. [DOI] [PubMed] [Google Scholar]
- 34.Lu J, Zhao Z, Zhang J, et al. Functional maps of direct electrical stimulation-induced speech arrest and anomia: a multicentre retrospective study. Brain 2021;144(8):2541–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tate MC, Herbet G, Moritz-Gasser S, Tate JE, Duffau H. Probabilistic map of critical functional regions of the human cerebral cortex: Broca’s area revisited. Brain 2014;137(Pt 10):2773–2782. [DOI] [PubMed] [Google Scholar]
- 36.Duffau H, Capelle L, Denvil D, et al. The role of dominant premotor cortex in language: a study using intraoperative functional mapping in awake patients. Neuroimage 2003; 20(4):1903–1914. [DOI] [PubMed] [Google Scholar]
- 37.Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 1937;60(4):389–443. [Google Scholar]
- 38.Richardson JD, Fillmore P, Rorden C, Lapointe LL, Fridriksson J. Re-establishing Broca’s initial findings. Brain Lang 2012;123(2):125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basilakos A, Rorden C, Bonilha L, Moser D, Fridriksson J. Patterns of poststroke brain damage that predict speech production errors in apraxia of speech and aphasia dissociate. Stroke 2015;46(6):1561–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dronkers NF. A new brain region for coordinating speech articulation. Nature 1996;384(6605):159–161. [DOI] [PubMed] [Google Scholar]
- 41.Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain 2004;127(Pt 7):1479–1487. [DOI] [PubMed] [Google Scholar]
- 42.Duffau H A personal consecutive series of surgically treated 51 cases of insular WHO Grade II glioma: advances and limitations. J Neurosurg 2009;110(4):696–708. [DOI] [PubMed] [Google Scholar]
- 43.Duffau H, Capelle L, Sichez N, et al. Intraoperative mapping of the subcortical language pathways using direct stimulations. An anatomo-functional study. Brain 2002; 125(Pt 1):199–214. [DOI] [PubMed] [Google Scholar]
- 44.Desmurget M, Bonnetblanc F, Duffau H. Contrasting acute and slow-growing lesions: a new door to brain plasticity. Brain 2007;130(Pt 4):898–914. [DOI] [PubMed] [Google Scholar]
- 45.Charras P, Herbet G, Deverdun J, et al. Functional reorganization of the attentional networks in low-grade glioma patients: a longitudinal study. Cortex 2015;63:27–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


